Abstract
Purpose
Breast cancer patients can switch hormonal therapy (HT) regimens due to treatment side effects or menopausal status change. We describe HT class switching from aromatase inhibitor (AI) to tamoxifen (TAM), and vice versa.
Methods
In a cohort of 3,265 women diagnosed with hormone-receptor positive breast cancer at Kaiser Permanente Northern California from 2005–2013, we analyzed prescription records, switching reasons, and treatment adherence post-switch by chart review, through December 31, 2014.
Results
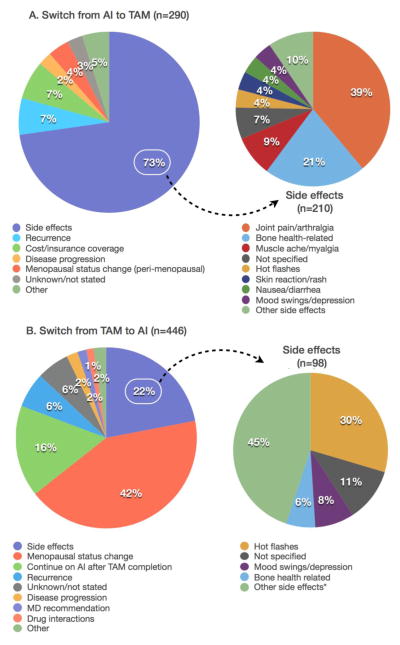
There were 290 women who switched from AI to TAM (AI switchers), including 130 (45%) switchers during the first year of treatment; and 446 women who switched from TAM to AI (TAM switchers), including 120 (27%) switchers within the first year. After the switch, 136 (47%) AI switchers and 260 (58%) TAM switchers finished or remained on the planned therapy; 69 (24%) AI switchers and 99 (22%) TAM switchers discontinued therapy. AI side effects (73%), specifically joint pain/arthralgia and bone health issues, were the most common reasons for switching from AI to TAM, whereas from TAM to AI, it was menopausal status change (42%).
Conclusions
Study findings highlight the need for better ways to control patient symptoms from HT to prevent discontinuation, and thus ensure best prognosis.
Keywords: breast cancer, cancer survivor, cohort study, aromatase inhibitors, tamoxifen, hormonal therapy
Introduction
Aromatase inhibitors (AIs) and tamoxifen (TAM) are the primary adjuvant hormonal therapies for women diagnosed with early-stage, hormone receptor (HR)-positive breast cancer. TAM is a selective estrogen receptor modulator used in pre- or post-menopausal women, whereas AIs interfere with the body’s ability to produce estrogen by suppressing aromatase enzyme activity and is effective in postmenopausal women [1]. Both therapies have been shown to be associated with improved disease-free and recurrence-free survival in women with HR-positive breast cancer [2–4].
Once patients initiate one hormonal therapy (HT), a proportion of women will switch to the other therapy during their treatment period. Reasons for the switch can include planned switching after completion of the initial regimen (2–3 or 5 years of TAM followed by extended AI therapy), and unplanned switching due to adverse events associated with the initial therapy, or cancer recurrence. While research on adherence or early discontinuation of HT and outcomes in breast cancer patients has been prominent [5–7], there is a paucity of information describing the sub-group of patients who switch from one therapy to another and reasons why they do so. To our knowledge, only one study to date has examined HT drug switching and associated side effects [8]. In this Swiss cohort of 400 patients treated from 1998–2008, 64 patients (16%) switched therapies due to drug-related side effects, whereas 27 patients (7%) completely stopped therapy due to drug-related side effects and other reasons.
To help fill this research gap, we describe HT switching from AI to TAM or TAM to AI, and subsequent continuation and discontinuation of treatment, in one of the largest U.S. cohorts of 3,265 breast cancer patients diagnosed from 2006–2013. We also examine reasons associated with the medication switch.
Materials and Methods
Study Population
The Pathways Study is a prospective study of 4,505 women with newly diagnosed invasive breast cancer who are members of Kaiser Permanente Northern California (KPNC), a large, integrated health care delivery system covering the San Francisco-Oakland Bay Area, Sacramento, and surrounding counties in CA. Recruitment was from January 2006 to April 2013 through rapid case ascertainment procedures designed to enroll women prior to initiation of chemotherapy, as described elsewhere [9]. Eligibility criteria included: KPNC female members at least 21 years of age; had no previous history of malignancy other than non-melanoma skin cancer; spoke English, Spanish, Cantonese, or Mandarin; and resided within a 65-mile radius of a field interviewer. The mean time from diagnosis to enrollment was 2.0 (± 0.7) months.
For this bone health sub-study, women were included if they had stage I-III disease and at least one HT prescription of an AI or TAM that was indicated for treatment of their first primary breast cancer based on patients’ outpatient pharmacy records. The final study population consisted of 3,265 eligible women. Based on complete HT prescription data through December 31, 2014, 2,122 (65%) were initial AI users, and 1,143 (35%) were initial TAM users.
Self-reported Participant Information
The baseline interview was conducted at enrollment into the cohort approximately two months post-diagnosis, and included interviewer and self-administered questionnaires on sociodemographics, lifestyle factors, established breast cancer risk factors, and health history.
Pharmacy Data
Prescription drug data for nearly 100% of KPNC enrollees are recorded in the KPNC pharmacy database, including drug name, National Drug Code, dosage and therapeutic class; prescription dates and cost; dispensing and refills; and prescribing physician, thus minimizing recall bias [11]. The pharmacy database was accessed to identify any outpatient prescriptions of AIs (anastrozole, letrozole, and exemestane) and TAM after breast cancer diagnosis, as of December 31, 2014. An AI switcher was defined as a woman whose first prescription was for an AI and a subsequent prescription was for TAM, whereas a TAM switcher was defined as a woman whose first prescription was TAM and a subsequent prescription was for an AI. The dispensing date of the new prescription was considered the date of the switch. Discontinuation of HT was defined as no refills before the end of the expected treatment course from analysis of pharmacy data and confirmation by chart review.
Reasons for Hormonal Therapy Switching
From the oncology notes in the electronic medical record (EMR), a trained medical record reviewer abstracted reasons for HT switching among the AI switchers and TAM switchers. She recorded primary and secondary reasons for the initial switch as noted by the oncologist, dates and reasons of any subsequent switches, and information on whether or not patients completed their last treatment as planned, or were still continuing as of last record in the EMR. The reasons were reviewed by J.M.R. and M.L.K., and classified into larger categories for switching such as side effects, menopausal status change, and completed treatment as planned.
Statistical Analysis
Descriptive frequencies and chi-square tests were calculated for the AI and TAM switcher groups, as well as the comparison groups of AI only and TAM only. Reasons for switching were enumerated. Statistical analyses were done using SAS 9.3 (Cary, NC).
Results
Among 2,122 breast cancer patients who started on AI therapy, 290 (13.7%) switched to TAM (i.e., AI switchers) with a mean time between last fill of old drug and first fill of new drug of 120 days (SD=196). Among 1,143 patients who started on TAM therapy, 446 women (39.0%) switched to AI (i.e., TAM switchers) with a mean time of 124 days (SD=180). The remaining women took AI only (n=1,832, 86.3%) or TAM only (n=697, 61.0%) during the study period. Within the first year of treatment start, 44.8% of the AI to TAM switching occurred compared to 26.9% of the TAM to AI switching. Among the AI switchers, 136 (46.9%) women continued with the new therapy after the initial switch from AI to TAM, which included 70 women (24.1%) finishing their TAM as planned and 66 (22.8%) still taking it at the time of the chart review. 70 women (24.1%) switched back to AI and 69 women (23.8%) completely discontinued HT prior to completion of therapy as planned. Similarly, among the TAM switchers, 260 (58.3%) women continued with the new therapy after the initial switch from TAM to AI, which included 128 women (28.7%) finishing their AI as planned and 132 (29.6%) still taking it. 61 women (13.7%) switched back to TAM and 99 women (22.2%) completely discontinued HT prior to completion of therapy as planned.
Comparison of characteristics among the AI and TAM users and their respective switchers are shown in Table 1. Because AI is primarily indicated for postmenopausal breast cancer, there were expected differences in age at diagnosis and baseline menopausal status between patients initially treated with AI and with TAM. The mean age at breast cancer diagnosis among AI switchers was lower than the AI only group (62.4 vs. 64.1 years), whereas the mean age among TAM switchers was higher than the TAM only group (51.5 vs. 49.1 years). Of note, from our chart review, the premenopausal women who were initially prescribed an AI (n=141) had peri-menopausal symptoms at the start of treatment and then some later resumed menses.
Table 1.
Characteristics of patients who switched from AI-TAM and TAM-AI compared to those who did not switch
| AI Only | AI Switchers | p-valuea | TAM Only | TAM Switchers | p-valuea | |
|---|---|---|---|---|---|---|
|
|
|
|||||
| N=1832 N (%) |
N=290 N (%) |
N=697 N (%) |
N=446 N (%) |
|||
| Age at BC Diagnosis (years) | 0.0004 | <0.0001 | ||||
| <50 | 58 (3.2) | 21 (7.2) | 432 (62.0) | 206 (46.2) | ||
| 50–59 | 546 (29.8) | 85 (29.3) | 154 (22.1) | 172 (38.6) | ||
| 60–69 | 748 (40.8) | 130 (44.8) | 62 (8.9) | 39 (8.7) | ||
| ≥70 | 480 (26.2) | 54 (18.6) | 49 (7.0) | 29 (6.5) | ||
| Mean (SD) | 64.1 (9.0) | 62.4 (9.2) | 49.1 (11.1) | 51.5 (9.9) | ||
| Menopausal Status at Baseline | 0.09 | 0.42 | ||||
| Premenopausal | 110 (6.0) | 31 (10.7) | 531 (76.2) | 315 (70.6) | ||
| Postmenopausal | 1722 (94.0) | 259 (89.3) | 166 (23.8) | 131 (29.4) | ||
| Race | 0.60 | 0.004 | ||||
| White | 1309 (71.5) | 210 (72.4) | 352 (50.5) | 286 (64.1) | ||
| African American | 109 (6.0) | 11 (3.8) | 58 (8.3) | 23 (5.2) | ||
| Asian | 186 (10.2) | 33 (11.4) | 146 (21) | 74 (16.6) | ||
| Hispanic | 190 (10.4) | 29 (10.0) | 123 (17.7) | 55 (12.3) | ||
| Other | 38 (2.1) | 7 (2.4) | 18 (2.6) | 8 (1.8) | ||
| Education at Baseline | 0.29 | 0.33 | ||||
| High School or less | 322 (17.6) | 37 (12.8) | 88 (12.6) | 48 (10.8) | ||
| Some College | 623 (34.0) | 108 (37.2) | 226 (32.4) | 132 (29.6) | ||
| College Graduate | 457 (25.0) | 83 (28.6) | 219 (31.4) | 158 (35.4) | ||
| Post-graduate | 424 (23.1) | 62 (21.4) | 162 (23.2) | 108 (24.2) | ||
| Unknown | 6 (0.3) | 0 (0) | 2 (0.3) | 0 (0) | ||
| Household Income ($) at Baseline | 1.00 | 0.07 | ||||
| <25,000 | 193 (10.5) | 28 (9.7) | 46 (6.6) | 19 (4.3) | ||
| 25,000–49,999 | 386 (21.1) | 58 (20.0) | 94 (13.5) | 66 (14.8) | ||
| 50,000–89,999 | 541 (29.5) | 86 (29.7) | 202 (29.0) | 118 (26.5) | ||
| >90,000 | 487 (26.6) | 84 (29.0) | 287 (41.2) | 204 (45.7) | ||
| Not reported | 225 (12.3) | 34 (11.7) | 68 (9.8) | 39 (8.7) | ||
| BMI at Baseline (kg/m2) | 0.10 | 0.34 | ||||
| <25 | 533 (29.4) | 99 (34.5) | 314 (45.5) | 194 (43.7) | ||
| 25–29.9 | 573 (31.6) | 93 (32.4) | 193 (28.0) | 144 (32.4) | ||
| ≥30 | 710 (39.1) | 95 (33.1) | 183 (26.5) | 106 (23.9) | ||
| Mean (SD) | 29.1 (6.5) | 28.2 (5.9) | 0.02 | 27.2 (6.7) | 27.1 (6.4) | 0.72 |
| Any Fracture before BC Diagnosis | 0.97 | 0.90 | ||||
| No | 1529 (83.5) | 245 (84.5) | 648 (93.0) | 408 (91.5) | ||
| Yes | 303 (16.5) | 45 (15.5) | 49 (7.0) | 38 (8.5) | ||
| AJCC Stage | 0.03 | 0.0008 | ||||
| I | 1029 (56.2) | 137 (47.2) | 388 (55.7) | 211 (47.3) | ||
| II | 631 (34.4) | 119 (41.0) | 248 (35.6) | 179 (40.1) | ||
| III | 172 (9.4) | 34 (11.7) | 61 (8.8) | 56 (12.6) | ||
NOTE: Pharmacy data through December 31, 2014
Chi-square test and age-adjusted except for age at breast cancer diagnosis
There was a higher proportion of White women in the TAM switcher group (64.1%) compared with the TAM only group (50.5%), whereas there were no substantial differences in race between the AI only and AI switcher groups. The mean BMI at baseline was slightly lower in the AI switcher than AI only group (28.2 vs. 29.1 kg/m2), but there was no difference between the TAM switcher and TAM-only group. Both switcher groups were more likely to be diagnosed with stage II or III disease compared with the non-switcher groups. There were no significant differences between the switcher and non-switcher groups by education, household income, or previous history of fracture.
The reasons for hormonal therapy switching are shown in Figure 1. Among AI switchers, side effects from AI was the primary reason for switching to TAM (top panel, n=211, 72.8%), primarily joint pain/arthralgia (28.3%), bone health-related side effects (15.5%), and muscle ache/myalgia (6.2%). Other common reasons for switching included cost/insurance coverage (6.9%), and recurrence after initial treatment (6.6%). For those who switched due to cost/insurance coverage, the reason for the switch was recorded as “too expensive”, and it appears that the switch occurred prior to AI medications becoming generic in June 2010 and subsequently being covered with lower copayment under the KPNC formulary (https://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/DrugandBiologicApprovalReports/ANDAGenericDrugApprovals/ucm218923.htm).
Figure 1. Reasons for initial switch from AI-TAM or TAM-AI.
Figure 1A – Top Panel (AI to TAM)
aOther side effects with n≤5: Neuropathy/pain; fatigue; edema; vaginal dryness/itching; bad taste/white tongue; body twitching; breast swelling; GERD; gout; insomnia
bOther reasons with n≤5: New breast cancer diagnosis; completed AI treatment as planned; patient preference; drug interactions
Figure 1B – Bottom Panel (TAM to AI)
aOther side effects with n≤5: Joint pain/arthralgia; vaginal bleeding/spotting; blood clot; skin reaction/rash; weight gain; fatigue; nausea/diarrhea; migraine/headache; muscle ache/myalgia; neuropathy/pain; effects on endometrium; shortness of breath; endometrial cancer; edema; elevated liver function test; hypertension; stroke; vaginal dryness/itching
bOther reasons with n≤5: Patient preference; new breast cancer diagnosis; cost/insurance coverage
Among TAM switchers, the most common reason for switching from TAM to AI was menopausal status change (bottom panel, n=189, 42.4%), as therapy-induced or natural amenorrhea made AI appropriate for them. Other common reasons included side effects (22.0%), completed TAM treatment as planned with continued AI treatment (16.4%), and cancer recurrence (6.3%).
We further identified reasons for switching back from TAM to AI or AI to TAM, or completely stopping HT prior to completion of therapy as planned (data not shown). AI switchers and TAM switchers most commonly switched back to AI or TAM, respectively, due to treatment-related side effects. Other reasons for switching back included disease progression (14.3% for AI switchers) and menopausal status change (16.4% for TAM switchers). Among the 69 AI switchers who completely discontinued HT, the most common reason was side effects (44.9%), whereas among the 99 TAM switchers who completely discontinued HT, it was a mixture of disease progression (23.2%) and side effects (21.2%).
Discussion
In a contemporary cohort of 3,265 breast cancer patients taking HT, 736 (22.5%) patients switched between classes of therapy. About 45% switched from AI to TAM and 27% switched from TAM to AI within the first year of treatment. Side effects was the most common reason for switching from AI to TAM (72.8%), with joint pain/arthralgia and bone health-related issues being the most prevalent side effects, whereas menopausal status change was the most common reason for switching from TAM to AI (42.4%).
To date, only one study has examined reasons for switching hormonal therapies using data from 400 postmenopausal breast cancer patients in Switzerland who initiated HT from 1998–2008 and reported how often the women had adverse side effects [8]. They further evaluated how many patients discontinued their treatment due to side effects. Among the 400 patients, 66.3% initiated TAM and 29.5% initiated AI, and 74.8% completed their initial therapy. Out of the 400 women who started HT, 37 (9.3%) completely discontinued their initiated therapy; out of these, 24 (64.9%) were due to therapy-related side effects, and 13 (35.1%) due to other reasons. In our study with over 8-times the study population of women on HT from 2005–2014, we had opposite trends of 35% initiating TAM and 65% initiating AI, and 5.1% completely discontinuing their initial therapy. These different proportions in initial treatment are most likely due to a combination of temporal trends in clinical care of AIs becoming first-line adjuvant therapy with superior outcomes in postmenopausal women, and the Swiss study only including postmenopausal patients.
A recent study reported that switching therapy from TAM to AI or AI to TAM during the first year of hormonal therapy was associated with increased risk of discontinuation during the next four years (HR, 1.50; 95% CI, 1.23 to 1.83) [12]. In our study, among those who had switched from AI to TAM or TAM to AI, 23.8% and 22.2%, respectively, completely discontinued hormonal therapy. The majority of this former group of discontinuers who switched from AI to TAM switched therapies during the first two years of hormonal therapy (40.6% and 31.9%, respectively). The majority of the latter group of discontinuers who switched from TAM to AI switched during the first three years of hormonal therapy (21.2%, 32.3%, and 20.2%, respectively). These proportions also suggest that switching therapies earlier, as opposed to later, relative to start of therapy could be associated with discontinuation.
Strengths of our study include a contemporary cohort of breast cancer patients diagnosed from 2006–2013 and treated within an integrated health care system, long-term mean follow-up from cancer diagnosis of 6.6 years and from HT initiation of 6.2 years, reliance on pharmacy data to more accurately capture medication use by patients, and detailed information from chart review on reason for switching hormonal therapies and subsequent continuation or completion of treatment. Future analyses will focus on calculating measures of adherence and discontinuation using our pharmacy data, determining predictors of these measures, and examining their associations with breast cancer outcomes such as recurrence and survival in this cohort.
In conclusion, adherence to HT is important to help ensure the best outcomes in eligible breast cancer patients. However, we found that therapy-related side effects was the dominant cause for patients to switch from AI to TAM, and also the second most common cause for patients to switch from TAM to AI. It is also the most common reason for AI switchers to completely discontinue HT after switching. These findings highlight the need for better ways to control patient symptoms from HT to prevent discontinuation to the current medication, and ultimately the best prognosis.
Acknowledgments
Funded by National Cancer Institute R01 CA166701 (M.L.K. and S.Y.) and R01 CA105274 (L.H.K).
Footnotes
Compliance with Ethical Standards
Conflict of Interest: The authors declare no conflicts of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Institutional Review Board of Kaiser Permanente Northern California.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- 1.Fabian CJ. The what, why and how of aromatase inhibitors: hormonal agents for treatment and prevention of breast cancer. Int J Clin Pract. 2007;61(12):2051–2063. doi: 10.1111/j.1742-1241.2007.01587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burstein HJ, Prestrud AA, Seidenfeld J, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, Giordano SH, Hudis CA, Malin J, Mamounas EP, Rowden D, Solky AJ, Sowers MR, Stearns V, Winer EP, Somerfield MR, Griggs JJ. American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28(23):3784–3796. doi: 10.1200/JCO.2009.26.3756. JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dowsett M, Cuzick J, Ingle J, Coates A, Forbes J, Bliss J, Buyse M, Baum M, Buzdar A, Colleoni M, Coombes C, Snowdon C, Gnant M, Jakesz R, Kaufmann M, Boccardo F, Godwin J, Davies C, Peto R. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol. 2010;28(3):509–518. doi: 10.1200/JCO.2009.23.1274. JCO.2009.23.1274. [DOI] [PubMed] [Google Scholar]
- 4.Early Breast Cancer Trialists’ Collaborative G. Dowsett M, Forbes JF, Bradley R, Ingle J, Aihara T, Bliss J, Boccardo F, Coates A, Coombes RC, Cuzick J, Dubsky P, Gnant M, Kaufmann M, Kilburn L, Perrone F, Rea D, Thurlimann B, van de Velde C, Pan H, Peto R, Davies C, Gray R. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386(10001):1341–1352. doi: 10.1016/S0140-6736(15)61074-1. [DOI] [PubMed] [Google Scholar]
- 5.Hershman DL, Kushi LH, Shao T, Buono D, Kershenbaum A, Tsai WY, Fehrenbacher L, Lin Gomez S, Miles S, Neugut AI. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28(27):4120–4128. doi: 10.1200/JCO.2009.25.9655. JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hershman DL, Shao T, Kushi LH, Buono D, Tsai WY, Fehrenbacher L, Kwan M, Gomez SL, Neugut AI. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2010 doi: 10.1007/s10549-010-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chlebowski RT, Kim J, Haque R. Adherence to endocrine therapy in breast cancer adjuvant and prevention settings. Cancer Prev Res (Phila) 2014;7(4):378–387. doi: 10.1158/1940-6207.CAPR-13-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guth U, Myrick ME, Schotzau A, Kilic N, Schmid SM. Drug switch because of treatment-related adverse side effects in endocrine adjuvant breast cancer therapy: how often and how often does it work? Breast Cancer Res Treat. 2011;129(3):799–807. doi: 10.1007/s10549-011-1668-y. [DOI] [PubMed] [Google Scholar]
- 9.Kwan ML, Ambrosone CB, Lee MM, Barlow J, Krathwohl SE, Ergas IJ, Ashley CH, Bittner JR, Darbinian J, Stronach K, Caan BJ, Davis W, Kutner SE, Quesenberry CP, Somkin CP, Sternfeld B, Wiencke JK, Zheng S, Kushi LH. The Pathways Study: a prospective study of breast cancer survivorship within Kaiser Permanente Northern California. Cancer Causes Control. 2008;19(10):1065–1076. doi: 10.1007/s10552-008-9170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oehrli MD, Quesenberry CP. Kaiser Permanente, Northern California Cancer Registry. 2013. 2013 Annual Report on Trends, Incidence, and Outcomes. [Google Scholar]
- 11.Selby JV, Smith DH, Johnson ES, Raebel MA, Friedman GD, McFarland BH. Kaiser Permanente Medical Care Program. In: BLS, editor. Pharmacoepidemiology. John Wiley & Sons, Ltd; West Sussex: 2005. pp. 241–259. [Google Scholar]
- 12.He W, Fang F, Varnum C, Eriksson M, Hall P, Czene K. Predictors of Discontinuation of Adjuvant Hormone Therapy in Patients With Breast Cancer. J Clin Oncol. 2015;33(20):2262–2269. doi: 10.1200/JCO.2014.59.3673. [DOI] [PubMed] [Google Scholar]