Abstract
BACKGROUND/OBJECTIVES
A high incidence of hypogonadism in men with type 2 diabetes (T2D) has been globally reported. This study aimed to determining the frequency of hypogonadism and related risk factors among men with T2D in a single-site hospital in Saudi Arabia.
DESIGN AND METHODS
A cross-sectional study was performed on 157 men with T2D (between 30 and 70 years of age). Using a prestructured questionnaire, the demographic features of these patients were gathered and their medical records were referred to gather information regarding the duration of the diabetes, smoking habits, and the presence of retinopathy, neuropathy, and nephropathy. Besides these, the biochemical parameters, total testosterone (TT), free testosterone, sex hormone–binding globulin, follicle-stimulating hormone, luteinizing hormone, prolactin, serum lipids, and glycosylated hemoglobin were also recorded. All the patients submitted the fully completed Androgen Deficiency in Aging Male (ADAM) questionnaire. The combination of symptoms (positive ADAM score) plus a TT level ⩽8 nmol/L constituted the condition of hypogonadism.
RESULTS
The total frequency of hypogonadism was 22.9% (36/157). Of the 157 total patients, 123 (78.3%) were shown to be ADAM positive, and of these, 90 (73.2%) exhibited decreased libido, 116 (94.3%) had weak erections, and 99 (80.5%) reported more than 3 symptoms of ADAM. Of these hypogonadic patients, 22.2% (n = 8) revealed primary hypogonadism, whereas 77.8% (n = 28) showed secondary hypogonadism. From the univariate analysis conducted, significant relationship was observed between treatment type, body mass index (BMI), and hypogonadism. The regression analysis showed BMI acting an independent risk factor of hypogonadism.
CONCLUSIONS
Saudi men with T2D revealed a high incidence of hypogonadism. Body mass index was identified as an independent risk factor for hypogonadism.
Keywords: Hypogonadism, type 2 diabetes, total testosterone, Saudi Arabia
Introduction
Apart from the current global report of 415 million adults with diabetes (which implies 1 in every 11 adults has the disease), another 318 million adults reveal impaired glucose tolerance, at high risk for developing the disease in full form relatively quickly.1 A subtle rise in the spread and occurrence of diabetes mellitus (DM) was observed in the wake of the escalating industrialization that swept across the Kingdom of Saudi Arabia, causing a rapid rise in the standard of living and the adoption of a more “Westernized” lifestyle.2 According to the World Health Organization ranking, Saudi Arabia stands second in the Middle East region and seventh globally for the incidence of diabetes.3
There is sufficient evidence to show that patients with type 2 diabetes (T2D) have a greater tendency to acquire several complications, including hypogonadism.4,5 Male hypogonadism is defined as a clinical condition in which the men exhibit the symptoms/signs and biochemical evidence of testosterone deficiency. The fact that T2D is linked to hypogonadism was first recognized about 20 years ago when a high frequency of men with T2D revealed low testosterone levels. The low testosterone levels have also been identified as reliable predictors of insulin resistance and the likelihood of developing T2D in the future.6 Furthermore, many studies have shown that the testosterone levels reported in men with diabetes was much lower than those of the nondiabetic ones.7,8
From the cross-sectional studies done, it is clear that between 20% and 64% of men with diabetes have hypogonadism; generally, there is a slow and continuous decrease in testosterone production among older population.9 Furthermore, the prevalence of hypogonadism varies between racial, ethnic groups.10 Hypogonadism can pose high risk for the development of diabetes and metabolic syndrome via several mechanisms including alterations in body composition, polymorphism in the androgen receptors, glucose transport, and decreased antioxidant effect.9–11 However, diabetes and the metabolic syndrome can be risk factors for hypogonadism via certain similar, but most often, well-defined mechanisms, such as body weight increase, drop in the levels of the sex hormone–binding globulin (SHBG), suppression of gonadotropin release or Leydig cell testosterone secretion, cytokine-mediated inhibition of the release of the testicular steroid, and a rise in the aromatase activity leading to a relative estrogen surplus.9–11
Type 2 diabetes–associated hypogonadism might exacerbate sexual dysfunction by reducing libido and mood and further compromising penile vascular reactivity and lipid metabolism. Hence, testing the circulating testosterone is strongly recommended in patients with type 2 DM with erectile dysfunction.12,13 Observational studies consistently show that men with diabetes have lower total testosterone (TT) levels compared with the nondiabetic controls.14 At least 25% of men with type 2 DM show evidence of secondary hypogonadism, and an additional 4% have primary hypogonadism. Given the strong correlation between diabetes and testosterone deficiency, the Endocrine Society recommends the routine measurement of testosterone levels in patients with type 2 DM. The relationship between testosterone and type 2 DM is complex and likely mediated by obesity.15
As the incidence of diabetes is steadily rising in all the age groups and a notable number will most probably be in their prime reproductive years, in fact that one-third of the men with T2D possess low testosterone causes a considerable public health burden with respect to deficient sexual function and potential infertility.16 In comparison with the developed countries, there is a paucity of research on hypogonadism in Saudi Arabia. Therefore, the aim of this study was to determine the occurrence of hypogonadism and its related risk factors present in the men with T2D in Saudi Arabia.
Methods
This cross-sectional study involved 157 men with type 2 diabetes, between 30 and 70 years, registered with the Diabetes Treatment Center, Prince Sultan Military Medical City (PSMMC), Riyadh, Saudi Arabia.
Patients with a history of hypopituitarism and chronic debilitating diseases such as renal failure, liver cirrhosis, malignancy, autonomic neuropathy, and those on testosterone replacement therapy were excluded from this study. Written consent to participate in the study was collected from each patient.
This study was conducted based on the Helsinki Declaration of 1975 (revised in 2000), and the study protocol received approval from the research ethics committee of PSMMC, Riyadh, Saudi Arabia.
Measures
Their demographic features were gathered during the patients’ routine visits to the center using a prestructured questionnaire. The duration of diabetes, smoking habits, presence of retinopathy, neuropathy, and nephropathy were noted from the medical records.
Calculation of the body mass index (BMI) was done by dividing the weight in kilograms by the square of the height in meters. Blood pressure was taken by a trained nurse, using a standardized sphygmomanometer with the patient in the sitting posture, keeping the arm at heart level, and repeated after 5 minutes of rest. Hypertension is defined as elevated systolic (⩾140 mm Hg) or diastolic (⩾90 mm Hg) blood pressure.5
After an overnight fast, a venous blood sample (20 mL) was drawn using a disposable syringe from the cubital fossa and/or dorsum of the hand veins from each patient, between 8:00 and 10:00 am. The blood sample was transferred into a plain complete blood count tube; once centrifugation was done, the serum was aliquoted and stored at −20°C to determine TT, free testosterone (FT), SHBG, follicle-stimulating hormone (FSH), luteinizing hormone (LH), prolactin (PRL), glycosylated hemoglobin A1c (HbA1c), total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and triglyceride. Laboratory technicians were blinded to the participants’ information. Radioimmunoassay was used to determine TT. AxSYM (Abbott Laboratories, USA) was employed to assess FT based on a microparticle enzyme immunoassay.8 Immunochemiluminometric assay was used to test SHBG. Chemiluminescent immunometric assays were used to determine LH, FSH, and PRL. High-performance liquid chromatography method (Bio-Rad, Hercules, CA, USA) enabled the analysis of HbA1c. Hemoglobin A1c–related information was recorded from tests performed in the PSMMC laboratory. Total cholesterol, triglyceride, HDL, and LDL were checked using an automated spectrophotometer, enzymatic colorimetric method, and COBAS INTEGRA with the commercial kits from Roche Diagnostics (Roche Diagnostics Limited, Switzerland). Bioavailable and FT levels were recorded using the TT and SHBG applying the Vermeulen equation.17
Diabetes mellitus was diagnosed based on the criteria of the American Diabetes Association.18 Nephropathy, according to the nephrologists (in view of the microalbuminuria present is defined as the concentration of microalbuminuria ⩾30-299 mg/24-hour urine collection sample or the presence of macroalbuminuria ⩾300 mg/24-hour urine collection sample)19 was taken from the patients’ records. Diabetic neuropathies are heterogeneous, either focal or diffuse, and affect various portions of the nervous system, producing various clinical manifestations.20 Retinopathy is one such example, defined according to the American Academy of Ophthalmology.21
Androgen Deficiency in the Aging Male Questionnaire
All the patients were required to complete an Arabic version of the Androgen Deficiency in Aging Male (ADAM) questionnaire.22 This 10-item screening questionnaire was employed to assess the androgen insufficiency in aging men. A positive response is based on lowered libido or erectile dysfunction or any 3 nonspecific queries that include fatigability, reduced muscle strength, mood alterations and height loss. As this questionnaire has 88% sensitivity and 60% specificity, it must be employed only when the testosterone level is low.23
Hypogonadism
Hypogonadism was defined as the combination of symptoms (positive ADAM score) along with TT level ⩽8 nmol/L. Primary hypogonadism implies FSH or LH ⩾10 IU/L, whereas secondary hypogonadism means FSH or LH < 10 IU/L.
Statistical Analysis
Data analysis was performed employing Microsoft Excel 2010 (Microsoft Corporation, Seattle, WA, USA) and the SPSS version 22 (SPSS Inc., Chicago, IL, USA). Besides the descriptive analysis, the χ2 test and independent “t” test were also done to identify the differences among the group. The variables associated with hypogonadism were verified using the logistic regression analysis. The P value of <.05 was accepted as having statistical significance.
Results
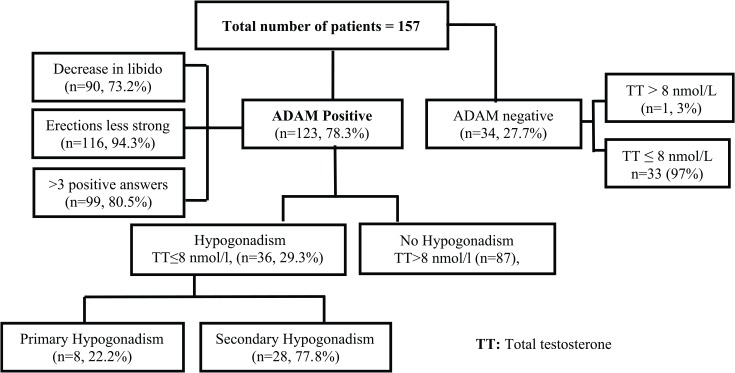
The description of the ADAM and TT level of the population is shown in Figure 1. The total frequency of hypogonadism was 22.9% (36/157). Of the total number of 157 patients (from 30 to 70 years), 123 (78.3%) revealed ADAM positive, whereas 34 (27.7%) showed ADAM negative. Among the ADAM positive, 90 (73.2%) showed a reduction in libido, 116 (94.3%) had weaker erections, and 99 (80.5%) showed more than 3 positive answers in ADAM. Of these hypogonadic patients, 22.2% (n = 8) revealed primary hypogonadism, whereas 77.8% (n = 28) showed secondary hypogonadism.
Figure 1.
Patient distribution.
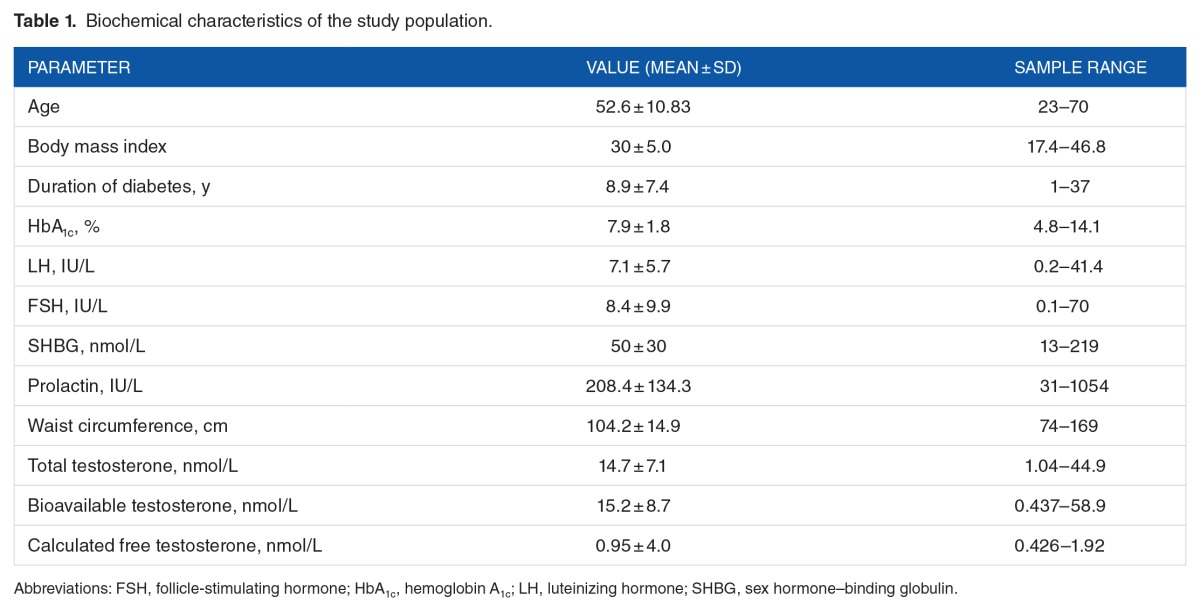
The demographic and clinical variables of the study population are shown in Table 1. The study cohort had a mean age of 52.6 ± 10.8 (mean ± SD) years. The duration of diagnosis of DM showed a mean of 8.9 ± 7.4 (mean ± SD). The mean values of the gonadal hormone levels were as follows: LH: 7.1, FSH: 8.4, SHBG: 50, PRL: 208.4, and TT: 14.7, with the bioavailable testosterone being 15.2 and calculated FT being 0.95.
Table 1.
Biochemical characteristics of the study population.
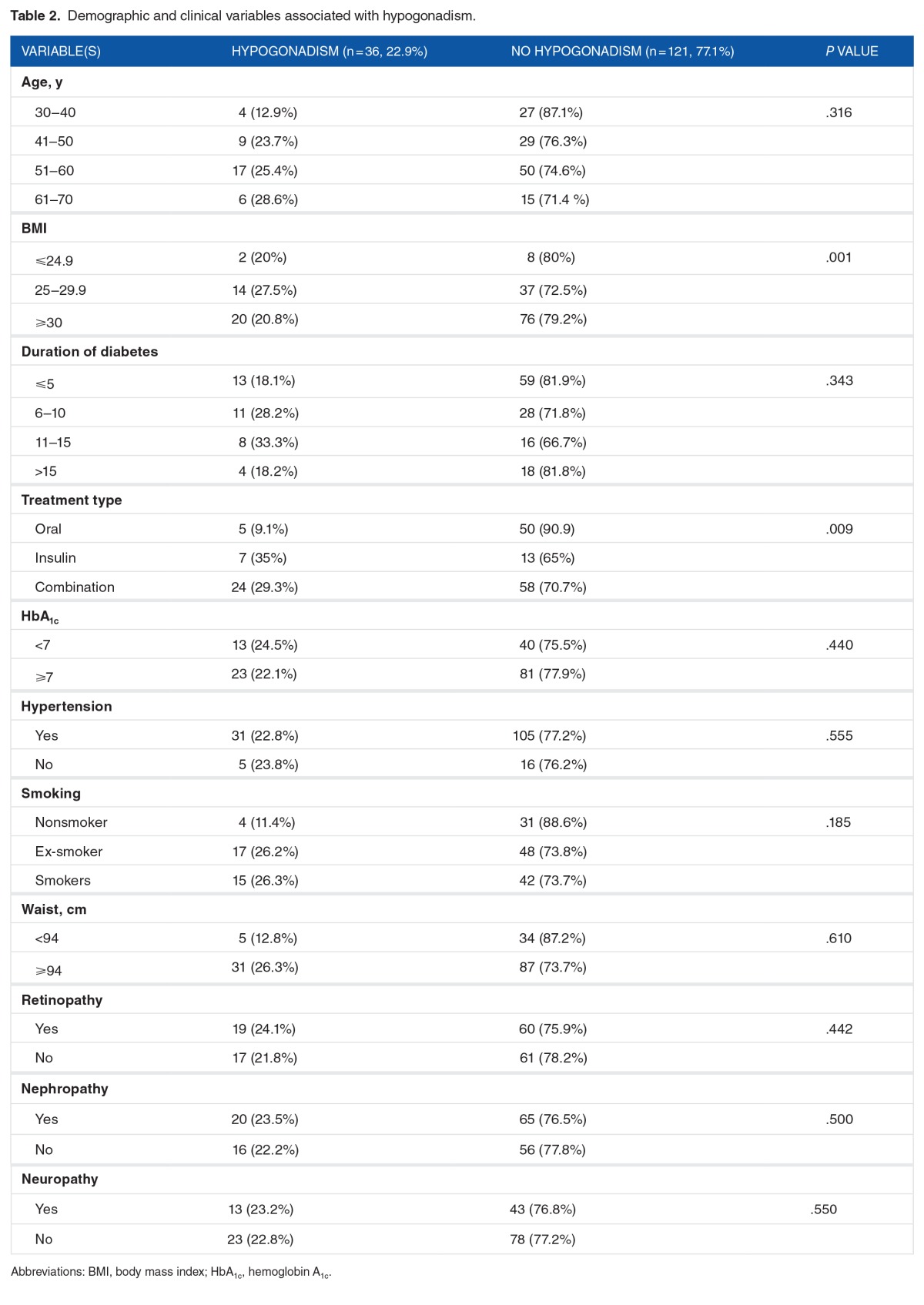
The variables associated with hypogonadism are demonstrated in Table 2. A major part of the study population, 67 patients (42.7%), belonged to the 51 to 60 years age category. No significant differences were identified in the age, duration of diabetes, HbA1c, hypertension, smoking, waist measurement, and diabetes-related impairments of retinopathy, nephropathy, and neuropathy. Significant differences were noted in the BMI and different treatment groups.
Table 2.
Demographic and clinical variables associated with hypogonadism.
The mean age of patients those with hypogonadism was 54.3 ± 10 years and those patients without hypogonadism was 47.2 ± 11 years. Similarly, the mean BMI of those patients with hypogonadism was 30.4 ± 5.19 and those patients without hypogonadism was 28.7 ± 4.16 (P < .042). Among patients with hypogonadism, the mean total cholesterol, triglycerides, LDL, and HDL were observed as 4.57 ± 1.2, 1.98 ± 1.37, 2.7 ± 0.95, and 1.09 ± 0.28, respectively. Among patients without hypogonadism, the mean total cholesterol, triglycerides, LDL, and HDL were observed as 4.14 ± 1.8, 2.44 ± 1.76, 2.3 ± 0.96, and 1.27 ± 1.8, respectively. No statistically significant differences were observed on lipid profile among the patients with and without hypogonadism (P > .05).
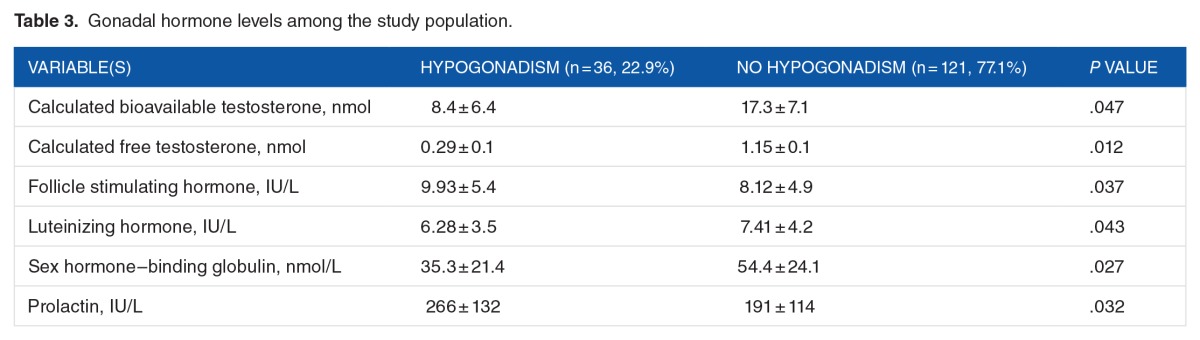
The gonadal hormone levels among the study population are shown in Table 3. In comparison with the normal patients, those with hypogonadism revealed significantly reduced differences in the calculated bioavailable testosterone, as well as the FT, LH, and SHBG. Compared with the normal patients, those with hypogonadism revealed significantly higher differences in the FSH and PRL levels.
Table 3.
Gonadal hormone levels among the study population.
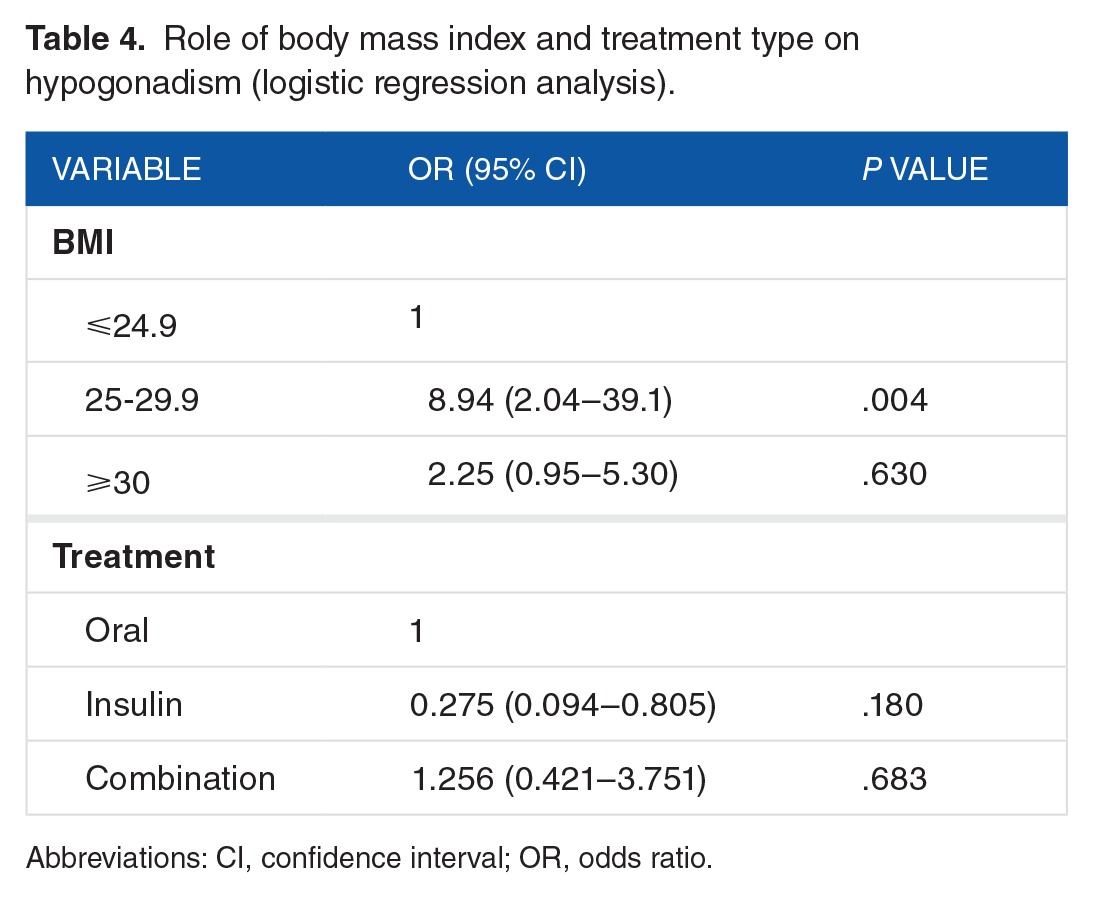
All the variables showing statistical significance in the “χ2” test were submitted to regression analysis, which demonstrated that BMI was the independent risk factor for hypogonadism (Table 4).
Table 4.
Role of body mass index and treatment type on hypogonadism (logistic regression analysis).
Discussion
It must be noted that the male patients with T2D have reduced serum testosterone and a greater tendency to develop hypogonadism than the nondiabetic ones, regardless of the metabolic disease control.24 The underlying mechanisms controlling the lowered testosterone could possibly be connected with age, obesity, and insulin resistance, commonly evident in the patients with T2D.24 The objective of this work attempted to determine hypogonadism in the men with T2D in the adult Saudi population.
From this study, the high occurrence of symptomatic hypogonadism is clear in men with T2D. Recent works have established beyond doubt that a minimum of 25% of the men with T2D have subnormal FT levels linked with inappropriately low LH and FSH levels.7,15 In light of this fact, the Endocrine Society has now recommended the routine verification of the testosterone concentrations in patients with T2D.15 These confirmatory tests have been recently done to include even the younger patients with T2D in the 18 to 35 years age category, who revealed hypogonadotropic hypogonadism at a rate of 33% as against the normal range for the middle-aged; also, 58% was the rate considered when the age-specific normal range for FT for the young was used.25 Further studies reported that generally there is a slow and continuous decrease in testosterone production among older population, and the prevalence of hypogonadism varies between racial, ethnic groups.9,10
Decreasing testosterone levels are often linked with aging even in healthy men.12,26,27 Findings from a longitudinal study on aging,8,12,19 and 28% of men aged >40, 50, 60, and 70 years, respectively, revealed serum TT levels under the normal range.28 However, in another study, the differences in the testosterone levels between the diabetic and nondiabetic patients were found to be remarkable in the sixth decade, although it disappeared later. In fact, in diabetic patients above 60 years, hypogonadism does not occur more frequently than in their counterparts.28 In this study, a higher occurrence of hypogonadism was observed in all the age groups barring those in the 60 years and above age group (12.9%, 23.7%, 29.8%, and 19.4% in the age groups of 30-40, 41-50, 51-60, and 61-70 years, respectively).
The mean total and bioavailable testosterone levels reported for the men with diabetes in this study were also lower, concurring with the findings of the earlier studies.28 Furthermore, this study discovered that men with low testosterone levels also appear to have a high tendency to express symptoms indicating hypogonadism such as libido (73.2%) and erectile dysfunction (94.3%) once again concurring with earlier findings.8,29 In a cross-sectional study, there was evidence to show a high incidence of low libido (64%), erectile dysfunction (74%), and fatigue (63%) in hypogonadal men with T2D.28 However, these symptoms were present in similarly high levels even in eugonadal men with T2D (48%, 65%, and 57%, respectively).8,15
It is noteworthy that no correlation was found between age, duration of diabetes, HbA1c, smoking status hypertension, waist circumference, retinopathy, nephropathy, and neuropathy in patients with hypogonadism and those without the disease. Other works have reported, similar to the findings in this work, that low testosterone levels have no bearing on glycosylated hemoglobin or duration of diabetes, although there was an association with obesity.15 In a study exploring the occurrence of low testosterone levels in a large number of obese men and men with diabetes, 44% of the men with diabetes and 33% of the age-matched nondiabetic ones revealed subnormal FT concentrations, respectively.30 Furthermore, 40% of the obese men and 50% of the obese diabetic ones revealed subnormal FT levels.30 This study also showed that patients with a higher BMI value had a significant link with hypogonadism. From the regression analysis done in this study, BMI was isolated as an independent risk factor for hypogonadism. It is therefore evident that BMI is connected with a high incidence of hypogonadism and having diabetes augments that risk. Similar to BMI, significant difference was noted among diabetes treatment groups of the study population by univariate analysis. However, regression analysis done in this study showed that type of diabetes treatment was not an independent risk factor for hypogonadism. Also, literature related to the relationship between diabetes treatment and hypogonadism are limited.
Testosterone occurs in the circulation in 3 major fractions: free (2%-3%), albumin-bound (20%-40%), and SHBG (60%-80%). Both the free and albumin-bound fractions include the bioavailable testosterone as they constitute the biologically active component, readily available to the tissues; the SHBG-bound testosterone, however, is strongly bound and inactive. Sex hormone–binding globulin regulates the bound serum testosterone. Therefore, it becomes essential to determine the bioavailable testosterone or FT in men with diabetes.31 In this study, patients with hypogonadism, when compared with the normal patients, revealed significantly lower differences in the calculated bioavailable testosterone, calculated FT, LH, and SHBG. On the contrary, when compared with the normal patients, the hypogonadic men exhibited significantly greater differences in the FSH and PRL; these findings concur with findings from earlier researches.31,32
There are a few limitations in this study including its cross-sectional nature and its performance in a single center. Furthermore, the lack of a control group against which to compare the study group restricts the results from being generalizable and applicable to real-life situations. More studies conducted on a larger scale/multicenter are required to overcome these limitations. In the face of these limitations, this study gives significant data for the occurrence of hypogonadism among Saudi men with T2D.
Conclusions
This study identified the fact that the testosterone levels are often low in men with T2D, and that most of these patients exhibit symptoms of hypogonadism. Body mass index was identified as the independent risk factor of hypogonadism in the T2D men. Therefore, more effective strategies in patient education and communication must be implemented to ensure sufficient attention being paid to sexual health issues in men with diabetes; besides, a multidisciplinary team of professionals is mandatory to render optimal comprehensive care. The endocrinologist has a vital part in screening patients for low testosterone, offering patient education, and raising the awareness of hypogonadism among those providing health care.
Footnotes
PEER REVIEW: Six peer reviewers contributed to the peer review report. Reviewers’ reports totaled 748 words, excluding any confidential comments to the academic editor.
FUNDING: The author(s) received no financial support for the research, authorship, and/or publication of this article.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions
AAAH, AAR, and MALD conceived and designed the experiments; wrote the first draft of the manuscript; and contributed to the writing of the manuscript. MALD made critical revisions and approved the final version. GA and HH agreed with manuscript results and conclusions. All authors reviewed and approved the final manuscript.
REFERENCES
- 1.Calderon B, Gomez-Martin JM, Vega-Pinero B, et al. Prevalence of male secondary hypogonadism in moderate to severe obesity and its relationship with insulin resistance and excess body weight. Andrology. 2016;4:62–67. doi: 10.1111/andr.12135. [DOI] [PubMed] [Google Scholar]
- 2.Al Dawish MA, Robert AA, Braham R, et al. Diabetes mellitus in Saudi Arabia: a review of the recent literature. Curr Diabetes Rev. 2016;12:359–368. doi: 10.2174/1573399811666150724095130. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . Preventing Chronic Diseases: A Vital Investment. Geneva, Switzerland: World Health Organization; 2005. Chapter 1. Chronic diseases: causes and health impacts; pp. 34–58. [Google Scholar]
- 4.Agarwal PK, Singh P, Chowdhury S, et al. A study to evaluate the prevalence of hypogonadism in Indian males with Type-2 diabetes mellitus. Indian J Endocr Metab. 2017;21:64–70. doi: 10.4103/2230-8210.196008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al Hayek AA, Khader YS, Jafal S, Khawaja N, Robert AA, Ajlouni K. Prevalence of low testosterone levels in men with type 2 diabetes mellitus: a cross-sectional study. J Family Community Med. 2013;20:179–186. doi: 10.4103/2230-8229.122006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asare-Anane H, Ofori E, Agyemang Y, et al. Obesity and testosterone levels in Ghanaian men with type 2 diabetes. Clin Diabetes. 2014;32:61–65. doi: 10.2337/diaclin.32.2.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhindsa S, Prabhakar S, Sethi M, Bandyopadhyay A, Chaudhuri A, Dandona P. Frequent occurrence of hypogonadotropic hypogonadism in type 2 diabetes. J Clin Endocrinol Metab. 2004;89:5462–5468. doi: 10.1210/jc.2004-0804. [DOI] [PubMed] [Google Scholar]
- 8.Kapoor D, Aldred H, Clark S, Channer KS, Jones TH. Clinical and biochemical assessment of hypogonadism in men with type 2 diabetes: correlations with bioavailable testosterone and visceral adiposity. Diabetes Care. 2007;30:911–917. doi: 10.2337/dc06-1426. [DOI] [PubMed] [Google Scholar]
- 9.Al Hayek AA, Khawaja NM, Khader YS, Jaffal SK, Ajlouni KM. The prevalence of hypogonadism among diabetic and non-diabetic men in Jordan. J Diabetes Complications. 2014;28:135–140. doi: 10.1016/j.jdiacomp.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Dandona P, Rosenberg MT. A practical guide to male hypogonadism in the primary care setting. Int J Clin Pract. 2010;64:682–696. doi: 10.1111/j.1742-1241.2010.02355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng R, Cao L, Cao W, et al. Risk factors for hypogonadism in male patients with type 2 diabetes. J Diabetes Res. 2016;2016:5162167. doi: 10.1155/2016/5162167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ugwu TE, Ikem RT, Kolawole BA, Ezeani IU. Clinicopathologic assessment of hypogonadism in men with type 2 diabetes mellitus. Indian J Endocr Metab. 2016;20:667–673. doi: 10.4103/2230-8210.190554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corona G, Mannucci E, Petrone L, et al. Association of hypogonadism and type II diabetes in men attending an outpatient erectile dysfunction clinic. Int J Impot Res. 2006;18:190–197. doi: 10.1038/sj.ijir.3901391. [DOI] [PubMed] [Google Scholar]
- 14.Grossmann M. Low testosterone in men with type 2 diabetes: significance and treatment. J Clin Endocrinol Metab. 2011;96:2341–2353. doi: 10.1210/jc.2011-0118. [DOI] [PubMed] [Google Scholar]
- 15.Dandona P, Dhindsa S. Update: hypogonadotropic hypogonadism in type 2 diabetes and obesity. J Clin Endocrinol Metab. 2011;96:2643–2651. doi: 10.1210/jc.2010-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beatrice AM, Dutta D, Kumar M, et al. Testosterone levels and type 2 diabetes in men: current knowledge and clinical implications. Diabetes Metab Syndr Obes. 2014;7:481–486. doi: 10.2147/DMSO.S50777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 18.American Diabetes Association Standards of medical care in diabetes—2007. Diabetes Care. 2007;30:S4–S41. doi: 10.2337/dc07-S004. [DOI] [PubMed] [Google Scholar]
- 19.Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28:164–176. doi: 10.2337/diacare.28.1.164. [DOI] [PubMed] [Google Scholar]
- 20.Boulton AJ, Vinik AI, Arezzo JC, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28:956–962. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 21.Wilkinson CP, Ferris FL, 3rd, Klein RE, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677–1682. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 22.Rabah DM, Arafa MA. Validation of an Arabic ADAM questionnaire for androgen deficiency screening in the Arab community. Aging Male. 2009;12:95–99. doi: 10.3109/13685530903265065. [DOI] [PubMed] [Google Scholar]
- 23.Morley JE, Charlton E, Patrick P, et al. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism. 2000;49:1239–1242. doi: 10.1053/meta.2000.8625. [DOI] [PubMed] [Google Scholar]
- 24.Costanzo PR, Knoblovits P. Male gonadal axis function in patients with type 2 diabetes. Horm Mol Biol Clin Investig. 2016;26:129–134. doi: 10.1515/hmbci-2016-0014. [DOI] [PubMed] [Google Scholar]
- 25.Chandel A, Dhindsa S, Topiwala S, Chaudhuri A, Dandona P. Testosterone concentration in young patients with diabetes. Diabetes Care. 2008;31:2013–2017. doi: 10.2337/dc08-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nieschlag E, Behre HM, Bouchard P, et al. Testosterone replacement therapy: current trends and future directions. Hum Reprod Update. 2004;10:409–419. doi: 10.1093/humupd/dmh035. [DOI] [PubMed] [Google Scholar]
- 27.Corona G, Bianchini S, Sforza A, Vignozzi L, Maggi M. Hypogonadism as a possible link between metabolic diseases and erectile dysfunction in aging men. Hormones. 2015;14:569–578. doi: 10.14310/horm.2002.1635. [DOI] [PubMed] [Google Scholar]
- 28.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 29.El Saghier EO, Shebl SE, Fawzy OA, Eltayeb IM, Bekhet LM, Gharib A. Androgen deficiency and erectile dysfunction in patients with type 2 diabetes. Clin Med Insights Endocrinol Diabetes. 2015;8:55–62. doi: 10.4137/CMED.S27700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dhindsa S, Miller MG, McWhirter CL, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care. 2010;33:1186–1192. doi: 10.2337/dc09-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mirzaei MR, Amini M, Aminorroaya A. The prevalence of hypogonadism in diabetic men in Isfahan Endocrine and Metabolism Research Center, Isfahan, Iran. J Res Med Sci. 2012;17:602–606. [PMC free article] [PubMed] [Google Scholar]
- 32.Sa EQ, Sa FC, Oliveira KC, Feres F, Verreschi IT. Association between sex hormone-binding globulin (SHBG) and metabolic syndrome among men. Sao Paulo Med J. 2014;132:111–115. doi: 10.1590/1516-3180.2014.1322666. [DOI] [PMC free article] [PubMed] [Google Scholar]