Abstract
Benzo[a]pyrene, a ubiquitous environmental pollutant, has been suggested to be capable of initiating and/or accelerating atherosclerosis. Accumulation of vascular smooth muscle cells (VSMCs) in vessel intima is a hallmark of atherosclerosis. Nitric oxide (NO) can suppress VSMCs proliferation and induce VSMCs apoptosis. NO plays a compensatory role in the vascular lesions to reduce proliferation and/or accelerate apoptosis of VSMCs. The aim of this study was to investigate whether benzo[a]pyrene can affect VSMCs growth and apoptosis induced by NO. Benzo[a]pyrene (1–30 μmol/L) did not affect the cell number and cell cycle distribution in VSMCs under serum deprivation condition. Sodium nitroprusside (SNP), a NO donor, decreased cell viability and induced apoptosis in VSMCs. Benzo[a]pyrene significantly suppressed SNP-induced cell viability reduction and apoptosis. VSMCs cultured in conditioned medium from cells treated with benzo[a]pyrene could also prevent SNP-induced apoptosis. Benzo[a]pyrene was capable of inducing the activation of nuclear factor (NF)-κB and phosphorylation of p38 mitogen-activated protein kinase (MAPK) in VSMCs. Both NF-κB inhibitor and p38 MAPK inhibitor significantly reversed the anti-apoptotic effect of benzo[a]pyrene on SNP-treated VSMCs. Incubation of VSMCs with benzo[a]pyrene significantly and dose-dependently increased interleukin (IL)-6 production. A neutralizing antibody to IL-6 effectively reversed the anti-apoptotic effect of benzo[a]pyrene on SNP-treated VSMCs. Taken together, these results demonstrate for the first time that benzo[a]pyrene activates IL-6 induction and protects VSMCs from NO-induced apoptosis. These findings propose a new mechanism for the atherogenic effect of benzo[a]pyrene.
Introduction
Vascular smooth muscle cells (VSMCs) are responsible for the structural characteristics of the vessel wall, which is important in development, growth, remodeling and repair [1,2]. Many vascular diseases including hypertension, atherosclerosis, post-angioplasty restenosis, and transplant arteriosclerosis are characterized by abnormal VSMCs proliferation and migration, causing VSMCs accumulation in the intima during vascular remodeling [2,3]. Vascular structure and remodeling have been suggested to be determined in large part by a balance between cell growth and cell death by apoptosis [4].
Epidemiological and experimental studies have shown that polycyclic aromatic hydrocarbons (PAHs) are associated with the progression of cardiovascular diseases, including atherosclerosis [5,6]. Benzo[a]pyrene, a major environmental pollutant and a PAH present in tobacco smoke, has been demonstrated to possess the potential of atherogenesis in experimental models [7–10]. The most of studies defining the pathology of benzo[a]pyrene in vascular disease have majorly focused on the abnormal regulation of cell growth/proliferation. However, in addition to changes in the regulation of cell growth, the regulation of cell death by apoptosis may be another important determinant of vessel structure and lesion formation.
Nitric oxide (NO), generated from L-arginine by nitric oxide synthase (NOS), plays diverse physiological functions, such as vascular tonus regulation, neurotransmission, and cytotoxicity [11,12]. NO can exert proapoptotic or anti-apoptotic effects for various cell types [13,14]. Low concentrations of NO (pmol/L-nmol/L) seem to favor cell proliferation/anti-apoptosis and higher concentrations of NO (μmol/L-mmol/L) favor cell cycle arrest or apoptosis in cardiovascular-related cells [13]. NO donors have been shown to affect the cell cycle and suppress proliferation in the aortic VSMCs [15]. In the blood vessels, it has been reported that NO induces apoptosis in vascular endothelial cells [16] and smooth muscle cells [17]. It has also been shown that apoptosis occurs during the process of vascular remodeling and lesion formation [13,18,19]. In vivo gene transfer of endothelial NOS resulted in a marked reduction of neointimal formation after balloon injury in rats by constitutively generation of endogenous NO [20]. Furthermore, it has been shown that expression of inducible NOS (iNOS) mRNA and protein is localized not only to macrophages and foam cells but also to VSMCs in atherosclerotic lesions and neointima after balloon angioplasty [21,22]. The iNOS-dependent NO production has been found to act as a survival signal in benzo[a]pyrene-treated rat hepatic epithelial F258 cells via an AhR-regulatory pathway [23]. These observations suggested that NOS expression in the vascular lesions might represent a compensatory mechanism to reduce proliferation and/or accelerate apoptosis of VSMCs through excess generation of NO.
Interleukin-6 (IL-6) is a pleiotropic cytokine. Several studies indicated that IL-6 has critical pathophysiological roles in cardiovascular diseases, such as atherosclerosis [24, 25] and congestive heart failure [26]. Nevertheless, it has been suggested that locally secreted IL-6 is involved in the VSMCs proliferation in response to platelet-derived growth factor (PDGF) [27]. IL-6 could also participate in the 15(S)-hydroxyeicosatetraenoic acid-induced VSMCs migration and neointima formation [28]. IL-6 has also been found to decrease the endothelial NOS activity in human vascular endothelial cells [29]. Moreover, benzo[a]pyrene at a concentration of 10 μmol/L was capable of stimulating the IL-6 secretion in human sebocytes via an AhR signaling pathway [30].
In this study, we hypothesized that benzo[a]pyrene possesses antagonistic potential against NO-related VSMCs death/apoptosis. We investigated the antagonistic effect of benzo[a]pyrene on NO donor-triggered death/apoptosis in a primary rat VSMCs culture model. We also determined whether IL-6 would be a survival mediator in the anti-cell death/apoptotic effect of benzo[a]pyrene on NO donor-treated VSMCs.
Materials and methods
The protocol for animal study was approved by the Institutional Animal Care and Use Committee, National Taiwan University, College of Medicine, Taipei, Taiwan.
Primary culture of vascular smooth muscle cells
VSMCs were isolated from the thoracic aortas of male Wistar rats (150–200 g) by the method described previously [31]. Wistar rats were purchased from BioLASCO (Taipei, Taiwan). The study was conducted in accordance with the guidelines of the Animal Research Committee of National Taiwan University, College of Medicine, for the care and use of laboratory animals. Before experiments began, rats were allowed at least 1 week acclimation period at animal quarters with air conditioning and constant humidity. The light was controlled automatically at an interval of 12 h per day. The animals were allowed free access to food and water. To prepare VSMCs, the thoracic aortas were cleaned of fat and adventitia, cut into small strips, and then digested with 1 mg/mL collagenase (Sigma) and 0.125 mg/mL elastase (Sigma) at 37°C for 60 min. Cells were cultured in DMEM containing 10% FCS at 37°C in a humidified atmosphere of 5% CO2/95% air. Cells exhibited characteristics of VSMCs were used between the third and sixth passages.
Analysis of cell number
Cells were seeded at 2×104 cells/well into 12-well plates and allowed to attach overnight. Cells were cultured in serum-free DMEM for 48 h, and then test compounds were added to medium for another 24 h. Cells were harvested, and a 50 μL aliquot was mixed with 0.04% trypan blue and counted twice on a hemocytometer.
Cell treatment and preparation of total cell lysates
Cells seeded in 6-well plates and grown to 60% to 80% confluence were serum-deprived in DMEM containing 0.1% bovine serum albumin (BSA) for 24–48 h, and treated with or without benzo[a]pyrene, in the presence or absence of sodium nitroprusside (SNP) for indicated time intervals. Cells were then harvested by scraping in 200 μL of ice-cold extraction buffer [50 mmol/L Tris-HCl (pH 7.4), 150 mmol/L NaCl, 10 mmol/L EDTA, 0.1% NP-40, 1 mmol/L orthovanadate, 1 mmol/L PMSF, 10 mmol/L sodium fluoride, 10 μg/mL leupeptin, and 10 μg/mL aprotinin], rotated for 15 min at 4°C, and centrifuged at 10000 × g for 20 min. The supernatant were collected, and stored at -80°C until use.
Cell cytotoxicity assay
The cytotoxicity was determined using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay (Sigma). Briefly, cells (1×104) were seeded into 96-well plates overnight and starved for 48 h. Then, the medium was aspirated and cells were cultured in serum-free DMEM with vehicle or various concentrations of benzo[a]pyrene solubilized in DMSO in the presence or absence of SNP for indicated time intervals. Subsequently the medium was removed and cells were incubated in medium with MTT (5 μg/mL) for 1 h at 37°C, which was metabolized to formazan, and then dissolved in DMSO and measured in an ELISA.
Detection of subdiploid DNA population
Cells were harvested and prepared single cell suspension in PBS at 1–2 x 106 cells/mL. Aliquoted 1 mL cells in a 15 mL polypropylene, V-bottomed tube and added 3 mL cold absolute ethanol forcibly in order to prevent clumping and cell loss. Cells were fixed for at least 1 h at 4°C. Cells were washed 2 times with PBS and added 1 mL of 50 μg/mL propidium iodide (PI) staining solution to cell pellet and mixed well. Added 50 μL of RNaseA stock solution (10 mg/mL) and incubate for 30 min at room temperature. Samples were stored at 4°C until analyzed by flow cytometry (Becton-Dickinson, San Jose, CA).
Annexin V Apoptosis Detection
The annexin V-FITC Apoptosis Detection Kit was used for flow cytometry experiment to detect apoptotic cells. VSMCs cultured in DMEM with test compounds were washed twice with cold PBS and then resuspended in 1x binding buffer at the concentration of 1 x 106 cells/mL. The cell suspension was transferred to a 5-ml culture tube and mixed with 5 μL of annexin V-FITC and 10 μL of PI. The cells were gently vortexed and incubated for 15 min at room temperature in the dark. Then 400 μL of 1x binding buffer was added to each tube and analyzed by flow cytometry with the use of a FACSCalibur flow cytometer (Becton-Dickinson, San Jose, CA).
Western blot analysis
Equal amounts of proteins (30 μg per lane) were subjected to 10% SDS-PAGE, transferred to nitrocellulose membranes (Amersham). The membranes were blocked with 5% fat-free milk in PBS containing 0.1% Tween 20 (PBST) for 1 h and followed by immunoblotting with antibodies for nuclear factor (NF)-κB, IκBα, phospho-p38 mitogen-activated protein kinase (MAPK), p38 MAPK, bcl-2, C23, or α-tubulin (Santa Cruz Biochemicals). Subsequently, membranes were washed three times with PBST, incubated with secondary horseradish peroxidase (HRP)-conjugated antibodies (Santa Cruz Biochemicals), and again followed by three washes. The signals were then visualized with an enhanced chemiluminescence detection system (Amersham). Exposures were recorded on X-film (Fuji).
IL-6 and NO assays
Cells (2 x 105 cells/mL) were serum-starved for 48 h and then treated with test compounds for 24 h. The medium was collected and centrifuged at 500 rpm for 1 min. The supernatant was stored at -70°C until assay. ELISA for rat IL-6 was performed with an ELISA kit (Pierce Endogene) according to the manufacturer’s instructions. Moreover, the NO (nitrite/nitrate) levels were determined using the nitrite/nitrate colorimetric assay kit (R&D Systems).
Statistical analysis
Data are expressed as mean ± SEM of a variable number of experiments or displayed as representative observations of at least three separate experiments. Statistical significance was assessed by one way analysis of variance (ANOVA) and Dunnett’s test. The significant difference is determined when p-value is less than 0.05.
Results
Benzo[a]pyrene suppressed NO-induced death and apoptosis in VSMCs
There was no change on total cell number between control and benzo[a]pyrene (1–30 μmol/L)-treated VSMCs under serum deprivation culture condition (S1A Fig). We next observed the cell cycle distribution in VSMCs under serum-free condition, and confirmed that no change between control and benzo[a]pyrene (10 μmol/L)-treated VSMCs (S1B Fig). These results indicated that benzo[a]pyrene did not cause cell death of VSMCs in serum-free condition.
In quiescent VSMCs, NO donor SNP (1 mmol/L) time-dependently suppressed cell viability by 28.5% (12 h) and 46% (24 h), respectively (Fig 1A). Co-incubation with benzo[a]pyrene (1–10 μmol/L) for 24 h dose-dependently reversed the inhibition of cell viability induced by SNP (Fig 1B). SNP (1–30 mmol/L) effectively increased the NO release in a dose-dependent manner (Fig 1C). Moreover, we also used another NO donor- streptozotocin [32], which is a glucosamine-nitrosourea compound, to confirm the effect of benzo[a]pyrene on VSMCs viability. As shown in Fig 1D, streptozotocin (30 mmol/L) effectively decreased the VSMCs viability, which could be significantly reversed by benzo[a]pyrene (10 μmol/L). Streptozotocin (1–30 mmol/L) could increase the NO release in a dose-dependent manner (Fig 1E).
Fig 1. Effect of NO donors on cell viability and NO release in the presence or absence of benzo[a]pyrene in VSMCs.
(A) VSMCs were cultured in serum-free DMEM in the presence or absence of sodium nitroprusside (SNP, 1 mmol/L) for 12 or 24 h. (B) VSMCs were treated with SNP (1 mmol/L) in the presence or absence of benzo[a]pyrene (10 μmol/L) for 24 h. (C) VSMCs were treated with SNP (1–30 mmol/L) for 24 h. (D) VSMCs were treated with streptozotocin (STZ, 30 mmol/L) in the presence or absence of benzo[a]pyrene (10 μmol/L) for 24 h. (E) VSMCs were treated with streptozotocin (STZ, 1–30 mmol/L) for 24 h. Cell viability was determined by MTT assay. Cell survival was expressed as % of untreated control. The NO (nitrite/nitrate) levels were determined using the nitrite/nitrate colorimetric assay kit. All data are represented as mean ± SEM from three independent experiments. *P < 0.05 as compared with the control. #P < 0.05 as compared with SNP alone (B) or STZ alone (D).
We next analyzed the subdiploid DNA population in VSMCs by flow cytometry. The subdiploid DNA content was markedly increased after SNP stimulation by 36% (Fig 2A and 2B). Benzo[a]pyrene (10 μmol/L) treatment significantly reversed SNP-increased subdiploid DNA levels (Fig 2A and 2B). The annexin V-FITC and PI staining was further used to analyze the percentage of apoptotic cells. As shown in Fig 2C and 2D, the late apoptotic cells and early apoptotic cells were increased from 0.9% to 4.1% or 4.8% to 43.3%, respectively, when cells were treated with SNP for 12 h. Total percentage of apoptotic cells was increased from 5.7% to 47.4%. Once benzo[a]pyrene (10 μmol/L) was co-incubated with SNP, total percentage of apoptotic cells shifted to 26.1% (Fig 2C and 2D). Moreover, SNP markedly decreased the protein expression of bcl-2 in VSMCs, which could be effectively reversed by benzo[a]pyrene (Fig 2E). These results indicated that benzo[a]pyrene was capable of inhibiting SNP-induced apoptosis of VSMCs.
Fig 2. Effect of NO donor on subdiploid DNA content and cell apoptosis in the presence or absence of benzo[a]pyrene in VSMCs.
VSMCs were cultured in serum-free DMEM. VSMCs were treated with SNP (1 mmol/L) in the presence or absence of benzo[a]pyrene (10 μmol/L) for 24 h (A and B) or 12 h (C and D). (A) and (B) Cells were fixed with 70% ethanol and stained with propidium iodide (PI), and the DNA content was analyzed by flow cytometry. Representative images are shown (A). The percentage of subdiploid DNA content in cells was calculated (B). (C) and (D) The cells were collected, and stained with Annexin V-FITC and PI. The percentage of annexin V-positive (annexin V (+)), PI-negative (PI (-)) or PI (+) cells was calculated from fluorescence-1 (FL1-H) / fluorescence-2 (FL-2-H) dot plots, and is shown in the respective upper right and lower right hand corner. Data are represented as mean ± SEM from six independent experiments. *P < 0.05 as compared with control. #P < 0.05 as compared with SNP alone. (E) VSMCs were treated with SNP (1 mmol/L) in the presence or absence of benzo[a]pyrene (10 μmol/L) for 6 or 12 h. The protein expression of bcl-2 and α-tubulin (internal control) was determined by Western blotting. One representative experiment of three is shown.
Role of IL-6 in anti-apoptotic effect of benzo[a]pyrene
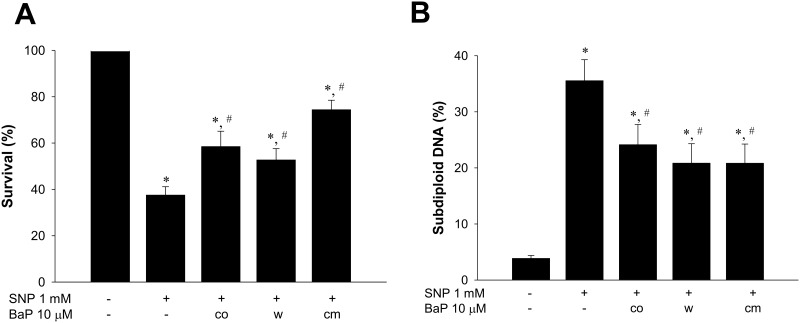
To study the signaling involved in the anti-apoptotic effect of benzo[a]pyrene, we collected conditioned media (cm) from cells treated with benzo[a]pyrene for 24 h and then added it to another cultured cells following treatment with SNP. The results showed that benzo[a]pyrene-condition media enabled to prevent decreased cell viability (Fig 3A) and increased subdiploid DNA content (Fig 3B) in VSMCs by SNP challenge. Besides, both decreased cell viability (Fig 3A) and increased subdiploid DNA content (Fig 3B) were also attenuated during the condition in which cells treated with benzo[a]pyrene for 24 h were then washed and changed to fresh culture media containing SNP alone (w). These results indicated that some mediators might be induced and secreted to media by which benzo[a]pyrene prevented cell death in an autocrine manner. Since IL-6 has been reported to prevent apoptosis in various cell types [33,34], we next investigated if benzo[a]pyrene was able to stimulate IL-6 release. As shown in Fig 4A, benzo[a]pyrene does-dependently increased the production of IL-6 in VSMCs. We next investigated the involvement of IL-6 in anti-apoptotic effect of benzo[a]pyrene. Blockade of IL-6 with the neutralizing antibody (2 μg/mL) abolished benzo[a]pyrene-reduced subdiploid DNA content and apoptosis in SNP-treated VSMCs (Fig 4B and 4C). These results showed that IL-6 produced by VSMCs contributed to anti-apoptotic effect of benzo[a]pyrene on NO-related VSMCs apoptosis.
Fig 3. Inhibition of cell viability by NO donor was partially suppressed by conditioned medium of benzo[a]pyrene-treated cells.
VSMCs were cultured in serum-free DMEM in the presence or absence of benzo[a]pyrene (10 μmol/L) for 24 h and then the condition medium was collected. Cells were refreshed with DMEM containing SNP (1 mmol/L) as “w”. The condition medium was added to another cell culture dish as “cm”. Cells were co-incubated with SNP and benzo[a]pyrene as “co”. After 24 h, the cell viability was determined by MTT assay (A) and the subdiploid DNA content was determined by flow cytometry (B). Data are represented as mean ± SEM from three independent experiments. *P < 0.05 as compared with control. #P < 0.05 as compared with SNP alone.
Fig 4. Role of interleukin-6 (IL-6) in the anti-apoptotic effect of benzo[a]pyrene on SNP-treated VSMCs.
(A) VSMCs were cultured in serum-free DMEM in the presence or absence of benzo[a]pyrene for 24 h. The IL-6 production was measured by ELISA. Data are represented as mean ± SEM from three independent experiments. *P < 0.05 as compared with the control. (B) VSMCs were treated with SNP (1 mmol/L) in the presence or absence of benzo[a]pyrene (10 μmol/L) for 24 h. The subdiploid DNA content was determined by flow cytometry. Representative images are shown. (C) VSMCs were treated with SNP (1 mmol/L) in the presence or absence of benzo[a]pyrene (10 μmol/L) for 12 h. The annexin V-FITC and PI staining was analyzed by flow cytometry. Data are represented as mean ± SEM from three independent experiments. #P < 0.05 as compared with SNP alone. $P < 0.05 as compared with SNP+benzo[a]pyrene.
Involvement of NF-κB and p38 MAPK in the anti-apoptotic effect of benzo[a]pyrene
Both NF-κB and p38 MAPK signals possess the ability to regulate VSMCs proliferation [35,36] and IL-6 induction [37]. To investigate the signaling involved in the anti-apoptotic effect of benzo[a]pyrene in VSMCs, we tested whether NF-κB and p38 MAPK were involved. As shown in Fig 5A-a, the nuclear NF-κB-p65 protein expression in VSMCs was time-dependently increased by benzo[a]pyrene (10 μmol/L). The protein expression of IκBα was time-dependently decreased by benzo[a]pyrene (10 μmol/L) (Fig 5A-b). The phosphorylation of p38 MAPK in VSMCs was also time-dependently increased by benzo[a]pyrene (10 μmol/L) (Fig 5B). Moreover, both NF-κB inhibitor PDTC (10 μmol/L) and p38 MAPK inhibitor SB203589 (3 μmol/L) significantly suppressed the benzo[a]pyrene-increased IL-6 production in VSMCs (Fig 5C). Both PDTC (10 μmol/L) and SB203589 (3 μmol/L) could also significantly inhibit the anti-apoptotic effect (Fig 6A) and survival enhancement (Fig 6B) of benzo[a]pyrene against SNP challenge. These results implicated that benzo[a]pyrene inhibited SNP-induced VSMCs apoptosis through the activation of NF-κB and p38 MAPK signals.
Fig 5. Benzo[a]pyrene induced the activation of both NF-κB and p38 MAPK, which were involved in the benzo[a]pyrene-increased IL-6 production in VSMCs.
VSMCs were cultured in serum-free DMEM in the presence or absence of benzo[a]pyrene (10 μmol/L) for 0.25–4 h (A) or 5–30 min (B). The protein expressions of NF-κB-p65 (nuclear protein) and IκBα (A) and phospho-p38/p38 MAPK (B) were determined by Western blotting. The protein fold changes, which were normalized to C23, α-tubulin, or p38, were shown below each blot. Experiments were repeated three times. One representative experiment is shown. (C) VSMCs were cultured in serum-free DMEM in the presence or absence of benzo[a]pyrene for 24 h. The IL-6 production was measured by ELISA. Data are represented as mean ± SEM from three independent experiments. *P < 0.05 as compared with control. #P < 0.05 as compared with benzo[a]pyrene alone.
Fig 6. Role of NF-κB and p38 MAPK in the anti-apoptotic effect of benzo[a]pyrene on SNP-treated VSMCs.
VSMCs were pretreated with SB203580 (3 μmol/L) or PDTC (10 μmol/L) followed by treatments of benzo[a]pyrene (10 μmol/L) and SNP (1 mmol/L) for 12 h. The annexin V-FITC and PI staining was analyzed by flow cytometry (A). Cell viability was determined by MTT assay (B). Data are represented as mean ± SEM from three independent experiments. *P < 0.05 as compared with the control. #P < 0.05 as compared with SNP alone. $P < 0.05 as compared with SNP+benzo[a]pyrene.
Discussion
The present study provides the first characterization of the effect of benzo[a]pyrene on the regulation of apoptosis in VSMCs. Our observations also suggest that the survival signal by benzo[a]pyrene is mediated from IL-6 release because the neutralizing antibody to IL-6 inhibits benzo[a]pyrene-induced anti-apoptotic effect.
Low-concentration NO is considered to regulate the physiological functions, but high-concentration NO may contribute to the pathological effects [13]. The physiological NO levels appear to be in the range from 1 μmol/L to 10 nmol/L with the short half-life (9 to 900 min) [38]. The range of NO levels in bloods of human or mammalian has been estimated to be 3 nmol/L up to 20 μmol/L [39]. The exhaled NO concentrations in acute asthma children were significantly higher (31.3 ± 4.2 ppb (μg/L)) than in healthy children (5.4 ± 0.4 ppb (μg/L)) [40]. The serum total concentrations of NO (NO3−/NO2−) in control subjects and squamous cell carcinoma of the oral cavity patients in IV stage were about 12 and 30 μmol/L, respectively [41]. In the present study, the NO levels in culture medium of control VSMCs and SNP (1 mmol/L)-treated VSMCs were about 4.5 and 15 μmol/L, respectively. Therefore, we used a NO donor at the released NO concentrations relevant to human exposure in bloods to test its cytotoxicity to VSMCs.
Apoptosis is known to as a physiological suicide pathway to maintain the homeostasis of tissue organs. VSMCs are major constituents of the medial layer of blood vessels and are involved in the development of atherosclerotic plaque by abnormal accumulation in intimal vessels [2,3]. NO-induced VSMCs apoptosis may be an important determinant to regulate cell number of normal arterial wall and is a feature of atherosclerosis pathology [13,18]. The complex mechanisms of NO-mediated apoptosis have been mentioned. NO donor has been shown to potentiate DNA damage and alter DNA repair in ionizing radiation-treated cells [42]. NO could also inhibit the catalytic activity of the 26S proteasome and regulate proteasomal subunit expression in VSMCs [15]. An increased susceptibility to NO-induced VSMC apoptosis has been observed in p53(-/-) cells, which could be effectively abrogated by antioxidant catalase [43]. Besides, the protein expression of anti-apoptotic protein was decreased under SNP exposure in VSMCs [44]. In the present study, we also found that SNP induced bcl-2 degradation, apoptosis, and cell death in primary rat VSMCs, which could be significantly reversed by benzo[a]pyrene. These results suggest that benzo[a]pyrene is capable of suppressing NO-induced apoptosis and cell death in VSMCs.
Atherosclerosis has been suggested to be an inflammatory disease [45,46]. A significant role of IL-6 in the pathophysiology of atherosclerosis has also been suggested [24,45]. VSMCs secrete copious IL-6 under stimulation conditions such as tumor necrosis factor (TNF)-α [47], IL-1β [48], platelet-derived thrombin [49], endothelin I [50], and lipopolysaccharide [37] that they may be involved in the pathogenesis of atherosclerosis. There are several important regulatory cis DNA elements in the promoter region of the IL-6 gene such as AP-1, CRE, NF-IL6, and NF-κB, which are conserved among species such as mice, rat and human, and regulate IL-6 gene expression in a cell-specific manner [51,52]. Recombinant interleukin-6 administration has been found to protect MIN6 β-cells from NO dependent cytokine-induced apoptosis and reduced bcl-2/bax protein ratio [53]. The pleiotropic action of IL-6 has also been found to improve the NO-induced cytotoxic CD8+ T cell dysfunction from chagasic patients [54]. IL-6 was capable of inducing bcl-2 expression to protecting cell functions in response to hyperoxia toxicity in human umbilical vein endothelial cells (HUVECs) [55]. It has been demonstrated that NO triggers cell death by regulating anti-apoptotic bcl-2 family members in mouse embryonic fibroblasts [56]. In the present study, we found that benzo[a]pyrene dose-dependently and significantly increased the IL-6 production and inhibited the reduced bcl-2 expression in SNP-treated VSMCs. IL-6 neutralizing antibody could significantly reverse the anti-apoptotic effect of benzo[a]pyrene on SNP-treated VSMCs. These findings suggest that IL-6 plays an important role in the atherogenic effect of benzo[a]pyrene.
The activation of NF-κB has been shown to play an important role in angiotensin II-dependent VSMCs proliferation [35]. Benzo[a]pyrene has been shown to induce rapid NF-κB activation via redox regulation in VSMCs [57,58]. Mehrhof et al. (2005) have suggested that NF-κB signaling may be as a regional regulator of VSMCs survival rather than a direct promoter of VSMCs proliferation [58]. Moreover, p38 MAPK signaling has also been demonstrated to be involved in the serum-induced VSMCs proliferation [36]. A p38 MAPK-dependent signaling pathway has been found to contribute to the perivascular adipose tissue-derived leptin-triggered VSMCs phenotypic switching [59]. On the other hand, transcriptional activation of cytokine genes commonly requires the induction of NF-κB [60]. It has been reported that pretreatment of human airway smooth muscle cells with p38 MAPK inhibitor SB203580 significantly inhibited the secretion of IL-6 after TNF-αstimulation [41]. TNF-α has also been found to induce p38-dependent IL-6 induction and protect cardiac myocytes from apoptosis [61]. The angiotensin II-induced IL-6 gene expression also depends on NF-κB activation in VSMCs [62]. Both NF-κB and p38 MAPK signals have also been shown to be involved in the lipopolysaccharide-induced IL-6 induction in VSMCs [37]. The intracellular signaling pathways by which benzo[a]pyrene leads to cell survival and IL-6 production in SNP-treated VSMCs are of interest. In the present study, we confirmed that benzo[a]pyrene in deed activated NF-κB to translocate to nucleus, and PDTC, an inhibitor of NF-κB activation, abolished the anti-apoptotic effect of benzo[a]pyrene. We also found that benzo[a]pyrene markedly increased the phosphorylation of p38 MAPK in VSMCs. SB203580 could also inhibit the benzo[a]pyrene-induced anti-apoptotic effect, suggesting that p38 MAPK signaling pathway is involved in the benzo[a]pyrene-induced anti-apoptotic effect. We further found that both NF-κB and p38 MAPK inhibitors significantly inhibit the benzo[a]pyrene-induced IL-6 production. The protein kinase C (PKC)-related signaling has also been shown to be involved in the IL-6 production induced by serotonin from human VSMCs [63]. However, Funakoshi et al reported that angiotensin II-induced IL-6 expression was dependent on intracellular Ca2+, tyrosine phosphorylation, and ERK activation, and independent of PKC and extracellular Ca2+ [64]. These findings suggest that regulation of IL-6 may be complex and needs more studies to understand the mechanisms by which benzo[a]pyrene induces IL-6 release. On the other hand, benzo[a]pyrene has been found to inhibit angiogenesis in HUVECs via an aryl hydrocarbon receptor (AhR)-dependent pathway [65]. The coplanar polychlorinated biphenyls (PCBs), the AhR agonists, can disrupt endothelial barrier function and promote IL-6 production in porcine endothelial cells; but PCB 153, which is not a ligand for the AhR, had no effect on endothelial function and IL-6 production [66]. Hu et al. recently showed that BaP induced IL-6 production and inhibited sebum production in human sebocytes via the activation of AhR signaling [30]. Therefore, in this study, benzo[a]pyrene induced IL-6 production in VSMCs may through an AhR signaling pathway.
Indeed, not only proliferation of VSMCs but also apoptosis is found in atherosclerotic lesions [2,18], suggesting that apoptosis may be a compensatory behavior to repair vascular injury. Dysfunction of the apoptosis process has been linked to pathogenesis of cancer and atherosclerosis [67,68]. The present study showed the ability of benzo[a]pyrene to suppress a death signal in VSMCs triggered by NO through an IL-6 signaling pathway (Fig 7). These findings propose a new mechanism for the atherogenic effect of benzo[a]pyrene. Benzo[a]pyrene may therefore not only alter VSMCs to a proliferative phenotype, but also exert an anti-apoptotic effect participating in vascular disease. It is conceivable that the ability of benzo[a]pyrene to inhibit NO-induced cell death may play a substantial role in atherosclerotic lesion formation. Further studies are necessary to define the anti-apoptotic effect of benzo[a]pyrene on the pathogenesis of vascular lesion in vivo.
Fig 7. A proposed model showed the ability of benzo[a]pyrene to suppress a death signal in VSMCs triggered by NO through an IL-6 signaling pathway.
Supporting information
(A) VSMCs were cultured in serum-free DMEM in the presence or absence of benzo[a]pyrene (1–30 μmol/L). After 72 h, cells were collected, stained with trypan blue, and counted by hemocytometry. Data are presented as mean ± SEM from three independent experiments. (B) VSMCs were cultured in serum-free DMEM in the presence or absence of benzo[a]pyrene (10 μmol/L) for 72 h. The DNA content was analyzed by flow cytometry. One representative experiment of three is shown.
(TIF)
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
This study was supported by the grant from the Ministry of Science and Technology of Taiwan (MOST103-2314-B-002-035). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Majesky MW, Schwartz SM. Smooth muscle diversity in arterial wound repair. Toxicol Pathol. 1990; 18: 554–559. [PubMed] [Google Scholar]
- 2.Wang G, Jacquet L, Karamariti E, Xu Q. Origin and differentiation of vascular smooth muscle cells. J Physiol. 2015; 593: 3013–3030. 10.1113/JP270033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andres V. Control of vascular smooth muscle cell growth and its implication in atherosclerosis and restenosis (review). Int J Mol Med. 1998; 2: 81–89. [DOI] [PubMed] [Google Scholar]
- 4.Irani K. Oxidant signaling in vascular cell growth, death, and survival: a review of the roles of reactive oxygen species in smooth muscle and endothelial cell mitogenic and apoptotic signaling. Circ Res. 2000; 87: 179–183. [DOI] [PubMed] [Google Scholar]
- 5.Bangia KS, Symanski E, Strom SS, Bondy M. A cross-sectional analysis of polycyclic aromatic hydrocarbons and diesel particulate matter exposures and hypertension among individuals of Mexican origin. Environ Health. 2015; 14:51 10.1186/s12940-015-0039-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curfs DM, Knaapen AM, Pachen DM, Gijbels MJ, Lutgens E, Smook ML, et al. Polycyclic aromatic hydrocarbons induce an inflammatory atherosclerotic plaque phenotype irrespective of their DNA binding properties. FASEB J. 2005; 19: 1290–1292. 10.1096/fj.04-2269fje [DOI] [PubMed] [Google Scholar]
- 7.Hough JL, Baird MB, Sfeir GT, Pacini CS, Darrow D, Wheelock C. Benzo(a)pyrene enhances atherosclerosis in White Carneau and Show Racer pigeons. Arterioscler Thromb. 1993; 13:1721–1727. [DOI] [PubMed] [Google Scholar]
- 8.Oesterling E, Toborek M, Hennig B. Benzo[a]pyrene induces intercellular adhesion molecule-1 through a caveolae and aryl hydrocarbon receptor mediated pathway. Toxicol Appl Pharmacol. 2008; 232: 309–316. 10.1016/j.taap.2008.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ou X, Ramos KS. Proliferative responses of quail aortic smooth muscle cells to benzo[a]pyrene: implications in PAH-induced atherogenesis. Toxicology. 1992; 74: 243–258. [DOI] [PubMed] [Google Scholar]
- 10.Ramos KS, Zhang Y, Sadhu DN, Chapkin RS. The induction of proliferative vascular smooth muscle cell phenotypes by benzo(a)pyrene is characterized by up-regulation of inositol phospholipid metabolism and c-Ha-ras gene expression. Arch Biochem Biophys. 1996; 332: 213–222. 10.1006/abbi.1996.0335 [DOI] [PubMed] [Google Scholar]
- 11.Kim YM, Bombeck CA, Billiar TR. Nitric oxide as a bifunctional regulator of apoptosis. Circ Res. 1999; 84: 253–256. [DOI] [PubMed] [Google Scholar]
- 12.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991; 43: 109–142. [PubMed] [Google Scholar]
- 13.Napoli C, Paolisso G, Casamassimi A, Al-Omran M, Barbieri M, Sommese L, et al. Effects of nitric oxide on cell proliferation: novel insights. J Am Coll Cardiol. 2013; 62: 89–95. [DOI] [PubMed] [Google Scholar]
- 14.Nicotera P, Brune B, Bagetta G. Nitric oxide: inducer or suppressor of apoptosis? Trends Pharmacol Sci. 1997; 18: 189–190. [PubMed] [Google Scholar]
- 15.Kapadia MR, Eng JW, Jiang Q, Stoyanovsky DA, Kibbe MR. Nitric oxide regulates the 26S proteasome in vascular smooth muscle cells. Nitric Oxide. 2009; 20: 279–288. 10.1016/j.niox.2009.02.005 [DOI] [PubMed] [Google Scholar]
- 16.Ramachandran A, Moellering DR, Ceaser E, Shiva S, Xu J, Darley-Usmar V. Inhibition of mitochondrial protein synthesis results in increased endothelial cell susceptibility to nitric oxide-induced apoptosis. Proc Natl Acad Sci USA. 2002; 99: 6643–6648. 10.1073/pnas.102019899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishio E, Fukushima K, Shiozaki M, Watanabe Y. Nitric oxide donor SNAP induces apoptosis in smooth muscle cells through cGMP-independent mechanism. Biochem Biophys Res Commun. 1996; 221: 163–168. 10.1006/bbrc.1996.0563 [DOI] [PubMed] [Google Scholar]
- 18.Isner JM, Kearney M, Bortman S, Passeri J. Apoptosis in human atherosclerosis and restenosis. Circulation. 1995; 91: 2703–2711. [DOI] [PubMed] [Google Scholar]
- 19.Geng YJ, Wu Q, Muszynski M, Hansson GK, Libby P. Apoptosis of vascular smooth muscle cells induced by in vitro stimulation with interferon-gamma, tumor necrosis factor-alpha, and interleukin-1 beta. Arterioscler Thromb Vasc Biol. 1996; 16: 19–27. [DOI] [PubMed] [Google Scholar]
- 20.von der Leyen HE, Gibbons GH, Morishita R, Lewis NP, Zhang L, Nakajima M, et al. Gene therapy inhibiting neointimal vascular lesion: in vivo transfer of endothelial cell nitric oxide synthase gene. Proc Natl Acad Sci USA. 1995; 92: 1137–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansson GK, Geng YJ, Holm J, Hardhammar P, Wennmalm A, Jennische E. Arterial smooth muscle cells express nitric oxide synthase in response to endothelial injury. J Exp Med. 1994; 180: 733–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan ZQ, Yokota T, Zhang W, Hansson GK. Expression of inducible nitric oxide synthase inhibits platelet adhesion and restores blood flow in the injured artery. Circ Res. 1996; 79: 38–44. [DOI] [PubMed] [Google Scholar]
- 23.Hardonnière K, Huc L, Podechard N, Fernier M, Tekpli X, Gallais I, et al. Benzo[a]pyrene-induced nitric oxide production acts as a survival signal targeting mitochondrial membrane potential. Toxicol In Vitro. 2015; 29: 1597–1608. 10.1016/j.tiv.2015.06.010 [DOI] [PubMed] [Google Scholar]
- 24.Seino Y, Ikeda U, Ikeda M, Yamamoto K, Misawa Y, Hasegawa T, et al. Interleukin 6 gene transcripts are expressed in human atherosclerotic lesions. Cytokine. 1994; 6: 87–91. [DOI] [PubMed] [Google Scholar]
- 25.Lee WY, Allison MA, Kim DJ, Song CH, Barrett-Connor E. Association of interleukin-6 and C-reactive protein with subclinical carotid atherosclerosis (the Rancho Bernardo Study). Am J Cardiol. 2007; 99: 99–102. 10.1016/j.amjcard.2006.07.070 [DOI] [PubMed] [Google Scholar]
- 26.Tsutamoto T, Hisanaga T, Wada A, Maeda K, Ohnishi M, Fukai D, et al. Interleukin-6 spillover in the peripheral circulation increases with the severity of heart failure, and the high plasma level of interleukin-6 is an important prognostic predictor in patients with congestive heart failure. J Am Coll Cardiol. 1998; 31: 391–398. [DOI] [PubMed] [Google Scholar]
- 27.Loppnow H, Libby P. Proliferating or interleukin 1-activated human vascular smooth muscle cells secrete copious interleukin 6. J Clin Invest. 1990; 85: 731–738. 10.1172/JCI114498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chava KR, Karpurapu M, Wang D, Bhanoori M, Kundumani-Sridharan V, Zhang Q, et al. CREB-mediated IL-6 expression is required for 15(S)-hydroxyeicosatetraenoic acid-induced vascular smooth muscle cell migration. Arterioscler Thromb Vasc Biol. 2009; 29: 809–815. 10.1161/ATVBAHA.109.185777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hung MJ, Cherng WJ, Hung MY, Wu HT, Pang JH. Interleukin-6 inhibits endothelial nitric oxide synthase activation and increases endothelial nitric oxide synthase binding to stabilized caveolin-1 in human vascular endothelial cells. J Hypertens. 2010; 28: 940–951. 10.1097/HJH.0b013e32833992ef [DOI] [PubMed] [Google Scholar]
- 30.Hu T, Pan Z, Yu Q, Mo X, Song N, et al. Benzo(a)pyrene induces interleukin (IL)-6 production and reduces lipid synthesis in human SZ95 sebocytes via the aryl hydrocarbon receptor signaling pathway. Environ Toxicol Pharmacol. 2016; 43: 54–60. [DOI] [PubMed] [Google Scholar]
- 31.Tzeng HP, Yang RS, Ueng TH, Lin-Shiau SY, Liu SH. Motorcycle exhaust particulates enhance vasoconstriction in organ culture of rat aortas and involve reactive oxygen species. Toxicol Sci. 2003; 75: 66–73. 10.1093/toxsci/kfg164 [DOI] [PubMed] [Google Scholar]
- 32.Kwon NS, Lee SH, Choi CS, Kho T, Lee HS. Nitric oxide generation from streptozotocin. FASEB J. 1994; 8: 529–533. [DOI] [PubMed] [Google Scholar]
- 33.Schwarze MM, Hawley RG. Prevention of myeloma cell apoptosis by ectopic bcl-2 expression or interleukin 6-mediated up-regulation of bcl-xL. Cancer Res. 1995; 55: 2262–2265. [PubMed] [Google Scholar]
- 34.Chen RH, Chang MC, Su YH, Tsai YT, Kuo ML. Interleukin-6 inhibits transforming growth factor-beta-induced apoptosis through the phosphatidylinositol 3-kinase/Akt and signal transducers and activators of transcription 3 pathways. J Biol Chem. 1999; 274: 23013–23019. [DOI] [PubMed] [Google Scholar]
- 35.Zahradka P, Werner JP, Buhay S, Litchie B, Helwer G, Thomas S. NF-κB activation is essential for angiotensin II-dependent proliferation and migration of vascular smooth muscle cells. J Mol Cell Cardiol. 2002; 34: 1609–1621. [DOI] [PubMed] [Google Scholar]
- 36.Zhao M, Liu Y, Bao M, Kato Y, Han J, Eaton JW. Vascular smooth muscle cell proliferation requires both p38 and BMK1 MAP kinases. Arch Biochem Biophys. 2002; 400: 199–207. 10.1016/S0003-9861(02)00028-0 [DOI] [PubMed] [Google Scholar]
- 37.Son YH, Jeong YT, Lee KA, Choi KH, Kim SM, Rhim BY, et al. Roles of MAPK and NF-kappaB in interleukin-6 induction by lipopolysaccharide in vascular smooth muscle cells. J Cardiovasc Pharmacol. 2008; 51: 71–77. 10.1097/FJC.0b013e31815bd23d [DOI] [PubMed] [Google Scholar]
- 38.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and the ugly. Am J Physiol. (Cell Physiol.) 1996; 271: C1424–C1437. [DOI] [PubMed] [Google Scholar]
- 39.Kelm M. Nitric oxide metabolism and breakdown. Biochim Biophys Acta. 1999; 1411: 273–289. [DOI] [PubMed] [Google Scholar]
- 40.Baraldi E, Azzolin NM, Zanconato S, Dario C, Zacchello F. Corticosteroids decrease exhaled nitric oxide in children with acute asthma. J Pediatr. 1997; 131: 381–385. [DOI] [PubMed] [Google Scholar]
- 41.Ratajczak-Wrona W, Jablonska E, Antonowicz B, Dziemianczyk D, Grabowska Stanislawa Zyta. Levels of biological markers of nitric oxide in serum of patients with squamous cell carcinoma of the oral cavity. Int J Oral Sci. 2013; 5: 141–145. 10.1038/ijos.2013.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mikhailenko VM, Muzalov II. Exogenous nitric oxide potentiate DNA damage and alter DNA repair in cells exposed to ionizing radiation. Exp Oncol. 2013; 35: 318–324. [PubMed] [Google Scholar]
- 43.Popowich DA, Vavra AK, Walsh CP, Bhikhapurwala HA, Rossi NB, Jiang Q, et al. Regulation of reactive oxygen species by p53: implications for nitric oxide-mediated apoptosis. Am J Physiol Heart Circ Physiol. 2010; 298: H2192–H2200. 10.1152/ajpheart.00535.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lau HK. Cytotoxicity of nitric oxide donors in smooth muscle cells is dependent on phenotype, and mainly due to apoptosis. Atherosclerosis. 2003; 166: 223–232. [DOI] [PubMed] [Google Scholar]
- 45.Bennett MR, Sinha S, Owens GK. Vascular Smooth Muscle Cells in Atherosclerosis. Circ Res. 2016; 118: 692–702. 10.1161/CIRCRESAHA.115.306361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hartman J, Frishman WH. Inflammation and atherosclerosis: a review of the role of interleukin-6 in the development of atherosclerosis and the potential for targeted drug therapy. Cardiol Rev. 2014; 22: 147–151. 10.1097/CRD.0000000000000021 [DOI] [PubMed] [Google Scholar]
- 47.Amrani Y, Ammit AJ, Panettieri RA Jr. Tumor necrosis factor receptor (TNFR) 1, but not TNFR2, mediates tumor necrosis factor-α-induced interleukin-6 and RANTES in human airway smooth muscle cells: role of p38 and p42/44 mitogen-activated protein kinases. Mol Pharmacol. 2001; 60: 646–655. [PubMed] [Google Scholar]
- 48.Alexander MR, Murgai M, Moehle CW, Owens GK. Interleukin-1β modulates smooth muscle cell phenotype to a distinct inflammatory state relative to PDGF-DD via NF-κB-dependent mechanisms. Physiol Genomics. 2012; 44: 417–429. 10.1152/physiolgenomics.00160.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tokunou T, Ichiki T, Takeda K, Funakoshi Y, Iino N, Shimokawa H, et al. Thrombin induces interleukin-6 expression through the cAMP response element in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2001; 21: 1759–1763. [DOI] [PubMed] [Google Scholar]
- 50.Browatzki M, Schmidt J, Kubler W, Kranzhofer R. Endothelin-1 induces interleukin-6 release via activation of the transcription factor NF-κB in human vascular smooth muscle cells. Basic Res Cardiol. 2000; 95: 98–105. [DOI] [PubMed] [Google Scholar]
- 51.Tanabe O, Akira S, Kamiya T, Wong GG, Hirano T, Kishimoto T. Genomic structure of the murine IL-6 gene. High degree conservation of potential regulatory sequences between mouse and human. J Immunol. 1988; 141: 3875–3881. [PubMed] [Google Scholar]
- 52.Sato S, Sakurai T, Ogasawara J, Shirato K, Ishibashi Y, Oh-ishi S, et al. Direct and indirect suppression of interleukin-6 gene expression in murine macrophages by nuclear orphan receptor REV-ERBα. ScientificWorldJournal. 2014; 2014: 685854 10.1155/2014/685854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park H, Ahn Y, Park CK, Chung HY, Park Y. Interleukin-6 protects MIN6 beta cells from cytokine-induced apoptosis. Ann N Y Acad Sci. 2003; 1005: 242–249. [DOI] [PubMed] [Google Scholar]
- 54.Sanmarco LM, Visconti LM, Eberhardt N, Ramello MC, Ponce NE, Spitale NB, et al. IL-6 improves the nitric oxide-induced cytotoxic CD8+ T-cell dysfunction in human chagas disease. Front Immunol. 2016; 7: 626 10.3389/fimmu.2016.00626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Waxman AB, Kolliputi N. IL-6 protects against hyperoxia-induced mitochondrial damage via bcl-2–induced bak interactions with mitofusions. Am J Respir Cell Mol Biol. 2009; 41: 385–396. 10.1165/rcmb.2008-0302OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Snyder CM, Shroff EH, Liu J, Chandel NS. Nitric oxide induces cell death by regulating anti-apoptotic BCL-2 family members. PLoS One. 2009; 4(9):e7059 10.1371/journal.pone.0007059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yan Z, Subbaramaiah K, Camilli T, Zhang F, Tanabe T, McCaffrey TA, et al. Benzo[a]Pyrene induces the transcription of cyclooxygenase-2 in vascular smooth muscle cells. Evidence for the involvement of extracellular signal-regulated kinase and NF-κB. J Biol Chem. 2000; 275: 4949–4955. [DOI] [PubMed] [Google Scholar]
- 58.Mehrhof FB, Schmidt-Ullrich R, Dietz R, Scheidereit C. Regulation of vascular smooth muscle cell proliferation: role of NF-κB revisited. Circ Res. 2005; 96: 958–964. 10.1161/01.RES.0000166924.31219.49 [DOI] [PubMed] [Google Scholar]
- 59.Li H, Wang YP, Zhang LN, Tian G. Perivascular adipose tissue-derived leptin promotes vascular smooth muscle cell phenotypic switching via p38 mitogen-activated protein kinase in metabolic syndrome rats. Exp Biol Med (Maywood). 2014; 239: 954–965. 10.1177/1535370214527903 [DOI] [PubMed] [Google Scholar]
- 60.Li Q, Verma IM. NF-κB regulation in the immune system. Nat Rev Immunol. 2002; 2: 725–734. 10.1038/nri910 [DOI] [PubMed] [Google Scholar]
- 61.Craig R, Larkin A, Mingo AM, Thuerauf DJ, Andrews C, McDonough PM, et al. p38 MAPK and NF-κB collaborate to induce interleukin-6 gene expression and release. Evidence for a cytoprotective autocrine signaling pathway in a cardiac myocyte model system. J Biol Chem. 2000; 275: 23814–23824. 10.1074/jbc.M909695199 [DOI] [PubMed] [Google Scholar]
- 62.Inanaga K, Ichiki T, Matsuura H, Miyazaki R, Hashimoto T, Takeda K, et al. Resveratrol attenuates angiotensin II-induced interleukin-6 expression and perivascular fibrosis. Hypertens Res. 2009; 32: 466–471. 10.1038/hr.2009.47 [DOI] [PubMed] [Google Scholar]
- 63.Ito T, Ikeda U, Shimpo M, Yamamoto K, Shimada K. Serotonin increases interleukin-6 synthesis in human vascular smooth muscle cells. Circulation. 2000; 102: 2522–2527. [DOI] [PubMed] [Google Scholar]
- 64.Funakoshi Y, Ichiki T, Ito K, Takeshita A. Induction of interleukin-6 expression by angiotensin II in rat vascular smooth muscle cells. Hypertension. 1999; 34: 118–125. [DOI] [PubMed] [Google Scholar]
- 65.Li CH, Cheng YW, Hsu YT, Hsu YJ, Liao PL, Kang JJ. Benzo[a]pyrene inhibits angiogenic factors-induced alphavbeta3 integrin expression, neovasculogenesis, and angiogenesis in human umbilical vein endothelial cells. Toxicol Sci. 2010; 118: 544–553. 10.1093/toxsci/kfq279 [DOI] [PubMed] [Google Scholar]
- 66.Hennig B, Meerarani P, Slim R, Toborek M, Daugherty A, Silverstone AE, et al. Proinflammatory properties of coplanar PCBs: in vitro and in vivo evidence. Toxicol Appl Pharmacol. 2002; 181: 174–183. [DOI] [PubMed] [Google Scholar]
- 67.Haunstetter A, Izumo S. Apoptosis: basic mechanisms and implications for cardiovascular disease. Circ Res. 1998; 82: 1111–1129. [DOI] [PubMed] [Google Scholar]
- 68.Xia P, Liu Y, Cheng Z. Signaling Pathways in Cardiac Myocyte Apoptosis. Biomed Res Int. 2016; 2016: 9583268 10.1155/2016/9583268 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) VSMCs were cultured in serum-free DMEM in the presence or absence of benzo[a]pyrene (1–30 μmol/L). After 72 h, cells were collected, stained with trypan blue, and counted by hemocytometry. Data are presented as mean ± SEM from three independent experiments. (B) VSMCs were cultured in serum-free DMEM in the presence or absence of benzo[a]pyrene (10 μmol/L) for 72 h. The DNA content was analyzed by flow cytometry. One representative experiment of three is shown.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.