Abstract
Objective: To investigate the impact of lithotripter focal width on stone fragmentation.
Materials and Methods: A modified reflector was used to reduce −6 dB beam size of the HM3 lithotripter, while increasing concomitantly peak pressure. Fragmentation in vitro was assessed with modified and original reflectors using BegoStone phantoms. A membrane holder was used to mimic lithotripsy in vivo, and a matrix holder was used to assess variations of fragmentation power in the focal plane of the lithotripter field. Stone fragmentation in vivo produced by the two reflectors was further compared in a swine model.
Results: Stone fragmentation in vitro after 500 (or 2000) shocks was ∼60% (or ∼82%) vs ∼40% (or ∼75%) with original and modified reflector, respectively (p ≤ 0.0016). Fragmentation power with the modified reflector was the highest on the lithotripter axis, but dropped rapidly in the lateral direction and became insignificant at radial distances >6.0 mm. Stone fragmentation with the original reflector was lower along the lithotripter axis, but fragmentation power decayed slowly in lateral direction, with appreciable fragmentation produced at 6.0 mm. Stone fragmentation efficiency in vivo after 500 (or 2000) shocks was ∼70% (or ∼90%) vs ∼45% (or ∼80%) with original and modified reflector, respectively (p ≤ 0.04).
Conclusions: A lithotripter field with broad beam size yields superior stone comminution when compared with narrow beam size under comparable effective acoustic pulse energy both in vivo and in vitro. These findings may facilitate future improvements in lithotripter design to maximize comminution efficiency while minimizing tissue injury.
Keywords: : shockwave lithotripsy, lithotripter, focal width, stone comminution, nephrolithiasis
Introduction
Shockwave lithotripsy (SWL) has been utilized clinically in the treatment of kidney and upper ureteral stones for more than two decades. Controversy remains with respect to the effect of lithotripter −6 dB focal width (or beam size) on stone fragmentation. Advancements in SWL technology have led to the replacement of first-generation HM3 lithotripters (characterized by broad beam size with low peak pressure) by second- and third-generation lithotripters (characterized by narrow beam size with concomitantly increased peak pressure). The initial changes in lithotripter design were aimed to better concentrate shockwave energy onto stones, therefore, decreasing injury to surrounding tissue, reducing patient discomfort, and possibly achieving anesthesia-free SWL.1 However, an increasing number of studies demonstrate that second- and third-generation lithotripters are less effective than original HM3 in stone comminution and have a greater predilection for stone recurrence, vascular injury, and soft-tissue injury.2–4
Although certain changes in lithotripter design (i.e., dry coupling) and practice patterns (i.e., faster pulse delivery rate) may partially influence the unimproved performance of modern lithotripters, it is unknown whether beam sizes of the third-generation lithotripters may directly impact comminution efficiency.5–8 The renewed interest in clinical shockwave lithotripters with broad beam size has heightened the awareness and urgency in addressing this issue.9–12 Previously, stone comminution produced by different types of lithotripters with various beam sizes has been studied at clinically relevant output settings.13–15 However, inherent lithotripter differences in acoustic fields, coupling methods, stone localization techniques, and output settings make it impossible for an objective and direct comparison.15
In an in vitro study, the original reflector of the HM3 was modified by an insert so that the acoustic field could be transformed into one with significantly amplified peak pressure and concomitantly reduced beam size.16 This modification allowed us to characterize the performance of two lithotripter fields with distinctively different beam sizes using the same energy source and output settings (i.e., 20 kV). In this follow-up in vivo study, we report that with equivalent effective acoustic pulse energy, a lithotripter field with low peak pressure and broad beam size yields significantly superior stone comminution in a swine model compared with a lithotripter field with high peak pressure and narrow beam size using the same generator, coupling medium, and stone localization technique with only slightly modified reflector geometry.
Materials and Methods
Lithotripter
This study was performed in an original Dornier HM3 lithotripter equipped with an ellipsoidal brass reflector and an 80-nF capacitor. The half-focal length was c = 114 mm, semiminor axis b = 77.5 mm, and semimajor axis a = 138 mm. The HM3 was operated at a clinically relevant output setting of 20 kV with 1 Hz pulse repetition frequency. To produce an acoustic field with narrow beam size and large peak pressure, a thin shell ellipsoidal brass reflector insert (c′ = 111.5 mm, b′ = 75 mm, and a′ = 134.4 mm) was engineered to fit closely into the original HM3 reflector (Fig. 1).16,17
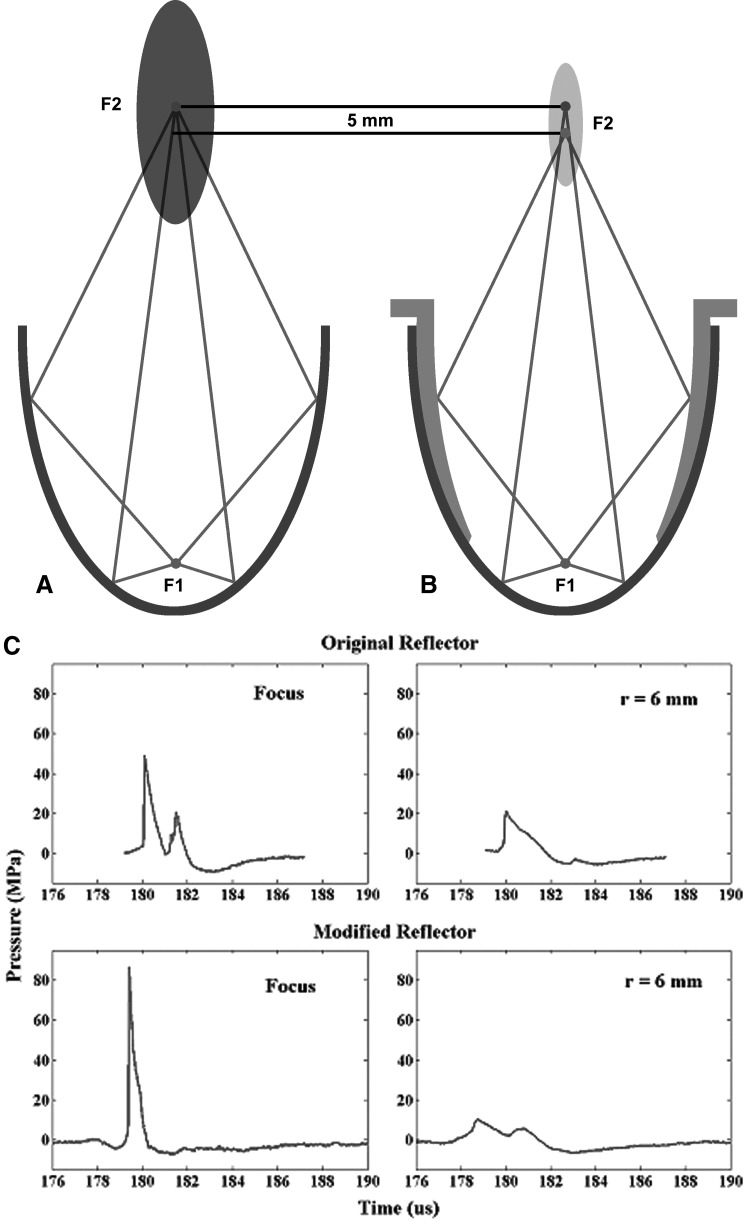
FIG. 1.
Schematic diagram of (A) original and (B) modified reflector configurations in HM3 lithotripter. (C) Average pressure waveform produced by the original and modified reflectors, respectively, at lithotripter focus and an off-axis position (r = 6 mm).
Pressure measurements, energy density, and acoustic pulse energy calculations
The acoustic field of the HM3 with either the modified or original reflector has been characterized by using a light spot hydrophone (University of Erlangen-Nuremberg, Erlangen, Germany) with a bandwidth of 40 MHz, a sensitivity of 10 mV/MPa, and spatial resolution of 100 μm, which is comparable to the fiber optic probe hydrophone.18 Typical pressure waveforms measured at the lithotripter focus and 6 mm radial distance off the central axis are shown in Figure 1 and key lithotripter field parameters are summarized in Table 1.
Table 1.
Characteristics of the Acoustic Fields Produced by HM3 at 20 kV Using the Original and Modified Reflectors
| Original reflector | Modified reflector | |
|---|---|---|
| Peak positive pressure (MPa) | 48.9 ± 1.3 | 86.9 ± 3.8 |
| Peak negative pressure (MPa) | −10.7 ± 0.6 | −10.6 ± 0.6 |
| −6 dB beam size (mm)a | 10.9 | 3.6 |
| Acoustic pulse energy (mJ)a | 42.9 | 37.4 |
Data were calculated based on the arithmetic mean of the value measured on the x and y axes.
Stone comminution in vitro
Although some comparisons of stone comminution generated by the HM3 with modified and original reflectors were carried out previously, we first performed additional in vitro experiments to further ascertain the difference between the two reflectors before proceeding to animal experiments. Artificial kidney stones prepared from BegoStone® (Bego USA, Smithfield, RI) with a powder-to-water mixing ratio of 15:3 were fabricated and treated in two different types of stone holders (Fig. 2) following an established protocol.16,19 The membrane holder (Fig. 2A) was used for treatment of 10 mm spherical stone phantoms at the lithotripter focus, which allows stone fragments to disperse laterally, mimicking lithotripsy procedures in vivo. In addition, a matrix holder (Fig. 2B) was used to treat 4 × 4 mm cylindrical stone phantoms at various radial distances from the lithotripter focus to assess changes in fragmentation power in the lithotripter field at 18 and 22 kV, respectively. After shockwave treatment, all residual fragments were collected and air-dried for 24 hours. The dry fragments were separated by sequential sieving method through a sequence of grids with sizes in 2, 2.8, and 4 mm. The separated fragments were weighed thereafter and efficiency of stone fragmentation was calculated by the weight percent of fragments with size <2 mm with respect to the original weight of the stone.
FIG. 2.
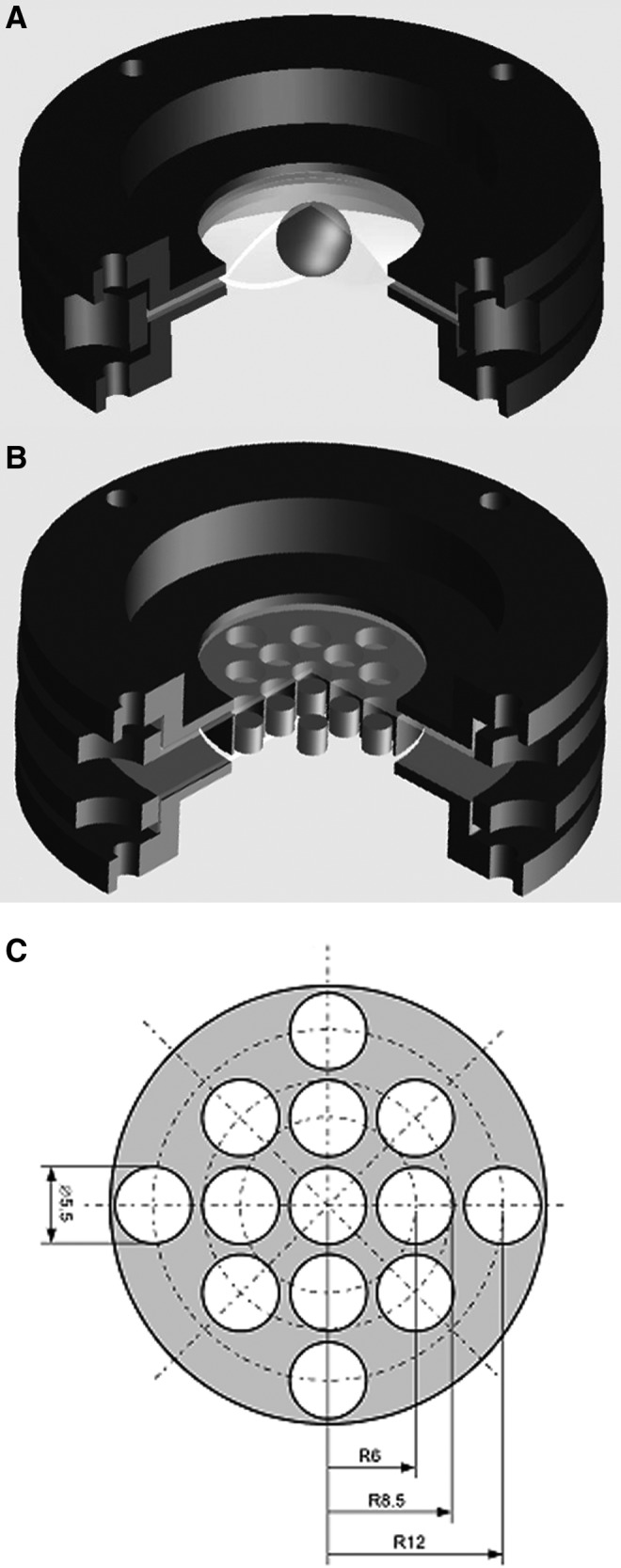
A schematic diagram of the membrane holder (A) that mimics lithotripsy procedures in vivo and allows stone fragments to disperse laterally. The matrix holder (B) assesses fragmentation power in the lithotripter focal plane at various radial distances. The size and location of individual holes in the matrix holder are shown in figure (C).
Stone comminution in vivo
Next, stone comminution was evaluated in a swine model with surgically implanted cylindrical BegoStone phantoms (5.5 × 10 mm, DxL). All experimental designs used in the study were reviewed and authorized by Institutional Animal Care and Use Committee of Duke University. Female farm pigs of nominal bodyweight of 80 lbs (36.3 kg) were randomly separated into two groups of equal size. One group underwent treatment using the modified reflector and the other by the original reflector. After induction of general anesthesia, surgical procedures were performed to introduce a cylindrical stone phantom into the lumen of both the left and right ureter. Each stone was subsequently advanced into the renal pelvis using lithotomy forceps through a 2-cm incision on anterior wall of the proximal ureter. A 6F polyurethane ureteral stent was then placed in both the right and left ureter and renal pelvis. The ureterotomy and midline incision were closed with a running suture. At least, six kidneys were treated under each condition after effective surgical preparation.
After SWL, the animal was euthanized with both kidneys harvested and bisected to recover residual fragments in the collecting system. The fragments were dried at room temperature (24 hours) and then sorted sequentially through mesh sizes of 4, 2, 1, and 0.5 mm. Fragmentation efficiency was determined by the analysis of percentage of fragments <2 mm. Standard analysis of variance and student's t-test analyses (two-tailed) were used to compare mean efficiencies of stone fragmentation.
Results
-
1. Stone comminution in the membrane holder after 500 and 2000 shocks (Fig. 3).
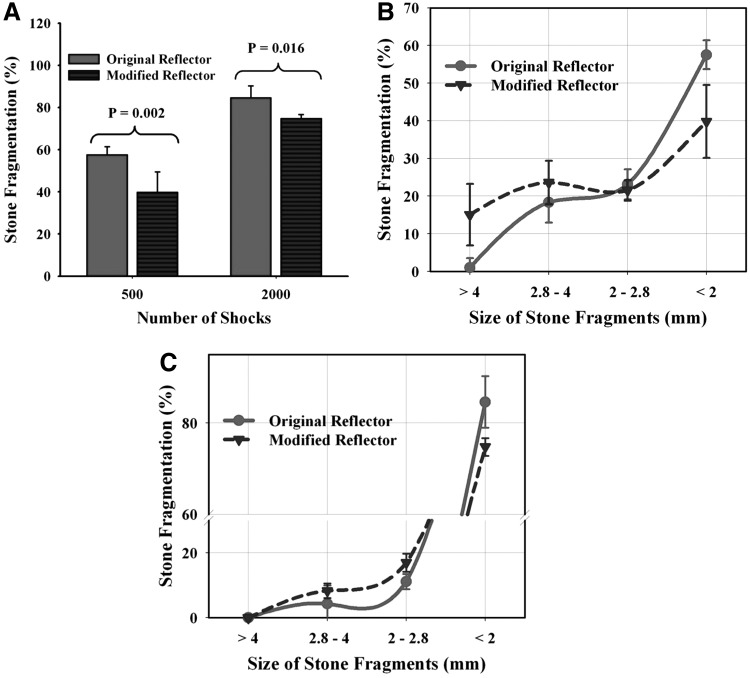
In vitro studies demonstrate that the original reflector with low peak pressure and broad beam size produces superior stone fragmentation than the modified reflector with narrow beam size and high peak pressure (Fig. 3). Stone fragmentation was ∼58% vs 39% after 500 shocks (p = 0.0002), and 82% vs 75% after 2000 shocks (p = 0.016) when using the original and the modified reflector, respectively. Fragmentation size distribution shows that after 500 shocks the original reflector produces more small fragments in the size range of <2 mm, while the modified reflector produces more large fragments in the size range of >4 mm and between 2.8 and 4 mm (Fig. 3B). This trend continues to 2000 shocks, except that all the fragments produced by the modified reflector were <4 mm with more residual fragments observed in the size range of between 2 and 2.8 mm in comparison with the original reflector (Fig. 3C).
-
2. Stone comminution in vitro in the matrix holder after 350 shocks treatment by the original and modified reflector (Fig. 4).
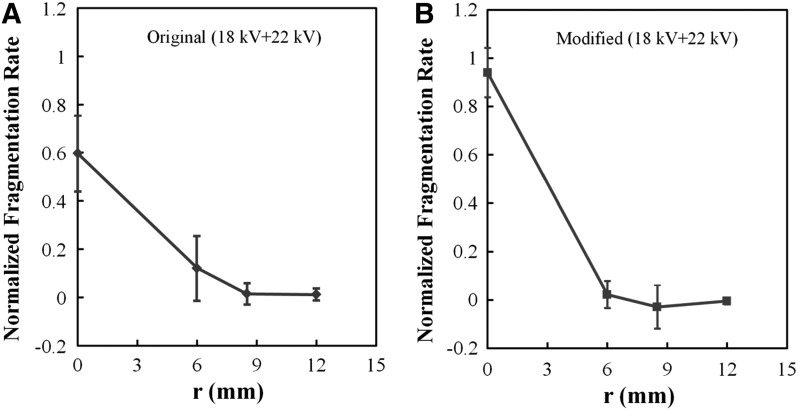
The efficiency of stone fragmentation was found to decrease with increased radial distance from the lithotripter axis. Using the matrix holder, the highest fragmentation was produced along the lithotripter axis by the modified reflector, which was used to normalize the results obtained at other locations, including the results produced by the original reflector. Fragmentation power produced by the modified reflector dropped off rapidly and became insignificant at radial distances >6.0 mm (Fig. 4). In comparison, stone fragmentation created by the original reflector is lower at the lithotripter focus, however, fragmentation power decayed more slowly away from the central axis of the lithotripter, with appreciable fragmentation produced at a radial distance of 6.0 mm.
-
3. Stone comminution in vivo—500 and 2000 results comparing original and modified reflector (Fig. 5).
The original reflector was found to produce consistently better stone comminution than the modified reflector under the same acoustic pulse energy in vivo (Fig. 5). Stone fragmentation efficiency was ∼70% vs 45% after 500 shocks (p < 0.001) and about 90% vs 80% after 2000 shocks (p = 0.04), when using the original and the modified reflector, respectively.
FIG. 3.
Stone fragmentation produced in the membrane holder by the HM3 lithotripter after 500 and 2000 shocks using the original and modified reflectors at 20 kV (A), fragmentation size distribution after 500 shocks (B), and fragmentation size distribution after 2000 shocks (C). The results are presented by mean + standard deviation.
FIG. 4.
Variations of stone fragmentation power in the focal plane at different radial distances off the lithotripter axis. In each test, 350 shocks generated by HM3 lithotripter using either the (A) original or (B) modified reflector at 18 and 22 kV were delivered to the cylindrical stones (4.0 × 4.0 mm, D × H) in the matrix holder. To represent an average effect produced at 20 kV, the combined results of 18 + 22 kV are presented in the form of mean + standard deviation.
FIG. 5.
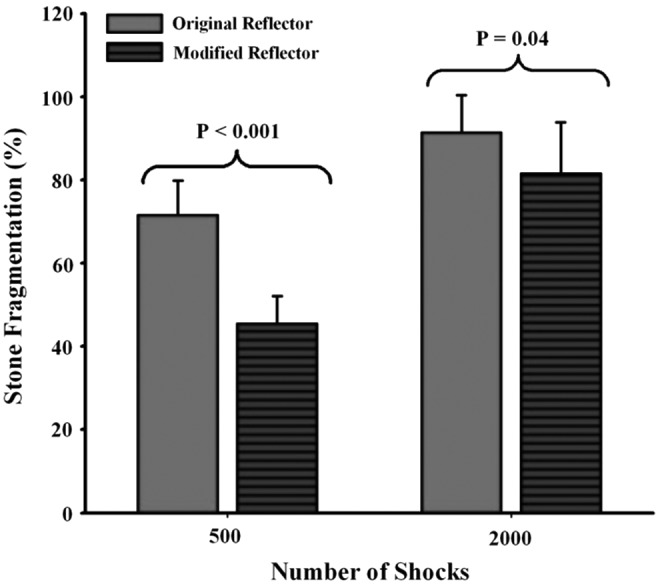
In vivo stone fragmentation produced in pig kidneys by the HM3 lithotripter using the original and modified reflectors at 20 kV. The results are presented by mean + standard deviation.
Discussion
Since the introduction of third-generation lithotripters, there has been controversy regarding the effect of beam size on SWL treatment outcomes.4,20 It has been previously demonstrated that some third-generation lithotripters, characterized by high peak pressure and narrow focal width, are inferior in stone fragmentation if there is significant artifact motion from respiration of the patient2,21 or movement of residual fragments3,16 during SWL. By comparison, the first-generation lithotripters and their contemporary counterparts, characterized by low peak pressure and broad focal width, have been found to be often more reliable, safe, and effective during SWL.4–6,9,10,12 These findings have been validated by recent investigations of the mechanisms responsible for stone comminution during SWL. Stone comminution in SWL is directly correlated with the local peak pressure, but not to the maximum peak pressure at the lithotripter focus.7 Therefore, a lithotripter with broad focal width and low peak pressure tends to produce a broader fragmentation zone without risking tissue injury than its counterpart with narrow focal width and high peak pressure. In clinical SWL, 30%–40% of highly focused shockwaves could miss the target stones due to respiratory motion with excursion distance up to 5 cm in patients under sedation4,6,22,23
Recently, we reported that a novel acoustic lens specifically designed for electromagnetic (EM) shockwave lithotripters, which produces superior stone comminution in vivo compared with the original lens of the lithotripter. This novel lens design has broadened the focal width while correcting concomitantly the drawbacks of misalignment in acoustic focus and nonidealized pulse profile, which are commonly seen in contemporary EM lithotripters.6–8
To further assess the impact of lithotripter focal width on stone fragmentation both in vitro and in vivo, we developed a novel reflector insert for the HM3, thereby producing a modified high peak pressure (∼87 MPa) and narrow beam size (∼4 mm) lithotripter field compared with the original reflector. The most unique advantage of this approach is that we can compare the impact of beam width on stone fragmentation with the same shockwave generator, coupling medium, and stone localization technique, and thus, effective acoustic pulse energy is delivered to the stone with only slightly modified reflector geometry. Moreover, our previous study using a mesh holder or a finger cot for phantom stone placement is limited in recapitulating the main features of in vivo stone comminution.16 The membrane holder (Fig. 2A) overcomes this limitation by allowing for stone fragment accumulation and lateral dispersion during SWL. In addition, the matrix holder (Fig. 2B) provides us a means to assess quantitatively the variations of fragmentation power along the radial direction in the lithotripter focal plane.
We observed that a broad beam size (11 mm) produces superior stone fragmentation than a narrow beam size (i.e., <4 mm) for an equivalent acoustic pulse energy both in vivo and in vitro under clinically relevant test conditions (i.e., in the membrane holder). Several factors may account for our findings.
It is postulated that a low pressure and broad beam size lithotripter field initiates fragmentation primarily by shear stresses produced on the lateral sides of the stone.9,10 By design, the damage propagates through multiple fracture planes and produces fragments with relatively homogeneous size.16 In comparison, however, a high pressure and narrow beam size lithotripter field tends to initiate fragmentation from the posterior surface of the stone. Through a combination of high pressure at the center and low pressure surrounding the stone, spallation mechanism may dominate, leading to uneven stone fracture.9,24–27 When the original spherical geometry of the stone is compromised, residual fragments with irregular geometry, are much more resistant to ensuing shockwaves, especially for a lithotripter field with narrow beam size.16 The only exception is a small stone (i.e., 4 mm) located precisely at the lithotripter focus (an unlikely clinical scenario), which will be fragmented more effectively by the high pressure-narrow beam size lithotripter field (Fig. 4).
More realistically, lateral displacement of stone fragments will occur during SWL and large residual fragments may disperse away from the high-pressure region around the lithotripter focus. Given this context, significant lateral dispersion of fragments may negatively impact stone comminution in a lithotripter field with high peak pressure and narrow beam width due to its smaller effective fragmentation zone compared with its counterpart with low peak pressure and broad beam width.6,16
Subtle differences in cavitation bubble dynamics produced by the two lithotripter fields also exist. High-speed imaging and modeling studies have demonstrated previously that although the maximum bubble size remains similar along beam axis, a lithotripter field with broad beam size may generate stronger cavitation potentials than its narrow beam size counterpart at off-axis locations.16 Therefore, a high-pressure and narrow beam size lithotripter field may perform efficiently when stones are aligned to the lithotripter focus, or when the residual fragments are confined in a small volume around the beam focus (i.e., under minimal respiratory motion). In contrast, a low-pressure and broad beam size lithotripter field will perform efficiently even when stones are less accurately aligned with the beam focus, and/or residual fragments are dispersed from lithotripter axis by acoustic radiation forces or respiratory motion.16
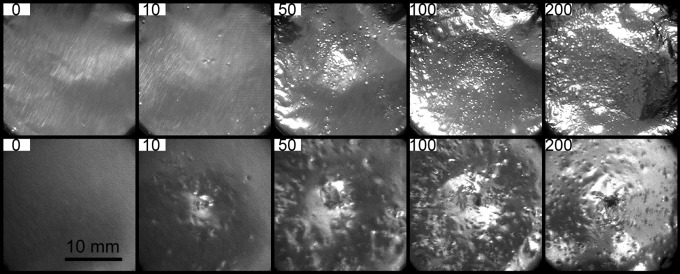
Furthermore, tissue injury primarily consists of vascular damage to endothelial cells, and rupture of small blood vessels is a safety concern in SWL.28 Cavitation19 and shear stress29,30 have been proposed to be responsible for injury during SWL. Using aluminum foils as test targets, we have observed deep pitting lesions produced by the narrow beam size and high peak pressure lithotripter field (Fig. 6), presumably due to strong lithotripter shockwave–bubble interaction with jet formation that leads to membrane poration.31,32 In contrast, only dispersed pitting with superficial lesions, yet no poration, was produced by the broad beam size and low peak pressure lithotripter field. This observation is consistent with other studies, which suggest that lithotripters with broad beam size and low peak pressure are less prone to producing tissue injury in SWL compared with lithotripters with narrow beam size and high peak pressure.6,10,12,33
FIG. 6.
Cavitation damage in aluminum foils after exposure to 0, 10, 50, 100, and 200 shocks generated by the HM3 lithotripter at 20 kV using the original (top row) and modified reflectors (bottom row). The scale bar is 10 mm.
Limitations in our experimental design should also be considered when interpreting our findings. Several types of phantom stones have been applied in previous studies, including Plaster-of-Paris, Z-brick, breeze blocks, glass marbles, and Iceland spar, and more recently, BegoStone. While BegoStones are artificial stones not entirely accurately representative of human stones, which can be mosaic in material properties, composition, and susceptibility to lithotripters, BegoStones possess performance characteristics that enable reliable SWL experimental design.34 Prior investigations have demonstrated that BegoStones have consistent reproducible fragmentation, and also resist abrasion and softening in urine, and furthermore, have acoustical properties comparable with those of calcium oxalate monohydrate stones.35 Although artificial stones composed of properties more comparable with natural counterparts have been developed, they were not used in our study due to lack of commercial availability, costly production, and need for skillful fabrications.
Similarly, although the swine model has been applied in urologic and endourologic research due to the similarity in swine renal anatomy and physiology to those found in man, our findings must still be interpreted cautiously, as the swine may not be indicative of the broad spectrum of clinical situations related to stone patients.
Abbreviations Used
- SWL
shockwave lithotripsy
- EM
electromagnetic
Acknowledgments
This work was supported by NIH grant Nos. R37DK052985-20. The authors acknowledge the technical support provided by Jun Qin, Victor Leitao, Nathan Smith, Eric Esch, Carson Moore, and John Mancini during the course of this study.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Lingeman JE. Extracorporeal shock wave lithotripsy. Development, instrumentation, and current status. Urol Clin North Am 1997;24:185–211 [DOI] [PubMed] [Google Scholar]
- 2.Gerber R, Studer UE, Danuser H. Is newer always better? A comparative study of 3 lithotriptor generations. J Urol 2005;173:2013–2016 [DOI] [PubMed] [Google Scholar]
- 3.Graber SF, Danuser H, Hochreiter WW, Studer UE. A prospective randomized trial comparing 2 lithotriptors for stone disintegration and induced renal trauma. J Urol 2003;169:54–57 [DOI] [PubMed] [Google Scholar]
- 4.Lingeman JE, McAteer JA, Gnessin E, Evan AP. Shock wave lithotripsy: Advances in technology and technique. Nat Rev Urol 2009;6:660–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiland D, Lee C, Ugarte R, Monga M. Impact of shockwave coupling on efficacy of extracorporeal shockwave lithotripsy. J Endourol 2007;21:137–140 [DOI] [PubMed] [Google Scholar]
- 6.Neisius A, Smith NB, Sankin G, et al. Improving the lens design and performance of a contemporary electromagnetic shock wave lithotripter. Proc Natl Acad Sci USA 2014;111:E1167–E1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith N, Zhong P. Stone comminution correlates with the average peak pressure incident on a stone during shock wave lithotripsy. J Biomech 2012;45:2520–2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith NB, Zhong P. A heuristic model of stone comminution in shock wave lithotripsy. J Acoust Soc Am 2013;134:1548–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisenmenger W. The mechanisms of stone fragmentation in ESWL. Ultrasound Med Biol 2001;27:683–693 [DOI] [PubMed] [Google Scholar]
- 10.Eisenmenger W, Du XX, Tang C, et al. The first clinical results of “wide-focus and low-pressure” ESWL. Ultrasound Med Biol 2002;28:769–774 [DOI] [PubMed] [Google Scholar]
- 11.Leistner R, Wendt-Nordahl G, Grobholz R, et al. A new electromagnetic shock-wave generator “SLX-F2” with user-selectable dual focus size: Ex vivo evaluation of renal injury. Urol Res 2007;35:165–171 [DOI] [PubMed] [Google Scholar]
- 12.Pishchalnikov YA, McAteer JA, Williams JC Jr., et al. Evaluation of the LithoGold LG-380 lithotripter: In vitro acoustic characterization and assessment of renal injury in the pig model. J Endourol 2013;27:631–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chuong CJ, Zhong P, Preminger GM. A comparison of stone damage caused by different modes of shock wave generation. J Urol 1992;148:200–205 [DOI] [PubMed] [Google Scholar]
- 14.Teichman JM, Portis AJ, Cecconi PP, et al. In vitro comparison of shock wave lithotripsy machines. J Urol 2000;164:1259–1264 [PubMed] [Google Scholar]
- 15.Cleveland RO, McAteer JA. The physics of shock wave lithotripsy. Oxford, UK: Wiley-Blackwell, 2007, pp. 529–558 [Google Scholar]
- 16.Qin J, Simmons WN, Sankin G, Zhong P. Effect of lithotripter focal width on stone comminution in shock wave lithotripsy. J Acoust Soc Am 2010;127:2635–2645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Y, Zhong P. Suppression of large intraluminal bubble expansion in shock wave lithotripsy without compromising stone comminution: Refinement of reflector geometry. J Acoust Soc Am 2003;113:586–597 [DOI] [PubMed] [Google Scholar]
- 18.Smith N, Sankin GN, Simmons WN, Nanke R, Fehre J, Zhong P. A comparison of light spot hydrophone and fiber optic probe hydrophone for lithotripter field characterization. Rev Sci Instrum 2012;83:014301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhong P, Zhou Y, Zhu S. Dynamics of bubble oscillation in constrained media and mechanisms of vessel rupture in SWL. Ultrasound Med Biol 2001;27:119–134 [DOI] [PubMed] [Google Scholar]
- 20.Rassweiler J, Rassweiler MC, Frede T, Alken P. Extracorporeal shock wave lithotripsy: An opinion on its future. Indian J Urol 2014;30:73–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cleveland RO, Anglade R, Babayan RK. Effect of stone motion on in vitro comminution efficiency of Storz Modulith SLX. J Endourol 2004;18:629–633 [DOI] [PubMed] [Google Scholar]
- 22.Leighton TG, Fedele F, Coleman AJ, et al. A passive acoustic device for real-time monitoring of the efficacy of shockwave lithotripsy treatment. Ultrasound Med Biol 2008;34:1651–1665 [DOI] [PubMed] [Google Scholar]
- 23.Sorensen MD, Bailey MR, Shah AR, Hsi RS, Paun M, Harper JD. Quantitative assessment of shockwave lithotripsy accuracy and the effect of respiratory motion. J Endourol 2012;26:1070–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cleveland RO, Sapozhnikov OA. Modeling elastic wave propagation in kidney stones with application to shock wave lithotripsy. J Acoust Soc Am 2005;118:2667–2676 [DOI] [PubMed] [Google Scholar]
- 25.Sapozhnikov OA, Maxwell AD, MacConaghy B, Bailey MR. A mechanistic analysis of stone fracture in lithotripsy. J Acoust Soc Am 2007;121:1190–1202 [DOI] [PubMed] [Google Scholar]
- 26.Gracewski SM, Dahake G, Ding Z, Burns SJ, Everbach EC. Internal stress wave measurements in solids subjected to lithotripter pulses. J Acoust Soc Am 1993;94:652–661 [DOI] [PubMed] [Google Scholar]
- 27.Xi X, Zhong P. Dynamic photoelastic study of the transient stress field in solids during shock wave lithotripsy. J Acoust Soc Am 2001;109:1226–1239 [DOI] [PubMed] [Google Scholar]
- 28.Evan A, McAteer J. Q-effects of shock wave lithotripsy. In: Kidney Stones: Medical and Surgical Management. New York: Raven, 1996, pp. 549–570 [Google Scholar]
- 29.Howard D, Sturtevant B. In vitro study of the mechanical effects of shock-wave lithotripsy. Ultrasound Med Biol 1997;23:1107–1122 [DOI] [PubMed] [Google Scholar]
- 30.Freund JB, Colonius T, Evan AP. A cumulative shear mechanism for tissue damage initiation in shock-wave lithotripsy. Ultrasound Med Biol 2007;33:1495–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Philipp A, Delius M, Scheffczyk C, Vogel A, Lauterborn W. Interaction of lithotripter-generated shock waves with air bubbles. J Acoust Soc Am 1993;93:2496–2509 [Google Scholar]
- 32.Sankin G, Simmons W, Zhu S, Zhong P. Shock wave interaction with laser-generated single bubbles. Phys Rev Lett 2005;95:034501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Connors BA, McAteer JA, Evan AP, et al. Evaluation of shock wave lithotripsy injury in the pig using a narrow focal zone lithotriptor. BJU Int 2012;110:1376–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carey RI, Kyle CC, Carey DL, Leveillee RJ. Preparation of artificial kidney stones of reproducible size, shape, and mass by precision injection molding. J Endourol 2008;22:127–132 [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Zhong P. BegoStone—A new stone phantom for shock wave lithotripsy research. J Acoust Soc Am 2002;112:1265–1268 [DOI] [PubMed] [Google Scholar]