The Indiana Clinical and Translational Science Institute (ICTSI) was designed with input from a wide variety of critical stakeholders across the entire state of Indiana to create a vibrant engine for “Translational Sciences” research and training. The Institute was established in 2008 with a CTSA grant from the National Institute of Health (NIH) awarded to the Indiana University (IU) School of Medicine and a consortium of three leading universities in the state that includes Indiana, Purdue and Notre Dame Universities. It brought together a wide array of the state’s government, hospital, industry, and foundation partners to engage all of the key stakeholders in health sciences research (see Figure 1). Thus, ICTSI is in a unique position to influence biomedical education in the state of Indiana, investigate the majority of Indiana’s population health data through medical informatics capabilities, and exert statewide influence in both public and private health services through our partnerships. In short, the ICTSI was created to be a true statewide laboratory to explore innovative methods aimed at transforming research in health economics, health care delivery, and health policy. Although the ICTSI is designed to meet all of the traditional goals of a CTSA award such as training and career development, providing research infrastructure to academic investigators including the Clinical Research Center (CRC), and community engagement programs, this report will specifically focus on some of the programs that illustrate our approach to developing successful public–private partnerships to advance translation research and training.
Figure 1.
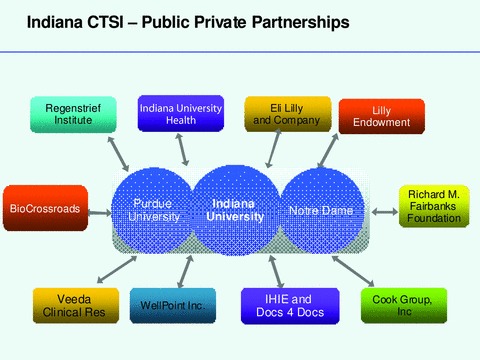
His schematic diagram illustrating some of the Public–Private partnerships of the Indiana CTSI. Regenstrief Institute—a nonprofit organization and nationally recognized medical informatics research; Indiana University Health—Indiana’s larget and the one of the nation’s largets hospital systems; Eli Lilly—large pharama based in Indianapolis; Lilly Endowment—one of the nation’s largest philanthropic organizations; BioCrossroads—Indiana’s economic development initiative; Richard M. Fairbanks Foundation—an Indianapolis based philanthropic organization; Veeda Clinical Research—a global CRO; WellPoint—the nation’s larget health insurer; IHIE—Indiana Health Information Exchange; Docs4Docs—a coomercial messaging system delivering test results to physician offices across Indiana; Cook Group—one of the nation’s largest device companies.
From the beginning, ICTSI was conceived as a partnership between academia and private stakeholders and many of our private partners were included in the earliest meetings as we were coalescing to write the grant. This early writing period spanned nearly a year of biweekly meetings where many of the representatives from our business and private partners were regular participants. These intense interactions lead to some key insights about what it takes to build successful public private partnerships:
-
1
Listen and understand each others’ perspectives and priorities to create meaningful communications with potential partners.
-
2
Identify mutually beneficial areas of collaboration all the while being sensitive to issues of ownership, conflicts of interest and intellectual property rights.
-
3
Develop clearly defined sets of goals, deliverables and metrics for the collaborative programs.
-
4
Engage the key personnel from all sides in leadership roles for the program.
Based on these principles, we have developed and are still developing a number of partnerships. We will describe examples of the major Academic‐Private Collaborations of the Indiana CTSI in areas of building research infrastructures and training physician scientists.
Many of ICTSI’s public–private collaborations span the full range of T1 through T4 translation areas. The concept of Translational Sciences that is envisioned for the ICTSI supports the smooth flow of ideas and discoveries along a series of steps within a “Translational Cycle” (see Figure 2). It starts with studies that take “bench” findings to controlled clinical trials at the “bedside” (Translation level 1); then moves to studies that test results obtained in controlled discovery settings to larger patient populations (Translation level 2); which leads to studies of translation of such findings of clinical effectiveness into practice across communities (Translation level 3); and finally bringing the knowledge gained from responses back to the “laboratory” for further study and refinement (Translation level 4), thus creating the reiterative process of transformation. The following are a few examples of the partnerships across this translational cycle.
Figure 2.
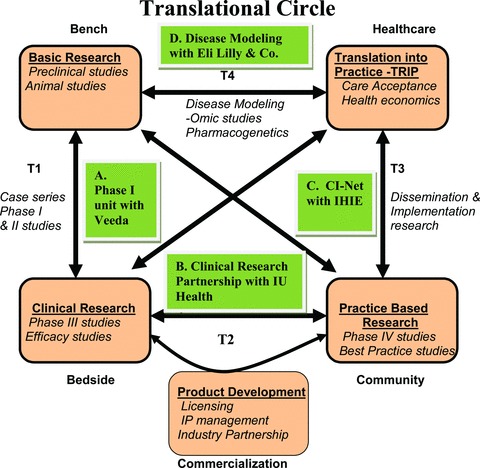
The schematic diagram represents the concept of the translational circle beginning from the bench, progressing through to the bedside, then community and eventually coming back as new knowledge for further research. Also shown are the four exemplary public–private partnerships of the Indiana CTSI at each of these steps.
T1 Translation: developing a phase I unit with a commercial clinical research organization (CRO)
One of the obstacles for translating many discoveries from academic laboratories into effective therapies is the inability to access a fully good clinical practice (GCP) compliant, commercial grade unit by academic investigators to conduct phase I studies that satisfy FDA registration. Currently these studies have to be conducted in commercial units that are expensive, requiring significant commercial fund raising that is beyond the scope of most academic researchers. This resource shortage is particularly acute in areas such as oncology where even early phase studies have to be conducted in patients with the relevant disease; even large established pharmaceutical companies are finding this to be a bottle neck for translation. Thus, there is great need for academic center based, commercial grade phase I units that enroll severely ill patients in phase I trials to discover new therapies rapidly. On the other side, from the CTSI perspective, while most CRCs run by CTSA institutions conduct clinical trials in patients, they do not typically have the financial resources to maintain the infrastructure to conduct GCP compliant, FDA registration phase I studies. Many of the CRCs are struggling even to recoup minimal costs back from the standard clinical studies they are supporting. Thus, a mutually beneficial approach may be to partner with a commercial CRO and large pharmaceutical companies to create a phase I unit at an academic medical center.
The ICTSI has been developing such a program to create a Phase I unit with an oncology focus at IU Hospital. We have partnered with a commercial CRO, Veeda Clinic Research/Veeda Oncology, which is a full service global CRO specializing in the early clinical development of drugs. Veeda has state‐of‐the‐art facilities in the United Kingdom, India and Malaysia. Veeda provides a full range of services in phase I and IIa clinical research, has won Frost and Sullivan’s “Partner of Choice” for phase I studies in 2007 and is a current contract vendor to Eli Lilly globally. Veeda brings CRO management expertise and an established global brand with a business development infrastructure that can be used to support the new clinic’s operations. This would be their first US‐based unit and their first academic partnership in the United States. We then specifically engaged the IU Simon Cancer Center researchers and physicians who wish to test novel therapies they would like to make available to their patients. These physicians have access to patients with cancer, an important and unique “asset” for early phase clinical studies, particularly in oncology. Thirdly, we brought in the hospital management to work out the space and service lease agreements. Fourth component of this was to engage a sponsor who could begin placing studies in such a unit. Here, we have been supported strongly by the Lilly oncology discovery group who see the benefits of creating an accessible, high quality unit like this near their headquarters in Indianapolis.
Finally, BioCrossroads, Indiana’s initiative to help grow Indiana’s life sciences economy, which provides commercial investment and grant funding to emerging life science opportunities, launches new life sciences businesses, and forms and expands collaborations and partnerships among Indiana’s life science institutions, played critical facilitator role in developing this program. For example, BioCrossroads provided a full time project manager to develop the phase I unit related business venture with ICTSI. Thus, by connecting a variety of key constituents with mutually beneficial goals, we are creating a unique model of a CRC. The primary focus will be to conduct trials of novel therapies. However, we have also designed the unit to have excess capacity so that many of the NIH clinical studies at IU School of Medicine can be conducted for minimal costs, providing significant relief to the traditional CRC, which is always resource constrained.
T2 Translation: creating clinical research infrastructure with Indiana University Health, the state’s largest hospital system
Since early 2010, Clarian Health Partners, the state’s largest and one of the nation’s largest hospital systems has been working with the IU School of Medicine to form what is now called IU Health. IU Health includes IU Hospital, Methodist Hospital, Riley Hospital for Children, Clarian West and North Hospitals, and 20 other hospitals statewide. This network has provided an exceptional opportunity for ICTSI to partner with IU Health and facilitate clinical research across Indiana. The leadership of IU Health has made research and education two of the key metrics of success for the entire health system. Through joint strategic planning with IU School of Medicine, IU Health will invest significant resources to help recruit patients into clinical trials, assist with biological specimen collection and create a single IRB infrastructure that will eventually cover all hospitals statewide. The CEO of IU health, Dan Evans has laid out a policy where predefined milestones for each hospital facility demonstrating research support such as the number of patients enrolling in clinical trials and donating biological specimens for research would in part determine the financial compensation of the hospital CEO and senior administrators.
T3 Translation: creating patient‐centered research capabilities with Regenstrief Institute and Indiana Health Information Exchange (IHIE)
The ICTSI, in collaboration with Regenstrief Institute, a private nonprofit organization that has been a national leader in medical informatics, has established the Central Indiana Innovation Network (CI‐Net) to provide researchers access to the Indiana CTSI’s integrated informatics network of networks found throughout the state of Indiana. The central goal of CI‐Net is to enhance subject recruitment from participating practices into a wide variety of studies by importing registration and contact data and upcoming appointments from registration and scheduling systems. Patients eligible for study recruitment are approached by research assistants who are anchored within and affiliated with each practice. This approach works extremely well for large practices with large numbers of patients; two‐thirds of study participants have been minorities. However, Indiana has thousands of smaller practices whose physicians are often interested in having their patients participate in the latest clinical research involving innovative interventions. Yet, it is not feasible to have a research assistant at each of these smaller practices.
To communicate with both small and large practices, Regenstrief Institute has developed a clinical messaging system called the DOCS4DOCS® (D4D) Service. This system, run by the not for profit business entity called Indiana Health Information Exchange (IHIE) in conjunction with Regenstrief Institute, delivers laboratory and other clinical results from the participating data sources (e.g., a hospital’s laboratory) to the intended responsible provider. Results can also be transmitted via Health Level Seven (HL7) directly to a practice’s electronic medical record or via fax. The D4D Service delivers electronic copies of discharge summaries, operative notes, EKGs, and radiology reports from over 35 hospitals in Indiana, and more are being added. Over 36 million messages have been delivered to nearly 19,000 physicians throughout Indiana.
The D4D System provides Regenstrief/IHIE with a direct connection to the physician’s practice. It is also a channel that can be used to provide other services. For example, Regenstrief Institute is currently developing the capability to annotate a laboratory report with patient‐specific reminders or comments about a new research result that may apply. Regenstrief Institute delivers adaptive turnaround documents to physicians that allow these physicians to provide feedback, complete forms within D4D, and deliver completed and verified forms to applications that can then extract data. Additionally, discussions with public health officials are underway to explore how the D4D Service could be used for public health alerts. The capability for a notification to all practices is currently built into the system and is used to make various announcements and deliver information to practices. D4D has a 24/7 Helpdesk to support the needs of practices. We are now expanding CI‐Nets reach to the 19,000 physicians in smaller practices; we posit this will greatly increase the yield of subject recruitment for clinical research in real world settings. We will develop an informatics‐based solution to enable access to engage the practices in D4D. This connectivity with a large number of physicians’ offices also creates an ideal platform for conducting “real life” comparative effectiveness research of many standard treatment protocols. By connecting all of the practices in IU Health to clinical trials and naturalistic health outcome studies, this program also provides a natural link to T2 and T3 research activities across our statewide health care system.
T4 Translation: training program in disease and quantitative modeling with Eli Lilly
We partnered with Eli Lilly to create a unique career development program for young faculty titled “Disease and Therapeutic Response Modeling Program.” The training curriculum was designed to generate quantitative models of disease and drug effect that can be used to catalyze the movement of new diagnostics and therapies along the translational continuum. This was organized as a career development academic program designed to train K scholars in the use of quantitative modeling tools to improve the effectiveness of translational medicine. Specifically, we expect that the models of disease, disease‐progression, and therapeutic effect generated by the faculty and fellows trained in this program will provide a unique body of data. These would include data that can be refined and improved over time to aid in the translation of multiple potentially valuable biomarkers of therapeutic effect into the clinic and to improve the rate of translation of targeted therapeutic agents.
The program involved identifying three major areas of mutually beneficial goals: increase both partners capability of disease state modeling by training individuals in specific competencies; develop major disease‐state and disease‐progression models; and make these models publicly available throughout the CTSI network. This would be a major benefit to the pharmaceutical industry in general, and was an attractive win‐win scenario for Eli Lilly and ICTSI. This new program was designed to bring the science of quantitative modeling to our efforts to translate new tests and therapies from the laboratory into the clinic and to the community. Its ultimate purpose is to catalyze effective translational medicine through the rigorous application of quantitative models at each stage of the translational process within the ICTSI. Implicit within this effort was the drive toward more personalized medicine using biomarkers that characterize disease severity and/or therapeutic response and particularly pharmacogenomics to target genetically identifiable response populations. We predict that the new models we develop in the laboratory, in the CRC and in the community will be used to improve existing and future therapies by generating the data that enable targeting them to susceptible populations and even individual patients who are likely to achieve the most benefit with the least adversity from specific treatments. One nationally recognized example of such a genetic information based recommendation to not use a treatment was the finding by Dr. David Flockhart at IUSM that some patients with breast cancer who have a certain gene do not benefit from a commonly used drug tamoxifen.
This program was made possible by institutional commitment from IU School of Medicine and Eli Lilly. Specifically, Eli Lilly agreed to support three new training positions per year and the recruitment of one new faculty member to direct the program who is supported fully for the first three years. The financial commitment by Lilly is approximately $1 million per year matched by in kind and other support from the CTSI. Work space for faculty and fellows is provided primarily at the IU School of Medicine in the Division of Clinical Pharmacology and flexible space is available at Lilly as well so that fellows will be able to regularly interact with faculty from both institutions. Eli Lilly and IU School of Medicine will collaborate to provide appropriate infrastructure that supports modeling and simulation analysis computing time. In addition, there is support for some pilot modeling projects by the fellows in collaboration with supervising faculty.
Partnership with philanthropy: physician scientists program with Lilly endowment
Another major partnership that has boosted the mission of the Indiana CTSI is a grant of $60 million that the Lilly Endowment provided at the end of 2009 to the IU School of Medicine (IUSM) to create a Physician Scientist Program. The major portion of these funds is to help recruit at least 20 physician scientists to IUSM. Another $10 Million will be utilized as an endowment for the NIH‐funded Medical Scientist Training Program (MSTP). This extraordinary support from the Lilly Endowment was guided by their keen awareness of the national crisis of the ever decreasing number of clinician‐scientists and the need to train, attract and retain physician scientists in Indiana. This was a mutually important mission to both IUSM and the Endowment. These discussions with the Endowment occurred under the leadership of Dr. D. Craig Brater, Dean, and Dr. David Wilkes, the Executive Associate Dean for Research at IUSM, who were able to convey this message of mutually important goals and convinced the philanthropic organization that it can be accomplished with a partnership. In addition, about 8 million will support a robust biorepository under the auspices of the Indiana CTSI. This ongoing longitudinal collection of biological specimen connected to phenotypic annotations and electronic medical record will be lasting resource for future physician scientists.
While these are some of the unique partnerships with Indiana CTSI, there are a number of other partnerships that are not listed here. For example, Richard M. Fairbanks Foundation is another philanthropic organization based in Indianapolis that has partnered with ICTSI toward enhancing health care and research. It has awarded grants of over $2.4 million to enhance the CTSI’s community activities and administrative operations. In summary, partnering with private corporations and philanthropic organizations is an important step in creating a successful CTSI. These partners bring not only enormous local resources to facilitate translational research, but also provide help setting clear goals and deliverables that are guided by their business practices. This level of discipline and metrics based resource allocation makes the CTSI much more effective in accomplishing its mission.
Acknowledgments
This publication was made possible, in part, with support from the Indiana Clinical and Translational Sciences Institute funded, in part by Grant RR025761 from the National Institutes of Health, National Center for Research Resources, Clinical and Translational Sciences Award.


