Abstract
Background
The live-attenuated Japanese encephalitis (JE) vaccine (JE-CV; IMOJEV) induces a protective response in children. A shift in circulating JE virus strains suggests that a genotype shift phenomenon may occur throughout Southeast Asia. We assessed the neutralization of wild-type (WT) JE virus isolates at distal time points after vaccination.
Methods
We analyzed serum samples from a subset of 47 children who had received a JE-CV booster after an inactivated JE vaccine primary immunization. We measured antibody titers (50% plaque reduction neutralization test) using a panel of WT JE strains at baseline, then after the booster at 28 days and 6 months in all subjects present at the time points and in a subset at year 5. Three additional recent isolates were tested at year 5.
Results
Of 47 subjects, 43 (91.5%) subjects had JE neutralizing antibody titers ≥10 (reciprocal serum dilution) against the homologous strain before JE-CV boost; all were seroprotected up to year 5 after the JE-CV boost. Baseline WT seroprotection ranged between 78.7% and 87.2%; all subjects were seroprotected against the 4 WT strains at 28 days and 6 months; year 5 seroprotection ranged between 95.7% and 97.9%. Similar rates of protection against 3 additional WT isolates were observed at year 5.
Conclusions
The long-term immune responses induced after a JE-CV booster dose in toddlers were able to neutralize WT viruses from various genotypes circulating in Southeast Asia and India.
Clinical Trials Registration
Keywords: Japanese encephalitis vaccines, wild-type strains, cross-neutralization, long-term protection, genotypes
Japanese encephalitis (JE) is a mosquito-borne viral infection that is considered a disease of major public health importance because of its high epidemic potential, high case-fatality rate, and the severity of sequelae among survivors. Despite the availability of effective vaccines for many decades, JE is still believed to cause >68 000 cases of encephalitis annually, resulting in approximately 15 000 deaths [1–3]. JE also causes major neurological sequelae, affecting more than one-third of survivors. An estimated 3 billion persons live in JE-endemic areas and are at risk if not vaccinated [3]. Expatriates and children living in these endemic areas are also at risk of contracting JE, as well as travelers and military personnel deployed overseas [4; 5, chap 4]. There is no specific treatment for JE and the virus cannot be eradicated given its animal reservoir. Hence, vaccination is the most effective approach to disease control [6]. New JE vaccines have been bringing significant improvement in terms of safety, immunogenicity, and modern updated production methods.
A single serotype of JE virus has been identified, although antigenic heterogeneity has been reported between different isolates. The existence of a single serotype is also supported by detailed phylogenetic analysis [7].
Sequence heterogeneity between the different isolates was used to define 4 main genotypes based on a 240-base nucleotide sequence stretch of viral premembrane (prM). This region was selected because it shows the largest sequence variation of the whole JE virus genome. A fifth JE virus genotype (V) recently reemerged in Asia (China and South Korea) [8–11].
Recent analyses of circulating strains have shown a shift from genotype III in favor of genotype I in Vietnam [12] and Thailand [13], suggesting that a genotype shift phenomenon may be occurring throughout Southeast Asia [9, 12]. There is no indication yet that this shift toward genotype I is affecting the efficacy of the JE vaccines in use, which are all derived from genotype III viruses (strains Nakayama, P3, Beijing-1, and SA14-14-2), although some studies have shown differences in the capability of the serum samples from vaccinees who received genotype III to neutralize in vitro wild-type (WT) strains from nonvaccine strains, namely genotype I isolates [14, 15]. In addition, most studies that have assessed the neutralization of WT strains in vaccinees have tested serum samples taken at the peak of the effector immune response, that is, 28 days after vaccination, when antibody titers are at their maximum [14–18]. We sought to assess whether responses at distal time points after vaccination were still able to neutralize recent WT isolates, including those from genotype I.
MATERIALS AND METHODS
Vaccine: JE-CV
JE-CV, JE live-attenuated vaccine (IMOJEV; Sanofi Pasteur), is constructed by replacing the premembrane and envelope coding sequences from the yellow fever vaccine virus (strain 17D) genome with the corresponding sequences from the JE SA14-14-2 virus strain, as described elsewhere [19, 20] JE-CV was developed by Acambis, now part of Sanofi Pasteur. The reconstituted vaccine was stored at 2°C to 8°C and a 0.5- L vaccine dose, containing 104 PFU, was administered within 4 hours of reconstitution by subcutaneous injection in the deltoid region.
Phase II Study
The study design, participants and procedures have been described before and the trial was registered with Clinicaltrials.gov (NCT 00621764) [17]. Briefly, this phase II, open-label crossover study of JE-CV booster vaccination with hepatitis A vaccine as a safety control was conducted in Thailand in 100 children aged 2–5 years who had been vaccinated at age 12–18 months with 2 doses of a mouse-brain derived inactivated JE vaccine (Beijing strain), according to the national immunization schedule at that time. Participants were randomly allocated to receive a single dose of JE-CV (0.5 mL) subcutaneously, followed 28 days later by hepatitis A vaccine (0.5 mL given intramuscularly), or vice versa, using an interactive voice response system based on randomization lists prepared by the study sponsor for each center and age stratum using the block method. Vaccine identity was not masked. The time between the primary immunization (inactivated JE vaccine) and JE-CV booster administration was 12.8 months (range, 5.5–38.9 months; interquartile range, 9.9–14.4 months). The immune responses against JE-CV virus were determined from serum samples obtained up to year 5 after JE-CV boost.
The protocol was approved by the ethics committee or institutional review board of the 4 participating centers and the study was conducted according to Good Clinical Practice guidelines. The child's parent or guardian provided signed informed consent to the study before exposure to any study procedures.
JE Viruses
Serum samples from all children were assessed for antibody responses against JE-CV virus, and selected samples were also tested for responses to WT JE viruses. The samples evaluated for the current analysis were taken at baseline, 28 days after JE-CV vaccination, then at either 6 or 7 months after the JE-CV vaccination (depending on administration sequence), and at year 5.
A panel of WT JE strains was used for antibody testing in the phase II study: a 1991 genotype I isolate from Korea (1991 TVP-8236), a 1983 genotype II isolate from Thailand (B1034/8), a 1949 genotype III isolate from China (Beijing), and a 1981 genotype IV isolate from Indonesia (JKT 9092/TVP-6265) [17]. WT assays were initially performed on samples taken at baseline and at 28 days, and 6 months after JE-CV vaccination in all subjects present at the corresponding time points. These 4 strains, as well as 3 additional ones, were also tested in a subset of subjects on serum samples obtained at the year 5 follow-up visit: a 2003 genotype I isolate from Thailand (JEV-SM1), a 1997 genotype III isolate from Vietnam (JEV-902/97), and a 2005 genotype III isolate from India (JEV-057434) [18]; these 3 WT strains were added to the testing schedule to assess cross-neutralization to strains more recent than the original 4 WT strains evaluated. Further details on the WT isolates are provided in Table 1. A serological correlate based on JE virus neutralizing antibody titers is accepted and recommended by a panel of experts assembled by the World Health Organization (WHO); a threshold of ≥1:10 using a 50% plaque reduction neutralization test (PRNT50) is accepted as evidence of protection [22, 23].
Table 1.
JE Virus Strains Used in the PRNT50 Test
| JE Virus | Genotype | Country of Origin | Year of Origin | Virus Origin | Passage History of Virus | Accession No. |
|---|---|---|---|---|---|---|
| TVP-8236 | I | Korea | 1991 | Culex tritaeniorhynchus | 6 passages in mosquito cell lines; 6 passages in Vero cells |
Not available |
| JEV-SM1 | I | Thailand | 2003 | Pig | 2 passages in mosquito cell lines; 3 passages in Vero cells |
GenBank: DQ087971.1 |
| B1034/8 | II | Thailand | 1983 | Pig | 2 passages in mosquito cell lines; 4 passages in Vero cells |
GenBank: D00997.1 |
| Beijing | III | China | 1949 | Human | 5 passages in Vero cells | GenBank: L48961 |
| JEV-902/97 | III | Vietnam | 1997 | Human | 1 passage C6/36 cells; 1 passage in suckling mouse; 3 passages in LLC-MK2 cells |
GenBank: JQ390453.1 |
| JEV-057434 | III | India | 2005 | Human | 2 passages in mosquito cell lines; 3 passages in Vero cells |
GenBank: EF623988.1 |
| JKT 9092/TVP-6265 | IV | Indonesia | 1981 | Culex vishnui mosquitoes | 1 passage in mosquito cell lines; 4 passages in Vero cells |
GenBank: U70409 |
| JE-CV | III | Chimeric virus, YF 17D with envelope sequence from SA14-14-2 [21] | 5 passages on Vero cells | Not applicable | ||
Abbreviations: JE, Japanese encephalitis; PRNT50, 50% plaque reduction neutralization test.
Neutralization Tests
Antibody titers were tested by PRNT50. For all WT JE strains, testing was conducted at the WHO Flavivirus Diagnostics Reference Laboratory for Asia at the Center for Vaccine Development (Mahidol University, Thailand) using serial 10-fold dilutions of the serum samples mixed with a constant challenge dose of each respective JE virus and inoculated in duplicate onto wells confluent with LLC-MK2 cells. The neutralizing antibody titer was calculated and expressed as the reciprocal serum dilution (1/dil) reducing the mean plaque count by 50%, as calculated by probit analysis. Antibody testing for JE-CV strain was performed at Focus Diagnostics using serial 2-fold dilutions of serum samples mixed with a constant challenge dose of JE-CV and inoculated in duplicate onto wells confluent with Vero cells. The assay had a lower limit of quantification titer of 10 (1/dil). Children with titers ≥10 (1/dil) are considered seroprotected against JE [23].
Statistical Methods
The analysis of neutralizing antibody to JE viruses was done using the geometric mean titer (GMT) and seroprotection rate (defined as the proportion of subjects with a JE PRNT50 neutralizing antibody titer ≥10). Assuming that log10 transformation of the titers would follow a normal distribution, the mean and 95% confidence intervals (CIs) were calculated for log10 titers, using the usual calculation for normal distribution, then antilog transformation was applied to the results of those calculations to provide final GMT and CI values. CIs for the single proportion were calculated using the exact binomial method (Clopper-Pearson method). The results are presented for the full analysis set (ie, for all participants present at the first vaccination visit who received ≥1 dose of vaccine and had serum samples available for WT testing at year 5).
RESULTS
Study Population
There were 100 children aged 2–5 years enrolled in the study. The subset was defined as the 47 subjects present at year 5 visits with remaining serum samples available for conducting WT assessments at this time point (these 47 subjects had been tested with JE-CV virus and the 4 original WT isolates at baseline before JE-CV immunization and at 28 days and 6 months after JE-CV vaccination). The subset of 47 was similar to the 100 enrollees in terms of age, sex, weight, height, and body mass index at enrollment (see Table 2). An equal number of subjects in the subset received JE-CV as first or at second vaccine administration in the crossover design.
Table 2.
Demographic Characteristics at Screening
| Characteristic | All (n = 47)a |
|---|---|
| Sex, No. (%) | |
| Male | 20 (42.6) |
| Female | 27 (57.4) |
| Age at screening, y | |
| Mean (SD) | 2.5 (0.5) |
| Range | 2.0–4.8 |
| Median (IQR) | 2.3 (2.2–2.7) |
| Weight at screening, kg | |
| Mean (SD) | 12.8 (1.6) |
| Range | 9.8–17.2 |
| Median (IQR) | 12.6 (11.6–13.6) |
| Height at screening, cm | |
| Mean (SD) | 89.5 (3.9) |
| Range | 83.0–102.0 |
| Median (IQR) | 89.0 (87.0–91.0) |
| BMI at screening, kg/m² | |
| Mean (SD) | 16.0 (1.5) |
| Range | 13.3–19.8 |
| Median (IQR) | 15.8 (15.1–16.5) |
| Ethnic origin, No. (%) Asian | 47 (100.0) |
Abbreviations: BMI, body mass index; IQR, interquartile range.
a Full analysis set with samples available for testing against wild-type strains at 5 years.
Seroprotection Rates
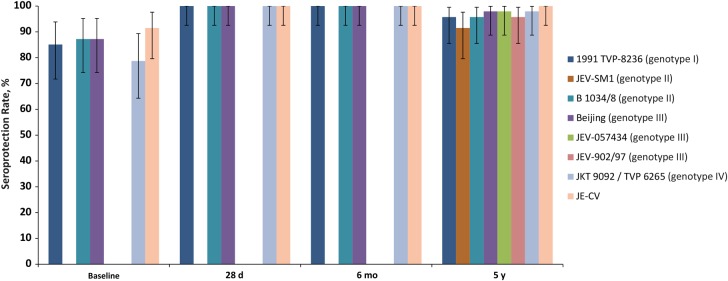
As measured by the immune response against JE-CV, 43 children (91.5%; 95% CI, 79.6%–97.6%) had protective JE neutralizing antibody titers before administration of the JE-CV booster, and all 47 (100%; 92.5%–100%) presented with protective JE neutralizing antibody titers after the JE-CV booster at 28 days, 6 months, and 5 years. Seroprotection at baseline ranged between 78.7% (95% CI, 64.3%–89.3%) for genotype IV and 87.2% (74.3%–95.2%) for genotypes II and III. All 47 children were seroprotected against the 4 WT strains tested at 28 days and 6 months; at year 5, seroprotection ranged between 95.7% (95% CI, 85.5%–99.5%) for genotypes I and II and 97.9% (88.7%–99.9) for genotypes III and IV (Figure 1).
Figure 1.
Seroprotection rates against 7 wild-type Japanese encephalitis (JE) virus strains up to 5 years after booster vaccination with JE-CV (n = 47).
Similar rates of protection were observed against the 3 “recent” WT isolates not tested before year 5: 91.5% (95% CI, 79.6%–97.6%) for genotype I and 95.7% (85.5%–99.5%) and 97.9% (88.7%–99.9%) for genotype III. More particularly, the seroprotection rates for the 3 genotype III strains tested (1949 Beijing, 1997 JEV-902/97, and 2005 JEV-057434 isolates) are similar, as are those for the 2 genotype I strains (1991 TVP-8236 and 2003 JEV-SM1 isolates) (Figure 1).
Immunogenicity
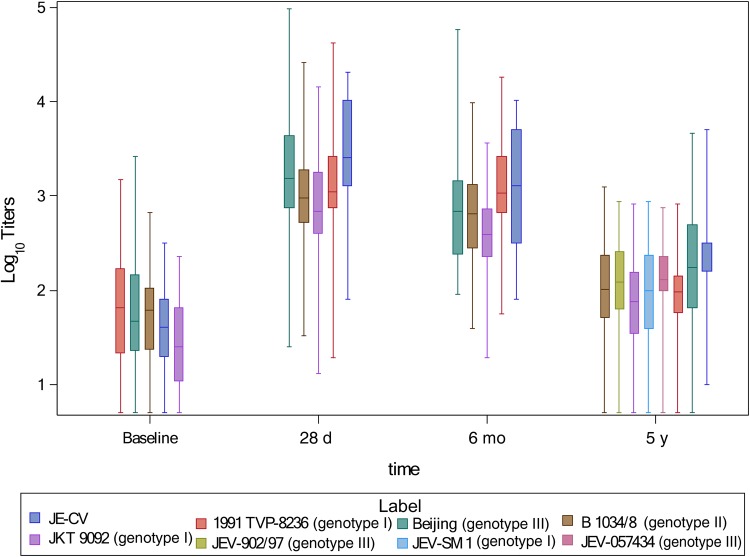
The GMTs (JE-CV virus assay) increased from 47.7 (95% CI, 34.3–66.4) before booster dose to 2838.4 (1919.5–4197.3) at 28 days after booster vaccination, remaining well above the protection threshold at 6 months (1072.4; 95% CI, 695.0–1654.7) and up to year 5 (205.6; 143.3–295.0). The pattern of change in neutralizing antibody titers for WT strains, when assessed, was the same as for JE-CV virus. The highest postdose GMT value against each strain was measured at 28 days, and the lowest at year 5. The year 5 value for each of the 4 strains tested from baseline to year 5 was approximately 10-fold lower than that at 28 days but remained well above the protective threshold, and was approximately 2–3 times the baseline value (Figure 2). At 28 days, the highest GMT value for the 4 original WT strains was 1699.4 (95% CI, 1153.0–2504.7) for genotype III Beijing strain and the lowest was 793.3 (552.3–1139.5) for genotype IV JKT 9092/TVP 6265; these 2 strains also achieved the highest and lowest GMTs at year 5, with values of 181.3 (124.0–264.9) and 69.5 (51.7–99.3), respectively. At year 5, the GMTs against the 3 more recent WT strains were of similar magnitude compared with the values observed against the other 4 WT strains: 88.3 (95% CI, 60.4–128.9) for genotype I JEV-SM1, and 120.2 (85.5–169.0) and 122.4 (90.8–165.0) for genotype III JEV-902/97 and JEV-057434, respectively.
Figure 2.
Box plots of log10 antibody titers (expressed as reciprocal serum dilution) against 7 wild-type (WT) Japanese encephalitis (JE) virus strains up to 5 years after booster vaccination with JE-CV (n = 47).
DISCUSSION
Previous publications have described the immune response to a JE-CV vaccination in pediatric populations when JE-CV was used as a single dose for primary immunization, and as a JE booster vaccination in subjects immunized earlier with a JE vaccine (either inactivated mouse brain-derived vaccine or JE-CV) [17, 24, 25] These studies also demonstrated that the seroprotective response after a JE-CV booster vaccination persisted in almost all vaccine recipients up to year 5, and neutralizing antibody titers remained well above the threshold for protection [26]. A modeling approach predicted that the seroprotection rate in children after a JE-CV booster vaccination at age 2–5 years would be high for ≥10 years [27]. In contrast, limited or no data have been reported or published on the cross-neutralization of circulating WT JE virus strains over time after JE vaccination. A previous article [15] reported the immune responses in terms of seroprotection rates and GMTs against JE-CV virus and WT strains up to 6 months after a JE-CV booster vaccination. Assessment of immune responses to WT strains from all 4 JE virus genotypes is recommended as part of the evaluation of new JE vaccines [22].
We report here the cross-neutralization of a range of different WT JE virus strains from the 4 main genotypes up to 5 years after JE-CV booster vaccination. In our sample of 47 children aged 2–5 years at the time of the booster dose, with serum samples available at year 5 for testing the 4 original WT strains previously assessed up to 6 months after JE-CV booster vaccination, as well as 3 more recent WT strains, seroprotection rates remained high 5 years after JE-CV booster vaccination, with 92%–96% of vaccinees protected against genotype I strains and 96%–98% protected against genotype III strains. These results compare with a 100% seroprotection rate against homologous JE-CV virus at all postbaseline time points.
The observation of a strong booster immune response, while many subjects still had titers above the 10 IU/mL WHO-supported correlate of protection, confirms that prior vaccination does not confer sterile immunity. The booster response was strong, multifold higher than the original response. Feroldi et al [25] previously described a memory response in children who received a primary vaccination of JE-CV and a JE-CV booster 12–24 months later; there was a measurable robust response within the first 7 days . This documented memory and boosting response can reassure us that the risks for clinically apparent infection with a WT virus after prior vaccination is unlikely, because it would take a few days for a neurotropic virus such as JE virus to enter the nervous system—the virus would be effectively neutralized. In fact, the analysis of vaccine viremia showed that no subjects had viremia in the boosted pediatric cohort while this was a common observation (though at very low titers) in naive adult or pediatric subjects vaccinated for the first time [17].
Two genotype I strains of different origins were tested, with similar seroprotection rates and GMTs at year 5 being observed for strains isolated in Thailand (2003 JEV-SM1) and in Korea (1991 TVP-8236). In addition, the year 5 cross-neutralization response to a newer genotype III strain (2005 JEV-057434 from India) was similar to the responses to strains isolated in China (1949 Beijing) or Vietnam (1997 JEV-902/97). The antibody titers at the 28-day and 6-month time points were similar for the 3 WT strains representing genotypes I–III, but those for a genotype IV strain were lower than all other WT isolates at 28 days, 6 months, and 5 years, although at all these time points the GMT values remained well above the protective threshold, and the seroprotection rate was high (97.9%–100%). However, for the recent WT isolates, no data before year 5 are available. As expected, and as already observed in adults [28], the lower GMTs were in response to the genotype IV strain, despite a high seroprotection rate.
All currently approved JE vaccines are based on a single strain, and all available epidemiological data suggest that they are able to induce a protective response against all circulating JE viruses [29]. The vaccine strain SA14-14-2 (a parent strain of the JE-CV virus) has been characterized antigenically in animal studies using panels of monoclonal and polyclonal antibodies and JE viruses; the results showed that antibodies elicited by SA14-14-2 were able to neutralize all the strains tested [30]. In addition, protection against JE viruses belonging to the 4 major genotypes was demonstrated after passive transfer of mouse serum samples raised against JE-CV [22, 28].
JE virus genotypes seem to have no relevance in terms of the elicited protection against disease but are useful for characterizing circulating JE viruses [8, 31]. There is no evidence for any correlation between a genotype and location, virulence or antigenicity. The functional significance of the prM nucleotide variation and of the genotypes remains to be established.
JE-CV (IMOJEV) is indicated in individuals 9 months of age or older. In pediatric populations, JE-CV is recommended as single dose for primary immunization, with a booster dose given preferably 12–24 months after primary vaccination. The cross-neutralization of WT JE strains up to 28 days after a single dose for primary immunization has been reported elsewhere [17]. Owing to the recommended immunization regimen combining a single dose primary immunization and a booster dose, the assessment of WT JE strains after primary vaccination was assessed only up to 6 months after JE-CV administration (data not shown), because it has been demonstrated that the administration of a JE-CV booster dose induces high antibody titers for long-lasting protection in children [26].
The results of the current analysis using individual serum samples are consistent with data on pooled serum samples from JE-vaccine-naive toddlers 28 days after primary immunization with JE-CV reported by Bonaparte et al [18], which showed effective cross-neutralization of the same recent WT strains from genotypes I and III that were tested in our study, and 3 other reference JE viruses. As of this writing, existing licensed and marketed JE vaccines are based on genotype III strains (eg, Nakayama, Beijing, SA14-14-2) but in contrast to JE-CV, the neutralization of WT strains in vaccinated children over time has not been assessed. Our study findings show that up to 5 years after vaccination JE-CV can neutralize WT virus strains of the 4 main genotypes circulating in Southeast Asia and India, where JE is endemic [14, 15].
In conclusion, the seroprotective response after a JE-CV booster vaccination persists in almost all vaccine recipients up to 5 years and neutralizing antibody titers remain well above the threshold for protection. The serum samples obtained from JE-CV vaccinees 28 days, 6 months, and 5 years after vaccination can neutralize WT viruses of the 4 main genotypes circulating in Southeast Asia and in India where JE is endemic. Our data are useful for decision making concerning JE vaccination strategy, keeping in mind that a shift from genotype III to genotype I strains has been increasingly observed in some Asian countries.
Supplementary Material
Notes
Acknowledgments. We thank and acknowledge the participation of the infants and parents in Bangkok, Thailand, as well as the contribution of investigational staffs at the Infectious Diseases Unit, Department of Pediatrics, Chulalongkorn Hospital, the Division of Infectious Diseases, Faculty of Medicine, Siriraj Hospital, Mahidol University, and the Department of Tropical Pediatrics, Faculty of Tropical Medicine, Mahidol University, all in Bangkok. We also acknowledge with thanks the contributions of Arunee Sabchareon, and of Céline Petit and Jason Smith from Sanofi Pasteur Global Clinical Immunology.
Author contributions. E. F., M. B., and A. B. conceived the experimental research and study design. K. C., U. T., and C. P. were the principal investigators at their respective sites. S. Y. carried out the WT serology. C. M. conducted the statistical analysis. E. F., C. M., and A. B. contributed to the data analysis and data interpretation. E. F. drafted the manuscript with assistance from Richard Glover, InScience Communications, Springer Healthcare. All authors participated in the development of the manuscript and approved the final version submitted.
Financial support. Funding for this study was provided by Sanofi Pasteur. The sponsor participated in the study design and managed all operational aspects of the study, including writing of this report.
Potential conflicts of interest. E. F., C. M., and A. B. are Sanofi Pasteur employees. M. B. was a Sanofi Pasteur employee at the time of the study and testing determination. The institutions of S. Y., K. C., U. T., and C. P. have received research grants from Sanofi Pasteur. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med 2004; 10(12 suppl):S98–109. [DOI] [PubMed] [Google Scholar]
- 2. Tsai TF. New initiatives for the control of Japanese encephalitis by vaccination: minutes of a WHO/CVI meeting, Bangkok, Thailand, 13-15 October 1998. Vaccine 2000; 18(suppl 2):1–25. [DOI] [PubMed] [Google Scholar]
- 3. Campbell GL, Hills SL, Fischer M et al. . Estimated global incidence of Japanese encephalitis: a systematic review. Bull World Health Organ 2011; 89:766–74, 74A-74E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tsai TF, Chang G-J, Yu YX. Japanese encephalitis vaccines. In: Plotkin SA, Orenstein WA, eds. Vaccines. 3rd ed, Philadelphia, PA: Saunders, 1999:672–710. [Google Scholar]
- 5. Centers for Disease Control and Prevention. Health information for international travel 2008. Atlanta, GA: US Department of Health and Human Services, Public Health Service, 2007. [Google Scholar]
- 6. Tauber E, Dewasthaly S. Japanese encephalitis vaccines—needs, flaws and achievements. Biol Chem 2008; 389:547–50. [DOI] [PubMed] [Google Scholar]
- 7. Tsarev SA, Sanders ML, Vaughn DW, Innis BL. Phylogenetic analysis suggests only one serotype of Japanese encephalitis virus. Vaccine 2000; 18(suppl 2): 36–43. [DOI] [PubMed] [Google Scholar]
- 8. Halstead SB, Jacobson J. Japanese encephalitis vaccines. In: Plotkin SA, Orenstein WA, eds. Vaccines. 5th ed Philadelphia: WB Saunders and Company, 2008:311–352. [Google Scholar]
- 9. Pan XL, Liu H, Wang HY et al. . Emergence of genotype I of Japanese encephalitis virus as the dominant genotype in Asia. J Virol 2011; 85:9847–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li MH, Fu SH, Chen WX et al. . Genotype v Japanese encephalitis virus is emerging. PLoS Negl Trop Dis 2011; 5:e1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Takhampunya R, Kim HC, Tippayachai B et al. . Emergence of Japanese encephalitis virus genotype V in the Republic of Korea. Virol J 2011; 8:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nga PT, del Carmen Parquet M, Cuong VD et al. . Shift in Japanese encephalitis virus (JEV) genotype circulating in northern Vietnam: implications for frequent introductions of JEV from Southeast Asia to East Asia. J Gen Virol 2004; 85(Pt 6):1625–31. [DOI] [PubMed] [Google Scholar]
- 13. Nitatpattana N, Dubot-Peres A, Gouilh MA et al. . Change in Japanese encephalitis virus distribution, Thailand. Emerg Infect Dis 2008; 14:1762–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Erra EO, Askling HH, Yoksan S et al. . Cross-protective capacity of Japanese encephalitis (JE) vaccines against circulating heterologous JE virus genotypes. Clin Infect Dis 2013; 56:267–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fan YC, Chen JM, Chiu HC et al. . Partially neutralizing potency against emerging genotype I virus among children received formalin-inactivated Japanese encephalitis virus vaccine. PLoS Negl Trop Dis 2012; 6:e1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jia L, Wang Z, Yu Y. Protection of SA14-14-2 live attenuated Japanese encephalitis vaccine against the wild-type JE viruses. Chin Med J (Engl) 2003; 116:941–3. [PubMed] [Google Scholar]
- 17. Chokephaibulkit K, Sirivichayakul C, Thisyakorn U et al. . Safety and immunogenicity of a single administration of live-attenuated Japanese encephalitis vaccine in previously primed 2- to 5-year-olds and naive 12- to 24-month-olds: multicenter randomized controlled trial. Pediatr Infect Dis J 2010; 29:1111–7. [DOI] [PubMed] [Google Scholar]
- 18. Bonaparte M, Dweik B, Feroldi E et al. . Immune response to live-attenuated Japanese encephalitis vaccine (JE-CV) neutralizes Japanese encephalitis virus isolates from South-East Asia and India. BMC Infect Dis 2014; 14:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Monath TP, Guirakhoo F, Nichols R et al. . Chimeric live, attenuated vaccine against Japanese encephalitis (ChimeriVax-JE): phase 2 clinical trials for safety and immunogenicity, effect of vaccine dose and schedule, and memory response to challenge with inactivated Japanese encephalitis antigen. J Infect Dis 2003; 188:1213–30. [DOI] [PubMed] [Google Scholar]
- 20. Monath TP, McCarthy K, Bedford P et al. . Clinical proof of principle for ChimeriVax: recombinant live, attenuated vaccines against flavivirus infections. Vaccine 2002; 20:1004–18. [DOI] [PubMed] [Google Scholar]
- 21. Chambers TJ, Nestorowicz A, Mason PW, Rice CM. Yellow fever/Japanese encephalitis chimeric viruses: construction and biological properties. J Virol 1999; 73:3095–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hombach J, Solomon T, Kurane I, Jacobson J, Wood D. Report on a WHO consultation on immunological endpoints for evaluation of new Japanese encephalitis vaccines, WHO, Geneva, 2–3 September, 2004. Vaccine 2005; 23:5205–11. [DOI] [PubMed] [Google Scholar]
- 23. World Health Organization. Japanese encephalitis vaccines. Wkly Epidemiol Rec 2015; 90:69–88.25726573 [Google Scholar]
- 24. Feroldi E, Pancharoen C, Kosalaraksa P et al. . Single-dose, live-attenuated Japanese encephalitis vaccine in children aged 12–18 months: randomized, controlled phase 3 immunogenicity and safety trial. Hum Vaccin Immunother 2012; 8:929–37. [DOI] [PubMed] [Google Scholar]
- 25. Feroldi E, Capeding MR, Boaz M, Gailhardou S, Meric C, Bouckenooghe A. Memory immune response and safety of a booster dose of Japanese encephalitis chimeric virus vaccine (JE-CV) in JE-CV-primed children. Hum Vaccin Immunother 2013; 9:889–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chokephaibulkit K, Sabchareon A, Thisyakorn U et al. . Long-term neutralizing antibody response to a booster dose of a novel live attenuated Japanese encephalitis vaccine (JE-CV – IMOJEV®) in a pediatric population. Presented at: Seventh Asian Congress of Pediatric Infectious Disease; 12–15 October 2014;Beijing, China. [Google Scholar]
- 27. Feroldi E, Bailleux F, Monfredo C, Bouckenooghe A. Modeling the long-term persistence of neutralizing antibodies after booster vaccination with a novel live attenuated Japanese encephalitis vaccine (JE-CV)—modeling Ab persistence in children. Presented at: Eighth World Congress of the World Society for Pediatric Infectious Diseases; 19–22 November 2013;Cape Town, South Africa. [Google Scholar]
- 28. Hombach J, Barrett AD, Cardosa MJ et al. . Review on flavivirus vaccine development: proceedings of a meeting jointly organised by the World Health Organization and the Thai Ministry of Public Health, 26–27 April 2004, Bangkok, Thailand. Vaccine 2005; 23:2689–95. [DOI] [PubMed] [Google Scholar]
- 29. Beasley DW, Li L, Suderman MT et al. . Protection against Japanese encephalitis virus strains representing four genotypes by passive transfer of sera raised against ChimeriVax-JE experimental vaccine. Vaccine 2004; 22:3722–6. [DOI] [PubMed] [Google Scholar]
- 30. Wills MR, Sil BK, Cao JX, Yu YX, Barrett AD. Antigenic characterization of the live attenuated Japanese encephalitis vaccine virus SA14-14-2: a comparison with isolates of the virus covering a wide geographic area. Vaccine 1992; 10:861–72. [DOI] [PubMed] [Google Scholar]
- 31. Paranjpe S, Banerjee K. Phylogenetic analysis of the envelope gene of Japanese encephalitis virus. Virus Res 1996; 42:107–17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.