Abstract
The experimental aims of this study were to determine: (1) whether nitric oxide‐mediated endothelium‐dependent vasodilation is blunted in adult humans with borderline high plasma low‐density lipoprotein (LDL)‐cholesterol compared with adults with optimal/near optimal LDL‐cholesterol levels; and, if so: (2) whether the magnitude of impairment in adults with borderline high LDL‐cholesterol is similar to adults with high LDL‐cholesterol. Forearm blood flow responses to intraarterial infusions of acetylcholine and sodium nitroprusside were measured in 50 middle‐aged (43–64 year) adults: 20 in the optimal/near optimal LDL‐cholesterol range (<130 mg/dL); 20 with borderline high LDL‐cholesterol (130–159 mg/dL); and 10 with high LDL‐cholesterol ($160 mg/dL). In addition, blood flow responses to acetylcholine were determined in the absence and presence of the endothelial nitric oxide synthase inhibitor NG‐monomethyl‐L‐arginine (L‐NMMA). Vasodilation to acetylcholine was ∼20% lower (p < 0.05) in the borderline high (from 4.3 ± 0.2 to 12.3 ± 0.8 mL/100 mL tissue/min) and high (from 4.3 ± 0.3 to 12.0 ± 0.5 mL/100 mL tissue/min) LDL‐cholesterol groups compared with the optimal/near optimal (from 4.4 ± 0.2 to 14.5 ± 0.5 mL/100 mL tissue/min) LDL‐cholesterol group. L‐NMMA significantly reduced (∼30%) the vasodilator response to acetylcholine in the optimal/near optimal LDL‐cholesterol group but not the borderline high or high LDL‐cholesterol groups. Borderline high LDL‐cholesterol is associated with impaired nitric oxide‐mediated endothelium‐dependent vasodilation. Clin Trans Sci 2012; Volume #: 1–6
Keywords: nitric oxide, LDL cholesterol, endothelium, vasodilation
Introduction
Low‐density lipoprotein (LDL)‐cholesterol is a major atherogenic lipoprotein constituent of total cholesterol. 1 Several epidemiological, clinical, and animal studies have demonstrated a strong link between LDL‐cholesterol and atherosclerotic vascular disease risk, development, and events. 2 , 3 , 4 Moreover, primary prevention trials have demonstrated that lowering LDL‐cholesterol reduces the risk of cardiovascular disease related events and limits the progression of coronary atherosclerosis. 1 , 5 , 6 , 7 The association between LDL‐cholesterol and risk of coronary heart disease, while continuous, is log‐linear demonstrating sharp rises in risk with increasing LDL‐cholesterol concentration. In fact, the relative risk for coronary heart disease is estimated to increase by ∼30% for every 30 mg/dL increase in LDL‐cholesterol levels above a baseline of 40 mg/dL. 1 As a result, the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adult Treatment Panels (ATP), including the most recent report (NCEP ATP III), continue to identify LDL‐cholesterol as the primary target in the treatment of hypercholesterolemia. 8 The increasing recognition that even modest elevations in plasma LDL‐cholesterol levels confer a significant increase in cardiovascular risk heightens the importance for early initiation of LDL‐cholesterol lowering therapy. Indeed, the relative risk of coronary heart disease is almost twofold higher in adults with borderline high LDL‐cholesterol (130–159 mg/dL) compared with adults with LDL‐cholesterol in a more optimal range (<130 mg/dL). 1 Considering it is estimated that 40% of the adults in the United States have LDL‐cholesterol levels in the borderline high range, a better understanding of the potential mechanisms underlying the increased vascular risk is important and may influence therapeutic strategies.
Vascular endothelial function is paramount to cardiovascular health. Located at the interface of the circulating blood and vascular smooth muscle, endothelial cells produce and release a myriad of substances that play prominent roles in the regulation of a variety of cardiovascular functions from vasomotor tone to the control of fibrinolysis. 9 Impaired endothelial function, particularly reduced nitric oxide (NO)‐mediated endothelium‐dependent vasodilation, is thought to occur early in the atherogenic process contributing to the initiation and progression of atherosclerotic vascular disease and acute vascular events. 10 , 11 LDL‐cholesterol has been shown to damage and impair endothelial cell function. 12 , 13 , 14 , 15 , 16 In humans, LDL‐cholesterol levels above 160 mg/dL are associated with diminished NO‐mediated endothelium‐dependent vasodilation. 14 Whether this impairment is apparent with borderline high LDL‐cholesterol (130–159 mg/dL) is not clear. If so, this may contribute to the increased cardiovascular risk with even moderate elevations in LDL‐cholesterol. Indeed, this appears to be the case with other risk factors such as hypertension and type 2 diabetes, where similar impairments in NO‐mediated endothelium‐dependent vasodilation have been observed in adults with prehypertension 17 and obesity/insulin resistance, 18 respectively.
Accordingly, the experimental aims of this study were to determine: (1) whether NO‐mediated endothelium‐dependent vasodilation is blunted in adult humans with borderline‐high plasma LDL‐cholesterol compared with adults with optimal/near optimal LDL‐cholesterol levels; and, if so: (2) whether the magnitude of impairment in adults with borderline high LDL cholesterol is similar to adults with LDL‐cholesterol levels in the high range ($160 mg/dL). To address these aims, we measured forearm blood flow (FBF) responses to intraarterial infusion of the endothelial agonist acetylcholine, in the absence and presence of the endothelial NO synthase inhibitor NG‐monomethyl‐L‐arginine (L‐NMMA), in adults with LDL‐cholesterol levels across the following ATP III classifications: optimal/near optimal, borderline high and high.
Methods
Subjects
Fifty sedentary middle‐aged adults (age range: 43–64 years) were studied: 20 in the optimal/near optimal LDL‐cholesterol range (<130 mg/dL); 20 with borderline high LDL‐cholesterol (130–159 mg/dL); and 10 with high LDL‐cholesterol (≥160 mg/dL). LDL‐cholesterol classifications were based on ATP III guidelines regarding plasma LDL‐cholesterol concentrations and cardiovascular risk. 8 All subjects were normotensive (arterial blood pressure < 140/90 mmHg), nonobese and free of overt disease as assessed by medical history, physical examination, and fasting blood chemistries. None of the subjects smoked or were taking medications. All subjects were further evaluated for clinical evidence of coronary artery disease with electrocardiograms and blood pressure at rest and during incremental exercise performed to exhaustion. All of the women were at least 1 year postmenopausal and had never taken or had discontinued use of hormone replacement therapy at least 1 year before the start of the study. Before participation, all of the subjects had the research study and its potential risks and benefits explained fully before providing written informed consent according to the guidelines of the University of Colorado at Boulder.
Body composition and metabolic measurements
Body mass was measured to the nearest 0.1 kg using a medical beam balance. Percent body fat was determined by dual energy x‐ray absorptiometry (Lunar Corp., Madison, WI, USA). Body mass index (BMI) was calculated as weight (kilograms) divided by height (meters) squared. Minimal waist circumference was measured according to published guidelines. 19 Fasting plasma lipid, lipoprotein, glucose, and insulin concentrations were determined using standard techniques. Plasma concentrations of C‐reactive protein (CRP) and oxidized‐LDL (oxLDL) were determined by enzyme immunoassay as previously described by our laboratory. 20
Habitual physical activity and maximum oxygen consumption (VO2 max)
Daily physical activity was assessed by the Stanford Physical Activity Questionnaire and used to document the sedentary status (i.e., absence of regular aerobic and other types of exercise) of the subjects. To assess aerobic fitness, subjects performed incremental treadmill exercise with a modified Balke protocol. Maximal oxygen consumption (VO2 max) was measured with on‐line computer‐assisted open circuit spirometry as described previously. 21
Intraarterial infusion protocol
All studies were performed between 7 am and 10 am in a temperature‐controlled room following a 12‐hour overnight fast as previously described. 22 Briefly, under local anesthesia (1% lidocaine), a 5‐cm, 20‐gauge catheter was inserted in the brachial artery of the nondominant arm. FBF was measured in both the experimental (nondominant) and contralateral (dominant) forearm using strain‐gauge venous occlusion plethysmography at baseline and in response to the various vasoactive agents. Acetylcholine (IOLAB Pharmaceuticals, Duluth, GA, USA) was infused intraarterially at a rates of 4.0, 8.0, 16.0 μg/100 mL tissue/min. Sodium nitroprusside (Abbott Laboratories, Chicago, IL, USA) was infused at rates of 1.0, 2.0, and 4.0 μg/100 mL tissue/min. The sequence of drug administration was randomized to avoid an order effect.
To determine the contribution of nitric oxide to acetylcholine‐mediated endothelium‐dependent vasodilation, FBF responses to acetylcholine were repeated with the coadministration of the endothelial nitric oxide synthase (eNOS) inhibitor L‐NMMA (Clinalfa) in 25 of the 50 subjects: 10 of the 20 subjects in the optimal/near optimal LDL‐C group (6 males/4 females); 10 of the 20 subjects in the borderline‐high LDL‐C group (5 males/5 females); and 5 of 10 subjects in the high LDL‐C group (4 males/1 female). After the initial infusion of acetylcholine at the doses noted above, and allowing for blood flow to return to resting levels, L‐NMMA was infused at 5 mg/min for 5 minutes. Immediately thereafter, the acetylcholine dose‐response was repeated with the continuous infusion of L‐NMMA.
Statistical analysis
Differences in subject characteristics were determined by analysis of variance (ANOVA). Group differences in FBF responses to acetylcholine, sodium nitroprusside, and acetylcholine + L‐NMMA were determined by repeated measures ANOVA. When indicated by a significant F value, a post hoc test using the Newman‐Keuls method was performed to identify differences among the groups. Pearson correlations were determined between variables of interest. There were no significant sex differences with respect to the main effect of LDL‐C with any of the key outcome variables; therefore, the data were pooled and are presented together. All data are presented as mean ± SEM. Statistical significance was set at p < 0.05.
Results
Selected subject characteristics are presented in Table 1. Anthropometric and hemodynamic characteristics were similar across the groups. By design, total cholesterol, and LDL‐C concentrations were different (p < 0.05) between the groups. Triglyceride levels were not significantly different in the optimal/near optimal and borderline high LDL‐C groups, but were significantly higher in the high LDL‐C compared with the other groups. There were no significant group differences in either plasma glucose or insulin concentrations. Plasma oxLDL, but not CRP concentrations, were higher (p < 0.05) in both the borderline high and high LDL‐C groups than the optimal/near optimal LDL‐C group. Ox‐LDL concentrations were similar between the borderline high and high LDL‐C groups.
Table 1.
Selected subject characteristics.
| Variable | Optimal/near optimal LDL‐C (n = 20) | Borderline high LDL‐C (n = 20) | High LDL‐C (n = 10) |
|---|---|---|---|
| Age (years) | 56 ± 1 | 55 ± 1 | 54 ± 2 |
| Males/Females | 12/8 | 13/7 | 7/3 |
| Body mass (kg) | 81.4 ± 2.9 | 81.3 ± 2.5 | 87.0 ± 5.5 |
| BMI (kg/m2) | 27.3 ± 0.7 | 27.2 ± 0.8 | 29.0 ± 1.6 |
| Body fat (%) | 31.8 ± 2.1 | 34.6 ± 1.8 | 33.9 ± 2.7 |
| Waist circumference (cm) | 92.6 ± 2.6 | 91.1 ± 1.9 | 98.5 ± 4.4 |
| Systolic BP (mmHg) | 119 ± 3 | 119 ± 2 | 124 ± 3 |
| Diastolic BP (mmHg) | 75 ± 2 | 77 ± 2 | 76 ± 2 |
| VO2max (mL/kg/min) | 31.4 ± 1.7 | 29.5 ± 1.2 | 29.0 ± 2.2 |
| Total cholesterol (mg/dL) | 184 ± 4 | 214 ± 4* | 253 ± 5*, † |
| LDL‐cholesterol (mg/dL) | 108 ± 3 | 143 ± 2* | 172 ± 2*, † |
| HDL‐cholesterol (mg/dL) | 56 ± 3 | 48 ± 3 | 51 ± 3 |
| Triglycerides (mg/dL) | 97 ± 10 | 114 ± 13 | 153 ± 18* |
| Glucose (mg/dL) | 94 ± 2 | 91 ± 2 | 94 ± 3 |
| Insulin (μU/mL) | 5.5 ± 0.5 | 6.0 ± 0.6 | 6.0 ± 1.0 |
| CRP (mg/L) | 1.9 ± 0.5 | 2.0 ± 0.5 | 1.8 ± 0.8 |
| oxLDL (U/L) | 46.6 ± 3.6 | 61.8 ± 4.0* | 69.0 ± 8.8* |
BMI = body mass index; BP = blood pressure; VO2max = maximal oxygen uptake; LDL = low‐density lipoprotein; HDL = high‐density lipoprotein; CRP = C‐reactive protein; oxLDL = oxidized low‐density lipoprotein. Values are mean ± SEM.*p < 0.05 versus normal weight; †p < 0.05 versus overweight.
Resting FBF in the noninfused arm and mean arterial pressure were unchanged throughout the infusion protocols (data not presented). Figure 1 shows the FBF responses to acetylcholine and sodium nitroprusside. Resting FBF was not significantly different between groups. However, the increase in FBF to acetylcholine was ∼20% less (p < 0.05) in the borderline‐high (from 4.3 ± 0.2 to 12.3 ± 0.8 mL/100 mL tissue/min) and high (from 4.3 ± 0.3 to 12.0 ± 0.5 mL/100 mL tissue/min) LDL‐cholesterol groups compared with the optimal/near optimal (from 4.4 ± 0.2 to 14.5 ± 0.5 mL/100 mL tissue/min) LDL‐cholesterol group. Of note, the vasodilator responses to acetylcholine were not significantly different between the borderline high and high LDL‐cholesterol groups. In fact, total blood flow response to acetylcholine (area under the curve) was almost identical between the borderline‐high (53.1 ± 3.8 mL/100 mL tissue) and high (56.4 ± 6.1 mL/100 mL tissue) LDL‐cholesterol groups with both significantly lower than the optimal/near optimal (73.8 ± 5.0 mL/100 mL tissue) LDL‐cholesterol group. There were no significant group differences in FBF responses to sodium nitroprusside.
Figure 1.
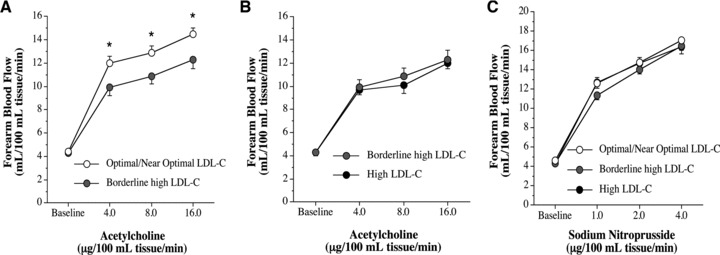
FBF responses to acetylcholine in the: optimal/near optimal and borderline‐high LDL‐cholesterol groups (Panel A); and borderline high and high LDL‐cholesterol (LDL‐C) groups (Panel B). Panel C shows the FBF responses to sodium nitroprusside amongst the groups. Values are mean ± SEM; *p < 0.05.
Infusion of L‐NMMA produced a significant reduction (∼35%) in resting FBF in all three groups ( Figure 2 ). Coinfusion of L‐NMMA significantly reduced the vasodilator response to acetylcholine in the optimal/near optimal LDL‐cholesterol group but not the borderline high or high LDL‐cholesterol groups ( Figure 3 ). For example, FBF at the peak dose of acetylcholine was ∼30% lower (p < 0.05) with L‐NMMA in the optimal/near optimal LDL‐cholesterol group (from 14.9 ± 0.7 to 10.5 ± 1.0 mL/100 mL tissue/min); whereas, peak FBF to acetylcholine was only marginally affected by L‐NMMA in both the borderline high (from 10.8 ± 1.3 to 9.0 ± 1.0 mL/100 mL tissue/min) and high (from 11.5 ± 0.4 to 10.0 ± 0.5 mL/100 mL tissue/min) LDL‐cholesterol groups. Moreover, total blood flow response to acetylcholine with L‐NMMA was markedly lower (∼30%; p < 0.05) in the optimal/near optimal LDL‐cholesterol group (79.7 ± 6.2 mL/100 mL tissue vs. 55.3 ± 9.8 mL/100 mL tissue) but not significantly different in either the borderline high LDL (47.8 ± 9.2 mL/100 mL tissue vs. 41.0 ± 6.9 mL/100 mL tissue) or high (52.0 ± 6.0 mL/100 mL tissue vs. 45.2 mL/100 mL tissue) LDL‐cholesterol groups.
Figure 2.
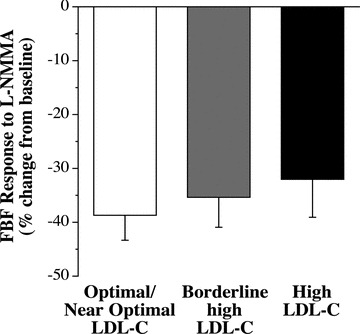
Resting FBF response to L‐NMMA in the optimal/near optimal, borderline high and high LDL‐cholesterol groups. Values are mean ± SEM.
Figure 3.
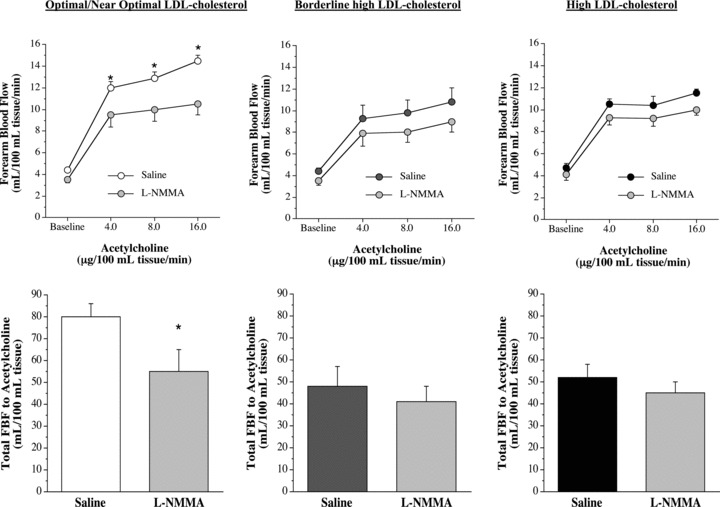
FBF responses and total FBF (area under the curve) to acetylcholine in the absence and presence of nitric oxide synthase inhibitor NG‐monomethyl‐L‐arginine (L‐NMMA) in the optimal/near optimal, borderline high and high LDL‐cholesterol groups. Values are mean ± SEM; *p < 0.05 versus saline.
In the overall study population, both total cholesterol (r=−0.39; p < 0.05) and LDL‐C (r=−0.43; p < 0.05) were inversely related to the peak FBF response to acetylcholine. There were no other significant univariate correlations between any anthropometric, hemodynamic, or metabolic variables and the FBF response to acetylcholine.
Discussion
The key findings of this study are as follows: (1) NO‐mediated endothelium‐dependent vasodilation is impaired in adults with borderline high plasma LDL‐cholesterol levels, independent of other cardiovascular risk factors; and (2) the degree of impairment in NO‐mediated endothelial vasodilation in adults with borderline high LDL‐cholesterol is similar to that observed in adults with high LDL‐cholesterol. Taken together, these results indicate that diminished NO‐mediated endothelium‐dependent vasodilation accompanies hypercholesterolemia and appears to develop with modest elevations in LDL‐cholesterol.
A recent study by Walker et al. 23 was the first to indicate that borderline high LDL‐cholesterol levels was associated with endothelial vasodilator dysfunction. Using ultrasound to measure brachial artery flow‐mediated dilation, they reported that the vasodilation response to hyperemia was 35% lower in sedentary, older men (66 ± 1 year) with borderline high LDL‐cholesterol (142 ± 2 mg/dL) compared with men of similar age with optimal/near optimal LDL‐cholesterol (105 ± 3.0 mg/dL) levels. The results of this study compliment and significantly extend these findings by demonstrating that vasodilation to the endothelial agonist acetylcholine is blunted in adults with borderline high LDL‐cholesterol and that this impairment is due, in large part, to decreased NO bioavailability. Indeed, compared with adults with optimal/near optimal LDL‐cholesterol levels the FBF response to acetylcholine was markedly lower (∼20%) in the borderline high‐cholesterol group. Moreover, in contrast to the optimal/near optimal group, the coinfusion of the NO synthase inhibitor L‐NMMA did not significantly affect the FBF responses to acetylcholine in the borderline high LDL‐cholesterol group; indicating that the contribution of NO to the vasodilator response to acetycholine was reduced in adults with borderline high LDL‐cholesterol. To our knowledge, this is the first study to assess the influence of borderline high LDL‐cholesterol levels on NO‐mediated endothelium‐dependent vasodilation. Deficient NO bioavailability, in addition to affecting vasodilator function, is associated with an endothelial phenotype that is less resistant to atherosclerosis and more prone to initiate thrombotic processes. 24 Considering the relative risk of coronary heart disease is approximately 70% higher with LDL‐cholesterol in the borderline high compared with optimal/near optimal range, reduction in NO bioactivity and diminished endothelial vasodilator function may contribute to this increased vascular risk.
Interestingly, the magnitude of impairment in NO‐mediated endothelium‐dependent vasodilation in the adults with borderline high LDL‐cholesterol was similar to that observed in the adults with high LDL‐cholesterol (>160 mg/dL). Both groups demonstrated ∼20% lower FBF responses to acetylcholine compared with the optimal/near optimal LDL‐cholesterol group and almost identical nonsignificant changes in acetylcholine‐stimulated vasodilation with L‐NMMA. It is important to note, that the degree of impairment in NO‐mediated endothelial vasodilation in our adults with high LDL‐cholesterol is consistent with previous studies employing the same isolated forearm model and pharmacological approach as in this study. 14 , 15 For example, Casino et al. 14 reported markedly blunted forearm vasodilation to acetylcholine as well as no significant effect of L‐NMMA on acetycholine‐mediated vasodilation in hypercholesterolemic (LDL‐cholesterol: 179 ± 33 mg/dL) adults. To our knowledge this study is the first to directly compare endothelial vasodilator function in adults with borderline high and high LDL‐cholesterol levels. Our results indicate that the NO‐related endothelial vasodilator dysfunction observed with high LDL‐cholesterol is already apparent at borderline high LDL‐cholesterol concentrations, suggesting that endothelial dysfunction occurs early with elevations in LDL‐cholesterol. It is important to emphasize that the subjects with borderline high LDL‐cholesterol were free of other cardiovascular risk factors that usually accompany dyslipidemia and are associated with endothelial dysfunction such as obesity and type 2 diabetes. If other risk factors were present it is quite possible the degree of vascular impairment would have been worse. Nevertheless, given the strong link between endothelial dysfunction and atherosclerosis, 11 therapeutic interventions that not only lower LDL‐cholesterol but also favorably influence endothelial function should be considered when formulating a treatment plan for any adult with borderline high LDL‐cholesterol. For example, in addition to its well‐described lipid lowering effects, statin therapy has been shown to increase endothelium‐dependent vasodilation via an increase in NO bioavailability. 25 Improved endothelial function may play a role in the success of primary prevention trials demonstrating the cardiovascular benefit of statin therapy targeting LDL‐cholesterol.
Although we demonstrate that the impairment in endothelial vasodilator function in adults with borderline high LDL‐cholesterol is mediated, at least in part, by diminished NO bioavailability the mechanisms underlying the apparent deficit in the NO system is not clear. There were no group differences in the resting FBF response to L‐NMMA indicating that basal production of NO is not altered by elevations in LDL‐cholesterol. This finding is consistent with those of Casino et al. 14 in a comparable population of adults with high LDL‐cholesterol levels. However, under stimulated conditions (i.e., acetylcholine infusion), NO bioavailability is depressed suggesting that the ability to increase production is impaired or potentially the release of NO is compromised. In adults with high LDL‐cholesterol the administration of L‐arginine, the primary substrate for endothelial NO synthesis by NO synthase, does not improve the vasodilator response to acetylcholine ruling out inadequate intracellular NO substrate availability. 26 Increases in oxLDL, which often accompany elevations in LDL‐cholesterol, may be a primary culprit underlying the cholesterol‐related reduction in NO bioavailability. In vitro studies have shown that oxLDL severely disrupts NO‐mediated responses. 27 , 28 In an elegant series of studies Blair et al. 27 demonstrated that oxLDL, but not native LDL, inhibits acetylcholine‐stimulated activation of eNOS by altering the ability of eNOS to associate with caveola membranes and the caveola protein, caveolin. The interaction of eNOS with caveolin helps to regulate the activity of the enzyme. oxLDL depletes caveola membranes of cholesterol causing a redistribution of eNOS to other intracellular membranes. The severing of the interaction between eNOS and caveola diminishes the activation of the enzyme to acetylcholine ultimately resulting in limited NO production. oxLDL also promotes superoxide anion formation creating an environment prone to oxidative inactivation of NO. 29 In this study, oxLDL concentrations were significantly higher in both the borderline high and high LDL‐cholesterol groups compared with the optimal/near optimal LDL‐cholesterol group. Although there was no significant relation between plasma oxLDL levels and the FBF responses to either acetylcholine or L‐NMMA in this study, we can not dismiss the potential impact of oxLDL on the vascular endothelium and, in turn, our results. There are currently no data on the influence of borderline high LDL‐cholesterol on the endothelin (ET)‐1 system. ET‐1 is a potent vasoconstrictor peptide produced by the endothelium that plays critical role in the regulation of vascular tone and has been implicated in the atherosclerotic process. 30 ET‐1‐mediated vasoconstrictor tone has been shown to be higher with hypercholesterolemia (LDL‐cholesterol >160 mg/dL) contributing to diminished NO‐mediated endothelium‐dependent vasodilation. 31 , 32 , 33 It is possible that ET‐1 vasoconstrictor activity is also enhanced with LDL‐cholesterol levels in the borderline high range. If so, the ET‐1 system may represent an important therapeutic target to improve vascular health and reduce cardiovascular risk in adults with borderline high LDL‐cholesterol. Studies are currently ongoing in our laboratory to address this issue.
There are a few experimental considerations regarding this study that deserve mention. First, considering our cross‐sectional study design, we cannot dismiss the possibility that genetic and/or lifestyle behaviors may have influenced the results. To minimize the influence of lifestyle behaviors, all subjects were nonsmokers, were not currently taking medication, and did not differ in habitual physical activity or aerobic fitness. In addition, all subjects were carefully screened to eliminate the confounding effects of clinically overt cardiovascular and metabolic disease. Second, we did not perform an analysis of LDL particle size or number, thus we are unable to comment on the possible influence of differences in these parameters on endothelial function. There are data to suggest that a preponderance of smaller LDL particles is more atherogenic and predictive of cardiovascular events. 34 However, these measures are not typically performed clinically and standard of care are not based upon these LDL subclasses.
In conclusion, the seminal finding of this study is that borderline high LDL‐cholesterol levels, independent of other cardiovascular risk factors, is associated with impaired NO‐mediated endothelium‐dependent vasodilation. In fact, the magnitude of impairment in endothelial vasodilator function in adults with borderline high LDL‐cholesterol is not different to that observed in adults with clinically high LDL‐cholesterol. Thus borderline high LDL‐cholesterol, even in adults with no other risk factors, is associated with a proatherogenic endothelial phenotype. Endothelial vasodilator dysfunction may contribute to the increase in atherosclerotic risk associated with LDL‐cholesterol in the borderline high range.
Acknowledgments
We would like to thank all of the subjects who participated in the study.
This study was supported by National Institutes of Health awards HL077450, HL076434, MOI RR00051, and 1 UL1 RR025780.
References
- 1. Grundy SM, Cleeman JI, Merz CN, Brewer HB Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC Jr, Stone NJ. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol. Aug 4 2004; 44(3): 720–732. [DOI] [PubMed] [Google Scholar]
- 2. Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. May 12 1998; 97(18): 1837–1847. [DOI] [PubMed] [Google Scholar]
- 3. Stamler J, Wentworth D, Neaton JD. Is relationship between serum cholesterol and risk of premature death from coronary heart disease continuous and graded? Findings in 356,222 primary screenees of the Multiple Risk Factor Intervention Trial (MRFIT). JAMA. Nov 28 1986; 256(20): 2823–2828. [PubMed] [Google Scholar]
- 4. The Lipid Research Clinics Coronary Primary Prevention Trial results. II. The relationship of reduction in incidence of coronary heart disease to cholesterol lowering. JAMA. Jan 20 1984; 251(3): 365–374. [PubMed] [Google Scholar]
- 5. Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, Langendorfer A, Stein EA, Kruyer W, Gotto AM Jr. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. May 27 1998; 279(20): 1615–1622. [DOI] [PubMed] [Google Scholar]
- 6. Sever PS, Dahlöf B, Poulter NR, Wedel H, Beevers G, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower‐than‐average cholesterol concentrations, in the Anglo‐Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT‐LLA): a multicentre randomised controlled trial. Lancet. Apr 5 2003; 361(9364): 1149–1158. [DOI] [PubMed] [Google Scholar]
- 7. Ferrieres J. Effects on coronary atherosclerosis by targeting low‐density lipoprotein cholesterol with statins. Am J Cardiovasc Drugs. 2009; 9(2): 109–115. [DOI] [PubMed] [Google Scholar]
- 8. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. Dec 17 2002; 106(25): 3143–3421. [PubMed] [Google Scholar]
- 9. Luscher T, Tanner F, Tschudi M, Noll G. Endothelial dysfunction in coronary artery disease. Ann Rev Med. 1993; 44: 395–418. [DOI] [PubMed] [Google Scholar]
- 10. Yasue H, Matsuyama K, Matsuyama K, Okumura K, Morikami Y, Ogawa H. Responses of angiographically normal human coronary arteries to intracoronary injection of acetylcholine by age and segment. Possible role of early coronary atherosclerosis. Circulation. 1990; 81: 482–490. [DOI] [PubMed] [Google Scholar]
- 11. Vanhoutte PM, Shimokawa H, Tang EH, Feletou M. Endothelial dysfunction and vascular disease. Acta Physiol (Oxf). Jun 2009; 196(2): 193–222. [DOI] [PubMed] [Google Scholar]
- 12. Holland JA, Meyer JW, Schmitt ME, Sauro MD, Johnson DK, Abdul‐Karim RW, Patel V, Ziegler LM, Schillinger KJ, Small RF, et al. Low‐density lipoprotein stimulated peroxide production and endocytosis in cultured human endothelial cells: mechanisms of action. Endothelium. 1997; 5(3): 191–207. [DOI] [PubMed] [Google Scholar]
- 13. Alderson LM, Endemann G, Lindsey S, Pronczuk A, Hoover RL, Hayes KC. LDL enhances monocyte adhesion to endothelial cells in vitro. Am J Pathol. May 1986; 123(2): 334–342. [PMC free article] [PubMed] [Google Scholar]
- 14. Casino P, Kilcoyne C, Quyyumi A, Hoeg J, Panza J. The role of nitric oxide in endothelium‐dependent vasodilation of hypercholesterolemic patients. Circulation. 1993; 88: 2541–2547. [DOI] [PubMed] [Google Scholar]
- 15. Casino PR, Kilcoyne CM, Cannon RO, 3rd , Quyyumi AA, Panza JA. Impaired endothelium‐dependent vascular relaxation in patients with hypercholesterolemia extends beyond the muscarinic receptor. Am J Cardiol. 1995; 75(1): 40–44. [DOI] [PubMed] [Google Scholar]
- 16. Chowienczyk P, Watts G, Cockcroft J, Ritter J. Impaired endothelium‐dependent vasodilation of forearm resistance vessels in hypercholesterolemia. Lancet. 1992; 340: 1430–1432. [DOI] [PubMed] [Google Scholar]
- 17. Weil BR, Stauffer BL, Greiner JJ, DeSouza CA. Prehypertension is associated with impaired nitric oxide‐mediated endothelium‐dependent vasodilation in sedentary adults. Am J Hypertens. Sep 2011; 24(9): 976–981. [DOI] [PubMed] [Google Scholar]
- 18. Steinberg H, Chaker H, Leaming R, Johnson A, Brechtel G, Baron A. Obesity/insulin resistance is associated with endothelial dysfunction. J Clin Invest. 1996; 97: 2601–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lohman T, Roche A, Mortorell R. Athropometric Standardization Reference Manual. Champaign , IL : Human Kinetics; 1988. [Google Scholar]
- 20. Van Guilder GP, Hoetzer GL, Greiner JJ, Stauffer BL, Desouza CA. Influence of metabolic syndrome on biomarkers of oxidative stress and inflammation in obese adults. Obesity (Silver Spring). Dec 2006; 14(12): 2127–2131. [DOI] [PubMed] [Google Scholar]
- 21. DeSouza C, Jones P, Seals D. Physical activity status and age‐related differences in coagulation and fibrinolytic factors in women. Arterioscler Thromb Vasc Biol. 1998; 18: 362–368. [DOI] [PubMed] [Google Scholar]
- 22. DeSouza C, Clevenger C, Greiner J, Smith D, Hoetzer G, Shapiro L, Stauffer B. Evidence for agonist‐specific endothelial vasodilator dysfunction with ageing in healhty humans. J Physiol (London). 2002; 542: 255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Walker AE, Eskurza I, Pierce GL, Gates PE, Seals DR. Modulation of vascular endothelial function by low‐density lipoprotein cholesterol with aging: influence of habitual exercise. Am J Hypertens. Mar 2009; 22(3): 250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. Jan 25 2005; 111(3): 363–368. [DOI] [PubMed] [Google Scholar]
- 25. Beckman JA, Creager MA. The nonlipid effects of statins on endothelial function. Trends Cardiovasc Med. Jul 2006; 16(5): 156–162. [DOI] [PubMed] [Google Scholar]
- 26. Casino PR, Kilcoyne CM, Quyyumi AA, Hoeg JM, Panza JA. Investigation of decreased availability of nitric oxide precursor as the mechanism responsible for impaired endothelium‐dependent vasodilation in hypercholesterolemic patients. J Am Coll Cardiol. Mar 15 1994; 23(4): 844–850. [DOI] [PubMed] [Google Scholar]
- 27. Blair A, Shaul PW, Yuhanna IS, Conrad PA, Smart EJ. Oxidized low density lipoprotein displaces endothelial nitric‐oxide synthase (eNOS) from plasmalemmal caveolae and impairs eNOS activation. J Biol Chem. Nov 5 1999; 274(45): 32,512–32,519. [DOI] [PubMed] [Google Scholar]
- 28. Wang W, Hein TW, Zhang C, Zawieja DC, Liao JC, Kuo L. Oxidized low‐density lipoprotein inhibits nitric oxide‐mediated coronary arteriolar dilation by up‐regulating endothelial arginase I. Microcirculation. Jan 2011; 18(1): 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chin JH, Azhar S, Hoffman BB. Inactivation of endothelial derived relaxing factor by oxidized lipoproteins. J Clin Invest. Jan 1992; 89(1): 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miyauchi T, Masaki T. Pathophysiology of endothelin in the cardiovascular system. Ann Rev Physiol. 1999; 61: 391–415. [DOI] [PubMed] [Google Scholar]
- 31. Cardillo C, Kilcoyne C, Cannon R, Panza J. Increased activity of endougenous endothelin in patients with hypercholesterolemia. J Am Coll Cardiol. 2000; 36: 1483–1488. [DOI] [PubMed] [Google Scholar]
- 32. Best P, mcKenna C, Hasdai D, Holmes D, Lerman A. Chronic endothelin‐receptor antagonism preserves coronary endothelial function in experimental hypercholesterolemia. Circulation. 1999; 99: 1747–1752. [DOI] [PubMed] [Google Scholar]
- 33. Best PJ, Lerman LO, Romero JC, Richardson D, Holmes DR, Jr. , Lerman A. Coronary endothelial function is preserved with chronic endothelin receptor antagonism in experimental hypercholesterolemia in vitro. Arterioscler Thromb Vasc Biol. Nov 1999; 19(11): 2769–2775. [DOI] [PubMed] [Google Scholar]
- 34. Superko HR, Gadesam RR. Is it LDL particle size or number that correlates with risk for cardiovascular disease? Curr Atheroscler Rep. Oct 2008; 10(5): 377–385. [DOI] [PubMed] [Google Scholar]


