Abstract
Introduction
Relapses of multiple sclerosis (MS) are usually treated with high-dose intravenous methylprednisolone (IVMP), given over 3–10 days. There is no consensus on the optimal duration of treatment. In this study, we aimed to investigate whether longer treatment provides additional short-term clinical benefits assessed by the change in plasma cytokine levels and EDSS scores in patients with relapsing–remitting MS (RRMS).
Methods
Forty RRMS patients during relapse were grouped into 3 and treated with 1 g/day of IVMP for either 5, 7, or 10 consecutive days.
Results
Levels of IL-10 and IL-12 were analyzed, and EDSS scores were noted before treatment, after treatment (on days 6, 8, or 11) and at the 4th week. IVMP treatment significantly induced anti-inflammatory IL-10 levels but had no effect on IL-12 levels. IVMP treatment for 7 or 10 consecutive days was not significantly different than that for 5 days in terms of the change in IL-12, IL-10 levels or clinical outcome.
Conclusion
In conclusion, pulse high-dose IVMP treatment enhances functional recovery in patients with acute relapses of RRMS. In addition, IVMP treatment significantly increases the levels of IL-10 but has no effect on the levels of IL-12 in the short term.
Keywords: Interleukin-10, intravenous methylprednisolone, glucocorticoids, relapsing-remitting multiple sclerosis, treatment duration
INTRODUCTION
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system (CNS) and relapsing–remitting MS (RRMS) is the most common clinical type. Relapses in MS are often associated with significant functional impairment in various neurological systems to various degrees. Importantly, they clearly impair the quality of life and affect future disabilities in MS (1). On rare occasions though, acute attacks of MS may be permanently and severely disabling (2). Therefore, the effective and adequate treatment of relapses is essential to hasten recovery and delay the progression of disability in MS.
Inflammation is the hallmark of an active MS lesion. The pathogenesis of MS is highly complex, even though roles of the proinflammatory cytokine interleukin (IL)-12 and anti-inflammatory cytokine IL-10 have been mentioned in several previous studies (3,4,5). Although the exact roles of Th1 and Th17 cells in the development of MS lesions have not yet been established, both these effector T-cell populations can induce CNS inflammation and demyelinating lesions in experimental and clinical settings (6,7). IL-12, which is mainly secreted by antigen-presenting cells during antigen presentation to naive T cells, are known to induce the differentiation of CD4+ T cells toward a Th1 phenotype and promote inflammation through the activation of the JAK/STAT signaling pathways (8,9). On the other hand, IL-10 acts in immunoregulation and inflammation and has the capability to downregulate the expression of Th1 cells and inhibit the synthesis of various pro-inflammatory cytokines. In two previous studies, lower serum levels of IL-10 have been been shown in patients with MS, and IL-10 levels have been found to increase during the remission phases of the disease (3,10).
Hence, treating relapses of MS with corticosteroids is a well-accepted approach due to their anti-inflammatory and immunosuppressive properties (11,12,13). The efficacy of different doses and routes of administration have been compared in previous studies (14,15,16,17). In most cases, 500–1000 mg of intravenous methylprednisolone (IVMP) is given for 3–5 days for acute relapses of MS (18).
However, there is no consensus on the optimal duration for treating relapses with IVMP (15,19). Usually, clinicians decide the treatment duration according to the severity of clinical symptoms and personal factors affecting the disease prognosis depending on the patient; in some practices, 7 or even 10 consecutive days of administration could be the decision. Till date, a study comparing the efficacy of IVMP treatment for 5, 7, or 10 days has not been conducted, and it is unknown if extending the treatment for few more days yields favorable outcomes.
The aim of this study was to investigate whether treating relapses with longer durations of IVMP therapy provides additional benefits by assessing effects on plasma cytokines and EDSS scores in patients with RRMS.
METHODS
Patients
A total of 40 patients (all Caucasians) between the ages of 18 and 58 years treated with a diagnosis of clinically definite RRMS according to McDonald’s criteria at our tertiary care Multiple Sclerosis Clinic were included. Inclusion criteria were as follows: 1) having an acute relapse of MS, which is defined as a new or worsening neurological deficit lasting for 24 h or more in the absence of fever or infection; 2) symptoms should have begun within 5 days prior to admission; and 3) the MRI scan should demonstrate a related lesion with gadolinium enhancement in neuroimaging. Exclusion criteria were having another systemic autoimmune disorder; abnormalities in the serum liver, renal or thyroid function tests; and being on medications other than immunosuppressive or immunomodulatory agents approved for RRMS treatment.
A detailed physical and neurological examination was performed, and the baseline EDSS score was calculated. Laboratory analysis for the complete blood count and measuring lipid and serum fasting glucose levels and liver, renal, and thyroid function tests were performed prior to IVMP treatment. Patients were hospitalized and grouped into 3, according to the relative severity of their clinical symptoms. Patients in the groups were treated with 1 g/day of IVMP for either 5 (n=12), 7 (n=14), or 10 (n=14) consecutive days. A neurological examination was repeated on the day after the IVMP treatment finished (post-treatment, i.e., day 6 for patients on the 5-day course, day 8 for patients on the 7-day course, and day 11 for patients on the 10-day course) and at the 4th week to assess the follow-up EDSS scores.
The study protocol was in accordance with the Helsinki declaration of human rights and was approved by The Regional Committee for Research Ethics, and all subjects provided written informed consent to participate.
IL-12 and IL-10 Analysis
All participants were examined by blood tests for plasma IL-12 and IL-10 levels at baseline (pre-treatment; Day 0), post-treatment, and at the 4th week evaluations. A peripheral venous blood sample for biomarker analysis was withdrawn in the morning during the resting state for at least 5 min, following the 12-h fasting period. Samples were immediately stored at 4°C for 10 min and centrifuged at 1500 rpm for 15 min, and the extracted plasma was stored at −80°C in the dark until they were assayed. The presence of IL-12 (Bender MedSystems GmbH, Austria) and IL-10 (Bender MedSystems GmbH, Austria) in plasma were detected by commercially available human-ELISA Kits according to the manufacturers’ instructions.
Statistical Analysis
Statistical analysis was performed using Statistical Package for Social Sciences v.15.0 (SPSS Inc., Chicago, IL, USA) for Windows. Categorical data were analyzed by means of the chi-square test. The Kolmogorov–Smirnov normality test was used for testing the Gaussian distribution of continuous data whenever required. Because the number of patients in each treatment group was less than 30, non-parametric tests were used. There were 3 parameters for each of the 3 treatment groups: IL-12, IL-10, and EDSS. First, changes in these parameters over time within each group were analyzed with the Friedman test, and differences among the treatment groups were analyzed using the Kruskal–Wallis H test. Then, post hoc analyses (testing two-samples at a time) for these 3 parameters among and within the treatment groups were performed as follows for significant parameters: the Wilcoxon signed ranks test was used for comparisons within the same group and Mann–Whitney U test was used for comparisons among the groups. P-values of <0.05 were considered significant. Bonferroni correction was applied for the results of post hoc analyses, and the adjusted significance level for multiple comparisons was calculated.
RESULTS
Demographic and clinical data of the RRMS patients are summarized in Table 1. Although the patients were grouped according to the relative severity of symptoms, no significant difference was found among the 3 treatment groups according to age, gender, duration of disease, and duration of symptoms during admission (p>0.05 in Kruskal–Wallis or chi-square tests, wherever applicable). However, we found that the 3 groups significantly differed according to the types of clinical symptoms. In the 5-day treatment group, majority of the patients had sensorial symptoms. On the contrary, patients in the 10-day treatment group had motor symptoms. Patients in the 7-day treatment group had relatively well-balanced according to the symptom types.
Table 1.
Characteristics of RRMS patients; data are presented as n (%) or mean±SD (range)
| Total | 5-day group | 7-day group | 10-day group | ||
|---|---|---|---|---|---|
| Age (years) | 36.9±10.0 (18.0–58.0) | 35.0±8.31 (26.0–57.0) | 39.1±9.9 (27.0–57.0) | 36.2±11.6 (18.0–58.0) | |
| Gender (F/M) | 28/12 | 6/6 | 12/2 | 10/4 | |
| Duration of symptoms at admission (days) | 2.3±0.9 (1.0–5.0) | 2.1±0.8 (1.0–3.0) | 2.6±0.8 (1.0–4.0) | 2.2±1.0 (1.0–5.0) | |
| Duration of disease (years) | 8.5±5.5 (1.0–25.0) | 7.7±2.8 (2.0–10.0) | 8.1±4.6 (1.0–15.0) | 9.5±7.7 (1.0–25.0) | |
| Number of Clinical Relapses | |||||
| 2 | 20 (50.0) | 7 (58.3) | 7 (50.0) | 6 (42.9) | |
| 3–5 | 18 (45.0) | 5 (41.7) | 7 (50.0) | 6 (42.9) | |
| >5 | 2 (5.0) | 0 | 0 | 2 (14.3) | |
| Clinical symptoms | |||||
| Sensory | 12 (30.0) | 8 (66.7) | 3 (21.4) | 1 (7.1) | |
| Motor | 18 (45.0) | 2 (16.7) | 5 (35.7) | 11 (78.6) | |
| Optical | 1 (2.5) | 0 | 1 (7.1) | 0 | |
| Cerebellar | 4 (10.0) | 1 (8.3) | 2 (14.3) | 1 (7.1) | |
| Brain stem | 5 (12.5) | 1 (8.3) | 3 (21.4) | 1 (7.1) | |
| EDSS | |||||
| Pre-treatment | <2.0 | 5 (12.5) | 1 (8.3) | 3 (21.4) | 1 (7.1) |
| 2.0–4.0 | 28 (70.0) | 10 (83.3) | 8 (57.1) | 10 (71.4) | |
| >4.0 | 7 (17.5) | 1 (8.3) | 3 (21.4) | 3 (21.4) | |
| Post-treatment | <2.0 | 24 (60.0) | 9 (75.0) | 8 (57.1) | 7 (50.0) |
| 2.0–4.0 | 15 (37.5) | 3 (25.0) | 6 (42.9) | 6 (42.9) | |
| >4.0 | 1 (2.5) | 0 | 0 | 1 (7.1) | |
| 4th week | <2.0 | 34 (85.0) | 10 (83.3) | 13 (92.9) | 11 (78.6) |
| 2.0–4.0 | 6 (15.0) | 2 (16.7) | 1 (7.1) | 3 (21.4) | |
| >4.0 | 0 | 0 | 0 | 0 | |
| RRMS Treatment | |||||
| Azathiopurine | 4 (10.0) | 1 (8.3) | 1 (7.1) | 2 (14.3) | |
| IFNβ or GA | 16 (40.0) | 4 (33.3) | 6 (42.9) | 6 (42.9) | |
| None | 20 (50.0) | 7 (58.3) | 7 (50.0) | 6 (42.9) | |
RRMS: relapsing–remitting multiple sclerosis; n: number of cases; SD: standard deviation; F: female; M: male; EDSS: Kurtzke Expanded Disability Status Scale; IFNβ: interferon-beta; GA: glatiramer acetate
The levels of both IL-12 and IL-10 were similar in all groups at the beginning of the treatment. Further, EDSS scores were not significantly different (Table 2). In addition, the analysis of cytokine levels and EDSS scores among the 3 groups revealed no any differences throughout the study (during the evaluations at post-treatment and at the 4th week). In other words, IL-12 and IL-10 levels were not significantly different among the 3 treatment groups at neither of the follow-up evaluations. In addition, this was the case for EDSS scores.
Table 2.
Comparison of the three treatment groups for the plasma levels of IL-10 and IL-12 and EDSS scores (mean±SD) of RRMS patients
| Treatment Duration with IVMP | ||||
|---|---|---|---|---|
| 5-day group (n=12) | 7-day group (n=14) | 10-day group (n=14) | p* | |
| IL-12 | ||||
| Pre-treatment | 1.36±0.97 | 1.68±2.06 | 0.94±0.51 | NS |
| Post-treatment | 1.13±0.97 | 1.13±1.23 | 1.03±1.19 | NS |
| 4th week | 1.03±0.66 | 1.39±1.52 | 1.05±0.97 | NS |
| IL-10 | ||||
| Pre-treatment | 1.76±1.06 | 1.51±0.71 | 2.08±0.87 | NS |
| Post-treatment | 2.55±1.59 | 2.44±0.69 | 3.80±1.91 | NS |
| 4th week | 1.80±0.58 | 2.12±1.18 | 2.52±0.82 | NS |
| EDSS | ||||
| Pre-treatment | 3.1±0.7 | 3.3±1.1 | 3.8±0.7 | NS |
| Post-treatment | 2.1±0.8 | 2.1±0.9 | 2.5±0.8 | NS |
| 4th week | 1.5±0.9 | 1.4±0.5 | 1.6±0.7 | NS |
RRMS: relapsing–remitting multiple sclerosis; n: number of cases; SD: standard deviation; p: significance level; NS: not significant; IVMP: intravenous methylprednisolone; EDSS: Kurtzke Expanded Disability Status Scale; IL: interleukin
Kruskal–Wallis test with significance level of <0.05.
The significance levels for the comparisons of the IL-12 and IL-10 levels and EDSS scores at different time points in each treatment group are listed in Table 3. There were no significant changes in IL-12 levels within the treatment groups at different time points (Figure 1). Conversely, there were immediate and significant increases in IL-10 levels at post-treatment measures in all 3 groups; however, the levels decreased during evaluation at the 4th week (Figure 2). The final levels of plasma IL-10 at the 4th week were not significantly different from the pre-treatment levels. EDSS scores significant-ly decreased in all 3 groups after IVMP treatments. The final EDSS score was significantly lower than the previously calculated 2 scores (Figure 3).
Table 3.
The significance levels of differences between the pre-treatment, post-treatment, and 4th week values of IL-10 and IL-12 levels and EDSS scores of RRMS patients in all treatment groups
| p* | |||
|---|---|---|---|
| Pre- vs. post- treatment | Post- treatment vs. 4th week | Pre- treatment vs. 4th week | |
| 5-days (n=12) | |||
| IL-12 | 0.347 | 0.456 | 0.695 |
| IL-10 | 0.008 | 0.028 | 0.099 |
| EDSS | 0.002 | 0.006 | 0.002 |
| 7-days (n=14) | |||
| IL-12 | 0.173 | 0.249 | 0.345 |
| IL-10 | 0.001 | 0.035 | 0.055 |
| EDSS | 0.001 | 0.007 | 0.001 |
| 10-days (n=14) | |||
| IL-12 | 0.778 | 0.925 | 0.638 |
| IL-10 | 0.001 | 0.001 | 0.035 |
| EDSS | 0.001 | 0.001 | 0.001 |
RRMS: relapsing–remitting multiple sclerosis; p: significance level; EDSS: Kurtzke Expanded Disability Status Scale; IL: interleukin
p-value of <0.017 was considered as significant after correction for multiple comparisons
Figure 1.
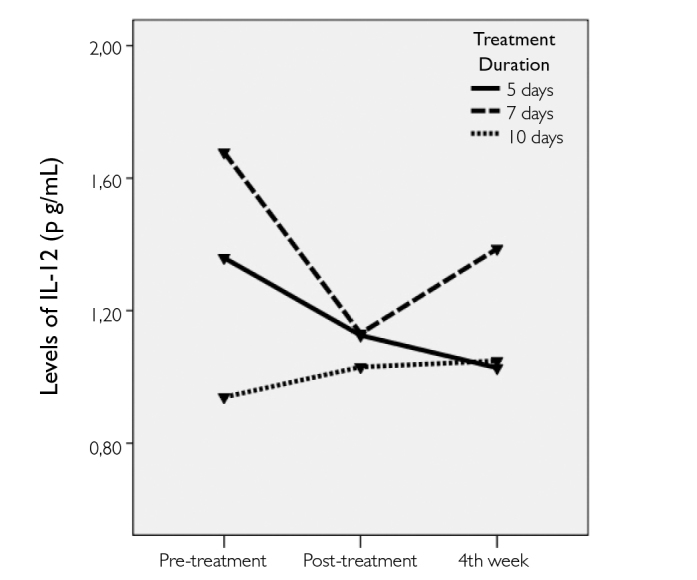
The change in the plasma levels of IL-12 before and after treatment with IVMP in all groups
Figure 2.
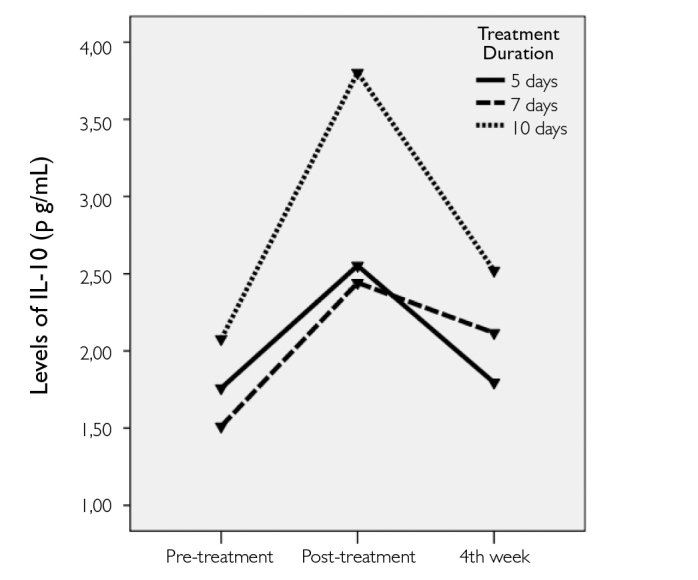
The change in plasma levels of IL-10 before and after treatment with IVMP in all groups
Figure 3.
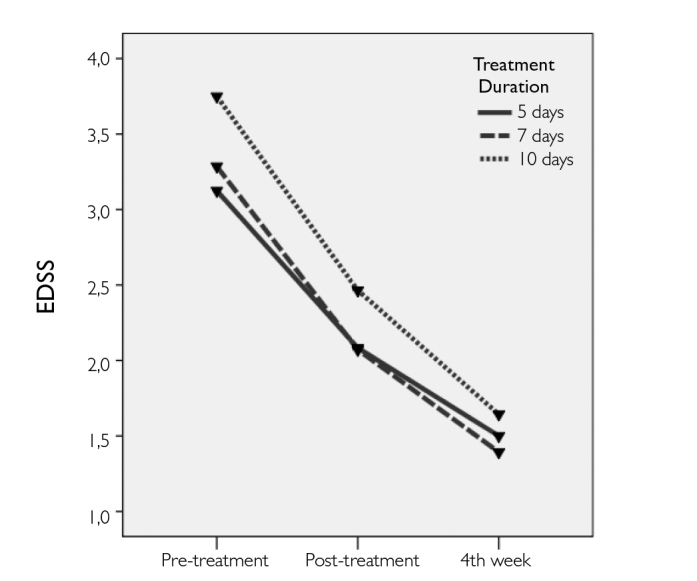
The change in EDSS scores before and after IVMP treatment in all groups
Furthermore, we divided the patients in each group into subgroups according to the usage of chronic MS therapies, i.e., the patients who were on immunomodulatory or immunosuppressive therapies and those who were treatment-naive. Analyses for the effect of RRMS therapies revealed no significant differences (data not shown).
DISCUSSION
To our knowledge, this is the first study to compare the effects of IVMP treatments given for different durations in relapses of MS relapses by measuring changes in serum IL-10 and IL-12 levels. The exact mechanisms responsible for the beneficial effects of IVMP in the relapses of MS have not been clearly identified; however, Rentzos et al. (20) have investigated the effects of IVMP treatment for 5 days on IL-12 and IL-10 levels and CCL2 chemokine expressions. They found no significant influence of the IVMP treatment on IL-12 levels. Similarly, we did not find any significant changes in IL-12 levels with the initiation of IVMP. Moreover, treatment duration did not influence the final results. However, this does not eliminate the potential effects of methylprednisolone on pro-inflammatory cytokines. One explanation could be the intricate cross-talk between various pro- and anti-inflammatory cytokines in the highly complex mechanisms in the pathogenesis of MS. The inhibitory effects of glucocorticoids on pro-inflammatory cytokines in ameliorating the inflammation have been previously mentioned (21,22). On the other hand, in accordance with the study by Rentzos et al. (20), we demonstrated that IVMP significantly induces IL-10 levels. This effect was immediate and significant on the day after the treatment had finished, but it was temporary because this significance was lost at the 4th week.
We found that the EDSS scores substantially and continuously decreased, starting immediately after the treatment, suggesting that the damaged tissue was provided a favorable environment for repair and remyelination to take place. The amelioration of inflammation might be due to the induced levels of anti-inflammatory cytokines to some extent.
Besides the numerous beneficial effects of corticosteroids in treating relapses, a number of side-effects (complications), some of which are dose-dependent, have been reported, including gastrointestinal disturbances, hypertension, palpitations and cardiac arrhythmia, diabetes, mood alterations, insomnia, psychosis, memory impairment, hot flashes, myopathy, osteoporosis/osteopenia, cataract, and endocrine disturbances (12). Although many of the common side-effects are not severe and could easily be manipulated by appropriate precautions, some rare, but serious, ones could be life-threatening. None of our patients suffered from a serious complication related to IVMP treatment during the study.
There are several limitations to this study. First and most importantly, patient selection bias could be an issue as patients were not randomized into the treatment groups. They were grouped according to the severity of their symptoms, which was decided by the treating clinician, at the beginning of the study. Motor symptoms were regarded as more severe due to the disability they cause, than the sensorial symptoms. The lesion sites and lesion loads were not considered during selection. As shown in Table 1, majority of the patients in the 5-day treatment group mainly suffered sensorial symptoms, whereas the patients in 10-day treatment group mostly had motor symptoms. Nevertheless and interestingly, groups were not statistically different according to baseline cytokine levels and EDSS scores, most probably due to the nature of the EDSS scoring system. Second, the effects of treatment duration in a single relapse were assessed; thus, effects on long-term parameters such as time to the next relapse, disability, and disease progression could not have been examined. Even so, the cytokine levels and EDSS scores at the 4th week provided substantial information. Finally, the number of patients in each treatment group was small. Randomized multi-center studies with larger patient groups could provide more credible results.
In conclusion, pulse high-dose IVMP treatment enhances functional recovery in patients with acute relapses of MS. In addition, IVMP treatment significantly increases IL-10 levels, but has no effect on IL-12 levels in the short term. More studies are needed to clarify the exact mechanisms of action of IVMP in treating relapses of MS.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of İzmir Tepecik Training and Research Hospital.
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - Y.Z.; Design - Y.Z., U.K.; Supervision - Y.Z., U.Ş., G.K.; Resource - Y.Z., G.K.; Materials - U.K., Y.Z., M.Ç., U.Ş.; Data Collection and/or Processing - U.K., M.Ç., G.T., G.K.; Analysis and/or Interpretation - U.K., B.I.T., Y.Z., U.Ş.; Literature Search - U.K., B.I.T., Y.Z.; Writing - B.I.T.; Critical Reviews - Y.Z., U.Ş.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Lublin FD, Baier M, Cutter G. Effect of relapses on development of residual deficit in multiple sclerosis. Neurology. 2003;61:1528–1532. doi: 10.1212/01.wnl.0000096175.39831.21. https://doi.org/10.1212/01.WNL.0000096175.39831.21. [DOI] [PubMed] [Google Scholar]
- 2.Bejaoui K, Rolak LA. What is the risk of permanent disability from a multiple sclerosis relapse? Neurology. 2010;74:900–902. doi: 10.1212/WNL.0b013e3181d55ee9. https://doi.org/10.1212/WNL.0b013e3181d55ee9. [DOI] [PubMed] [Google Scholar]
- 3.Imitola J, Chitnis T, Khoury SJ. Cytokines in multiple sclerosis: from bench to bedside. Pharmacol Ther. 2005;106:163–177. doi: 10.1016/j.pharmthera.2004.11.007. https://doi.org/10.1016/j.pharmthera.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Carrieri PB, Ladogana P, Di SG, de Leva MF, Petracca M, Montella S, Buonavolonta L, Florio C, Postiglione L. Interleukin-10 and interleukin-12 modulation in patients with relapsing-remitting multiple sclerosis on therapy with interferon-beta 1a: differences in responders and non responders. Immunopharmacol Immunotoxicol. 2008;30:1–9. doi: 10.1080/08923970802302753. https://doi.org/10.1080/08923970802302753. [DOI] [PubMed] [Google Scholar]
- 5.Kallaur AP, Oliveira SR, Colado Simao AN, Delicato de Almeida ER, Kaminami MH, Lopes J, de Carvalho Jennings Pereira WL, Marques AR, Muliterno PL, Donizete BS, Kaimen-Maciel DR, Reiche EM. Cytokine profile in relapsingremitting multiple sclerosis patients and the association between progression and activity of the disease. Mol Med Rep. 2013;7:1010–1020. doi: 10.3892/mmr.2013.1256. [DOI] [PubMed] [Google Scholar]
- 6.Comabella M, Balashov K, Issazadeh S, Smith D, Weiner HL, Khoury SJ. Elevated interleukin-12 in progressive multiple sclerosis correlates with disease activity and is normalized by pulse cyclophosphamide therapy. J Clin Invest. 1998;102:671–678. doi: 10.1172/JCI3125. https://doi.org/10.1172/JCI3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balashov KE, Smith DR, Khoury SJ, Hafler DA, Weiner HL. Increased interleukin 12 production in progressive multiple sclerosis: induction by activated CD4+ T cells via CD40 ligand. Proc Natl Acad Sci U S A. 1997;94:599–603. doi: 10.1073/pnas.94.2.599. https://doi.org/10.1073/pnas.94.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lovett-Racke AE, Yang Y, Racke MK. Th1 versus Th17: are T cell cytokines relevant in multiple sclerosis? Biochim Biophys Acta. 2011;1812:246–251. doi: 10.1016/j.bbadis.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun L, He C, Nair L, Yeung J, Egwuagu CE. Interleukin 12 (IL-12) family cytokines: Role in immune pathogenesis and treatment of CNS autoimmune disease. Cytokine. 2015;75:249–255. doi: 10.1016/j.cyto.2015.01.030. https://doi.org/10.1016/j.cyto.2015.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ozenci V, Kouwenhoven M, Huang YM, Xiao B, Kivisakk P, Fredrikson S, Link H. Multiple sclerosis: levels of interleukin-10-secreting blood mononuclear cells are low in untreated patients but augmented during interferon-beta-1b treatment. Scand J Immunol. 1999;49:554–561. doi: 10.1046/j.1365-3083.1999.00546.x. https://doi.org/10.1046/j.1365-3083.1999.00546.x. [DOI] [PubMed] [Google Scholar]
- 11.Schweingruber N, Reichardt SD, Luhder F, Reichardt HM. Mechanisms of glucocorticoids in the control of neuroinflammation. J Neuroendocrinol. 2012;24:174–182. doi: 10.1111/j.1365-2826.2011.02161.x. https://doi.org/10.1111/j.1365-2826.2011.02161.x. [DOI] [PubMed] [Google Scholar]
- 12.Goodin DS. Glucocorticoid treatment of multiple sclerosis. Handb Clin Neurol. 2014;122:455–464. doi: 10.1016/B978-0-444-52001-2.00020-0. https://doi.org/10.1016/B978-0-444-52001-2.00020-0. [DOI] [PubMed] [Google Scholar]
- 13.Frohman EM, Shah A, Eggenberger E, Metz L, Zivadinov R, Stuve O. Corticosteroids for multiple sclerosis: I. Application for treating exacerbations. Neurotherapeutics. 2007;4:618–626. doi: 10.1016/j.nurt.2007.07.008. https://doi.org/10.1016/j.nurt.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oliveri RL, Valentino P, Russo C, Sibilia G, Aguglia U, Bono F, Fera F, Gambardella A, Zappia M, Pardatscher K, Quattrone A. Randomized trial comparing two different high doses of methylprednisolone in MS: a clinical and MRI study. Neurology. 1998;50:1833–1836. doi: 10.1212/wnl.50.6.1833. https://doi.org/10.1212/WNL.50.6.1833. [DOI] [PubMed] [Google Scholar]
- 15.Mirowska-Guzel D, Gromadzka G, Kurowska K, Czlonkowski A, Czlonkowska A. Long-term effect of high doses glucocorticosteroids on mRNA expression for IL-6 and IL-8 in relapsed multiple sclerosis patients. Immunopharmacol Immunotoxicol. 2010;32:416–421. doi: 10.3109/08923970903486625. https://doi.org/10.3109/08923970903486625. [DOI] [PubMed] [Google Scholar]
- 16.Alam SM, Kyriakides T, Lawden M, Newman PK. Methylprednisolone in multiple sclerosis: a comparison of oral with intravenous therapy at equivalent high dose. J Neurol Neurosurg Psychiatry. 1993;56:1219–1220. doi: 10.1136/jnnp.56.11.1219. https://doi.org/10.1136/jnnp.56.11.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fierro B, Salemi G, Brighina F, Buffa D, Conte S, La BV, Piazza A, Savettieri G. A transcranial magnetic stimulation study evaluating methylprednisolone treatment in multiple sclerosis. Acta Neurol Scand. 2002;105:152–157. doi: 10.1034/j.1600-0404.2002.1o369.x. https://doi.org/10.1034/j.1600-0404.2002.1o369.x. [DOI] [PubMed] [Google Scholar]
- 18.Sellebjerg F, Barnes D, Filippini G, Midgard R, Montalban X, Rieckmann P, Selmaj K, Visser LH, Sorensen PS. EFNS guideline on treatment of multiple sclerosis relapses: report of an EFNS task force on treatment of multiple sclerosis relapses. Eur J Neurol. 2005;12:939–946. doi: 10.1111/j.1468-1331.2005.01352.x. https://doi.org/10.1111/j.1468-1331.2005.01352.x. [DOI] [PubMed] [Google Scholar]
- 19.Frequin ST, Lamers KJ, Barkhof F, Borm GF, Hommes OR. Follow-up study of MS patients treated with high-dose intravenous methylprednisolone. Acta Neurol Scand. 1994;90:105–110. doi: 10.1111/j.1600-0404.1994.tb02688.x. https://doi.org/10.1111/j.1600-0404.1994.tb02688.x. [DOI] [PubMed] [Google Scholar]
- 20.Rentzos M, Nikolaou C, Rombos A, Evangelopoulos ME, Kararizou E, Koutsis G, Zoga M, Dimitrakopoulos A, Tsoutsou A, Sfangos C. Effect of treatment with methylprednisolone on the serum levels of IL-12, IL-10 and CCL2 chemokine in patients with multiple sclerosis in relapse. Clin Neurol Neurosurg. 2008;110:992–996. doi: 10.1016/j.clineuro.2008.06.005. https://doi.org/10.1016/j.clineuro.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Reichardt HM, Gold R, Luhder F. Glucocorticoids in multiple sclerosis and experimental autoimmune encephalomyelitis. Expert Rev Neurother. 2006;6:1657–1670. doi: 10.1586/14737175.6.11.1657. https://doi.org/10.1586/14737175.6.11.1657. [DOI] [PubMed] [Google Scholar]
- 22.Tischner D, Reichardt HM. Glucocorticoids in the control of neuroinflammation. Mol Cell Endocrinol. 2007;275:62–70. doi: 10.1016/j.mce.2007.03.007. https://doi.org/10.1016/j.mce.2007.03.007. [DOI] [PubMed] [Google Scholar]


