Abstract
Background: Abalones are large marine snails in the family Haliotidae and the genus Haliotis belonging to the class Gastropoda of the phylum Mollusca. The family Haliotidae contains only one genus, Haliotis, and this single genus is known to contain several species of abalone. With 18 additional subspecies, the most comprehensive treatment of Haliotidae considers 56 species valid [1]. Abalone is an economically important fishery and aquaculture animal that is considered a highly prized seafood delicacy. The total global supply of abalone has increased 5-fold since the 1970s and farm production increased explosively from 50 mt to 103 464 mt in the past 40 years. Additionally, researchers have recently focused on abalone given their reported tumor suppression effect. However, despite the valuable features of this marine animal, no genomic information is available for the Haliotidae family and related research is still limited. To construct the H. discus hannai genome, a total of 580-G base pairs using Illumina and Pacbio platforms were generated with 322-fold coverage based on the 1.8-Gb estimated genome size of H. discus hannai using flow cytometry. The final genome assembly consisted of 1.86 Gb with 35 450 scaffolds (>2 kb). GC content level was 40.51%, and the N50 length of assembled scaffolds was 211 kb. We identified 29 449 genes using Evidence Modeler based on the gene information from ab initio prediction, protein homology with known genes, and transcriptome evidence of RNA-seq. Here we present the first Haliotidae genome, H. discus hannai, with sequencing data, assembly, and gene annotation information. This will be helpful for resolving the lack of genomic information in the Haliotidae family as well as providing more opportunities for understanding gastropod evolution.
Keywords: Abalone genome, Halotidae, Haliotis discus hannai
Introduction
Abalone is one of the most important marine gastropod mollusks that inhabits various coastal regions of the world. It is well known that abalone habitation impacts algal communications connected with the reef ecosystem, so they are often utilized for ecological research [2]. Among many abalone species, Haliotis discus hannai is a widely used ingredient in East Asian cuisine and is a valuable food resource due to its richness in protein and other nutrients (Fig. 1) [3, 4]. It is considered an important fishery industry animal. The total global supply of abalone has increased 5-fold since the 1970s. To prevent indiscreetly fishing abalones, legal landings from abalone fisheries have made fishery production decrease gradually from 19 720 mt to 7486 mt but have made farm productions increase explosively from 50 mt to 103 464 mt in the past 40 years [5]. Additionally, researchers have recently focused on H. discus hannai given its reported tumor suppression effect [6–8]. However, despite the valuable features of this marine animal, no genomic information is available. Therefore, the first draft genome in family Haliotidae has the potential to be utilized as a valuable resource for many researchers.
Figure 1.
Example of a H. discus hannai, the pacific abalone.
A single wild abalone (H. discus hannai) was collected from the brood stock at the Genetic and Breeding Research Center of the National Fisheries Research & Development Institute on Geoje Island, Korea for sampling. Hemolymph (10 ml) was withdrawn from the sole side foot muscle using a syringe. For genomic DNA extraction, hemocytes were harvested from fresh hemolymph by centrifugation at 3000 × rpm for 5 min at 4°C. Genomic DNA was extracted using a DNeasy Animal Mini Kit (Qiagen, Hilden, Germany). A total 39.38 μg of DNA was quantified using the standard procedure of Quant-iT PicoGreen dsDNA Assay Kit (Molecular Probes, Eugene, OR, USA) with Synergy HTX Multi-Mode Reader (Biotek, Winooski, VT, USA). Quality of DNA was also checked using ND-1000 spectrophotometer (Thermo Scientifc, Wilmington, DE, USA).
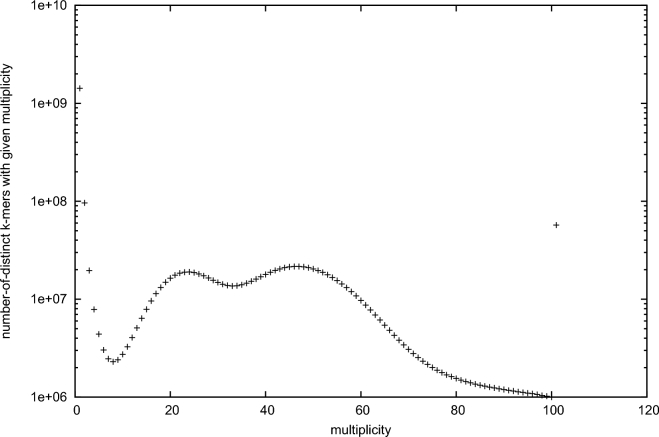
For whole genome shotgun sequencing and draft genome assembly, we used multiple sequencing platforms (Illumina Hiseq2000, Nextseq500 and Pacbio RS II) with seven different libraries. First, two paired-end libraries with insert sizes of 250 and 350 bp were constructed using Illumina TruSeq DNA Sample Prep. Kit (Illumina, San Diego, CA, USA). Mate pair libraries with insert sizes of about 3, 5, 8, and 10 k were constructed for scaffolding process using Illumina Nextera mate-pair library construction protocol (Illumina). For high-quality genome assembly, long mate pair library with insert size over 40 kb is essential. We tried to construct a long mate pair library using 40 kb fosmid clone. However, efficiency of fosmid library construction was very low and we could not retain enough amount of clone. Therefore, Pacbio system was employed for final scaffolding process using long read. Pacbio long reads were generated using P6-C4 chemistry of Pacbio RS II system. Detailed information about the constructed library and generated sequencing data is provided in Table 1. Quality control process of generated raw data was conducted for downstream analysis. Quality of raw data was checked using FASTQC [9] and adapter sequences were removed via Trimmomatic [10], for paired-end libraries, and Nxtrim [11], for mate-pair libraries. K-mer frequency analysis of the abalone genome was conducted using a paired-end library with 350-bp insert size and the jellyfish [12] command-line program. The K-mer distribution of the paired-end library provides valuable information about the target genome. As a result, 19-mer distribution of H. discus hannai genome was generated (Fig. 2). Genome size estimation based on the 19-mer distribution was conducted through “Estimate genome size.pl” code (https://github.com/josephryan/estimate_genome_size.pl/wiki/Estimate-genome-size.pl”). The estimated genome size of H. discus hannai using 19-mer distribution was about 1.65 Gb. Based on the 19-mer distribution of paired-end reads, there was a second peak located in the half x-axis of the main peak. This result indicates that the H. discus hannai genome had high heterozygous genetic character or probable DNA contamination from other organisms. Therefore, before genome assembly, raw reads from Hiseq2000 and Nextseq500 paired-end and mate pairs were preprocessed by bacterial sequences, duplicates, and ambiguous nucleotides. To remove the contaminant sequence, clean reads without adapter and low quality bases were mapped to bacterial and ocean metagenome databases downloaded from NCBI by applying the default setting run (-s 0.8 –l 0.5) of clc_mapper (https://www.qiagenbioinformatics.com/). After that, duplicates and ambiguous nucleotides were filtered out using clc_remove_duplicates (https://www.qiagenbioinformatics.com/). The resulting high-quality sequences were used in subsequent assembly. Error correction and initial contig assembly was conducted using clc_assembler within the CLC Assembly Cell (https://www.qiagenbioinformatics.com/products/clc-assembly-cell/) software pipeline. Scaffolds were then built using the mate-pairs and Pacbio RS II reads sequentially by SSPACE [13] and PBJelly2 [14]. After scaffolding, we iteratively conducted gap filling process using Gapcloser [15] using -l 155 and -p 31 parameter option. Summary statistics for final assembly is provided in Table 2.
Table 1.
Summary statistics of generated whole genome shotgun sequencing data.
| Library name | Library type | Insert size | Platform | Read length | No. read | Total bp |
|---|---|---|---|---|---|---|
| 250 bp | Paired-end | 250 | Nextseq500 | 150 | 876 529 480 | 131 440 418 087 |
| 350 bp | Paired-end | 350 | Hiseq2000 | 101 | 1 413 620 786 | 142 775 699 386 |
| 3 k | Mate-pair | 3000 | Nextseq500 | 150 | 580 064 464 | 85 689 154 056 |
| 5 k | Mate-pair | 5000 | Nextseq500 | 150 | 468 432 888 | 69 966 139 205 |
| 8 k | Mate-pair | 8000 | Nextseq500 | 150 | 335 132 792 | 50 109 845 012 |
| 10 k | Mate-pair | 10 000 | Nextseq500 | 150 | 569 376 096 | 85 080 237 236 |
| 20 k | P6-C4 | 20 000 | Pacbio RS II | 10 094 | ||
| (average) | 1,573,020 | 15 879 626 978 | ||||
| Total | 580 941 119 960 |
Figure 2.
19-mer distribution of using jellyfish with 350-bp paired-end whole genome sequencing data.
Table 2.
Summary statistics for the H. discus hannai draft genome (>2 kb).
| Assembled genome | |
|---|---|
| Size (1n) | 1.80 Gb |
| GC level | 40.51% |
| No. scaffolds | 35 450 |
| N50 of scaffolds (bp) | 211 346 |
| N bases in scaffolds (%) | 116 Mb (6.45%) |
| Longest (shortest) scaffolds (bp) | 2 207 537 (2000) |
| Average scaffold length (bp) | 50 870.65 |
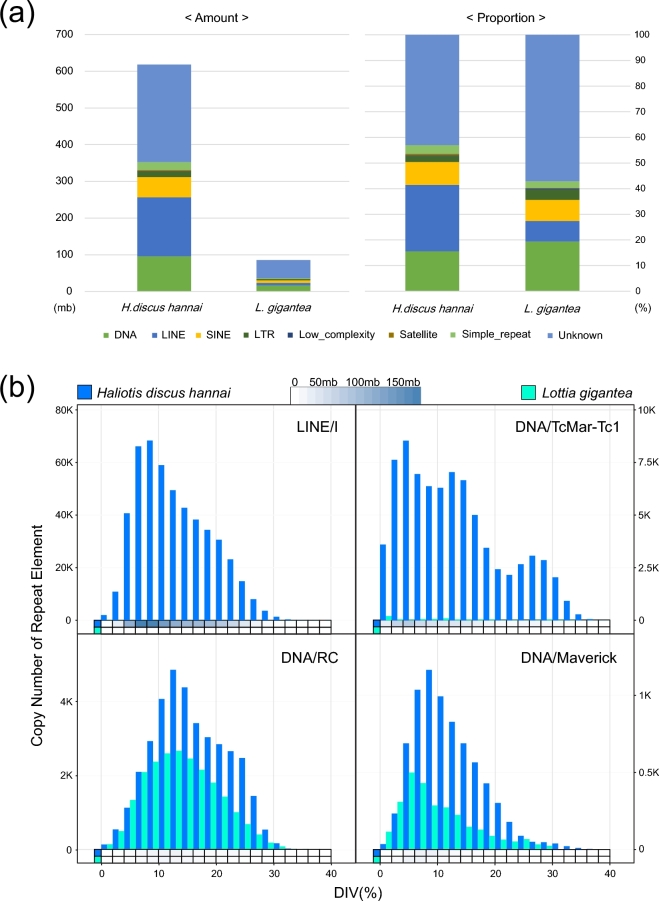
Before conducting gene prediction using the assembled sequence, repeat elements were identified using RepeatMasker [16] with Repbase [17]. RepeatModeler, which includes RECON [18], RepeatScout [19], and TRF [20], was used to create a custom database of H. discus hannai. After custom library construction, RepeatMasker with RMBlast was used for each genome with ‘no_is’ option, using repeat libraries from RepeatModeler and Repbase. Identified mobile elements are summarized in Table 3. Identified repeat elements were parsed for identifying more detailed information using a perl code named “One code to find them all” [21] and Fig. S1 shows the proportion of each mobile element. The genome size of H. discus hannai was 1.86 Gb, and this is the biggest genome among known gastropods. It is 5.31 and 2.02 times larger than genomes size of Lottia gigantea (0.35 Gb) and Aplysia californica (0.92 Gb) in the same Gastropoda class. In animals, the increase of genome size is commonly driven by transposable element, and this is a known genetic adaption mechanism to stressful environments [22]. Therefore, we conducted comparative analysis of repeat element against L. gignatea, a similar marine gastropod with large genome size difference from that of H. discus hannai, to identify the reason for this large difference. Fig. 3a shows the amount and proportion of identified repeat element from two marine gastropods. The proportion of identified total repeat elements in H. discus hannai and L. gigantea is 30.76% and 22.25%, respectively. And the total amount of identified repeat elements in the H. discus hannai genome is almost six times larger than that of L. gigantea same as genome size. Such a linear relationship between genome size and the total proportion of repeat elements is consistent with a previous study [23]. The proportion, copy number, and divergence of each mobile element were identified and compared (Figs S2–6) for a deeper understanding of mobile elements in the two species. From the comparison, a notable finding has been observed on mobile elements: DNA transposable element, a Class II transposable element, exists in diverse forms in both species; however, retrotransposon element, a Class I transposable element, is much more abundant in H. discus hannai genome than in L. gigantea genome. Especially, the number of a non-LTR retrotransposon called LINE Element was exceptionally high. Fig. 3b illustrates the difference between the two species, using two signature mobile elements (H. discus hannai: LINE/I, DNA/TcMar-Tc1, L. gigantea: DNA/RC, DNA/Maverick) in each genome. DNA/RC and DNA/Maverick, two major mobile elements in L. gigantea genome, are observed in H. discus in somewhat similar distribution. On the other hand, the two signature mobile elements of H. discus hannai genome, LINE/I and DNA/TcMar-Tc1, are specifically abundant in H. discus hannai and seems to have expanded recently diverged compared to other elements. In sum, species specificity can be inferred from the distinctive patterns of repeat element expansion between the two species and the increased genome size of H. discus hannai may be associated with the non-LTR elements (especially LINE/I) contribution, in parallel to the human genome [23].
Table 3.
Summary of identified repeat elements in the Haliotis discus hannai genome.
| Repeat element | No. element | Length (%) |
|---|---|---|
| SINE | 284 485 | 96 155 199 (5.11%) |
| LINE | 700 245 | 160 387 248 (8.53%) |
| LTR element | 383 770 | 55 149 794 (2.93%) |
| DNA element | 58 022 | 14 563 432 (0.77%) |
| Small RNA | 20 997 | 1 537 853 (0.08%) |
| Simple repeat | 161 246 | 32 547 245 (1.73%) |
| Low complexity | 326 399 | 21 446 303 (1.14%) |
| Unclassifed | 1 522 272 | 265 603 066 (14.1%) |
Figure 3.
Repeat element information of H. discus hannai compared to L. gigantean. (a) Total amount and ratio of identified repeat element classified into eight classes (DNA, LINE, SINE, LTR, Low complexity, Satellite, Simple repeat, and Unknown) from each genome. (b) Distribution of gene copy number of the two highly possessed repeat elements in each genome based on the divergence. Heat maps indicate the total amount of repeat element divided into 20 levels based on the divergence.
Genes were predicted through three different algorithms: ab initio, RNA-seq transcript based, and protein homology-based. For RNA-seq transcript based prediction, transcriptome data from six organ tissues (Table 4) were aligned to the assembled genome sequence using Tophat [24], and transcript structure was predicted through Cufflinks [25]. The homology-based method employs complete protein sequences from diverse taxonomical genomes, which is fit to our model. For H. discus hannai, the following eight species were utilized: L. gigantea, Crassostrea gigas, A. california, Strongylocentrtus purpuratus, Branchiostoma floridae, Danio rerio, Oncorhynchus mykiss, and Homo sapiens. Those protein sequences were aligned to the H. discus hannai genome using TBASTN (E-value ≤ 1E-4) [26]. Next, the homologous genome sequences were aligned to the matched proteins using Exonerate [27] to predict the accurate spliced alignments. Table 5 summarizes the alignment results of known proteins in various species. For ab initio gene prediction, Augustus [28] was trained using RNA-seq data and known proteins by using the complete transcriptome as training matrix for HMM. Fgenesh [29] and Geneid [30] were also used. The parameters used and the number of predicted genes is provided in Table 6. Gene prediction data from each method was combined using EVM (Evidence Modeler) [31] to build a consensus gene set for the abalone genome. All gene models were converted to EVM compatible GFF3 format and merged to a consensus gene set. After consensus gene annotation was generated from EVM, manual curation was conducted for abandon genes from EVM to build a final consensus gene set of H. discus hannai. Manual curation was performed based on the genomic DNA mapping position of the RNA-seq sequence and the protein sequence of the related species. To determine the exon-intron edge of the gene, the genome mapping information of the transcriptome sequence was firstly reflected, and if not, the mapping information of the protein sequence of the related species was referred to secondarily to confirm the gene model. Finally, genes that were not translated into protein sequences in the final gene model were removed. A total of 29 449 genes was predicted in the H. discus hannai genome and summary statistics for the consensus gene set is provided in Table 7. To evaluate the quality of the H. discus hannai draft genome, we conducted paired-end read remapping and BUSCO (Benchmarking Universal Single-Copy Orthologs) analysis. 94.89% of paired-end reads with a 350-bp insert size were successfully mapped to the assembled genome and assembled genome contains 609 complete and 130 fragmented genes in BUSCO analysis. The detailed information of BUSCO analysis is summarized in Table 8.
Table 4.
Summary statistics of generated transcriptome data for six organ tissues using Illumina platform.
| Library name | Library type | Platform | Read length | No. read | Total bp |
|---|---|---|---|---|---|
| Blood | Paired-end | Hiseq2000 | 101 | 53 525 950 | 5 406 120 950 |
| Digestive duct | Paired-end | Hiseq2000 | 101 | 56 485 666 | 5 705 052 266 |
| Gill | Paired-end | Hiseq2000 | 101 | 66 415 882 | 6 708 004 082 |
| Hepatopancreas | Paired-end | Hiseq2000 | 101 | 58 467 176 | 5 905 184 776 |
| Mantle | Paired-end | Hiseq2000 | 101 | 65 741 776 | 6 639 919 376 |
| Ovary | Paired-end | Hiseq2000 | 101 | 60 997 100 | 6 160 707 100 |
| Total | 36 524 988 550 |
Table 5.
Summary statistics of protein alignment using tBlastn for protein based evidence gene structure.
| Total | Count/ | Total | Mean | Genome | |||
|---|---|---|---|---|---|---|---|
| Species | Type | Element | count | gene | length, bp | length, Bp | coverage, % |
| Homo sapiens | Protein | Transcript | 18 792 | 109 068 639 | 5803.99 | 5.80 | |
| (69 002) | Exon | 77 320 | 4.11 | 12 667 395 | 163.83 | 0.67 | |
| Danio rerio | Protein | Transcript | 11 605 | 68 796 463 | 5928.17 | 3.66 | |
| (42 474) | Exon | 47 300 | 4.08 | 7 978 167 | 168.67 | 0.42 | |
| Oncorhynchus mykiss | Protein | Transcript | 15 901 | 55 043 032 | 3461.61 | 2.93 | |
| (53 876) | Exon | 46 040 | 2.90 | 7 567 059 | 164.36 | 0.40 | |
| Lottia gigantea | Protein | Transcript | 29 345 | 177 851 531 | 6060.71 | 9.47 | |
| (23 851) | Exon | 118 165 | 4.03 | 20 583 999 | 174.20 | 1.10 | |
| Crassostrea gigas | Protein | Transcript | 32 978 | 231 175 282 | 7009.98 | 12.30 | |
| (28 027) | Exon | 140 784 | 4.27 | 23 649 828 | 167.99 | 1.26 | |
| Aplysia californica | Protein | Transcript | 10 570 | 67 396 621 | 6376.22 | 3.59 | |
| (29 096) | Exon | 45 737 | 4.33 | 7 797 503 | 170.49 | 0.42 | |
| Strongylocentrotus purpuratus | Protein | Transcript | 9116 | 46 270 640 | 5075.76 | 2.46 | |
| (38 730) | Exon | 34 572 | 3.79 | 5 627 082 | 162.76 | 0.30 | |
| Branchiostoma floridae | Protein | Transcript | 27 438 | 125 307 206 | 4566.92 | 6.67 | |
| (58 493) | Exon | 92 426 | 3.37 | 15 483 164 | 167.52 | 0.82 |
Table 6.
Summary statistics for ab initio gene prediction results using various programs and parameters.
| Total | Count/ | Total | Mean | Genome | |||
|---|---|---|---|---|---|---|---|
| Program | Matrix | Element | count | gene | length, bp | length, bp | coverage, % |
| Augustus | Custom parameter (RNAseq) | Gene | 88 825 | 3.92 | 367 066 732 | 4132.47 | 19.54 |
| CDS | 348 528 | 76 388 076 | 219.17 | 4.07 | |||
| Custom parameter (H.discus hannai IsoSeq) | Gene | 90 396 | 4.11 | 395 511 710 | 4375.32 | 21.05 | |
| CDS | 371 487 | 78 508 401 | 211.34 | 4.18 | |||
| Custom parameter (H.discus discus IsoSeq) | Gene | 84 322 | 3.97 | 346 455 180 | 4108.72 | 18.44 | |
| CDS | 335 103 | 72 527 841 | 216.43 | 3.86 | |||
| Custom parameter (BUSCO) | Gene | 111 058 | 4.24 | 626 749 935 | 5643.45 | 33.36 | |
| CDS | 470 839 | 84 333 972 | 179.11 | 4.49 | |||
| Custom parameter (CEGAM) | Gene | 76 504 | 4.95 | 393 121 657 | 5138.58 | 20.92 | |
| CDS | 378 485 | 63 424 677 | 167.58 | 3.38 | |||
| Custom parameter (Protein) | Gene | 22 420 | 3.43 | 184 289 721 | 8219.88 | 9.81 | |
| CDS | 76 848 | 20 291 739 | 264.05 | 1.08 | |||
| Fgenesh | Custom parameter | Gene | 184 051 | 3.46 | 1 366 924 540 | 7426.88 | 72.75 |
| CDS | 636 568 | 98 055 591 | 154.04 | 5.22 | |||
| Geneid | Ciona intestinalis | Gene | 789 540 | 1.41 | 436 990 370 | 553.47 | 23.26 |
| CDS | 1 112 959 | 140 976 492 | 126.67 | 7.50 |
Table 7.
Summary statistics for the consensus gene set of Haliotis discus hannai genome.
| Element | No. elements | Exon/transcript | Avg. length | Total length | Genome coverage |
|---|---|---|---|---|---|
| Gene | 29 449 | – | 2705 | 79 661 536 | 4.2% |
| Exon | 74 745 | 2.54 | 280 | 20 985 298 | 1.1% |
| Intron | 45 296 | 1.54 | 1295 | 58 676 238 | 3.1% |
Table 8.
Summary statistics of Benchmarking Universal Single-Copy Orthologs (BUSCO) analysis for H. discus hannai genome based on Metazoans DB.
| Categories | #Genes | Percentage |
|---|---|---|
| Complete single-copy BUSCOs | 609 | 72.2 |
| Complete duplicate BUSCOs | 48 | 5.7 |
| Fragmented BUSCOs | 130 | 15.4 |
| Missing BUSCOs | 104 | 12.3 |
In summary, here we report the first annotated Haliotidae genome of H. discus hannai based on various genetic evidence. We expect that the H. discus hannai genome presented here, which is the first genome to be sequenced in the family Haliotidae, will provide useful genomic information for many researchers. H. discus hannai is a cold-water abalone breed that has difficulties dealing with the change in their inhabitable latitude, which is due to global warming and the resulting increase in the rate of sudden perishing. Genomic information of abalone is essential information that can be used for genetic breeding to improve productivity and genetic engineering for the heat resistance breed. It can also provide valuable information for future genomic studies, because only limited genome information about marine animals and mollusks is currently available. Evolutionary signatures recorded in the abalone genome can be identified through future comparative genomic studies and we expect our result will provide more insight into Haliotidae and marine mollusk evolution.
Availability of supporting data
Raw data is available in project accession PRJNA317403 in the NCBI database. Further supporting data can be found in the GigaScience GigaDB [32].
List of abbreviations
EVM - Evidence Modeler
BUSCO - Benchmarking Universal Single-Copy Orthologs
Competing interests
All authors report no competing interests.
Author contributions
Sampling - Bo-Hye Nam, Young-Ok Kim, Dong-Gyun Kim
Sequencing - Bo-Hye Nam, Hee Jeong Kong, Woo-Jin Kim, Jeong-Ha Kang, Ji-Young Moon, Choul Ji Park, Duk Kyung Kim
Genome assembly - Bo-Hye Nam, Woori Kwak, Jae Woong Yu, Joon Yoon, SaetByeol Lee, Samsun Sung, Chul Lee, Sojeong Ka, Kelsey Caetano-Anolles
Repeat element analysis - Woori Kwak, Minseok Seo, Kwondo Kim
Gene prediction - Woori Kwak, Younhee Shin, Myunghee Jung, Byeong-Chul Kang, Ga-hee Shin
Funding and experimental design – Jung Youn Park, Cheul Min An, Seoae Cho, Heebal Kim
Additional files
Figure S1. Tree map for sum of repeat element for H. discus hannai.
Figure S2. Comparison of SINE element distribution of H. discus hannai and L. gigantea.
Figure S3. Comparison of LINE element distribution of H. discus hannai and L. gigantea.
Figure S4. Comparison of LTR element distribution of H. discus hannai and L. gigantea.
Figure S5. Comparison of DNA transposon element distribution of H. discus hannai and L. gigantea.
Supplementary Material
Figure S1. Tree map for sum of repeat element for H. discus hannai.
Figure S2. Comparison of SINE element distribution of H. discus hannai and L. gigantea.
Figure S3. Comparison of LINE element distribution of H. discus hannai and L. gigantea.
Figure S4. Comparison of LTR element distribution of H. discus hannai and L. gigantea.
Figure S5. Comparison of DNA transposon element distribution of H. discus hannai and L. gigantea.
Acknowledgements
This work was supported by a grant from the National Institute of Fisheries Science (R2016024).
References
- 1. Appeltans W, Bouchet P, Boxshall G et al. World Register of Marine Species. 2012. http://www.marinespecies.org (28 February 2014, date last accessed). [Google Scholar]
- 2. Hamer P, Jenkins G, Womersley B et al. , Understanding the ecological role of abalone in the reef ecosystem of Victoria. Fish Res Rep. 2010; 132p. [Google Scholar]
- 3. Elliott NG. Genetic improvement programmes in abalone: what is the future? Aqua Res. 2000;31(1):51–9. [Google Scholar]
- 4. Gordon HR, Cook PA. World abalone fisheries and aquaculture update: supply and market dynamics. J Shellfish Res. 2004;23(4):935–40. [Google Scholar]
- 5. Cook PA. The worldwide abalone industry. Modern Econ. 2014;5(13):1181. [Google Scholar]
- 6. Suleria HR, Masci P, Gobe G et al. Therapeutic potential of abalone and status of bioactive molecules: a comprehensive review. Crit Rev Food Sci Nutr. 2015;57:1742–48. [DOI] [PubMed] [Google Scholar]
- 7. Lim SY. Cytotoxic and antioxidant activities of abalone (Haliotis discus hannai) extracts. J Life Sci. 2014;24(7):737–42. [Google Scholar]
- 8. Lee C-G, Kwon HK, Ryu JH et al. Abalone visceral extract inhibit tumor growth and metastasis by modulating Cox-2 levels and CD8+ T cell activity. BMC Complement Alt Med. 2010;10(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Andrews S. FastQC a quality-control tool for high-throughput sequence data. 2014. http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ 2 April 2015, date last access. [Google Scholar]
- 10. Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014:btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O’Connell J, Schulz-Trieglaff O, Carlson E et al. NxTrim: optimized trimming of Illumina mate pair reads. Bioinformatics. 2015. 31(12):2035–37. [DOI] [PubMed] [Google Scholar]
- 12. Marçais G, Kingsford C. A fast, lock-free approach for efficient parallel counting of occurrences of k-mers. Bioinformatics. 2011;27(6):764–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boetzer M, Henkel CV, Jansen HJ et al. Scaffolding pre-assembled contigs using SSPACE. Bioinformatics. 2011;27(4):578–79. [DOI] [PubMed] [Google Scholar]
- 14. English AC, Richards S, Han Y et al. Mind the gap: upgrading genomes with Pacific Biosciences RS long-read sequencing technology. PloS One. 2012;7(11):e47768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luo R, Liu B, Xie Y et al. SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. GigaScience. 2012;1(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tarailo‐ Graovac M, Chen N. Using RepeatMasker to identify repetitive elements in genomic sequences. Curr Protoc Bioinformatics. 2009:4.10. 1–4.10. 14. [DOI] [PubMed] [Google Scholar]
- 17. Jurka J, Kapitonov VV, Pavlicek A et al. Repbase Update, a database of eukaryotic repetitive elements. Cyt Genome Res. 2005;110(1–4):462–67. [DOI] [PubMed] [Google Scholar]
- 18. Bao Z, Eddy SR. Automated de novo identification of repeat sequence families in sequenced genomes. Genome Res. 2002;12(8):1269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Price AL, Jones NC, Pevzner PA. De novo identification of repeat families in large genomes. Bioinformatics. 2005;21(suppl 1):i351–i358. [DOI] [PubMed] [Google Scholar]
- 20. Benson G. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Res. 1999;27(2):573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bailly-Bechet M, Haudry A, Lerat E. “One code to find them all”: a perl tool to conveniently parse RepeatMasker output files. Mobile DNA. 2014; 5(1):1.24382139 [Google Scholar]
- 22. Chénais B, Caruso A, Hiard S et al. The impact of transposable elements on eukaryotic genomes: from genome size increase to genetic adaptation to stressful environments. Gene. 2012;509(1):7–15. [DOI] [PubMed] [Google Scholar]
- 23. Kidwell MG. Transposable elements and the evolution of genome size in eukaryotes. Genetica. 2002;115(1):49–63. [DOI] [PubMed] [Google Scholar]
- 24. Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25(9):1105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Trapnell C, Roberts A, Goff L et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protocols. 2012;7(3):562–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Altschul SF, Madden TL, Schäffer AA et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Slater GS, Birney E. Automated generation of heuristics for biological sequence comparison. BMC Bioinformatics. 2005;6(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stanke M, Diekhans M, Baertsch R et al. Using native and syntenically mapped cDNA alignments to improve de novo gene finding. Bioinformatics. 2008;24(5):637–44. [DOI] [PubMed] [Google Scholar]
- 29. Solovyev V, Kosarev P, Seledsov I et al. Automatic annotation of eukaryotic genes, pseudogenes and promoters . Genome Biol. 2006;7(Suppl 1):S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Blanco E, Parra G, Guigó R. Using geneid to identify genes. Curr Protoc Bioinformatics. 2007;4.3. 1–4.3. 28. [DOI] [PubMed] [Google Scholar]
- 31. Haas BJ, Salzberg SL, Zhu W et al. Automated eukaryotic gene structure annotation using EVidenceModeler and the Program to Assemble Spliced Alignments. Genome Biol. 2008;9(1):R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nam B, Kwak W, Kim Y et al. Supporting data for “Genome sequence of pacific abalone (Haliotis discus hannai): the first draft genome in family Haliotidae” GigaScience Database. 2017. http://dx.doi.org/10.5524/100281. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Tree map for sum of repeat element for H. discus hannai.
Figure S2. Comparison of SINE element distribution of H. discus hannai and L. gigantea.
Figure S3. Comparison of LINE element distribution of H. discus hannai and L. gigantea.
Figure S4. Comparison of LTR element distribution of H. discus hannai and L. gigantea.
Figure S5. Comparison of DNA transposon element distribution of H. discus hannai and L. gigantea.