Abstract
Although stem cell therapy is not a new field, the field was limited to transplantation of hematopoietic stem cells. Such transplantation has provided invaluable information for the emerging field with new stem cells. Mesenchymal stem cells (MSCs) are an attractive source for therapy; reduced ethical concern, ease in expansion, as off‐the‐shelf stem cells. MSCs exert immune suppressive properties, providing them with the potential for immune suppressive therapy such as autoimmunity, asthma, allergic rhinitis and graft versus host disease. In addition, MSCs, as well as other stem cells, can be applied for bone and cartilage repair, cardiovascular disease, and neural repair/protection. The data thus far with MSCs are mixed. This review discusses the immune‐enhancing properties of MSCs to explain the possible confounds of inflammatory microenvironment in the MSCs therapy. Although this review focuses on MSCs, the information can be extrapolated to other stem cells. The review summarizes the biology of MSCs, including multilineage differentiation potential, transdifferentiation capability, and immunological effects. We emphasize the key concepts that may predict the use of these cells in medicine, namely, the application of these cells from the bench to the bedside. Prospects on immunotherapy, neuroregeneration, and cardiovascular repair are used as examples of tissue repair. Clin Trans Sci 2011; Volume 4: 380–386
Keywords: bone marrow, cytokines, graft versus host disease, stem cell transplantation
Mesenchymal Stem Cells (MSCs)
MSCs appear to comprise of heterogeneous cell subsets, as indicated by the varied functional outcome. A major advantage of studying MSCs is that they appear to be similar by phenotype and exhibit multilineage differentiation. The question is whether MSCs are indeed heterogeneous, or if the differences in functions are attributed to the source and/or the method of culture.
MSCs are ubiquitously present and can be isolated in both adult and fetal tissues. 1 , 2 , 3 MSCs are also found in the amniotic fluid with the highest frequency during the first trimester. 4 Stem cells from the placenta, although not designated as MSCs, seem to be comparable with the functions and phenotype of MSCs. 5 The frequency of MSCs in umbilical cord blood is low. 6 However, the Wharton jelly of umbilical cord appears to be a more “rich” source of MSCs. 6
In adults, bone marrow and adipose tissues are major sources of MSCs. In bone marrow, MSCs surround blood vessels, are in contact with the trabeculae and might be also located close to the endosteum. 2 , 7 The adipose tissue is a major source of MSCs, 8 making adipose tissues as a significant source of MSCs in future treatment. While some laboratories use MSCs from bone marrow, in other laboratories, investigations are reported with adipose‐derived MSCs. It is unclear if the source of tissues for propagating MSCs is linked to social issues of the country and/or other issues, such as the lack of collaborations with clinical personnel to obtain the bone marrow aspirates or adipose tissues. Regardless, the data from each source of MSCs will require careful consideration to determine if the source of MSCs can influence the cells’ functions. These types of analyses will be relevant for the effective translation of MSCs to patients. The discussed concern is underscored by a report showing differences in chondrogenesis between MSCs from the bone marrow and from adipose tissue. 9 However, there are reports that describe little differences in the functions of MSCs from bone marrow and adipose tissues. 10 We propose that robust studies are required to compare MSCs from bone marrow and adipose tissues. Answers to the functions of different sources of MSCs might not be limited to different tissues, but within areas of an organ. MSCs have been reported at regions close to the endosteum, in addition to regions surrounding blood vessels. Therefore, studies might be required to compare adipose‐derived MSCs with those from different regions of the bone marrow.
MSCs differentiate along distinct lineages to generate stroma, adipocytes, chondrocytes, osteogenic cells, and cartilage, among other lineages 11 ( Figure 1A ). Currently, expanded MSCs suggest that there are multiple subsets and each subset might be primed to form a distinct specialized cell. This premise is mostly based on the expression of specific differentiation genes. 12 These studies are relevant because it would be possible to select particular subsets of MSCs for specific cell therapy. For example, a population of CD146‐expressing subendothelial cells can form osteogenic and stromal cells, suggesting that they could be osteoprogenitors. 13 Since both osteoblast and stroma are hematopoietic‐supporting cells, the information on CD146‐expressing MSCs would be significant in clinical intervention for hematopoietic regulation, such as bone marrow transplantation.
Figure 1.
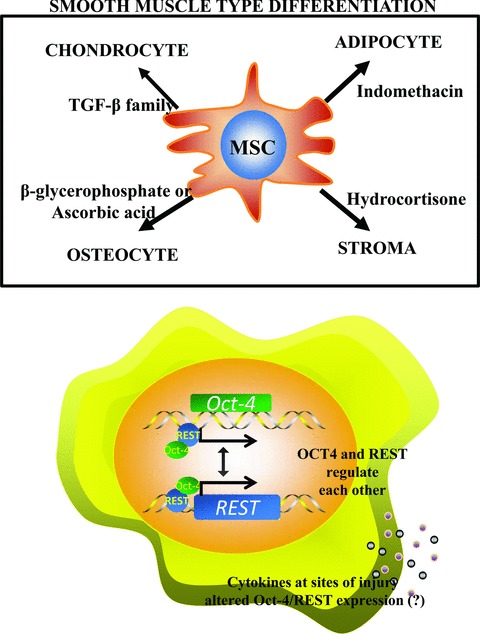
(A) MSCs show lineage differentiation to smooth muscle type cells, in the presence of distinct growth factors. (B) Shown is the maintenance of stemness by Oct4 and REST, regulating the expression of each other. Stemness can be challenged by cytokines, which could alter the expressions of REST and Oct4.
Morphologically, MSCs are symmetrical cells with fibroblastoid appearance. 14 Among the markers found on MSCs are, CD44, CD29, CD105, CD73, CD90, and CD166. MSCs do not express markers of hematopoietic cells such as CD45. 15 The embryonic origin of MSCs is unclear. Reports suggest that MSCs may be of mesodermal and neuroepithelial origins. 11 , 16 MSCs show structures comparable to smooth muscle and are generally referred as stem cells of mesodermal origin. The designation, neuroepithelial versus mesodermal, is fundamental to appreciate the limit of MSCs with regard to their ability to generate specialized cells. If the MSCs are indeed mesodermal, the ability to form neurons is significant because this underscores the cells’ plasticity to traverse germ layer: mesoderm to ectoderm; generally referred as transdifferentiation. On the other hand, if the source of MSCs is indeed neuroepithelial, this would explain the ease by which they form functional neurons and other neuronal cells. 17 , 18 , 19 , 20 , 21 , 22
A major concern for the clinical application of MSCs is their safety with regard to tumor formation and immune rejection. Since MSCs are already in the clinic, 23 , 24 the limited data suggest that these stem cells could be safe, and more importantly can be delivered across allogeneic barriers. 25 If MSCs can be used as off‐the‐shelf sources, this makes them particularly attractive for clinical application. In addition, MSCs can easily form other cell types, making them attractive for application to treat multiple disorders, to repair and protect tissues.
The potential use of MSCs in clinical application is linked to their immune properties. Although mostly considered as immunosuppressors, 14 , 26 MSCs can also exert immune‐enhancing properties. These two broad functions are expected to be at the forefront when MSCs are considered for any type of clinical application. Therefore, the next section has an expanded discussion on the biological properties of MSCs, with a focus on the cells; immune functions.
The results of early clinical trials for graft versus host disease with MSCs are mixed. The contradictory outcome of these trials cannot be explained. However, one can extrapolate, based on the properties biology of MSCs. Regardless of how MSCs behave in the laboratory, in vivo, these cells will be exposed to a milieu of factors within any microenvironment. This will cause functional crosstalk between the MSCs and mediators within the microenvironment. MSCs express several receptors for cytokines, such as interferon γ and TGF‐β and chemokines. 27 , 28 , 29 Their placement within a milieu of inflammatory mediators could result in predicted outcomes since MSCs are known suppressors of immune cell functions: dendritic, natural killer and T and B cells. 30 However, the activation of cytokine receptors is likely to activate MSCs for immune responses, such as antigen presentation and autoimmune responses. 27 , 31 , 32 Together, these findings suggest that MSCs and generated specialized cells might establish communications with inflammatory mediators within the areas of tissue damage through a network that encompasses cytokines and their receptors, in addition to the recruitment of other immune cells.
Immune Biology of MSCs
MSCs show functional plasticity with regard to their immune properties by exerting both immune suppressor and enhancer functions. 33 In addition, MSCs might be instructive cells to macrophages as a mechanism of tissue repair. 34 MSCs produce varied cytokines that can mediate autocrine and/or paracrine stimulation. 2 Dominci et al. 3 , 35 suggested major histocompatibility complex‐II (MHC‐II) expression to be included among the minimal requirements for cells designated as MSCs. However, there are several reports of cells that show phenotype similar to MSCs that exhibit plastic adherence, with multilineage capabilities, but with undetectable MHC‐II. It is possible that a population of MSCs expresses MHC‐II and its expression does not require prior stimulation with inflammatory mediators. 14 , 32 The expression of MHC‐II provides the cells with the ability to act as antigen presenting cells (APCs). 27 , 36
Despite the functional similarities between MSCs and other APCs, they differ with regard to MHC‐II expression. 27 Although there are no direct studies that link MHC‐II with immune functions, the APC functions of MSCs and, subsequent decrease in MHC‐II at high interferon gamma (IFNγ) level indirectly link MHC‐II with immune functions. The differences in MHC‐II expression are significant to an understanding of the responses by stem cells at sites of tissue injury with immune cells. In a milieu of inflammatory mediators, MSCs show a bimodal expression of MHC‐II with high densities at low levels of IFNγ, and decrease at high IFNγ levels. 37 This contrasts macrophages where MHC‐II expression is proportional to IFNγ levels. 38 Based on these differences, it is apparent that the levels of IFNγ and perhaps other cytokines that are involved in the regulation of MHC‐II could be fundamental in determining the responses of MSCs. To be specific, MHC‐II expression would determine if the MSCs will respond as immune suppressor or enhancer. The immune suppressor functions would prevent the host from responding to MSCs as allogeneic cells.
The molecular mechanisms in MHC‐II expression in MSCs appear to be regulated at the level of the transcription factor CIITA. 37 In vitro studies showed MHC‐II decrease on MSC‐derived neurons, but reversion in the presence of IFNγ. 27 This finding is highly significant since future therapies with MSCs need to address the possibility that there could be immune rejection of the implanted cells by the host immune system. Reexpression of MHC‐II could occur at times long after implantation. At that time, their tolerance to the host’s cells might be nonexistent since MHC‐II was not expressed. Differentiated MSCs will need to be studied, in vivo, to determine if, and how, MHC‐II could be reexpressed. If MHC‐II is reexpressed, then future therapies will need to consider methods that induce tolerance to the implanted cells.
Pro‐ and Antiinflammatory Mediators and MSCs
Sites of tissue injuries are complex with the presence of multiple soluble and insoluble mediators as well as varied immune cell subsets. This complexity is compounded by timeline and regional changes of mediators at sites of tissue insult. For example, the levels of particular cytokines would be different, depending on the time after injury. In addition, at a specific time, the levels could be different, depending on the distance from the focus of injury.
This review briefly discusses two cytokines, interleukin‐1α (IL‐1α) and transforming growth factor‐beta (TGF‐β). IL‐1 is selected because it could regulate other cytokines with positive and negative effects. 39 TGF‐β is discussed due to its role as a pro‐ and antiinflammatory mediator. 40 Another reason to discuss TGF‐β is due to its association with oncogenesis. The oncogenic role of TGF‐β is significant if MSCs and other stem cells are introduced directly for therapy. 40 The placement of stem cells within a milieu of inflammatory mediators will establish crosstalk between the cells and the microenvironment, which would alter the functions and also the biology of the implanted stem cells. The efficiency of MSCs or other stem cells in clinical application would be achieved with detailed studies on the effects of cytokines on genes associated with pluripotency because most of those genes are linked to oncogenesis and tumor suppression.
IL‐1α belongs to the family of IL‐1 cytokines. 41 This family is central to inflammation and host defense. IL‐1α and IL‐1β appear to exhibit similar effects through the type I IL‐1 receptor. IL‐1 could be considered significant in an understanding of stem cell responses to tissue factors because of its ability to induce the expression of other inflammatory mediators. Since IL‐1 can be induced by tissue insults such as infectious agents or hypoxia, it is likely that this cytokine can regulate the expression of inflammatory mediators at the sites of tissue injury. 41 In this regard, IL‐1 can exert its effects on stem cells could be direct or indirect through other cytokines. 42 , 43 In addition, IL‐1 could cause indirect effects on neurons by inducing other cytokines in the surrounding cells, which in turn can bind to neurons. 42 , 44 , 45 , 46
TGF‐β1 belongs to a superfamily of proteins that include the activins, inhibins, and bone morphogenic proteins. 47 TGF‐β receptors are ubiquitously expressed by various cell types, including malignant cells. 48 , 49 TGF‐β1 interacts with receptor subtypes I and II. 50 Type I signaling involves four members of the Smad transcription factors. 51 , 52 , 53 The type II receptor activates the type I form. 54 The family of TGF‐β proteins has been linked to developmental processes such as embryogenesis and neurogenesis. 47 , 55 TGF‐β1 is an immune modulator, inhibits cell proliferation and affects differentiation and apoptosis. 56 , 57 TGF‐β1 has been determined to exhibit both tumor‐suppressor and oncogenic properties and can therefore inhibit cell proliferation and promote malignancy. 58 TGF‐β1 has been reported to activate the differentiation of MSCs to myoblasts, which can support tumor growth. 59 This property of TGF‐β on MSC is significant to future therapy if the recipient has an underlying/undiagnosed tumor.
Microenvironment of Tissue Injury in MSC Therapy
The advent of stem cell therapy, other than the well‐established therapy with hematopoietic stem cells, brings us the question of how the cells should be applied: as pluripotent cells versus partially or fully differentiated cells. These questions are difficult to answer, in the absence of adequate experimental studies. Most important, regardless of the maturational stage of the cells, they are expected to be delivered within tissues of complex microenvironment. Going forward, the answers to these questions are critical for successful treatment of human disease.
MSCs offer the potential to treat complex disease for which no definitive solution has been obtained. These include neurological diseases such as Parkinson’s disease, insulin‐dependent diabetes, traumatic brain injury, Huntington’s disease, and multiple sclerosis. 60 , 61 , 62 , 63 Unlike in vitro conditions of the investigative laboratory; sites of injury and trauma present a vast number of proinflammatory mediators and cytokines. For example, IL‐1 if produced in an inflammatory microenvironment would be able to interact with MSC‐derived neurons. 64 Genes associated with stem cell pluripotency and tumorogenicity such as Repressor Element‐1 Silencing Transcription factor (REST) and Oct4 may be influenced by the presence of these microenvironmental molecules, as well as regulating the expression of each other, based on our bioinformatics analyses ( Figure 1B ). We focus on Oct4 and REST, although there are other stem cell‐associated genes that are involved. Oct4 and REST represent prototypical stem cell genes that can be influenced by microenvironmental factors to change the functions and perhaps the maturation of stem cells.
REST, also known as Neuron Restrictive Silencing Factor, is a DNA‐binding protein that exerts both tumor‐suppressor and oncogenic properties. 65 REST assembles a repressor complex to modify histone acetylation, chromosomal methylation, and DNA phosphorylation in promoter regions of a wide array of genes. 66 , 67 , 68 , 69 , 70 , 71 , 72 Since REST is a tumor‐suppressor gene 73 its discussion might explain the risks of cytokines, at sites of tissue injuries, in predisposing stem cells to transformation. Consider that REST is involved in maintaining pluripotency as well as suppressing tumor formation. We will discuss the possible changes triggered by alterations in REST expression, in response to varied cytokine levels. Similar discussion could occur with Oct4, which is linked to both oncogenesis and pluripotency 74 ( Figure 1B ).
Oct4, also referred as octamer‐binding transcription factor (also Pou5F1), is expressed in adult and embryonic stem cells. However, the expression of Oct4 comes with controversies since others have argued against its expression in somatic cells and its involvement in the pluripotency of adult stem cells. Recent studies have identified different isoforms of Oct4 that might account for the seeming differences in the literature. 75 Regardless, as cells differentiate, Oct4 expression is decreased, underscoring its link to pluripotency. 76
IL‐1 could be a master regulator of other cytokines. TGF‐β1 could negatively affect inflammatory responses. IL‐1α has been shown to cause a rapid decrease in REST expression in MSCs. 17 While this increase could be an advantage to tissue repair, the rapid decrease in REST expression could predispose the cell to transformation. This assumption is based on other studies showing a tumor‐suppressor role of REST. 73 , 77
To explain the possible crosstalk between stem cells and microenvironmental factors, we incorporate two neurotransmitter genes with the role of REST in their expressions as MSCs develop into mature neurons ( Figure 2 ). Stem cells are nonneural cells and are therefore expected to repress neural genes. However, when stem cells mature to neurons, the neural genes are de‐repressed while nonneuronal genes should be repressed. REST expression is critical in the expression of neural and nonneural genes. Indeed, the regulatory regions of the neurotransmitter TAC1 gene and tyrosine hydroxylase gene have binding sites for REST. 17 , 78 As expected, REST acts as a repressor for TAC1 transcription in nonneuronal cells. 17 During the development of MSCs to neurons, REST expression is gradually decreased, leading to TAC1 expression. 17 Stimulation of MSCs or the early neuronal differentiated MSCs with IL‐1 led to rapid decrease in REST with concomitant increase in TAC1 expression. 17 This increase in the neurotransmitter gene is consistent with a repressor function of REST. These findings, if placed in the context of MSCs at sites of tissue injuries, could explain why the response of implanted cells could be unpredictable. First, it is unclear what cytokines will be at the region of tissue injury. Second, the differences in receptors at varied maturational stage of MSCs. Regardless, it is expected that crosstalk would be established with the MSCs and cytokines ( Figure 2 ). At this time it would be difficult to predict the physiological responses unless there are further investigations that provide insights on the role of inflammatory mediators such as cytokines in the molecular changes leading to altered expressions of neuronal genes.
Figure 2.

Shown are comparisons of MSC responses, with regard to neurotransmitter genes, in the presence of noninflammatory region and with a milieu of inflammatory microenvironment. Left: REST represses the expression of neurotransmitter genes; Right: IL‐1a decreases the expression of REST to de‐repress neurotransmitter expression, and also enhanced neurogenesis.
Regarding predisposition to transformation, we refer to the derivation of inducible pluripotent stem cells in which Oct4 is among the four genes that can convert adult fibroblasts to cells to embryonic‐like cells. 79 In addition, Oct4 expression is increased in various tumors, 80 , 81 in addition to its presence in cancer‐initiating cells. 82 The latter finding is highly significant because if Oct4 is involved in transforming implanted cells to cancer‐initiating cells, this will be difficult to identify. We propose that this role of Oct4 should not be trivialized since this could be fundamental to safety of stem cell therapy.
The future of stem cell therapy would require in‐depth investigation on the role of cytokines and other inflammatory mediators in Oct4 expression, in addition to other pluripotent genes. If cytokines can regulate Oct4 expression, this would lead to insights on the behavior of stem cells through changes in genes regulated by Oct4, as well as those that regulate Oct4 expression. This information would not be important only for stem cells, but also during the maturation to specialized cells. Overall, REST and Oct4, as discussed, are among the genes that could provide insights on the complex interactions that might occur in vivo. We propose that the crosstalk that could begin with stem cells and the differentiated cells with tissue microenvironment could be an advantage for tissue repair. It is possible to develop effective therapies by taking advantage of the tissue microenvironment. Currently, these studies are fragmented, but are fundamental for effective therapy.
Application: Cardiac Repair as an Example
Currently, cardiovascular disease ranks as the leading cause of death in the United States, accounting for greater mortality than cancer, accidents, and pulmonary disease. It is also a significant cause of morbidity worldwide. 83 The incidence of myocardial ischemia increases with age and declining health, as cardiomyocytes become susceptible to ischemia‐induced death, leading to scar formation and diminished contractile function. Myocardial repair and regeneration has been a topic of interest in the field of stem cell biology, as MSCs have been shown to differentiate into functional myocytes when subject to the proper microenvironment or chemical induction cocktail. 84 , 85 There are reviews on MSCs in cardiac repair. 86 MSCs are attractive stem cells in cardiac repair showing differentiation to cardiac, vascular muscle, and endothelial cells. 87 This section presents few examples of MSC differentiation into cardiac myocytes that will be highlighted in this section.
Induction of MSCs into various lineages requires specific growth factors, such as β‐glycerophosphate/ascorbic acid for osteogenesis, hydrocortisone for stroma, TGF‐β family members for chondrogenesis, and indomethacin for adipogenesis ( Figure 1A ). Cardiogenic induction has been demonstrated by treating MSCs with 5‐azacytidine, which is a DNA‐demethylating agent that resulted in demethylation of the glycogen synthase kinase‐3β promoter. 84 Cocultures of MSCs with cardiac myocytes resulted in the expression of cardiomyocyte‐specific genes. 88 Although this effect was enhanced by hepatocyte growth factor and insulin like growth factor‐1, 89 other studies show a role for notch 1 in bone marrow‐derived MSCs in cardiac repair. 90
As discussed above, prior to the administration of MSCs for cardiac repair, the hurdle of delivery as well as other confounds must be overcome. Intravenous delivery of MSCs has been used in clinical trials thus far. 91 Intravenous administration is an attractive strategy because it is not invasive and also allows for repeated administration. 91 The migratory ability of MSCs to cardiac tissue is one of the reasons why these cells may be efficient for cardiac repair. However, a significant obstacle posed by MSC therapy is the low percentage of MSCs that ultimately migrates to the injured heart. 91 Coadministration of MSCs and granulocyte colony‐stimulating factor in rats with myocardial infarction resulted in improved efficiency. 91 The mechanism governing this effect may involve the stromal cell‐derived factor‐1 (SDF‐1)/CXCR4 axis on MSCs, which normally functions in homing and mobilization. 91 Research into the optimization of SDF‐1/CXCR4 interaction may lead to facilitation of MSC delivery to ischemic cardiac tissue. 91 , 92
Another significant drawback to stem cell therapy using MSCs for cardiovascular disease is that MSCs have fastidious growth conditions, leading to cell death if the stem cells are not properly maintained. The phosphatidylinositol 3 kinase pathway has been shown to play an essential role in the survival of MSCs. MSCs that were transduced with Akt, or protein kinase B, resulted in improved ventricular remodeling and establishment of functional cardiac improvement within a few days. 92 The transduced cells appear to have a growth/trophic factor signature that may play a role in maintaining MSC survival; these include fibroblast growth factor‐2, HGF, vascular endothelial growth factor (VEGF), and insulin growth factor (IGF‐1). 92 VEGF and IGF‐1 can protect cardiac myocytes from apoptosis while enhancing endothelial cell function. Studies have identified the trophic factor‐mediated JAK‐STAT signaling pathway to be relevant to the protection of MSCs during cardiac repair. 93 MSCs could be engineered to express chemokines to improve the cells’ functions in cardiac repair. 94 , 95 Together, the discussed literature indicates that contact‐independent factors play important roles in maintaining MSC viability in the context of stem cell therapy for cardiovascular disease.
Upon homing to target tissues, MSCs can secrete a variety of cytokines, such as TGF‐β, which has been shown to recruit immunosuppressive T‐cell subsets. 96 Through the secretion of TGF‐β, MSCs may be able to enhance cardiac repair through recruitment of regulatory T lymphocytes (Tregs). 96 , 97 In any case, it is critical to further explore the effects of cytokines and MSCs within the cardiac tissue microenvironment in order to understand the benefits and limitations of stem cell therapy. The recruitment of Tregs could be important for long‐term engraftment of MSCs since studies have shown immunogenic responses to differentiated MSCs in cardiac repair. 98
In summary, scientists have demonstrated that by placing MSCs in a microenvironment containing the appropriate cues, MSCs can give rise to functional myocardial tissue, allowing them to serve as a valuable source in regenerative medicine. MSCs can exert paracrine effects through secretion of various cytokines that lead to suppression of inflammation. Despite these in vitro findings, the clinical significance in terms of treatment of patients remains to be tested. Further complicating the picture is the idea that there is no clear consensus regarding the myocardial regenerative capacity of MSCs. As research in this area progresses, the future may see broader applications for MSCs for treatment of cardiac injury.
Conclusion
This review summarizes the complex network that could develop with stem cells if placed within a milieu of tissue injury. Although we selected MSCs to discuss the complex issues, it should be stressed that similar mechanism could occur by any stem cell. The review attempted to bring attention to the potential that undetermined responses could occur when stem cells are placed in vivo. We suggest that in‐depth experimental systems are required for defined animal models. The plastic properties of MSCs provide these cells with the ability to interact with mediators at different microenvironment. These properties, in addition to the wide application of MSCs indicate that effective translation of MSCs will require global standardizations. 99 Together, these studies will enable efficient clinical trials with any stem cells since it might be better to predict outcomes. It is paramount to consider the cytokines, other proinflammatory and antiinflammatory mediators as well as resident cells that could establish a crosstalk with the implanted stem cells or their differentiated cells. Crosstalk between the cells and mediators in a microenvironment can change rapidly, depending on the rate of differentiation. Furthermore, if the stem cells are dispersed this would indicate that each stem cell would be in its own microenvironment. This would indicate that within the site of tissue injury, there could be a lack of synchrony in the types of receptors on each cell. Although not discussed in this review, a major issues with MSC treatment is their role in supporting and/or protecting tumors. 97 The fast‐growing field of cancer stem cells brings to the surface, the safety of treating patients with MSCs. The presence of cancer stem cells might not correlate with clinical detection of the cancer, leaving the treatment with MSCs in a subject with cancer stem cells. 100
Conflict of Interest
The authors have no conflicts of interest to disclose.
Acknowledgment
This work was supported by the FM Kirby Foundation.
References
- 1. Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first‐trimester fetal blood, liver, and bone marrow. Blood. 2001; 98: 2396–2402. [DOI] [PubMed] [Google Scholar]
- 2. Castillo M, Liu K, Bonilla LM, Rameshwar P. The immune properties of mesenchymal stem cells. Int J Biomed Sci. 2007; 3(100): 100–104. [PMC free article] [PubMed] [Google Scholar]
- 3. Dominici M, Paolucci P, Conte P, Horwitz EM. Heterogeneity of multipotent mesenchymal stromal cells: from stromal cells to stem cells and vice versa. Transplantation. 2009; 87: S36–S42. [DOI] [PubMed] [Google Scholar]
- 4. You Q, Tong X, Guan Y, Zhang D, Huang M, Zhang Y, Zheng J. The biological characteristics of human third trimester amniotic fluid stem cells. J Int Med Res. 2009; 37: 105–112. [DOI] [PubMed] [Google Scholar]
- 5. Parolini O, Alviano F, Bergwerf I, Boraschi D, De Bari C, De Waele P, Dominici M, Evangelista M, Falk W, Hennerbichler S, et al Toward cell therapy using placenta‐derived cells: disease mechanisms, cell biology, preclinical studies, and regulatory aspects at the round table. Stem Cells Dev. 2009; 19: 143–154. [DOI] [PubMed] [Google Scholar]
- 6. Zeddou M, Briquet A, Relic B, Josse C, Malqaise MG, Gothot A, Lechanteur C, Beguin Y. The umbilical cord matrix is a better source of mesenchymal stem cells (MSC) than the umbilical cord blood. Cell Biol Int. 2010; 34: 693–701. [DOI] [PubMed] [Google Scholar]
- 7. Sakaguchi Y, Sekiya I, Yagishita K, Ichinose S, Shinomiya K, Muneta T. Suspended cells from trabecular bone by collagenase digestion become virtually identical to mesenchymal stem cells obtained from marrow aspirates. Blood. 2004; 104: 2728–2735. [DOI] [PubMed] [Google Scholar]
- 8. Tucker HA, Bunnell BA. Characterization of human adipose‐derived stem cells using flow cytometry. Methods Mol Biol. 2011; 702: 121–131. [DOI] [PubMed] [Google Scholar]
- 9. Puetzer JL, Petitte JN, Loboa EG. Comparative review of growth factors for induction of three‐dimensional in vitro chondrogenesis in human mesenchymal stem cells isolated from bone marrow and adipose tissue. Tissue Eng Part B Rev. 2010; 16: 435–444. [DOI] [PubMed] [Google Scholar]
- 10. Mosna F, Sensebe L, Krampera M. Human bone marrow and adipose tissue mesenchymal stem cells: a user’s guide. Stem Cells and Dev. 2010; 19: 1449–1470. [DOI] [PubMed] [Google Scholar]
- 11. Caplan AI. The mesengenic process. Clin Plast Surg. 1994; 21: 429–435. [PubMed] [Google Scholar]
- 12. Delorme B, Ringe J, Pontikoglou C, Gaillard J, Langonne A, Sensebe L, Noel D, Jorgensen C, Haupl T, Charbord P. Specific lineage‐priming of bone marrow mesenchymal stem cells provides the molecular framework for their plasticity. Stem Cells. 2009; 27: 1142–1151. [DOI] [PubMed] [Google Scholar]
- 13. Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, et al Self‐renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007; 131: 324–336. [DOI] [PubMed] [Google Scholar]
- 14. Potian JA, Aviv H, Ponzio NM, Harrison JS, Rameshwar P. Veto‐like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. J Immunol. 2003; 171: 3426–3434. [DOI] [PubMed] [Google Scholar]
- 15. Blanc KL, Pittenger MF. Mesenchymal stem cells: progress toward promise. Cytotherapy. 2005; 7: 36–45. [DOI] [PubMed] [Google Scholar]
- 16. Takashima Y, Era T, Nakao K, Kondo S, Kasuga M, Smith AG, Nishikawa SI. Neuroepithelial cells supply an initial transient wave of MSC differentiation. Cell. 2007; 129: 1377–1388. [DOI] [PubMed] [Google Scholar]
- 17. Greco SJ, Smirnov S, Rameshwar P. Synergy between RE‐1 silencer of transcription (REST) and NFkB in the repression of the neurotransmitter gene Tac1 in human mesenchymal stem cells: implication for microenvironmental influence on stem cell therapies. J Biol Chem. 2007; 282: 30039–30050. [DOI] [PubMed] [Google Scholar]
- 18. Greco SJ, Zhou C, Ye JH, Rameshwar P. An interdisciplinary approach and characterization of neuronal cells transdifferentiated from human mesenchymal stem cells. Stem Cells Dev. 2007; 16: 811–826. [DOI] [PubMed] [Google Scholar]
- 19. Trzaska KA, Kuzhikandathil EV, Rameshwar P. Specification of a dopaminergic phenotype from adult human mesenchymal stem cells. Stem Cells. 2007; 25: 2797–2808. [DOI] [PubMed] [Google Scholar]
- 20. Trzaska KA, Reddy BY, Munoz JL, Li KY, Ye JH, Rameshwar P. Loss of RE‐1 silencing factor in mesenchymal stem cell‐derived dopamine progenitors induces functional maturity. Mol Cell Neurosci. 2008; 39: 285–290. [DOI] [PubMed] [Google Scholar]
- 21. Trzaska KA, King CC, Li KY, Kuzhikandathil EV, Nowycky MC, Ye JH, Rameshwar P. Brain‐derived neurotrophic factor facilitates maturation of mesenchymal stem cell‐derived dopamine progenitors to functional neurons. J Neurochem. 2009; 110: 1058–1069. [DOI] [PubMed] [Google Scholar]
- 22. Brohlin M, Mahay D, Novikov LN, Terenghi G, Wiberg M, Shawcross SG, Novikova LN. Characterisation of human mesenchymal stem cells following differentiation into Schwann cell‐like cells. Neurosci Res. 2009; 64: 41–49. [DOI] [PubMed] [Google Scholar]
- 23. Wagner J, Kean T, Young R, Dennis JE, Caplan AI. Optimizing mesenchymal stem cell‐based therapeutics. Curr Opin Biotechnol. 2009; 20: 531–536. [DOI] [PubMed] [Google Scholar]
- 24. Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, et al Mesenchymal stem cells for treatment of steroid‐resistant, severe, acute graft‐versus‐host disease: a phase II study. Lancet. 2008; 371: 1579–1586. [DOI] [PubMed] [Google Scholar]
- 25. Potian JA, Aviv H, Ponzio NM, Harrison JS, Rameshwar P. Veto‐like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. J Immunol. 2003; 171: 3426–3434. [DOI] [PubMed] [Google Scholar]
- 26. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284: 143–147. [DOI] [PubMed] [Google Scholar]
- 27. Chan JL, Tang KC, Patel AP, Bonilla LM, Pierobon N, Ponzio NM, Rameshwar P. Antigen‐presenting property of mesenchymal stem cells occurs during a narrow window at low levels of interferon‐gamma. Blood. 2006; 107: 4817–4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Razmkhah M, Jaberipour M, Erfani N, Habibagahi M, Talei Ar, Ghaderi A. Adipose derived stem cells (ASCs) isolated from breast cancer tissue express IL‐4, IL‐10 and TGF‐[beta]1 and upregulate expression of regulatory molecules on T cells: do they protect breast cancer cells from the immune response? Cell Immunol. 2011; 266: 116–122. [DOI] [PubMed] [Google Scholar]
- 29. da Silva ML, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008; 26: 2287–2299. [DOI] [PubMed] [Google Scholar]
- 30. Salem HK, Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 2010; 28: 585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heng BC, Cowan CM, Davalian D, Stankus J, Duong‐Hong D, Ehrenreich K, Basu S. Electrostatic binding of nanoparticles to mesenchymal stem cells via high molecular weight polyelectrolyte chains. J Tissue Eng Regen Med. 2009; 3: 243–254. [DOI] [PubMed] [Google Scholar]
- 32. Romieu‐Mourez R, Francois M, Boivin MN, Stagg J, Galipeau J. Regulation of MHC class II expression and antigen processing in murine and human mesenchymal stromal cells by IFN‐{gamma}, TGF‐beta, and cell density. J Immunol. 2007; 179: 1549–1558. [DOI] [PubMed] [Google Scholar]
- 33. Patel SA, Sherman L, Munoz J, Rameshwar P. Immunological properties of mesenchymal stem cells and clinical implications. Arch Immunol Ther Exp. 2008; 56: 1–8. [DOI] [PubMed] [Google Scholar]
- 34. Kim J, Hematti P. Mesenchymal stem cell‐educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol. 2009; 37: 1445–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dominici M, Le BK, Mueller I, Slaper‐Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006; 8: 315–317. [DOI] [PubMed] [Google Scholar]
- 36. Stagg J. Immune regulation by mesenchymal stem cells: two sides to the coin. Tissue Antigens. 2007; 69: 1–9. [DOI] [PubMed] [Google Scholar]
- 37. Tang KC, Trzaska KA, Smirnov S, Kotenko SV, Schwander SK, Ellner JJ, Rameshwar P. Down‐regulation of MHC‐II in mesenchymal stem cells at high IFN‐ can be partly explained by cytoplasmic retention of CIITA. J Immunol. 2008; 180: 1826–1833. [DOI] [PubMed] [Google Scholar]
- 38. Herrero C, Sebastian C, Marquqs L, Comalada M, Xaus J, Valledor AF, Lloberas J, Celada A. Immunosenescence of macrophages: reduced MHC class II gene expression. Exp Gerontol. 2002; 37: 389–394. [DOI] [PubMed] [Google Scholar]
- 39. Dinarello CA. Immunological and inflammatory functions of the interleukin‐1 family. Annu Rev Immunol. 2009; 27: 519–550. [DOI] [PubMed] [Google Scholar]
- 40. Yang L. TGFbeta, a potent regulator of tumor microenvironment and host immune response, implication for therapy. Curr Mol Med. 2010; 10: 374–380. [DOI] [PubMed] [Google Scholar]
- 41. Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin‐1 and neuronal injury. Nat Rev Immunol. 2005; 5: 629–640. [DOI] [PubMed] [Google Scholar]
- 42. Moore MA. Cytokine and chemokine networks influencing stem cell proliferation, differentiation, and marrow homing. J Cell Biochem Suppl. 2002; 38: 29–38. [DOI] [PubMed] [Google Scholar]
- 43. Laver J, Moore MAS. Clinical use of recombinant human hematopoietic growth factors. J Natl Cancer Inst. 1989; 81: 1370–1382. [DOI] [PubMed] [Google Scholar]
- 44. Dinarello CA. Blocking IL‐1 in systemic inflammation. J Exp Med. 2005; 201: 1355–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dinarello CA. Biologic basis for interleukin‐1 in disease. Blood. 1996; 87: 2095–2147. [PubMed] [Google Scholar]
- 46. Bagby GC. Interleukin‐1 and hematopoiesis. Blood Rev. 1989; 3: 152–161 [DOI] [PubMed] [Google Scholar]
- 47. Roberts AB, Sporn MB. Physiological actions and clinical applications of transforming growth factor‐beta (TGF‐beta). Growth Factors. 1993; 8: 1–9. [DOI] [PubMed] [Google Scholar]
- 48. Massague J, Andres J, Attisano L, Cheifetz S, Lopez‐Casillas F, Ohtsuki M, Wrana JL. TGF‐beta receptors. Mol Reprod Dev. 1992; 32: 99–104. [DOI] [PubMed] [Google Scholar]
- 49. Massague J, Weis‐Garcia F. Serine/threonine kinase receptors: mediators of transforming growth factor beta family signals. Cancer Surv. 1996; 27: 41–64. [PubMed] [Google Scholar]
- 50. Shi Y, Massague J. Mechanisms of TGF‐[beta] signaling from cell membrane to the nucleus. Cell. 2003; 113: 685–700. [DOI] [PubMed] [Google Scholar]
- 51. Massague J, Wotton D. Transcriptional control by the TGF‐beta/Smad signaling system. EMBO J. 2000; 19: 1745–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A. Targeted disruption of TGF‐beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest. 2003; 112: 1486–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mehra A, Wrana JL. TGF‐beta and the Smad signal transduction pathway. Biochem Cell Biol. 2002; 80: 605–622. [DOI] [PubMed] [Google Scholar]
- 54. Wrana JL. Regulation of Smad activity. Cell. 2000; 100: 189–192. [DOI] [PubMed] [Google Scholar]
- 55. Golestaneh N, Mishra B. TGF‐beta, neuronal stem cells and glioblastoma. Oncogene. 2005; 24: 5722–5730. [DOI] [PubMed] [Google Scholar]
- 56. Sokol JP, Schiemann WP. Cystatin C antagonizes transforming growth factor {beta} signaling in normal and cancer cells. Mol Cancer Res. 2004; 2: 183–195. [PubMed] [Google Scholar]
- 57. Downing JR. TGF‐{beta} signaling, tumor suppression, and acute lymphoblastic leukemia. N Engl J Med. 2004; 351: 528–530. [DOI] [PubMed] [Google Scholar]
- 58. Kim SJ, Letterio J. Transforming growth factor‐beta signaling in normal and malignant hematopoiesis. Leukemia. 2003; 17: 1731–1737. [DOI] [PubMed] [Google Scholar]
- 59. Mishra PJ, Banerjee D. Activation and differentiation of mesenchymal stem cells. Methods Mol Biol. 2011; 717: 245–253. [DOI] [PubMed] [Google Scholar]
- 60. Trzaska KA, Rameshwar P. Current advances in the treatment of Parkinson’s disease with stem cells. Curr Neurovasc Res. 2007; 4: 99–109. [DOI] [PubMed] [Google Scholar]
- 61. Karussis D, Karageorgiou C, Vaknin‐Dembinsky A, Gowda‐Kurkalli B, Gomori JM, Kassis I, Bulte JWM, Petrou P, Ben‐Hur T, Abramsky O, et al Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010; 67: 1187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Heile AMB, Wallrapp C, Klinge PM, Samii A, Kassem M, Silverberg G, Brinker T. Cerebral transplantation of encapsulated mesenchymal stem cells improves cellular pathology after experimental traumatic brain injury. Neurosci Lett. 2009; 463: 176–181. [DOI] [PubMed] [Google Scholar]
- 63. Fotino C, Ricordi C, Lauriola V, Alejandro R, Pileggi A. Bone marrow‐derived stem cell transplantation for the treatment of insulin‐dependent diabetes. Rev Diabet Stud. 2010; 7: 144–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Greco SJ, Rameshwar P. Enhancing effect of IL‐1a on neurogenesis from adult human mesenchymal stem cells: implication for inflammatory mediators in regenerative medicine. J Immunol. 2007; 179: 3342–3350. [DOI] [PubMed] [Google Scholar]
- 65. Coulson JM. Transcriptional regulation: cancer, neurons and the REST. Curr Biol. 2005; 15: R665–R668. [DOI] [PubMed] [Google Scholar]
- 66. Fiskerstrand CE, Newey P, McGregor GP, Gerrard L, Millan F, Quinn JP. A role for octamer binding protein motifs in the regulation of the proximal preprotachykinin‐a promoter. Neuropeptides. 2000; 34: 348–354. [DOI] [PubMed] [Google Scholar]
- 67. Quinn JP, Bubb VJ, Marshall‐Jones ZV, Coulson JM. Neuron restrictive silencer factor as a modulator of neuropeptide gene expression. Reg Peptides. 2002; 108: 135–141. [DOI] [PubMed] [Google Scholar]
- 68. Su X, Kameoka S, Lentz S, Majumder S. Activation of REST/NRSF target genes in neural stem cells is sufficient to cause neuronal differentiation. Mol Cell Biol. 2004; 24: 8018–8025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yeo M, Lee SK, Lee B, Ruiz EC, Pfaff SL, Gill GN. Small CTD phosphatases function in silencing neuronal gene expression. Science. 2005; 307: 596–600. [DOI] [PubMed] [Google Scholar]
- 70. Bruce AW, Donaldson IJ, Wood IC, Yerbury SA, Sadowski MI, Chapman M, Gottgens B, Buckley NJ. Genome‐wide analysis of repressor element 1 silencing transcription factor/neuron‐restrictive silencing factor (REST/NRSF) target genes. Proc Natl Acad Sci. 2004; 101: 10458–10463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wood IC, Belyaev ND, Bruce AW, Jones C, Mistry M, Roopra A, Buckley NJ. Interaction of the repressor element 1‐silencing transcription factor (REST) with target genes. J Mol Biol. 2003; 334: 863–874. [DOI] [PubMed] [Google Scholar]
- 72. Belyaev ND, Wood IC, Bruce AW, Street M, Trinh JB, Buckley NJ. Distinct RE‐1 silencing transcription factor‐containing complexes interact with different target genes. J Biol Chem. 2004; 279: 556–561. [DOI] [PubMed] [Google Scholar]
- 73. Reddy BY, Greco SJ, Patel PS, Trzaska KA, Rameshwar P. RE‐1‐çosilencing transcription factor shows tumor‐suppressor functions and negatively regulates the oncogenic TAC1 in breast cancer cells. Proc Natl Acad Sci. 2009; 106: 4408–4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Trosko JE. From adult stem cells to cancer stem cells: Oct‐4 gene, cell‐cell communication, and hormones during tumor promotion. Ann N Y Acad Sci. 2006; 1089: 36–58. [DOI] [PubMed] [Google Scholar]
- 75. Wang X, Dai J. Concise review: isoforms of Oct4 contribute to the confusing diversity in stem cell biology. Stem Cells. 2010; 28: 885–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Greco SJ, Liu K, Rameshwar P. Functional similarities among genes regulated by Oct4 in human mesenchymal and embryonic stem cells. Stem Cells. 2007; 25: 3143–3154. [DOI] [PubMed] [Google Scholar]
- 77. Majumder S. REST in good times and bad: roles in tumor suppressor and oncogenic activities. Cell Cycle. 2006; 5: 1929–1935. [DOI] [PubMed] [Google Scholar]
- 78. Kim SM, Yang JW, Park MJ, Lee JK, Kim SU, Lee YS, Lee MA. Regulation of human tyrosine hydroxylase gene by neuron‐restrictive silencer factor. Biochem Biophys Res Commun. 2006; 346: 426–435. [DOI] [PubMed] [Google Scholar]
- 79. Tweedell KS. New paths to pluripotent stem cells. Curr Stem Cell Res Ther. 2008; 3: 151–162. [DOI] [PubMed] [Google Scholar]
- 80. Karoubi G, Gugger M, Schmid R, Dutly A. Oct4 expression in human non‐small cell lung cancer: implications for therapeutic intervention. Interact CardioVasc Thorac Surg. 2009; 8: 393–397. [DOI] [PubMed] [Google Scholar]
- 81. Sotomayor P, Godoy A, Smith GJ, Huss WJ. Oct4A is expressed by a subpopulation of prostate neuroendocrine cells. Prostate. 2009; 69: 401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Levings PP, McGarry SV, Currie TP, Nickerson DM, McClellan S, Ghivizzani SC, Steindler DA, Gibbs CP. Expression of an exogenous human Oct‐4 promoter identifies tumor‐initiating cells in osteosarcoma. Cancer Res. 2009; 69: 5648–5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Writing Group , Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, et al., and for the American Heart Association Statistics Committee and Stroke Statistics Subcommittee: Heart Disease and Stroke Statistics–2008 Update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008; 117: e25–e146. [DOI] [PubMed] [Google Scholar]
- 84. Cho J, Rameshwar P, Sadoshima J. Distinct roles of glycogen synthase kinase (GSK)‐3beta and GSK‐3beta in mediating cardiomyocyte differentiation in murine bone marrow‐derived mesenchymal stem cells. J Biol Chem. 2009; 284: 36647–36658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ohnishi S, Ohgushi H, Kitamura S, Nagaya N. Mesenchymal stem cells for the treatment of heart failure. Int J Hematol. 2007; 86: 17–21. [DOI] [PubMed] [Google Scholar]
- 86. Boyle AJ, McNiece IK, Hare JM. Mesenchymal stem cell therapy for cardiac repair. Methods Mol Biol. 2010; 660: 65–84. [DOI] [PubMed] [Google Scholar]
- 87. Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbaum GS, Rodriguez JE, Valdes D, Pattany PM, Zambrano JP, Hu Q, McNiece I, et al Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci. 2009; 106: 14022–14027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wang T, Xu Z, Jiang W, Ma A. Cell‐to‐cell contact induces mesenchymal stem cell to differentiate into cardiomyocyte and smooth muscle cell. Int J Cardiol. 2006; 109: 74–81. [DOI] [PubMed] [Google Scholar]
- 89. Li Z, Gu TX, Zhang YH. Hepatocyte growth factor combined with insulin like growth factor‐1 improves expression of GATA‐4 in mesenchymal stem cells cocultured with cardiomyocytes. Chin Med J (Engl). 2008; 121: 336–340. [PubMed] [Google Scholar]
- 90. Li Y, Hiroi Y, Ngoy S, Okamoto R, Noma K, Wang CY, Wang HW, Zhou Q, Radtke F, Liao R, et al Notch1 in bone marrow‐derived cells mediates cardiac repair after myocardial infarction. Circulation. 2011; 123: 866–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Cheng Z, Ou L, Zhou X, Li F, Jia X, Zhang Y, Liu X, Li Y, Ward CA, Melo LG, et al Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol Ther. 2008; 16: 571–579. [DOI] [PubMed] [Google Scholar]
- 92. Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, et al Evidence supporting paracrine hypothesis for Akt‐modified mesenchymal stem cell‐mediated cardiac protection and functional improvement. FASEB J. 2006; 20: 661–669. [DOI] [PubMed] [Google Scholar]
- 93. Shabbir A, Zisa D, Lin H, Mastri M, Roloff G, Suzuki G, Lee T. Activation of host tissue trophic factors through JAK‐STAT3 signaling: a mechanism of mesenchymal stem cell‐mediated cardiac repair. Am J Physiol Heart Circ Physiol. 2010; 299: H1428–H1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Xu J, Chen Q, Shi C, Yin Z. Overexpression of CXCR1/CXCR2 on mesenchymal stromal cells may be an effective treatment for acute myocardial infarction. Cytotherapy. 2009; 11: 990–991. [DOI] [PubMed] [Google Scholar]
- 95. Huang J, Zhang Z, Guo J, Ni A, Deb A, Zhang L, Mirotsou M, Pratt RE, Dzau VJ. Genetic modification of mesenchymal stem cells overexpressing CCR1 increases cell viability, migration, engraftment, and capillary density in the injured myocardium. Circ Res. 2010; 106: 1753–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Casiraghi F, Azzollini N, Cassis P, Imberti B, Morigi M, Cugini D, Cavinato RA, Todeschini M, Solini S, Sonzogni A, et al Pretransplant infusion of mesenchymal stem cells prolongs the survival of a semiallogeneic heart transplant through the generation of regulatory T cells. J Immunol. 2008; 181: 3933–3946. [DOI] [PubMed] [Google Scholar]
- 97. Patel SA, Meyer JR, Greco SJ, Corcoran KE, Bryan M, Rameshwar P. Mesenchymal stem cells protect breast cancer cells through regulatory T cells: role of mesenchymal stem cell‐derived TGF‐beta. J Immunol. 2010; 184: 5885–5894. [DOI] [PubMed] [Google Scholar]
- 98. Huang XP, Sun Z, Miyagi Y, McDonald Kinkaid H, Zhang L, Weisel RD, Li RK. Differentiation of allogeneic mesenchymal stem cells induces immunogenicity and limits their long‐term benefits for myocardial repair. Circulation. 2010; 122: 2419–2429. [DOI] [PubMed] [Google Scholar]
- 99. Garcia‐Gomez I, Elvira G, Zapata AG, Lamana ML, Ramirez M, Castro JG, Arranz MG, Vicente A, Bueren J, Garcia‐Olmo D. Mesenchymal stem cells: biological properties and clinical applications. Expert Opin Biol Ther. 2010; 10: 1453–1468. [DOI] [PubMed] [Google Scholar]
- 100. Alison MR, Lim SM, Nicholson LJ. Cancer stem cells: problems for therapy? J Pathol. 2011; 223: 147–161. [DOI] [PubMed] [Google Scholar]


