Abstract
In this study, we evaluated whether the protective potential of resveratrol (RSV; 3,5,4′-trihydroxy-trans-stilbene) against γ-radiation caused damages in peripheral blood lymphocyte of mice. Resveratrol as a polyphenolic compound scavenges free radicals. Various doses of RSV were administered intraperitoneally 2 hours to adult male mice before a single dose of whole-body γ-irradiation (2 Gy). To assess the protective ability of RSV, the alkaline comet assay in blood lymphocyte of mice was performed and the total comet score was evaluated. The results of the alkaline comet assay showed that RSV significantly inhibited radiation-induced DNA damage. We observed that RSV protects blood lymphocyte against radiation-induced damage in mice.
Keywords: resveratrol, radiation, comet assay, lymphocyte, radioprotective
Introduction
Radiotherapy, using ionizing radiation for cancer therapy, is a usual treatment modality in modern medicine.1 Although ionizing radiation is an effective tool for tumor treatment, the side effects that damage normal tissues around the tumor limit therapeutic gain.2 The main purpose of radiotherapy is to gain an optimum balance between therapy efficacy and ill effects of radiation.3 To protect normal tissues and delivery of higher doses to tumor tissues, radioprotective agents have received significant interest.4
Researches in the discovery of radioprotective agents have discovered many chemical and biological compounds.5 A large number of natural and synthetic compounds, for example, antioxidants, cytoprotective agents, immunomodulators, vitamins, and DNA-binding molecules, have been studied extensively for this purpose in both in vitro and in vivo models.6 Because of toxicity and ill effects at effective radioprotective doses, many of the synthetic agents have not been used in clinical applications. Hence, studies were shifted toward the natural compounds.7
Resveratrol (RSV) is a potent antioxidant, chemoprotective, anti-inflammatory, and anticancer agent that is found in grapes, nuts, fruits, and wine.8 As a polyphenolic compound, RSV scavenges hydroxyl, superoxide, and metal-induced radicals and promotes the activities of antioxidant enzymes, for example, superoxide dismutase and catalase.9 Reduction of these free radicals by RSV provides a kind of protection against ionizing radiation injuries.10
The single-cell electrophoresis, the comet assay, is a new technique for evaluating DNA damage.11 The basic principle of the comet assay is the migration of DNA out of the nucleus under electrophoretic condition in an agarose matrix. The image of the cell under microscope resembles a comet with a head (the nuclear region) and a tail containing DNA fragments or strand migration.12 Comet assay is widely used over other methods for the detection of DNA damage because it is rapid and simple and requires small number of cells in each sample and collection of data at the individual cell level.13 The aim of this study is to evaluate, in vivo, the radioprotective activity of RSV in peripheral blood lymphocyte of mice.
Materials and Methods
Chemicals
Resveratrol, low-melting-point agarose, normal-melting-point agarose (NMA), propidium iodide, NaCl, Na-EDTA, Tris, NaOH, Triton X-100, ethanol, EDTA, and dimethyl sulfoxide (DMSO) were obtained from Merk (Darmstadt, Germany) and Sigma-aldrich (Steinheim, Germany).
Animal
Six-week-old male NMRI mice weighing 25 ± 3 g were housed in a good condition. Animals were given standard pellet and water and kept under controlled lighting condition (light:dark, 12:12 hours) and temperature (22°C ± 1°C).
Treatment
Experimental animals were randomly divided into 8 groups (Table 1). Resveratrol was dissolved in 96% ethanol to a concentration of 50 mg/mL. A 0.1 mL of RSV/ethanol was diluted in 1.9 mL distilled water to gain concentration of 2.5 mg/mL. Various doses of RSV were injected intraperitoneally to adult male mice 2 hours before a single dose (50 and 100 mg/kg) of whole-body γ-irradiation (2 Gy).
Table 1.
Experimental Animals Were Randomly Divided Into 8 Groups.
| Group | Treatment |
|---|---|
| 1 | Sham control (no treatment) |
| 2 | Vehicle control (4.8% ethanol) |
| 3 | Radiation (2 Gy) |
| 4 | Vehicle control (4.8% ethanol) + radiation (2 Gy) |
| 5 | Resveratrol (50 mg/kg) |
| 6 | Resveratrol (100 mg/kg) |
| 7 | Resveratrol (50 mg/kg) + radiation (2 Gy) |
| 8 | Resveratrol (100 mg/kg) + radiation (2 Gy) |
Irradiation
Animals were placed in Plexiglas cages and irradiated at 2 Gy using a 6-MV γ-radiation linear accelerator unit (Oncor; Siemens). The source to sample distance was 100 cm.
Blood Sample
After irradiation, blood samples were collected from orbital sinus of anesthetized animals using heparinized tubes.
Comet Assay
We used the technique of alkaline comet assay of Singh et al14 with some modifications15 to analyze the DNA damage in blood, liver, and spleen cells. Microscopic slides were coated with NMA, immediately coverslipped and kept at 4°C for 10 minutes to get the agarose solidified. After removal of the cover slip, 10 µL of treated cells was mixed with 200 µL of low-melting agarose in phosphate-buffered saline and layered on slides, spread out with a cover slip, and then kept at 4°C for 10 minutes. After solidification, the coverslips were removed, and the slides were immersed in cold lysing solution ([2.5 M NaCl, 100 mM Na2 EDTA, 10 mM Tris, pH 10] with 1% Triton X-100 and 10% DMSO added just before use); after lysing, the slides were placed in a horizontal gel electrophoresis tank filled with alkaline buffer (300 mM NaOH and 1 mM EDTA, pH 13) for 40 minutes to allow the unwinding of the DNA and the expression of alkali-labile damage. Electrophoresis was performed for 30 minutes at 300 mA and 25 V. After electrophoresis, the slides were washed 3 times with neutralizing buffer (0.4 M Tris, pH 7.5) and stained for 10 minutes with 20 µm/mL ethidium bromide.
Microscopic Analysis and Scoring
A total of 100 randomly captured cells per slide were visually analyzed under a fluorescent microscope (Nikon Eclipse 800) at 400 magnification according to comet appearance. The fluorescent microscope was equipped with an excitation filter of 546 nm and a barrier filter of 590 nm. Each cell was classified into 5 classes, that is, from class 0 (no DNA migration) to class 4 (maximum DNA migration). Total comet score (TCS) was calculated according to the formula: TCS = 0 (n0) +1 (n1) + 2 (n2) + 3 (n3) + 4 (n4), where “n” indicates the number of cells in each class. Damaged cells (DCs) were calculated as the sum of cells with class 2, 3, and 4 damages. Percentage of DCs (%DC) were calculated as: ([n2 + n3 + n4]/[n0 + n1 + n2 + n3 + n4]) × 100.
For estimating the level of radiation protection, we used the following equation: protection magnitude (%) = ([TCSIR − TCSRSV + IR]/TCSIR) × 100.
Statistical Analysis
Data are represented as mean ± standard error (SE). Since comet assay data did not show a normal distribution, the nonparametric test (Mann-Whitney U test) was used for analyzing comet data.
Results
Mean frequency of every comet class for study groups is shown in Table 2. Comet class 3 and class 4 were observed more frequently in the radiation groups than in the control groups, while comet class 0 was observed more frequently in the control groups as compared to the radiation groups (Figure 1).
Table 2.
Mean Frequency of Every Comet Class Per 100 Cells (±SE) Result in Comet Assay With RSV for Study Groups.
| Groups | Treatment | Class 0 | Class 1 | Class 2 | Class 3 | Class 4 |
|---|---|---|---|---|---|---|
| 1 | Sham control (no treatment) | 67.6 ± 3.74 | 15.6 ± 2.43 | 7.2 ± 1.02 | 5.5 ± 0.62 | 4.4 ± 0.6 |
| 2 | Vehicle control (4.8% ethanol) | 67.7 ± 3.45 | 15.3 ± 2.41 | 7.5 ± 0.99 | 5.4 ± 0.64 | 5.1 ± 0.53 |
| 3 | Radiation (2 Gy) | 5.4 ± 0.64a | 10.2 ± 1.37 | 11.4 ± 1.20 | 23.8 ± 1.98a | 49.2 ± 4.30a |
| 4 | Vehicle control (4.8% ethanol) + radiation (2 Gy) | 6.2 ± 0.7b | 10.7 ± 1.21 | 11.8 ± 1.20 | 24.3 ± 2.11b | 47.0 ± 3.94b |
| 5 | Resveratrol (50 mg/kg) | 67.1 ± 3.79 | 15.4 ± 2.43 | 8.0 ± 1.09 | 5.7 ± 0.65 | 3.8 ± 0.51 |
| 6 | Resveratrol (100 mg/kg) | 65.3 ± 3.89 | 17.7 ± 2.84 | 7.1 ± 0.82 | 5.4 ± 0.65 | 4.5 ± 0.54 |
| 7 | Resveratrol (50 mg/kg) + radiation (2 Gy) | 18.1 ± 1.66c | 22.7 ± 2.40c | 15.5 ± 1.43 | 16.2 ± 1.62 | 27.5 ± 2.78c |
| 8 | Resveratrol (100 mg/kg) + radiation (2 Gy) | 21.4 ± 1.75c | 24.7 ± 1.94c | 17.6 ± 1.84 | 14.0 ± 1.11 | 22.3 ± 2.49c |
Abbreviations: RSV, resveratrol; SE, standard error.
a P < .001 comparison of group 3 with group 1.
b P < .001 comparison of group 4 with group 2.
c P < .001 comparison of groups 7 and 8 with group 3.
Figure 1.
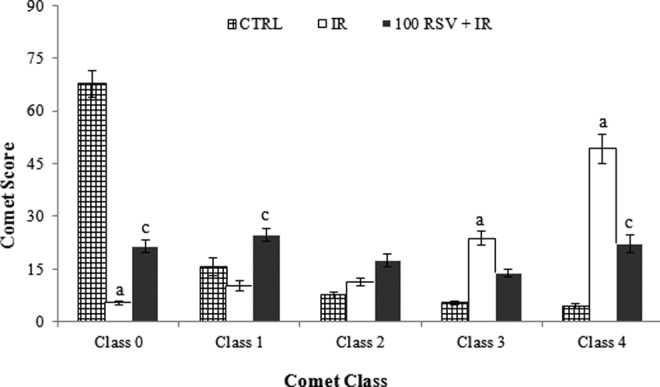
Mean frequency of every comet class per 100 cells (±SE) result in comet assay with RSV for study groups. a P < .001 comparison of group 3 with group 1. b P < .001 comparison of group 4 with group 2. c P < .001 comparison of groups 7 and 8 with group 3. RSV indicates resveratrol; SE, standard error.
Changes in TCS are shown in Figure 2; among the study groups, the lowest TCS value was observed in the control groups, and TCS was significantly increased in exposed groups in comparison with the control group (P < .001).
Figure 2.
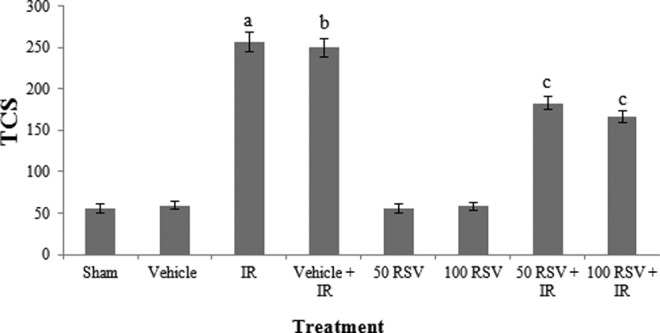
The TCS result in comet assay with RSV in study groups. a P < .001 compared with sham group. b P < .001 compared with vehicle group. c P < .001 compared with vehicle + RT, Radiotherapy. RSV indicates resveratrol; TCS, Total Comet Score.
Injection of RSV 2 hours prior to irradiation significantly decreased the TCS (P < .001) compared to the radiation groups. The results indicated that the TCS increased with γ-irradiation and treatment with RSV decreased the TCS. In RSV groups, there was no significant difference in TCS between 50 and 100 mg/kg.
As shown in Figure 3, the %DC was significantly increased in the exposed groups (2 Gy) in comparison with the control groups and treatment with RSV before irradiation decreased the %DC in comparison with only irradiated groups (P < .001). The magnitude of radioprotection ranged from 28.72 to 35.27 for RSV (Table 3).
Figure 3.
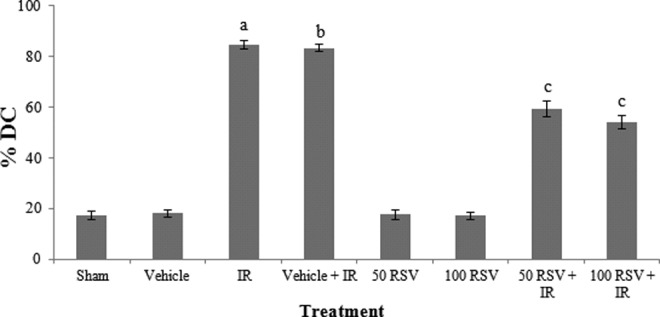
Percentage of damaged cells result in comet assay with RSV in study groups. a P < .001 compared with sham group. b P < .001 compared with vehicle group. c P < .001 compared with vehicle + RT. RSV indicates resveratrol.
Table 3.
Percentage of Damaged Cells and the Total Comet Score (TCS) Result in Comet Assay With RSV in Study Groups.
| Groups | Treatment | TCS (Mean ± SE) | % DC (Mean ± SE) | PM (%) |
|---|---|---|---|---|
| 1 | Sham control (no treatment) | 56.1 ± 5.74 | 17.1 ± 1.75 | |
| 2 | Vehicle control (4.8% ethanol) | 59.1 ± 4.95 | 18.0 ± 1.57 | |
| 3 | Radiation (2 Gy) | 256.6 ± 12.24a | 84.4 ± 1.81a | |
| 4 | Vehicle control (4.8% ethanol) + radiation (2 Gy) | 249.6 ± 11.14b | 83.1 ± 1.46b | |
| 5 | Resveratrol (50 mg/kg) | 55.3 ± 5.82 | 17.5 ± 1.94 | |
| 6 | Resveratrol (100 mg/kg) | 58.3 ± 4.94 | 17.0 ± 1.4 | |
| 7 | Resveratrol (50 mg/kg) + radiation (2 Gy) | 182.9 ± 8.08c | 59.2 ± 3.10c | 28.72 |
| 8 | Resveratrol (100 mg/kg) + radiation (2 Gy) | 166.1 ± 7.55c | 53.9 ± 2.53c | 35.27 |
Abbreviations: DC, damaged cell; PM, protection magnitude; RSV, resveratrol; SE, standard error; TCS, Total Comet Score.
a P< .001 comparison of group 3 with group 1.
b P < .001 comparison of group 4 with group 2.
c P < .001 comparison of groups 7 and 8 with group 3.
The results indicated a significant increase in TCS in the radiation groups when compared to RSV (50 and 100 mg/kg), and pretreatment groups with RSV modulated the effects of radiation. In RSV-alone groups, we observed no significant increase in the comet formation when compared to the control group.
Discussion
The use of ionizing radiation is a part of cancer therapy in modern medicine. Radiation toxicity to biological systems leads to immediate and widespread oxidative damages. The most important target of radiation is the cellular DNA. Ionizing radiation induces DNA damages via direct deposition of energy to DNA and indirect interactions with water within the cells that result in the formation of free radicals.16 Although living cells to protect themselves from reactive oxygen species (ROS) are well equipped with endogenous cellular and enzymatic compounds such as superoxide dismutase, glutathione (GSH) peroxidase, and catalase and nonenzymatic compounds such as vitamin C, uric acid, and GSH,17 when the generation of ROS increases, the endogenous cellular compounds are not able to remove them, thus ROS scavenger ability is critical for the development of radioprotective agents.18
Various compounds with radioprotective potential have been the subject of recent studies.19,20 Unfortunately, there are no ideal strategies that can be used for occupational or clinical improvements to protect those exposed to ionizing radiation. Radiation-induced cellular damage is attributed to the toxicity effects of free radicals. Consequently, molecules with radical scavenging effects are specially promising as radioprotectors in current literatures.21 In the past few years, many compounds have been investigated for clinically radioprotective agents, but toxicity and efficiency for human usage are still the main concern.
There are natural compounds that are able to inhibit carcinogenesis at different stages, among them RSV, a phytoalexin from the group of stilbene compounds,22 is synthesized by many plants in response to injury, stress, and ultraviolet irradiation.23 Resveratrol is present in human diet, that is, in fruits such as grapes, strawberry and also in red wine.
It has been previously shown that RSV and a novel RSV analogue, HS-1793 have radioprotective effects against genotoxicity induced by ionizing radiation.24 The studies on radioprotective agents showed that pretreatment with hesperidin,25,26 chlorogenic, and quinic acid27 decreases DNA strand breaks in irradiated human lymphocyte. Other studies showed that RSV has antioxidant activity and specially inhibits cellular growth and induces DNA damage in cancer cells but not in normal human cell lines.28 Our results revealed that treatment with RSV before irradiation decreases TCS and %DC, showing that RSV prevented DNA damage.
Many methods have been used to detect and quantify damages to DNA caused by genotoxicity agents such as ionizing radiation. Comet assay or microgel electrophoresis assay is a biochemical technique that uses neutral pH conditions for the lysis and electrophoresis of cells, which permits observing double breaks in DNA strands. The comet assay was used to assess in vivo the radioprotective effects of RSV. This test allows analyzing the cell damage induced by γ-irradiation in the presence or absence of RSV, and injury induced by high concentrations of RSV in the absence of γ-radiation.
Exposure to ionizing radiation may lead to changes in the deoxyribose ring and base structures and may cause intrastrand and interstrand DNA–DNA cross-links, DNA single-strand breaks, DNA double-strand breaks, and DNA–protein cross-links.29 The exposure to γ-irradiation of lymphocytes increased DNA damage, as assessed by the alkaline comet assay. The increase in tail size observed in the present study is due to DNA strand breaks. Treatment with RSV prior to irradiation decreased the TCS and the %DC, indicating that RSV prevented DNA damage. In our study, RSV (50 and 100 mg/kg) increased the frequency of DNA damage, suggesting that phenolic compound has genotoxicity effects. It has already been noted that ionizing radiation radiolysis the water, the most abundant intracellular material, and generates ROS in cells. We found that 50 and 100 mg/kg of RSV offers the best protection against 2 Gy γ-irradiation, causing a decrease in the TCS, as shown in the alkaline comet assay. Free radicals generated by ionizing radiation disturb macromolecules such as DNA leading to cell death in normal tissues. Resveratrol protects normal tissues against these effects.30
In agreement to our results, Dobrzynska et al31 reported that irradiation and coadministration of RSV induced reduction in micronuclei in bone marrow and reticulocytes. Decrease in the DNA damage in mice reticulocytes might be attributed to proposed antioxidant mechanisms of RSV. Resveratrol as a hydrophobic molecule can pass easily via cell membranes and directly scavenge the free radicals generated by γ-irradiation. The radioprotective properties of RSV at DNA level could be expressed, if so it scavenges the free radical produced in those water molecules that are extremely near the DNA double helix, in spite of the fact that it is a hydrophobic.
Also Carsten et al28 showed the radioprotective efficiency of this polyphenol in mouse lymphocytes can be expressed due to RSV directly scavenging oxygen species as a presence of hydroxyl molecules in its structure.32,33 Resveratrol increased hemeoxygenase I expression and extracellular GSH.34 Yen et al35 reported that RSV is able to restore the levels of intracellular antioxidants such as GSH peroxidase, superoxide dismutase, and catalase activity, resulting in indirect prevention of damaging to DNA molecule by free radicals. Further to the antioxidant effect, RSV inhibits interleukin 1, beta expression induced by irradiation.36
Other antioxidant such as quercetin also has radioprotective effects maintaining antioxidant levels or even increasing them as a case of lycopene.37,38
Our results show that pretreatment with RSV is an efficient radioprotective procedure, protecting nontumorigenic human lymphocytes from the damaging effects of γ-irradiation. Future studies should assess the radioprotective effects of RSV in whole organisms using rodent genotoxicity test systems.
Conclusion
From the results obtained, we could suggest that RSV is an effective radioprotective against lymphocyte damage caused by γ-radiation in the mice. Resveratrol acts against γ-radiation in the mice. Resveratrol as a natural agent is useful for personnels and patients are exposed to radiation. Further studies should assess the radioprotective effects of RSV in all organisms. Resveratrol is nontoxic and available in pills and could be efficiently used in the radioprotection with preventive and/or therapeutic effects. The antioxidant effects and ability to cause apoptosis and cell cycle block and lack of toxicity make RSV an agreeable candidate for the protection of normal cells against radiation and prevention of cancer.
It is important to protect the germ cells during radiological accidents and treatment of cancer patients too. In case of creation of germ cell mutations following DNA damage, such mutations can be passed to the offspring of irradiated person and onward through future generations. For this reason, usage of RSV is also promising. Results of another literature showed that RSV decreases harmful effects of radiation, so it looks to be promising for radioprotection.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was conducted with financial support of Isfahan University of Medical Sciences (IRAN) with grant number 394667.
References
- 1. Hogle W. Cytoprotective agents used in the treatment of patients with canver. Semin Oncol Nurs. 2007;23(3):213–224. [DOI] [PubMed] [Google Scholar]
- 2. Bump E, Malaker K. Radioprotectors: Chemical, Biological and Clinical Perspectives. London, United Kingdom: CRC Press; 1998. [Google Scholar]
- 3. Zhou X, Phadtare S, Schmidt J, Agrawal K, Kishore V. Synthesis and radioprotective effects of new phosphorothioate esters of WR-2721, WR-3689 and WR-151327. Bioorg Med Chem Lett. 1997;7(6):693–698. [Google Scholar]
- 4. Nair C, Parida D, Nomura T. Radioprotectors in radiotherapy. J Radiat Res. 2001;42(1):21–37. [DOI] [PubMed] [Google Scholar]
- 5. Arora R, Guota D, Chawla R, et al. Radioprotection by plant products: present status and future prospects. Phytoter Res. 2005;19(1):1–22. [DOI] [PubMed] [Google Scholar]
- 6. Weiss J, Landauer M. Radioprotection by antioxidants. Ann N Y Acad Sci. 2000;899:44–60. [PubMed] [Google Scholar]
- 7. Hosseinimehr S, Mahmoudzadeh A, Ahmadi A, Ashrafi S, Shafaghati N, Hedayati N. The radioprotective effect of Zataria multiflora against genotoxicity induced by gamma irradiation in human blood lymphocytes. Cancer Biother Radiopharm. 2011;26(3):325–329. [DOI] [PubMed] [Google Scholar]
- 8. Burns J, Yokota T, Ashihara H, Lean M, Crozier A. Plant foods and herbal sources or resveratrol. J Agric Food Chem. 2002;50(11):3337–3340. [DOI] [PubMed] [Google Scholar]
- 9. Sinorelli P, Ghidoni T. Resveratrol as an anticancer nutrient: molecular basis, open questions and promises. J Nutr Biochem. 2005;16(8):449–466. [DOI] [PubMed] [Google Scholar]
- 10. Losa G. Resveratrol modulates apoptosis and oxidation in human blood mononuclear cells. Eur J Clin Invest. 2003;33(9):818–823. [DOI] [PubMed] [Google Scholar]
- 11. Anderson D, Yu T, McGregor D. Comet assay responses as indicators of carcinogen exposure. Mutagenesis. 1998;13(6):539–555. [DOI] [PubMed] [Google Scholar]
- 12. Rojas E, Lopez MC, Valvarde M. Single cell gel electrophoresis assay: methodology and applications. J Chromatogr B Biomed Sci Appl. 1999;722(1-2):225–254. [DOI] [PubMed] [Google Scholar]
- 13. Olive P. DNA damage and repair in individual cells: applications of the comet assay in radiobiology. Int J Radiat Biol. 1999;75(4):395–405. [DOI] [PubMed] [Google Scholar]
- 14. Singh N, McCoy M, Tice R, Schneider L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175(1):184–191. [DOI] [PubMed] [Google Scholar]
- 15. Maurya D, Salvi V, Krishnan Nair C. Radioprotection of normal tissues in tumor-bearing mice by troxerutin. J Radiat Res 2004;45(2):221–228. [DOI] [PubMed] [Google Scholar]
- 16. De Bont R, Larabeke N. Endogenous DNA damage in human: a review of quantitative data. Mutagenesis. 2004;19(3):169–185. [DOI] [PubMed] [Google Scholar]
- 17. Chen L, Liu Y, Dong L, Chen X. Edaravone protects human peripheral blood lymphocytes from γ-irradiation-induced apoptosis and DNA damage. Cell Stress Chaper. 2015;20(2):289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jeong MH, Park YS, Jeong DH, et al. In vitro evaluation of Cordyceps militaris as a potential radioprotective agent. Int J Mol Med. 2014;34(5):1349–1357. [DOI] [PubMed] [Google Scholar]
- 19. Jagetia G, Rajanikant G, Rao S. Evaluation of the effect of ascorbic acid treatment on wound healing in mice exposed to different doses of fractionated gamma radiation. Radiat Res. 2003;159(3):371–380. [DOI] [PubMed] [Google Scholar]
- 20. Mauryaa D, Adhikari S, Nair C, Devasagayam T. DNA protective properties of vanillin against gamma-radiation under different conditions: possible mechanisms. Mutat Res. 2007;634(1-2):69–80. [DOI] [PubMed] [Google Scholar]
- 21. Tiwari P, Kumar A, Balakrishnan S, Kushwahaa H, Mishra K. Radiation-induced micronucleus formation and DNA damage in human lymphocytes and their prevention by antioxidant thiols. Mutat Res. 2009;676(1-2):62–68. [DOI] [PubMed] [Google Scholar]
- 22. Jeandet P, Douillet-Breuil A, Bessis R, Debord S, Sbaghi M, Adrian M. Phytoalexin from the Vitaceae: biosynthesis, phytoalexin gene expression in transgenic plants, antifungal activity, and metabolism. J Agric Food Chem. 2002;50(10):2731–2741. [DOI] [PubMed] [Google Scholar]
- 23. Van Etten H, Mansfield J, Bailey J, Farmer E. Two classes of plant antibiotics: phytoalexins versus “phytoanticipins”. Plant Cell. 1994;6(9):1191–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jeong M, Yang K, Jeong D, et al. Protective activity of a novel resveratrol analoge, HS-1793, against DNA damage in Cs-irradiated CHO-K1 cells. J Radiat Res. 2014;55(3):464–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kalpana K, Devipriya M, Menon V. Investigation of the radioprotective efficacy of hesperidin against gamma-radiation induced cellular damage in cultured human pripheral blood lymphocytes. Mutat Res. 2009;676(1-2):54–61. [DOI] [PubMed] [Google Scholar]
- 26. Hosseinimehr S, Ahmadi A, Beiki D. Protective effect of hesperidin against genotoxicity induced by (99 m) Tc-MIBI in human cultured lymphocyte cells. Nucl Med Biol. 2009;36(7):863–867. [DOI] [PubMed] [Google Scholar]
- 27. Cinkilic N, Cetintas S, Zorlu T, et al. Radioprotection by two phenolic compounds: chlorogenic and quinic acid, on X-ray induced DNA damage in human blood lymphocyte in vitro. Food Chem Toxicol. 2013;53:359–363. [DOI] [PubMed] [Google Scholar]
- 28. Carsten R, Bachand S, Bailey S, Ullrich R. Resvertrol reduces radiation-induced chromosome aberration frequencies in mouse bone marrow cells. Radiat Res. 2008;169(6):633–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Burnet N, Wurm R, Peacock J. Low dose-rate fibroblast radiosensitivity and the prediction of patient response to radiotherapy. Int J Radiat Biol. 1996;70(3):289–300. [DOI] [PubMed] [Google Scholar]
- 30. Zhang H, Zhai Z, Wang Y, et al. Resveratrol ameliorates irradiation-induced long-term hematopoietic stem cell injury in mice. Free Radic Biol Med. 2013;54:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dobrzynska M, Gajowik A, Radzikowska J. The effect of in vivo resveratrol supplementation in irradiated mice on the induction of micronuclei in peripheral blood and bone marrow reticulocytes. Mutagenesis. 2016;31(4):393–399. [DOI] [PubMed] [Google Scholar]
- 32. Frombaum M, Clanche S, Bonnefont-Rousselot D, Borderie D. Antioxidant effects of resveratrol and other stilbene derivatives on oxidative stress and NO bioavailability: potential benefits to cardiovascular diseases. Biochimie. 2012;94(2):269–276. [DOI] [PubMed] [Google Scholar]
- 33. Iuga C, Alvarez-Idaboy J, Russo N. Antioxidant activity of trans-resveratrol toward hydroxyl and hydroperoxyl radicals: a quantum chemical and computational kinetics study. J Org Chem. 2012;77(8):3868–3877. [DOI] [PubMed] [Google Scholar]
- 34. Quincozes-Santos A, Bobermin LD, Latini A, et al. Resveratrol protects C6 astrocyte cell line against hydrogen peroxideinduced oxidative stress through heme oxygenase 1. PLoS One. 2013;8(5):e64372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yen GC, Duh PD, Lin CW. Effects of resveratrol and 4-hexylresorcinol on hydrogen peroxide-induced oxidative DNA damage in human lymphocytes. Free Radical Res. 2003;37(5):509–514. [DOI] [PubMed] [Google Scholar]
- 36. Fu Y, Wang Y, Du L, et al. Resveratrol inhibits ionising irradiation-induced inflammation in MSCs by activating Sirt1 and limiting NLRP-3 inflammasome activation. Int J Mol Sci. 2013;14(7):14105–14118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Srinivasan M, Devipriya N, Kalpana K, Menon V. Lycopene: an antioxidant and radioprotector against γ-radiation-induced cellular damages in cultured human lymphocytes. Toxicology. 2009;262(1):43–49. [DOI] [PubMed] [Google Scholar]
- 38. Devipriya N, Sudheer A, Srinivasan M, Menon V. Quercetin ameliorates gamma radiation-induced DNA damage and biochemical changes in human peripheral blood lymphocytes. Mutat Res. 2008;654(1):1–7. [DOI] [PubMed] [Google Scholar]


