Abstract
Objective: The aim of this literature review is to study the effect of photodynamic antimicrobial chemotherapy (PACT) on mono- and multi-species cariogenic biofilms. Methods: To this purpose, the database, PubMed, was searched using the descriptors, photodynamic therapy, antimicrobial photodynamic chemotherapy, and photoinactivation, associated with the mandatory presence of the word biofilm. A total of 98 references published from 2003 to 2016 were selected. Moreover, literature reviews (15), investigations that did not have biofilms related to dental caries (65), and those that did not have Streptococcus mutans count as an outcome (7) were excluded, yielding a final amount of 11 publications. Results: The results revealed that Toluidine Blue O was the most used photosensitizer. Among the sources of light, light-emitting diode was the choice, and the biofilm models varied between in vitro and in situ. Multi-species biofilms were more resistant to the antimicrobial effects of PACT due to the thickness and complexity they have, which impede the penetration of the photosensitizer. This fact may also be associated with the type of photosensitizer used as well as with the light exposure time since the antimicrobial effect seems to be dose dependent. Despite this, in all the included publications, the therapy was effective in reducing S. mutans count. Conclusions: This review demonstrated that under different conditions, PACT is effective in reducing S. mutans count in monospecies biofilms. Multi-species biofilms were more resistant to the antimicrobial action of the therapy, possibly due to their thickness and complexity.
Keywords: : antimicrobial photodynamic chemotherapy, caries, photodynamic therapy, photoinactivation, Streptococcus mutans
Introduction
Dental caries is a biofilm-dependent oral disease that requires the consumption of fermentable carbohydrates as the main environmental factors involved in its appearance and progress.1,2 Nevertheless, it is frequently recognized as a multi-factorial condition, considering the disease process also involves other factors such as sugar consumption, presence of acidogenic microorganisms, differential properties of different teeth, salivary flow, and the role of fluoride, among others. Dental caries is also a result of interaction between dietary sugars and specific oral bacteria within a biofilm. These bacteria produce an acid through fermentation of carbohydrates consumed by the host, which causes the pH of the oral cavity to have a sustained decrease. Consequently, the pH of the enamel also falls, leading it to experience mineral dissolution.3
The treatments available for patients suffering from oral diseases mediated by biofilms involve mechanical removal, use of antiseptics, and antibiotic therapy. However, the increasing antibiotic resistance has led to significant research efforts to discover novel alternative antimicrobial treatments. Photodynamic antimicrobial chemotherapy (PACT), which is characterized by the association of a photosensitizer agent (PS) with a complementary wavelength of light,4,5 could be a desired approach.
PACT consists in photosensitizing microbial or cellular components, which are led to an excited state when exposed to a complementary wavelength of light. This change stimulates electrons to move out to higher energy levels, promoting the release of photons. The triplet PS has a sufficiently long lifetime to allow it to undergo chemical reaction.6 In this excited state, the photosensitizer can interact with molecular oxygen, initiating the formation of highly reactive singlet oxygen (photoprocess type II). Likewise, it can interact with other molecules acting as an electron receptor, which results in the production of hydroxyl and other organic radicals (photoprocess type I).7,8 The products of these photochemical reactions may irreversibly affect the cell metabolic activities as well as damage essential cell components, such as the cytoplasmic membrane, resulting in bacterial death.6,9
The result of the interaction between the light source and the bacterial cell depends on the wavelength of light, the potency, the irradiation time, the diameter of the probe, the light emission mode (continuous or pulsed), and if the irradiation is focused or defocused. Some bacterial factors may also affect the results, especially the ones regarding the physiological characteristics of cells, their growth stage, and density in suspension or in biofilm.10 PACT has several advantages when compared with traditional antimicrobial therapies, including, but not limited to, the ability to kill a wide range of microbes, including bacteria, yeasts, fungi, and protozoa, as well as inactivate viruses; the low chance to develop photoresistant species even after multiple treatments; the ability to design the therapy in a way that it can present selectivity for microbes over host cells and tissue; the low risk of inducing mutations; and the short time needed to kill microbial cells (min), while traditional antibiotics can take days to work. Since PSs are topically delivered, it can be effective when the blood supply is compromised, which would prevent the antibiotics from reaching the microbes. Further, PACT can be effective against biofilms that are usually more resistant to antibiotics. Last, it is inexpensive.11
Previous investigations have demonstrated the antimicrobial effect of PACT on oral bacteria in planktonic cultures,12 unorganized13 and organized biofilms.14 The effects of PACT on in vitro15 and in situ16 dentin caries lesions, when the appropriate combination of photosensitizer and light is used, have also been demonstrated. Even though a number of in vitro and in situ investigations have demonstrated the effectiveness of PACT, there is no scientific evidence that supports the clinical use of this therapy against dental biofilms. Hence, the aim of this study is to conduct a review of the literature on the action of PACT on mono and multi-species biofilms formed on dental surfaces.
Methods
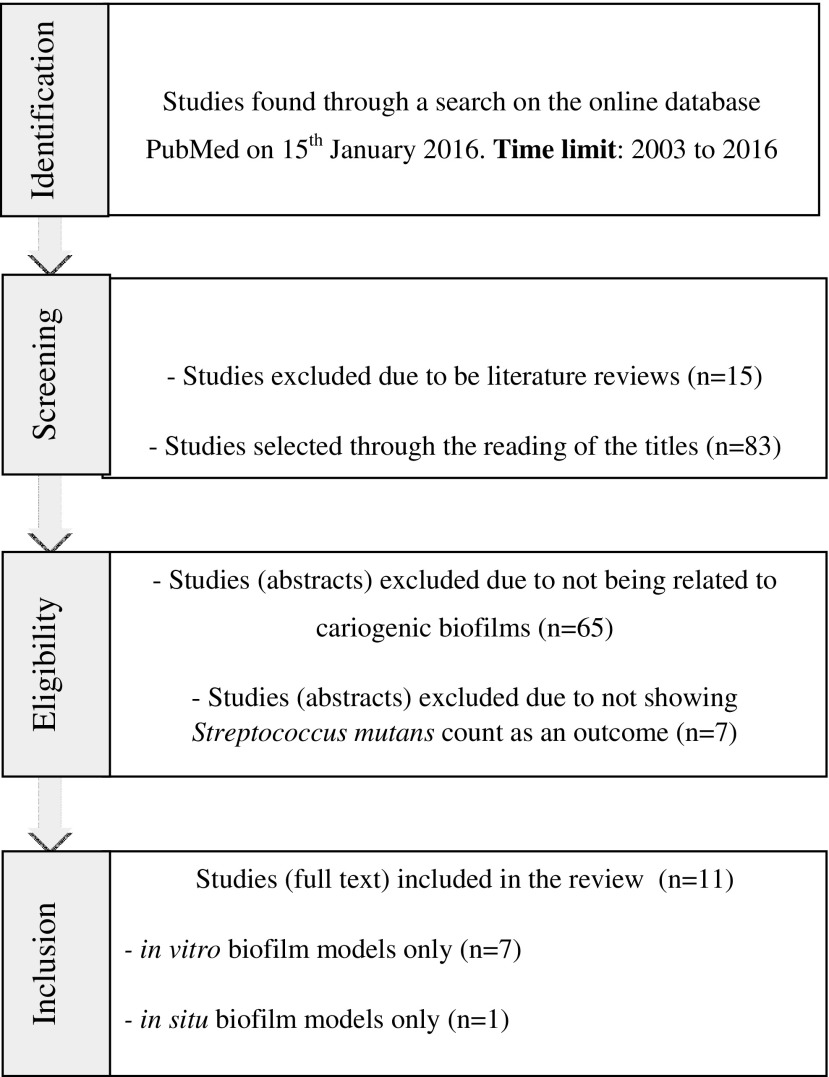
The electronic database, PubMed, was searched on January 15, 2016, to identify eligible studies using the descriptors, photodynamic therapy, antimicrobial photodynamic chemotherapy, photoinactivation, and biofilm, combined together according to the description shown in Fig. 1. The time limit used for searching the articles was 2003–2016. The use of the word biofilm was mandatory to the search. The search resulted in the selection of 98 articles that underwent an initial analysis, through which were eliminated 15 literature reviews. Then, the titles of the 83 remaining articles were read, and with this strategy, 65 studies that were not related to cariogenic biofilms were excluded, which led us to an amount of 18 articles. After reading the abstracts, only those investigations that had Streptococcus mutans count as an outcome were chosen, yielding a result of 11 items that had their full text read.
FIG. 1.
Flowchart of the search strategy of the literature review.
Results
Table 1 presents a summary of the selected data. The publications used to compose this systematic literature review showed some variables in regard to the components of PACT such as the photosensitizer, the type of light, the time of irradiation, and the model of biofilm cultivated. Apropos of the photosensitizer, Toluidine Blue O (TBO), and erythrosine were not only the most used ones, but the studies also mentioned curcumin, Photofrin®, methylene blue (MB), photodithazine, and chlorin E6. Among the types of lights, the light-emitting diode (LED) was the most frequently used, being mentioned by 5 of the 11 studies selected. The fractional lights, helium–neon laser, conventional halogen lights, the YAG laser, and the visible light plus water-filtered A (VIS+wIRA), which is a halogen light associated with an infrared water filter, were also approaches present in the elected articles. The in vitro biofilm model was the most used, followed by the in situ. Monospecies models were the most prevalent, counting 9 of the 11 references used, whereas the 2 remaining ones used multi-species models.
Table 1.
Summary of Contents on Each of the Selected Publications
| Author | Photosensitizer features (type/concentration) | Converted photosensitizer concentrationa(mg/mL) | Type and wavelength of light (nm) | Irradiation parameters | Biofilm model |
|---|---|---|---|---|---|
| Zanin et al.26 | TBO 100 mg/L | 0.1 | LED/638.8 | Power: 32 mW for both types of lights | In vitro |
| He/Ne/632.8 | Energy density: 49 J/cm2 (300 sec), 147 J/cm2 (900 sec), 294 J/cm2 (180 sec) for both types of lights | ||||
| Therapy applied at 72, 120, and 168 h | |||||
| Zanin et al.4 | TBO 0.1 mg/mL | 0.1 | LED/638.8 | Power: 32 mW | In vitro |
| Energy density: 85.7 J/cm2 (420 sec) | |||||
| Therapy applied at 72, 120, and 168 h | |||||
| Wood et al.17 | Erythrosine 1 mg/mL | 1.0 | White/500–550 | Power: 40 W | In vitro |
| Power density: 22.7 mW/cm2 (900 sec), 22.5 mW/cm2 (900 sec) | |||||
| Therapy applied at 48, 120, 168, 216, and 288 h | |||||
| MB 1 mg/mL | 1.0 | 600–650 | |||
| Photofrin® 1 mg/mL | 1.0 | ||||
| Metcalf et al.24 | Eriyhrosine 22 μgM | 1.0 | Fractional/500–550 | Power density: 22.7 mW/cm2 | In vitro |
| Energy density: 6.75 J/cm2 after 300 sec | |||||
| Continuous irradiation for 60, 120, 300, 600, 900, or 1800 sec | |||||
| Therapy applied at 48, 120, 168, 216, and 288 h | |||||
| Karygianni et al.27 | TBO 100 μg/mL | 0.1 | VIS_wIRA/570–1400 | Power density: 200 mW/cm2 (VIS 48 mW/cm2/wIRA 152 mW/cm2) | In situ |
| Chlorin e6 100 μg/mL | Irradiation time: 300 sec | ||||
| Therapy applied at 2 and 72 h | |||||
| Lee et al.25 | Eriyhrosine 1 mM | 0.017 | Halogen/400–520 | Power density: 600 mW/cm2 | In vitro |
| Irradiation time: 30 sec | |||||
| Therapy applied at 8 and 12 h | |||||
| Mang et al.18 | Photofrin 125 μg/mL | 0.025 | YAG Laser/630 | Power density: 100 mW/cm2 | In vitro |
| 0.125 | Energy density: 30 J/cm2 | ||||
| Irradiation time: 300 sec | |||||
| Therapy applied at 48 h | |||||
| Frequency: 48, 120, 168, 216, and 288 h | |||||
| Teixeira et al.14 | TBO 100 μg/mL | 0.1 | LED/638.8 | Power: 40 mW | In vitro |
| Energy density: 55 J/cm2 after 5 min | In situ | ||||
| Irradiation time: 900 sec | |||||
| Therapy applied at 120 h | |||||
| Araújo et al.5 | Curcumin | 0.75 | LED/450 | Power density: 19 mW/cm2 | In vitro |
| Energy density: 5.7 J/cm2 | In situ | ||||
| 0.15 | Irradiation time: 300 sec | ||||
| 0.003 | Therapy applied at 168 h | ||||
| 0.004 | |||||
| 0.005 | |||||
| Quishida et al.19 | Photodithazine® | 0.1 | LED/660 | Power density: 71 mW/cm2 | In vitro |
| Energy density: 37.5 J/cm2 | |||||
| 0.15 | Irradiation time: 540 min | ||||
| 0.175 | Therapy applied at 48 h | ||||
| 0.2 | |||||
| 0.25 | |||||
| Manoil et al.22 | Curcumin | 0.0184 | Halogen/360–550 | Power density: 450 mW/cm2 | In vitro |
| Energy density: not informed | |||||
| 0.0368 | Irradiation time: 120 sec | ||||
| 0.0736 | Therapy applied at 72 h | ||||
| 0.014 | |||||
| 0.022 |
The concentration units of the photosensitizers were converted to facilitate the comparison among the investigations.
LED, light-emitting diode; MB, methylene blue; TBO, Toluidine Blue O; Vis+wIRA, visible light plus water-filtered A.
Discussion
Photosensitizer
Wood et al.17 compared the photosensitizers, MB, Photofrin, and erythrosine, using a tungsten light to treat S. mutans biofilms of different formation times (48, 120, 168, 216, and 288 h). Erythrosine was significantly more effective (p < 0.01) than both Photofrin and MB for all the times studied, which might have happened because MB acts modifying the DNA of the bacterial cell and, to a lesser extent, the outer cellular membrane. S. mutans is primarily photoinactivated through membrane damage because of lipid peroxidation, which is known to be the erythrosine mechanism of action. Photofrin, a mixture of porphyrin oligomers that has been used to treat established tumors, demonstrated the lowest effectiveness among the tested photosensitizers. Photofrin was also used in another study18 at the concentrations of 0.025 and 0.125 mg/mL, having the best results at the concentration of 125 mg/mL.
Photodithazine is a second-generation photosensitizer derivate from chlorin E6 that has been considered a potent drug to be used for photodynamic therapy since it shows low toxicity and high quantum yield of singlet oxygen. Quishida et al.19 used LED and photodithazine at the concentrations of 0.1, 0.15, 0.175, 0.2, and 0.25 mg/mL to evaluate the efficacy of PACT in a multi-species biofilm of Candida albicans, Candida glabrata, and S. mutans. The results demonstrated a significant reduction of the three types of microorganisms when comparing the controls with the treatment groups using 0.175 and 0.2 mg/mL of the photosensitizer associated with an LED. Nevertheless, the highest reduction of cell viability was noticed in the group that was treated with 0.2 mg/mL of photodithazine, which exhibited a decrease of 1.21 log10, 1.19 log10, and 2.39 log10 in the colony-forming units (CFUs) of Candida albicans, C. glabrata, and S. mutans, respectively. Photodithazine, however, has been investigated to be used for photodynamic therapy to treat cancer cells, thus more investigations are necessary on its antimicrobial efficacy.20
Curcumin is a natural compound isolated from the plant, Curcuma longa, and has been used for centuries as a remedy and dietetic pigment. It has a variety of pharmacological applications such as the treatment of liver diseases, wounds, inflamed joints, as well as in blood purification, and as an antimicrobial substance. Araújo et al.5 used curcumin as a photosensitizer to evaluate the effects of the PDT on multi-species biofilms of S. mutans and Lactobacillus acidophilus on dentin caries lesions. The investigation was performed using concentrations of 0.75, 1.5, 3.0, 4.0, and 5.0 mg/mL of the photosensitizer associated with an LED light. The exposure of the biofilm to PACT was effective in all the concentrations tested, exhibiting a reduction of 100% of the number of viable cells when concentrations of 4.0 and 5.0 mg/mL were applied. The same was not observed when PACT was applied on the dentin caries lesion, a result that showed a significant reduction (69.4%) only when the highest concentration (5.0 mg/dL) was used. Possibly, the effects of PACT on oral microorganisms present in demineralized dentin are reduced due to the lower penetration depth of the photosensitizer.21
Manoil et al.22 used curcumin at concentrations of 0.0184, 0.0368, 0.0736, 0.014, and 0.022 mg/mL performing immersion times of 5 and 10 min in association with a conventional halogen light irradiation on planktonic cell cultures of S. mutans and on biofilms formed on hydroxyapatite discs. The results showed a dose-dependent reduction in the bacterial viability for both the planktonic culture (50%) and the biofilm (95.5%) when the concentration of 60 μM was used. The authors attributed these results to the fact that curcumin is poorly soluble in water-based solvents or due to the fact that the polymeric extracellular matrix inhibits the spread of the photosensitizer. Although the incubation times tested have not shown differences in the results, longer times may be required for diffusion of the photosensitizer through the extracellular matrix and through deeper layers of the biofilm. Further, the use of chelating agents such as EDTA can increase the permeability of the photosensitizer into the polysaccharide matrix.
The other studies analyzed performed the PACT using TBO and erythrosine without association with any other photosensitizer. According to Teixeira et al.,14 they used TBO because of its photophysical, chemical, and biological characteristics, such as the possibility of local application to the infected area, the antimicrobial selective toxicity, the effectiveness at low concentrations, and the diffusion capacity. Bevilacqua et al.23 assured that TBO is able to penetrate easily through the bacterial membrane because it has a transmembrane permeability coefficient greater than other photosensitizing solutions, a fact that possibly makes TBO more effective in bacterial destruction.
Teixeira et al.14 used TBO associated with an LED light to treat biofilms in vitro and in situ. The results demonstrated a significant reduction of CFU counts in biofilms grown in vitro, which was not observed in situ. According to the authors, these results can be justified because the in situ biofilms are multi-species, which result in a more complex microbiota and a lower susceptibility to the therapy.13
Metcalf et al.24 and Lee et al.25 used erythrosine as a photosensitizer in their studies and both showed significant reduction (p ≤ 0.05) in the count of viable microorganisms when PACT was applied. Other studies have reported that erythrosine has antimicrobial activity against gram-positive and gram-negative oral bacteria, as well as a well-documented ability to initiate photochemical reactions.17 Moreover, erythrosine demonstrates advantages when compared with other photosensitizers since it does not cause toxicity to the host, which explains the fact that it has been used for dental biofilm detection.17
Types of lights
In the investigations conducted by Teixeira et al.,14 Zanin et al.,4,26 Araújo et al.,5 and Quishida et al.,19 an LED light was chosen as the light source. The wavelengths of light went from 450 to 660 nm, and the spectrum of colors varied from blue to red. The option to use this type of light instead of laser was due to the physical characteristics of LED light: easy to use and has a low cost. Other important reason for choosing this kind of light is the lack of a perfect collimation and coherence of LEDs, which results in broader emission bands and provides light emission throughout the whole sensitizer absorption spectrum.14
In the study of Zanin et al.,26 two different types of lights were used, an LED light and a helium/neon laser, both associated with the photosensitizer TBO. Both types of lights were effective, and the results did not show any significant differences when they were compared. Thus, the study demonstrated that when an LED is used as light a source, the technology of PACT can be simplified and the cost of treatment can be reduced.
Wood et al.,17 Metcalf et al.,24 Manoil et al.,22 and Lee et al.25 used the conventional halogen light in their studies. The difference among them was the way that the light was applied since Metcalf et al.24 used it fractionated. Three of these four authors chose erythrosine, whereas one of them used curcumin as a photosensitizer, and they all agreed that halogen lights are not an ideal light source because of the low potency and the low energy flow.
The results of Wood et al.17 showed that the use of photodynamic therapy was effective in reducing CFU, particularly in older biofilms, which corroborates with the results of Metcalf et al.24 and Lee et al.25 However, Metcalf et al.24 observed that the therapy is more effective when the halogen lights are fractionated than when they are continuous, which can be explained because of the resupplying of oxygen molecules during dark periods.
Mang et al.18 used a YAG laser of wavelength of 630 nm that proved to be effective in association with Photofrin in biofilms of S. mutans. Karygianni et al.27 used a visible light in association with a infrared water filter (VIS+wIRA) of wavelengths of 570 and 1400 nm and an irradiation time of 5 min. This investigation found that PACT significantly decreased the viable counts of oral microorganisms during initial adhesion after application of the therapy using VIS+wIRA in the presence of TBO and chlorin E6. Technically, VIS+wIRA is a broadband heat radiation generated by a halogen light, which does not polarize light emission within the range of 570–1400 nm. The radiation goes through a water filter that absorbs or decreases harmful radiation so that no harmful infrared rays can penetrate deeply into the target tissue with a low thermal conductivity.
Biofilm models
Zanin et al.,26 Wood et al.,17 and Metcalf et al.24 used the constant-depth film fermenter as a laboratory model for the growth of S. mutans on hydroxyapatite discs. Wood et al.17 obtained a reduction of 2.2 log10 for the 48-h biofilm, while Zanin et al.4 obtained a 3.2 log10 reduction for the 72-h biofilm, both authors irradiated the discs for 15 min. These results helped us understand that the combination of TBO with an LED light demonstrated better results once Zanin et al.26 showed higher reduction ratios, even testing older biofilms.
Zanin et al.4 and Teixeira et al.14 used in vitro batch culture biofilm models. Although Araújo et al.5 have also used a similar experimental design, their results were not included in this comparison because they used biofilms with different ages when they compared the other research publications and due to the different combinations of light and photosensitizer that were tested in their experiment. Zanin et al.4 showed a reduction of 7.45 × 107 (control) to 3.75 × 106 (group submitted to PACT) in S. mutans count after 7 min of irradiation. Teixeira et al.14 showed a reduction of 3.78 × 109 (control) to 1.40 × 104 (group submitted to photodynamic therapy) after 15 min of irradiation. It could be hypothesized that the best results were found in the study of Teixeira et al.14 due to the substrate used. Teixeira et al.14 used hydroxyapatite discs, and Zanin et al.4 used bovine enamel fragments. The difference found can also be associated with longer irradiation times used by Teixeira et al.14
Araújo et al.5 and Quishida et al.19 used in vitro multi-species biofilm models. In both studies, the therapy was effective, but Araújo et al.5 obtained greater efficacy with the use of curcumin as a photosensitizer, associated with an LED light than Quishida et al.19 A reduction of 100% in the number of microorganisms was observed when the highest studied concentrations of curcumin (4.0 and 5.0 mg/mL) were applied. Quishida et al.19 also demonstrated significant reduction in S. mutans counts, showing a decrease of 2.39 log10. These data corroborate with the study of Karygianni et al.,27 which demonstrated a reduction in the bacterial count for both aerobic and anaerobic bacteria. When comparing the two photosensitizers used, there was a slight difference between chlorin E6 and TBO. The first one was able to reduce 10% of the viable bacterial cells, while TBO reduced 17%. It was also observed that the chlorin E6 was able to penetrate deeper within the biofilm than the TBO.
In contrast, with an investigation on multi-species biofilms, Teixeira et al.14 demonstrated that the therapy was able to cause only a slight reduction in the number of microorganisms. These results corroborate with Müller et al.,28 who tested different forms of antimicrobial treatments in in vitro multi-species biofilms and the therapy was not effective.
Final Considerations
The results demonstrated that PACT under different conditions is effective in reducing S. mutans count in monospecies biofilms. Additionally, they also showed that multi-species biofilms were more resistant to the antimicrobial action of the therapy, possibly due to their thickness and complexity. This outcome may also be associated with the type of photosensitizer used as well as with the time of application of the therapy. The times used might have been insufficient to remove the bacteria since the antimicrobial effects of the therapy seem to be dose dependent. The solution to this standoff is the use of alternative approaches such as a photosensitizer that are able to penetrate through the biofilm matrix or photomechanical waves to push the photosensitizer deeper into the biofilms.
Acknowledgments
This research was supported by grants of the National Council for Science and Technology Development (CNPq 487587/2012-0) and by the Ceara Foundation of Support for Scientific and Technological Development (FUNCAP BPI-0067-00049.01.00/12). The first and second authors received a scholarship from CNPq.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Paes Leme AF, Koo H, Bellato CM, Bedi G, Cury JA. The role of sucrose in cariogenic dental biofilm formation—new insight. J Dent Res 2006;85:878–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sheiham A, James WPT. Diet and dental caries: the pivotal role of free sugars reemphasized. J Dent Res 2015;94:1341–1347 [DOI] [PubMed] [Google Scholar]
- 3.Tenuta LMA, Cury JA. Laboratory and human studies to estimate anticaries efficacy of fluoride toothpastes. Monogr Oral Sci 2013;23:108–124 [DOI] [PubMed] [Google Scholar]
- 4.Zanin ICJ, Lobo MM, Rodrigues LKA, Pimenta LAF, Höfling JF, Gonçalves RB. Photosensitization of in vitro biofilms by toluidine blue O combined with a light-emitting diode. Eur J Oral Sci 2006;114:64–69 [DOI] [PubMed] [Google Scholar]
- 5.Araújo NC, Fontana CR, Bagnato VS, Gerbi MEM. Photodynamic antimicrobial therapy of curcumin in biofilms and carious dentine. Lasers Med Sci 2014;29:629–635 [DOI] [PubMed] [Google Scholar]
- 6.de Melo WCMA, Avci P, de Oliveira MN, et al. . Photodynamic inactivation of biofilm: taking a lightly colored approach to stubborn infection. Expert Rev Anti Infect Ther 2013;11:669–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacRobert AJ, Bown SG, Phillips D. What are the ideal photoproperties for a sensitizer? Ciba Found Symp 1989;146:4–12; discussion 12–16 [DOI] [PubMed] [Google Scholar]
- 8.Huang L, Huang Y-Y, Mroz P, et al. . Stable synthetic cationic bacteriochlorins as selective antimicrobial photosensitizers. Antimicrob Agents Chemother 2010;54:3834–3841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malik Z, Hanania J, Nitzan Y. Bactericidal effects of photoactivated porphyrins—an alternative approach to antimicrobial drugs. J Photochem Photobiol B 1990;5:281–293 [DOI] [PubMed] [Google Scholar]
- 10.Wilson M. Lethal photosensitisation of oral bacteria and its potential application in the photodynamic therapy of oral infections. Photochem Photobiol Sci 2004;3:412–418 [DOI] [PubMed] [Google Scholar]
- 11.Sharma SK, Dai T, Kharkwal GB, et al. . Drug discovery of antimicrobial photosensitizers using animal models. Curr Pharm Des 2011;17:1303–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paschoal MA, Santos-Pinto L, Lin M, Duarte S. Streptococcus mutans photoinactivation by combination of short exposure of a broad-spectrum visible light and low concentrations of photosensitizers. Photomed Laser Surg 2014;32:175–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fontana CR, Abernethy AD, Som S, et al. . The antibacterial effect of photodynamic therapy in dental plaque-derived biofilms. J Periodontal Res 2009;44:751–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teixeira AH, Pereira ES, Rodrigues LKA, Saxena D, Duarte S, Zanin ICJ. Effect of photodynamic antimicrobial chemotherapy on in vitro and in situ biofilms. Caries Res 2012;46:549–554 [DOI] [PubMed] [Google Scholar]
- 15.Melo MAS, de-Paula DM, Lima JPM, et al. . In vitro photodynamic antimicrobial chemotherapy in dentine contaminated by cariogenic bacteria. Laser Phys 2010;20:1504–1513 [Google Scholar]
- 16.Lima JPM, Sampaio de Melo MA, Borges FMC, et al. . Evaluation of the antimicrobial effect of photodynamic antimicrobial therapy in an in situ model of dentine caries. Eur J Oral Sci 2009;117:568–574 [DOI] [PubMed] [Google Scholar]
- 17.Wood S, Metcalf D, Devine D, Robinson C. Erythrosine is a potential photosensitizer for the photodynamic therapy of oral plaque biofilms. J Antimicrob Chemother 2006;57:680–684 [DOI] [PubMed] [Google Scholar]
- 18.Mang TS, Tayal DP, Baier R. Photodynamic therapy as an alternative treatment for disinfection of bacteria in oral biofilms. Lasers Surg Med 2012;44:588–596 [DOI] [PubMed] [Google Scholar]
- 19.Quishida CCC, Carmello JC, Mima EGDO, Bagnato VS, Machado AL, Pavarina AC. Susceptibility of multispecies biofilm to photodynamic therapy using Photodithazine®. Lasers Med Sci 2015;30:685–694 [DOI] [PubMed] [Google Scholar]
- 20.Wen LY, Bae S-M, Chun H-J, Park K-S, Ahn WS. Therapeutic effects of systemic photodynamic therapy in a leukemia animal model using A20 cells. Lasers Med Sci 2012;27:445–452 [DOI] [PubMed] [Google Scholar]
- 21.Williams JA, Pearson GJ, Colles MJ, Wilson M. The photo-activated antibacterial action of toluidine blue O in a collagen matrix and in carious dentine. Caries Res 2004;38:530–536 [DOI] [PubMed] [Google Scholar]
- 22.Manoil D, Filieri A, Gameiro C, et al. . Flow cytometric assessment of Streptococcus mutans viability after exposure to blue light-activated curcumin. Photodiagnosis Photodyn Ther 2014;11:372–379 [DOI] [PubMed] [Google Scholar]
- 23.Bevilacqua IM, Nicolau RA, Khouri S, et al. . The impact of photodynamic therapy on the viability of Streptococcus mutans in a planktonic culture. Photomed Laser Surg 2007;25:513–518 [DOI] [PubMed] [Google Scholar]
- 24.Metcalf D, Robinson C, Devine D, Wood S. Enhancement of erythrosine-mediated photodynamic therapy of Streptococcus mutans biofilms by light fractionation. J Antimicrob Chemother 2006;58:190–192 [DOI] [PubMed] [Google Scholar]
- 25.Lee Y-H, Park H-W, Lee J-H, Seo H-W, Lee S-Y. The photodynamic therapy on Streptococcus mutans biofilms using erythrosine and dental halogen curing unit. Int J Oral Sci 2012;4:196–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zanin ICJ, Gonçalves RB, Junior AB, Hope CK, Pratten J. Susceptibility of Streptococcus mutans biofilms to photodynamic therapy: an in vitro study. J Antimicrob Chemother 2005;56:324–330 [DOI] [PubMed] [Google Scholar]
- 27.Karygianni L, Ruf S, Follo M, et al. . Novel broad-spectrum antimicrobial photoinactivation of in situ oral biofilms using visible light plus water-filtered infrared-A (VIS + wIRA). Appl Environ Microbiol 2014;80:7324–7336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Müller P, Guggenheim B, Schmidlin PR. Efficacy of gasiform ozone and photodynamic therapy on a multispecies oral biofilm in vitro. Eur J Oral Sci 2007;115:77–80 [DOI] [PubMed] [Google Scholar]