Summary
Using longitudinal data from geographically diverse community cohorts, this study informs disease etiology by describing hepatitis C virus (HCV) incidence temporal trends and related exposure behaviors, highlighting the impact early and sustained harm reduction strategies can have on HCV trends.
Keywords: hepatitis C virus (HCV), incidence trends, epidemiology, people who inject drugs, harm reduction strategies.
Abstract
Background.
We determined temporal trends (1985–2011) in hepatitis C virus (HCV) incidence and associated behavioral exposures for people who inject drugs (PWID) from the United States (Boston, Baltimore, and San Francisco), Canada (Montreal), the Netherlands (Amsterdam), and Australia (Sydney and Melbourne).
Methods.
Using population-based cohort data from HCV-negative PWID, we calculated overall and within-city HCV incidence trends, HCV rates by study enrollment period (1985–2011), and temporal trends in exposure behaviors. Poisson regression models estimated trends in HCV incidence over calendar-time. Survival models identified risk factors for HCV incidence across cities and estimated independent effects of city and calendar period on HCV infection risk.
Results.
Among 1391 initially HCV-negative participants followed prospectively (1644.5 person-years of observation [PYO]), 371 HCV incident infections resulted in an overall incidence of 22.6 per 100 PYO (95% confidence interval [CI], 20.4–25.0). Incidence was highest and remained elevated in Baltimore (32.6/100 PYO), San Francisco (24.7/100 PYO), and Montreal (23.5/100 PYO), lowest in Melbourne and Amsterdam (7.5/100 PYO and 13.1/100 PYO, respectively), and moderate (21.4/100 PYO) in Sydney. Higher rates of syringe and equipment sharing and lower prevalence of opioid agonist therapy were associated with HCV incidence in cities with the highest incidence. Risk for infection dropped by 18% for every 3-year increase in calendar-time (adjusted hazard ratio, 0.8 [95% CI, .8–.9]) in the multivariable model.
Conclusions.
Differences in prevention strategies and injecting contexts may explain the ongoing high HCV incidence in these North American cities and emphasize the need for scale-up of opioid agonist therapy and increased coverage of needle and syringe programs in North America.
More than 10 million people who inject drugs (PWID) live with chronic hepatitis C virus (HCV) infection globally, and HCV prevalence is estimated to be between 10% and 90% in this population [1, 2]. Injection drug use continues to be the major mode of HCV transmission in high- and middle-income countries since the implementation of effective blood supply screening in the early 1990s [3]. To date, meta-analyses and systematic reviews of HCV have focused on prevalence, with analyses of data from the same studies [1, 4, 5]. Only 2 meta-studies included HCV incidence estimates, and no prior studies compared HCV incidence across geographic regions or time periods [6, 7]. Robust data on HCV incidence are essential for describing disease etiology and developing effective public health responses, including prevention and treatment.
Longitudinal studies enable the observation and analysis of HCV transmission trends over time and produce insights into drivers of the epidemic. The International Collaboration of Incident HIV and HCV in Injecting Cohorts (InC3) study brings together well-characterized longitudinal cohorts of PWID in the United States (Boston, Baltimore, and San Francisco), Canada (Montreal), the Netherlands (Amsterdam), and Australia (Sydney and Melbourne) [8] (https://ctsc.health.unm.edu/apps/inc3/). Each cohort systematically collected prospective serologic and behavioral data from HCV antibody–negative PWID recruited from the community, and together, span >2 decades (1985–2011). City-level differences in harm reduction policies and intervention coverage are difficult to directly measure. We leveraged epidemiological data collected at the individual level to (1) estimate overall incidence of HCV infection and temporal (1985–2011) trends in incidence; (2) examine behavioral predictors of HCV infection; and (3) describe and compare temporal trends in injecting exposures within a subset of InC3 cities (Baltimore, San Francisco, Montreal, Sydney, Amsterdam, and Melbourne). This study provides findings on HCV etiology and inferences about drivers of infection trends within a high-income country context, globally.
METHODS
Study Population
Participants were selected from pooled epidemiological data collected from prospective cohort studies in Baltimore [9], San Francisco [10], Montreal [11], Sydney [12, 13], Amsterdam [14], and Melbourne [15]. Study eligibility criteria required PWID to be HCV antibody (anti-HCV) negative and HCV RNA negative at enrollment, with at least 1 follow-up serologic visit, and to self-report recent injection drug use (Table 1). Two InC3 cohorts (Boston Boston Acute HCV Study: Transmission, Immunity, and Outcome Network (BAHSTION) cohort and the Sydney Australian Trial in Acute Hepatitis C (ATAHC) cohort) were excluded because only participants with acute or early (within 2 years of exposure) HCV infection were enrolled. A third InC3 cohort (Australian HITS-p cohort) recruited from prisons, rather than community samples, and thus was excluded [8].
Table 1.
Summary of Inclusion and Study Procedures Applicable to Study Analysis
| Cohort Name | City | Sample Size | Inclusion Criteria | No. of Sites | Recruitment Method(s) | Enrollment Perioda | Follow-up Interval for Serologyb |
|---|---|---|---|---|---|---|---|
| Amsterdam Cohort Studies (ACS) | Amsterdam | 48 | Active drug users (both PWID and non-PWID) using hard drugs at least 3 times/wk; ≥18 y of age; HIV negative and anti-HCV negative | 1 | Community-based outreach; open enrollment | 1984–present | 4-mo intervals (until 2003) then 6-mo intervals |
| Baltimore Before and After Acute Study in Hepatitis (BBAASH)c | Baltimore | 288 | Active PWID aged 18–65 y; anti-HCV negative | 1 | Community-based outreach; open enrollment | 1996–present | Monthly |
| Networks Study (N2) | Melbourne | 199 | Injection drug use in past 6 mo; ≥18 years of age; anti-HCV negative | 6 | Community- based outreach and respondent-driven sampling; open enrollment | 2005–2012 | 3-mo intervals |
| St Luc Cohort (HEPCO) | Montreal | 244 | Injection drug use in past 6 mo; ≥14 y of age; anti-HCV negative | 1 | Community-based outreach; open enrollment | 2004–present | 3–6-mo intervals |
| The UFO Study (UFO) | San Francisco | 398 | Injection drug use in past mo; <30 y of age at enrollment; anti-HCV negative | 1 | Community-based outreach; open enrollment | 2000–present | Monthly |
| Hepatitis C Virus Cohort (CU) | Sydney | 257 | Injection drug use within the past 6 mo; anti-HCV negative | 3 | Community-based outreach; open enrollment | 1999–2002 | 3–6-mo intervals |
| Hepatitis C Incidence and Transmission Study- Community (HITS-c) | Sydney | 134 | Injection drug use within past 12 mo; ≥16 y of age; anti- HCV negative | 5 | Community-based outreach; open enrollment | 2008–2012 | 3-, 6-, 9-, 12-, 15-, 18-, 24-mo intervals |
All cohorts enrolled participants prospectively.
Abbreviations: anti-HCV, hepatitis C virus antibody; HCV, hepatitis C virus; HIV, human immunodeficiency virus; PWID, people who inject drugs.
aAs of March 2015.
bAntibody and RNA testing were performed at the time of acute HCV detection. Anti-HCV testing was performed using the following assays: HCV enzyme immunoassay (EIA) 2.0 (Abbott Laboratories, Abbott Park, Illinois), EIA-3 (Ortho Clinical Diagnostics, Raritan, New Jersey), or Abbott Architect anti-HCV. Very little difference in antibody detection has been demonstrated between HCV EIA 2.0 and 3.0 [18]. Qualitative HCV RNA testing was performed using the following assays: Versant TMA (Bayer, Australia; <10 IU/mL), COBAS AmpliPrep/COBAS TaqMan (Roche, Branchburg, New Jersey; <15 IU/mL), COBAS Amplicor HCV Test v2.0 (Roche Diagnostics, Mannheim, Germany; <50 IU/mL), or discriminatory HCV transcription-mediated amplification component of the Procleix HIV-1/HCV (Gen-Probe, San Diego, California; <12 copies/mL). Quantitative HCV RNA testing was performed using the Versant HCV RNA 3.0 (Bayer; <615 IU/mL), COBAS Amplicor HCV Monitor 2.0 (Roche Diagnostics; <600 IU/mL), COBAS AmpliPrep/COBAS TaqMan (Roche; <15 IU/mL), or an in-house polymerase chain reaction (<1000 IU/mL).
cBBAASH recruits and monitors young PWID for HCV infection, with a focus on serological data to assess the clinical characteristics of acute infection and reinfection. Because research goals focus on understanding questions related to immunology and virology, behavioral data were not systematically collected between 2000 and 2012. The BBAASH data server was compromised in 2005, leading to the loss of data on age and biological sex for some participants. Investigators preformed genome-wide association studies to obtain sex for participants who acquired HCV infection, but because age is not a genetic trait, age information was not recovered.
Data Collection and Procedures
Cohorts systematically collected self-reported data on sociodemographic characteristics, injecting behaviors, and serological data on anti-HCV and HCV RNA. All cohorts also provided loss-to-follow-up data.
HCV Testing
HCV testing methods varied by cohort, including anti-HCV, HCV RNA, and genotype testing, but were consistent within each site (Figure 1). The majority of HCV tests occurred during regular study data collection periods, with some retrospective testing on frozen serum or plasma specimens to fill in data gaps.
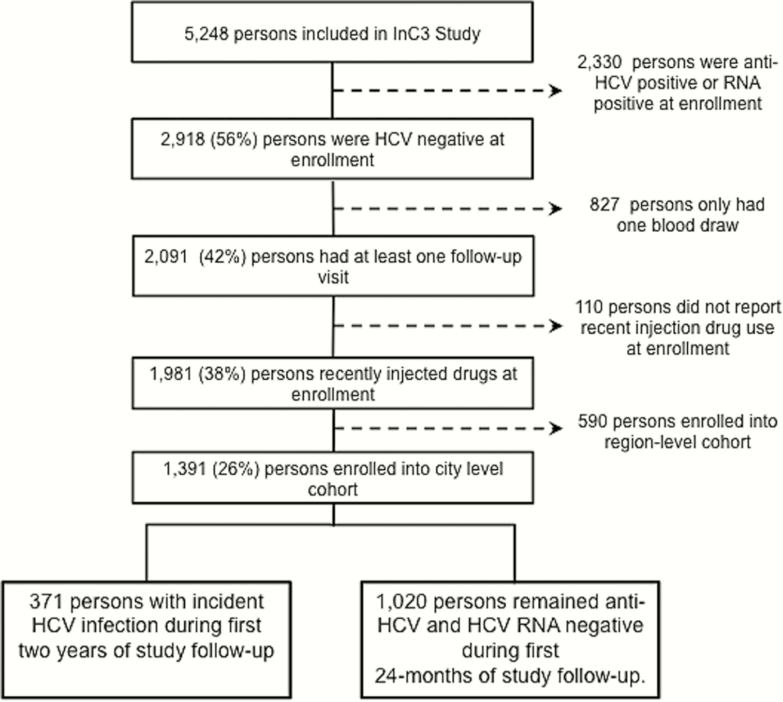
Figure 1.
Participant inclusion flowchart. Abbreviations: HCV, hepatitis C virus; InC3, International Collaboration of Incident HIV and HCV in Injecting Cohorts.
HCV Infection Outcome
In this analysis, primary HCV incident infection (hereafter referred to as HCV incident infection) was defined as either (1) positive anti-HCV and HCV RNA positive, or (2) positive HCV RNA test following a previously documented negative anti-HCV and HCV RNA test (Figure 1). Because a few cohorts stopped following participants at the 24-month mark (Baltimore and Sydney), we censored the follow-up period of all participants eligible for this analysis at 24 months.
The date of HCV infection was estimated as either (1) the midpoint between the last negative and first positive anti-HCV test dates for those defined as having anti-HCV seroconversion, or (2) the date of the first HCV RNA–positive visit minus 28 days for those whose infection was identified via an RNA-positive/anti-HCV–negative test [16]. The median duration of observed time to incident HCV was 12 months (interquartile range [IQR], 6–20).
Statistical Analyses
To examine potential bias due to differential loss to follow-up, we used Pearson χ2 or Kruskal-Wallis tests to compare distributions of selected demographic and injecting risk behavior variables between participants with follow-up visits and those with only baseline visits.
We used descriptive analyses to characterize the study population overall and by city (Baltimore, San Francisco, Montreal, Sydney, Amsterdam, and Melbourne). Primary analyses employed life-table methods to construct HCV incidence curves by city and calculate HCV infection rates within the first 24 months of follow-up (presented as rates per 100 person-years of observation [PYO] with 95% confidence intervals [CIs]). Next, we conducted analyses to describe overall and within-city HCV incidence trends. To compare HCV infection rates across cities, we used Kaplan-Meier estimates and log-rank tests (see Supplementary Figure 1). The same approach was used to compare HCV rates by period of study enrollment (1985–1990 and in 3-year intervals from 1991 to 2011) across cities. We used Poisson regression to test for the trend in HCV incidence over calendar-time (presented as incidence rate ratios [IRRs] per 3-year intervals and 95% CIs).
We fit bivariate Poisson regression models to examine possible explanations for differences in HCV incidence trends across cities. Next, using self-reported data collected at enrollment, we plotted temporal trends in the proportion of persons reporting the following recent injecting behaviors: (1) main or most frequent/often illicit drug injected (heroin, methamphetamine/amphetamine, cocaine, other opiates (ie, prescription opioids), or heroin and cocaine in combination); (2) receptive syringe sharing (RSS) (yes/no); (3) opioid agonist therapy (OAT), including methadone and buprenorphine (yes/no); and (4) access to sterile syringes through safe sources (needle syringe program, chemist/pharmacist, vending machine, mobile outreach) (yes/no). Supplementary Table 1 provides details on time frames for risk factor measures by cohort. Proportions were calculated for 1985–1990 and 3-year intervals from 1991 to 2011. Analyses did not include time-varying covariates due to variability in follow-up intervals and data availability. Last, we fit a stratified multivariable Cox propositional hazards model, allowing unique baseline hazards by city, to assess the independent relationship between recent RSS, OAT, and calendar-time with risk for HCV incidence, while controlling for biological sex, age, and city. Sensitivity analyses assessed multivariable model fit and main effects when Baltimore was included (Supplementary Table 2). All analyses were performed using Stata software version 13.0 (College Station, Texas).
RESULTS
General Characteristics
In total, 827 HCV-negative participants did not complete follow-up visits. These participants were not significantly different from those with at least 1 follow-up visit with respect to biological sex, ethnicity, main or most frequent drug injected, frequency of injection, recent OAT, and history of incarceration. However, participants lost to follow-up were significantly younger (mean age, 23 vs 26 years; P ≤ .001), more educated (53% vs 41% completed secondary school; P ≤. 001), and more likely to report RSS (40% vs 19%; P ≤. 001) than those who were retained. Supplementary Table 3 details loss to follow-up rates by city.
Of the 1391 persons included in this study, 49% were in the United States, 18% in Canada, 30% in Australia, and 3% in the Netherlands. Overall, 64% were male, and the median age at enrollment was 25 years (IQR, 21–29 years). The most frequently injected drug at baseline was heroin (53%), followed by meth/amphetamine (21%), cocaine (13%), other opiates (8%), and heroin and cocaine in combination (4%); Baltimore did not collect data on drug use. Table 2 shows all participant characteristics, including loss to follow-up, at enrollment by city.
Table 2.
Frequencies and Hepatitis C Virus Incidence Estimates for Baseline Characteristics by City (N = 1391)
| Characteristic | Baltimore (n = 288) | San Francisco (n = 398) | Montreal (n = 244) | Sydney (n = 294) | Amsterdam (n = 48) | Melbourne (n = 119) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. (%) | IR | No. (%) | IR | No. (%) | IR | No. (%) | IR | No. (%) | IR | No. (%) | IR | |
| Overall HCV incidence | 108 (37) | 32.6 (26.8–39.8) | 137 (34) | 24.7 (20.4–29.9) | 94 (38) | 23.5 (18.8–29.4) | 66 (22) | 21.4 (16.7–27.3) | 17 (35) | 13.1 (7.0–24.3) | 32 (27) | 7.5 (4.6–12.3) |
| Median No. of study visits (IQR) | 13 (5–23) | … | 4 (3–7) | … | 8 (4–15) | … | 4 (2–5) | … | 17 (5–29) | … | 4 (3–8) | … |
| Loss to follow-up before 24 mo (n = 1020) | 112 (59) | … | 201 (68) | … | 61 (36) | … | 181 (78) | … | 9 (24) | … | 39 (38) | … |
| Biological sex | ||||||||||||
| Male | 141 (49) | 41.5 (32.0–54.0) | 265 (67) | 22.2 (17.5–29.3) | 197 (81) | 24.6 (19.3–31.3) | 200 (68) | 24.6 (19.3–31.3) | 35 (73) | 14.6 (7.3–29.3) | 78 (66) | 9.2 (5.3–15.8) |
| Female | 78 (27) | 60.0 (44.3–81.0) | 133 (33) | 30.4 (22.2–41.6) | 47 (19) | 19.0 (10.8–33.4) | 94 (32) | 19.0 (10.8–33.4) | 13 (27) | 9.2 (2.3–36.6) | 41 (35) | 4.2 (1.4–13.0) |
| Unknowna | 69 (24) | 1.0 (0.1–7.2) | 0 | … | 0 (0) | … | 0 (0) | … | 0 (0) | … | 0 (0) | … |
| Median age, y (IQR) | 25 (23–29) | 22 (20–26) | 31 (26–39) | 24 (20–28) | 27 (24–31) | 25 (22–28) | ||||||
| Education level | ||||||||||||
| High school or below | 0 (0) | … | 173 (44) | 25.7 (19.6–33.7) | 64 (26) | 29.0 (19.4–43.2) | 117 (40) | 29.0 (19.4–43.2) | 3 (6) | … | 31 (26) | 3.1 (0.8–12.3) |
| Completed high school or above | 0 (0) | … | 219 (55) | 22.4 (16.9–29.7) | 180 (74) | 21.7 (16.5–29.3) | 177 (60) | 21.7 (16.5–28.3) | 0 (0) | … | 16 (13) | 5.8 (1.5–23.4) |
| Unknowna | 288 (100) | … | 6 (1) | … | 0 (0) | … | 0 (0) | … | 45 (94) | … | 72 (61) | 10.6 (6.0–18.6) |
| Ethnicity | ||||||||||||
| White/ Caucasian | 147 (51) | … | 298 (75) | 25.7 (20.8–31.9) | 220 (90) | 24.1 (19.1–30.4) | 218 (74) | 24.1 (19.1–30.4) | 39 (81) | 12.6 (6.3–25.2) | 94 (79) | 5.9 (3.2–11.1) |
| Black/African | 39 (14) | … | 33 (8) | 20.2 (9.6–42.3) | 2 (1) | 40.2 (5.7–285.6) | 54 (18) | 40.2 (5.7–285.6) | 0 (0) | … | 16 (13) | 17.3 (7.2–41.1) |
| Indigenous/ Native American | 0 (0) | … | 9 (2) | 20.8 (5.2–83.3) | 8 (3) | 26.4 (6.6–105.6) | 13 (4) | 26.4 (5.6–105.6) | 0 (0) | … | 6 (5) | … |
| Mixed or other | 2 (1) | … | 52 (13) | 24.0 (13.9–42.2) | 6 (2) | 23.5 (5.9–93.9) | 7 (2) | 23.5 (5.9–93.9) | 5 (10) | 13.2 (1.9–93.8) | 0 (0) | … |
| Unknowna | 100 (45) | … | 6 (1) | … | 8 (3) | 7.0 (1.0–49.9) | 2 (1) | … | 4 (8) | 18.5 (2.6–131.4) | 3 (3) | 21.7 (3.1–154.0) |
| History of prison sentence | ||||||||||||
| No | 0 (0) | … | 79 (20) | 22.1 (14.1–34.6) | 64 (26) | 21.1 (13 .3–33 .5) | 78 (27) | 13.8 (8.0–23.8) | 0 (0) | … | 87 (73) | 8.4 (4.9–14.5) |
| Yes | 0 (0) | … | 316 (80) | 24.6 (19.8–30.5) | 180 (73) | 24.4 (18 .9–31 .4) | 56 (19) | 4.5 (1.5–13.9) | 20 (42) | 2.6 (0.4–18.6) | 32 (27) | 5.1 (1.6–15.8) |
| Unknowna | 288 (100) | … | 3 (<1) | … | 0 (0) | … | 160 (54) | 34.7 (26.2–46.1) | 28 (58) | 23.5 (12.2–45.2) | 0 (0) | … |
| Recent unstable housing | ||||||||||||
| No | 0 (0) | … | 59 (15) | 17.6 (9.2–33.8) | 91 (37) | 18.6 (12.4–27.8) | 100 (34) | 9.0 (5.0–16.3) | 26 (54) | 7.0 (2.3–21.8) | 76 (63.9) | 7.93 (4.4–14.3) |
| Yes | 0 (0) | … | 329 (82.7) | 24.9 (20.3–30.6) | 153 (62.7) | 26.7 (20.4–35.0) | 194 (66) | 30.0 (22.9–39.2) | 17 (35.4) | 6.7 (1.7–26.7) | 43 (36.1) | 6.6 (2.8–16.2) |
| Unknowna | 288 (100) | … | 10 (2) | … | 0 (0) | … | 0 (0) | … | 5 (10) | … | 0 (0) | … |
| Recent unemployment | ||||||||||||
| No | 0 (0) | … | 94 (24) | 25.4 (16.9–38.2) | 153 (63) | 26.3 (20.0–34.5) | 77 (26) | 28.5 (18.2–44.6) | 0 (0) | … | 49 (41) | 3.5 (1.1–10.7) |
| Yes | 0 (0) | … | 286 (72) | 23.9 (19.1–30.0) | 72 (30) | 15.7 (9.8–25.3) | 83 (28) | 40.6 (28.2–58.4) | 0 (0) | … | 69 (58) | 11.3 (6.6–19.5) |
| Unknowna | 288 (100) | … | 19 (5) | 32.9 (15.7–69.0) | 19 (8) | 37.1 (18.5–74.1) | 134 (46) | 9.6 (6.1–16.2) | 48 (0) | … | 1 (<1) | … |
| HIV status | ||||||||||||
| Positive | 0 (0) | 7 (2) | 20.4 (5.1–81.6) | 1 (1) | … | 0 (0) | … | 0 (0) | … | 0 (0) | … | |
| Negative | 0 (0) | 265 (67) | 23.7 (18.8–29.9) | 241 (98) | 23.3 (18.6–29.2) | 134 (46) | 10.0 (6.1–16.2) | 35 (73) | 5.0 (1.6–15.6) | 67 (56) | 8.4 (4.4–16.2) | |
| Unknowna | 288 (100) | … | 126 (31) | 27.5 (19.6–38.7) | 2 (1) | 84.4 (11.9–598.8) | 160 (54) | 24.9 (19.0–32.5) | 13 (27) | 41.5 (19.8–87.2) | 52 (44) | 6.6 (3.2–13.9) |
| Median (IQR) duration of injection drug use, y | 4 (1–7) | 7 (3–13) | 4 (2–8) | 4 (1–8) | 6 (3–10) | |||||||
| ≤2 y | 0 (0) | … | 105 (26) | 24.3 (16.7–35.5) | 34 (14) | 35.3 (20.9–59.5) | 58 (20) | 35.3 (20.9–59.5) | 17 (35) | 19.9 (8.3–47.7) | 14 (12) | 13.7 (4.4–42.5) |
| >2 y | 0 (0) | … | 293 (73) | 24.8 (19.9–31.0) | 210 (86) | 21.9 (17.1–28.0) | 236 (80) | 21.9 (17.1–28.0) | 31 (65) | 9.7 (4.1–23.4) | 104 (87) | 7.2 (4.2–12.4) |
| Drug injected most often | … | |||||||||||
| Heroin | 0 (0) | … | 214 (54) | 27.4 (21.2–35.6) | 68 (28) | 12.4 (7.0–21.8) | 203 (69) | 25.3 (18.7–34.1) | 18 (38) | 17.9 (7.5–43.1) | 76 (64) | 8.3 (4.6–15.0) |
| Amphetamine/ methamphetamine | 0 (0) | … | 126 (32) | 17.5 (11.8–25.9) | 1 (1) | … | 70 (24) | 7.8 (3.4–17.3) | 5 (10) | … | 19 (16) | 10.4 (3.4–32.0) |
| Cocaine | 0 (0) | … | 9 (2) | 16.4 (4.1–65.5) | 121 (49) | 19.3 (13.5–27.6) | 6 (2) | 56.9 (31.5–102.8) | 5 (10) | 12.0 (1.7–85.5) | 1 (1) | … |
| Other opiates | 0 (0) | … | 3 (1) | 32.7 (4.6–232.1) | 50 (21) | 51.3 (34.4–76.6) | 14 (5) | 6.6 (0.9–46.8) | 0 (0) | … | 20 (17) | 5.5 (1.4–22.0) |
| Heroin + cocaine (combined) | 0 (0) | … | 28 (7) | 35.3 (19.6–63.8) | 5 (2) | … | 0 (0) | … | 17 (35) | 16.1 (6.1–43.0) | 0 (0) | … |
| Unknowna | 288 (100) | … | 18 (4) | … | 6 (2) | 38.7 (21.4–69.8) | 1 (<1) | 12.6 (7.4–21.2) | 3 (6) | … | 3 (2) | … |
| Recent receptive syringe sharing | ||||||||||||
| No | 0 (0) | … | 231 (58) | 18.4 (14.0–24.3) | 166 (68) | 22.5 (17.1–29.5) | 226 (77) | 22.5 (17.1–29.5) | 28 (58) | 9.3 (3.9–22.4) | 97 (82) | 6.3 (2.5–11.4) |
| Yes | 0 (0) | … | 147 (37) | 34.6 (26.1–45.8) | 76 (31) | 26.0 (17.6–38.5) | 68 (23) | 26.0 (17.6–38.5) | 7 (15) | 39.0 (16.2–93.7) | 22 (18) | 13.7 (5.7–33.0) |
| Unknown a | 288 (100) | … | 20 (5) | 71.6 (29.8–171.9) | 2 (1) | … | 0 (0) | … | 13 (27) | … | 0 (0) | … |
| Recent equipment sharing | ||||||||||||
| No | 0 (0) | … | 67 (17) | 15.8 (9.2–22.0) | 139 (57) | 22.5 (16.7–30.4) | 233 (79) | 22.5 (16.7–30.4) | 0 (0) | … | 47 (40) | 4.8 (2.0–11.5) |
| Yes | 0 (0) | … | 257 (65) | 28.1 (22.6–34.9) | 103 (42) | 24.9 (17.8–34.8) | 61 (21) | 24.9 (17.8–34.8) | 0 (0) | … | 1 (1) | … |
| Unknowna | 288 (100) | … | 74 (19) | 19.6 (10.6–36.5) | 2 (1) | … | 0 (0) | … | 48 (100) | 13.1 (7.0–24.3) | 71 (60) | 11.2 (6.2–20.3) |
| Recent opioid agonist therapy, including methadone and buprenorphine | ||||||||||||
| No | 0 (0) | … | 342 (86) | 24.2 (18.7–31.3) | 168 (69) | 26.4 (20.7–33.7) | 157 (53) | 26.4 (20.7–33.7) | 23 (48) | 19.9 (9.5–41.6) | 44 (37) | 8.4 (4.5–15.6) |
| Yes | 0 (0) | … | 50 (13) | 11.8 (3.8–36.6) | 75 (31) | 15.3 (8.9–26.3) | 137 (46) | 15.3 (8.9–26.3) | 25 (52) | 7.3 (2.3–22.6) | 75 (63) | 5.4 (2.2–12.9) |
| Unknowna | 288 (100) | … | 6 (2) | … | 1 (1) | … | 0 (0) | … | 0 (0) | … | 0 (0) | … |
| Recently obtained any needles/syringes from safe sourceb | ||||||||||||
| No | 0 (0) | … | 47 (12) | 6.9 (2.3–18.4) | 21 (8) | 8.6 (2.8–26.8) | 14 (5) | … | 0 (0) | … | 3 (3) | … |
| Yes | 0 (0) | … | 350 (88) | 27.7 (22.8–33.6) | 223 (92) | 25.3 (20.1–31.7) | 115 (39) | 10.8 (6.5–18.0) | 44 (92) | 13.0 (6.8–25.0) | 53 (45) | 4.5 (1.9–10.9) |
| Unknown a | 287 (100) | … | 1 (<1) | … | 0 (0) | … | 165 (56) | 24.9 (19.1–32.5) | 4 (8) | 13.7 (1.9–97.4) | 63 (53) | 11.3 (6.3–20.5) |
Incidence rates are shown as IR per 100 person-years (95% confidence interval), calculated using the quadratic approximation to the Poisson log likelihood for the log-rate parameter. Boldface values indicate significant difference at P < .05.
Abbreviations: HCV, hepatitis C virus; HIV, human immunodeficiency virus; IQR, interquartile range; IR, incidence rate; PY, person-years.
aData not collected by cohort or not reported by participant; the Baltimore cohort recruits and monitors young people who inject drugs for HCV infection, with a focus on serological data to assess the clinical characteristics of acute infection and reinfection. Because research goals focus on understanding questions related to immunology and virology, behavioral data were not systematically collected between 2000 and 2012. The Baltimore data server was compromised in 2005, leading to the loss of data on age and sex for some participants. Investigators preformed genome-wide association studies to obtain sex for participants who acquired HCV infection, but because age is not a genetic trait, age information was not recovered.
bSafe source defined as needle syringe program, needle exchange program, chemist/pharmacist, vending machine, mobile outreach.
HCV Incidence: Overall, Geographic, and Temporal Trends
Overall Incidence
Over 1644.5 PYO, 371 persons had a HCV incident infection for an overall estimated incidence of 22.6/100 PYO (95% CI, 20.4–25.0). Approximately one-half (n = 187) of incident infections were identified via an RNA-positive test. Overall, incidence decreased from 24.6/100 PYO (95% CI, 21.8–27.8) in the first year of observation to 18.8/100 PYO (95% CI, 15.5–22.7) during the second study year. Compared with Melbourne, which had the lowest incidence rate of HCV (7.5/100 PYO [95% CI, 4.6–12.3/100 PYO]), IRRs were significantly elevated for Baltimore (IRR, 1.89 [95% CI, 1.1–3.14]), San Francisco (IRR, 2.6 [95% CI, 1.5–4.4]), Montreal (IRR, 2.6 [95% CI, 1.5–4.4]), and Sydney (IRR, 2.2 [95% CI, 1.3–3.8]). Incidence in Amsterdam was not significantly elevated compared with Melbourne (IRR, 1.6 [95% CI, .7–3.6]). Table 2 describes overall HCV incidence rates by city.
Temporal Trends in HCV Incidence
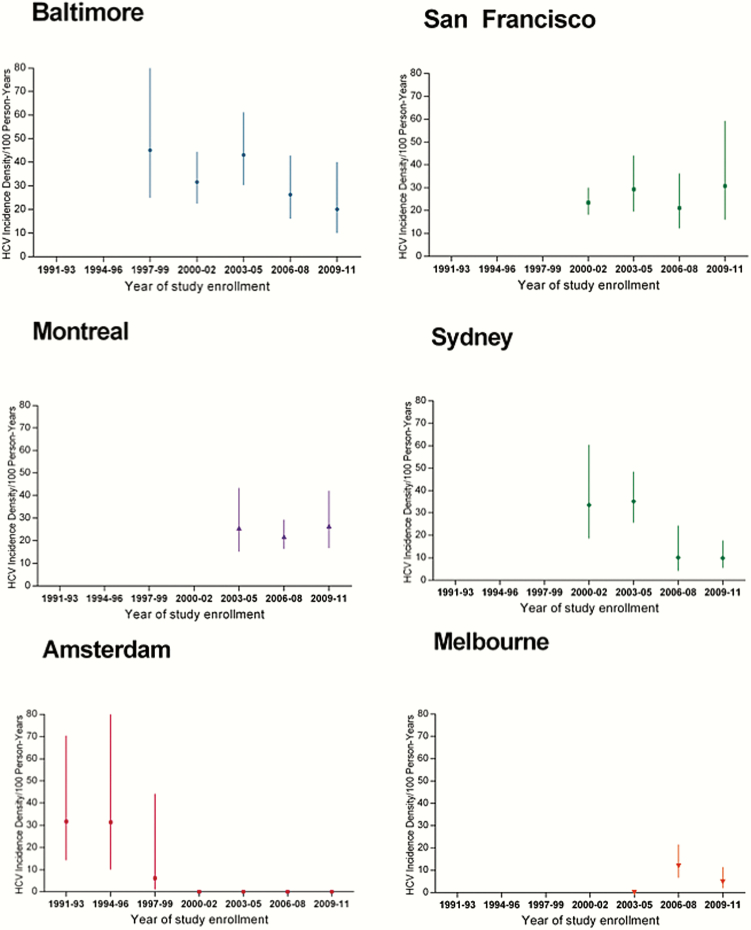
We observed differences in the magnitude and direction of HCV incidence trends across cities (Figure 2). In Baltimore, HCV incidence was initially very high at 45.1/100 PYO during 1997–1999, but then significantly declined to 20.1/100 PYO during 2009–2011 (IRR, 0.7 per 3 years [95% CI, .6–.9]) (Figure 2A). In San Francisco, incidence was 23.4/100 PYO during 2000–2002, remaining high and relatively constant across calendar-time with an increase to 30.8/100 PYO during 2009–2011 (IRR, 1.0 [95% CI, .8–1.2]). In Montreal, incidence remained high (range, 20.9–26.6) across 2003–2011 (IRR, 1.1 [95% CI, .8–1.6]). In Sydney, incidence was high during the early 2000s (33.4/100 PYO) with a statistically significant decline in 2009–2011 (9.9/100 PYO) (IRR, 0.7 [95% CI, .6–.8]). In Amsterdam, incidence was initially high during 1986–1993, but quickly declined to 9.0/100 PYO during 1994–1996 with no new infections identified in subsequent years (IRR, 0.4 [95% CI, .3–.8]). Last, in Melbourne, incidence was low and remained low across all years (IRR, 0.8 [95% CI, .4–1.5]).
Figure 2.
Trends in hepatitis C virus (HCV) incidence density (per 100 person-years) across calendar period, by city. Vertical lines represent 95% confidence intervals. Abbreviation: HCV, hepatitis C virus.
Geographical Differences in Behaviors/Characteristics
Table 2 displays HCV incidence estimates by demographic and behavioral variables for each city.
Temporal Trends in Injection-Related Exposures
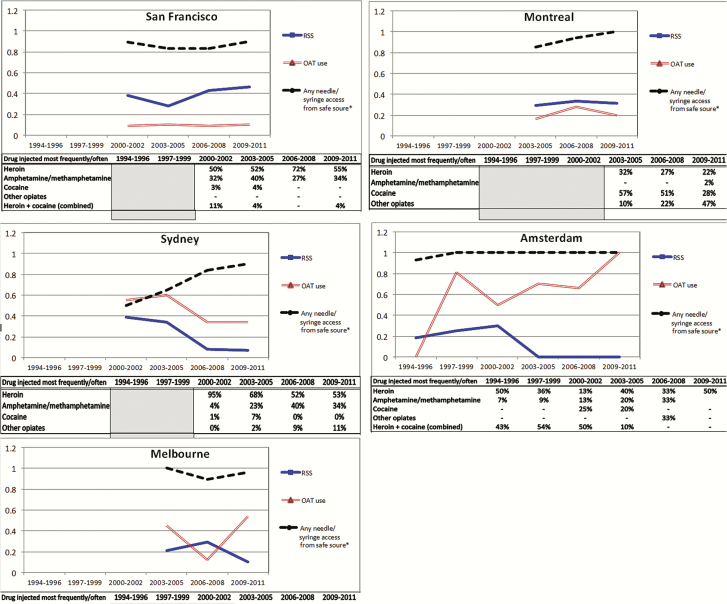
To further contextualize observed differences in HCV incidence trends across cities, Figure 3 illustrates temporal trends in the most frequent drug injected, recent RSS, recent OAT use, and recent access to sterile syringes through safe sources reported at enrollment. For San Francisco, where the majority of persons were injecting heroin, levels of RSS remained between 35% and 43%, with few (8%–12%) reporting recent OAT across all time periods. In Montreal, cocaine was the most frequently injected drug in the 2003–2005 period, but by 2009–2011 had declined and was primarily superseded by “other opiates.” Additionally, consistent levels (36%–38%) of RSS and OAT (19%–37%) were observed. In Sydney, reports of heroin as the most frequent drug injected were varying (53%–95%) over time, with methamphetamine injecting increasing from 4% to 40% from 2000 to 2008; a sharp decline in the prevalence of RSS from 39% in 2000–2002 to 7% in 2009–2011 was coupled with increasing levels (34%–60%) of OAT. In Amsterdam, levels of heroin injection fluctuated across 1994–2008, with levels of methamphetamine injection steadily increasing from 5% during 1994–1999 to 38% in 2006–2008; RSS declined as OAT levels increased from 3% in 1994–1996 to almost 100% in 2009–2011. In Melbourne, during 2003–2011, the majority of individuals reported injecting heroin, and RSS levels decreased from 20% to 11%; levels of OAT were consistent (42%–58%). Obtaining sterile syringes from needle syringe provision (NSP), pharmacies, vending machines, or through outreach was high (>80%) across all time periods and in all cities, with the exception of Sydney, where access levels increased from 50% in 2000–2002 to 90% in 2009–2011.
Figure 3.
Trends in proportions of self-reported exposures across calendar period, by city. Measurement of selected exposure behaviors collected at enrollment. Proportions were calculated for 1985–1990 and 3-year intervals from 1991 to 2011. Note that the Baltimore (Maryland) cohort does not collect survey data. Abbreviations: OAT, opioid agonist therapy; RSS, receptive syringe sharing.
Independent Differences in Risk for HCV Incidence
After adjusting for age, biological sex, and city, recent RSS was independently associated with an elevated risk for HCV infection (adjusted hazard ratio [aHR], 1.5 [95% CI, 1.2–2.0]), whereas recent OAT use reduced risk (aHR, 0.5 [95% CI, .4–.7]). Risk of infection dropped by approximately 18% for every 3-year increase in calendar-time (aHR, 0.7 [95% CI, .6–.8]). No significant effect was observed for biological sex or age at enrollment (Table 3).
Table 3.
Association Between Recent Receptive Syringe Sharing, Opioid Agonist Therapy, and Enrollment Year After Adjusting for Biological Sex, Age, and City (n = 1137)
| Variable | Adjusted HR (95% CI) |
|---|---|
| City | |
| Amsterdam | … |
| Sydney | … |
| Montreal | … |
| San Francisco | … |
| Baltimore | … |
| Biological sex | |
| Female | 1.15 (.88–1.50) |
| Unknown | … |
| Age at enrollment (per 1-y increase) | 0.98 (.96–1.00) |
| Recent receptive syringe sharing | |
| Yes | 1.53 (1.19–1.97) |
| Unknown | 1.17 (.43–3.23) |
| Recent OAT, including methadone and buprenorphine | |
| Yes | 0.50 (.36–.70) |
| Unknown | 2.18 (1.40–3.42) |
| Enrollment year (per 3-y increase) | 0.72 (.62–.83) |
Boldface text indicates P ≤ .05. Stratified Cox models satisfied proportional hazards assumptions. Model stratified by city to allow a unique baseline hazard by city.
Abbreviations: CI, confidence interval; HR, hazard ratio; OAT, opioid agonist therapy.
DISCUSSION
To our knowledge, this is the first multicity study of trends in HCV incidence and injecting behavior over the past 25 years. Overall HCV incidence was high (21.5/100 PYO), and the risk for HCV infection decreased an average of 18% per 3 years from a high of 31.3/100 PYO in 1994–1997, independent of city, OAT, RSS, age, and biological sex. While overall HCV incidence was high, we identified differences in infection trends across continents and cities. High infection rates persisted in the North American cities of Baltimore, San Francisco, and Montreal. In contrast, incidence was lower in Australian cities (Sydney and Melbourne), with Sydney showing a significant reduction in the HCV incidence over time. The most significant change was observed in Amsterdam, where a sharp decline in HCV incidence was experienced early in the epidemic (1985–1996).
The lower HCV incidence in Amsterdam and Melbourne, and the downward trend in Sydney, likely reflect an early and sustained implementation of harm reduction services. The Netherlands and Australia were global leaders in scaling up harm reduction programs to include NSP services. NSP services first became available in the Netherlands in 1981; they were expanded by the Dutch government in 1984 to include the provision of sterile injecting equipment, healthcare, and health information and were available at pharmacies and community centers and through outreach [17, 18]. Similarly, Australia established free and legal government-funded NSP programs in the late 1980s. By 1991, 6.3 million syringes were being distributed annually to an estimated 62 000 regular injectors across Australia [19]. Our data show a high (≥80%) level of NSP program access across cities. Given the equivalent NSP access levels across cities, observed differences in HCV trends likely reflect city-specific receptive RSS behaviors. HCV incidence was highest within the North American cities of Baltimore, San Francisco, and Montreal, where the prevalence of RSS was consistently >40%. In contrast, the epidemic remained relatively stable in cities like Melbourne, where RSS levels were substantially lower. Our findings expand on the results of other studies showing the relationship between high RSS on increased HCV incidence [20–23]. Similar to previous individual human immunodeficiency virus (HIV) and HCV incidence studies among PWID, we found a relationship between NSP access and HCV infection [22–27], which is understandable considering that persons seeking NSP services are more likely to experience homelessness and inject drugs more frequently than those not accessing NSP services.
Overall, HCV incidence rates were lower in Sydney, Melbourne, and Amsterdam where OAT uptake was higher than in San Francisco. This finding is consistent with recent evidence from the United States, Canada, and Australia demonstrating the strong protective effect of OAT against HCV infection [13, 28, 29]. Important differences in OAT policies and subsequent OAT availability exist across cities. Compared to the long waiting periods for entry and high-threshold restrictions associated with OAT service access in the United States, Australia and the Netherlands have lower thresholds for participation and higher coverage rates [30, 31]. Our results are consistent with other, usually single-site, studies showing the synergistic effect of both NSP and OAT on reducing HCV infection among PWID [32], an approach that, if sustained with high coverage, has the potential to also reduce HCV prevalence [33, 34]. Studies show that full participation in methadone maintenance therapy (a form of OAT) combined with obtaining 100% coverage of syringes from sterile distribution sources reduces HCV infection risk by 80% compared with no participation [7, 31].
In Montreal, HCV incidence rates remained high (approximately 20/100 PYO) even though NSP, OAT, and RSS levels were comparable to the lower-HCV-incidence settings of Sydney and Melbourne. Montreal’s high prevalence of cocaine injection suggests limited impact of OAT on HCV incidence in settings where opioids are not the primary drug. When analyses are restricted to Montreal (participants who mainly injected heroin/opiates), HCV incidence was significantly lower among those reporting recent OAT compared with no recent OAT (IRR, 0.4 [95% CI, .2–.8]; data not shown). Given that opioids are not the only drug injected, expanded prevention strategies to meet the needs of non–opioid users are needed. For example, cocaine injectors have larger injecting networks, inject more frequently, and engage in riskier injecting practices than do opioid injectors [35, 36], and therefore, require increased coverage of sterile injecting equipment. Last, given the efficacy of OAT in reducing HCV incidence by decreasing injection frequency [13, 28, 32], a need remains for efficacious pharmacotherapies for cocaine and methamphetamine dependence.
Our study is distinguished from previous work by its international scope and its use of participant-level data collected from well-characterized cohorts of PWID. However, several limitations exist. First, heterogeneity in cohort protocols for behavioral data collection resulted in an inability to assess time-varying predictors of HCV infection. Although this lack of harmonization is challenging, we were able to map behavioral trends over time by using baseline data on key exposure measures. Differing cohort start dates limited our ability to compare cities prior to 1990. Last, the lack of city-level measures for overall and individual harm reduction intervention coverage limited our ability to assess participant-level differences while controlling for city-level variability. Subsequent studies leveraging city-level measures of harm reduction and health service access on city-level HCV infection rates are vital to better understanding how structural-level factors, such as changes in access to sterile injecting equipment, interact with individual-level factors to drive HCV epidemics.
CONCLUSIONS
Contextual differences in the timing and level of harm reduction programs reflect observed city heterogeneity in behavioral patterns and HCV incidence trends. A sustained commitment to fund evidence-based harm reduction programs is necessary to maintain low incidence in Amsterdam, Sydney, and Melbourne; in contrast, in San Francisco, Baltimore, and Montreal, where HCV incidence remains high, an aggressive public health approach is urgently needed. In December 2015, the US Congress approved the Consolidated Appropriations Act 2016, which allows the use of government funds for NSP services [37]. For this decision to result in a tangible impact, an expanded budget dedicated to NSP services is imperative. NSP programs provide a window of opportunity to engage with populations who are often underserved by traditional healthcare services. Capitalizing on the high (>80%) level of NSP engagement among PWID across cities, scaling up resources for NSP services can offer long-term impact. For example, new HCV direct-acting antiviral (DAA) therapies offer a highly effective tool for controlling the HCV epidemic [38]. Expanding current NSP services to inform and introduce DAA treatment both for cure and as prevention has the potential to drive down incidence [39] while also improving health by resolving infection [40].
Supplementary Material
Notes
Author contributions. M. D. M. and K. P. were responsible for study concept and design, and M. D. M., K. P., J. H., and L. M. were responsible for revising the manuscript and interpretation of findings. M. D. M. and S. S. analyzed the data. M. D. M. wrote the first draft; all authors provided input on the design, interpretation, and development of the manuscript. All authors read and approved the final version of the manuscript before submission.
Financial support. This work was supported by the National Institutes of Health (NIH), National Institute on Drug Abuse (NIDA) (R01DA599901). M. D. M. is supported by an NIH/NIDA career development award (K01DA037802) and the NIH National Center for Advancing Translational Sciences (KL2TR000143). Research support for the individual cohorts include The Netherlands National Institute for Public Health and the Environment to the Amsterdam Cohort Study; Baltimore Before and After Study (NIH U19 AI088791); BAHSTION Boston Acute HCV Study: Transmission, Immunity and Outcomes Network (NIH U19 AI066345);Sydney HITS-c – UNSW Hepatitis C Vaccine Initiative and NHMRC Project (grant number 630483); Melbourne Networks/MIX – National Health and Medical Research Council, Australia (NHMRC) Project (grant numbers 331312 and 545891) and the Victorian Operational Infrastructure Support Programme (Department of Health, Victoria, Australia); Montreal HepCo–the Canadian Institutes of Health Research (MOP-103138 and MOP-106468); San Francisco (UFO) (NIH/NIDA R01 DA016017).
Potential conflicts of interest. J. G. is a consultant/advisor and has received grants from Merck and Gilead. G. D. is a consultant/advisor and has received research grants from Roche, Merck, Janssen, Gilead, and Bristol-Myers Squibb. M. H. has received research grants from Gilead and AbbVie. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Aceijas C, Rhodes T. Global estimates of prevalence of HCV infection among injecting drug users. Int J Drug Policy 2007; 18:352–8. [DOI] [PubMed] [Google Scholar]
- 2. Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology 2013; 57:1333–42. [DOI] [PubMed] [Google Scholar]
- 3. Patti AM, Santi AL, Pompa MG, et al. Viral hepatitis and drugs: a continuing problem. Int J Epidemiol 1993; 22:135–9. [DOI] [PubMed] [Google Scholar]
- 4. Hagan H, Des Jarlais DC, Stern R, et al. HCV synthesis project: preliminary analyses of HCV prevalence in relation to age and duration of injection. Int J Drug Policy 2007; 18:341–51. [DOI] [PubMed] [Google Scholar]
- 5. Sievert W, Altraif I, Razavi HA, et al. A systematic review of hepatitis C virus epidemiology in Asia, Australia and Egypt. Liver Int 2011; 31(suppl 2):61–80. [DOI] [PubMed] [Google Scholar]
- 6. Pouget ER, Hagan H, Des Jarlais DC. Meta-analysis of hepatitis C seroconversion in relation to shared syringes and drug preparation equipment. Addiction 2012; 107:1057–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Turner KM, Hutchinson S, Vickerman P, et al. The impact of needle and syringe provision and opiate substitution therapy on the incidence of hepatitis C virus in injecting drug users: pooling of UK evidence. Addiction 2011; 106:1978–88. [DOI] [PubMed] [Google Scholar]
- 8. Grebely J, Morris MD, Rice TM, et al. ; InC Study Group Cohort profile: the international collaboration of incident HIV and hepatitis C in injecting cohorts (InC3) study. Int J Epidemiol 2013; 42:1649–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cox AL, Netski DM, Mosbruger T, et al. Prospective evaluation of community-acquired acute-phase hepatitis C virus infection. Clin Infect Dis 2005; 40:951–8. [DOI] [PubMed] [Google Scholar]
- 10. Page K, Hahn JA, Evans J, et al. Acute hepatitis C virus infection in young adult injection drug users: a prospective study of incident infection, resolution, and reinfection. J Infect Dis 2009; 200:1216–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bruneau J, Roy E, Arruda N, Zang G, Jutras-Aswad D. The rising prevalence of prescription opioid injection and its association with hepatitis C incidence among street-drug users. Addiction 2012; 107:1318–27. [DOI] [PubMed] [Google Scholar]
- 12. Maher L, Jalaludin B, Chant KG, et al. Incidence and risk factors for hepatitis C seroconversion in injecting drug users in Australia. Addiction 2006; 101:1499–508. [DOI] [PubMed] [Google Scholar]
- 13. White B, Dore GJ, Lloyd AR, Rawlinson WD, Maher L. Opioid substitution therapy protects against hepatitis C virus acquisition in people who inject drugs: the HITS-c study. Med J Aust 2014; 201:326–9. [DOI] [PubMed] [Google Scholar]
- 14. van den Berg CH, Smit C, Bakker M, et al. Major decline of hepatitis C virus incidence rate over two decades in a cohort of drug users. Eur J Epidemiol 2007; 22:183–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sacks-Davis R, Daraganova G, Aitken C, et al. Hepatitis C virus phylogenetic clustering is associated with the social-injecting network in a cohort of people who inject drugs. PLoS One 2012; 7:e47335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Page-Shafer K, Pappalardo BL, Tobler LH, et al. Testing strategy to identify cases of acute hepatitis C virus (HCV) infection and to project HCV incidence rates. J Clin Microbiol 2008; 46:499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Buning EC. Effects of Amsterdam needle and syringe exchange. Int J Addict 1991; 26:1303–11. [DOI] [PubMed] [Google Scholar]
- 18. Witteveen E, Schippers G. Needle and syringe exchange programs in Amsterdam. Subst Use Misuse 2006; 41(6–7):835–6. [DOI] [PubMed] [Google Scholar]
- 19. Razali K, Thein HH, Bell J, et al. Modelling the hepatitis C virus epidemic in Australia. Drug Alcohol Depend 2007; 91(2–3):228–35. [DOI] [PubMed] [Google Scholar]
- 20. Schechter MT, Strathdee SA, Cornelisse PG, et al. Do needle exchange programmes increase the spread of HIV among injection drug users? An investigation of the Vancouver outbreak. AIDS 1999; 13:F45–51. [DOI] [PubMed] [Google Scholar]
- 21. Hahn JA, Page-Shafer K, Lum PJ, Ochoa K, Moss AR. Hepatitis C virus infection and needle exchange use among young injection drug users in San Francisco. Hepatology 2001; 34:180–7. [DOI] [PubMed] [Google Scholar]
- 22. Hahn JA, Vranizan KM, Moss AR. Who uses needle exchange? A study of injection drug users in treatment in San Francisco, 1989–1990. J Acquir Immune Defic Syndr Hum Retrovirol 1997; 15: 157–64. [DOI] [PubMed] [Google Scholar]
- 23. Bruneau J, Lamothe F, Franco E, et al. High rates of HIV infection among injection drug users participating in needle exchange programs in Montreal: results of a cohort study. Am J Epidemiol 1997; 146:994–1002. [DOI] [PubMed] [Google Scholar]
- 24. Patrick DM, Tyndall MW, Cornelisse PG, et al. Incidence of hepatitis C virus infection among injection drug users during an outbreak of HIV infection. CMAJ 2001; 165:889–95. [PMC free article] [PubMed] [Google Scholar]
- 25. Miller CL, Tyndall M, Spittal P, Li K, Palepu A, Schechter MT. Risk-taking behaviors among injecting drug users who obtain syringes from pharmacies, fixed sites, and mobile van needle exchanges. J Urban Health 2002; 79:257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Strathdee SA, Patrick DM, Currie SL, et al. Needle exchange is not enough: lessons from the Vancouver injecting drug use study. AIDS 1997; 11: F59–65. [DOI] [PubMed] [Google Scholar]
- 27. Hagan H, McGough JP, Thiede H, Weiss NS, Hopkins S, Alexander ER. Syringe exchange and risk of infection with hepatitis B and C viruses. Am J Epidemiol 1999; 149:203–13. [DOI] [PubMed] [Google Scholar]
- 28. Tsui JI, Evans JL, Lum PJ, Hahn JA, Page K. Association of opioid agonist therapy with lower incidence of hepatitis C virus infection in young adult injection drug users. JAMA Intern Med 2014; 174:1974–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nolan S, Dias Lima V, Fairbairn N, et al. The impact of methadone maintenance therapy on hepatitis C incidence among illicit drug users. Addiction 2014; 109:2053–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Substance Abuse and Mental Health Services Administration. Federal guidelines for opioid treatment programs. Rockville, MD: SAMHSA, 2015. [Google Scholar]
- 31. Van Den Berg C, Smit C, Van Brussel G, Coutinho R, Prins M; Amsterdam Cohort Full participation in harm reduction programmes is associated with decreased risk for human immunodeficiency virus and hepatitis C virus: evidence from the Amsterdam Cohort Studies among drug users. Addiction 2007; 102:1454–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hagan H, Pouget ER, Des Jarlais DC. A systematic review and meta-analysis of interventions to prevent hepatitis C virus infection in people who inject drugs. J Infect Dis 2011; 204:74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Martin NK, Hickman M, Hutchinson SJ, Goldberg DJ, Vickerman P. Combination interventions to prevent HCV transmission among people who inject drugs: modeling the impact of antiviral treatment, needle and syringe programs, and opiate substitution therapy. Clin Infect Dis 2013; 57(suppl 2):S39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Platt L, Reed J, Minozzi S, et al. Effectiveness of needle/syringe programmes and opiate substitution therapy in preventing HCV transmission among people who inject drugs. Cochrane Database Syst Rev 2016; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rice E, Milburn NG, Rotheram-Borus MJ, Mallett S, Rosenthal D. The effects of peer group network properties on drug use among homeless youth. Am Behav Sci 2005; 48:1102–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. De P, Jolly A, Cox J, Boivin JF. Characterizing the drug-injecting networks of cocaine and heroin injectors in Montreal. Can J Public Health 2006; 97:207–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. 114th Congress of the United States. Consolidated Appropriations Act, 2016. Available at: https://www.congress.gov/bill/114th-congress/house-bill/2029/text Accessed 3 January 2017. [Google Scholar]
- 38. Kohli A, Shaffer A, Sherman A, Kottilil S. Treatment of hepatitis C: a systematic review. JAMA 2014; 312:631–40. [DOI] [PubMed] [Google Scholar]
- 39. Martin NK, Vickerman P, Grebely J, et al. Hepatitis C virus treatment for prevention among people who inject drugs: modeling treatment scale-up in the age of direct-acting antivirals. Hepatology 2013; 58:1598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Smith DJ, Combellick J, Jordan AE, Hagan H. Hepatitis C virus (HCV) disease progression in people who inject drugs (PWID): a systematic review and meta-analysis. Int J Drug Policy 2015; 26:911–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.