Abstract
B cell functional defects are associated with delayed neutralizing antibody development in pathogenic lentivirus infections. However, the timeframe for alterations in the antibody repertoire and somatic hypermutation (SHM) remains unclear. Here, we utilized the SIV/rhesus macaque (RM) model to investigate the dynamics of immunoglobulin VH gene diversity and SHM following infection. Three RMs were infected with SIVmac239 and VH1, VH3 and VH4 genes were amplified from peripheral blood at 0, 2, 6, 24 and 36 weeks post-infection for next-generation sequencing. Analysis of over 3.8 million sequences against currently available RM germline VH genes revealed a highly biased VH gene repertoire in outbred RMs. SIV infection did not significantly perturb the predominant IgG1 response, but overall immunoglobulin SHM declined during the course of SIV infection. Moreover, SHM at the AID deamination hotspot, WRC, rapidly decreased and was suppressed throughout SIV infection. In contrast, a transient increase in mutations at the APOBEC3G deamination hotspot, CCC, coincided with a spike in APOBEC3G expression during acute SIV infection. The results outline a timetable for altered VH gene repertoire and IgG SHM in the SIV/RM model and suggest a burst of APOBEC3G-mediated antibody SHM during acute SIV infection.
INTRODUCTION
Restoring immune function in HIV-1 infected individuals requires a detailed understanding of dysfunctional components of the immune response. HIV-1 infection causes significant functional T cell defects, as exemplified by CD4+ T cell depletion and chronic CD8+ T cell activation. In addition, HIV-1 infection is associated with significant humoral immune dysfunction. Immunoglobulin (Ig) levels are elevated and this has been linked to polyclonal B cell activation (Lane et al. 1983). In addition, many B cell phenotypic perturbations have been described in HIV-infected individuals (Moir and Fauci 2009). These B cell perturbations during HIV-1 infection may in part explain why neutralizing antibody responses (NAb) develop late during infection and do not temporally correlate with control of viremia.
Antibodies are composed of IgH (heavy) and IgL (light) chains that are combinatorially assembled in the bone marrow. For IgH, this involves linking VH, DH and JH genes, whereas IgL is assembled from VL and JL genes. Successful V(D)J rearrangement results in mature, naïve cells that patrol various compartments for foreign antigens. Subsequently, naïve B cells that have encountered antigen could undergo class-switching from IgM to IgG and become memory cells to efficiently respond to antigen re-exposure. Notably, peripheral B cells from HIV+ individuals show an immature/transitional CD10+ phenotype (Malaspina et al. 2006), and CD27+ memory B cells are gradually depleted (De Milito et al. 2001; Nagase et al. 2001). These B cell perturbations may alter the Ab repertoire during HIV infection. Since specific VH genes have been associated with reactivity to certain antigens, an altered VH repertoire may differentially impact humoral immunity to a variety of pathogens and vaccines (Bekker et al. 2006; Kroon et al. 1999; Malaspina et al. 2005). Alterations in the Ab repertoire may also predispose HIV-infected individuals to opportunistic infections (Hart et al. 2007; Janoff et al. 1993; Pitzurra et al. 2003).
Upon encountering antigen, B cells rapidly proliferate and Ab genes mutate to increase their affinity to the cognate antigen through somatic hypermutation (SHM). Ig SHM is primarily driven by Activation Induced Deaminase (AID), an enzyme which deaminates deoxycytidines to deoxyuridines in Ig genes (Muramatsu et al. 2000; Revy et al. 2000). During Ig SHM, AID catalyzes C→T (forward strand) or G→A (reverse strand) mutations. This is followed by other error-prone repair processes which result in additional mutations (Peled et al. 2008). While Ig SHM primarily occurs in germinal centers (GCs) in secondary lymphoid organs, dysregulated IgG SHM, particularly in the VH3 complementarity determining regions (CDR), has been detected in peripheral blood of HIV-1-viremic individuals (Bowers et al. 2014). However, the timeframe for SHM perturbations during HIV-1 infection remains unknown.
AID belongs to a diverse family of deaminases that include APOBEC3 (Conticello et al. 2007). APOBEC3 could potently restrict retroviruses by inhibiting reverse transcription and instigating G→A mutations in retroviral reverse transcripts (Malim 2009). Interestingly, APOBEC3 improved retrovirus NAb responses in a mouse model of retrovirus infection (Santiago et al. 2008; Smith et al. 2011; Tsuji-Kawahara et al. 2010). We recently obtained evidence for a direct mechanism for this genetic link (Halemano et al. 2014). Similar to WT mice, APOBEC3-deficient mice exhibited normal levels of VH mutations at preferred sites of AID deamination, WRC (Pham et al. 2003; Rogozin and Kolchanov 1992; Zheng et al. 2005) (W=Weak base=A or T; R=puRine=A or G). However, APOBEC3-deficient mice showed significantly lower levels of TYC mutations (Y=pYrimidine=C or T) – the deamination hotspot for mouse APOBEC3 (Langlois et al. 2009; Petit et al. 2009; Yu et al. 2004) – in VH genes associated with the retrovirus-specific B cell response. These data suggest that APOBEC3 may edit Ig genes during retrovirus infection in vivo. While mice encode only one APOBEC3 gene, humans and RMs express 7 APOBEC3 proteins (A3A, A3B, A3C, A3D, A3F, A3G and A3H) that together mutate retroviral reverse transcripts in the preferred YC context (pYrimidine=C or T) (Hultquist et al. 2011; Jern et al. 2009; Schmitt et al. 2011). However, it remains unclear if primate APOBEC3 genes could edit Ig genes, and if mutations in APOBEC3 sequence hotspots are dysregulated during pathogenic lentivirus infections.
In general, HIV-1 specific NAbs neutralize prior, but not contemporary HIV-1 strains in an infected individual (Richman et al. 2003; Wei et al. 2003). Thus, the humoral immune response may not have the capacity to cope with the rapid pace of HIV-1 evolution. The slow NAb response to newly-evolved HIV-1 strains may be exacerbated by an altered Ab repertoire and impaired SHM. However, understanding Ab repertoire diversity and SHM defects may require the longitudinal tracking of Ig genes. This undertaking is logistically difficult in humans due to the rarity of samples pre- and post-infection relative to a defined transmission time point. In contrast, the timing, route and dose of infection can be controlled and samples at defined stages of the disease can be obtained in SIV-infected rhesus macaques (RMs). Importantly, the SIV/RM model recapitulates many aspects of both T and B cell dysfunction documented in HIV-1 disease (Das et al. 2011; Kuhrt et al. 2010; Titanji et al. 2010; Zhang et al. 2007). However, to date, it remains unknown how B cell dysfunction during the course of SIV infection of RMs influenced Ab repertoire diversity and SHM.
Quantifying Ab repertoire diversity and SHM rates in RMs would require tools that capture the unique RM Ig repertoire. Here, we utilized available RM VH germline sequences (Andris et al. 1997; Bible et al. 2003; Helmuth et al. 2000; Olivieri and Gambon-Deza 2015; Sundling et al. 2012) primarily mined from the RM genome sequence (Gibbs et al. 2007) and adopted a next-generation sequencing (NGS) strategy to quantify Ig SHM. We provide, to our knowledge, the first longitudinal analysis of IgG VH gene distribution and SHM rates of RMs infected with SIV using an NGS strategy. The data provides a timetable for alterations in VH gene repertoire and SHM and suggest that A3G may drive early IgG SHM in pathogenic lentivirus infections.
MATERIALS AND METHODS
Ethics statement
The RMs used in the study were obtained from certified vendors and housed in an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International-approved animal facility at the University of Kansas Medical Center (KUMC). RMs were housed in compliance with standard cage dimensions as certified by AAALAC International and with additional sources of enrichment including objects for perching and other manipulanda. RMs were given 5038 primate chow with access to water ad libitum. All RMs were handled in accordance with the recommendations in the NIH Guide for the Care and Use of Laboratory Animals through an Animal Care Use Protocol (ACUP) approved by the KUMC Institutional Animal Care and Use Committee (IACUC) with protocol number ACUP 2009-1789. The SIVmac239 stock was obtained from the NIH AIDS Reagent Program. All infections and blood collections were performed with the RMs under ketamine anesthesia using appropriate efforts to minimize suffering.
Viral stock and inoculation
SIVmac239 virus stocks were prepared in CEMx174 cells. RMs were inoculated i.v. with 104 TCID50 of virus stock. RMs 2H2 and 8G5 were 2 years 8 months old and RM 4440 was 2 years 6 months old at the time of inoculation.
Processing of blood samples
EDTA-treated blood was collected weekly for 4 weeks, then at 2-week intervals until 4 months and monthly intervals until 40 to 42 weeks, and sent overnight by courier to the University of Colorado Denver. PBMCs were obtained using Ficoll-Hypaque gradients, and cryopreserved at 106 cells/ml.
Plasma viral load
For each time point, 1 ml of plasma was pelleted using ultracentrifugation (Beckman SW55Ti, 250,000×g, 2 h) and RNA was extracted using the QIAamp viral RNA extraction kit (Qiagen). Quantitative reverse-transcriptase PCR was performed using SIV gag primers and probe as previously described (Hofmann-Lehmann et al. 2000). Standard curves were prepared using six 10-fold dilutions of SIV gag RNA of known concentration. The assay had a limit of detection of 100 copies/ml.
Quantification of RM IgG
ELISAs were performed at room temperature and with 100 μl/well volumes unless otherwise indicated. Mouse-adsorbed goat anti-human κ-light chain and goat anti-human λ-light chain (MyBiosource) were coated into Immulon-4 HBX plates (Thermo Scientific) at 100 ng/well each and incubated overnight at 4°C. Plates were blocked with 200 μl/well SuperBlock (Pierce) for 1 hr and washed once with phosphate buffered saline (PBS) with 0.05% Tween-20 (PBS-T). Serial 2-fold dilutions of purified RM IgG (NIH Nonhuman Primate Reagent Resource/NPRR) in PBS, starting at 0.2 ng/μl, were added to the plate to produce a standard curve. Serial 2-fold dilutions of plasma in PBS, starting at 1:5000, were added to the plate and incubated for 2 hrs at 37°C. After 5 washes with PBS-T, 1:6000 HRP-conjugated mouse anti-human IgG (G18-145, BD Pharmingen; this mAb cross-reacts with RM IgG as noted in the NIH NPRR) was added and incubated for 1 hr. Following 6 PBS-T washes, 100 μl/well of TMB substrate (BioFX Laboratories) was added and incubated in the dark for 10 min. The reaction was stopped with 0.3N sulfuric acid and absorbances were read at 450 nm in a Victor X5 plate reader (Perkin Elmer).
Flow cytometry
PBMCs were thawed and incubated in Fc receptor block (Innovex Biosciences) for 20 min at 4°C. Cells were resuspended in 1 ml PBS per 1×106 cells and stained with 1 μl/ml Live/Dead Aqua viability stain (Invitrogen) for 30 min at 4°C. Cells were resuspended in FACS buffer (PBS, 1% fetal bovine serum) and stained with CD3-V450 (Clone SP34-2, BD Horizon), CD4-APC-H7 (L200, BD Pharmingen), CD8-Alexa Fluor 700 (RPA-T8, BD Pharmingen), CD20-Alexa Fluor 700 (2H7, BD Pharmingen). The stained cells were analyzed in an LSR-II flow cytometer (BD Biosciences) and analyzed using the Flowjo software (Treestar).
Quantitative PCR for A3G expression
Total RNA was extracted from RM PBMCs using the RNAEasy kit (Qiagen). cDNA was synthesized using the QuantiTect reverse transcription kit (Qiagen). The cDNA was used for triplicate quantitative PCR (qPCR) reactions (25 μl) with RM A3G (rhA3G) specific primers: 5′-GCCATTTAAGCCTTGGAACA-3′ (sense), 5′-GAGCCTGGTTGCGTAGAAAG-3′, and probe: 5′-[6-FAM]AACCTTGGGTCAGTGGACAG[TAMRA]-3′. GAPDH was used as the housekeeping gene using specific primers: 5′-CCCATGTTCGTCATGGGTGT-3′ (sense), 5′-TGGTCATGAGTCCTTCCACGAT-3′ (antisense), and probe: 5′-[6-FAM]CTGCACCACCAACTGCTTAGCACCC[TAMRA]-3′. All qPCR were performed in a CFX-96 real-time PCR machine (Biorad). Briefly, 5 μl of cDNA was mixed with 12.5 μl of 1× TaqMan Gene Expression Master Mix (Applied Biosystems), 10 pmol of primers and 5 pmol of probe and subjected to 40 cycles of 15 s denaturation at 95°C and 45 s of annealing/elongation at 60.5°C and 64.5°C for rhA3G and GAPDH, respectively. Cloned cDNA sequences were used as standards for the absolute quantification. The rhA3G qPCR assay had a limit of detection of 5 copies per reaction, efficiency of 99% and R2 value of 99%.
Amplification of IgH sequences
Total RNA was extracted from RM PBMCs (106 cells) using the RNAEasy kit (Qiagen), cDNA was synthesized using the QuantiTect reverse transcription kit (Qiagen) according to the manufacturer’s instructions. Amplicons were produced using Phusion Hi-Fidelity DNA Polymerase (New England Biolabs) and 10 pmole of VH1, VH3 or VH4 forward and IgG or IgM reverse primers. Three previously described human VH1, VH3, and VH4 forward primers were found to be highly conserved in the leader regions (Tiller et al. 2008) were also highly conserved in counterpart RM VH1, VH3, and VH4 genes. These primers were modified to include Illumina MiSeq adapters and barcodes. The reverse primer was located 180 bp within the IgG constant region to include the SNPs required for determination of IgG subclass. To increase diversity in the sample, a four-nucleotide random sequence (NNNN) was added 5′ of the 6 bp barcode (or index) sequence. The primers were purchased from Eurofins MWG Operon. The sequences (5′-3′) are shown below:
-
VH1_forward:
AATGATACGGCGACCACCGAGATCTACACTCTTTCCCTACACGACGCTCTTCCGATCT NNNN INDEX AGGTGCCCACTCCCAGGTGCAG
-
VH3_forward:
AATGATACGGCGACCACCGAGATCTACACTCTTTCCCTACACGACGCTCTTCCGATCT NNNN INDEX AAGGTGTCCAGTGTGARGTGCAG
-
VH4_forward:
AATGATACGGCGACCACCGAGATCTACACTCTTTCCCTACACGACGCTCTTCCGATCT NNNN INDEX ATGGGTCCTGTCCCAGGTGCAG
-
IgHG_reverse:
CAAGCAGAAGACGGCATACGAGATGTGACTGGAGTTCAGACGTGTGCTCTTCCGAT CT NNNN INDEX GMGCCTGAGTTCCACGACACG
-
IgHM_reverse:
CAAGCAGAAGACGGCATACGAGATGTGACTGGAGTTCAGACGTGTGCTCTTCCGAT CT NNNN INDEX GGGAATTCTCACAGGAGACGA
Amplification was performed in a S1000 thermo-cycler (Biorad) using the following conditions: 98°C for 30 min, 35 cycles of 98°C for 10 s, 62°C for 15 s, and 72°C for 25 s. Amplicons were gel-extracted using the QIAquick gel extraction kit (Qiagen). Amplicons were quantified by Quant-IT PicoGreen dsDNA assay kit (Invitrogen) and mixed at equimolar amounts for paired-end 2×250 bp sequencing on the Illumina MiSeq.
Illumina sequencing and quality scores
FASTQ output files from the MiSeq were sorted into samples using 6-bp barcodes. The quality score (Q score) for each base was used to filter out low scoring segments of sequence. Using custom Perl scripts, segments of 10 bases (10-base sliding window) were tested at time. If the average Q score of the segment fell below 20, which corresponds to 1 error in 100 bases, the segment was discarded along with the preceding (reverse read) or succeeding (forward read) bases. If the length of a quality-filtered sequence was less than 100 bp, the sequence read was considered too short and also discarded. Afterwards, gene-specific primer sequences were removed from the ends of sequences called ‘Read1’ (forward). Subsequently, the filtered Read1 sequences, which include majority of the VH region, were collapsed into unique sequences while keeping track of the count numbers. The Read2 sequences were used for IgG subclass classification. Read1 sequences that occurred only once were excluded from further analysis to decrease error rates based on optimization studies (Supplementary Fig. 1). Raw NGS sequence data were deposited in the Sequence Read Archive Bioproject PRJNA267125.
Calculation of VH repertoire changes
For each RM, the percentage of each VH1, VH3 and VH4 gene relative to the total number of VH1, VH3 and VH4 counts were computed, respectively. The ‘percent change’ for each infection time point (2, 6, 24 and 36 wpi) was computed relative to the pre-infection (0 wpi) time point. For example, if the percentage of VH3.58 among the total VH3 counts at 0 wpi was 0.18%, and at 2 wpi, it was 0.40%, the calculated change was [100 × (0.40% – 0.18% divided by 0.18%) = +122% change]. Note that the change in representation could either be a negative or positive value depending on whether the VH gene was downregulated or upregulated. To evaluate overall gene repertoire perturbations within the VH1, VH3 and VH4 clades, the absolute percent change values relative to 0 wpi were obtained for each RM at 2, 6, 24 and 36 wpi. The absolute percent change from each RM served as biological replicates for each VH gene per time point. Repeated-measures ANOVA was used to evaluate if there were significant changes in the gene repertoire within the VH1, VH3 and VH4 clades (Graphpad Prism).
Mutational frequencies
Unique sequences were matched and aligned against all RM VH genes mined from the RM genome (Olivieri and Gambon-Deza 2015; Sundling et al. 2012), King’s College RM Ig Gene Database, and IMGT Database using USEARCH (Edgar 2010), then classified according to the nomenclature in Supplementary Table 1. Custom Perl scripts were used to compare each unique sequence to the aligned reference sequence in order to determine the mutation frequency and to identify mutations as AID-type or APOBEC3-type based on their dinucleotide or trinucleotide context (Supplementary Fig. 2). For some analyses, we utilized IgM sequences from each RM as the reference, similar to retroviral quasispecies comparisons that we recently described (Barrett et al. 2014). To quantify the frequencies of AID and APOBEC3-type mutations, we utilized the total number of bp analyzed as denominator. To quantify the percentage of AID and APOBEC3-type deaminations, the counts were normalized to the number of G→A and C→T mutations.
Phylogenetic analysis
Human and RM VH nucleotide sequences were aligned and unrooted phylogenetic trees were constructed using the neighbor-joining method (Saitou and Nei 1987) excluding gaps as implemented in the CLUSTAL_X interface (Thompson et al. 1997). Clades supported by >90% bootstrap after 1000 replicates were considered significant.
Statistical analysis
All analyses were performed using Prism 5.0 (GraphPad). Comparisons between two groups at 2 time points were performed using a paired 2-tailed Student’s t test. For analysis of longitudinal data with time as an independent variable, repeated measures ANOVA was performed followed by Tukey’s multiple comparison posttest. Data for each RM served as biological replicates for inferring longitudinal changes in distribution and mutation rates across well-represented VH genes. Comparisons of multiple groups were performed using Kruskall-Wallis ANOVA with Dunn’s multiple comparison posttest. P values less than 0.05 were considered significant.
RESULTS
RM VH germline reference sequences
For this longitudinal study, we focused on VH sequences since the sequence length provides more information for molecular classification. The analysis of Ig VH repertoire diversity and SHM in humans and mice are made possible by the availability of annotated germline VH sequences in the Immunogenetics (IMGT) database (http://www.imgt.org) (Lefranc et al. 2009). However, the number of functional RM germline VH sequences in the IMGT database (n=23) was disproportionately low compared to the human sequences (n=53; Supplementary Table 2). Mining of the currently available RM IgH locus (Gibbs et al. 2007) revealed 61 RM VH genes (Sundling et al. 2012), but functional VH germline sequences (n=89) from several studies (Andris et al. 1997; Bible et al. 2003; Helmuth et al. 2000) were also compiled in the King’s College RM Ig Gene Database (http://www.kcl.ac.uk/immunobiology/Mac_ig/).
Preliminary analyses of RM VH genes from these multiple sources revealed substantial sequence identities. To consolidate the currently available set of RM VH sequences, we utilized known allelic variations in human VH genes as a guide. In the IMGT human VH compilation, the maximum pairwise difference between human VH alleles averaged 3.01 ± 2.23% (mean ± SD; Supplementary Table 2). We considered RM VH gene sequences that differed by >7.5% from genome-mined sequences as distinct VH genes, as a 2× SD difference could reliably distinguish the human VH genes. This resulted in the inclusion of 7 additional VH genes (Supplementary Table 1). Phylogenetic analyses showed that these 7 VH genes were distinct from other VH genes but belonged to one of the 7 major primate VH clades, VH1 to VH7 (Fig. 1). Similar to humans, the RM VH3 clade is the most diverse with 39 genes, followed by VH4 (n=12) and VH1 (n=8).
Fig. 1.
Phylogeny of human versus RM VH germline genes. RM VH genes mined from the RM genome sequence (Sundling et al. 2012) were consolidated with sequences compiled in IMGT and the King’s College RM Ig gene databases. Database sequences that differed by >7.5% in nucleotide sequence relative to the mined germline sequences from the RM genome were considered as distinct genes (arrows and grey circles). RM VH gene names were adapted from (Sundling et al. 2012). The 7 major VH clades were conserved between human and RMs. Trees were generated by neighbor-joining method and evaluated using 1000 bootstrap replicates.
Humoral immunity during SIV infection of RMs
To investigate the dynamics of VH gene distribution and SHM during SIV infection, we infected 3 RMs with SIVmac239. Blood samples were obtained prior to and at regular intervals following SIV infection. Viral loads showed the characteristic peak in viremia before 4 weeks post-infection (wpi) and the establishment of a viral load set-point shortly after (Fig. 2A). Gradual inversion of the CD4:CD8 ratio was observed, indicating progressive CD4+ T cell depletion (Fig. 2B, upper). We observed an ~2-fold decrease in B cell percentages during acute infection in line with other studies (Das et al. 2011; Kuhrt et al. 2010; Titanji et al. 2010; Zhang et al. 2007), but these differences did not reach statistical significance, possibly due to low numbers of RMs analyzed (Fig. 2B, lower). Of note, IgG levels gradually increased (Fig. 2C), consistent with polyclonal B cell activation. However, none of the plasma samples at any time point neutralized the parental SIVmac239, consistent with previous reports (Sato et al. 2008; Yeh et al. 2010).
Fig. 2.
Profiles of SIVmac239-infected RMs. (A) Plasma viral load as measured by quantitative PCR. (B) T cell levels. The average value for SIV-infected RMs (n=3) at the indicated timeframes were computed and shown ± SEM. (C) Total plasma IgG levels were determined by ELISA. Dots correspond to means and error bars correspond to SD.
IgG1 is the predominant IgG subclass independent of SIV infection
Analogous to humans, RM IgG can be classified into 4 subclasses: IgG1, IgG2, IgG3 and IgG4 (Scinicariello et al. 2004). The relative proportion of these IgG subclasses during SIV infection remains unknown. While it is feasible to quantify IgG subclass distribution by qPCR, this would require the independent validation of each qPCR assay and necessitate absolute quantifications to obtain distribution information. We therefore pursued a paired-end NGS strategy to determine the relative transcript abundance of RM IgG subclasses (Fig. 3A). Conserved reverse primers were designed to capture single nucleotide polymorphisms in the Cγ region that would distinguish the 4 IgG subclasses. Since 5′ VH sequences were not conserved across RM VH clades, separate forward primers were designed in the conserved leader regions within VH1, VH3 and VH4 (Fig. 3A) (Sundling et al. 2012), respectively. VH1, VH3 and VH4 are the most prevalent VH clades in RMs (Margolin et al. 1997), as well as in SIV-specific antibodies (Kuwata et al. 2011; Kuwata et al. 2013) (Supplementary Fig. 3). IgG VH genes were PCR-amplified from RNA extracted from 106 unstimulated PBMCs prior to infection and at 2, 6, 24 and 36 wpi. VH sequences with stop codons (3.1%) were considered non-functional and excluded from subsequent analyses. Over 3.8 million sequence reads were analyzed (Fig. 3A). Prior to SIV infection, majority (>90%) of sequence reads were IgG1, independent of VH clade (Fig. 3B). IgG2 was lower but significantly represented (7.1%), whereas IgG3 and IgG4 each represented less than 0.3% of IgG transcript reads. This IgG subclass distribution remained consistent throughout SIV infection, although transient perturbations in IgG1/IgG2 ratios were observed during chronic SIV infection (Supplementary Fig. 4, arrows). In general, however, IgG1 dominated the VH transcript pool before and during SIV infection.
Fig. 3.
IgG subclass and VH gene distribution in RMs evaluated by NGS. (A) NGS strategy. Forward primers were positioned at leader (*) and 5′VH sequences conserved within the VH1, VH3 and VH4 clades, respectively, whereas reverse primers were positioned at Cγ regions conserved between the IgG subclasses. The 250-bp paired-end reads would not form a contig due to the length, but VH sequences can be matched to the corresponding Cγ sequence. Both reads yielded an average of 240 bp. The number of reads obtained by NGS for each VH dataset are shown. (B) IgG1 subclass distribution in uninfected RMs. Independent of VH clade, IgG1 accounted for over 90% of Cγ reads. Error bars correspond to SD. (C) Baseline VH gene distribution in uninfected RMs. VH1, VH3 and VH4 genes that accounted for majority (>60%) of read counts were shown in color. (D) Overall changes in VH gene usage. The average net change from Figure S5 were computed. Error bars correspond to SEM. Data were analyzed using repeated measures ANOVA followed by Tukey’s multiple comparison posttest. *, p<0.05.
Dynamics of VH gene distribution during SIV infection
We next evaluated the relative proportions of individual VH genes within the VH1, VH3 and VH4 clades. There was substantial bias in VH gene distribution in uninfected RM IgG1 transcripts, since a relative minority of VH genes accounted for majority of observed sequences. Remarkably, VH gene usage bias was consistent across the 3 RMs studied (Fig. 3C). Two VH1 genes (VH1.23 and VH1.16) accounted for >80% of VH1 genes, and 6 VH3 genes and 4 VH4 genes accounted for majority (>60%) of VH3 and VH4 genes, respectively. These findings demonstrate a biased IgG1 VH gene repertoire that is conserved between outbred RMs.
We next evaluated whether IgG1 VH gene distribution was altered during the course of the SIV infections. Increasing, decreasing and ‘blip’ patterns were observed in several VH genes, but these trends were not consistent (Supplementary Fig. 5). To determine if there were overall changes in VH gene distribution within the VH1, VH3 and VH4 clades, the absolute percent changes at 2, 6, 24 and 36 wpi relative to the 0 wpi time point were calculated and analyzed by repeated measures ANOVA as described in the Materials and Methods (‘Calculation of VH repertoire changes’). These analyses revealed that: (1) significant perturbations in VH3 gene distribution occurred at 2 wpi relative to later post-infection time points; (2) VH1 gene distribution was altered at 2 wpi but remained stable thereafter; and (3) VH4 gene distribution was relatively unchanged from baseline (Fig. 3D). Thus, the data showed alterations in the gene distribution of IgG1 VH3 and VH1, but not VH4, particularly during acute SIV infection. We also evaluated IgG2 VH gene usage in time points when IgG2 accounted for >25% of the total IgG transcripts (Supplementary Fig. 4, arrows). The sharp increase in VH1 IgG2 at 24 wpi was >70% due to VH1.23, the most prevalent VH1 gene. However, the sharp increase in VH3 IgG2 at 6 wpi was >90% due to a less prevalent VH3 gene, VH3.27. Thus, blips in IgG1/IgG2 subclass distribution could be due to the induction of a specific VH gene.
SIV infection decreased overall VH SHM frequencies
The identification of germline counterparts of RM VH sequences provided an opportunity to determine if SIV infection could influence overall Ig SHM. To gain an overall perspective of IgG SHM during SIV infection, we consolidated all IgG sequences regardless of subclass designation. To quantify SHM, we first removed sequence reads that were found only once in the dataset to minimize NGS errors (Supplementary Fig. 1). As we previously described for mice (Halemano et al. 2014), we ‘collapsed’ the remaining data into unique sequences per RM, since the expansion of specific B cell clones (and corresponding VH sequences) may bias the SHM frequencies obtained. SHM was quantified as the number of mutations relative to germline, divided by the number of nucleotides analyzed per VH gene × 100%. Prior to infection, the average SHM frequency per IgG VH gene was 6.43% and were not significantly different between the RMs studied (Fig. 4A).
Fig. 4.
Impaired antibody SHM during SIV infection. SHM were calculated dividing the number of mutations relative to germline to the number of bp analyzed × 100%. The analyses were limited to 41 different VH genes with sequence data in at least 2 RMs for the 5 time points. The average SHM per RM was calculated for each VH gene. (A) Baseline (or pre-infection, 0 wpi) total IgG SHM rates in uninfected RMs. Each dot corresponds to a VH sequence and lines correspond to means. Data were analyzed using a Kruskall-Wallis ANOVA followed by Dunn’s multiple comparison posttest. (B) Box-and-whiskers plot showing median and 25–75 percentiles as hinges. Error bars correspond to 10–90 percentile ranges. (C) Paired analysis of SHM in 41 VH genes at 0 and 24 wpi. Data were analyzed using a paired 2-tailed Student’s t test. (D) Comparison of SHM in the 3 major VH clades. Values correspond to mean ± SEM. Data in panels B and D were analyzed using repeated measures ANOVA followed by Tukey’s multiple comparison posttest. *, p<0.05; **, p<0.01; ***, p<0.001.
We next evaluated longitudinal SHM rates up to 36 wpi in 41 different VH genes that were well-represented in the 3 RMs. This analysis involved 1.3 million sequence reads with an average of 6,331 reads per IgG VH gene per time point (Supplementary Table 3). Of note, the average number of reads obtained per time point were not significantly different from each other (Supplementary Table 3), suggesting that the number of B cell lineages sampled were equivalent despite a modest reduction in B cell percentages during acute SIV infection (Fig. 3B). For each VH gene, the mean SHM frequency between the SIV-infected RMs at each time point was computed. In other words, data from individual RMs were used as biological replicates to obtain a single SHM value per VH gene at 0, 2, 6, 24 and 36 wpi. We then tested whether each VH gene showed a similar pattern of increased or decreased SHM during SIV infection using repeated measures ANOVA. This analysis revealed a progressive decline in IgG VH SHM (Fig. 4B). The lowest SHM levels were observed at 24 wpi (Fig. 4B), corresponding to a 22% reduction in SHM. At 24 wpi, nearly all VH genes had a decrease in SHM relative to baseline (Fig. 4C).
Decreased SHM frequencies were observed in VH4, and to a lesser extent, VH3 genes at various time points following infection (Fig. 4D). By 36 wpi, SHM frequencies returned to baseline levels (Fig. 4B), primarily due to increased SHM in VH1 genes (Fig. 4D). The data indicated that overall IgG VH SHM was impaired during the first 6 months of SIV infection.
Decreased mutations at AID deamination hotspots during SIV infection
The progressive decrease in Ab SHM during SIV infection could be due to the dysregulation of various factors in the SHM machinery. Previously, it was shown that patients with established (chronic) HIV-1 infection exhibited decreased VH3 mutations that fit the preferred AID deamination context, WRC (Bowers et al. 2014). However, it remains unknown if this phenomenon extends to SIV infection of RMs and if so, at which infection timeframe WRC mutations decline. To fill this knowledge gap, we evaluated mutations that fit the preferred deamination context for AID, WRC (Pham et al. 2003; Rogozin and Kolchanov 1992; Zheng et al. 2005). Deamination in the WRC context of either DNA strand results in 8 possible C→T or G→A mutations (Supplementary Fig. 2). To calculate WRC frequencies, the number of WRC mutations were counted, and divided by the number of base pairs analyzed × 100%. This analysis revealed that the frequency of WRC deaminations were similar between the uninfected RMs (Fig. 5A).
Fig. 5.
Mutations at the AID deamination hotspot. Mutation frequencies were calculated by dividing the number of WRC mutations to the total number of bp analyzed × 100%. The analyses were limited to 41 different VH genes with sequence data in at least 2 RMs per time point. The average WRC mutational frequencies per RM were calculated for each VH gene. (A) Baseline WRC mutation frequences in 4 uninfected RMs. Each dot corresponds to a VH sequence and lines correspond to means. Data were analyzed using Kruskall-Wallis ANOVA followed by Dunn’s multiple comparison posttest. (B) Box-and-whiskers plot (25–75th percentile) showing median WRC mutational frequencies at defined time points following SIV infection and 10–90 percentile as error bars. The data were analyzed using repeated measures ANOVA, and groups were compared using Tukey’s multiple comparison test. (C) Paired analysis of SHM in 41 VH genes at 0 and 6 wpi. Data were analyzed using a paired 2-tailed Student’s t test. (D) Comparison of WRC mutational frequencies in the 3 major VH clades. The data were analyzed using repeated measures ANOVA followed by Tukey’s multiple comparison posttest. Values correspond to mean ± SEM. *, p<0.05; **, p<0.01; ***, p<0.001.
We next evaluated the frequency of WRC mutations during the course of SIV infection. RM VH genes exhibited decreased percentages of WRC mutations beginning at 2 wpi, and this was maintained during the course of SIV infection (Fig. 5B). This was further exemplified in Fig. 5C, which showed that the majority of VH genes had reduced WRC mutation frequencies at 6 wpi. These data revealed that reduced mutational frequencies at WRC sites contributed to the decline in overall SHM rates during the course of SIV infection. Decreased WRC mutational frequencies were most pronounced in the VH3 and VH4 genes (Fig. 5D).
Temporal correlation between A3G expression and A3G-type VH mutations during acute SIV infection
Our recent study demonstrating that mouse APOBEC3 could edit Ig genes during retrovirus infection (Halemano et al. 2014) raised the possibility that the primate APOBEC3 proteins may also edit IgG genes during HIV/SIV infection. Interestingly, the APOBEC3 proteins deaminate deoxycytidines with a distinct sequence context from AID. Primate A3A, A3D, A3F and A3H preferentially mutate in the YTC context, whereas A3G proteins prefer to mutate in the CCC context (Supplementary Fig. 2) (Beale et al. 2004; Bishop et al. 2004; Harris et al. 2003; Hultquist et al. 2011; Liddament et al. 2004; Refsland et al. 2012; Schmitt et al. 2011; Schmitt et al. 2009; Wiegand et al. 2004). We therefore investigated the frequency of APOBEC3-type mutations in VH genes during the course of SIV infection. Prior to infection, RMs had similar YTC and CCC mutation frequencies (Kruskall-Wallis ANOVA, p=0.3931 and 0.2818, respectively). Mutations in the YTC context remained unchanged (Fig 6A). Surprisingly, we observed a significant increase in the frequency of CCC mutations at 2 wpi (Fig. 6B), particularly for the VH3 genes, with a 2.4-fold increase relative to baseline (Fig. 6C).
Fig. 6.
Mutations at APOBEC3-deamination hotspots. (A–C) Mutation frequencies were calculated by dividing the number of mutations at APOBEC3 deamination hotspots to the total number of bp analyzed × 100%. To facilitate longitudinal analyses, the dataset was limited to 41 different VH genes that were represented in at least 2 RMs per time point. The average APOBEC3-type mutations per RM were calculated for each VH gene. Box-and-whiskers (25–75th percentile) plot showing median (A) TYC and (B) CCC mutational frequencies at defined time points following SIV infection and Tukey error bars. (C) CCC mutations in 24 different VH3 genes. Values correspond to mean ± SEM. The data were analyzed using repeated measures ANOVA followed by Tukey’s multiple comparison posttest; *, p<0.05; **, p<0.01; ***, p<0.001. (D) A3G mRNA expression levels in PBMCs during SIV infection. A3G/GAPDH copies were normalized to 1 at the pre-infection time point per RM.
It was previously shown that RM PBMC A3G levels were transiently induced during the first 2 weeks of SIV infection (Mussil et al. 2011). Consistent with these findings, we detected a 4 to 6-fold induction of A3G mRNA levels during the first 2 weeks of SIV infection (Fig. 6D). At 6 wpi, the frequency of A3G-type mutations returned to baseline, while A3G expression levels were suppressed from 3 to 20 wpi. A3G expression increased again at 24 to 36 wpi (Fig. 6D), but VH CCC mutations did not during these time points (Figs. 6B and 6D). Thus, there was a temporal correlation between a spike in A3G expression and a burst in A3G-type CCC mutations in Ig VH genes during acute, but not chronic, SIV infection.
Transient changes in the sequence context of VH deaminations during acute SIV infection
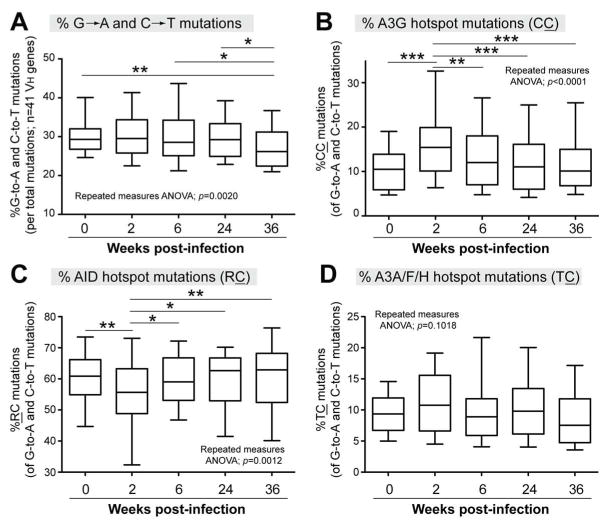
The previous analyses revealed quantitative changes in mutations at different sequence contexts, but do not provide insights on qualitative changes in deamination profiles. Of note, mutations (G→A and C→T) due to AID, A3G and other APOBEC3 members were also associated with the less stringent dinucleotide contexts RC, CC and TC, respectively (Supplementary Fig. 2). To determine if there were qualitative changes in the dinucleotide context of deamination mutations, we tallied the dinucleotide context of 576,147 G→A and C→T mutations, which accounted for ~30% of all VH mutations throughout SIV infection (Fig. 7A). As expected, over half of G→A and C→T mutations fit the AID dinucleotide hotspot RC at baseline (Fig. 7B), but CC mutations were common, accounting for an average of 11% (range: 2 to 26%) of deaminations (Fig. 7B). By 2 wpi, CC mutations significantly increased to 17% (range: 5 to 35%) of deaminations (Fig. 7B), the percentage of RC mutations significantly decreased (Fig. 7C) and TC mutations had no change (Fig. 7D). Subsequently, both CC and RC deamination profiles returned to baseline (Figs. 7B–C). No significant changes in TC mutations were observed during SIV infection (Fig. 7D). Thus, based on the relative proportions of RC versus CC mutations, acute SIV infection likely altered the deamination profile of VH genes.
Fig. 7.
Dinucleotide contexts of G→A and C→T mutations during the course of SIV infection. The box-and-whiskers plot corresponds to the median and 25–75 percentile values, with 10–90 percentiles as error bars. The data corresponds to the aggregate analysis of 41 different VH genes. (A) Percent G→A and C→T mutations, normalized to the number of mutations × 100%. The percentage of (B) CC; (C) RC and (D) TC mutations relative to the number of G→A and C→T mutations were computed. These contexts correspond to dinucleotide deamination hotspots for A3G, AID and other APOBEC3 proteins, respectively. The data were analyzed using a repeated measures ANOVA followed by Tukey’s multiple comparison posttest; *, p<0.05; **, p<0.01; ***, p<0.001.
Mutational profiles using pre-infection IgM as reference sequences
Ig SHM rates for humans, mice and RMs are routinely calculated using reference sequences from a representative genome sequence. However, with the exception of laboratory mice (which are inbred), it is theoretically possible that genomic DNA polymorphisms in VH genes may influence mutational rate calculations. We therefore investigated the impact of potential allelic polymorphisms by obtaining IgM sequences from each RM by NGS. The rationale is that IgM is induced during the primary Ab response and should not have undergone extensive SHM. In other words, IgM sequences should have higher sequence identity to the germline compared to IgG. We focused our analyses on VH3 genes as it is the most diverse and accounted for majority of VH genes in our mutational analyses in Figs. 4–7. We obtained 11,555 unique IgM VH3 sequences from the pre-infection time point, translating to an average of 168 unique IgM reference sequences per VH3 gene per RM. At the pre-infection time point, significant mutational frequencies were observed in IgM sequences, suggesting that we captured not only potential germline polymorphisms, but also memory IgM+ B cells that have undergone SHM. However, as expected, when compared against the standard RM VH gene reference database, these IgM sequences had significantly lower SHM relative to IgG sequences (Fig. 8A). We next utilized the pre-infection IgM sequences of each RM as reference sequences to evaluate IgG mutational frequencies for that particular RM. In effect, this approach would cancel any IgG mutation found in the pre-infection IgM repertoire, and would therefore provide a more conservative estimate of IgG SHM. Using this approach, AID-type WRC VH3 mutations declined (Fig. 8B), but A3G-type CCC mutations spiked at 2 wpi (Fig. 8C), similar to our results using the standard RM VH gene database (Figs. 5D and 6C, respectively). Moreover, the proportion of deamination mutations in the CC context increased whereas RC mutations decreased by 2 wpi (Fig. 8D), similar to Fig. 7B and 7C, respectively.
Fig. 8.
IgG VH3 mutational analyses using individualized IgM repertoire sequences. (A) Comparison of IgG versus IgM mutation frequencies. The average mutation frequency for each VH3 gene relative to the standard RM gene database was computed. The resulting values were compared using a 2-tailed paired Student’s t test. Mutational frequencies at (B) WRC and (C) CCC sites were evaluated using the corresponding IgM repertoire for each RM. The box-and-whiskers (25–75th percentile) plots show medians at defined time points following SIV infection and Tukey error bars. The data were analyzed using repeated measures ANOVA followed by Tukey’s multiple comparison posttest. (D) Dinucleotide contexts of G→A and C→T mutations using IgM reference sequences customized for each RM. The percentage of (left) RC and (right) CC mutations relative to the number of G→A and C→T mutations were computed. The data were analyzed using a paired 2-tailed Student’s t-test. For all statistical tests, *, p<0.05; **, p<0.01; ***, p<0.001.
DISCUSSION
HIV-1 infection is characterized by humoral immune dysfunction that is mirrored during SIV infection of RMs. However, acute HIV-1 infection studies are logistically difficult, thus highlighting the importance of the SIV-RM model in understanding early pathogenic events relevant to HIV-1 infection. Notably, HIV-1 infection is associated with altered VH3 gene usage and reduced SHM in the CDR regions compared to uninfected individuals (Bowers et al. 2014). However, the impact of SIV infection on the overall RM Ab repertoire remains unknown. Here, we utilized RM VH genes mined from published sources (Andris et al. 1997; Bible et al. 2003; Helmuth et al. 2000; Sundling et al. 2012) as well as pre-infection IgM sequences to quantify VH gene repertoire changes and mutation rates. Using the SIV-RM model, we now provide a potential timetable for SHM changes during pathogenic lentivirus infection.
Our analysis of SHM mutation rates suggested a decrease in AID-associated SHM that begins during acute SIV infection. Although the magnitude of this decrease in SHM can be interpreted as numerically modest, the biological impact of these changes may be compounded by additional B cell perturbations already documented in the SIV/RM model. We speculate on possible mechanisms for the observed decrease in AID-associated IgG SHM. Acute SIV and HIV-1 infection were associated with decreased numbers of GCs (Levesque et al. 2009; Zhang et al. 2007), the primary sites for AID activity. Thus, the lack of SHM may be a direct consequence of early GC dysfunction. SIV infection of follicular helper T cells (Folkvord et al. 2005; Petrovas et al. 2012; Xu et al. 2013), which prime GC B cells to proliferate, may potentially alter cytokine production by these cells and stall Ab SHM processes. In addition, unusually high levels of B cell activation may result in premature expansion of class-switched B cells with lower SHM levels. Although we controlled for the contribution of B cell or plasma cell expansion in this study by analyzing 41 VH genes and focusing on nonredundant IgG sequences per RM time point to capture as much VH diversity as possible, we could not exclude that B cell lineage bias may have contributed to our analyses. The decline in AID-associated IgG SHM may also be a direct effect of HIV-1/SIV on AID function. AID was up-regulated in B cells following co-incubation with HIV-1 (Perise-Barrios et al. 2012), especially if host CD40L was incorporated in virions (Imbeault et al. 2011). The consequence of HIV-1 induced AID expression on SHM remains to be determined, but AID overexpression may lead to reduced activity due to sequestration of AID into inactive cytoplasmic complexes (Hasler et al. 2012). Further studies would be required to confirm if AID function is indeed impaired during pathogenic lentivirus infections.
The results from this study enrich current models of B cell dysfunction in pathogenic lentivirus infections. It was therefore surprising that CCC or CC mutations significantly increased during acute SIV infection, despite the fact that overall SHM frequencies were on a downward trend. These CCC or CC mutations could be due to coldspot AID activity, but AID hotspot mutation frequencies (WRC or RC) actually decreased at 2 wpi. While we cannot entirely rule out that some of these mutations may be due to other APOBEC3 members, these deaminases preferentially mutate in the YTC or TC context. In contrast, A3G mutates retroviral transcripts preferably in the CCC (or CC) context (Bishop et al. 2004; Harris et al. 2003; Liddament et al. 2004; Wiegand et al. 2004; Yu et al. 2004). Moreover, there was a marked induction of A3G expression during acute SIV infection. A3G is endogenously expressed in activated B cells, and A3G may have generated CCC mutations in single-stranded Ig gene substrates following co-incubation with nuclear tonsil B cell lysates in vitro (Pham et al. 2008). Our studies with APOBEC3-deficient mice also provided evidence that mouse APOBEC3 could edit VH genes in vivo (Halemano et al. 2014). Thus, the simplest explanation for our results is that A3G directly mutated multiple IgG VH genes during acute SIV infection. Interestingly, we did not observe a correlation between A3G induction and VH-CC mutations during chronic SIV infection. One possibility is that the cell types where A3G was induced during chronic SIV infection were not B cells, but due to limited blood samples available for the various analyses, we were unable to analyze A3G levels in purified B cells at various time points. Alternatively, during chronic SIV infection, mechanisms supporting A3G-mediated SHM may have been impaired, or inhibitory mechanisms may have been induced. Moreover, it is feasible that a different mutational profile will be obtained in lymphoid compartments (Briney et al. 2014), particularly in germinal centers which are the primary sites of SHM. Studies are ongoing to evaluate how A3G function in B cells is regulated and correlate with IgG SHM patterns in lymphoid tissues of RMs with acute versus chronic SIV infection (Connick et al. 2014).
A spike in A3G-mediated SHM during acute SIV infection may provide an initial boost to diversify the Ab repertoire. We speculate that some of these A3G-mutated B cells may be used to initiate virus-specific Ab responses. Several SIV-specific rhMAbs (Kuwata et al. 2013) harbored CC mutations relative to germline (K. Guo, T. Kuwata, M. Santiago, unpublished), but it remains to be determined if these mutations emerged during acute SIV infection. It will be of interest to determine if spikes in VH-CC mutations occur in other viral infections. Finally, the transient nature of the A3G-deamination mutations suggested a tightly regulated mechanism. One potential mechanism driving A3G induction and VH-CC mutational spike is the type I Interferon (IFN) response. IFNα could regulate APOBEC3 activity in vivo (Harper et al. 2013; Mehta et al. 2012; Pillai et al. 2012) and IFNα exhibited rapid, transient expression during acute HIV-1 and SIV infection (Malleret et al. 2008; Rahmberg et al. 2013; Stacey et al. 2009). Further understanding of mechanisms that regulate A3G-mediated SHM during acute SIV infection may provide approaches to augment vaccine regimens that require eliciting antibodies with high levels of SHM, such as the highly sought-after broadly-neutralizing antibodies against HIV-1 infection (Burton et al. 2012; Klein et al. 2013).
Supplementary Material
Acknowledgments
We thank the NIH AIDS Reagent Program and the NIH Nonhuman Primate Reagent Resource for reagents. We also thank Edward Janoff, Liz Connick, Daniel Frank, Brent Palmer (UCD) and Vanessa Hirsch (NIH) for scientific insights and technical advice.
This work was supported by the National Institutes of Health [AI090795 to M.L.S., AI108391 to E.B.S.] and the University of Colorado Department of Medicine Early Career Scholar Program [to M.L.S.]. K.H. was the recipient of the Robert D. Watkins Predoctoral Fellowship from the American Society for Microbiology and M.S.H. was partially supported by an endowment from the Tim Gill Foundation to the Division of Infectious Diseases.
References
- Andris JS, Miller AB, Abraham SR, Cunningham S, Roubinet F, Blancher A, Capra JD. Variable region gene segment utilization in rhesus monkey hybridomas producing human red blood cell-specific antibodies: predominance of the VH4 family but not VH4–21 (V4-34) Mol Immunol. 1997;34:237–53. doi: 10.1016/s0161-5890(97)00021-7. [DOI] [PubMed] [Google Scholar]
- Barrett BS, Guo K, Harper MS, Li SX, Heilman KJ, Davidson NO, Santiago ML. Reassessment of murine APOBEC1 as a retrovirus restriction factor in vivo. Virology. 2014;468–470C:601–608. doi: 10.1016/j.virol.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale RC, Petersen-Mahrt SK, Watt IN, Harris RS, Rada C, Neuberger MS. Comparison of the differential context-dependence of DNA deamination by APOBEC enzymes: correlation with mutation spectra in vivo. J Mol Biol. 2004;337:585–96. doi: 10.1016/j.jmb.2004.01.046. [DOI] [PubMed] [Google Scholar]
- Bekker V, Westerlaken GH, Scherpbier H, Alders S, Zaaijer H, van Baarle D, Kuijpers T. Varicella vaccination in HIV-1-infected children after immune reconstitution. Aids. 2006;20:2321–9. doi: 10.1097/QAD.0b013e3280113f29. [DOI] [PubMed] [Google Scholar]
- Bible JM, Howard W, Robbins H, Dunn-Walters DK. IGHV1, IGHV5 and IGHV7 subgroup genes in the rhesus macaque. Immunogenetics. 2003;54:867–73. doi: 10.1007/s00251-003-0536-2. [DOI] [PubMed] [Google Scholar]
- Bishop KN, Holmes RK, Sheehy AM, Davidson NO, Cho SJ, Malim MH. Cytidine deamination of retroviral DNA by diverse APOBEC proteins. Curr Biol. 2004;14:1392–6. doi: 10.1016/j.cub.2004.06.057. [DOI] [PubMed] [Google Scholar]
- Bowers E, Scamurra RW, Asrani A, Beniguel L, MaWhinney S, Keays KM, Thurn JR, Janoff EN. Decreased mutation frequencies among immunoglobulin G variable region genes during viremic HIV-1 infection. PLoS One. 2014;9:e81913. doi: 10.1371/journal.pone.0081913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briney BS, Willis JR, Finn JA, McKinney BA, Crowe JE., Jr Tissue-specific expressed antibody variable gene repertoires. PLoS One. 2014;9:e100839. doi: 10.1371/journal.pone.0100839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton DR, Ahmed R, Barouch DH, Butera ST, Crotty S, Godzik A, Kaufmann DE, McElrath MJ, Nussenzweig MC, Pulendran B, Scanlan CN, Schief WR, Silvestri G, Streeck H, Walker BD, Walker LM, Ward AB, Wilson IA, Wyatt R. A Blueprint for HIV Vaccine Discovery. Cell Host Microbe. 2012;12:396–407. doi: 10.1016/j.chom.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connick E, Folkvord JM, Lind KT, Rakasz EG, Miles B, Wilson NA, Santiago ML, Schmitt K, Stephens EB, Kim HO, Wagstaff R, Li S, Abdelaal HM, Kemp N, Watkins DI, MaWhinney S, Skinner PJ. Compartmentalization of Simian Immunodeficiency Virus Replication within Secondary Lymphoid Tissues of Rhesus Macaques Is Linked to Disease Stage and Inversely Related to Localization of Virus-Specific CTL. J Immunol. 2014 doi: 10.4049/jimmunol.1401161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conticello SG, Langlois MA, Yang Z, Neuberger MS. DNA deamination in immunity: AID in the context of its APOBEC relatives. Adv Immunol. 2007;94:37–73. doi: 10.1016/S0065-2776(06)94002-4. [DOI] [PubMed] [Google Scholar]
- Das A, Veazey RS, Wang X, Lackner AA, Xu H, Pahar B. Simian immunodeficiency virus infection in rhesus macaques induces selective tissue specific B cell defects in double positive CD21+CD27+ memory B cells. Clin Immunol. 2011;140:223–8. doi: 10.1016/j.clim.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Milito A, Morch C, Sonnerborg A, Chiodi F. Loss of memory (CD27) B lymphocytes in HIV-1 infection. Aids. 2001;15:957–64. doi: 10.1097/00002030-200105250-00003. [DOI] [PubMed] [Google Scholar]
- Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–1. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- Folkvord JM, Armon C, Connick E. Lymphoid follicles are sites of heightened human immunodeficiency virus type 1 (HIV-1) replication and reduced antiretroviral effector mechanisms. AIDS Res Hum Retroviruses. 2005;21:363–70. doi: 10.1089/aid.2005.21.363. [DOI] [PubMed] [Google Scholar]
- Gibbs RA, Rogers J, Katze MG, Bumgarner R, Weinstock GM, Mardis ER, Remington KA, Strausberg RL, Venter JC, Wilson RK, Batzer MA, Bustamante CD, Eichler EE, Hahn MW, Hardison RC, Makova KD, Miller W, Milosavljevic A, Palermo RE, Siepel A, Sikela JM, Attaway T, Bell S, Bernard KE, Buhay CJ, Chandrabose MN, Dao M, Davis C, Delehaunty KD, Ding Y, Dinh HH, Dugan-Rocha S, Fulton LA, Gabisi RA, Garner TT, Godfrey J, Hawes AC, Hernandez J, Hines S, Holder M, Hume J, Jhangiani SN, Joshi V, Khan ZM, Kirkness EF, Cree A, Fowler RG, Lee S, Lewis LR, Li Z, Liu YS, Moore SM, Muzny D, Nazareth LV, Ngo DN, Okwuonu GO, Pai G, Parker D, Paul HA, Pfannkoch C, Pohl CS, Rogers YH, Ruiz SJ, Sabo A, Santibanez J, Schneider BW, Smith SM, Sodergren E, Svatek AF, Utterback TR, Vattathil S, Warren W, White CS, Chinwalla AT, Feng Y, Halpern AL, Hillier LW, Huang X, Minx P, Nelson JO, Pepin KH, Qin X, Sutton GG, Venter E, Walenz BP, Wallis JW, Worley KC, Yang SP, Jones SM, Marra MA, Rocchi M, Schein JE, Baertsch R, Clarke L, Csuros M, Glasscock J, Harris RA, Havlak P, Jackson AR, Jiang H, et al. Evolutionary and biomedical insights from the rhesus macaque genome. Science. 2007;316:222–34. doi: 10.1126/science.1139247. [DOI] [PubMed] [Google Scholar]
- Halemano K, Guo K, Heilman KJ, Barrett BS, Smith DS, Hasenkrug KJ, Santiago ML. Immunoglobulin somatic hypermutation by APOBEC3/Rfv3 during retroviral infection. Proc Natl Acad Sci U S A. 2014;111:7759–7764. doi: 10.1073/pnas.1403361111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper MS, Barrett BS, Smith DS, Li SX, Gibbert K, Dittmer U, Hasenkrug KJ, Santiago ML. IFN-alpha treatment inhibits acute Friend retrovirus replication primarily through the antiviral effector molecule Apobec3. J Immunol. 2013;190:1583–90. doi: 10.4049/jimmunol.1202920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RS, Bishop KN, Sheehy AM, Craig HM, Petersen-Mahrt SK, Watt IN, Neuberger MS, Malim MH. DNA deamination mediates innate immunity to retroviral infection. Cell. 2003;113:803–9. doi: 10.1016/s0092-8674(03)00423-9. [DOI] [PubMed] [Google Scholar]
- Hart M, Steel A, Clark SA, Moyle G, Nelson M, Henderson DC, Wilson R, Gotch F, Gazzard B, Kelleher P. Loss of discrete memory B cell subsets is associated with impaired immunization responses in HIV-1 infection and may be a risk factor for invasive pneumococcal disease. J Immunol. 2007;178:8212–20. doi: 10.4049/jimmunol.178.12.8212. [DOI] [PubMed] [Google Scholar]
- Hasler J, Rada C, Neuberger MS. The cytoplasmic AID complex. Semin Immunol. 2012;24:273–80. doi: 10.1016/j.smim.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Helmuth EF, Letvin NL, Margolin DH. Germline repertoire of the immunoglobulin V(H)3 family in rhesus monkeys. Immunogenetics. 2000;51:519–27. doi: 10.1007/s002510000170. [DOI] [PubMed] [Google Scholar]
- Hofmann-Lehmann R, Swenerton RK, Liska V, Leutenegger CM, Lutz H, McClure HM, Ruprecht RM. Sensitive and robust one-tube real-time reverse transcriptase-polymerase chain reaction to quantify SIV RNA load: comparison of one- versus two-enzyme systems. AIDS Res Hum Retroviruses. 2000;16:1247–57. doi: 10.1089/08892220050117014. [DOI] [PubMed] [Google Scholar]
- Hultquist JF, Lengyel JA, Refsland EW, LaRue RS, Lackey L, Brown WL, Harris RS. Human and rhesus APOBEC3D, APOBEC3F, APOBEC3G, and APOBEC3H demonstrate a conserved capacity to restrict Vif-deficient HIV-1. J Virol. 2011;85:11220–34. doi: 10.1128/JVI.05238-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbeault M, Ouellet M, Giguere K, Bertin J, Belanger D, Martin G, Tremblay MJ. Acquisition of host-derived CD40L by HIV-1 in vivo and its functional consequences in the B-cell compartment. J Virol. 2011;85:2189–200. doi: 10.1128/JVI.01993-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoff EN, O’Brien J, Thompson P, Ehret J, Meiklejohn G, Duvall G, Douglas JM., Jr Streptococcus pneumoniae colonization, bacteremia, and immune response among persons with human immunodeficiency virus infection. J Infect Dis. 1993;167:49–56. doi: 10.1093/infdis/167.1.49. [DOI] [PubMed] [Google Scholar]
- Jern P, Russell RA, Pathak VK, Coffin JM. Likely role of APOBEC3G-mediated G-to-A mutations in HIV-1 evolution and drug resistance. PLoS Pathog. 2009;5:e1000367. doi: 10.1371/journal.ppat.1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein F, Diskin R, Scheid JF, Gaebler C, Mouquet H, Georgiev IS, Pancera M, Zhou T, Incesu RB, Fu BZ, Gnanapragasam PN, Oliveira TY, Seaman MS, Kwong PD, Bjorkman PJ, Nussenzweig MC. Somatic mutations of the immunoglobulin framework are generally required for broad and potent HIV-1 neutralization. Cell. 2013;153:126–38. doi: 10.1016/j.cell.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroon FP, van Dissel JT, Ravensbergen E, Nibbering PH, van Furth R. Impaired antibody response after immunization of HIV-infected individuals with the polysaccharide vaccine against Salmonella typhi (Typhim-Vi) Vaccine. 1999;17:2941–5. doi: 10.1016/s0264-410x(99)00167-x. [DOI] [PubMed] [Google Scholar]
- Kuhrt D, Faith SA, Leone A, Rohankedkar M, Sodora DL, Picker LJ, Cole KS. Evidence of early B-cell dysregulation in simian immunodeficiency virus infection: rapid depletion of naive and memory B-cell subsets with delayed reconstitution of the naive B-cell population. J Virol. 2010;84:2466–76. doi: 10.1128/JVI.01966-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwata T, Katsumata Y, Takaki K, Miura T, Igarashi T. Isolation of potent neutralizing monoclonal antibodies from an SIV-Infected rhesus macaque by phage display. AIDS Res Hum Retroviruses. 2011;27:487–500. doi: 10.1089/AID.2010.0191. [DOI] [PubMed] [Google Scholar]
- Kuwata T, Takaki K, Yoshimura K, Enomoto I, Wu F, Ourmanov I, Hirsch VM, Yokoyama M, Sato H, Matsushita S. Conformational epitope consisting of the V3 and V4 loops as a target for potent and broad neutralization of simian immunodeficiency viruses. J Virol. 2013;87:5424–36. doi: 10.1128/JVI.00201-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane HC, Masur H, Edgar LC, Whalen G, Rook AH, Fauci AS. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1983;309:453–8. doi: 10.1056/NEJM198308253090803. [DOI] [PubMed] [Google Scholar]
- Langlois MA, Kemmerich K, Rada C, Neuberger MS. The AKV murine leukemia virus is restricted and hypermutated by mouse APOBEC3. J Virol. 2009;83:11550–9. doi: 10.1128/JVI.01430-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefranc MP, Giudicelli V, Ginestoux C, Jabado-Michaloud J, Folch G, Bellahcene F, Wu Y, Gemrot E, Brochet X, Lane J, Regnier L, Ehrenmann F, Lefranc G, Duroux P. IMGT, the international ImMunoGeneTics information system. Nucleic Acids Res. 2009;37:D1006–12. doi: 10.1093/nar/gkn838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque MC, Moody MA, Hwang KK, Marshall DJ, Whitesides JF, Amos JD, Gurley TC, Allgood S, Haynes BB, Vandergrift NA, Plonk S, Parker DC, Cohen MS, Tomaras GD, Goepfert PA, Shaw GM, Schmitz JE, Eron JJ, Shaheen NJ, Hicks CB, Liao HX, Markowitz M, Kelsoe G, Margolis DM, Haynes BF. Polyclonal B cell differentiation and loss of gastrointestinal tract germinal centers in the earliest stages of HIV-1 infection. PLoS Med. 2009;6:e1000107. doi: 10.1371/journal.pmed.1000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddament MT, Brown WL, Schumacher AJ, Harris RS. APOBEC3F properties and hypermutation preferences indicate activity against HIV-1 in vivo. Curr Biol. 2004;14:1385–91. doi: 10.1016/j.cub.2004.06.050. [DOI] [PubMed] [Google Scholar]
- Malaspina A, Moir S, Ho J, Wang W, Howell ML, O’Shea MA, Roby GA, Rehm CA, Mican JM, Chun TW, Fauci AS. Appearance of immature/transitional B cells in HIV-infected individuals with advanced disease: correlation with increased IL-7. Proc Natl Acad Sci U S A. 2006;103:2262–7. doi: 10.1073/pnas.0511094103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaspina A, Moir S, Orsega SM, Vasquez J, Miller NJ, Donoghue ET, Kottilil S, Gezmu M, Follmann D, Vodeiko GM, Levandowski RA, Mican JM, Fauci AS. Compromised B cell responses to influenza vaccination in HIV-infected individuals. J Infect Dis. 2005;191:1442–50. doi: 10.1086/429298. [DOI] [PubMed] [Google Scholar]
- Malim MH. APOBEC proteins and intrinsic resistance to HIV-1 infection. Philos Trans R Soc Lond B Biol Sci. 2009;364:675–87. doi: 10.1098/rstb.2008.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malleret B, Maneglier B, Karlsson I, Lebon P, Nascimbeni M, Perie L, Brochard P, Delache B, Calvo J, Andrieu T, Spreux-Varoquaux O, Hosmalin A, Le Grand R, Vaslin B. Primary infection with simian immunodeficiency virus: plasmacytoid dendritic cell homing to lymph nodes, type I interferon, and immune suppression. Blood. 2008;112:4598–608. doi: 10.1182/blood-2008-06-162651. [DOI] [PubMed] [Google Scholar]
- Margolin DH, Reimann KA, Sodroski J, Karlsson GB, Tenner-Racz K, Racz P, Letvin NL. Immunoglobulin V(H) usage during primary infection of rhesus monkeys with chimeric simian-human immunodeficiency viruses. J Virol. 1997;71:8582–91. doi: 10.1128/jvi.71.11.8582-8591.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta HV, Jones PH, Weiss JP, Okeoma CM. IFN-alpha and Lipopolysaccharide Upregulate APOBEC3 mRNA through Different Signaling Pathways. J Immunol. 2012 doi: 10.4049/jimmunol.1200777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir S, Fauci AS. B cells in HIV infection and disease. Nat Rev Immunol. 2009;9:235–45. doi: 10.1038/nri2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–63. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- Mussil B, Sauermann U, Motzkus D, Stahl-Hennig C, Sopper S. Increased APOBEC3G and APOBEC3F expression is associated with low viral load and prolonged survival in simian immunodeficiency virus infected rhesus monkeys. Retrovirology. 2011;8:77. doi: 10.1186/1742-4690-8-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase H, Agematsu K, Kitano K, Takamoto M, Okubo Y, Komiyama A, Sugane K. Mechanism of hypergammaglobulinemia by HIV infection: circulating memory B-cell reduction with plasmacytosis. Clin Immunol. 2001;100:250–9. doi: 10.1006/clim.2001.5054. [DOI] [PubMed] [Google Scholar]
- Olivieri DN, Gambon-Deza F. V genes in primates from whole genome sequencing data. Immunogenetics. 2015;67:211–28. doi: 10.1007/s00251-015-0830-9. [DOI] [PubMed] [Google Scholar]
- Peled JU, Kuang FL, Iglesias-Ussel MD, Roa S, Kalis SL, Goodman MF, Scharff MD. The biochemistry of somatic hypermutation. Annu Rev Immunol. 2008;26:481–511. doi: 10.1146/annurev.immunol.26.021607.090236. [DOI] [PubMed] [Google Scholar]
- Perise-Barrios AJ, Munoz-Fernandez MA, Pion M. Direct phenotypical and functional dysregulation of primary human B cells by human immunodeficiency virus (HIV) type 1 in vitro. PLoS One. 2012;7:e39472. doi: 10.1371/journal.pone.0039472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit V, Guetard D, Renard M, Keriel A, Sitbon M, Wain-Hobson S, Vartanian JP. Murine APOBEC1 is a powerful mutator of retroviral and cellular RNA in vitro and in vivo. J Mol Biol. 2009;385:65–78. doi: 10.1016/j.jmb.2008.10.043. [DOI] [PubMed] [Google Scholar]
- Petrovas C, Yamamoto T, Gerner MY, Boswell KL, Wloka K, Smith EC, Ambrozak DR, Sandler NG, Timmer KJ, Sun X, Pan L, Poholek A, Rao SS, Brenchley JM, Alam SM, Tomaras GD, Roederer M, Douek DC, Seder RA, Germain RN, Haddad EK, Koup RA. CD4 T follicular helper cell dynamics during SIV infection. J Clin Invest. 2012;122:3281–94. doi: 10.1172/JCI63039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham P, Bransteitter R, Petruska J, Goodman MF. Processive AID-catalysed cytosine deamination on single-stranded DNA simulates somatic hypermutation. Nature. 2003;424:103–7. doi: 10.1038/nature01760. [DOI] [PubMed] [Google Scholar]
- Pham P, Zhang K, Goodman MF. Hypermutation at A/T sites during G.U mismatch repair in vitro by human B-cell lysates. J Biol Chem. 2008;283:31754–62. doi: 10.1074/jbc.M805524200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai SK, Abdel-Mohsen M, Guatelli J, Skasko M, Monto A, Fujimoto K, Yukl S, Greene WC, Kovari H, Rauch A, Fellay J, Battegay M, Hirschel B, Witteck A, Bernasconi E, Ledergerber B, Gunthard HF, Wong JK. Role of retroviral restriction factors in the interferon-alpha-mediated suppression of HIV-1 in vivo. Proc Natl Acad Sci U S A. 2012;109:3035–40. doi: 10.1073/pnas.1111573109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitzurra L, Perito S, Baldelli F, Bistoni F, Vecchiarelli A. Humoral response against Cryptococcus neoformans mannoprotein antigens in HIV-infected patients. Clin Exp Immunol. 2003;133:91–6. doi: 10.1046/j.1365-2249.2003.02181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmberg AR, Neidermyer WJ, Jr, Breed MW, Alvarez X, Midkiff CC, Piatak M, Jr, Lifson JD, Evans DT. Tetherin upregulation in simian immunodeficiency virus-infected macaques. J Virol. 2013;87:13917–21. doi: 10.1128/JVI.01757-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refsland EW, Hultquist JF, Harris RS. Endogenous origins of HIV-1 G-to-A hypermutation and restriction in the nonpermissive T cell line CEM2n. PLoS Pathog. 2012;8:e1002800. doi: 10.1371/journal.ppat.1002800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revy P, Muto T, Levy Y, Geissmann F, Plebani A, Sanal O, Catalan N, Forveille M, Dufourcq-Labelouse R, Gennery A, Tezcan I, Ersoy F, Kayserili H, Ugazio AG, Brousse N, Muramatsu M, Notarangelo LD, Kinoshita K, Honjo T, Fischer A, Durandy A. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2) Cell. 2000;102:565–75. doi: 10.1016/s0092-8674(00)00079-9. [DOI] [PubMed] [Google Scholar]
- Richman DD, Wrin T, Little SJ, Petropoulos CJ. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc Natl Acad Sci U S A. 2003;100:4144–9. doi: 10.1073/pnas.0630530100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogozin IB, Kolchanov NA. Somatic hypermutagenesis in immunoglobulin genes. II. Influence of neighbouring base sequences on mutagenesis. Biochim Biophys Acta. 1992;1171:11–8. doi: 10.1016/0167-4781(92)90134-l. [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–25. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Santiago ML, Montano M, Benitez R, Messer RJ, Yonemoto W, Chesebro B, Hasenkrug KJ, Greene WC. Apobec3 encodes Rfv3, a gene influencing neutralizing antibody control of retrovirus infection. Science. 2008;321:1343–6. doi: 10.1126/science.1161121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Yuste E, Lauer WA, Chang EH, Morgan JS, Bixby JG, Lifson JD, Desrosiers RC, Johnson WE. Potent antibody-mediated neutralization and evolution of antigenic escape variants of simian immunodeficiency virus strain SIVmac239 in vivo. J Virol. 2008;82:9739–52. doi: 10.1128/JVI.00871-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt K, Guo K, Algaier M, Ruiz A, Cheng F, Qiu J, Wissing S, Santiago ML, Stephens EB. Differential virus restriction patterns of rhesus macaque and human APOBEC3A: implications for lentivirus evolution. Virology. 2011;419:24–42. doi: 10.1016/j.virol.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt K, Hill MS, Ruiz A, Culley N, Pinson DM, Wong SW, Stephens EB. Mutations in the highly conserved SLQYLA motif of Vif in a simian-human immunodeficiency virus result in a less pathogenic virus and are associated with G-to-A mutations in the viral genome. Virology. 2009;383:362–72. doi: 10.1016/j.virol.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scinicariello F, Engleman CN, Jayashankar L, McClure HM, Attanasio R. Rhesus macaque antibody molecules: sequences and heterogeneity of alpha and gamma constant regions. Immunology. 2004;111:66–74. doi: 10.1111/j.1365-2567.2003.01767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DS, Guo K, Barrett BS, Heilman KJ, Evans LH, Hasenkrug KJ, Greene WC, Santiago ML. Noninfectious retrovirus particles drive the APOBEC3/Rfv3 dependent neutralizing antibody response. PLoS Pathog. 2011;7:e1002284. doi: 10.1371/journal.ppat.1002284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey AR, Norris PJ, Qin L, Haygreen EA, Taylor E, Heitman J, Lebedeva M, DeCamp A, Li D, Grove D, Self SG, Borrow P. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol. 2009;83:3719–33. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundling C, Li Y, Huynh N, Poulsen C, Wilson R, O’Dell S, Feng Y, Mascola JR, Wyatt RT, Karlsson Hedestam GB. High-resolution definition of vaccine-elicited B cell responses against the HIV primary receptor binding site. Sci Transl Med. 2012;4:142ra96. doi: 10.1126/scitranslmed.3003752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–82. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiller T, Meffre E, Yurasov S, Tsuiji M, Nussenzweig MC, Wardemann H. Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J Immunol Methods. 2008;329:112–24. doi: 10.1016/j.jim.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titanji K, Velu V, Chennareddi L, Vijay-Kumar M, Gewirtz AT, Freeman GJ, Amara RR. Acute depletion of activated memory B cells involves the PD-1 pathway in rapidly progressing SIV-infected macaques. J Clin Invest. 2010;120:3878–90. doi: 10.1172/JCI43271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji-Kawahara S, Chikaishi T, Takeda E, Kato M, Kinoshita S, Kajiwara E, Takamura S, Miyazawa M. Persistence of viremia and production of neutralizing antibodies differentially regulated by polymorphic APOBEC3 and BAFF-R loci in friend virus-infected mice. J Virol. 2010;84:6082–95. doi: 10.1128/JVI.02516-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, Komarova NL, Nowak MA, Hahn BH, Kwong PD, Shaw GM. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307–12. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- Wiegand HL, Doehle BP, Bogerd HP, Cullen BR. A second human antiretroviral factor, APOBEC3F, is suppressed by the HIV-1 and HIV-2 Vif proteins. EMBO J. 2004;23:2451–8. doi: 10.1038/sj.emboj.7600246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Weatherall C, Bailey M, Alcantara S, De Rose R, Estaquier J, Wilson K, Suzuki K, Corbeil J, Cooper DA, Kent SJ, Kelleher AD, Zaunders J. Simian immunodeficiency virus infects follicular helper CD4 T cells in lymphoid tissues during pathogenic infection of pigtail macaques. J Virol. 2013;87:3760–73. doi: 10.1128/JVI.02497-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh WW, Rahman I, Hraber P, Coffey RT, Nevidomskyte D, Giri A, Asmal M, Miljkovic S, Daniels M, Whitney JB, Keele BF, Hahn BH, Korber BT, Shaw GM, Seaman MS, Letvin NL. Autologous neutralizing antibodies to the transmitted/founder viruses emerge late after simian immunodeficiency virus SIVmac251 infection of rhesus monkeys. J Virol. 2010;84:6018–32. doi: 10.1128/JVI.02741-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Konig R, Pillai S, Chiles K, Kearney M, Palmer S, Richman D, Coffin JM, Landau NR. Single-strand specificity of APOBEC3G accounts for minus-strand deamination of the HIV genome. Nat Struct Mol Biol. 2004;11:435–42. doi: 10.1038/nsmb758. [DOI] [PubMed] [Google Scholar]
- Zhang ZQ, Casimiro DR, Schleif WA, Chen M, Citron M, Davies ME, Burns J, Liang X, Fu TM, Handt L, Emini EA, Shiver JW. Early depletion of proliferating B cells of germinal center in rapidly progressive simian immunodeficiency virus infection. Virology. 2007;361:455–64. doi: 10.1016/j.virol.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Zheng NY, Wilson K, Jared M, Wilson PC. Intricate targeting of immunoglobulin somatic hypermutation maximizes the efficiency of affinity maturation. J Exp Med. 2005;201:1467–78. doi: 10.1084/jem.20042483. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.