Abstract
Abdominal aortic aneurysm (AAA) is a common condition with high mortality when ruptured. Most clinicians agree that small AAAs are best managed by ultrasonographic surveillance. However, it has been stated in recent reviews that a serum/plasma biomarker that predicts AAA rupture risk would be a powerful tool in stratifying patients with small AAA. Identification of such circulating biomarkers has been to date unsuccessful. In this study, we used a proteomic approach to find new, potential plasma AAA biomarker candidates. Prefractionated plasma samples were analyzed by two‐dimensional differential in‐gel electrophoresis to identify differentially expressed proteins between four patients with small AAA and four controls without aneurysm. Protein spots that differed significantly between patients and controls were selected and identified by mass spectrometry. Three protein spots had significantly different expression between patients and controls. The most interesting finding was that patients with small AAA had increased levels of the enzyme glycosylphosphatidylinositol‐specific phospholipase D (GPI‐PLD) compared with the controls without aneurysm. In conclusion, by using a proteomic approach, this pilot‐study provides evidence of GPI‐PLD as a novel potential plasma biomarker for AAA. Clin Trans Sci 2012; Volume 5: 56–59
Keywords: aortic aneurysm, proteomics, biomarker, glycosylphosphatidylinositol‐specific phospholipase D
Introduction
Abdominal aortic aneurysm (AAA) is a common condition with a prevalence of around 5% in men over 50 years of age. 1 , 2 The condition is often asymptomatic until the catastrophic onset of hemorrhagic shock due to aneurysm rupture. Despite advances in surgical and anesthetic techniques the perioperative mortality remains high in patients with ruptured AAA. 1 A presymptomatic elective repair in appropriately selected individuals will prevent rupture and thereby increase life expectancy. AAA screening programs have been introduced in an attempt to reduce mortality due to ruptured AAA in the general population. However, most clinicians agree that due to a very low rupture rate, small AAAs are best managed by ultrasonographic surveillance and that an AAA diameter above 5.0–5.5 cm generally justifies elective repair. Two studies have shown the safety of surveillance until a diameter of the AAA reaches 5.5 cm among male patients. 3 , 4 The expansion pattern of AAAs is estimated to be about 10% per annum. 1 However, there are large individual variations in expansion patterns. Episodes of rapid expansion maybe followed by periods of slower, or even cessation of, expansion. 1 , 2 Finding a biomarker for identifying aneurysms with progressive growth, that indicates a necessity for treatment, seems important. Biomarker discovery will allow identification of aneurysms more likely to rupture and stratify high‐risk patients.
Human plasma is one of the most important proteomes from a clinical and medical point of view 5 However, plasma is also the most complex human‐derived sample for proteomic analysis because it contains the widest dynamic range of cellular proteins. 5 Recent advances in proteomic technologies, including two‐dimensional differential in‐gel electrophoresis (2D‐DIGE) and improved mass spectrometry (MS), have provided new opportunities for identifying biomarkers. 5 , 6 2D‐DIGE is effective in separating complex protein samples and in quantifying protein levels between samples. 7 By loading an internal common standard sample labeled with different fluorescent dyes, gel‐to‐gel variation is cancelled out, and quantitative proteomic profiling can be achieved across multiple samples. 7 Proteins of interest can then be identified by tandem mass spectrometry (MS/MS). 5 , 6 Moreover, recent studies that focus on combining fractionation with 2D gel analysis and liquid chromatography (LC) coupled with MS have shown improved protein identification. 6 A major problem in proteome studies which use plasma and serum samples is that high abundance proteins mask low abundance proteins. 8 Several depletion and fractionation technologies have been developed to remove highly abundant proteins such as albumin and immunoglobulin G. Recently, a novel sample enrichment tool (ProteoMiner; Bio‐Rad Taboratories, Hercules, CA, USA) has been proposed as a promising and powerful alternative to common immuno‐subtraction tools. 8 This protein enrichment tool is based on the interaction of complex protein sample with a large, highly diverse library of hexapeptides bound to a chromatographic support where each unique hexapeptide binds to a unique protein sequence. Treatment of samples with the ProteoMiner (Bio‐Rad) kit causes partial depletion of high‐abundance proteins and simultaneous concentration of low‐abundance proteins, resulting in dynamic range compression of samples. 8
Previous AAA biomarker research has focused of one or few possible markers in each study. 9 A proteomic approach to screen for new biomarker candidates has been suggested. 9 , 10 , 11 Proteomic analysis allows a simultaneous detection of changes in hundreds of proteins in each study and thus provides invaluable insight to mechanisms of the AAA disease. 5 Thus, proteomic analysis is a convenient method to monitor changes in protein expression without prior knowledge of what those changes might be.
In this study, we aimed to find potential biomarker candidates for the AAA disease. We used the ProteoMiner technology (Bio‐Rad) for plasma enrichment followed by 2D‐DIGE analysis combined with nanoliquid chromatography‐Fourier transform ion cyclotron resonance MS for detection of differences in the protein profile between patients with small AAA and controls without aneurysm.
Methods
Subjects and blood sampling
The study was performed in accordance with the principles of the Declaration of Helsinki. Patients and control subjects signed an informed consent form approved by the local ethics committee. Ultrasonography investigation of the abdominal aorta was performed in AAA patients and controls. Patients and controls were stratified by sex (male), age, and smoking habit (nonsmoker). Four male AAA patients and four male controls without AAA were included. The average age was 72 (69–75) years in the AAA group and 71 (64–77) years in the control group. All subjects were nonsmokers but three subjects in each group were former smokers. The AAA patients had small infrarenal aneurysm (aortic diameter: A1 = 3.8 cm, A2 = 3.6 cm, A3 = 4.2 cm, A4 = 4.2 cm) and the controls had normal infrarenal aortic diameter (defined as diameter <3.0 cm). None of the subjects had coexisting malignant disease, uremia, diabetes, neither statin, nor anticoagulant therapy. Peripheral venous blood samples were taken from controls and patients. Samples were centrifuged within 30 minutes at 2000 g for 20 minutes and aliquots of citrated plasma were frozen and stored at −70°C until analysis.
Sample preparation and CyDye labeling
The plasma samples were treated with a large, highly diverse bead‐base library of combinatorial peptide ligands (ProteoMiner; Bio‐Rad) in accordance with manufacturer's instructions. This reduced the dynamic range of protein concentrations while maintaining representatives of all proteins within the original sample. The comparison between AAA patients and controls was performed by 2D‐DIGE analyses across four gels, using the same pooled‐sampled internal standard, the equimolecular mixture of all the samples, in all gels. The samples were CyDye labeled according to the manufacture's standard protocol (GE Healthcare), using 400 pmol of dye reagent for every 50 μg of sample protein. Individual samples were labeled with Cy3 or Cy5 dyes using dye switching, and the internal standard was always Cy2 labeled.
Two‐dimensional electrophoresis and image analysis
Isoelectric focusing was done in 24 cm pH 3–11 Nonlinear Imobiline Dry Strip (GE Healthcare, Uppsala, Sweden) on an Ettan IPGphore. The second dimension were run on an Ettan DALT II in in‐house made 1 mm polyacrylamide (T= 11%, C= 2.6%) Bis‐Tris gel with standard MOPS cathode buffer and acetic acid/diethanol amine anode buffer. After 2D electrophoresis, gels were scanned using the 2920 Master Imager (Amersham Bioscience, Uppsala, Sweden) using excitation/emission wavelengths specific for the different CyDyes. Gel images were analyzed using the Progenesis Same Spots software version 3.3 (Nonlinear Dynamics, Durham, NC, USA) for spot detection, spot quantification, intergel matching, and statistics. Spots were selected for spot picking and further identification by MS analysis.
Spot picking and in‐gel protein digestion
Selected protein spots, on a preparative gel of pooled samples to a total protein concentration of 450 μg stained with SYPRO Ruby, were picked and trypsinated in the Ettan Spot‐handling Workstation (GE Healthcare). The method for in‐gel protein digestion with trypsin described by Shevchenko et al 12 was applied with some minor modifications. Briefly, the gel pieces were destained by washing three times in 25 mM NH4HCO3 in 50% CH3 OH and once in 70% CH3CN. Gel pieces were dried and incubated with digestion buffer (50 mM NH4HCO3, 10 ng/μL trypsin) at 37°C for 3 hours. Peptides were extracted in 50% CH3CN/0.5% TFA and the supernatant was evaporated to dryness. Before MS analysis, the peptides were reconstituted in 0.2% HCOOH.
Protein identification
Sample injections were made with an HTC‐PAL auto‐sampler (CTC Analytics AG, Zwingen, Switzerland) connected to an Agilent 1100 binary pump (Agilent Technologies, Palo Alto, CA, USA). The peptides were trapped on a precolumn (45 × 0.075 mm i.d.) and separated on a reversed phase column, 200 × 0.050 mm. Both columns are packed in‐house with 3 μm Reprosil‐Pur C18‐AQ particles. The flow‐through to the analytical column was reduced by a split of approximately 100 nL/min. A 40 minute gradient 10–50% CH3CN in 0.2% COOH was used for separation of the peptides. For more details see Carlsohn et al. 13
The nanoflow LC‐MS/MS was performed on a hybrid linear ion trap‐FTICR mass spectrometer equipped with a 7 T ICR magnet (LTQ‐FT; Thermo Electron, Bremen, Germany). The spectrometer was operated in data‐dependent mode, automatically switching to MS/MS mode. MS‐spectra were acquired in the FTICR, whereas MS/MS‐spectra were acquired in the LTQ‐trap. For each scan of FTICR, the three most intense, doubly or triply charged, ions were sequentially fragmented in the linear trap by collision‐induced dissociation. All the tandem mass spectra were searched by MASCOT (Matrix Science, London, UK) against all species in the NCBI database.
The search parameters were set to: MS accuracy 5 ppm, MS/MS accuracy 0.5 Da, one missed cleavage by trypsin allowed, fixed propionamide modification of cysteine and variable modification of oxidized methionine.
For protein identification, the minimum criteria were; one tryptic peptide matched at or above the 99% level of confidence and one additional peptide match at the 95% level.
Results
Image analysis
Three spots, of 1234 detected, with significant confidence (ANOVA p < 0.05) were selected from the gel image analysis according to Table 1 . The location of the protein spots on the gel can be seen in Figure 1 . The variation in protein expression for spot #151 is illustrated in Figure 2 . A clear up‐regulation in small AAA can be seen in all gels except for gel 2 where the control has a quite high level.
Table 1.
Identification of proteins in selected gel spots by MS analysis and database search.
| Spot | Fold change* | Anova (p) | Protein | Accesion | Score | No. peptides† | Coverage (%)‡ | |
|---|---|---|---|---|---|---|---|---|
| 151 | + 1.4 | 0.0405 | Phosphatidylinositol‐glycan‐specific phospholipase D | PHLD_HUMAN | P80108 | 699 | 16 | 21 |
| ″ | Inter‐alpha‐trypsin inhibitor heavy chain H4 | ITIH4_HUMAN | Q14624 | 256 | 6 | 6 | ||
| 211 | −1.7 | 0.0368 | Ig mu chain C region | IGHM_HUMAN | P01871 | 361 | 12 | 38 |
| ″ | Gelsolin | GELS_HUMAN | P06396 | 112 | 2 | 4 | ||
| 379 | + 1.6 | 0.0290 | Ig gamma‐1 chain C region | IGHGI_HUMAN | P01857 | 236 | 13 | 43 |
| ″ | Ig gamma‐2 chain C region | IGHG2_HUMAN | P01859 | 150 | 9 | 29 | ||
*Up‐regulated (+), down‐regulated (‐).
†Number of unique peptides identified.
‡Total protein sequence coverage.
Figure 1.
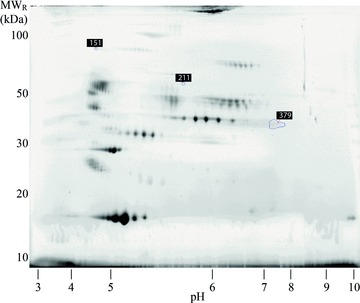
Reference image of 2D electrophoresis gels used in this study. Selected spots for MS analysis are marked with their spot number.
Figure 1.
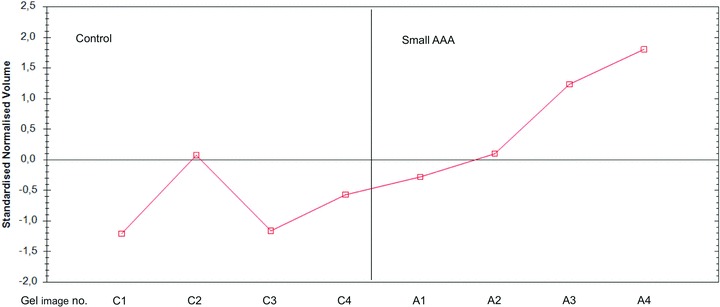
Expression profile of spot 151. Anotation on the x‐axis: C, control samples; A, small AAA samples; numbers 1–4, gel number. C1 and A1, etc. are images from the same gel.
Protein identification
Identification of proteins in the three spots from the gel ( Figure 1 ) is summarized in Table 1 . For spot 151, the main protein was found to be phosphatidylinositol‐glycan‐specific phospholipase D (P80108). Sixteen peptides were identified with a total protein sequence cover rate of 21%. A small amount of inter‐alpha‐trypsin inhibitor (Q14624) was also present in spot 151 (six peptides and a coverage of 6%). The other two spots contained mainly immunoglobulins, see Table 1 for details.
Discussion
Recent reviews state that a serum/plasma biomarker predicting aortic rupture risk would be a powerful tool to stratify patients with small screen detected aneurysms. 9 , 10 , 11 Identification of such circulating biomarkers has been unsuccessful and proteomic techniques to screen for new biomarker candidates is currently suggested. Because male gender, increasing age, and smoking are the dominant risk factors for AAA 1 we used a control group matched by age, gender, and smoking habits to the AAA patient group in this study to eliminate possible bias in accordance with the guidelines given by Grimes and Schulz. 14 By using a proteomic approach the present pilot‐study has identified three proteins which are significantly altered between patients with small AAA and controls without aneurysm. The regulation of biological events by enzymatic activity is a common paradigm in both normal and pathological states. From this point of view the most interesting of the identified proteins is the enzyme glycosylphosphatidylinositol‐specific phospholipase D (GPI‐PLD, spot 151), which is increased in patients with small AAA, compared with controls without aneurysm. As spot 151 also contains smaller amount of inter‐alpha‐trypsin inhibitor the possible contribution from this protein to the upregulation cannot be completely ruled out. The immunoglobulins (spots 211 and 379) are awkward to evaluate due to their diverse function in inflammation, etc.
The amount of gelsolin in spot 211 is low but the contribution to the down‐regulation might not be infinitesimal. No known function of gelsolin is interpreted to have connection to AAA. The enzyme GPI‐PLD was isolated and characterized 20 years ago by Hoener et al. 15 The function of this enzyme is poorly understood. However, it has been suggested that GPI‐PLD might participate in regulating inflammation in atherosclerosis. 16 Furthermore, a recent study shows that GPI‐PLD improves glucose tolerance; an interesting fact since the connection between arteriosclerosis and AAA has been questioned because arteriosclerosis is associated with diabetes in contrast to AAA. 17 , 18 , 19
In conclusion, by using a non–hypothesis‐driven proteomic approach to find new clinical useful biomarkers for AAA, this study provides evidence of GPI‐PLD as a possible biomarker for AAA. Furthermore, statin therapy may be a possible management to reduce the small AAA progression because Deeg et al. in a recent report show that statin reduces the serum level of GPI‐PLD. 20 Finally, the present results have shed new light on the negative association between AAA and diabetes. The findings in the present pilot‐study warrant urgent new investigations.
Acknowledgments
This study was presented (in part) by the first author (JW) at the 2nd International Meeting on Aortic Diseases: New insights into an old problem. September 30–October 1–2, 2010. Congress centre, Liège, Belgium. The Proteomics Core Facility at Sahlgrenska Academy, University of Gothenburg, was funded by a grant from the Knut and Alice Wallenberg Foundation. The authors acknowledge the financial support from Emil Andersson Foundation for Medical Research. We would also like to thank Mrs. Nikki Stephensen Nyberg for helpful linguistic comments on this manuscript.
References
- 1. Sakalihasan N, Limet R, Defawe OD. Abdominal aortic aneurysm. Lancet. 2005; 365: 1577–1589. [DOI] [PubMed] [Google Scholar]
- 2. Lederle FA, Johnson GR, Wilson SE, Chute EP, Hye RJ, Makaroun MS, Barone GW, Bandyk D, Moneta GL, Makhoul RG. The aneurysm detection and management study screening program. Arch Intern Med. 2000; 160: 1425–1430. [DOI] [PubMed] [Google Scholar]
- 3. The UK Small Aneurysm Trial Participants . Mortality results for randomized controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. Lancet. 1998; 352: 1649–1655. [PubMed] [Google Scholar]
- 4. Lederle FA, Wilson SE, Johnson GR, Reinke DB, Littooy FN, Acher CW, Ballard DJ, Messina LM, Gordon IL, EP Chute, al et. Detection and Management Veterans Affairs Cooperative Study Group. Immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002; 346: 1437–1444. [DOI] [PubMed] [Google Scholar]
- 5. Anderson NL, Anderson NG. The human plasma proteome: history, character, and diagnostic prospects. Mol Cell Proteomics. 2002; 11: 845–867. [DOI] [PubMed] [Google Scholar]
- 6. Wilkins MR, Appel RD, Van Eyk JE, Chung MCM, Görg A, Hecker M, Huber LA, Langen H, Link AJ, YK Paik, al et. Guidelines for the next 10 years of proteomics. Proteomics. 2006; 6: 4–8. [DOI] [PubMed] [Google Scholar]
- 7. Marouga R, David S, Hawkins E. The development of the DIGE system: 2D fluorescence difference gel analysis technology. Anal Bioanal Chem. 2005; 382: 669–678. [DOI] [PubMed] [Google Scholar]
- 8. Boschetti E, Lomas L, Citterio A, Righetti PG. Romancing the “hidden proteome,” Anno Domini two zero zero seven. J Chromatogr A. 2007; 1153: 277–290. [DOI] [PubMed] [Google Scholar]
- 9. Urbonavicius S, Urbonaviciene G, Honoré B, Henneberg EW, Vorum H, Lindholt JS. Potential circulating biomarkers for abdominal aortic aneurysm expansion and rupture—a systematic review. Eur J Vasc Endovasc Surg. 2008; 36: 273–280. [DOI] [PubMed] [Google Scholar]
- 10. Golledge J, Tsao PS, Dalman RL, Norman PE. Circulating markers of abdominal aortic aneurysm presence and progression. Circulation. 2008; 118: 2382–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nordon I, Brar R, Hinchliffe R, Cockerill G, Loftus I, Thompson M. The role of proteomic research in vascular disease. J Vasc Surg. 2009; 49: 1602–1612. [DOI] [PubMed] [Google Scholar]
- 12. Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver‐stained polyacrylamide gels. Anal Chem. 1996; 68: 850–858. [DOI] [PubMed] [Google Scholar]
- 13. Carlsohn E, Nyström J, Karlsson H, Svennerholm AM, Nilsson CL. Characterization of the outer membrane protein profile from disease‐related Helicobacter pylori isolates by subcellular fractionation and nano‐LC FT‐ICR MS analysis. J Proteome Res. 2006; 5: 3197–3204. [DOI] [PubMed] [Google Scholar]
- 14. Grimes DA, Schulz KF. Compared to what? Finding controls for case‐control studies. Lancet. 2005; 365:1429–1433. [DOI] [PubMed] [Google Scholar]
- 15. Hoener MC, Stieger S, Brodbeck U. Isolation and characterization of a phosphatidylinositolglycan‐anchor‐specific phospholipase D from bovine brain. Eur J Biochem. 1990; 190: 593–601. [DOI] [PubMed] [Google Scholar]
- 16. O'Brien KD, Pineda C, Chiu WS, Bowen R, Deeg MA. Glycosylphosphatidylinositol‐specific phospholipase D is expressed by macrophages in human atherosclerosis and colocalizes with oxidation epitopes. Circulation. 1999; 99: 2876–2882. [DOI] [PubMed] [Google Scholar]
- 17. Raikwar NS, Bowen‐Deeg RF, Du XS, Low MG, Deeg MA. Glycosylphosphatidylinositol‐specific phospholipase D improves glucose tolerance. Metabolism. 2010; 59: 1413–1420. [DOI] [PubMed] [Google Scholar]
- 18. Norman PE, Davis TM, Le MT, Golledge J. Matrix biology of abdominal aortic aneurysms in diabetes: mechanisms underlying the negative association. Connect Tissue Res. 2007; 48: 125–131. [DOI] [PubMed] [Google Scholar]
- 19. Shantikumar S, Ajjan R, Porter KE, Scott DJA. Diabetes and the abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2010; 39: 200–207. [DOI] [PubMed] [Google Scholar]
- 20. Deeg MA, Raikwar NS, Johnson C, Williams CD. Statin therapy reduces serum levels of glycosylphosphatidylinositol‐specific phospholipase D. Transl Res. 2007; 150: 153–157. [DOI] [PubMed] [Google Scholar]


