Abstract
Principal investigators who received Clinical and Translational Science Awards created academic homes for biomedical research. They developed program‐supported websites to offer coordinated access to a range of core facilities and other research resources. Visitors to the 60 websites will find at least 170 generic services, which this review has categorized in the following seven areas: (1) core facilities, (2) biomedical informatics, (3) funding, (4) regulatory knowledge and support, (5) biostatistics, epidemiology, research design, and ethics, (6) participant and clinical interaction resources, and (7) community engagement. In addition, many websites facilitate access to resources with search engines, navigators, studios, project development teams, collaboration tools, communication systems, and teaching tools. Each of these websites may be accessed from a single site, http://www.CTSAcentral.org. The ability to access the research resources from 60 of the nation's academic health centers presents a novel opportunity for investigators engaged in clinical and translational research. Clin Trans Sci 2012; Volume #: 1–5
Keywords: clinical trials, translational research, biostatistics, phase I, phase II, tests, core services, regulatory knowledge, novel methodologies, research navigation, research resources
The Purpose of This Report
The primary purpose of this report is to increase awareness of the coordinated access to research resources supported by the Clinical and Translational Science Award (CTSA) Program. Before 2006, no single‐point, facilitated access to these resources existed at academic health centers. With support from the CTSA Program, each awardee created a website that provides coordinated access to its resources. All of these websites may be accessed from the CTSA Program home page, http://www.CTSAcentral.org.
The Goal and Vision of the Clinical and Translational Science Program
In 2006, the National Institutes of Health (NIH) initiated the CTSA Program as a means of increasing the efficiency by which ideas and products might move between the research laboratory, the clinical testing arena, and the community to improve health and health care delivery. 1 , 2 The CTSA Program reached its full complement of 60 sites in 2011 with an annual budget of approximately $500 million. Investigators who participate in the CTSA Program share a vision of making each participating academic health center a home for clinical and translational science. 3 A functional home for research includes services to support investigators. The services provided by the CTSA Program sites have been categorized for this review in the following seven areas: (1) core facilities, (2) biomedical informatics, (3) funding, (4) regulatory knowledge and support, (5) biostatistics, epidemiology, research design, and ethics, (6) participant and clinical interaction resources, and (7) community engagement.
Methods
A review of core facilities and other research resources in August 2011 derived information from the 60 CTSA Program site web pages. At least 400 separate service, facility, product, and system items were found in the publicly accessible areas at those sites. The list of separate services was simplified by condensing related services into 170 generic titles (see Table 1). No attempt was made to review the password‐protected categorical items located at 22 of the sites.
Table 1.
Core facilities and other research resources provided by 60 CTSAs.
| Facilities and Services for Key Functions |
|---|
| Translational technologies |
| Amino acid analysis |
| Animal resources support, behavior, surgery, pathology, imaging, physiology, radiation therapy, target identification, model development, embryo preservation |
| Behavioral phenotyping, neuroscience core |
| Biophysical analysis |
| Biorepository, human tissue and fluid specimens, animal, microbiological |
| Body composition |
| Bone studies |
| Cell manufacture, study, biosurgery |
| cGMP facilities |
| Confocal microscopy |
| Cryopreservation of embryos, IVF |
| Cyclotron |
| Cytokine analysis |
| Device development |
| DNA analysis, sequencing, PCR |
| Drosophila |
| Electron microscopy |
| Electrophysiology core |
| Embryonic stem cells |
| Epigenomics |
| Fabrication, engineering, 3‐D printing |
| Flow cytometry |
| Gene therapy |
| Genomics core |
| High throughput screening (small molecules, RNAi, drug libraries) |
| High pressure liquid chromatography |
| Imaging cores |
| Immunology core |
| Intracytoplasmic sperm injection, in vitro fertilization |
| Mass spectrometry |
| Monoclonal antibody |
| Mouse core |
| Mutational analysis |
| Nanotechnology |
| Peptide and proteomics |
| Pharmaceutical core |
| Phenotyping |
| Pulmonary core |
| RNA expression, RNAi assays |
| Structural biology |
| Synchrotron |
| Synthetic chemistry |
| Therapeutic manufacture |
| Tissue bank |
| Toxicology |
| Transgene research |
| Vaccine core |
| Vector production |
| Virology |
| Xenografting |
| X‐ray diffraction crystallography |
| Zebrafish |
| Biomedical informatics |
| Application hosting, training |
| Behavioral data analysis |
| Biospecimen locator |
| Budget development support systems |
| Classification, language, ontology |
| Clinical data capture |
| Cohort/usergroup/data source identification |
| Communication tools, research, point of care |
| Computational biology |
| Computer facility, equipment, software packages |
| Crowd sourcing |
| CTSpedia |
| Customized software |
| Data acquisition, entry, analysis, management, quality control, sharing, transformation |
| Database development |
| Drug discovery |
| Feasibility, recruitment, |
| Genetic information sharing |
| Genomics analysis, genotype, array, sequencing |
| Geographic coding |
| Honest broker |
| Informatics for integrating biology and bedside |
| Image repository |
| Interfaces, internet applications |
| Motif finding |
| Novel analysis techniques |
| Privacy, security, HIPAA compliance |
| Proteomics |
| Registration for trials: ResearchMatch, others |
| Research Electronic Data Capture (REDCap) |
| RNA expression analysis |
| Sharing tools and consultation |
| Small molecule core analysis |
| Specialized (immuno, imaging) |
| Statistical packages |
| Supercomputing, cloud computing, warehousing, archiving, networks |
| Pilot funding |
| CTSA pilots |
| Institutional pilots |
| NIH institutional and center funding |
| Trainee and Scholar program |
| Other Federal agencies |
| Industry partners, private, not for profit |
| Regulatory knowledge |
| Advertisement, publicity, promotion |
| Adverse event reporting (AE, SAE) |
| Auditing, compliance measures |
| Budget review and development, training in good clinical practice and standard operating procedures |
| Clinical study monitoring |
| Clinical trials registry |
| http://www.ClinicalTrials.gov |
| Collaboration support, team building |
| Communication tool generation; newsletters, posters, fliers, mixed media, video, audio |
| Conflicts of interest management |
| Conflicts resolution |
| Contracts |
| Cost accounting, financial management, medical |
| coverage |
| Case report form prototypes |
| Data and Safety Monitoring Board |
| Document storage |
| Electronic forms processing |
| Emergency use of investigational drugs |
| Environmental health and safety |
| FDA guidance, IND, IDE support, management, reporting, meeting, communication |
| HIPAA compliance, IACUC, training programs in Responsible Conduct of Research, HSP |
| Informed consent document preparation |
| IRB prereview, review, waivers, agreements, triage |
| Legal advice |
| Office of research services |
| Protocol development team, studios, navigator, concierge |
| Protocol preparation, special cases, language |
| Public private partnership support |
| Recruitment and retention |
| Sponsored project handbook |
| Unanticipated risk evaluation |
| Workshop support |
| Biostatistics, epidemiology, research design, and ethics |
| Abstract, poster, table, figure, report, review assistance, manuscript preparation assistance |
| Adaptive (alternative) trial design, statistical models Bayesian analysis |
| Biostatistical team building |
| Comparative effectiveness research CTSpedia |
| Data analysis, reporting, coordination, mining |
| Detailed questionnaire preparation and review |
| Dose finding study methodology |
| Epidemiology assessment of risks, benefits, adherence |
| Ethics and genetics, team participation, cultures, communities |
| Ethics consultation, special topics |
| Gene environment interactions |
| Grant application support |
| Health services analysis |
| IRB application support |
| Multicenter coordination |
| Mu‐Stat and other special statistical analytic tools, outliers and distributional problems, missing data, model‐based methods, etc. |
| Randomization, blinding |
| Special assessments: quality of life, sample size, genetics |
| Study design |
| Participant and clinical interaction resources |
| Access, e‐application, e‐case report form completion, e‐tracking and reporting, |
| Calendar and scheduling of research‐related activities, meetings, reporting timelines |
| Case report form development, reporting compliance |
| Communication |
| Compliance with protocol, quality assurance, monitoring |
| Coordinator support, handbook, training |
| Cost recovery planning |
| Exercise and other functional testing facilities |
| Good Clinical Practice performance with standard operating procedures Standard |
| Operating Procedures, training, guidance |
| Diet, nutrition, energy consumption, body composition, metabolic kitchen, menus, special measurements |
| Phase I program |
| Phenotyping |
| Research nurse |
| Research Pharmacy |
| Research subject advocate |
| Review, reporting, oversight |
| Sleep study equipment, monitoring, testing |
| Community engagement |
| Adult literacy assessment, support |
| Community‐based consultation, advisory board, collaboration |
| Community‐wide IRB |
| Cultural competency training |
| Open forum to discuss research proposals, elicit suggestions |
| Public database |
| Relationships, trust, cultural advisor program |
| Research participation |
| Translation into community setting |
Core Facilities and Other Research Resources
Principal investigators who successfully competed for the CTSA Program awards all provide research resources to laboratory and clinical investigators at their institutions. Each CTSA Program site offers enhanced and coordinated access to its core facilities, which are often called translational technologies and novel methodologies, many of which were established before the CTSA Program awards were funded. As noted elsewhere, 4 NIH provides about $900 million per year in funds for core facilities, but these funds do not support coordination of access to the cores. The CTSA recipients also offer access to other research resources, identified as key functions. To coordinate access to their resources, awardees display research specific content and linkages on their CTSA Program websites. In addition to providing specific content, the websites use a variety of means to facilitate access to their services such as search engines, navigators, studios, project development teams, collaboration tools, communication systems, and teaching CTSA Programs. The websites support electronic systems for the following: (1) communication between investigators, consultants, and support staff, (2) requests for consultations and services, (3) libraries of policies, guidances, and forms, (4) templates for protocols, informed consent documents, and case report forms, (5) links to specific resources, collaborators, and funding, and (6) calendars for scheduling, meetings, tutorials, and presentations.
Core facilities
Investigators may access core facilities within categories identified as translational technologies, novel methodologies, and core laboratory services, although the terms are not constant between sites. CTSA Program‐supported investigators may request clinical and translational services in at least 52 generic areas; some are relatively traditional biomedical research cores, others are novel or rapidly evolving. Examples of novel methodologies include genome manipulation, specific embryonic stem cell lines, in vitro fertilization, intracytoplasmic sperm injection, frozen embryo transfer, nanotechnology, mass spectroscopy, epigenomics, and gene therapy.
Other research resources
Biomedical Informatics is a broad discipline that encompasses all aspects of information science as it applies to medical research. The increase in amount of raw data generated in the performance of clinical and translational science has resulted in a dependency on an informed application of bioinformatics for the successful conduct of many lines of research in the field. Services are categorized in a variety of ways but include at least 38 separate topic areas such as support systems, acquisition, management, analysis, interpretation, communication, sharing, storage, retrieval, and distribution of information. Informatics support is also provided for laboratory and clinical data acquisition, mining, and translation. Investigators are invited to interact with informaticians to explore novel models of computation and refined approaches to scientific, mathematic, and engineering problems in medical research. Access to education programs in bioinformatics is also offered at some sites. Many sites contain cross‐linkages between disciplines, schools, institutions, CTSA Program sites, and networks.
Funding for pilot studies in clinical and translational science is integral to CTSA Program applications. CTSA Program sites provide pilot funding for translational, clinical, comparative effectiveness, and community engagement research projects. CTSA Program websites direct applicants to funding opportunities and provide guidance in obtaining funding from their academic institutions, NIH institutes, and centers in addition to the CTSA Program, other Federal agencies, industrial partners, not‐for‐profit organizations, and other private foundations.
Regulatory knowledge and support services provide access to staff with specialized skills needed to help investigators comply with guidance, regulations, policies, and oversight committees as they conduct clinical research. Services are listed under at least 32 generic topic areas. Expert staff members invite investigators to use their services to (1) create, obtain approval for, and activate clinical research protocols, (2) negotiate and execute contracts, (3) budget, finance, and manage clinical research, (4) comply with FDA requirements, (5) conduct feasibility studies and recruit study participants, and (6) meet institutional, NIH, and other Federal requirements for the safe, ethical, and responsible conduct of clinical research.
The CTSA Program statistical functions include services in at least 21 generic areas for planning, conducting, analyzing, interpreting, and describing research. At many CTSA Program websites, biostatisticians encourage early consultation and/or collaboration to promote sound research designs and statistical plans. Some sites specify, for example, that statisticians can collaborate to develop adaptive trial designs, but that this efficient approach requires detailed planning and collaboration before a trial is initiated. Statistical services also include access to novel statistical tools, communication support, and trial support. Research ethics, often grouped with statistics, provides consultation, collaboration, support for protocol preparation and review, and guidance with specific topics such as genetic studies, risky interventions, cultural issues, and vulnerable and/or impaired study populations.
The management of active clinical trials begins with protocol development and ends with trial closure and reporting. Web pages show at least 16 generic services that include pretrial consultation, scheduling, facilities and equipment, protocol specific training, and intrastudy support in the clinical research unit, the outpatient suite, elsewhere in the institution, and in mobile units. Services are also offered in research pharmacy, nutrition, and core support for research coordinators, research nurses, and data managers.
Community Engagement Services include a complete spectrum of support for bidirectional interactions between the communities served by the institutions associated with the CTSA Program. CTSA Program websites offer at least eight generic services in community engagement. Investigators may participate with staff to enhance research participation, enrollment and retention, feasibility assessment, staff training, community outreach, prescreening processes, and marketing. Additional resources include institutional web pages for the public, registries for potential participants, and information about clinical trials.
Discussion
The linkage within and between websites permits the CTSA Program sites to provide an academic home for research that responds to the complex demands for access to technologies, management of data, conduct of research, and support for collaboration, networking, data sharing, and communication associated with the current pursuit of clinical and translational science. As suggested in Figure 1, the availability of centralized access to research support services could accelerate informed, effective research in academic health centers by biomedical investigators and by individuals in nonmedical disciplines who might not otherwise be able to participate fully in health‐related research. It would be of interest to determine whether enhanced access to resources has increased the efficiency of the development of drugs, devices, and biological agents at academic health centers and whether it has had an impact on the prevention, evaluation, and management of human disease. Before the CTSA Program was created, investigators lacked centralized, coordinated, and facilitated access to research resources, even when those resources were available to them at their institutions. The CTSA Program provides access to those resources at a single, common website, http://www.CTSAcentral.org. The facilitated access creates a novel opportunity for investigators engaged in clinical and translational research.
Figure 1.
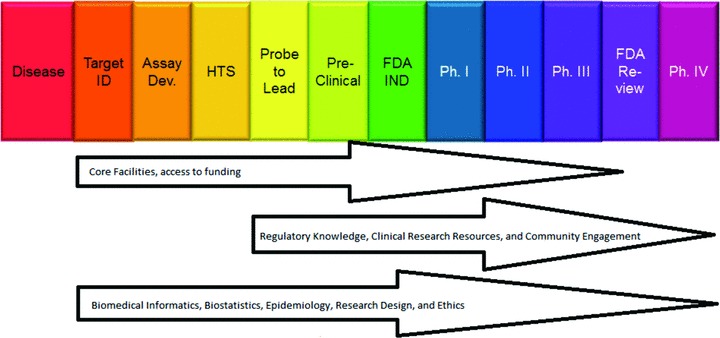
Relationship of core facilities and other research resources to clinical and translational science‐supported product development. Not shown are comparative effectiveness and development of devices, vaccines, behavioral interventions, and best practices, all of which constitute areas of CTSA‐supported research. NIH product development scheme as depicted by Francis S. Collins, M.D., Ph.D., Director, NIH.
Disclaimer
The information and opinions expressed in this publication are those of the author and do not reflect the policies of the United States Government.
Acknowledgments
The author gratefully acknowledges the careful review and valuable suggestions made by Barry Coller, M.D. (The Rockefeller University), Gary Rosenthal, M.D. (University of Iowa), Robert Sherwin, M.D. (Yale University), Barbara Alving, M.D. (Past Director of NCRR) and Anthony Hayward, M.D., Ph.D. (NCATS).
The information used in this report was derived from review of CTSA services, which, in turn, are funded by the National Center for Advancing Translational Sciences (NCATS).
References
- 1. Zerhouni EA. Translational and clinical science—time for a new vision. N Engl J Med. 2005; 353: 1621–1623. [DOI] [PubMed] [Google Scholar]
- 2. Zerhouni EA, Alving B. Clinical and Translational Science Awards: a framework for a national research agenda. Transl Res. 2006; 148: 4–5. [DOI] [PubMed] [Google Scholar]
- 3. Rosenblum D, Alving B. The role of the Clinical and Translational Science Awards Program in improving the quality and efficiency of clinical research. Chest. 2011; 140: 764–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Farber, GK , Weiss, L . Core facilities: maximizing the return on investment. Sci Transl Med. 2011; 3(95): 95cm21. [DOI] [PMC free article] [PubMed] [Google Scholar]


