Abstract
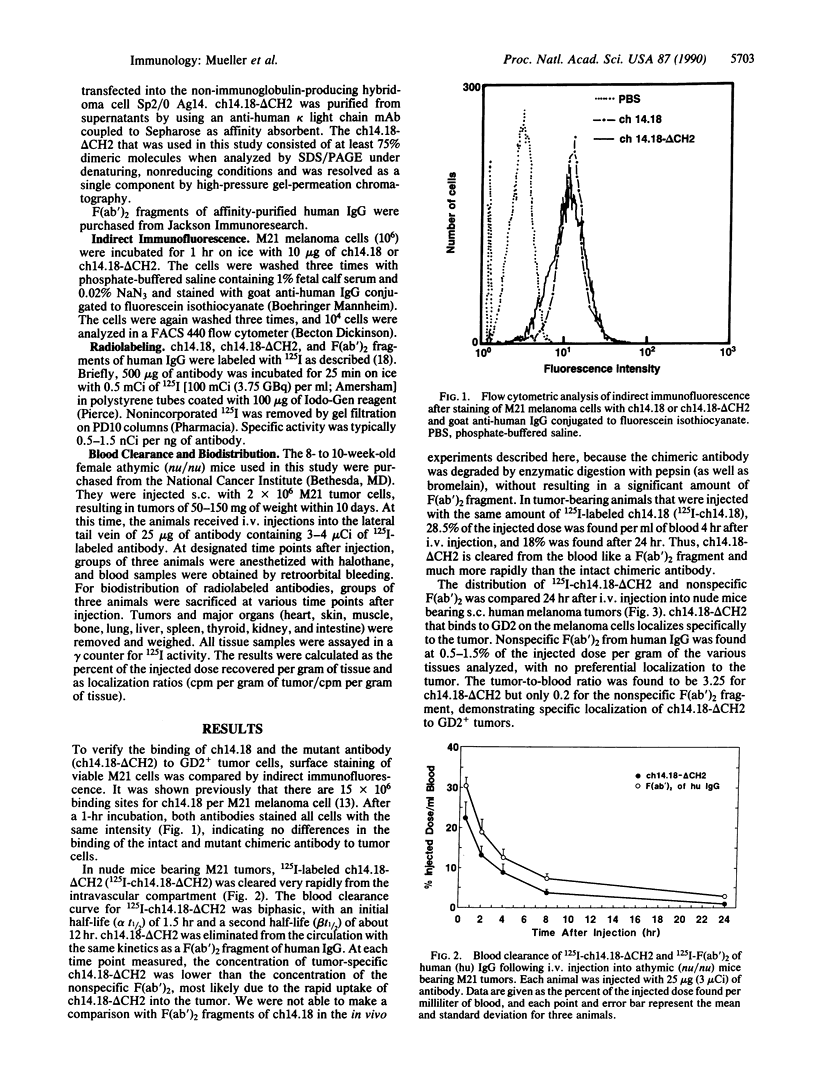
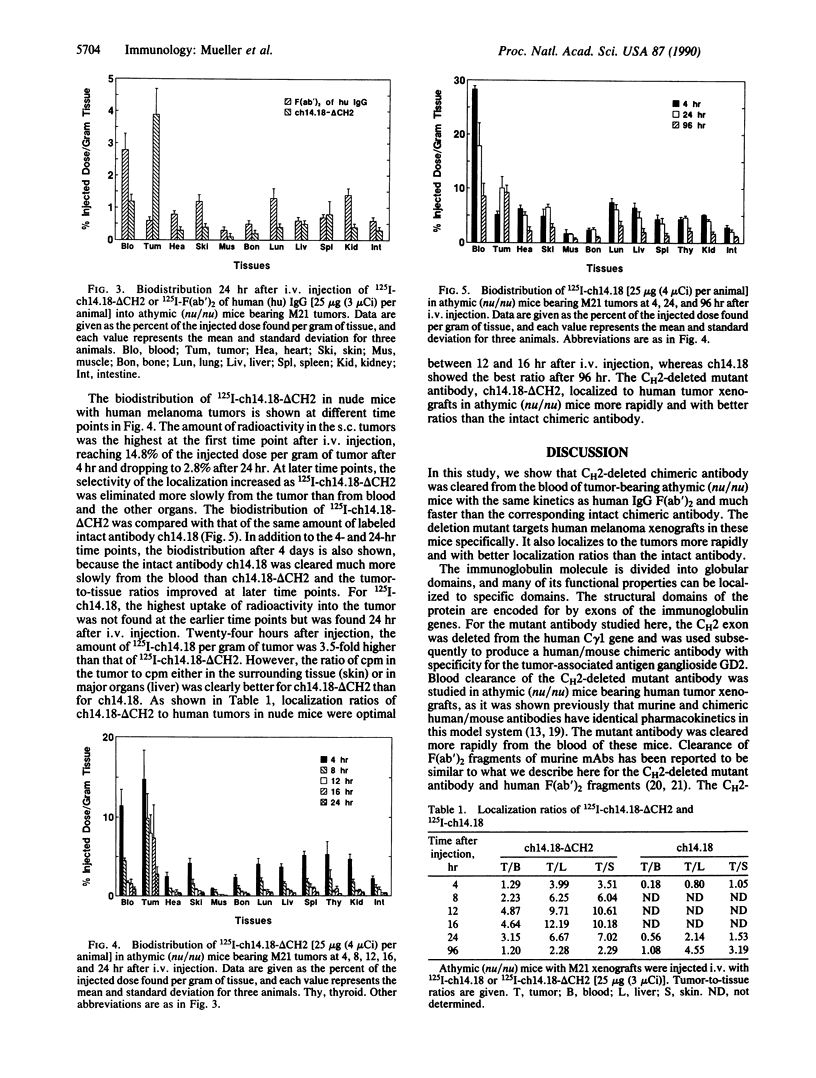
Recombinant techniques allow one to engineer an antibody molecule and, in this way, manipulate its properties and functions. We engineered a chimeric human/mouse antibody to the tumor-associated antigen ganglioside GD2, with the aim of decreasing its serum half-life, maintaining its full antigen-binding capacity, and deleting its effector functions, thus making it a potentially useful reagent for the radioimaging of tumors. To this end, the constant region of the human gamma 1 chain was mutated by deleting the second domain (CH2). Here we show that the CH2-deleted antibody (ch14.18-delta CH2) was cleared from the blood of athymic (nu/nu) mice bearing human melanoma tumors with the same kinetics as human IgG F(ab')2. At a beta t1/2 of 12 hr, 0.9% of the injected dose of 125I-labeled ch14.18-delta CH2 was found per milliliter of blood 24 hr after i.v. injection. In biodistribution experiments, 125I-labeled ch14.18-delta CH2 targeted specifically to melanoma xenografts, achieving optimal tumor-to-tissue ratios 12-16 hr after i.v. injection. ch14.18-delta CH2 was localized to the melanoma tumors more rapidly and with better localization ratios than the intact chimeric antibody ch14.18. Sixteen hours after i.v. injection, the tumor-to-blood and tumor-to-liver ratios of ch14.18-delta CH2 were 5 and 12, respectively, while optimal localization ratios obtained for ch14.18 were 1 and 5, respectively, but 96 hr after injection. A reagent such as ch14.18-delta CH2 should be useful for radioimmunodetection of human tumors because of reduced immunogenicity, increased targeting specificity, and rapid clearance from circulation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boguslawski S. J., Ledden D. J., Fredrickson R. A. Improved procedure for preparation of F(ab')2 fragments of mouse IgGs by papain digestion. J Immunol Methods. 1989 Jun 2;120(1):51–56. doi: 10.1016/0022-1759(89)90288-3. [DOI] [PubMed] [Google Scholar]
- Buchegger F., Mach J. P., Leonnard P., Carrel S. Selective tumor localization of radiolabeled anti-human melanoma monoclonal antibody fragment demonstrated in the nude mouse model. Cancer. 1986 Aug 1;58(3):655–662. doi: 10.1002/1097-0142(19860801)58:3<655::aid-cncr2820580310>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Colapinto E. V., Humphrey P. A., Zalutsky M. R., Groothuis D. R., Friedman H. S., de Tribolet N., Carrel S., Bigner D. D. Comparative localization of murine monoclonal antibody Me1-14 F(ab')2 fragment and whole IgG2a in human glioma xenografts. Cancer Res. 1988 Oct 15;48(20):5701–5707. [PubMed] [Google Scholar]
- Colcher D., Milenic D., Roselli M., Raubitschek A., Yarranton G., King D., Adair J., Whittle N., Bodmer M., Schlom J. Characterization and biodistribution of recombinant and recombinant/chimeric constructs of monoclonal antibody B72.3. Cancer Res. 1989 Apr 1;49(7):1738–1745. [PubMed] [Google Scholar]
- Colcher D., Zalutsky M., Kaplan W., Kufe D., Austin F., Schlom J. Radiolocalization of human mammary tumors in athymic mice by a monoclonal antibody. Cancer Res. 1983 Feb;43(2):736–742. [PubMed] [Google Scholar]
- Fritzberg A. R., Abrams P. G., Beaumier P. L., Kasina S., Morgan A. C., Rao T. N., Reno J. M., Sanderson J. A., Srinivasan A., Wilbur D. S. Specific and stable labeling of antibodies with technetium-99m with a diamide dithiolate chelating agent. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4025–4029. doi: 10.1073/pnas.85.11.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies S. D., Lo K. M., Wesolowski J. High-level expression of chimeric antibodies using adapted cDNA variable region cassettes. J Immunol Methods. 1989 Dec 20;125(1-2):191–202. doi: 10.1016/0022-1759(89)90093-8. [DOI] [PubMed] [Google Scholar]
- Goldenberg D. M. Current status of cancer imaging with radiolabeled antibodies. J Cancer Res Clin Oncol. 1987;113(3):203–208. doi: 10.1007/BF00396374. [DOI] [PubMed] [Google Scholar]
- Goldenberg D. M., Goldenberg H., Sharkey R. M., Higginbotham-Ford E., Lee R. E., Swayne L. C., Burger K. A., Tsai D., Horowitz J. A., Hall T. C. Clinical studies of cancer radioimmunodetection with carcinoembryonic antigen monoclonal antibody fragments labeled with 123I or 99mTc. Cancer Res. 1990 Feb 1;50(3 Suppl):909s–921s. [PubMed] [Google Scholar]
- Harwood P. J., Boden J., Pedley R. B., Rawlins G., Rogers G. T., Bagshawe K. D. Comparative tumour localization of antibody fragments and intact IgG in nude mice bearing a CEA-producing human colon tumour xenograft. Eur J Cancer Clin Oncol. 1985 Dec;21(12):1515–1522. doi: 10.1016/0277-5379(85)90247-0. [DOI] [PubMed] [Google Scholar]
- Lamoyi E., Nisonoff A. Preparation of F(ab')2 fragments from mouse IgG of various subclasses. J Immunol Methods. 1983 Jan 28;56(2):235–243. doi: 10.1016/0022-1759(83)90415-5. [DOI] [PubMed] [Google Scholar]
- Larson S. M., Brown J. P., Wright P. W., Carrasquillo J. A., Hellström I., Hellström K. E. Imaging of melanoma with L-131-labeled monoclonal antibodies. J Nucl Med. 1983 Feb;24(2):123–129. [PubMed] [Google Scholar]
- Liu A. Y., Robinson R. R., Murray E. D., Jr, Ledbetter J. A., Hellström I., Hellström K. E. Production of a mouse-human chimeric monoclonal antibody to CD20 with potent Fc-dependent biologic activity. J Immunol. 1987 Nov 15;139(10):3521–3526. [PubMed] [Google Scholar]
- LoBuglio A. F., Wheeler R. H., Trang J., Haynes A., Rogers K., Harvey E. B., Sun L., Ghrayeb J., Khazaeli M. B. Mouse/human chimeric monoclonal antibody in man: kinetics and immune response. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4220–4224. doi: 10.1073/pnas.86.11.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach J. P., Chatal J. F., Lumbroso J. D., Buchegger F., Forni M., Ritschard J., Berche C., Douillard J. Y., Carrel S., Herlyn M. Tumor localization in patients by radiolabeled monoclonal antibodies against colon carcinoma. Cancer Res. 1983 Nov;43(11):5593–5600. [PubMed] [Google Scholar]
- Macklis R. M., Kinsey B. M., Kassis A. I., Ferrara J. L., Atcher R. W., Hines J. J., Coleman C. N., Adelstein S. J., Burakoff S. J. Radioimmunotherapy with alpha-particle-emitting immunoconjugates. Science. 1988 May 20;240(4855):1024–1026. doi: 10.1126/science.2897133. [DOI] [PubMed] [Google Scholar]
- Morrison S. L. Transfectomas provide novel chimeric antibodies. Science. 1985 Sep 20;229(4719):1202–1207. doi: 10.1126/science.3929380. [DOI] [PubMed] [Google Scholar]
- Mueller B. M., Romerdahl C. A., Gillies S. D., Reisfeld R. A. Enhancement of antibody-dependent cytotoxicity with a chimeric anti-GD2 antibody. J Immunol. 1990 Feb 15;144(4):1382–1386. [PubMed] [Google Scholar]
- Neuberger M. S., Williams G. T., Fox R. O. Recombinant antibodies possessing novel effector functions. Nature. 1984 Dec 13;312(5995):604–608. doi: 10.1038/312604a0. [DOI] [PubMed] [Google Scholar]
- Oldham R. K., Foon K. A., Morgan A. C., Woodhouse C. S., Schroff R. W., Abrams P. G., Fer M., Schoenberger C. S., Farrell M., Kimball E. Monoclonal antibody therapy of malignant melanoma: in vivo localization in cutaneous metastasis after intravenous administration. J Clin Oncol. 1984 Nov;2(11):1235–1244. doi: 10.1200/JCO.1984.2.11.1235. [DOI] [PubMed] [Google Scholar]
- Roberts S., Cheetham J. C., Rees A. R. Generation of an antibody with enhanced affinity and specificity for its antigen by protein engineering. Nature. 1987 Aug 20;328(6132):731–734. doi: 10.1038/328731a0. [DOI] [PubMed] [Google Scholar]
- Tao M. H., Morrison S. L. Studies of aglycosylated chimeric mouse-human IgG. Role of carbohydrate in the structure and effector functions mediated by the human IgG constant region. J Immunol. 1989 Oct 15;143(8):2595–2601. [PubMed] [Google Scholar]
- Wahl R. L., Parker C. W., Philpott G. W. Improved radioimaging and tumor localization with monoclonal F(ab')2. J Nucl Med. 1983 Apr;24(4):316–325. [PubMed] [Google Scholar]
- Wiley H. S., Cunningham D. D. The endocytotic rate constant. A cellular parameter for quantitating receptor-mediated endocytosis. J Biol Chem. 1982 Apr 25;257(8):4222–4229. [PubMed] [Google Scholar]
- Yasmeen D., Ellerson J. R., Dorrington K. J., Painter R. H. The structure and function of immunoglobulin domains. IV. The distribution of some effector functions among the Cgamma2 and Cgamma3 homology regions of human immunoglobulin G1. J Immunol. 1976 Feb;116(2):518–526. [PubMed] [Google Scholar]
- Zalutsky M. R., Garg P. K., Friedman H. S., Bigner D. D. Labeling monoclonal antibodies and F(ab')2 fragments with the alpha-particle-emitting nuclide astatine-211: preservation of immunoreactivity and in vivo localizing capacity. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7149–7153. doi: 10.1073/pnas.86.18.7149. [DOI] [PMC free article] [PubMed] [Google Scholar]


