Abstract
In a subanalysis on the metformin, arterial function, intima‐media thickness, and nitroxidation in the metabolic syndrome (MEFISTO)8 (an open‐label fashion, with 1 year of 850 mg daily of metformin) subjects’ samples, we measured the paraoxonase 1 (PON1) activity in 39 patients that finished the study and relate values with high density lipoprotein (HDL). The comparative PON1 activities at the beginning and at the end of the study were 5.528 ± 0.588 and 4.743 ± 0.619 nmol/mg protein/min (NS) for control group and 3.229 ± 0.403 and 5.135 ± 0.585 nmol/mg protein/min (p < 0.02) for the metformin group. Our data showed an enhance of PON1 activity in patients with metabolic syndrome treated with metformin, although in them, the raise of HDL concentration was less than control patients, suggesting that the increase in quality (measured here as PON1 activity) could be at least as important as an increase in its concentration. Our results point out that there is a relationship among PON1 activity and the reduction of carotideal intima‐media thickness. Clin Trans Sci 2012; Volume #: 1–4
Keywords: metabolic syndrome, metformin, paraoxonase, HDL cholesterol, carotid intima‐media thickness
Introduction
Low levels of high density lipoprotein (HDL) cholesterol (HDL‐C) are involved in the development of atherosclerosis, mainly impairing the reverse transport of cholesterol, disrupting normal endothelial function and increasing the oxidative processes in subendothelial trapped lipoproteins. 1 Hypoalphalipoproteinemia is the most common lipid disorder found in Mexico nowadays, isolated or associated to hypertrygliceridemia. 2 On the other hand, overweight and obesity affect more than 75% of the adult urban population of both genders in Mexico, 3 increasing the prevalence of diabetes mellitus type 2, high blood pressure, and cardiovascular diseases. 4 In a country with high prevalence of both diabetes and obesity, 5 is not surprising the great prevalence of the so‐called metabolic syndrome (MS), 6 a cluster of cardiometabolic abnormalities, including lipid triad, the combination of increased levels of triglycerides and LDL cholesterol (LDL‐C), as well as low levels of HDL‐C. 7
Recently, we reported the results of the metformin, arterial function, intima‐media thickness, and nitroxidation in the metabolic syndrome (MEFISTO) study, demonstrating the effects of a modest dose of metformin on several cardiometabolic and nitroxidation variables in patients with MS. 8 Contrary to many other previous observations, we did not find any convincing effect of the drug on lipid profile, including the level of HDL‐C. However, the study showed a small but statistically significant reduction of carotid intima‐media thickness (c‐IMT) along with a remarkable reduction of nitroxidation molecule markers. 8 It is already established that increasing the amount of HDL‐C is not the only important therapeutic target, but also its functionality (its ability to induce reverse transport and antioxidation). 9 The latter function of HDL‐C is mainly due to several antioxidant enzymes like paraoxonase 1 (PON1), platelet activating factor acetylhydrolase, and glutathione peroxidase. 1 All of them inhibit the oxidation of LDL‐C, and its biodegradation and formation of molecules that activate endothelial cells, phenomenon which promotes the adhesion, recruiting and transmigration of monocytes in the vessel wall, the first step leading to atherosclerosis development. 10 Looking for an explanation of the reduction of IMT observed in the MEFISTO patients, in the absence of significant lowering of LDL‐C or major increases in HDL, we performed a subanalysis, measuring the PON1 activity in both the control and metformin‐treated groups.
Materials and Methods
The characteristics of the MEFISTO study have been described elsewhere. 8 In summary, the study enrolled patients with at least three of the Adult Treatment Panel III (ATP III) criteria for the diagnosis of MS, 11 without clinical evidence of vascular diseases. Sixty patients were randomly assigned in two groups receiving the same dietary and exercise counseling as well as antihypertensive medication, if needed, with a modulator agent of the renin angiotensin system. One group, medicated in an open‐label fashion, with 850 mg daily of metformin was the experimental one, whereas the other served as control. BMI, waist circumference, blood pressure, serum lipids, fasting glucose, nitroxidant metabolites (free carbonyls, malondialdehyde, dityrosines, and protein oxidation advanced products), nitrates, C reactive protein, and c‐IMT and vascular stiffness were determined in all patients, at the beginning of the study and after 1 year of follow‐up. Exploring the possibility that metformin could enhance the quality of HDL‐C, we measured the activity of PON1 in 39 patients that finished the study (17 patients in the control group and 22 in the metformin‐treated group), as follows: the rate of formation of p‐nitrophenol was measured using 1 mmol/L paraoxon in 50 mmol/L glycine buffer, pH 10.5, with 1.0 mmol/L CaCl2, 1 mol/L NaCl. The reaction was initiated by 20 μL of diluted plasma (1:20 in 25 mmol/L triethanolamine‐hydrochloride, pH 7.4, 1.0 mmol/L CaCl2) to 360 μL working reagent. The rate of p‐nitrophenol formation was measured at 405 nm over 200 seconds with a 25 seconds lag time. 12 The activity was expressed in nmol/mg protein/min. These results were correlated with the HDL value of each patient (index PON1 activity/HDL) and finally, this index was in turn normalized by the basal PON1/HDL value (control), to obtain the percent of change. Finally, to correlate the reduction of IMT with the PON1 activity, the values of the index IMT/PON1 activity were estimated at the beginning and at the end of the study.
Results
As previously described, 8 in both groups minor reductions in BMI, blood pressure, LDL‐C, and triglycerides were noticed. Contrariwise, although in the intergroup comparison, HDL‐C was similar in both groups at the end of the study, it increased more in the control than in the metformin group (basal vs. final values). Metformin reduced significantly nitroxidation marker molecules, mainly free carbonlys, reactive agents involved in the formation of advanced glycation end‐products and, in protein oxidation and tissue damage and inflammation. 13 The effect of metformin on carbonyls was one of the main findings of this work, as well as a small but significant reduction in c‐IMT. 8
In Table 1 are shown the data of HDL values, the comparative PON1 activity at the beginning and at the end of the study, as well as the individual normalized indices (PON1 activity/HDL) and the percentage of change (PON1 activity/HDL/basal values) at the beginning and at the end of the study. Figure 1 shows the changes in the PON1 activity/HDL indices, comparing the basal versus final values, in both control and metformin groups. This activity did not change significantly in controls, but increased ostensibly and significantly in metformin treated patients. Figure 2 shows a modest and no significant increase of 14% in control group and a remarkable 96% (p 5 0.02) increase in the metformin group.
Table 1.
Paraoxonase activity and HDL‐C in patients with metabolic syndrome.
| Control group | Metformin group | ||||
|---|---|---|---|---|---|
| Base | 1 year | p | Base | 1 year | p |
| Paraoxonase activity (nmol/mg protein/min) | |||||
| 5.528 ± 0.588 | 4.743 ± 0.619 | NS | 3.229 ± 0.403 | 5.135 ± 0.585 | 0.002 |
| Paraoxonase activity/HDL | |||||
| 0.1110 ± 0.08369 | 0.1023 ± 0.08111 | NS | 0.08003 ± 0.05092 | 0.1216 ± 0.06705 | 0.028 |
| Percentage of paraoxonase activity/HDL/control | |||||
| 100% | 114.1 ± 105% | NS | 100% | 196.7 ± 119% | 0.028 |
| HDL‐C | |||||
| 41.9 ± 7.49 | 50.1 ± 9.3 | 0.004 | 41.17 ± 10.94 | 44.96 ± 11.5 | NS |
Figure 1.
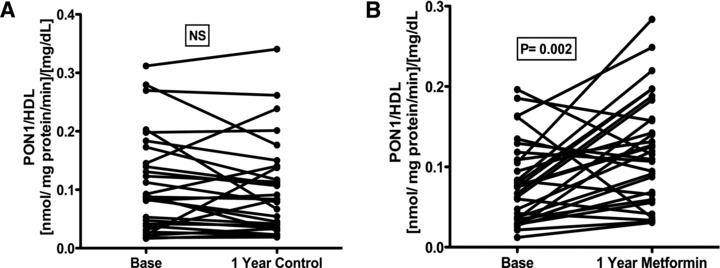
Analysis of the relation PON1 activity/HDL. (A) Control group, (B) metformin group. Significant differences (paired t‐test) were observed base versus after 1 year of treatment with metformin.
Figure 2.
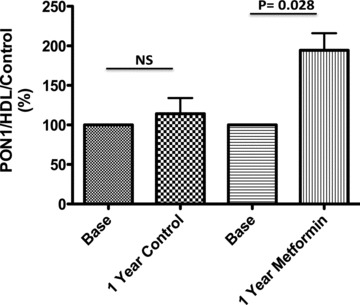
Analysis of the relation PON1 activity/HDL/Control values. In control and metformin group, each subject was normalized against basal values. Significant differences were observed base versus after 1 year of treatment with metformin.
Figure 3 shows the relationship between IMT/PON1 in both control and metformin treated groups at the base and at the end of the study. Although in controls no significative change of this index was noticed, in metformin treated subjects, it was significantly reduced (p= 0.002).
Figure 3.
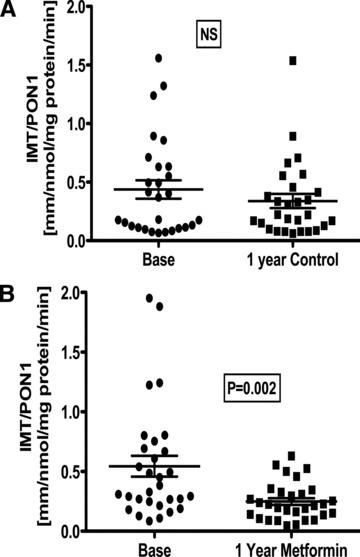
Analysis of the relation carotid Intima‐media Thickness (IMT)/PON1 activity. (A) Control group, (B) metformin group. Significant differences (paired t‐test) were observed base versus after 1 year of treatment with metformin.
Discussion
The family of paraoxonases (PON1, PON2, and PON3) are lactonase enzymes involved in the inactivation of organophosphates (in fact, the enzyme took its name because its capacity to hydrolyze paraoxon, a toxic compound of the well‐known insecticide parathion), but also play important roles in drug metabolism and on the inhibition of lipid oxidation. 14 , 15 PON1 is the main antioxidant enzyme contained in the HDL structure. 14 In fact, it has been proposed that the functionality of the enzyme is more important as an antiatherogenic mechanism than genotype characteristics. 14 Several concomitant factors can increase or decrease PON1 activity. Alcohol consumption, content of polyphenols and monounsaturated oils in the diet, and antioxidant vitamins can enhance PON status, whereas diabetes mellitus, advanced age, cigarette smoking, and great amount of saturated fat cause the opposite effect. 15 Recently, some reports have been published concerning the salutary effect of metformin in PON1 activity in VIH patients with lipodistrophy 16 and polycystic ovary syndrome. 17 Our data showed a striking enhance of PON1 activity in patients with MS treated 1 year with metformin, although in them, the raise of HDL concentration was less than control patients, suggesting that the increase in quality (measured here as PON1 activity) could be at least as important as an increase in its concentration. On the other hand, our original data showed a reduction of carotideal IMT, a marker of subclinical atherosclerosis, which predicts the development not only of carotid atherosclerotique plaque, but also cerebrovascular and coronary outcomes. 18 , 19 Results from the present work point out that there is a relationship among PON1 activity and the reduction of IMT. We believe that this fact reinforces the possible explanation of the vascular protective effect of metformin, recently described by others. 20 Altogether, the striking effect on carbonyl stress (inhibiting the generation of proinflammatory and profibrotic AGEs) and the enhancement of PON1 activity observed in metformin treated patients can explain the effect of metformin on subclinical atherosclerosis.
Acknowledgment
Source of Funding
This work was supported by Conacyt‐Mexico grant #129889.
References
- 1. Assman G, Gotto AM, Jr . Atherosclerosis: evolving vascular biology and clinical implications. HDL cholesterol and protective factors in atherosclerosis. Circulation. 2004; 109: III‐8–III‐14. [DOI] [PubMed] [Google Scholar]
- 2. Aguilar‐Salinas CA, Gómez‐Pérez FJ, Rull J, Villalpando S, Barquera S, Rojas R. Prevalence of dyslipidemias in the Mexican National Health and Nutrition Survey 2006. Salud Pública Méx. 2010; 52(Suppl 1): 44–53. [DOI] [PubMed] [Google Scholar]
- 3. Meaney E, Lara‐Esqueda A, Ceballos‐Reyes GM, Asbun J, Vela A, Martínez‐Marroquín Y. Cardiovascular risk factors in the urban Mexican population: the FRIMEX study. Public Health. 2007; 121: 378–384. [DOI] [PubMed] [Google Scholar]
- 4. Sánchez‐Castillo CP, Velásquez‐Monroy O, Lara‐Esqueda A, Berber A, Sepúlveda J, Tapia‐Conyer R, James WP. Diabetes and hypertension increases in a society with abdominal obesity: results of the Mexican National Health Survey 2000. Public Health Nutr. 2004; 8: 53–60. [DOI] [PubMed] [Google Scholar]
- 5. Olaiz‐Fernández G, Rivera‐Dommarco J, Shamah‐Levy T, Rojas R, Villalpando‐Hernández S, Hernández‐Avila M, Sepulveda‐Amor J. Encuesta Nacional de Salud y Nutrición 2006. Cuernavaca , México : Instituto Nacional de Salud Pública; 2006. [Google Scholar]
- 6. Rojas R, Aguilar‐Salinas CA, Jiménez‐Corona A, Shamah‐Levy T, Rauda J, Ávila‐Burgos L, Villalpando S, Lazcano E. Metabolic syndrome in Mexican adults. Results from the National Health and Nutrition Survey 2006. Salud Pública Méx. 2010; 52(Suppl 1): 11–18. [DOI] [PubMed] [Google Scholar]
- 7. Nesto RW. Beyond low‐density lipoprotein: addressing the atherogenic lipid triad in type 2 diabetes mellitus and the metabolic syndrome. Am J Cardiovasc Drugs. 2005; 5: 379–387. [DOI] [PubMed] [Google Scholar]
- 8. Meaney E, Vela A, Samaniego V, Meaney A, Asbún J, Zempoalteca JC, Zarate E, Mendoza E, Guzman M, Hicks JJ, et al Metformin, arterial function, intima–media, thickness and nitroxidation in metabolic syndrome: the MEFISTO study. Clin Exp Pharmacol Physiol. 2008; 35: 895–903. [DOI] [PubMed] [Google Scholar]
- 9. Nicholls SJ, Nissen SE. New targets of high density lipoprotein therapy. Curr Opin Lipidol. 2007; 18: 421–426. [DOI] [PubMed] [Google Scholar]
- 10. Navab M, Hama SY, Anantharamaiah GM, Hassan K, Hough G, Watson A, Reddy S, Sevanian A, Foranow G, Fogelman A. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: steps 2 and 3. J Lipid Res. 2000; 41: 1495–1508. [PubMed] [Google Scholar]
- 11. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001; 285: 2486–2497. [DOI] [PubMed] [Google Scholar]
- 12. Browne R, Koury S, Marion S, Wilding G, Muti P, Trevisan M. Accuracy and biological variation of human serum paraoxonase I activity and polymorphism (Q192R) by kinetic enzyme assay. Clin Chem. 2007; 53: 310–317. [DOI] [PubMed] [Google Scholar]
- 13. Alison Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products sparking the development of diabetic vascular injury. Circulation. 2006; 114: 597–605. [DOI] [PubMed] [Google Scholar]
- 14. Gupta N, Gill K, Singh S. Paraoxonases: structure, gene plymorphism & role in coronary artery disease. Indian J Med Res. 2009; 130: 361–368. [PubMed] [Google Scholar]
- 15. Costa LG, Vitallone A, Cole TB, Furlong CE. Modulation of paraoxonase (PON1) activity. Biochem Pharmacol. 2005; 69: 541–550. [DOI] [PubMed] [Google Scholar]
- 16. Coll B, Van Wijk JPH, Parra S, Castro Cabezas M, Hoepelman IM, Alonso‐Villaverde C, de Koening E, Camps J, Ferre N, Raberlink T, et al Effects of rosiglitazone and metformin on postprandial paraoxonase‐1 and monocyte chemoattractant protein‐1 in human immunodeficiency virus‐infected patients with lipodystrophy. Eur J Pharmacol. 2006; 544: 104–110. [DOI] [PubMed] [Google Scholar]
- 17. Koçer D, Muhtaroulu S, Bayram F. Effects of metformin on malondialdehyde level and paraoxonase 1 activity in patients with polycystic ovary syndrome. Erciyes Med J. 2008; 30: 6570. [Google Scholar]
- 18. Cece H, Yazgan P, Karakas E, Karakas O, Demirkol A, Toru I, Aksoy N. Carotid intima‐media thickness and paraoxonase activity in patients with ankylosing spondylitis. Clin Invest Med. 2011; 34: E225. [DOI] [PubMed] [Google Scholar]
- 19. Hurst RT, Ng DW, Kendall C, Khandheria B. Clinical use of carotid intima‐media thickness: review of the literature. J Am Soc Echocardiogr. 2007; 20: 907–914. [DOI] [PubMed] [Google Scholar]
- 20. Yang W‐I, Lee S‐H, Ko Y‐G, Kang S‐M, Choi D, Ha J‐W, Honh M‐K, Chung N, Shim W‐H, Cho S‐Y, et al. Relationship between paraoxonase‐1 activity, carotid intima‐media thickness and arterial stiffness in hypertensive patients. J Hum Hypertens. 2010; 24: 492–494. [DOI] [PubMed] [Google Scholar]


