Abstract
Objective: To investigate the expression of neuropeptides and their receptors that play a role in cardiac homeostasis in the right atrium of nondiabetic and diabetic patients undergoing coronary artery bypass graft surgery.
Background: The cardioactive neuropeptides and their receptors investigated in this study were Neuropeptide Y (NPY), and its receptors, NPY Receptor1 (NPY1R), NPY Receptor2 (NPY2R), NPY Receptor5 (NPY5R) and Substance P (SP) and its receptor, Neurokinin1R (NK1R).
Methods: The gene and protein expression of NPY, NPY1R, NPY2R, NPY5R, SP and NK1R from the atrial tissue of 10 nondiabetic and diabetic patients undergoing coronary artery bypass grafting (CABG) was assessed by Q‐RTPCR, immunohistochemistry, Western blot, and ELISA.
Results: Gene expression of NPY2R, NPY5R, preproTachykinin A (SP gene), and NK1R and their respective protein expression were significantly reduced whereas that of NPY and NPY1R were unchanged in the right atrium of diabetic patients compared to nondiabetic patients.
Conclusions: These results demonstrate that the expression of neuropeptides and their receptors in the diabetic heart is significantly impaired, and may be the link between neuropathy and cardiac complications. Further studies are warranted to delineate pathophysiologic mechanisms associated with dysregulation of the cardiac neuropeptide system and the relationship to cardiac complications in diabetes. Clin Trans Sci 2011; Volume 4: 346–350
Keywords: neuropeptide, diabetes, diabetic neuropathy, heart
Introduction
Cardiac complications are a major cause of the excess mortality of diabetes and are associated with diabetic neuropathy, microvascular, and macrovascular complications. 1 , 2 The cardioprotective neuropeptides and their receptors such as Substance P (SP), its receptor, Neurokinin 1R (NK1R), Neuropeptide Y (NPY), and its receptors, NPY Receptor 1 (NPY1R), NPY Receptor 2 (NPY2R), and NPY Receptor 5 (NPY5R) are vasomodulators present in the peripheral and/or autonomic nervous system that may influence cardiac function or response to injury.
NPY is 36‐amino acid peptide produced by cleavage from a larger precursor, preproNPY. It is one of the most abundantly distributed neurotransmitters and vasoconstrictors in the central and peripheral nervous system. In the heart, the major sources of NPY are the sympathetic nerve endings. 3 In experimental models, NPY is also known to be released by the parasympathetic neurons of the intrinsic cardiac ganglia. 4 Following injury, autonomic sensory neurons also produce significant amounts of NPY. 5 Nonneuronal cells such as endothelial and smooth muscle cells also produce NPY. 6 , 7 NPY exerts its action through 6, G‐protein coupled receptors (GPCR) subtypes including NPY1R, NPYR2, NPY3R, NPY4R, NPY5R, and NPY6R. 8 NPY and its receptors NPY1R, NPY2R, and NPY5R appear to be involved in the pathophysiology of a number of diseases including diabetes, heart failure, hypertension, peripheral arterial disease, and congestive feeding disorders. 9 NPY has vastly differential effects, for example, when signaling through the combination of NPY2R and NPY5R, it plays an imperative role in ischemic revascularization and wound healing. 10 Alternatively, when NPY acts through its NPY1R and NPY5R receptors the result is cardiac hypertrophy and vasoconstriction of coronary vessels. 11
SP, an 11‐amino acid peptide functions as a vasodilator and neurotransmitter and is released from the autonomic sensory nerve fibers. It signals through Neurokinin 1 receptor (NK1R), a member of the tachykinin subfamily of GPCR. NK1R is expressed on a variety of cell types including epithelial cells, endothelial cells, glial cells, immune cells, and neurons. Experimental and clinical studies have demonstrated that SP expression is dysregulated in type 1 and 2 diabetes. 12 Pertinent to this study, recent reports have shown that SP enhances the release of nitric oxide, which blunts the norepinephrine effects thereby preserving heart function. 13
Cardiac complications in diabetes associated with neuropathy, 14 may be due to the dysregulation of the neuropeptide system. 5 The goal of this study is to investigate the gene and protein expression of NPY and SP and their receptors NPY1R, NPY2R, and NPY5R, and NK1R, respectively, in the atrial muscle of diabetic and nondiabetic patients undergoing CABG surgery.
Materials and Methods
Human subjects and tissue sampling
Atrial tissue samples were collected from 10 consecutive nondiabetic and diabetic patients undergoing on‐pump elective CABG surgery. All research involving human participants is approved by the Beth Israel Deaconess Medical Center Institutional Review Board. A written informed consent was obtained from the patients and data are analyzed anonymously. At the time of CABG, tissue flaps are routinely removed to insert the backflow tube of the bypass and during this cannulation a small piece of right atrium is routinely excised and discarded and this discarded tissue was collected for this study. Patient characteristics are summarized in Table 1. Patients undergoing any valve surgeries or with cancer were excluded from this study.
Table 1.
Patient characteristics.
| Nondiabetic | Diabetes | |
|---|---|---|
| (n = 10) | (n = 10) | |
| Age (median) | 65 (45–77) | 68 (53–76) |
| Diabetes status | 0 | 8 type‐2 diabetic |
| 2 type‐1 diabetic | ||
| % Hemoglobin A1C (median) | 5.4 (5.1–5.9) | 8.0 (6.9–8.6) |
| Hypertension | 8 | 10 |
| Myocardial infarction | 1 | 3 |
| Hyperlipidemia | 8 | 9 |
| Left ventricular ejection fraction(median) | 60 (45–75) | 51 (30–65) |
| Left ventricular hypertrophy | 1 | 0 |
| Kidney Disease | 2 | 8 |
| BMI >30.0 | 7 | 3 |
| Medications | ||
| Insulin | 0 | 7 |
| Insulin secretagogue | 0 | 2 |
| Metformin | 0 | 2 |
| Beta blockers | 6 | 4 |
| Calcium channel blockers | 0 | 2 |
| ACE inhibitors | 2 | 3 |
| Diuretics | 0 | 1 |
| HMG‐CoA reductase inhibitors | 8 | 9 |
Gene expression
Quantitative real‐time RT‐PCR (qRT‐PCR) was used to determine specific RNA transcripts from atrial muscle samples as previously described. 8 For quantitative analysis, target gene levels were normalized to β‐actin levels and target and β‐actin gene amplification reactions were performed using Brilliant SYBR Green QPCR Reagent (Stratagene, La Jolla, CA, USA) in triplicate for each cDNA sample using 1 μL cDNA per reaction. Primers were obtained from Super Array Biosciences, Frederick, MD, USA.
Immunohistochemistry (IHC)
Tissue samples were fixed in formalin and embedded in paraffin. IHC was performed as described previously 8 with primary antibodies for NPY, NPY1R, NPY5R, and NK1R (Abcam, Cambridge, MA, USA), NPY2R (Imgenex, San Diego, CA, USA) and SP (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). The IHC staining was quantified by Improvision Volocity (Perkin Elmer, Waltham, MA, USA) software. Data are presented in the form of representative pictures along with pixel count analysis.
Western blot
Western blotting was performed using standard techniques with commercially available primary antibody NK1R (Abcam). Densitometry was performed using NIH Image J 1.34 with protein levels corrected to β‐Actin (Sigma, St. Louis, MO, USA) levels. Data are presented in the form of representative pictures of the blot along with densitometric analysis.
ELISA
For measurements of SP expression in the atrial muscle, an SP ELISA kit was obtained from (R&D System, Minneapolis, MN, USA). Manufacturer’s protocol was strictly followed. Data are represented as fold change over the nondiabetic values in pg/mL.
Statistics
All quantitative data are expressed as fold change in the expression in the diabetic tissue compared to the nondiabetic tissue. All data were analyzed using unpaired t‐test on GraphPad Prism (GraphPad, La Jolla, CA, USA). Data are presented as means ± SD. The value p < 0.05 was considered significant.
Results
Gene expression of preproNPY (NPY gene), preproTachykinin A (SP gene), and their receptors, NPY1R, NPY2R and NPY5R, and NK1R respectively:
The gene expression of NPY2R (0.575 ± 0.252 vs. 1.526 ± 1.41, p < 0.05), NPY5R (0.51 ± 0.282 vs. 1.39 ± 1.14, p < 0.05), preproTachykinin A (0.158 ± 0.103 vs. 3.631 ± 5.7, p < 0.05), and NK1R (0.248 ± 0.211 vs. 3.532 ± 5.09, p < 0.05) was significantly reduced in the diabetic patients compared to that of the nondiabetic patients. The gene expression of preproNPY (0.757 ± 0.766 vs. 2.01 ± 2.48 p > 0.05) and NPY1R (1.315 ± 2.14 vs. 1.544 ± 2.20, p > 0.05) was not different between diabetic and nondiabetic patients, (Figure 1).
Figure 1.
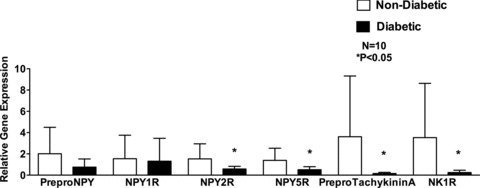
Gene expression of neuropeptides Y (preproNPY) and its receptors, NPY1R, NPY2R, and NPY5R and Substance P (preproTachykinin A), and its receptor NK1R in human right atrial muscle. Fold change in gene expression in diabetic patients compared to nondiabetic patients. Data are expressed as mean ± SD (N = 10).
Protein expression of NPY and its receptors, NPY2R and NPY5R:
As determined by IHC and similar to gene expression, protein expression of NPY2R (7.08×104 ± 7.92 × 104 vs. 1.82 × 105 ± 1.33 × 105, p < 0.05) and NPY5R (2.82 × 105 ± 2.59 × 105 vs. 5.67 × 105 ±3.87 × 105, p < 0.05) was significantly reduced whereas that of NPY (2.73 × 105 ± 1.95 ×105 vs. 4.12 × 105 ± 3.05 × 105, p > 0.05) and NPY1R (1.75 × 104 ± 7.03 × 103 vs. 1.94 × 104 ± 9.25 × 103, p > 0.05) was unchanged, in the diabetic patients compared to that of the nondiabetic patients (Figures 2A and B).
Figure 2.
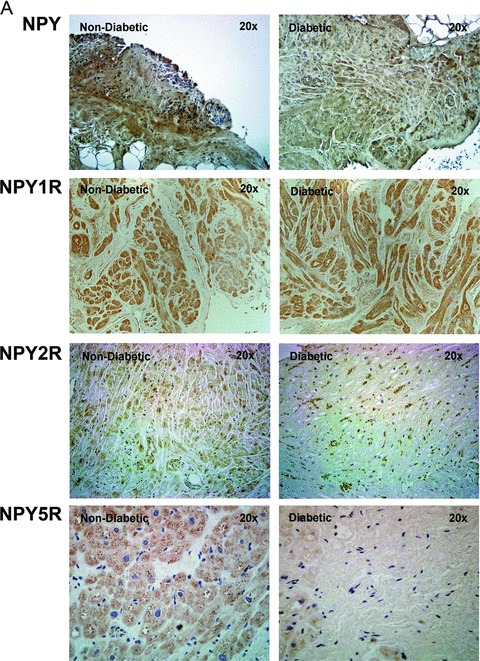
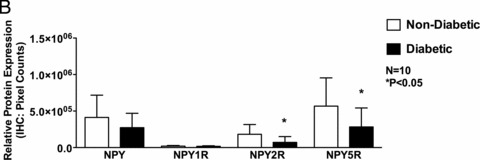
Protein expression of neuropeptides Y (NPY) and its receptor receptors, NPY1R, NPY2R, and NPY5R in human right atrial muscle. (A) Representative IHC images of NPY, NPY1R, NPY2R, and NPY5R of nondiabetic and diabetic atrial tissue (magnification 20 ×). (B) Quantification of NPY, NPY1R, NPY2R, and NPY5R IHC protein expression using Volocity software. Data are expressed as mean ± SD (N = 10).
Protein expression of SP and its receptor NK1R: Similar to gene expression, protein expression of SP (1.96 × 105 ± 2.98 × 105 vs. 1.17 × 106 ± 1.41 × 106, p < 0.05) and NK1R (2.42 × 105 ± 1.27 × 105 vs. 4.37 × 105 ± 3.05 × 105, p < 0.05) as determined by IHC was significantly reduced in the diabetic patients compared to that of the nondiabetic patients.
Changes in protein expression of SP when confirmed with ELISA (128 ± 49.43 pg/mL vs. 355.8 ± 102.5 pg/mL, p < 0.01) and of NK1R when confirmed with western blot (NPY (3.69 ± 1.89 vs. 8.497 ± 1.73, p < 0.01), once again showed a significant decrease in diabetic patients compared to nondiabetic patients (Figures 3A and B).
Figure 3.
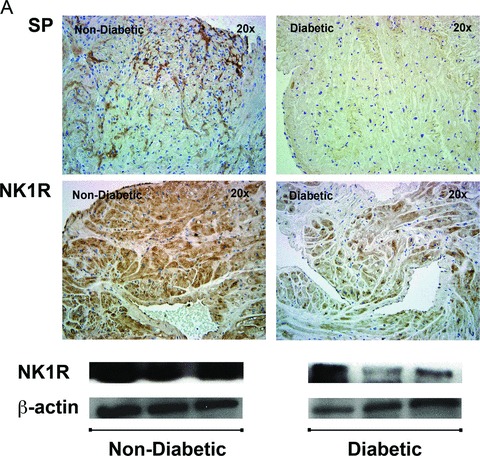
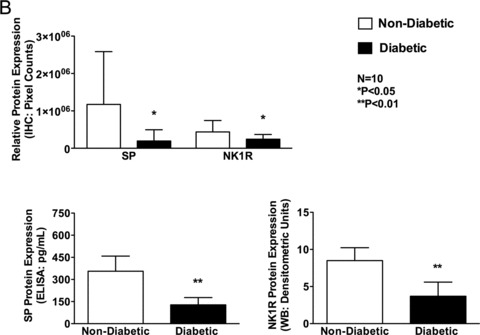
Protein expression of neuropeptides Y (NPY) and its receptor receptors, NPY1R, NPY2R, and NPY5R in human right atrial muscle. (A) Representative IHC images of NPY, NPY1R, NPY2R, and NPY5R of nondiabetic and diabetic atrial tissue (magnification 20 ×). (B) Quantification of NPY, NPY1R, NPY2R, and NPY5R IHC protein expression using Volocity software. Data are expressed as mean ± SD (N = 10).
Discussion
This study is focused on comparing the expression and translation of neuropeptides and their receptors in atrial muscle biopsies as related to diabetes status. These results, for the first time, clearly demonstrate that both gene and protein expression of SP (PreproTachykinin A) and its receptor NK1R and of NPY receptors, NPY2R and NPY5R, are significantly decreased in the right atrium of diabetic patients. Although there was a trend toward a reduction in NPY gene and protein expression in diabetic patients, this difference did not achieve statistical significance. The gene and protein expression of NPY1R was not different between the nondiabetic and diabetic patients.
The patient characteristics clearly indicate that there were no differences in medications that the diabetic and non‐diabetic patients were prescribed. Although there was no difference in the lipid profile and hypertension status, there were more diabetic patients who had kidney disease than non‐diabetic patients. Another interesting observation of this study is that the non‐diabetic patients had a higher BMI compared to the diabetic patients. The left ventricular ejection fraction was lower in diabetic patients compared to nondiabetic patients. Although we did not specifically assess patients for cardiac autonomic neuropathy (CAN), the reduced expression of neuropeptides in the diabetic atrial muscle maybe probably due in part to diabetic autonomic neuropathy. It has been shown that the prevalence of CAN is very high in diabetes and risk increases with longer duration of diabetes. 15 This is almost certainly true for SP, which is produced only by nerve endings and does not have a known non‐neuronal source.
In this study NPY expression is modestly reduced, whereas NPY2R and NPY5R expressions are significantly reduced with no change in NPY1R expression in the right atrium of diabetic patients compared to nondiabetic patients. The reason for the unchanged expression in NPY levels between diabetic and non‐diabetic patients might be due to the production of NPY from non‐neuronal sources such as the cardiomyocytes. Therefore, although CAN is associated with reduction and/or loss of neuronal activation in diabetes, its effect on NPY neuronal loss could be overridden by the local extra‐neuronal production of NPY. 16 – 18 NPY1R is expressed throughout the atria in humans and in rodents. In rodents, it is the most abundant of the NPY receptors whereas its distribution in the human heart is not clear. 5 , 19 NPY, acting via its NPY1R plays a pathogenic role by inducing cardiac hypertrophy and vasoconstriction of coronary vessels. 11 , 20 , 21 , 22 , 23 , 24 In this study, NPY1R expression is unchanged in the right atrium of the diabetic patients compared to nondiabetic patients and it does not seem to be the dominant of NPY receptors in the human right atrium. Moreover, in this study only one nondiabetic patient presented with left ventricular hypertrophy suggesting that NPY1R did not play a significant role in the right atria of these patients irrespective of their diabetic status. When NPY acts via the NPY2R receptor in concert with NPY5R receptor, NPY induces angiogenesis and this mechanism has been shown to be important for ischemic revascularization and wound healing. 25 In the atria, NPY2R is expressed on the intrinsic cardiac NPY producing neurons where it acts as a prejunctional inhibitory feed back receptor, inhibiting norepinephrine release. Thus, although NPY2R expression is more abundant in the atria due to the presence of intrinsic neurons, the feedback mechanism that it exerts can play an important role in ventricular function. 5 Given these mechanisms by which NPY2R and NPY5R exert a protective effect in the heart, any change, especially a reduction in NPY2R and NPY5R expression may alter the ventricular function.
The expression of SP and its receptor NK1R is significantly reduced in the atrial muscle of diabetic patients compared to that of nondiabetic patients. Clinically, it has been shown that serum SP level is significantly decreased in diabetic patients. 26 The reduced expression of SP and NK1R in diabetes may be due to the impairment in the sensory nervous system. In the heart, SP is released from the afferent nerves and diabetic neuropathy damages the myocardial sensory afferent fibers, leading to the impaired expression of SP. 17 SP preserves heart function via the release of nitric oxide and this is the only known mechanism through which SP affects the heart. 13 During myocardial ischemia, transient receptor potential vanilloid type 1 leads to the release of SP, which can cause coronary vasodilatation and protect the heart from injury. 27 Thus, any change in SP release and its receptor expression in diabetes can result in impaired cardiac response to ischemia.
This impaired expression of SP and its receptor and NPY receptors in diabetes may result in impaired ischemic revascularization, 11 impaired contractility, 28 and dysregulation in protein kinase C (PKC) activation. 29
In summary, the gene and protein expression of SP, and SP and NPY receptors are significantly impaired in diabetes, most probably due to CAN. This study is observational and whether the changes we find are a direct cause of the excess cardiac morbidity and mortality of diabetes has yet to be determined. This work justifies and encourages delineating the relationship between neuropeptide function and cardiac complications in diabetes.
Acknowledgment
This work in part is supported by the William J. von Liebig Foundation funds to FWL. The authors have no conflict of interest.
References
- 1. Fonarow GC. An approach to heart failure and diabetes mellitus. Am J Cardiol. 2005; 96(4A): 47E–52E. [DOI] [PubMed] [Google Scholar]
- 2. Ninds FS. Peripheral Neuropathy. Publication No. 04–4853 , 2009, http://www.ninds.nih.gov/disorders/peripheralneuropathy/detail_peripheralneuropathy.htm. Accessed June 27, 2011.
- 3. Blomqvist AG, Herzog H. Y‐receptor subtypes–how many more? Trends Neurosci. 1997; 20(7): 294–298. [DOI] [PubMed] [Google Scholar]
- 4. Richardson RJ, Grkovic I, Anderson CR. Immunohistochemical analysis of intracardiac ganglia of the rat heart. Cell Tissue Res. 2003; 314(3): 337–350. [DOI] [PubMed] [Google Scholar]
- 5. Chottova Dvorakova M, Wiegand S, Pesta M, Slavikova J, Grau V, Reischig J, Kuncova J, Kummer W. Expression of neuropeptide Y and its receptors Y1 and Y2 in the rat heart and its supplying autonomic and spinal sensory ganglia in experimentally induced diabetes. Neuroscience. 2008; 151(4): 1016–1028. [DOI] [PubMed] [Google Scholar]
- 6. Jacques D, Sader S, Perreault C, Fournier A, Pelletier G, Beck‐Sickinger AG, Descorbeth M. Presence of neuropeptide Y and the Y1 receptor in the plasma membrane and nuclear envelope of human endocardial endothelial cells: modulation of intracellular calcium. Can J Physiol Pharmacol. 2003; 81(3): 288–300. [DOI] [PubMed] [Google Scholar]
- 7. Zukowska Z, Pons J, Lee EW, Li L. Neuropeptide Y: a new mediator linking sympathetic nerves, blood vessels and immune system? Can J Physiol Pharmacol. 2003; 81(2): 89–94. [DOI] [PubMed] [Google Scholar]
- 8. Pradhan L, Cai X, Wu S, Andersen ND, Martin M, Malek J, Guthrie P, Veves A, Logerfo FW. Gene expression of pro‐inflammatory cytokines and neuropeptides in diabetic wound healing. J Surg Res. 2009; 23: 336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pedrazzini T, Pralong F, Grouzmann E. Neuropeptide Y: the universal soldier. Cell Mol Life Sci. 2003; 60(2): 350–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meit Björndahl RC, Luxun Xue, Yihai Cao. NPY‐Induced Angiogenesis in Retinopathy and Wound Healing. Stockholm : Birkhäuser Basel; 2006. [Google Scholar]
- 11. Kuo LE, Abe K, Zukowska Z. Stress, NPY and vascular remodeling: implications for stress‐related diseases. Peptides. 2007; 28(2): 435–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Iwasaki H, Kajimura M, Osawa S, Kanaoka S, Furuta T, Ikuma M, Hishida A. A deficiency of gastric interstitial cells of Cajal accompanied by decreased expression of neuronal nitric oxide synthase and substance P in patients with type 2 diabetes mellitus. J Gastroenterol. 2006; 41(11): 1076–1087. [DOI] [PubMed] [Google Scholar]
- 13. Paulus WJ. The role of nitric oxide in the failing heart. Heart Fail Rev. 2001; 6(2): 105–118. [DOI] [PubMed] [Google Scholar]
- 14. Aneja A, Tang WH, Bansilal S, Garcia MJ, Farkouh ME. Diabetic cardiomyopathy: insights into pathogenesis, diagnostic challenges, and therapeutic options. Am J Med. 2008; 121(9): 748–757. [DOI] [PubMed] [Google Scholar]
- 15. Pappachan JM, Sebastian J, Bino BC, Jayaprakash K, Vijayakumar K, Sujathan P, Adinegara LA. Cardiac autonomic neuropathy in diabetes mellitus: prevalence, risk factors and utility of corrected QT interval in the ECG for its diagnosis. Postgrad Med J. 2008; 84(990): 205–210. [DOI] [PubMed] [Google Scholar]
- 16. Ralevic V, Aberdeen JA, Burnstock G. Acrylamide‐induced autonomic neuropathy of rat mesenteric vessels: histological and pharmacological studies. J Auton Nerv Syst. 1991; 34(1): 77–87. [DOI] [PubMed] [Google Scholar]
- 17. Pittenger G, Vinik A. Nerve growth factor and diabetic neuropathy. Exp Diabesity Res. 2003; 4(4): 271–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bolinder J, Sjoberg S, Persson A, Ahren B, Sundkvist G. Autonomic neuropathy is associated with impaired pancreatic polypeptide and neuropeptide Y responses to insulin‐induced hypoglycaemia in type I diabetic patients. Diabetologia. 2002; 45(7): 1043–1044. [DOI] [PubMed] [Google Scholar]
- 19. Jonsson‐Rylander AC, Nordlander M, Svindland A, Ilebekk A. Distribution of neuropeptide Y Y1 and Y2 receptors in the postmortem human heart. Peptides. 2003; 24(2): 255–262. [DOI] [PubMed] [Google Scholar]
- 20. Taylor JC, Yang HT, Laughlin MH, Terjung RL. Alpha‐adrenergic and neuropeptide Y Y1 receptor control of collateral circuit conductance: influence of exercise training. J Physiol. 2008; 586(Pt 24): 5983–5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nilsson T, Cantera L, Edvinsson L. Presence of neuropeptide Y Y1 receptor mediating vasoconstriction in human cerebral arteries. Neurosci Lett. 1996; 204(3): 145–148. [DOI] [PubMed] [Google Scholar]
- 22. Gullestad L, Bjuro T, Aaberge L, Apelland T, Skardal R, Kjekshus E, Nordlander M, Ablad B, Pernow J. The effect of a neuropeptide Y Y1 receptor antagonist in patients with angina pectoris. Eur Heart J. 2003; 24(12): 1120–1127. [DOI] [PubMed] [Google Scholar]
- 23. Ekstrand AJ, Cao R, Bjorndahl M, Nystrom S, Jonsson‐Rylander AC, Hassani H, Hallberg B, Nordlander M, Cao Y. Deletion of neuropeptide Y (NPY) 2 receptor in mice results in blockage of NPY‐induced angiogenesis and delayed wound healing. Proc Natl Acad Sci U S A. 2003; 100(10): 6033–6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zukowska‐Grojec Z, Karwatowska‐Prokopczuk E, Rose W, Rone J, Movafagh S, Ji H, Yeh Y, Chen WT, Kleinman HK, Grouzmann E, et al Neuropeptide Y: a novel angiogenic factor from the sympathetic nerves and endothelium. Circ Res. 1998; 83(2): 187–195. [DOI] [PubMed] [Google Scholar]
- 25. Pradhan L, Nabzdyk C, Andersen ND, LoGerfo FW, Veves A. Inflammation and neuropeptides: the connection in diabetic wound healing. Expert Rev Mol Med. 2009; 11(e2): 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kunt T, Forst T, Schmidt S, Pfutzner A, Schneider S, Harzer O, Lobig M, Engelbach M, Goitom K, Pohlmann T, et al Serum levels of substance P are decreased in patients with type 1 diabetes. Exp Clin Endocrinol Diabetes. 2000; 108(3): 164–167. [DOI] [PubMed] [Google Scholar]
- 27. Wharton J, Gulbenkian S, Mulderry PK, Ghatei MA, McGregor GP, Bloom SR, Polak JM. Capsaicin induces a depletion of calcitonin gene‐related peptide (CGRP)‐immunoreactive nerves in the cardiovascular system of the guinea pig and rat. J Auton Nerv Syst. 1986; 16(4): 289–309. [DOI] [PubMed] [Google Scholar]
- 28. Khawaja AM, Rogers DF. Tachykinins: receptor to effector. Int J Biochem Cell Biol. 1996; 28(7): 721–738. [DOI] [PubMed] [Google Scholar]
- 29. Sieburth D, Madison JM, Kaplan JM. PKC‐1 regulates secretion of neuropeptides. Nat Neurosci. 2007; 10(1): 49–57. [DOI] [PubMed] [Google Scholar]


