Abstract
Regulation of the immune response during active tuberculosis (TB) has been partly deciphered. In pulmonary TB there is transient systemic immunosuppression due to overexpression of transforming growth factor beta and interleukin‐10. This is superimposed on a primary T‐cell defect. Locally there is intense inflammation (lung, pleural fluid) with overexpression of immunosuppressive factors (bronchoalveolar lavage) and extensive apoptosis. These observations suggest that immune therapies should be aimed at neutralizing the negative regulatory factors rather than accentuating an already intense immune response. Also a partially effective vaccine carries the potential risk of exacerbating disease. Clin Trans Sci 2010; Volume 3: 23–28
Keywords: tuberculosis, immunoregulation, immuno‐pathogenesis
Introduction
There are approximately 9.2 million cases and 1.8 million deaths due to tuberculosis (TB) annually. Ninety‐five% of the cases and 99% of the deaths occur in resource‐poor settings, a disproportionate number in Sub‐Saharan Africa. The global health problem of TB aggravated by the HIV pandemic and the spread of drug‐resistant disease has heightened interest in the development of new diagnostics, preventions, and interventions. The current vaccine, Mycobacterium bovis BCG has shown partial protection in adults and has had little impact on the public health problem of TB. Developing an effective vaccine has been hampered by limited understanding of the immunopathogenesis of TB and protective immunity in humans. In fact, TB poses a number of paradoxes to the immunologist that are relevant both to strategies to develop a preventive vaccine as well as immunotherapy.
Systemic Response in TB
The natural history of TB begins with infection with M. tuberculosis (Mtb). The normal host contains this infection and enters a period of clinical latency that may last years or decades. During this period, the tuberculin skin test (TST) and interferon‐gamma release assays are positive but the person has no symptoms. Progression from infection to disease is more likely when there is overt immunosuppression or medical comorbidities (diabetes mellitus, chronic renal failure, silicosis, old age) but often activation of the TB (termed “re‐activation”) occurs without a clear precipitating event or condition. The first paradox is that TB develops despite the presence of delayed type hypersensitivity and a protective immune response that has held the latent infection in abeyance for years. The second paradox is that at the time of the diagnosis of TB there is depression of the TST in 20–25% of patients with pulmonary TB. Negative TSTs are even more common in miliary TB and extra‐pulmonary disease involving serosal surfaces (meningitis, pleural disease, peritonitis). In pulmonary TB the likelihood of a negative TST and the extent of suppression of in vitro responses varies with the extent of disease. It is greater in moderately and far‐advanced TB graded radiographically than in minimal TB where immunosuppression is rarely seen. With treatment of TB, the TST usually reverts to positive.
The immunologic basis of the transient immunosuppression that is a concomitant of active TB has been deciphered. The depression of the TST is associated with selective depression of in vitro responses to antigens derived from Mtb. This includes PPD or Mtb‐culture filtrate‐induced blastogenesis, 1 production of IL‐2 2 and interferon‐gamma. 3 , 4 Partial restitution of T‐cell responses occurs following removal of adherent cells (mostly blood monocytes) by adherence to plastic surfaces and nylon wool columns. 1 Suppression also can be reconstituted in cell mixing experiments by adding back plastic adherent cells. 1 , 5 , 6 The finding that blood monocytes participated in a regulatory circuit that was antigen specific led to experiments designed to define the basis for antigen specificity given the fact that monocytes do not express antigen receptors. Several observations are pertinent. First, various constituents of Mtb culture filtrate provide a direct activating signal to human monocytes. 7 , 8 Second, stimulation through Mtb culture filtrate antigen 85B proceeds through binding of cell surface fibronectin. 8 And third, monocytes express Fcgamma receptors that are increased in TB 9 and modify cellular interactions in response to antigen bound in immune complexes. 10
The molecular mediators of immunosuppression in active TB are transforming growth factor (TGF)—beta 3 , 4 , 11 , 12 , 13 and IL‐10. 4 These cytokines are overexpressed in TB patients compared to TST positive healthy controls when peripheral blood mononuclear cells (PBMC) are stimulated with Mtb antigens and suppression is abrogated in part by neutralizing antibodies to either of these factors. 4 Sequential studies in a cohort of HIV‐uninfected patients with pulmonary TB showed that the overexpression of TGF‐beta and IL‐10 was transient; values had largely normalized at the end of 3 months of treatment ( Figure 1 ). Suppression of T‐cell response by these down‐modulatory cytokines was superimposed on a primary T‐cell defect. There was a decrease in the frequency of Mtb specific interferon‐gamma producing cells and a protracted depression of the expression of interferon‐gamma. Even 18 months after diagnosis and 12 months after completion of treatment for TB, PBMC responses to Mtb antigens failed to achieve the levels seen in healthy TST positive control subjects.
Figure 1.
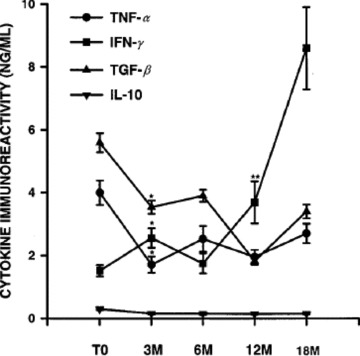
Cytokine profiles at diagnosis of tuberculosis and during and after completion of antituberculous chemotherapy. Peripheral blood mononuclear cells (PBMC) from human immunodeficiency virus (HIV) uninfected patients with tuberculosis (n= 24) were obtained at time of diagnosis (TO) and at 3–18 months (M) thereafter. Purified protein derivative‐induced cytokine production (by ELISA) was assessed in PBMC (I × 107 mL) cultures. Results represent mean ± SE of cytokine immunoreactivities. *p≤ 0.04. when compared with baseline immunoreactivity **p≤ 0.001. when compared with interferon (IFN)‐γ levels at baseline evaluation. IL‐10 = interleukin‐10; TGF‐/J = transforming growth factor‐p; TNF‐a = tumor necrosis factor‐a. Reprinted from Reference 4 with permission.
TGF‐beta and IL10 also were overproduced in TB patients with HIV infection ( Table 1 ). In this case, the primary T‐cell defect was profound. Neutralizing antibody failed to reconstitute a response.
Table 1.
PPD‐stimulated production of cytokines and markers of immune activation in HIV‐infected and ‐uninfected patients with TB and in PPD‐positive control subjects and modulation of IFN‐γ production by coculture with neutralizing antibodies to TGF‐β and IL‐10 or (r)‐IL‐12. Reprinted from Reference 4 with permission.
| TB patients | HIV/TB patients | Control subjects | |
|---|---|---|---|
| Levels of cytokines and markers of immune activation | |||
| IFN‐γ (ng/mL) | 1.53 ± 0.28a | 1.03 ± 0.33a | 8.00 ± 1.05 |
| TNF‐α (ng/mL) | 3.91 ± 0.37b | 4.00 ± 0.39c | 2.78 ± 0.35 |
| TGF‐β (ng/mL) | 5.56 ± 0.30a | 4.20 ± 0.28d | 3.20 ± 0.34 |
| IL‐10 (ng/mL) | 0.30 ± 0.03e | 0.53 ± 0.09a | 0.19 ± 0.02 |
| TNF‐RII (ng/mL) | 4.20 ± 0.50 | 7.30 ± 0.60a | 2.40 ± 0.30 |
| Neopterin (FU/mL) | 10.4 ± 2.40 | 16.3 ± 2.20a | 4.50 ± 1.60 |
| Modulation of PPD‐stimulated IFN‐y production by coculture with neutralizing antibodies to TGF‐p and 1 L‐10 or (r)‐IF‐12 | |||
| PPD only | 1.53 ± 0.28 | 1.03 ± 0.33 | 8.00 ± 1.05 |
| PPD +α TGP‐β | 5.02 ± 1.09f | 1.66 ± 0.20g | 14.3 ± 2.35 |
| PPD +α IL‐10 | 6.45 ± l.3lh | 2.43 ± 0.74i | 15.04 ± 2.88 |
| PPD + (r)‐IL‐12 | 11.39 ± 1.70j | 10.85 ± 2.13i | 28.83 ± 0.59 |
Neopterin and TNF‐RII levels were assessed in scrum, and cytokinc immunoreactivities were measured in PPD‐stimulated culture supernatants. Results are presented as mean ± SE. PPD = purified protein derivative; HIV = human immunodeficiency virus; TB = tuberculosis; IFN = interferon; TGF = transforming growth factor; IL = interleukin; TNF = tumor necrosis factor; TNF‐RII = TNF receptor type 2; PBMC = peripheral blood mononuclear cells; a = P < .001, when compared with levels of cytokines/markers of immune activation in supernatants/serum from control subjects; b = P < .02, when compared with TNF‐a immunoreactivity in supernatants from control subjects; c = P < .01, when compared with TNF‐α levels in supernatants from healthy control subjects; d = P < .03, when compared with cytokine immunoreactivity in culture supernatants from control subjects; e = P < .008, when compared with levels of markers of activation in serum from healthy control subjects; f = P < .004, when compared with IFN‐y production in supernatants of PBMC cultured without antibody to TGF‐J3; g = P < .003, when compared with IFN‐y production in supernatants of PBMC cultured without antibody to TGF‐J3; h = P < .05, when compared with levels of IFN‐y in cultures without IL‐10 antibody; i = P < .001, when compared with IFN‐y levels in cultures containing PPD alone; j = P < .001, when compared with IFN‐y immunoreactivity in cultures containing PPD alone.
Programmed cell death may contribute to the primary T‐cell abnormality in TB. The immune activation in TB is associated with increased spontaneous and Mtb antigen induced apoptosis of T cells. 14 , 15 This appears to proceed through several pro‐apoptotic ligands. 15 Apoptosis involved decreased levels of bcl‐2 and increased TNFalpha, TGFbeta, and FasL. Apoptosis is observed locally 21 as well as systemically. It may be that sites of inflammation in TB are “killing fields” where there is selective elimination of antigen responsive T cells.
Recently, well‐defined regulatory T‐cell populations have been reported that can suppress or more appropriately regulate an immune response. These regulatory T cells control the magnitude of response of both T 1 and T 2 cells. There is, in fact, a renewed interest in understanding how the immune response is regulated, particularly when challenged with a replicating pathogen. Tregs can be defined phenotypically (CD4+CD25high+ T cells) and functionally (expression of IL‐10, TGFbeta). 16 Tregs have an important role in the maintenance of tolerance to self and have been shown to abrogate immune pathologies and auto‐immune diseases in vivo. 17 Recently, two groups reported the presence of enhanced NatTregs in the peripheral blood of TB patients. 18 , 19 Wi t h increasing interest in regulatory T cells (Tregs), which produce TGF beta and IL‐10, their contribution vis‐à‐vis monocytes to immunosuppression in TB needs to be further evaluated.
The observations to this point clarify the basis for depressed immune responses seen systemically in TB patients. It is of great interest, however, to explore the local immune concomitants of active TB.
Local Response—TB Pleurisy
The immune response in pleural fluid is of particular interest to immunologists because in the absence of treatment, “self‐cure” is possible. Early studies indicated that the pleural immune response was upregulated and unregulated compared to blood, in part because immunosuppressive adherent monocytes were not found in the pleural compartment. 20 Pleural fluid contains high concentrations of cytokines, activated T cells, and pro‐aptotic molecules and there is an abundance of spontaneous apoptosis. 21 In HIV‐uninfected TB patients, Mtb organisms are scarce. The pleural effusion represents an in situ delayed‐type hypersensitivity reaction to Mtb antigens released into the pleural space. By comparison, in the HIV‐infected, there still is an upregulated and unregulated response; however, Mtb organisms are present.
Local Response—The Lung
Bronchoalveolarlavage (BAL) showed that TB was associated with an alveolitis restricted to involved segments of lung. 22 T cell numbers were increased and T cells expressed activation markers. The BAL also contained increased numbers of immature peroxidase positive macrophages ( Figure 2 ). These are most likely blood monocytes that are drawn by chemotaxins to the inflammatory focus. In contrast to the blood, the frequency of interferon‐gamma producing cells as determined by ELISPOT assay was increased compared to controls. 22 , 23 Multiple cell types produced interferon‐gamma in TB patients although the predominant cell was CD4+CD45RO+. 24 Mtb‐induced IL‐12p70 was increased in bronchoalveolar cells from TB patients. 24 Constitutive expression of IL12R beta1 and beta2 was found in both TB patients and controls. It appears, therefore, that interferon‐gamma induction, per se and consequent to IL12R engagement are unimpaired in TB patients. The implication is either that the immune response in the lung dissociates from that in the blood and represents a vigorous immune response, or that locally active immunosuppressive mechanisms and mediators may undermine immunity. As will be seen, recent evidence supports the latter scenario.
Figure 2.
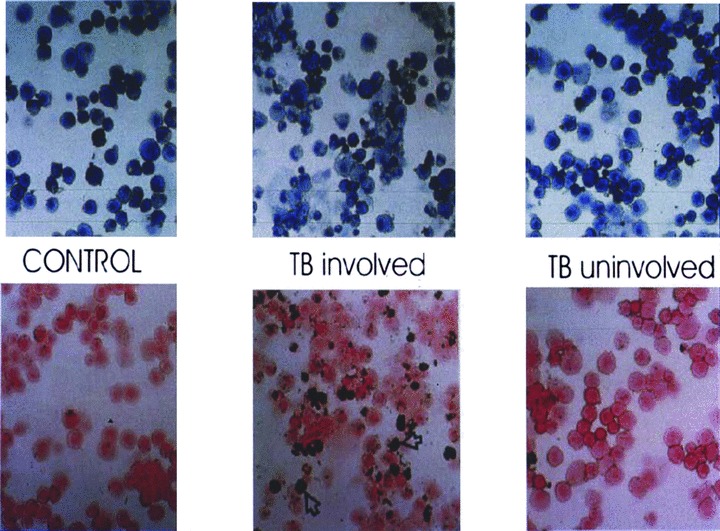
Giemsa (upper panel) and peroxidase (lower panel) stains of bronchoalveolar lavage (BAL) from TB patient and control. BAL from the involved segment shows lymphocytic alveolitis. Approximately 40% of the bronchoalveolar cells are peroxidase positive moncyte‐derived immature macrophages. Reprinted from Reference 22 with permission.
Studies of induced and expectorated sputum provide additional insights into immunoregulation locally. There are high levels of interferon‐gamma and tumor necrosis factor— alpha in sputum. 25 These decline rapidly with treatment. In fact, there is a good correlation between interferon‐gamma levels in the sputum and quantitative bacterial counts ( Figure 3 ). Recently, multiplexed RT‐PCR has been performed on induced sputum from TB patients. 26 Both genes for positive and negative regulators of the immune response and effector molecules were upregulated in TB ( Figure 4 ). Thirty days after the initiation of treatment, many of the suppressive mediators declined to control levels, whereas T 1‐type and innate immune mediators rose above pretreatment levels. The net result appeared to be an overlayer of immunosuppression as indicated by decreased alveolar macrophage production of the mediator NOS2 that can be considered, in this context, the composite indicator of immune reactivity.
Figure 3.
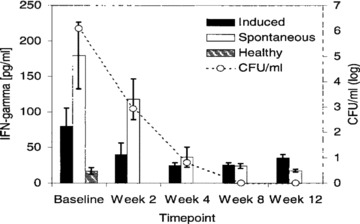
Correlation between IFN‐γ levels and mycobacterial load in sputum from TB patients during the first 12 weeks of anti‐Mtb treatment. Duplicate aliquots of spontaneous and induced sputum from TB patients (n= f 5) were used for assessment of IFN‐7 levels by ELISA and quantitative mycobacterial culture. Results are expressed as picograms of IFN‐7/mL (left v axis) and log CFU (CFU, right y axis). Sputum IFN‐7 levels from healthy PPD‐positive controls (n= 10) are included for comparison. Cytokine data are presented as mean ± SEM. ▪= induced sputum from TB patients; D= spontaneous sputum from TB patients; on= induced sputum from healthy controls. Reprinted from Reference 25 with permission.
Figure 4.
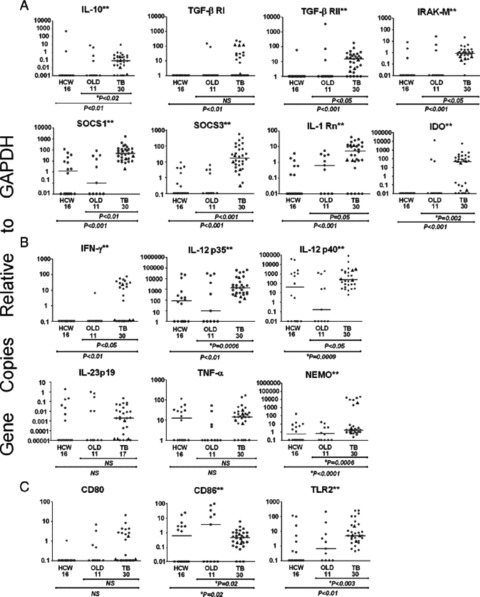
Lung immune response of TB cases, patients with occupational lung disease (OLD) and clinically well health care workers (HCW). Illustrated are the mRNA expression levels in log10 scale of the lung cells from patients with TB. patients with infectious OLD. and clinically well HCW quantified by real‐time PCR relative to GADPH. The scale of the horizontal axis for gene expression relative to GAPDH for each mediator was set to illustrate the lowest to the highest recorded levels. Linear bars represent the median of the group. The findings were segregated by mediators that impair Th I‐type immunity (A), promote Thl‐type immunity (B). or affect both Thl‐type or Th2‐type cells (C). Each symbol represents the value of one subject and the triangles designate HIV‐1‐coinfected patients. The two asterisks (**) alongside the name of the gene indicate a significant difference between TB cases and OLD and HCW was observed (Kruskal–Wallis or Fishers’ exact test). The p values for the comparison between groups are shown below the horizontal axis. A *p indicates that Fishers’ exact test was used for six of the 17 genes. Reproduced from Reference 26 with permission.
Another interesting question is the impact of the immune and inflammatory response in the lung of patients with pulmonary TB on the response to antituberculous chemotherapy. It is of interest that clinical trials in HIV‐infected and also when corticosteroids are used as adjunctive therapy are associated with accelerated sterilization of the sputum. 29 Therefore, once TB ensues the local protective immune response that held the latent focus in quiescence no longer promotes bacterial clearance. In fact, it may impede responses to TB treatment leading to the requirement for longer duration of therapy and greater risk of treatment failure.
Trials of Immunotherapies
There is renewed interest in immune therapies because of the advent of extensively drug resistant (XDR)—TB. A treatment shortening role in drug susceptible TB also is possible. However, trials in drug susceptible TB are complicated because of the narrow window to observe an effect—that is in rates of sputum sterilization. In a trial of heat‐killed Mycobacterium vaccae as an adjunct to therapy, there appeared to be acceleration of both sputum and radiographic clearance. 27 However, this was not confirmed in other trials. 30 Nor was there modulation of multiple immunologic parameters that were studied. 27 A recent trial of Mycobacterium vaccae for treatment of latent TB infection appears to show efficacy. 31 This trial used 5 intradermal doses of M. vaccae and was intended to boost the response to prior vaccination with BCG. Unfortunately, the product is unstandardized and may not be available as an intervention.
A controlled trial of subcutaneous IL‐2 as an adjunct to treatment of pulmonary TB failed to show efficacy. 28 A trial was conducted of aerosolized interferon‐gamma in MDR‐TB although the results have not been published presumably because of the lack of efficacy. An additional trial is in progress.
Conclusions
Dr. Izuo Tsuyuyguchi once characterized the immune response in TB “like an orchestra without a conductor.” Indeed, pulmonary TB is characterized by activation of the immune system and concomitant activation of immunoregulatory circuits. Immune modulation may be important in limiting the damage to the lung or preventing adult respiratory distress syndrome (SIRS). Mediators such as the pro‐f brotic TGF‐beta may lead to immunopathology. The net result, however, is progressive infection unchecked by the immune system.
Consequences and Implications
It seems unlikely that cytokine or immunomodulatory therapy aimed at increasing interferon‐gamma in the lung will be successful. The pathophysiology of TB is characterized by abundant T 1 cytokines but their activity is masked by immunosuppression. What are the implications for immunotherapy of TB? Interventions aimed at modifying the immunoregulatory circuits deserve consideration. In this regard, it is of note that naturally occurring inhibitors of TGFbeta exist (latency associate peptide) and that calcium channel blockers (Losarten) also may downregulate TGFbeta production.
What are the implications for vaccine development? Mtb is a facultative intracellular organism with a complex life cycle in the infected host. The natural history of TB includes a long period of stand‐of between the host immune response and bacterial replication known as clinical latency. The immune response may be a double‐edged sword conferring protection against activation of a latent focus but contributing to the immunopathogenesis and immunopathology of active disease. The cautionary note is that a partially effective vaccine could produce more local inflammation leading to pathology, morbidity, and suboptimal response to chemotherapy.
There are unanswered questions. Is immunosuppression that occurs in TB the cause of consequence of the disease? The antigen specificity of some of the regulatory circuits bespeaks the cause. What is the basis for the prolonged T‐cell dysfunction during and after treatment of TB? The cells repopulating the host immune and inflammatory response may be protective. This acquired immunodeficiency may contribute both to relapse of TB and to reinfection disease. The latter is known to occur more frequently after TB is treated. Do genetic factors determine immunoregulatory activity, thereby influencing risk of TB?
Lastly, a great deal of effort appropriately is directed toward identifying correlates of protective immunity. Leads may be possible in the immune reconstitution that occurs when TB is treated with chemotherapy and when TB/HIV is treated with antiretroviral therapy.
Acknowledgments
I would like to acknowledge my steady collaborators, Drs. Zahra Toossi, Christina Hirsch, Stephan Schwander, Roy Mugerwa, Eduardo Sada and Elizabeth Rich (deceased).
References
- 1. Ellner JJ. Suppressor adherent cells in human tuberculosis. J Immunol. 1978; 121(6): 2573–2579. [PubMed] [Google Scholar]
- 2. Toossi Z, Kleinhenz ME, Ellner JJ. Defective interleukin 2 production and responsiveness in human pulmonary tuberculosis. J Exp Med. 1986; 163(5): 1162–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hirsch CS, Hussain R, Toossi Z, Dawood G, Shahid F, Ellner JJ. Cross‐modulation by transforming growth factor beta in human tuberculosis: suppression of antigen‐driven blastogenesis and interferon gamma production. Proc Natl Acad Sci U S A. 1996; 93(8): 3193–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hirsch CS, Toossi Z, Othieno C, Johnson JL, Schwander SK, Robertson S, Wallis RS, Edmonds K, Okwera A, Mugerwa R, Peters P, Ellner JJ. Depressed T‐cell interferon‐gamma responses in pulmonary tuberculosis: analysis of underlying mechanisms and modulation with therapy. J Infect Dis. 1999; 180(6): 2069–2073. [DOI] [PubMed] [Google Scholar]
- 5. Kleinhenz ME, Ellner JJ. Immunoregulatory adherent cells in human tuberculosis: radiation‐sensitive antigen‐specific suppression by monocytes. J Infect Dis. 1985; 152(1): 171–176. [DOI] [PubMed] [Google Scholar]
- 6. Kleinhenz ME, Ellner JJ. Antigen responsiveness during tuberculosis: regulatory interactions of T cell subpopulations and adherent cells. J Lab Clin Med. 1987; 110(1): 31–40. [PubMed] [Google Scholar]
- 7. Wallis RS, Amir‐Tahmasseb M, Ellner JJ. Induction of interleukin 1 and tumor necrosis factor by mycobacterial proteins: the monocyte western blot. Proc Natl Acad Sci U S A. 1990; 87(9): 3348–3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aung H, Toossi Z, Wisnieski JJ, Wallis RS, Culp LA, Phillips NB, Phillips M, Averill LE, Daniel TM, Ellner JJ. Induction of monocyte expression of tumor necrosis factor alpha by the 30‐kD alpha antigen of Mycobacterium tuberculosis and synergism with fibronectin. J Clin Invest. 1996; 98(5): 1261–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vanham G, Edmonds K, Qing L, Hom D, Toossi Z, Jones B, Daley CL, Huebner B, Kestens L, Gigase P, Ellner JJ. Generalized immune activation in pulmonary tuberculosis: co‐activation with HIV infection. Clin Exp Immunol. 1996; 103(1): 30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hussain R, Shiratsuchi H, Ellner JJ, Wallis RS. PPD‐specific IgG1 antibody subclass upregulate tumour necrosis factor expression in PPD‐stimulated monocytes: possible link with disease pathogenesis in tuberculosis. Clin Exp Immunol. 2000; 119(3): 449–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Toossi Z, Ellner JJ. The role of TGF beta in the pathogenesis of human tuberculosis. Clin Immunol Immunopathol. 1998; 87(2): 107–114. [DOI] [PubMed] [Google Scholar]
- 12. Toossi Z, Gogate P, Shiratsuchi H, Young T, Ellner JJ. Enhanced production of TGF‐beta by blood monocytes from patients with active tuberculosis and presence of TGF‐beta in tuberculous granulomatous lung lesions. J Immunol. 1995; 154(1): 465–473. [PubMed] [Google Scholar]
- 13. Hirsch CS, Ellner JJ, Blinkhorn R, Toossi Z. In vitro restoration of T cell responses in tuberculosis and augmentation of monocyte effector function against Mycobacterium tuberculosis by natural inhibitors of transforming growth factor beta. Proc Natl Acad Sci U S A. 1997; 94(8): 3926–3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hirsch CS, Toossi Z, Vanham G, Johnson JL, Peters P, Okwera A, Mugerwa R, Mugyenyi P, Ellner JJ. Apoptosis and T cell hyporesponsiveness in pulmonary tuberculosis. J Infect Dis. 1999; 179(4): 945–953. [DOI] [PubMed] [Google Scholar]
- 15. Hirsch CS, Johnson JL, Okwera A, Kanost RA, Wu M, Peters P, Muhumuza M, Mayanja‐Kizza H, Mugerwa RD, Mugyenyi P, Ellner JJ, Toossi Z. Mechanisms of apoptosis of T‐cells in human tuberculosis. J Clin Immunol. 2005; 25(4): 353–364. [DOI] [PubMed] [Google Scholar]
- 16. O’Garra A, Vieira PL, Vieira P, Goldfeld AE. IL‐10‐producing and naturally occurring CD4+ Tregs: limiting collateral damage. J Clin Invest. 2004; 114(10): 1372–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Paust S, Cantor H. Regulatory T cells and autoimmune disease. Immunol Rev. 2005; 204: 195–207. [DOI] [PubMed] [Google Scholar]
- 18. Ribeiro‐Rodrigues R, Resende Co T, Rojas R, Toossi Z, Dietze R, Boom WH, Maciel E, Hirsch CS. A role for CD4+CD25+ T cells in regulation of the immune response during human tuberculosis. Clin Exp Immunol. 2006; 144(1): 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guyot‐Revol V, Innes JA, Hackforth S, Hinks T, Lalvani A. Regulatory T cells are expanded in blood and disease sites in patients with tuberculosis. Am J Respir Crit Care Med. 2006; 173(7): 803–810. [DOI] [PubMed] [Google Scholar]
- 20. Ellner JJ. Pleural fluid and peripheral blood lymphocyte function in tuberculosis. Ann Intern Med. 1978; 89(6): 932–933. [DOI] [PubMed] [Google Scholar]
- 21. Hirsch CS, Toossi Z, Johnson JL, Luzze H, Ntambi L, Peters P, McHugh M, Okwera A, Joloba M, Mugyenyi P, Mugerwa RD, Terebuh P, Ellner JJ. Augmentation of apoptosis and interferon‐gamma production at sites of active Mycobacterium tuberculosis infection in human tuberculosis. J Infect Dis. 2001; 183(5): 779–788. [DOI] [PubMed] [Google Scholar]
- 22. Schwander SK, Sada E, Torres M, Escobedo D, Sierra JG, Alt S, Rich EA. T lymphocytic and immature macrophage alveolitis in active pulmonary tuberculosis. J Infect Dis. 1996; 173(5): 1267–1272. [DOI] [PubMed] [Google Scholar]
- 23. Schwander SK, Torres M, Sada E, Carranza C, Ramos E, Tary‐Lehmann M, Wallis RS, Sierra J, Rich EA. Enhanced responses to Mycobacterium tuberculosis antigens by human alveolar lymphocytes during active pulmonary tuberculosis. J Infect Dis. 1998; 178(5): 1434– 1445. [DOI] [PubMed] [Google Scholar]
- 24. Herrera MT, Torres M, Nevels D, Perez‐Redondo CN, Ellner JJ, Sada E, Schwander SK. Compartmentalized bronchoalveolar IFN‐gamma and IL‐12 response in human pulmonary tuberculosis. Tuberculosis (Edinb). 2009; 89(1): 38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ribeiro‐Rodrigues R, Resende Co T, Johnson JL, Ribeiro F, Palaci M, Sa RT, Maciel EL, Pereira Lima FE, Dettoni V, Toossi Z, Boom WH, Dietze R, Ellner JJ, Hirsch CS. Sputum cytokine levels in patients with pulmonary tuberculosis as early markers of mycobacterial clearance. Clin Diagn Lab Immunol. 2002; 9(4): 818–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Almeida AS, Lago PM, Boechat N, Huard RC, Lazzarini LC, Santos AR, Nociari M, Zhu H, Perez‐Sweeney BM, Bang H, Ni Q, Huang J, Gibson AL, Flores VC, Pecanha LR, Kritski AL, Lapa e Silva JR, Ho JL. Tuberculosis is associated with a down‐modulatory lung immune response that impairs Th1‐type immunity. J Immunol. 2009; 183(1): 718–731. [DOI] [PubMed] [Google Scholar]
- 27. Johnson JL, Kamya RM, Okwera A, Loughlin AM, Nyole S, Hom DL, Wallis RS, Hirsch CS, Wolski K, Foulds J, Mugerwa RD, Ellner JJ. Randomized controlled trial of Mycobacterium vaccae immunotherapy in non‐human immunodeficiency virus‐infected ugandan adults with newly diagnosed pulmonary tuberculosis. The Uganda‐Case Western Reserve University Research Collaboration. J Infect Dis. 2000; 181(4): 1304–1312. [DOI] [PubMed] [Google Scholar]
- 28. Johnson JL, Ssekasanvu E, Okwera A, Mayanja H, Hirsch CS, Nakibali JG, Jankus DD, Eisenach KD, Boom WH, Ellner JJ, Mugerwa RD. Randomized trial of adjunctive interleukin‐2 in adults with pulmonary tuberculosis. Am J Respir Crit Care Med. 2003; 168(2): 185–191. [DOI] [PubMed] [Google Scholar]
- 29. Mayanja‐Kizza H, Jones‐Lopez E, Okwera A, Wallis RS, Ellner JJ, Mugerwa RD, Whalen CC, Uganda‐Case Western Research Collaboration . Immunoadjuvant prednisone therapy for HIV‐associated tuberculosis: a phase 2 clinical trial in Uganda. J Infect Dis. 2005; 191(6): 856–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mwinga A, Nuun A, Ngwira B, Chintu C, Warndoff D, Fine P, Darbyshire J, Zumia A; LUSKAR collaboration . Mycobacterium vaccae (SRL172) immunotherapy as an adjunct to standard antituberculosis treatment in HIV‐infected adults with pulmonary tuberculosis: a randomized placebo‐controlled trial. Lancet. 2002; 360(9339): 1032–1033. [DOI] [PubMed] [Google Scholar]
- 31. Von Reyn CF, Mtei L, Arbeit RD, Waddell R, Cole B, Mackenzie T, Matee M, Bakari M, Tvaroha S, Adams LV, Horsburgh CR, Pallangyo K; the DarDar Study Group . Prevention of tuberculosis in Bacille Calmette‐Guérin‐primed, HIV infected adults boosted with an in activated whole‐cell mycobacterial vaccine. AIDS. 2010. In press, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]


