Abstract
We reviewed 216 consecutive patients with MDS and abnormal karyotype treated with hypomethylating agents between 4/04 and 10/12. Median follow-up was 17 months. Using IWG criteria, best responses were complete response (CR) in 79 patients (37%), partial response (PR) in 4 (2%), and hematologic improvement (HI) in 10 (5%). Cytogenetic response (CyR) was achieved in 78 patients (36%): complete (CCyR) in 62 (29%) and partial in 16 (7%). CyR was achieved in 48 of 79 patients (61%) with CR, 1 of 14 (7%) with PR/HI, and in 29 of the 123 (24%) with no morphologic response. Median overall survival (OS) and leukemia-free survival (LFS) for patients with and without CCyR were 21 and 13 months (p=0.007), and 16 and 9 months (p=0.001), respectively. By multivariate analysis, the achievement of CCyR was predictive for better OS (HR=2.1; p<0.001). In conclusion, CyR occurs at a rate of 36% (complete in 29%) in patients with MDS treated with HMA and is not always associated with morphological response. The achievement of CCyR is associated with survival improvement and constitutes a major predictive factor for outcome particularly in patients without morphologic response. Therefore, the achievement of CCyR should be considered a milestone in the management of patients with MDS.
Keywords: Myelodysplastic syndromes, cytogenetic response, survival
Introduction
The myelodysplastic syndromes (MDS) are a group of heterogeneous hematopoietic stem cell disorders characterized by peripheral blood cytopenias, bone marrow dysplasia affecting one or more of the hematopoietic stem cell lines, and increased risk of transformation into acute myeloid leukemia (AML).1 Approximately 20–30% of patients with MDS are at risk for developing AML and many patients suffer from complications related to cytopenias.2
Therapy with hypomethylating agents (HMA) is now the standard of care for patients with myelodysplastic syndromes (MDS) and chronic myelomonocytic leukemia (CMML).3–6 Therapy with decitabine and 5-azacytidine has resulted in complete response (CR) rates of 7% to 35%, median response durations of 9 to 10 months, and median survival of 20 to 24 months.3–6
Recently, large studies have demonstrated the prognostic relevance of chromosomal defects in predicting outcome in MDS.7 Similarly, outcome of patients with acute myeloid leukemia is highly predictable by the baseline chromosomal abnormalities.8 We and others have reported on small series cytogenetic response (CyR) rates in the ranges of 30 to 40% following treatment with HMA.3–6,9
In patients with chronic myeloid leukemia, the achievement of CyR, particularly complete, has proven to correlate with better outcome.10–11 This was reported initially in patients receiving interferon and later on in patients treated with tyrosine kinase inhibitors. Patients with complete cytogenetic response (CCyR) had less transformation and better overall survival. Similarly, patients with acute myeloid leukemia and cytogenetic abnormalities at the time of complete response had significantly shorter relapse-free and overall survival compared with patients with normal cytogenetics at the time of achieving a complete response.12 However, this phenomenon has not been examined systematically in patients with MDS, where the criteria of response are still predominantly based on morphologic basis.13
The aim of this study was to assess the impact of achieving a cytogenetic response on outcome of patients with MDS treated with HMA.
Patients and Methods
We reviewed 460 consecutive patients who were referred to MD Anderson Cancer Center (MDACC) and treated with HMA between April 2004 and October 2012, on protocols approved by the Institutional Review Board after informed consent was obtained according to institutional guidelines. Of the 460 patients reviewed, 216 (47%) patients had abnormal baseline karyotype identified and therefore were evaluable for analysis. Treatments included decitabine (20 mg/m2 daily for 5 days) or azacitidne (75 mg/m2 daily for 5–7 days) as single agents or in combination with valproic acid14 and or all trans-retinoic acid.15
Response was based on the International Working Group (IWG) 2006 criteria.13 Routine cytogenetic analysis was conducted in the Clinical Cytogenetics laboratory at MDACC. Cytogenetic analyses were conducted on unstimulated bone marrow cells after culture (24–72 hr), and G-banding analysis was performed according to standard techniques at MDACC. At least 20 metaphases were analyzed for each case. Moreover, chromosomal abnormality was defined by structural change or gain in at least two metaphases and loss in three metaphases. Fluorescent in situ hybridization (FISH) was conducted to confirm the clonality of the cytogenetic abnormalities in patients exhibiting structural change or gain in less than two metaphases or loss in less than three metaphases.
Baseline cytogenetics were classified according to the revised International Prognostic Scoring System (R-IPSS).16 CCyR was defined as the achievement of a diploid karyotype among at least 20 metaphases analyzed. Partial cytogenetic response (PCyR) was defined as the reduction of 50% of the abnormal metaphases without the acquisition of any new abnormality among at least twenty metaphases analyzed.13
Differences among variables were evaluated by the Chi-square test and Mann–Whitney U test for categorical and continuous variables, respectively. Leukemia-free survival (LFS) was defined as the time interval between the start of therapy date and the date of transformation or death date, whichever occurred first. Leukemic transformation was defined when blast cells in the bone marrow exceeded more than 20%.17 Patients who were alive and without transformation were censored at the last follow-up date. Overall survival (OS) was defined as the time interval between the start of therapy date and death date, whichever occurred first. Patients who were alive were censored at the last follow-up date. The probabilities of LFS and OS were estimated using the method of Kaplan and Meier. Univariate and multivariate analyses were performed to identify potential factors associated with the achievement of CyR and survival. Factors retaining significance in the multivariate model were interpreted as being independently predictive of the achievement of CyR. Multivariate analysis of response used logistic regression model and survival used the Cox proportional hazard regression model.18–20
In a confirmatory second step, we performed a landmark analysis, using as start point the date of achievement of cytogenetic response (either complete or partial) for those who achieved it and the date of cytogenetic assessment for those who did not. This way, the time from therapy start to cytogenetic response, which is the confounding factor, was taken off the analysis. In an additional analysis, and to limit the effect of allogeneic stem cell transplantation (ASCT) on outcome, we performed univariate and multivariate analyses censoring at the time of ASCT.
Results
Patients Characteristics
Of the 460 patients treated at our institution with HMA therapy, 216 (47%) patients had abnormal baseline karyotype and therefore eligible for this study. Clinical characteristics of the study group (N = 216) are detailed in Table 1. Median age was 66 years (range, 21 to 90 years). By the R-IPSS,16 54% were very high-risk, 28% high-risk, 10% intermediate, 6% low-risk, and 2% very low-risk disease. Cytogenetic analysis revealed a complex karyotype in 24%, abnormalities involving chromosomes 5 or 7 only in 37%, and other abnormalities in 39%. By R-IPSS, cytogenetics were very poor, poor, intermediate, good, and very good, in 55%, 17%, 22%, 4% and 2% of the patients respectively. By the MD Anderson Global Scoring System (MDGSS),21 51% of the patients were of high-risk disease, 26% intermediate-2, 15% intermediate-1, and 8% low-risk disease. One hundred and forty-nine (69%) patients were treated with decitabine based therapy [of these 149 patients, 24 (16%) received a combination of decitabine and valproid acid13]; sixty-seven (31%) patients were treated with 5-azacitidine [of these 67 patients, 12 (18%) received a combination of azacitidine, all-trans retinoic acid and valproic acid14], both on clinical trials and predefined marrow evaluations.
Table 1.
Patient characteristics N=216
| Parameter | Number (%); Median [range] | |
|---|---|---|
| Age (years) | 66 [20–90] | |
| White Blood Cell Count (x 109/L) | 3.1 [0.7–106] | |
| Absolute Neutrophil Count (x 109/L) | 1.2 [0.1–40] | |
| Hemoglobin (g/dL) | 9.6 [5.7–14.7] | |
| Platelets (x 109/L) | 54 [9–987] | |
| Bone marrow blasts (%) | 7 [0–26] | |
| Prior malignancy | 116 (44) | |
| Prior chemotherapy | 92 (43) | |
| Prior radiotherapy | 56 (26) | |
| Cytogenetics13 | Very good | 5 (2) |
| Good | 9 (4) | |
| Intermediate | 48 (22) | |
| Poor | 36 (17) | |
| Very poor | 118 (55) | |
| WHO | RA | 24 (11) |
| RARS | 9 (4) | |
| RCMD | 19 (9) | |
| RAEB | 133 (62) | |
| MDS-U | 8 (4) | |
| CMML | 23 (10) | |
| IPSS | Low | 7 (3) |
| Intermediate-1 | 41 (19) | |
| Intermediate-2 | 107 (50) | |
| High | 61 (28) | |
| R-IPSS | Very low | 5 (2) |
| Low | 14 (6) | |
| Intermediate | 22 (10) | |
| High | 61 (28) | |
| Very high | 114 (54) | |
| MDGSS | Low | 18 (8) |
| Intermediate-1 | 33 (15) | |
| Intermediate-2 | 57 (26) | |
| High | 108 (51) | |
| Type of HMA | Azacitidine | 67 (31) |
| Decitabine | 149 (69) |
Among the 216 patients analyzed, 60 (28%) patients were referred and received ASCT after a median of 7 (4.5–9.5) months from diagnosis.
Overall Clinical Response
Responses are summarized in Table 2. Overall, 93 patients (43%) responded to hypomethylating agent. Best clinical responses were complete response (CR) in 79 (37%) patients, partial response (PR) in 4 (2%), and hematologic improvement (HI) only in 10 (5%). CyR was achieved in 78 patients (36%), being complete and partial in 62 (29%) and 16 (7%) patients, respectively. There was no difference in morphologic and cytogenetic response rates between the two hypomethylating agents used at the standard doses as single agents or in combination. Among the 216 patients analyzed, 60 patients were referred and received ASCT after a median 7 (4.5–9.5) months from diagnosis. Of those patients transplanted, 21 (35%) were in morphologic response and 20 (33%) were in cytogenetic response at the time of transplant.
Table 2.
Overall response*
| Cytogenetic | ||||
|---|---|---|---|---|
| Morphologic | Overall | Complete | Partial | |
| Response | N (%) | N (%) | ||
| Overall | 93 (43) | 78 (36) | 62 (29) | 16 (7) |
| CR | 79 (37) | 48 (61) | 42 (54) | 6 (7) |
| PR | 4 (2) | 1 (25) | 1 (25) | 0 |
| HI | 10 (5) | 0 | 0 | 0 |
| Non response | 123 (57) | 29 (24) | 19 (16) | 10 (8) |
CR=complete response; PR=partial response; HI=hematologic improvement
Response criteria were based on the International Working Group (IWG) 2006.12
The median time to cytogenetic and morphologic response was 3 (range, 1 to 27) and 3 (range, 1 to 45) months, respectively. Of note, only three patients received therapy beyond 6 cycles. Two of them achieved a cytogenetic response after 7 cycles and one after 13 cycles of therapy. The median duration of cytogenetic and morphologic response was 8 (range, 1 to 59) and 6 (range, 1 to 48) months respectively.
We explored the correlation between morphologic response and CyR: 48 of the 79 patients (61%) with CR achieved a CyR; of these, CCyR was achieved in 42 (54%) and PCyR in 6 (8%). One of the 4 patients (25%) with PR achieved a CCyR, and none of the 10 patients (0%) with HI achieved a CyR. Of note, CyR was observed in 29 (24%) among the 123 patients refractory to therapy. The rates of CCyR and PCyR were 16% and 8% in these patients, respectively.
Supplemental Table 1 summarizes correlation between patients’ clinical characteristics and CyR. The rate of CyR in patients with very poor cytogenetics was high (46%) as the rate of CyR in patients with very good cytogenetic (40%) (p=not significant). Out of the 54 cytogenetic responses noted among patients with very poor cytogenetics, only 9 (17%) were partial.
Outcome
With a median follow-up of 17 months (range, 1 to 72 months) from the date of diagnosis, 176 (81%) of the 216 patients developed leukemia or died. Fifty patients (23%) are still alive. Median LFS in the entire cohort was 11 months (95% CI: 9–12). Median OS in the entire cohort was 15 months (95% CI: 13–16). The 2-year LFS and OS rates were 18% and 25%, respectively (Supplemental Figure 1).
The median LFS for patients with and without CCyR were 16 and 9 months (Figure 1A; p=0.001). The median OS for patients with and without CCyR were 21 and 13 months (Figure 1B; p=0.007), respectively. Furthermore, the survival benefit was mostly observed among patients who had achieved CCyR (21 months) compared with patients who achieved PCyR only. The median LFS and OS for patients with CCyR and PCyR were 16 and 8 months (Figure 1C; p=0.03) and 21 and 17 months (Figure 1D; p=0.02), respectively.
Figure 1.
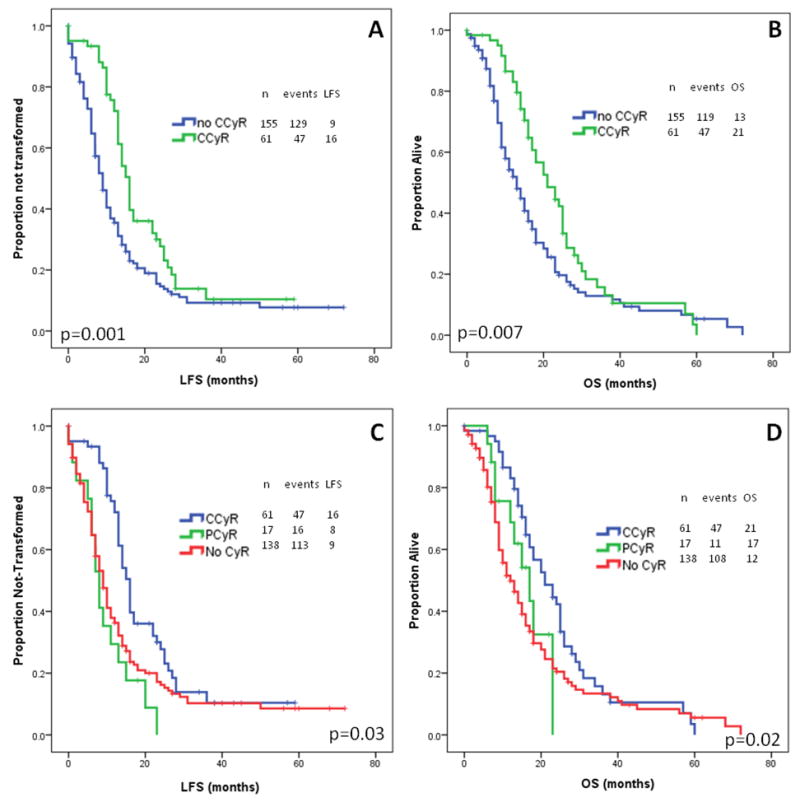
Overall and leukemia-free survival by cytogenetic response
In patients who achieved any morphologic response, there was no difference in LFS and OS whether a CCyR was achieved or not in addition to their morphologic response. The median LFS and OS were 15 and 12 months (p=0.98) and 21 and 18 months (p=0.57), respectively (Supplemental Figures 2A and 2C). In contrast, the achievement of a CCyR had a significant impact among patients considered not responding according to the IWG 2006 criteria. Among patients with no morphologic response those who achieved a CCyR had a better LFS and OS compared to those who did not with a median LFS and OS of 16 versus 8 months (p<0.001) and 21 versus 10 months (p=0.02), respectively (Supplemental Figures 2B and 2D).
Multivariate analysis for survival
We performed univariate and multivariable analysis to determine predictors of LFS and OS (Table 3). In the univariate analysis for LFS, presence of anemia, prior chemotherapy, higher-risk R-IPSS, IPSS or MDGSS categories, and lack of achievement of a complete cytogenetic response did correlate with a worse LFS. In the univariate analysis for OS, prior malignancy, prior chemotherapy, and high-risk MDGSS in addition to lack of achievement of a complete cytogenetic response were predictive of a worse OS. In the multivariate analysis, the significant predictive factors of poor LFS were higher-risk R-IPSS categories, and lack of achievement of a complete cytogenetic response. The independent predictive factors for a poor OS were higher-risk MDGSS and lack of achievement of a complete cytogenetic response [p<0.001; HR=2.1 (95% CI: 1.5–3)].
Table 3.
Univariate and multivariate analysis for transformation and overall survival
| Median | UVA | MVA | Median | UVA | MVA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| LFS (months) | P | HR | [95% CI] | P | OS (months) | P | HR | [95% CI] | P | ||
| Age | > 66 years | 11 | 14 | ||||||||
| < 66 years | 11 | 0.97 | 17 | 0.59 | |||||||
| Sex | Males | 12 | 16 | ||||||||
| Females | 10 | 0.22 | 15 | 0.24 | |||||||
|
| |||||||||||
| WBC | > 3.1 (109/L) | 11 | 15 | ||||||||
| < 3.1 (109/L) | 11 | 0.87 | 16 | 0.09 | |||||||
| ANC | > 1.2 (x 109/L) | 11 | 14 | ||||||||
| < 1.2 (x 109/L) | 11 | 0.53 | 16 | 0.48 | |||||||
| Hb | > 9.6 (g/dL) | 13 | 16 | ||||||||
| < 9.6 (g/dL) | 10 | 0.03 | 1 | 0.7–1.5 | 0.82 | 14 | 0.1 | ||||
| PLT | > 54 (x 109/L) | 13 | 17 | ||||||||
| < 54 (x 109/L) | 10 | 0.13 | 15 | 0.33 | |||||||
| BM blasts | > 7% | 10 | 15 | ||||||||
| < 7% | 12 | 0.16 | 18 | 0.26 | |||||||
|
| |||||||||||
| Prior Malignancy | Yes | 10 | 14 | ||||||||
| No | 13 | 0.06 | 17 | 0.05 | 0.7 | 0.4–1.2 | 0.25 | ||||
|
| |||||||||||
| Prior Chemotherapy | Yes | 10 | 13 | ||||||||
| No | 13 | 0.04 | 0.8 | 0.6–1.1 | 0.18 | 17 | 0.03 | 1 | 0.6–1.7 | 0.93 | |
|
| |||||||||||
| Prior Radiotherapy | Yes | 10 | 12 | ||||||||
| No | 12 | 0.19 | 16 | 0.11 | |||||||
|
| |||||||||||
| Cytogenetics | Very good | 23 | 0.16 | 21 | 0.19 | ||||||
| Good | 9 | 9 | |||||||||
| Intermediate | 13 | 18 | |||||||||
| Poor | 14 | 17 | |||||||||
| Very poor | 10 | 13 | |||||||||
|
| |||||||||||
| WHO | RA | 14 | 0.15 | 14 | 0.57 | ||||||
| RARS | 14 | 14 | |||||||||
| RCMD | 10 | 12 | |||||||||
| RAEB | 10 | 15 | |||||||||
| MDS-U | 10 | 12 | |||||||||
| CMML | 15 | 18 | |||||||||
|
| |||||||||||
| IPSS | Low | 16 | 0.002 | 0.4 | 0.1–1.7 | 0.24 | 23 | 0.23 | |||
| Intermediate-1 | 11 | 1 | 0.5–1.8 | 0.92 | 16 | ||||||
| Intermediate-2 | 12 | 0.7 | 0.5–1 | 0.07 | 14 | ||||||
| High | 8 | 0.16 | 14 | ||||||||
|
| |||||||||||
| R-IPSS | Very low | 23 | 0.4 | 0.2–1 | 0.06 | 23 | |||||
| Low | 15 | 0.5 | 0.2–1 | 0.06 | 18 | ||||||
| Intermediate | 15 | 0.002 | 0.4 | 0.2–0.9 | 0.05 | 18 | 0.32 | ||||
| High | 14 | 0.7 | 0.4–1 | 0.02 | 16 | ||||||
| Very high | 9 | 0.06 | 13 | ||||||||
|
| |||||||||||
| MDGSS | Low | 14 | 0.001 | 0.5 | 0.2–1.2 | 0.11 | 23 | 0.002 | 0.3 | 0.2–0.7 | 0.003 |
| Intermediate-1 | 16 | 0.5 | 0.3–1 | 0.30 | 24 | 0.4 | 0.2–0.7 | 0.001 | |||
| Intermediate-2 | 11 | 0.9 | 0.6–1.3 | 0.47 | 14 | 0.6 | 0.4–0.9 | 0.01 | |||
| High | 9 | 0.11 | 13 | <0.001 | |||||||
|
| |||||||||||
| Morphologic Response | Yes | 12 | 15 | ||||||||
| No | 10 | 0.34 | 15 | 0.6 | |||||||
|
| |||||||||||
| Morphologic CR | Yes | 12 | 16 | ||||||||
| No | 10 | 0.54 | 14 | 0.86 | |||||||
|
| |||||||||||
| CCyR | Yes | 16 | 21 | ||||||||
| No | 9 | 0.01 | 2.3 | 1.6–3.3 | <0.001 | 13 | 0.007 | 2.1 | 1.5–3 | <0.001 | |
WBC=White Blood Cells=; ANC=Absolute Neutrophil Count; Hb=Hemoglobin; PLT=Platelets; BM=Bone marrow; CG=cytogenetics; IPSS=International Prognostic Scoring System; MDGSS=MD Anderson Global Scoring System; CR=complete remission; CCyR=complete cytogenetic response; TFS=transformation-free survival; OS=overall survival; UVA=univariate analysis; MVA=multivariate analysis; HR=hazard ratio; CI=confidence interval.
To further confirm the impact of the achievement of complete cytogenetic response on outcome, we performed a landmark analysis, using as start point the date of achievement of cytogenetic response (either complete or partial) for those who achieved it and the date of cytogenetic assessment for those who did not. This way, the time from therapy start to cytogenetic response, which is the confounding factor, was taken off the analysis. In this analysis, the achievement of complete cytogenetic response remains independent predictive factor for survival [p=0.008; HR=1.6 (95% CI, 1.1–2.4)] and leukemia-free survival [p=0.003; HR=1.8 (95% CI, 1.2–2.6)] (Supplemental Figures 3A and 3B).
To understand the effect of allogeneic stem cell transplantation, we performed a subsequent univariate and multivariate analyses censoring at the time of ASCT. In this analysis, the achievement of CCyR remained a major predictive factor for LFS [p=0.002; HR=1.9 (95% CI; 1.3–2.9)] and overall survival [p=0.001; HR=2.1 (95% CI; 1.4–3.2)] (Supplemental Figures 4A and 4B).
Discussion
In this report, we show that in patients with MDS treated with HMA, cytogenetic response occurs at in 36% of patients, being complete in 29%. We have additionally shown that the achievement of a complete cytogenetic response is significantly associated with survival improvement in patients with MDS and no morphological response. Our CyR rates were similar to the rates reported in other independent studies using HMA.3–6 Similarly to the study reported by Li et al, the achievement of a CyR was associated with survival improvement, with a median survival of 20 months compared to 12 months in patients without a CyR.9
It has been clearly established that the achievement of a CyR, particularly complete, is the most important prognostic factor for long-term outcome in patients with chronic and acute myeloid leukemia.10–12 Furthermore, the depth of the response is crucial for an improvement of event-free and overall survival.11 Our current study suggests similar phenomenon in MDS and as such the achievement of a CCyR should be considered a milestone in the management of these patients.
Of importance, there was no relationship between morphological response and CyR. In fact, in our study, among the 123 patients considered not responding according to the IWG criteria, 24% had a CyR (complete in 15%). This is similar to the experience with lenalidomide in patients with MDS and chromosome 5q abnormalities, where the CCyR rate was 44%, whereas the morphologic CR rate was only 27%.22 Furthermore, outcome of patients with CyR and lack of morphologic response was similar to patients achieving a morphologic response after HMA therapy. There are several hypothetical explanations to this phenomenon. One possibility could be purely subjective due to the pathologist reviewing the slides. We have previously reported, among pathologists, a discordance rate of 12% in the diagnosis of patients with MDS.23 A consensus assessment of response by multiple experienced pathologists and integration of genetic and molecular markers in the response assessment will significantly decrease this gap. Another possibility could be the existence of a lag phase between early progenitors and later dividing precursors; repeating the assessment a week or two later can further elucidate the gap. Finally, that could be explained by the heterogeneity of the disease, where patients can harbor different clones with and without abnormal karyotype that can coexist at the same time. As such in patients with no morphologic response, a clone with abnormal karyotype could be suppressed while other disease clones with somatic point mutations and normal karyotype were not.
There are several limitations to our study. Because of the retrospective nature of the study design, schedule and frequency of bone marrow exams were not controlled throughout the cohort. We have previously reported on the importance of a close cytogenetic monitoring of such patients where the acquisition of a new cytogenetic clone could reflect genomic instability and imminent transformation,24 and as such we are implementing prospectively a close monitoring with marrow samples for morphologic cytogenetic and molecular assessment every 3 the first year and then every 6 months thereafter. Second, in our study, we did not take into account for the increasingly identified molecular alterations and somatic point mutations and its correlation with long-term outcome.25–26 It is of great interest to investigate association between these molecular markers, response to therapy (minimal residual disease), and subsequent disease progression and survival. With all these limitations, the data presented here indicate that the achievement of a CCyR is a major milestone in the management of patients with MDS.
In conclusion, the achievement of a CyR, particularly complete, in patients with MDS and cytogenetic abnormalities treated with HMA occurs in close to a third of the patients and is the major parameter associated with survival improvement, particularly among patients not in morphologic response. As such this constitutes a major milestone and should be added to the morphologic criteria already in use. Sequential cytogenetic analyses may allow the identification of subsets of patients with MDS at higher risk for progression and thus might guide treatment decisions in the future.
Supplementary Material
Acknowledgments
Funding: This research is supported in part by the following grants: The University of Texas MD Anderson Cancer Center Support Grant (CCSG) CA016672; the MDS Clinical Research Consortium sponsored by the Aplastic Anemia and MDS International Foundation (made possible by funding from the Evans Foundation); and generous philanthropic contributions to the MD Anderson Moon Shots Program.
Footnotes
Authorship
Contributions: E.J. and G.G.M. designed and performed the research and analyzed the data; P.S., Q.W., M.C., J.H., and N.J.S. analyzed the data; H.K., J.C., S.O., F.R., C.B.R., S.A.A., C.D., N.D., T.K., W.W., Y.W., S.C., G.B., and E.Z. provided the materials and analytical tools; and E.J. and G.G.M. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References
- 1.Tefferi A, Vardiman JW. Myelodysplastic syndromes. N Engl J Med. 2009;361:1872–1885. doi: 10.1056/NEJMra0902908. [DOI] [PubMed] [Google Scholar]
- 2.Dayyani F, Conley AP, Strom SS, Stevenson W, Cortes JE, Borthakur G, Faderl S, O’Brien S, Pierce S, Kantarjian H, Garcia-Manero G. Cause of death in patients with lower-risk myelodysplastic syndrome. Cancer. 2010;116:2174–2179. doi: 10.1002/cncr.24984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silverman LR, Demakos EP, Peterson BL, Kornblith AB, Holland JC, Odchimar-Reissig R, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20:2429–2440. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 4.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: A randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kantarjian H, Issa JP, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106:1794–1803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 6.Kantarjian H, Oki Y, Garcia-Manero G, et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 2007;109:52–57. doi: 10.1182/blood-2006-05-021162. [DOI] [PubMed] [Google Scholar]
- 7.Schanz J, Tuchler H, Sole F, et al. New comprehensive cytogenetic scoring system for primary myelodysplastic syndromes (MDS) and oligoblastic acute myeloid leukemia after MDS derived from an international database merge. J Clin Oncol. 2012;30:820–829. doi: 10.1200/JCO.2011.35.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jabbour EJ, Estey E, Kantarjian HM. Acute myeloid leukemia. Mayo Clin Proc. 2006;81(2):247–260. doi: 10.4065/81.2.247. [DOI] [PubMed] [Google Scholar]
- 9.Li X, Chang C, He Q, et al. Cytogenetic response based on revised IPSS cytogenetic risk stratification and minimal residual disease monitoring by FISH in MDS treated with low-dose decitabine. Leukemia research. 2013 doi: 10.1016/j.leukres.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Kantarjian H, O’Brien S, Cortes J, et al. Survival advantage with imatinib mesylate therapy in chronic-phase chronic myelogenous leukemia (CML-CP) after IFN-alpha failure and in late CML-CP, comparison with historical controls. Clin Cancer Res. 2004;10:68–75. doi: 10.1158/1078-0432.ccr-1035-3. [DOI] [PubMed] [Google Scholar]
- 11.Jabbour E, Kantarjian H, O’Brien S, et al. The achievement of an early complete cytogenetic response is a major determinant for outcome in patients with early chronic phase chronic myeloid leukemia treated with tyrosine kinase inhibitors. Blood. 2011;118(17):4541–4546. doi: 10.1182/blood-2011-04-348110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Cortes J, Estrov Z, et al. Persistence of cytogenetic abnormalities at complete remission after induction in patients with acute myeloid leukemia: prognostic significance and the potential role of allogeneic stem-cell transplantation. J Clin Oncol. 2011;29(18):2507–2513. doi: 10.1200/JCO.2010.34.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Issa JP, Garcia-Manero G, Huang X, et al. Results of Phase II Randomized Study of Low-Dose Decitabine with or without Valproic Acid in Patients with Myelodysplastic Syndrome and Acute Myelogenous Leukemia. Cancer. 2014 doi: 10.1002/cncr.29085. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soriano AO, Yang H, Faderl S, et al. Safety and clinical activity of the combination of 5-azacytidine, valproic acid, and all-trans retinoic acid in acute myeloid leukemia and myelodysplastic syndrome. Blood. 2007;110(7):2302–2308. doi: 10.1182/blood-2007-03-078576. [DOI] [PubMed] [Google Scholar]
- 15.Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108:419–425. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 16.Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–2465. doi: 10.1182/blood-2012-03-420489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292–2302. doi: 10.1182/blood-2002-04-1199. [DOI] [PubMed] [Google Scholar]
- 18.Agresti A. Categorical Data Analysis. 2. John Wiley & Sons Inc; Hoboken, NJ: 1990. [Google Scholar]
- 19.Kaplan EL, Maier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1965;53:457–481. [Google Scholar]
- 20.Cox DR. Regression models and life tables. J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 21.Kantarjian H, O’Brien S, Ravandi F, et al. Proposal for a new risk model in myelodysplastic syndrome that accounts for events not considered in the original International Prognostic Scoring System. Cancer. 2008;113:1351–1361. doi: 10.1002/cncr.23697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.List A, Dewald G, Bennett J, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355(14):1456–1465. doi: 10.1056/NEJMoa061292. [DOI] [PubMed] [Google Scholar]
- 23.Naqvi K, Jabbour E, Bueso-Ramos C, et al. Implications of discrepancy in morphologic diagnosis of myelodysplastic syndrome between referral and tertiary care centers. Blood. 2011;118(17):4690–4693. doi: 10.1182/blood-2011-03-342642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jabbour E, Takahashi K, Wang X, et al. Acquisition of cytogenetic abnormalities in patients with IPSS defined lower-risk myelodysplastic syndrome is associated with poor prognosis and transformation to acute myelogenous leukemia. Am J Hematol. 2013;88(10):831–837. doi: 10.1002/ajh.23513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bejar R, Stevenson KE, Caughey BA, et al. Validation of a prognostic model and the impact of mutations in patients with lower-risk myelodysplastic syndromes. J Clin Oncol. 2012;30:3376–3382. doi: 10.1200/JCO.2011.40.7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bejar R, Stevenson K, Abdel-Wahab O, et al. Clinical effect of point mutations in myelodysplastic syndromes. N Engl J Med. 2011;364:2496–2506. doi: 10.1056/NEJMoa1013343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


