Abstract
PURPOSE
Disturbances of emotional regulation and social difficulties are common in children and adolescents with traumatic brain injury (TBI). Recent research suggests that developments within “socio-emotional” brain systems during early adolescence and more protracted development of “cognitive control” systems have implications for emotional and behavioral regulation during adolescence. However, few functional neuroimaging studies have directly examined the interaction of these neuropsychological processes in adolescents with TBI. The current study examined how affective processing might modulate inhibitory processing in an Emotional Go/NoGo paradigm.
METHOD
The study uses a cross-sectional, age, gender, and maternal education matched design. A response inhibition paradigm (i.e., the Go/NoGo task with emotional faces) was used to examine emotional-cognition interaction in 11 adolescents with complicated mild to moderate TBI, at least 12 months post injury, and 14 typically-developing (TD) adolescents using functional magnetic resonance imaging (fMRI). Participants saw adult facial expressions of emotions (happy, sad, fearful, and angry) and were instructed to respond (“go”) on all expressions other than angry (“no-go”).
RESULTS
Preliminary results (p = 0.001 uncorrected, cluster size = 50) showed higher levels of inhibition-related activation in TD adolescents than in adolescents with TBI in several brain regions including anterior cingulate and motor/premotor regions.
CONCLUSION
These results suggest that TBI in adolescence might alter brain activation patterns and interrupt the development of brain networks governing emotion-cognition interactions.
Keywords: Childhood brain disorder, brain imaging, executive functions, head injury, emotion, adolescence
1. Introduction
Traumatic brain injury (TBI) is a leading cause of acquired disability in childhood and often results in deficits of cognition and behavior [1–3]. Estimates suggest an elevated incidence of TBI between the ages of approximately 15 and 24 [3,4]. Adolescence is a period of heightened vulnerability to negative outcomes after TBI, because many of the same brain regions that are vulnerable to TBI undergo extended and continual development throughout childhood. The prefrontal, temporal, and parietal cortices continue to increase in gray matter volumes into adolescence; similarly, white matter connections expand and increase in volume well into adolescence [5]. These changes are believed to enable the development of more efficient cognitive processing and improved executive functions during adolescence [6,7]. The more protracted development during early adolescence of “cognitive control” systems underlying executive functions may also impact development within the “socio-emotional” brain system during adolescence [1,8–10]. Frontal, temporal, and subcortical regions are particularly important for affective and social processing [11–13], while cognitive-control involves the prefrontal and inferior parietal cortices [7,8]. According to some models, (e.g., the social information processing network (SIPN)) [9], both networks undergo reorganization and functional changes during adolescence. Whereas changes to portions of the socio-emotional network (i.e., the “affective node” per SIPN model) occur fairly quickly and dramatically as a result of hormonal processes accompanying puberty, the maturation and development of the prefrontal cortex underlying cognitive-control (i.e., “cognitive-regulatory node”) are slower and occur over many years based on environmental learning and exposure. This mismatch between a highly responsive affective system and incompletely developed cognitive-regulation makes adolescents vulnerable to risky decision making and emotional and behavioral difficulties. With further development and life experience, these regions become more functionally connected, facilitating top-down processing by the prefrontal lobes for better regulatory control.
Disturbances of emotional regulation and social difficulties are quite common in children after TBI [14]. Children with TBI are often more impulsive and have less emotional awareness; they are also more likely to show reduced emotion regulation and increases in externalizing behavior problems (particularly following severe TBI) [14–18], putting them at increased risk for developing emotional disorders [9,19]. Deficits in executive functions are common following TBI as well [20], as many of the brain regions implicated in TBI mediate socio-emotional processing as well as executive functions. Furthermore, these difficulties in emotional regulation and management tend to persist and cause greater distress for children and their families than other acquired cognitive deficits. Studies have documented limited recovery of social function after TBI, and frequently outcomes worsen over time [15,21,22]. To date, however, the precise way in which TBI in adolescence impacts the development of brain networks governing cognitive control and socio-emotional functioning remains unclear.
Neuroimaging research on adolescent populations with TBI is scarce; however, recent neuroimaging studies have begun to reveal both structural [23–25] and functional differences [26–29] in individuals with TBI compared with healthy controls. Using functional magnetic resonance imaging (fMRI), Tlustos and colleagues [26] reported heightened levels of brain activation within the medial prefrontal, right dorsolateral prefrontal, and right parietal regions for adolescents with TBI compared with typically-developing controls (TD) while completing a verbal Counting Stroop task. These results suggest that TBI may alter the neural basis of cognitive control in adolescents, despite relatively comparable behavioral performance on the inhibitory task. Newsome and colleagues [27] found that adolescents with TBI showed higher levels of brain activation while completing a perspective-taking paradigm in social-cognitive regions (i.e., in left lingual gyrus, posterior cingulate cortex (PCC), cuneus and parahippocampal gyrus) compared with TD adolescents, and speculated that this was due to disrupted fronto-parietal networks from traumatic axonal injury (TAI). Hanten and colleagues [28] found that adolescents with TBI showed impaired social problem-solving on a virtual reality paradigm. Further, task performance differentially related to cortical thickness for participants with TBI compared with TD adolescents in a variety of regions within the social-cognitive network, including the orbitofrontal cortex (OFC), frontal pole, cuneus, and temporal pole. Data from these studies suggest that adolescent TBI may impact neural networks subserving socio-emotional functions. While the neural bases for cognitive control and socio-emotional processing have been studied independently, few neuroimaging studies to date have investigated their interaction in adolescence with TBI. Optimal decision making requires the timely and efficient integration of emotional and cognitive information [30,31], and this is especially challenging in the tumultuous period of adolescence [32]. Given that problems with emotional dysregulation and deficits of cognitive control are both prominent after TBI, adolescents with TBI may be particularly susceptible to responding impulsively and making poor choices in real-world social situations. This important interaction may highlight why individuals with TBI may perform within normal limits on neuropsychological tests of executive function, but demonstrate significant functional impairment in daily life.
Studies in healthy adults have shown that emotional information modulates neural activity during inhibitory tasks, recruiting regions typically activated in traditional inhibition paradigms as well as regions known to be important for socio-emotional processing. Goldstein and colleagues [33] used an emotional linguistic Go/No-Go for fMRI, with trials consisting of words intended to elicit positive or negative emotions as well as neutral words. Results showed common inhibition-related activations across emotional conditions including fronto-limbic regions (e.g., OFC and amygdala), with greater activation for the inhibition of negative emotions within medial OFC, dorsolateral prefrontal cortex (DLPFC), anterior cingulate cortex (ACC), amygdala, paralimbic cortex, and parietal cortex. Elliott and colleagues [34], in a similar Go/No-Go paradigm using words with emotional content, found that inhibition elicited activation within hippocampal gyrus, right insula, and ACC when compared to inhibition based on neutral words and highlighted the important roles these regions may play in the integration of emotional and cognitive information. In contrast to linguistic stimuli, several recent fMRI studies in adults have used more ecologically relevant stimuli such as faces expressing different kinds of emotions [31,32,35–39]. As an example, Shafritz and colleagues [31] compared response inhibition in healthy adults between different versions of Go/No-Go tasks. One version required participants to respond to all letters except one (i.e., Letters Go/NoGo), whereas another version used happy and sad faces and required participants to only respond to faces with one type of emotion (e.g., Happy Go/Sad NoGo or Sad Go/Happy NoGo). In both versions of the Go/NoGo task, inhibiting responses elicited activation in brain regions typically associated with response inhibition (i.e., DLPFC, ACC, premotor cortex, dorsal striatum, and thalamus); however, the Emotional Go/No-Go task additionally elicited activation in inferior frontal, inferior parietal, and anterior insular cortex. These authors concluded that response inhibition within an emotional context recruits a set of brain regions distinct from traditional cognitive control tasks that may be responsible for modulating emotional valence. Using an event-related fMRI Go/NoGo paradigm with fearful, happy and calm faces, Hare and colleagues [32] reported higher levels of activation in healthy adolescents (age 13–18) compared to children (age 7–12) and young adults (age 19–32) in some brain regions such as the amygdala.
The current study builds on these extant studies by examining the neural correlates of response inhibition with affective stimuli in adolescents with complicated-mild to moderate TBI [26]. A block-periodic emotional Go/No-Go task for fMRI was used. We hypothesized that adolescents with TBI would show poorer performance on the emotion Go/No-Go task and differential levels of activation within socio-emotional and cognitive control systems relative to matched TD participants.
2. Methods
2.1. Participants
A convenience sample of twenty-two adolescents between the ages of 13 and 17 years with confirmed complicated mild to severe TBI were invited to participate in this study from a pool of families that had participated in ongoing intervention studies. However, of the 4 participants with severe TBI we approached, only 3 consented to participate, and fMRI data from 1 of the 3 were not usable due to excessive motion and data from another was lost to equipment malfunction. We therefore excluded the third participant with severe TBI with usable fMRI data from the final sample to better characterize the resulting sample of participants as having complicated mild to moderate TBI. Three other participants with complicated mild TBI and one TD participant also declined participation, and 1 participant with moderate TBI was deemed ineligible. All 14 remaining participants in the TBI group who consented were at least 12-months post-injury at assessment to ensure that acute recovery was complete. Typically-developing (TD) adolescents with negative history for TBI and other neurological insult were recruited from the local community. Participants were matched as closely as possible on age, gender, and maternal education. Exclusion criteria for both groups included significant developmental delay, psychiatric or behavior disturbance, and vision or hearing impairments (all by parent reports during the phone screening). All participants came from families where English is the primary language spoken in the home and met all MRI eligibility requirements. This imaging study was approved by the local Institutional Review Board.
A total of 29 children (15 TD and 14 with complicated mild to moderate TBI) completed informed consent to participate in the study, and 25 (i.e., 86%) yielded usable fMRI data. Of the 14 participants with non-severe TBI, 3 were excluded due to excessive motion, leaving 11 with usable fMRI data. Of the 15 TD control participants, 1 was excluded due to excessive motion, leaving 14 with usable fMRI data. Comparisons on demographic data between those with and without usable fMRI data were conducted. Results indicated that those participants with TBI with usable fMRI data were not significantly different in age at the time of assessment, age at injury, or time since injury (all p > 0.13) from those without usable fMRI data; nor did they differ in terms of general cognitive ability, injury severity, or parent-report of EF skills (all p > 0.20). However, the group with usable fMRI data did self -report lower levels of EF skills than those excluded (Musable = 56.5, sd = 9.1; Mexcluded = 43.5, sd = 9.1; t[12] = 2.44, p = 0.03). The final TBI sample included nine participants with complicated-mild TBI characterized by GCS scores of 13–15 with abnormal imaging [40] and two with moderate TBI (GCS 9–12) with no abnormalities on clinical CT or MRI at the time of injury. Of the 9 participants with abnormal imaging, 3 had subarachnoid hemorrhage, 2 had epidural hematoma, 1 had extra-axial hemorrhage, 1 had subcutaneous hematoma, 1 had frontal lobe shear hemorrhage, and 1 had hemorrhagic contusion. Average time since injury was 1.8 years (sd = 0.50).
2.2. Procedure
Data were collected during a single 3-hour session. After providing consent, participants completed a brief neuropsychological battery and a neuroimaging session including four paradigms measuring aspects of executive functioning, which included the present Emotion Go/NoGo paradigm.
2.2.1. Neuropsychological measures
General cognitive ability was estimated using two measures that correlate highly with traditional intelligence tests. Single word reading skills, which have been used as a proxy for pre-injury cognitive functioning [41] were assessed using the Word Reading subtest of the Wide Range Achievement Test, Fourth Edition (WRAT-IV, 41). Receptive vocabulary was assessed using the Peabody Picture Vocabulary Test, Fourth Edition (PPVT-IV). Several aspects of executive functions believed to be vulnerable to TBI were probed; these include (1) Working Memory and Processing Speed, using the Working Memory (WMI) and Processing Speed (PSI) Indices from the Weschler Intelligence Scale for Children, Fourth Edition (WISC-IV, 42); and (2) Behavioral Manifestations of Executive Abilities, using the Behavior Rating Inventory of Executive Function (BRIEF) parent rating scales (BRIEF, 43) and self-report (BRIEF-SR, 44). The Global Executive Composite (GEC), which provides an indication of overall executive functioning in everyday environments, was used to characterize the sample. Finally, Emotional Labeling was assessed using the Diagnostic Assessment of Nonverbal Abilities (DANVA-2), Adult Faces subtest. The subtest consists of 24 photographs of adults displaying facial expressions of emotions (6 photographs for each of the 4 emotions: happy, sad, fearful, and angry). Overall emotion labeling accuracy (percent correct, collapsed across all emotion conditions) was used for a general behavioral measure of emotion recognition.
2.2.2. Measure of emotionally-mediated response inhibition using fMRI
In this Emotional Go/NoGo paradigm, 5 cycles of 3 conditions were presented in a fixed order: 1) Go blocks, where all trials elicited a “go” response, 2) Inhibit (“No-Go”) blocks, where 50% of trials elicited a “go” response and 50% elicited a “no-go” response intermixed randomly, and 3) Rest blocks where participants were instructed to passively view a row of “Xs,” which expanded and contracted in length within the block, presented in a horizontal row on the screen. To minimize the possibility that participants relied on single and/or incidental perceptual features of the faces for one category of emotion as a basis for their recognition, we modified the task developed by Shafritz and colleagues [31] to include multiple emotions in Go blocks and NoGo blocks respectively in each periodic cycle. Participants were asked to respond by button presses to faces with any emotions (i.e., happy, sad, fearful) except one (i.e., angry). Participants were not informed about block orders, nor were they informed about the trial structure within blocks. Presentation of Go blocks prior to NoGo blocks allowed for participants to develop a “go” response tendency, necessary for measuring inhibitory ability in Go/No-Go paradigms [31]. Go and NoGo blocks each consisted of ten 2-second trials. Rest blocks lasted 12 seconds each. Participants saw faces with happy, sad, fearful, or angry expressions from the Penn Emotion Recognition Test [45] presented one at a time and were instructed to withhold responding or “no-go” on angry faces but “go” on all other emotions. Stimuli were selected based on display of emotions at mild- and extreme-intensities, and to ensure ethnic variability across faces. The 3 expressions other than anger were presented roughly equally often across blocks. Anger was used for the inhibit condition because 1) research has shown that individuals with TBI may be impaired in their ability to recognize and respond to angry emotional expressions [46], and 2) the consequence of mis-identification of anger seems to be more severe than mis-identification of the other emotional expressions and warrants special attention.
Response accuracy (percent correct and errors of omission and commission) and reaction time were recorded to serve as behavioral measures of inhibitory ability. Stimulus administration and response logging were accomplished using the experimental software E-Prime [47] with magnet compatible goggles and a response system. In order to obtain an estimate of behavioral inhibitory ability while accounting for performance related to other task-dependent factors (e.g., emotion discrimination ability in go blocks), an interference-susceptibility score was calculated. For each participant, accuracy during the NoGo condition (i.e., appropriate omission of responding to angry faces plus responding to other faces) was subtracted from accuracy during the Go condition. Accuracy for the NoGo block is computed from correct responses to the 50% Go trials and correct withholding of responding to the other 50% of the NoGo trials.
2.2.3. fMRI data acquisition and analysis
Data were acquired on a 3T Phillips Achieva System MR scanner. A T1-weighted, three-dimensional MPRAGE whole-brain anatomical scan (TR/TE = 8.2/3.7 ms, FOV = 25 × 25, matrix = 250 × 250, slice thickness = 1 mm) lasting 6 minutes was acquired for the purposes of anatomical coregistration prior to the functional scans. A T2*-weighted, gradient EPI sequence was used for functional runs/scans (TR/TE = 2000/30 msec, FOV = 24 × 24 cm, matrix = 80 × 80, slice thickness = 4 mm, flip angle = 90 degree). Forty-one transverse slices were acquired at 142 time points. The total duration of this run was less than 5 minutes (284 seconds), consisting of an initial 12 second fixation period (screen display “Ready?”), followed by five cycles of the three conditions, and ended with a final 12 second period (screen display “Task complete now”). The initial four volumes (8 seconds) acquired at the beginning of the run were excluded from statistical analyses of fMRI data to accommodate for T1 relaxation effects as were data from the final “task complete” period. Data from fMRI were analyzed using SPM8 (Wellcome Department of Cognitive Neurology, London UK). The EPI images were reconstructed by built-in software on the 3T Philips Scanner. Pre-processing included realignment of functional images to the mean image to correct for head motion, normalization to the standardized space of the Montreal Neurological Institute (MNI), and spatial filtering using a 6 mm FWHM Gaussian filter. Excessive motion, targeting participants for potential exclusion from analyses, was defined as a median voxel displacement of 4.5 mm from reference for > 10% of the EPI data set [26]. Then, a general linear model (GLM) of block design (i.e., 1st level analysis) was fitted to each voxel for each subject, which used the convolution of 6 regressors (i.e., Go (20 s), NoGo (20 s), and Rest (12 s) blocks together with their respective linear interaction with time) with a canonical hemodynamic response function and 6 motion correction parameters (as nuisance covariates) as regressors. Between-Group analyses were then performed on relevant contrast images constructed from individual participants (e.g., main effect of Inhibit minus Go) in the form of random-effects GLM (i.e., 2nd level analysis). Clusters of voxels from individual t-maps meeting threshold (p = 0.001 uncorrected and cluster threshold = 50 unless otherwise stated) were identified. This level of statistical threshold was chosen to be consistent with the preliminary nature of the study [26,48]. A one-sample t-test (i.e., within-group analyses) identified the areas with significantly different levels of activation in the Inhibit and Go conditions across all participants. Then, two-sample t-tests (i.e., between-group analyses) were conducted on these contrast images to examine differential patterns of inhibition-related activation for adolescents with TBI compared to TD adolescents using the same threshold. Results were displayed on a high-resolution template anatomical MRI (i.e., MNI152) using the program xjview (http://www.alivelearn.net/xjview8). Clusters of activation were identified and visually checked for accuracy. Each cluster, together with the MNI coordinates of the pixel that showed the maximum F or t value (i.e., the maxima) is reported. MNI coordinates were later translated into Talairach coordinate, which facilitated subsequent Brodmann Areas (BA) identification.
3. Results
Fourteen TD adolescents (8 males, 6 females; Mage = 15.5 yrs) were compared with eleven adolescents with confirmed complicated-mild or moderate TBI (5 males, 6 females; Mage = 15.5 yrs). Group characteristics on demographic, cognitive, and behavioral measures are presented in Table 1. Groups were generally comparable with respect to age, sex, family income, race, and parent education. Groups were also comparable on neuropsychological measures including PPVT-4, WRAT-4, and the WISC PSI and WMI. Adolescents with TBI did have higher levels of parent-rated executive dysfunction on the BRIEF (MTBI = 55.5, sd = 13.6; MTD = 43.1, sd = 7.8; p < 0.005). However, we urge caution in interpreting this difference, given that means for both groups were within the normal range, and that this difference was obtained in the context of multiple comparisons.
Table 1.
Demographics and group characteristics on neuropsychological measures (means and s.d. given unless otherwise noted)
| Measure | Group
|
t/Chi square | p | Cohen’s d | |
|---|---|---|---|---|---|
| TBI (n = 11) | Control (n = 14) | ||||
| Age in years | 15.5 (1.3) | 15.5 (1.4) | 0 | 0.99 | |
| Male Sex (%) | 5 (45%) | 8 (57%) | 0.34 | 0.56 | 0.13 |
| Caucasian Race (%) | 8 (73%) | 12^ (92%) | 1.65 | 0.20 | 0.53 |
| Right Handedness | 11 (100%) | 10^ (77%) | 2.9 | 0.089 | 0.72 |
| Family Income† | 5.5 (4.5) | 8.3 (2.8) | 1.94 | 0.065 | 0.81 |
| Informant Education, N > High School (%) | 7 (64%) | 9 (64%) | ≪ 1 | 0.99 | |
| PPVT-4 | 101 (12) | 106 (9) | 1.36 | 0.188 | 0.57 |
| WRAT-4 Reading | 101 (18) | 114 (17) | 1.76 | 0.091 | 0.73 |
| WISC-IV PSI | 105 (18.1) | 106 (15.8) | ≪ 1 | 0.98 | |
| WISC-IV WMI | 98 (11.0) | 105 (14.9) | 1.18 | 0.251 | 0.49 |
| BRIEF Parent GEC* | 55.5 (13.6) | 43.1 (7.8) | −2.87 | 0.009 | −1.20 |
| BRIEF Self Report GEC | 56.5 (9.1) | 50.5 (10.8) | −1.46 | 0.157 | −0.61 |
| DANVA Total Errors | 5.3 (2.3) | 5.0 (2.1) | 0.31 | 0.758 | −0.13 |
| Emotional Go-No-Go | |||||
| median RT (ms) “Go” | 770 (104) | 717 (91) | −1.36 | 0.187 | −0.57 |
| median RT (ms) “No-Go” | 799 (135) | 731 (94) | −1.48 | 0.154 | −0.62 |
| % Correct “Go” | 95 (2.7) | 95 (4.8) | 0 | 0.99 | |
| % Correct “No-Go” | 79 (6.1) | 82 (5.3) | 1.22 | 0.240 | 0.51 |
| Interference Score % Correct (Go – No-Go) | 16 (6.7) | 13 (4.0) | −1.23 | 0.230 | −0.51 |
Note. PPVT-4 = Peabody Picture Vocabulary Test, 4th Edition; WRAT-4 = Wide Range Achievement Test, 4th Edition; WISC-IV = Wechsler Intelligence Scale for Children, 4th Edition; PSI = Processing Speed Index; WMI = Working Memory Index; BRIEF = Behavior Rating Inventory of Executive Functions; DANVA = Diagnostic Assessment of Nonverbal Abilities, 2nd Edition; RT = median Reaction Time for Go trials only; % Correct “No-Go” included both responding to Go trials and absence of responding to NoGo trials, which was in a 50–50 mix for a NoGo block.
Significant at p < 0.05.
Data for child’s race is missing on 1 participant and was excluded from the analysis.
Family income coded on 1–10 scale in $10,000 increments, where 1 = <$20,000 and 10=> / =$120,000.
3.1. Emotional Go/NoGo: Behavioral and fMRI results
Performance data for the Emotional Go/NoGo task were recorded during the fMRI scan. All participants showed higher accuracy for the Go blocks than the NoGo blocks. Contrary to our expectation, we did not find any significant group differences in accuracy during either condition. This, however, is consistent with the nearly identical performance levels in another behavioral test of emotion recognition, the DANVA, for both groups. Median RT for the Go and the NoGo blocks also did not differ systematically, for either group or both groups together (all p > 0.3). Median RT also was not different across groups for the Go or the Inhibit Blocks respectively. The groups were also not significantly different with regard to interference-susceptibility (i.e., 15.6% for the TBI group and 13.0% for the TD group respectively). Groups were similar with regard to omission errors, commission errors, and the patterns of commission errors for the various emotion types (e.g., 42% happy, 40% sad, and 18% fearful for the TBI group; 37% happy, 40% sad, and 23% fearful for the control group) when they were made.
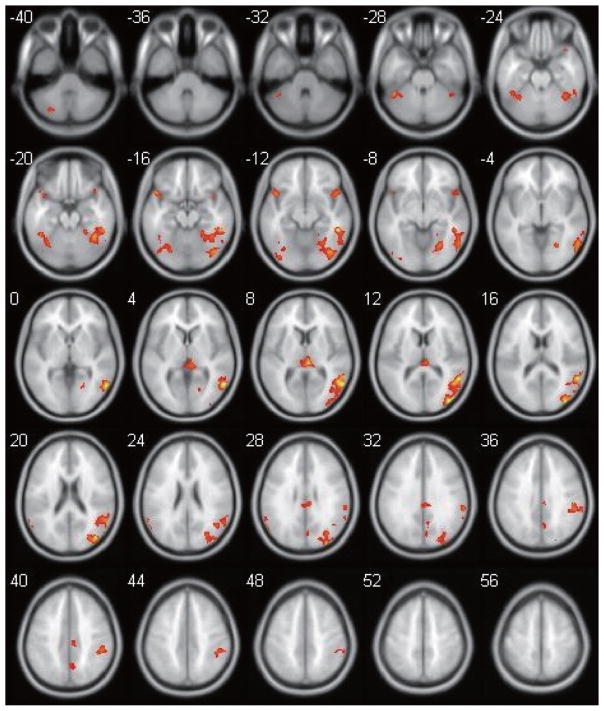
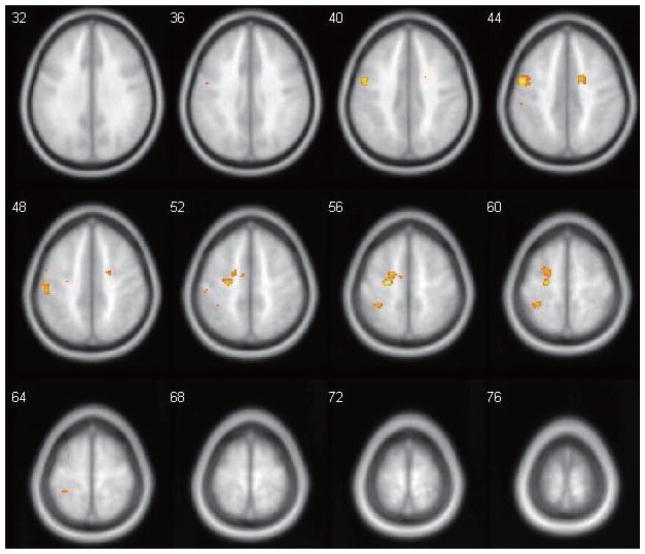
Figure 1 shows the main contrast of interest, the composite t-score map of brain regions that were significantly more active during the Inhibit condition compared to the Go condition in the entire sample of 25 participants. Inhibition-related activation (Inhibit > Go) was observed bilaterally within the cerebellum, supramarginal gyri, and inferior frontal gyri (IFG), as well as midline structures such as the thalamus and the cingulate cortex. Inhibition-related activation was also seen in fusiform gyrus, cuneus/precuneus, and other occipital/temporal areas predominantly in the right hemisphere (see Table 2). No regions met the same threshold for showing higher levels of activation in the Go condition than the Inhibit condition. Between-groups analyses revealed that there were no regions in which participants in the TBI group showed higher levels of inhibition-related activation. The participants in the TD group demonstrated greater inhibition-related activation than the TBI group in several left-hemisphere regions including the pre/post-central gyrus, precuneus, medial frontal gyrus, as well as right-hemispheric anterior (ACC, BA32) and middle sections of the cingulate cortex (BA 24). To examine whether between-group differences in the contrast between Inhibit and Go conditions were spurious and related solely to group differences in Go-related activation alone (i.e., relative to the Rest condition as baseline), the above analyses were repeated for the Go > Rest contrast. At the same thresholds, analyses revealed no regions of significant differences in the Go > Rest contrast between groups.
Fig. 1.
Brain activation map depicting the results of a one-sample t-test for the entire group of 25 participants (positive inhibition-related (No-Go > Go) activation; no negative activation survived the threshold). Images are from z = −40 to z = +56, with the following parameters: p-threshold = 0.001 uncorrected, cluster size threshold = 50. (Colours are visible in the online version of the article; http://dx.doi.org/10.3233/PRM-150350)
Table 2.
Regions showing inhibition-related activation (No-Go > Go) for all 25 participants. MNI (x,y,z) coordinates for the voxel with peak activation within each region of interest (ROI) are listed. There are no negative (No-Go < Go) activation foci that survived the same threshold
| ROI | Brodmann areas | x | y | z | Peak t |
|---|---|---|---|---|---|
| L. Cerebellum | −24 | −72 | −44 | 5.62 | |
| R. Thalamus | 6 | −28 | 10 | 6.57 | |
| R. Midline Cingulate Gyrus | 23/31 | 8 | −28 | 28 | 4.69 |
| R Fusiform gyrus/Middle Temporal Gyrus | 37/19 | 50 | −42 | −12 | 7.75 |
| R Insula/Inferior Frontal Gyrus/Superior Temporal Gyrus | 13/47/22 | 46 | 14 | −10 | 4.89 |
| L Inferior Frontal Gyrus | 47 | −44 | 14 | −14 | 6.13 |
| R. Inferior Frontal Gyrus | 9 | 54 | 10 | 18 | 3.99 |
| R. Middle/Superior Frontal Gyrus | 6 | 4 | 18 | 52 | 4.22 |
| L Supra-marginal gyrus/Superior Temporal Gyrus | 40/39 | −58 | −64 | 26 | 5.52 |
| R Supra-marginal gyrus/Inferior Parietal Lobule | 40 | 50 | −36 | 38 | 5.66 |
| R. Cuneus/Precuneus | 7 | 4 | −72 | 30 | 4.23 |
| L. Cuneus/Precuneus | 7 | −12 | −78 | 56 | 4.44 |
4. Discussion
We sought to investigate the neural correlates of emotionally-mediated response inhibition in adolescents with complicated-mild or moderate TBI compared with TD adolescents using an Emotional Go/No-Go paradigm in this preliminary study. Inhibition-related activation for all participants, shown in the Inhibit > Go analysis, included regions such as bilateral IFG, bilateral inferior and superior parietal lobes, and midline thalamus, known to be important for cognitive control [26,32,36–38], as well as other areas such as the insula known to be important for affective processing [31,37], and the right fusiform gyrus and other occipital and temporal areas known to be important for face processing [9,37]. These findings are consistent with the idea that successful performance in the Inhibit condition relative to the Go condition required greater utilization of face and affective processing as well as cognitive control. Taken together, these results are broadly consistent with previous findings in the context of an emotional Go/NoGo fMRI paradigm.
At the same time and in contrast to prior studies, many regions important for emotional processing such as the amygdala, orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), medial prefrontal cortex, and ventral striatum [31,33,34] did not show higher levels of activation in the Inhibit > Go contrast. ACC and dorsolateral prefrontal cortex (DLPFC), two regions known to be important for inhibitory processing and that have been shown to be recruited for cognitive inhibitory processing in adolescents with TBI [26], were also not activated more in the Inhibit than the Go condition in the current study. These differences may be partially attributable to the fact that the fMRI paradigm used in the current study used faces with different emotional expressions both in the Inhibit and in the Go condition: this procedure might have minimized the chance of observing differences in activation level related to affective processing given that it is involved in both the Inhibit and the Go conditions. Prior studies have compared stimuli eliciting only positive or only negative emotions of interest with neutral stimuli to highlight emotional processing regions. Hare and colleagues [32,35] also demonstrated that activity within the amygdala during completion of an Emotional Go/No Go paradigm was attenuated over time, demonstrating habituation. Thus, the lack of amygdala activation after exposure to exclusively emotional faces in the current study may further reflect habituation to emotional stimuli.
Granted that the present study has a limited sample size, adolescents with TBI showed subtle differences in neural responses on the Emotional Go/No-Go compared to TD adolescents. TD adolescents had greater activation in motor/pre-motor (BA 4, 6) and parietal (precuneus & IPL; BA 7/40) areas primarily in the left hemisphere, and two foci in the cingulate region on the right (ACC, BA 32, and middle portions of the cingulate gyrus, BA 24). Higher activation in the inhibitory node for TD adolescents than those with TBI is in contrast to recent brain imaging studies of executive functions in adolescents with TBI [26,27,49–52] that have found higher levels of activation in participants with TBI compared with controls. Increases in activation within brain regions are often thought to reflect increased processing load or “mental effort.” Many of these studies have suggested that higher activation in TBI groups may reflect “compensatory” processes. However, other studies have revealed a more complicated relationship between levels of brain activation on executive functioning tasks, which may depend on task difficulty or awareness of task performance. For example, Newsome et al. [48] found that adolescents with TBI showed varying levels of brain activation during a working memory (WM) paradigm, depending on the specific task demands, possibly reflecting a combination of compensatory processes (“over-recruitment”) and inefficiencies in processing (“hypoactivation”). Similarly, McAllister and colleagues [50] found varying levels of activation in adults within one month after mild TBI depending on WM load during an N-back task. Adults with TBI showed heightened activation relative to controls in moderate WM load conditions (2-back), but lower activation during high WM load conditions (4-back), suggesting that the task may have become too difficult for TBI participants during the high WM load condition. The authors concluded that TBI altered participants’ ability to modulate WM processes after injury.
How the aforementioned explanation might help explain the present findings, however, is less clear. Despite the limited sample size, adolescents with TBI and TD adolescents were well matched on performance on neuropsychological tasks, DANVA, and fMRI in-scanner performance, both in accuracy and in RT, for both the Go condition and the Inhibit condition. There is no clear evidence to support the idea that adolescents with TBI in our sample, relative to TD adolescents, had given up on task performance because of high task difficulty since fMRI task performance was well matched across groups. In addition, the finding of a greater Inhibit > Go contrast in the control group over a patient group is not unprecedented. Tamm and colleagues [53] used a letter Go/NoGo task and found that ADHD adolescents showed a smaller Inhibit > Go contrast than TD adolescents, in the right anterior/mid-cingulate cortex (BA 24, with local maxima at 10, 8, 40) and right supplementary/premotor areas (BA 6).
One possibility, admittedly of a post-hoc nature, is that the TBI group might be showing decreased error monitoring ability in the Emotional Go/No-Go task compared to the TD group. Specifically, adolescents with TBI might have been less aware that they made many errors in the Inhibit condition relative to TD adolescents, or that TD adolescents may be more aware than their TBI counterparts that they made errors (hence the higher activation) even though they were unable to correct those errors in time (hence the equivalent levels of performance). Studies have implicated heightened activation in the medial aspects of the frontal cortex such as the anterior cingulate, reflecting individuals’ realization that they made an error [54–56], and activation in this region has also been shown to be associated with “error-related negativities” (ERNs), a characteristic pattern observed on EEG after an individual has made an incorrect response to a task [57]. Thus, group differences observed in the current study may be related to better awareness or detection of errors by the TD participants. Clearly, performance in the Inhibit condition was far from ceiling for both groups of participants. Such an explanation would require an assumption that TD adolescents were more aware than adolescents with TBI that they were making errors in the Emotional Go/NoGo task, but they were still unable to alter their behavior to achieve better performance than the adolescents with TBI. Because participants participated in multiple fMRI paradigms in the current study, we did not specifically seek to query their memory/knowledge on how many errors they thought they made in various tasks. This explanation, therefore, awaits future confirmation and at this point remains only a speculative possibility.
An additional hypothesis for the basis of the lack of expected finding of increased activation in the TBI group is that the TBI group may be more heterogeneous in activation patterns used for successful task completion.1 If there is increased variability (hence noise) in fMRI data in participants with TBI relative to more consistent level of activation in the control group, this may serve to obscure small group differences. Because of the small sample size in the current study and the limited and comparable range of performance in the Inhibit condition for both participant groups, brain-behavior correlational analyses failed to detect brain regions whose activation level co-varied with higher or lower accuracy scores. Whether one or more of the aforementioned hypotheses are tenable await further research.
The current study has several inherent limitations. First, the small sample and low statistical power limit the generalizability of the current findings to the broader TBI population. The sample was composed primarily of adolescents with complicated-mild injuries, with GCS scores of 13–15 with abnormalities on imaging. Neuroimaging studies on executive abilities after TBI have shown an important relationship between brain activation and injury severity [58]. However, this could not be examined in the current study, as the small sample prohibits further analyses based on injury details. Further, we recruited participants on average 1.8 years post-injury in order to generalize the findings to adolescents outside of the range of acute recovery, but given the relatively mild nature of the TBI in the current study, this procedure may attenuate findings and contribute to the lack of strong associations in the current study. Moreover, although most measures of cognitive performance fell within the average range overall, there was considerable variability across participants. The effect size estimates (Cohen’s d) for some neuropsychological and behavioral measures, including estimates of overall cognitive ability, were in the “moderate” to “large” range, which suggests that statistically significant differences between groups might have emerged with a larger sample size. Additionally, the paradigm used in the current study appears to be more difficult than paradigms used in prior studies, as indicated by lower accuracy for both TD and TBI groups for the Inhibit condition (compared with 97% for sad no-go and 96% for happy no-go conditions reported in 31) than have previously been reported. Clearly, this difficulty reflects the fact that participants in our study had to recognize faces with different emotions across trials before initiating a Go response or withholding it. Moreover, as most reports documenting an effect of TBI on behavioral performance showed a significant effect of injury severity, with deficits often being minimal in the mild or even moderate group, further research is needed to determine whether the results will be the same in groups with more serious injuries. Finally, all functional neuroimaging studies are limited in their generalizability –fMRI results are truly generalizable only to those participants from any clinical groups who can successfully finish the task paradigm(s) in the scanner. Those participants with TBI in our study successfully finished imaging, had a higher BRIEF-SR score than those who did not finish imaging, and are indistinguishable from TD participants on all aspects of the Go/NoGo task, itself a measure of executive functions. All these factors serve to minimize the difference between our participant groups. With these considerations in mind, the positive finding of altered brain activation, however limited in scope they may be, do suggest that TBI in adolescence may impact the development of neural networks underlying cognitive control. We also note that the differences between groups primarily are seen in brain areas thought to be involved in cognitive control more than affective processing.
The present study is one of a few studies that investigated the interaction between socio-emotional and cognitive-control processes in adolescents with TBI. Individuals with TBI often show deficits in emotional and social cognition, as well as executive functions, which may have important implications for effective decision-making and social functioning [59,60]. Between-group differences in inhibition-related activation indicate that TD adolescents show higher levels of activation than adolescents with TBI in primarily frontal areas related to cognitive-control. These results suggest that adolescents with TBI could show subtle inefficiencies in neural processing in these regions, perhaps as the result of alterations to the fronto-parietal networks from TBI. However, the current study is preliminary, and further research is needed to understand the impact of TBI on emotional and inhibitory processing. There is still surprisingly little research investigating the neural basis for social cognition and executive functions in adolescence, despite this being a critical period for the development of these systems. Cross-sectional and longitudinal studies will be important for better understanding how disruptions to networks during this critical period of development impact both neural connectivity and the development of functional abilities. Furthermore, it will be important to understand how these differences in neural response are related to cognitive and socio-emotional outcomes after TBI in adolescence.
Fig. 2.
Between-group comparison (TD > TBI) of inhibition-related activation. Positive activation regions are where TD controls demonstrated greater activation than TBI (TD > TBI). Images are from z = +32 to z = +76, with the same threshold parameters as in Fig. 1. (Colours are visible in the online version of the article; http://dx.doi.org/10.3233/PRM-150350)
Table 3.
Regions showing higher levels of inhibition-related activation (No-Go > Go) in TD participants than in TBI participants. Other conventions as in Table 2
| ROI | Brodmann areas | x | y | z | Peak t |
|---|---|---|---|---|---|
| L Pre-central gyrus | 4 | −22 | −16 | 58 | 8.34 |
| R Pre-central gyrus | 4/5 | 50 | −10 | 52 | 4.42 |
| L Medial Frontal Gyrus | 6 | −18 | −6 | 56 | 5.06 |
| L Post-central gyrus/Inferior Parietal Lobule | 2/40 | −52 | −24 | 48 | 4.94 |
| L. Precuneus | 7 | −32 | −40 | 60 | 5.28 |
| R Cingulate Gyrus | 24 | 18 | 2 | 42 | 4.99 |
| R Anterior Cingulate Cortex | 32 | 12 | 34 | 16 | 4.00 |
Acknowledgments
This work was supported in part by 1) NIH grant RO1-MH073764 from the National Institute of Mental Health; 2) H1336050239 from the National Institute on Disability and Rehabilitation Research in the Department of Education, and 3) EMS/Trauma grant from the Ohio Department of Public Safety. The information in this manuscript is an original contribution to the literature and has not previously been published, electronically or in print. The order of the first two authors was determined by a coin toss. We wish to thank Krista Lisdahl Medina, Ph.D. and Jeff Epstein, Ph.D. for their many helpful comments on earlier drafts of this manuscript.
Footnotes
We thank an anonymous reviewer for suggesting this alternative hypothesis.
Conflict of interest
The authors have no conflicts of interest, and we adhere to the ethical guidelines of the Helsinki Declaration.
References
- 1.Casey BJ, Jones RM, Hare TA. The adolescent brain. Ann N Y Acad Sci. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Youth risk behavior surveillance: United States 2009 (Surveillance Summaries MMWR 2010; 59,No. SS-#5) [Internet] Atlanta, GA: Centers for Disease Control and Prevention; 2010. [cited 2014]. Available from: http://www.cdc.gov/HealthyYouth/yrbs/index.htm. [Google Scholar]
- 3.Langlois JA, Rutland-Brown W, Thomas KE. Traumatic brain injury in the United States: emergency department visits, hospitalization, and deaths. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2006. [cited 2014]. Available from: www.cdc.gov/ncipc/pub-res/tbi.../tbi%20in%20the%20us_jan_2006.pdf. [Google Scholar]
- 4.Bruns J, Jr, Hauser A. The epidemiology of traumatic brain injury: a review. Epilepsia. 2003;44(Suppl 20):2–10. doi: 10.1046/j.1528-1157.44.s10.3.x. [DOI] [PubMed] [Google Scholar]
- 5.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 6.Blakemore SJ. The social brain in adolescence. The social brain in adolescence. Nat Rev Neurosci. 2008;9:267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- 7.Luna B, Sweeney JA. The emergence of collaborative brain function: FMRI studies of the development of response inhibition. Ann NY Acad Sci. 2004;1021:296–309. doi: 10.1196/annals.1308.035. [DOI] [PubMed] [Google Scholar]
- 8.Blakemore SJ, Choudhury S. Development of the adolescent brain: implications for executive function and social cognition. J Child Psychol Psyc. 2006;47:296–312. doi: 10.1111/j.1469-7610.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- 9.Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol Med. 2005;35:163–174. doi: 10.1017/S0033291704003915. [DOI] [PubMed] [Google Scholar]
- 10.Steinberg L. A social neuroscience perspective on adolescent risk-taking. Dev Rev. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adolphs R. The neurobiology of social cognition. Curr Opin Neurobiol. 2001;11:231–239. doi: 10.1016/S0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- 12.Frith U, Frith C. The biological basis of social interaction. Curr Dir Psychol Sci. 2001;10:151–155. doi: 10.1111/1467-8721.00137. [DOI] [Google Scholar]
- 13.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol Psychiat. 2003;54:504–514. doi: 10.1016/S0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 14.Yeates KO, Bigler ED, Dennis M, Gerhardt CA, Rubin KH, Stancin T, et al. Social outcomes in childhood brain disorder: a heuristic integration of social neuroscience and developmental psychology. Psychol Bull. 2007;133:535–556. doi: 10.1037/0033-2909.133.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chapman LA, Wade SL, Walz NC, Taylor HG, Stancin T, Yeates KO. Clinically significant behavior problems during the initial 18 months following early childhood traumatic brain injury. Rehabil Psychol. 2010;55:48–57. doi: 10.1037/a0018418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganesalingam K, Sanson A, Anderson V, Yeates KO. Self-regulation and social and behavioral functioning following childhood traumatic brain injury. J Int Neuropsychol Soc. 2006;12:609–621. doi: 10.1017/S1355617706060796. [DOI] [PubMed] [Google Scholar]
- 17.Ganesalingam K, Yeates KO, Taylor HG, Walz NC, Stancin T, Wade S. Executive functions and social competence in young children 6 months following traumatic brain injury. Neuropsychol. 2011;25:466–476. doi: 10.1037/a0022768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warriner EM, Velikonja D. Psychiatric disturbances after traumatic brain injury: neurobehavioral and personality changes. Curr Psychiatry Rep. 2006;8:73–80. doi: 10.1007/s11920-006-0083-2. [DOI] [PubMed] [Google Scholar]
- 19.Dahl RE. Affect regulation, brain development, and behavioral/emotional health in adolescence. CNS Spectr. 2001;6:60–72. doi: 10.1017/s1092852900022884. [DOI] [PubMed] [Google Scholar]
- 20.Levin HS, Hanten G. Executive functions after traumatic brain injury in children. Pediatr Neurol. 2005;33:79–93. doi: 10.1016/j.pediatrneurol.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Yeates KO, Swift E, Taylor HG, Wade SL, Drotar D, Stancin T, Minich N. Short- and long-term social outcomes following pediatric traumatic brain injury. J Int Neuropsychol Soc. 2004;10:412–426. doi: 10.1017/S1355617704103093. doi:10.10170S1355617704103093. [DOI] [PubMed] [Google Scholar]
- 22.Bornhofen C, McDonald S. Emotion perception deficits following traumatic brain injury: a review of the evidence and rationale for intervention. J Int Neuropsychol Soc. 2008;14:511–525. doi: 10.1017/S1355617708080703. [DOI] [PubMed] [Google Scholar]
- 23.Wilde EA, Bigler ED, Haider JM, Chu A, Levin HS, Li X, et al. Vulnerability of the anterior commissure in moderate to severe pediatric traumatic brain injury. J Child Neruol. 2006;21:769–776. doi: 10.1177/08830738060210090201. [DOI] [PubMed] [Google Scholar]
- 24.Wilde EA, Bigler ED, Hunter JV, Fearing MA, Scheibel RS, Newsome MR, et al. Hippocampus, amygdala, and basal ganglia morphometrics in children after moderate-to-severe traumatic brain injury. Dev Med Child Neurol. 2007;49:294–299. doi: 10.1111/j.1469-8749.2007.00294.x. [DOI] [PubMed] [Google Scholar]
- 25.Wilde EA, Hunter JV, Newsome MR, Scheibel RS, Bigler ED, Johnson JL, et al. Frontal and temporal morphometric findings on MRI in children after moderate to severe traumatic brain injury. J Neurotraum. 2005;22:333–344. doi: 10.1089/neu.2005.22.333. [DOI] [PubMed] [Google Scholar]
- 26.Tlustos SJ, Chiu C-YP, Walz NC, Holland SK, Bernard L, Wade SL. Neural correlates of interference control in adolescents with traumatic brain injury: functional magnetic resonance imaging study of the counting stroop task. J Int Neuropsych Soc. 2011;17:181–189. doi: 10.1017/S1355617710001414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newsome MR, Scheibel RS, Hanten G, Steinberg JL, Lu H, Cook, et al. Brain activation while thinking about the self from another person’s perspective after traumatic brain injury in adolescence. Neuropsychology. 2010;24:139–147. doi: 10.1037/a0017432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanten G, Cook L, Orsten K, Chapman SB, Li X, Wilde EA, et al. Effects of traumatic brain injury on a virtual reality social problem solving task and relations to cortical thickness in adolescence. Neuropsychologia. 2011;49:486–497. doi: 10.1016/j.neuropsychologia.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newsome MR, Scheibel RS, Mayer AR, Chu ZD, Wilde EA, Hanten G, et al. How functional connectivity between emotion regulation structures can be disrupted: preliminary evidence from adolescents with moderate to severe traumatic brain injury. J Int Neuropsych Soc. 2013;19:911–924. doi: 10.1017/S1355617713000817. [DOI] [PubMed] [Google Scholar]
- 30.Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- 31.Shafritz KM, Collins SH, Blumberg HP. The interaction of emotional and cognitive neural systems in emotionally guided response inhibition. Neuroimage. 2006;31:468–475. doi: 10.1016/j.neuroimage.2005.11.053. [DOI] [PubMed] [Google Scholar]
- 32.Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiat. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldstein M, Brendel G, Tuescher O, Pan H, Epstein J, Beutel M, et al. Neural substrates of the interaction of emotional stimulus processing and motor inhibitory control: an emotional linguistic go/no-go fMRI study. Neuroimage. 2007;36:1026–1040. doi: 10.1016/j.neuroimage.2007.01.056. [DOI] [PubMed] [Google Scholar]
- 34.Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ. Selective attention to emotional stimuli in a verbal go/no-go task: an fMRI study. Brain Imaging. 2000;11:1739–1744. doi: 10.1097/00001756-200006050-00028. [DOI] [PubMed] [Google Scholar]
- 35.Hare TA, Tottenham N, Davidson MC, Glover GH, Casey BJ. Contributions of amygdala and striatal activity in emotion regulation. Biol Psychiat. 2005;57:624–632. doi: 10.1016/j.biopsych.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 36.Roberts G, Green MJ, Breakspear M, McCormack C, Frankland A, Wright A, et al. Reduced inferior frontal gyrus activation during response inhibition to emotional stimuli in youth at high risk of bipolar disorder. Biol Psychiat. 2013;74:55–61. doi: 10.1016/j.biopsych.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 37.Schulz KP, Clerkin SM, Halperin JM, Newcorn JH, Tang CY, Fan J. Dissociable neural effects of stimulus valence and preceding context during the inhibition of responses to emotional faces. Hum Brain Mapp. 2009;30:2821–2833. doi: 10.1002/hbm.20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schulz KP, Bedard A-CV, Fan J, Clerkin SM, Dima D, Newcorn JH, et al. Emotional bias of cognitive control in adults with childhood attention-deficit/hyperactivity disorder. Neuroimage: Clinical. 2014;5:1–9. doi: 10.1016/j.nicl.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wesse M, Houenou J, Paillere-Martinot M-L, Berthoz S, Artiges E, Leboyer M, et al. Frontostriatal overactivation in euthymic bipolar patients during an emotional go/nogo task. Am J Psychiat. 2007;164:638–646. doi: 10.1176/appi.ajp.164.4.638. [DOI] [PubMed] [Google Scholar]
- 40.Williams DH, Levin HS, Eisenberg HM. Mild head injury classification. Neurosurgery. 1990;27:422–428. doi: 10.1227/00006123-199009000-00014. [DOI] [PubMed] [Google Scholar]
- 41.Wilkinson GS, Robertson GJ. Wide range achievement test (WRAT-4) 4. Lutz, FL: Psychological Assessment Resources, Inc; 2006. [Google Scholar]
- 42.Wechsler D. Wechsler intelligence test for children (WISC-IV) 4. San Antonio, TX: Psychological Corporation; 2003. [Google Scholar]
- 43.Gioia GA, Isquith PK, Guy SC. Behavior rating of executive function (BRIEF) Child Neuropsychol. 2000;6:235–238. doi: 10.1076/chin.6.3.235.3152. 0929-7049/00/0603-235. [DOI] [PubMed] [Google Scholar]
- 44.Guy SC, Isquith PK, Gioia GA. BRIEF-SR: behavior rating inventory of executive function–self-report version. Lutz, FL: Psychological Assessment Resources; 2004. [Google Scholar]
- 45.Gur RC, Sara R, Hagendoorn M, Marom O, Hughett P, Macy L, et al. A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. J Neurosci Meth. 2002;115:137–143. doi: 10.1016/S0165-0270(02)00006-7. [DOI] [PubMed] [Google Scholar]
- 46.Croker V, McDonald S. Recognition of emotion from facial expression following traumatic brain injury. Brain Injury. 2005;19:787–799. doi: 10.1080/02699050500110033. [DOI] [PubMed] [Google Scholar]
- 47.E-Prime [Computer software] Sharpsburg, PA: Psychological Software Tools, Inc; [Google Scholar]
- 48.Newsome MR, Steinberg JL, Scheibel RS, Troyanskaya M, Chu Z, Hanten G, et al. Effects of traumatic brain injury on working memory-related brain activation in adolescents. Neuropsychology. 2008;22:419–425. doi: 10.1037/0894-4105.22.4.419. [DOI] [PubMed] [Google Scholar]
- 49.Kramer ME, Chiu C-YP, Walz NC, Holland SK, Yuan W, Karunanayaka P, et al. Long-term neural processing of attention following early childhood traumatic brain injury: fMRI and neurobehavioral outcomes. J Int Neuropsych Soc. 2008;14:424–435. doi: 10.1017/S1355617708080545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McAllister TW, Sparling MB, Flashman LA, Guerin SJ, Mamourian AC, Saykin AJ. Differential working memory load effects after mild traumatic brain injury. Neuroimage. 2001;14:1004–1012. doi: 10.1006/nimg.2001.0899. [DOI] [PubMed] [Google Scholar]
- 51.Sanchez-Carrion R, Fernandez-Espejo D, Junque C, Falcon C, Bargallo N, Roig T, et al. A longitudinal fMRI study of working memory in severe TBI patients with diffuse axonal injury. Neuroimage. 2008;43:421–429. doi: 10.1016/j.neuroimage.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 52.Newsome MR, Steinberg JL, Scheibel RS, Troyanskaya M, Chu Z, Hanten G, et al. Effects of traumatic brain injury on working memory-related brain activation in adolescents. Neuropsychology. 2008;22:419–425. doi: 10.1037/0894-4105.22.4.419. [DOI] [PubMed] [Google Scholar]
- 53.Tamm L, Menon V, Ringel J, Reiss AL. Event-related FMRI evidence of frontotemporal involvement in aberrant response inhibition and task switching in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2004;43:1430–1440. doi: 10.1097/01.chi.0000140452.51205.8d. [DOI] [PubMed] [Google Scholar]
- 54.Badgaiyan RD, Posner MI. Mapping the cingulate cortex in response selection and monitoring. Neuroimage. 1998;7:255–260. doi: 10.1006/nimg.1998.0326. [DOI] [PubMed] [Google Scholar]
- 55.Rubia K, Russell T, Overmeyer S, Bramer MJ, Bullmore ET, Sharma T, … Taylor E. Mapping motor inhibition: conjunctive brain activations across different versions of Go/No-Go and Stop Tasks. Neuroimage. 2001;13:250–261. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- 56.Wagner TD, Sylvester C-YC, Lacey SC, Nee DE, Franklin M, Jonides J. Common and unique components of response inhibition revealed by fMRI. Neuroimage. 2005;27:323–340. doi: 10.1016/j.neuroimage.2005.01.054. [DOI] [PubMed] [Google Scholar]
- 57.Taylor SF, Stern ER, Gehring WJ. Neural systems for error monitoring: recent findings and theoretical perspectives. Neuroscientist. 2007;13:160–172. doi: 10.1177/1073858406298184. [DOI] [PubMed] [Google Scholar]
- 58.Scheibel RS, Newsome MR, Troyanskaya M, Steinberg JL, Goldstein FC, Mao H, et al. Effects of severity of traumatic brain injury and brain reserve on cognitive-control related brain activation. J Neurotraum. 2009;26:1447–1461. doi: 10.1089/neu.2008.0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gouick J, Gentleman D. The emotional and behavioral consequences of traumatic brain injury. Trauma. 2004;6:285–292. doi: 10.1191/1460408604ta323oa. [DOI] [Google Scholar]
- 60.Green REA, Turner GR, Thompson WF. Deficits in emotion perception in adults with recent traumatic brain injury. Neuropsychologia. 2004;42:133–141. doi: 10.1016/j.neuropsychologia.2003.07.005. [DOI] [PubMed] [Google Scholar]