Abstract
Background
The ratio of the diameter of the pulmonary artery (PA) to the diameter of the aorta (PA:A) on computed tomography (CT) imaging is associated with both COPD exacerbation and pulmonary hypertension. The mechanisms of PA enlargement in COPD are poorly understood.
Methods
In this retrospective, single center study we evaluated pulmonary function, CT scans, right heart catheterizations, and echocardiography in 88 subjects with mild-to-moderately severe COPD. A sensitivity analysis was performed in 43 subjects in whom CT scan and echocardiogram were performed within 50 days of each other. To evaluate the association between PA:A ratio and echocardiographic parameters and hemodynamics, we performed simple correlations and multivariable linear regression analysis adjusting for lung function, age, sex, race, and diastolic function.
Results
All subjects had preserved left ventricular (LV) systolic function (LV ejection fraction 62.7%±5.5%). Among them, 56.8% had evidence of diastolic dysfunction. There was no association between PA:A ratio and the presence of diastolic dysfunction. In a multivariable model, PA:A ratio was associated with right ventricular (RV) chamber size (β=0.015; P<0.003), RV wall thickness (β=0.56; P<0.002), and RV function (−0.49; P=0.05). In the subgroup of subjects with testing within 50 days, the association with RV chamber size persisted (β=0.017; P=0.04), as did the lack of association with diastolic function. PA:A ratio was also associated with elevated PA systolic pressures (r=0.62; P=0.006) and pulmonary vascular resistance (r=0.46; P=0.05), but not pulmonary arterial wedge pressure (r=0.17; P=0.5) in a subset of patients undergoing right heart catheterization.
Conclusion
In patients with mild-to-moderately severe COPD and preserved LV function, increased PA:A ratio occurs independent of LV diastolic dysfunction. Furthermore, the PA:A ratio is associated with right heart structure and function changes, as well as pulmonary hemodynamics. These findings indicate that PA:A ratio is a marker of intrinsic pulmonary vascular changes rather than impaired LV filling.
Keywords: COPD, diastolic dysfunction, pulmonary artery
Introduction
COPD is the third leading cause of death in the United States, and is the only leading cause of death that is increasing in prevalence.1–3 The associations between established lung diseases such as COPD and cardiovascular disease and poor health outcomes have been well described.4–10 COPD exacerbations are important events in patients with COPD, as they account for significant morbidity and mortality. Serologic markers of cardiac injury and stretch have been noted to be elevated around the time of exacerbation.11,12 It is reasonable to think that at least a subset of COPD exacerbations could be the result of overt or subclinical cardiovascular disease.13 Pulmonary hypertension (PH) occurs frequently in patients with advanced COPD, and has been associated with exacerbation risk, as well as decreased functional status and increased mortality independent of severity of lung function impairment.14,15 Although most descriptions of PH in COPD are in patients with advanced COPD awaiting lung transplant,16,17 there is evidence that pathologic changes to the pulmonary vasculature consistent with PH occur across all stages of COPD severity.18 Exactly how the pulmonary vasculature is mechanistically linked to exacerbation risk remains unknown.
The ratio of the diameter of the pulmonary artery (PA) to the diameter of the aorta (PA:A ratio) on computed tomography (CT) scan can identify patients at an increased risk of COPD exacerbation and hospitalization.19 It has also been shown to outperform echocardiogram at predicting PH in patients with severe COPD.20 There are several potential mechanisms of PA enlargement in COPD. PA enlargement may occur with cardiac comorbidities such as systolic or diastolic dysfunction, parenchymal lung disease with loss of the pulmonary capillary bed (as occurs with emphysema), as well as underlying pulmonary vascular disease, all of which have been linked to exacerbation risk. In order to clarify mechanisms of PA enlargement in COPD, we sought to determine the relationships between PA:A ratio and diastolic function of the heart in a clinical population of COPD patients with preserved left ventricular (LV) systolic function and mild-to-moderately severe airflow limitation (forced expiratory volume in 1 second/forced vital capacity [FEV1/FVC] ratio <0.7 and FEV1 >50%).
Methods
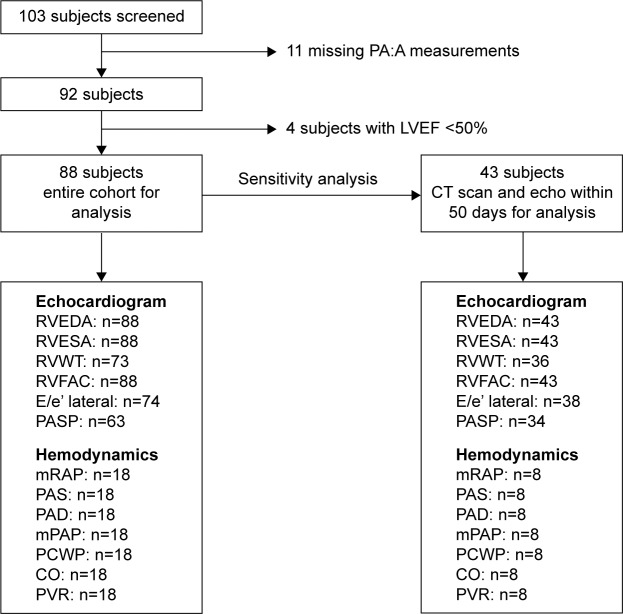
We studied 103 consecutive patients who had clinically indicated echocardiograms, lung CT scans, and pulmonary function data and were seen in the Northwestern Asthma and COPD program. Eleven patients were excluded for inability to measure PA:A ratio and four were excluded for ejection fraction below 50%, leaving 88 subjects in the analysis cohort. Given the wide time spread in some subjects between their echocardiogram and CT scan, a sensitivity analysis of 43 subjects who had these tests within 50 days of each other was performed (Figure 1). The Northwestern University Institutional Review Board reviewed and approved the study, given the retrospective nature of the study and reviewing only de-identified data ensuring patient data confidentiality, a waiver of written informed consent was granted by the Northwestern University Institutional Review Board. Data collected included general demographic information, CT scan of chest, echocardiographic parameters, and full pulmonary function testing.
Figure 1.
Description of analysis cohort.
Note: E/e’ lateral indicates ratio of the early mitral inflow velocity to tissue Doppler early lateral diastolic longitudinal velocity.
Abbreviations: CT, computed tomography; PASP, pulmonary artery systolic pressure; LVEF, left ventricular ejection fraction; CO, cardiac output; echo, echocardiogram; PA:A, pulmonary artery: aorta; RVEDA, right ventricular end diastolic area; RVESA, right ventricular end systolic area; RVWT, right ventricular end diastolic area; RVFAC, right ventricular fractional area change; mRAP, mean right atrial pressure; PAS, pulmonary artery systolic; PAD, pulmonary artery diastolic; mPAP, mean pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance.
Pulmonary function testing including spirometry, lung volumes, and diffusion capacity for carbon monoxide (DLCO) was performed by trained technicians according to American Thoracic Society/European Respiratory Society (ATS/ERS) standards and guidelines.21–23 We selected patients with mild, moderate, and moderately severe COPD based on ATS guidelines: mild was defined as FEV1/FVC ratio <0.7 and FEV1 percent predicted >70%, moderate was defined as FEV1/FVC ratio <0.7 and FEV1 percent predicted 60%–69%, and moderately severe was defined as FEV1/FVC ratio <0.7 and FEV1 percent predicted 50%–59%.24 Lung volumes were measured using body plethysmography in 97% (85/88) of patients. Six minute walk testing was performed by trained technicians according to ATS guidelines.25
All echocardiograms were performed on either Philips (ie33) or GE (Vivid7) machines and analyzed using a systematic protocol by a single cardiologist blinded to all other data. The data collected for analysis included: LV end diastolic dimension, biplane LV end-diastolic volume, biplane LV end-systolic volume, LV mass, LV ejection fraction, cardiac output (CO), left atrial volume, right ventricular (RV) basilar diameter, RV longitudinal dimension, RV end diastolic area, RV wall thickness, RV outflow track diameter, right atrial area, early mitral inflow velocity (E), late mitral inflow velocity (A), tissue Doppler early septal diastolic longitudinal velocity (e’), diastolic function grade, peak tricuspid regurgitation (TR) velocity, PA systolic pressure, tricuspid annular plane systolic excursion (TAPSE), and RV fractional area change.26,27
All echocardiographic parameters were measured and calculated according to previously published guidelines.26,28 Diastolic function was graded by analyzing mitral inflow patterns, tissue Doppler E’ (septal) velocity, and echocardiographic estimation of LV filling pressure (E/e’ ratio) using a previously published method.29 Normal diastolic function was defined as a mitral E/A ratio >0.75 but <1.5 and E/e’ septal <10. Grade 1 diastolic dysfunction was defined as a mitral E/A ratio of <0.75. Grade 2 diastolic dysfunction was defined as mitral E/A ratio >0.75 but <1.5 and an E/e’ septal ratio >10. Grade 3 diastolic dysfunction was defined as mitral E/A ratio >1.5 and either an E/e’ septal ratio >10 or E deceleration time <140. Patients were categorized as “indeterminate diastolic function” if they could not be classified based on the aforementioned diastolic function parameters.
Axial CT scan images were analyzed by two independent reviewers blinded to a subject’s clinical characteristics. The reviewers measured the diameter of the main PA at the level of its bifurcation, and measured the diameter of the ascending aorta in its maximum dimension using the same images.19 The correlation between reviewers’ measurements was 0.93.
Statistics
Data are presented as mean ± standard deviation or frequency and percent. A significant P-value is defined as being <0.05. Data were visually assessed for normality of distribution. Comparison of means was done through Student’s t-tests. Multiple means were compared using analysis of variance (ANOVA) with Bonferroni adjustment for multiple comparisons. Univariable and multivariable analyses were performed to evaluate whether PA:A ratio predicted echocardiographic parameters or hemodynamics. Variables included in statistical models were pre-specified based on a known or hypothesized association with PA:A measurements, echocardiographic parameters or hemodynamics in COPD. Statistical analysis was completed using STATA software (STATA 10; StataCorp LP, College Station, TX, USA).
Results
Patient population
Subjects were predominantly Caucasian (72.1%) with a mean age of 72.9±10.4 years and a mean body mass index (BMI) of 28.1±6.7 kg/m2. There was an equal sex distribution. All subjects had preserved LV systolic function on echocardiogram with a mean LV ejection fraction of 62.7%±5.5%. All subjects had mild-to-moderately severe obstructive lung disease by ATS criteria with a mean FEV1 % predicted of 65.0%±11.9%. Lung volume measurements revealed a mean total lung capacity of 98.2%±14.6% and mean residual volume was 122.4%±31.5%. The mean DLCO was 54.6%±16.6% and resting oxygen saturation was 96.1%±1.8%. Of the patients, 2.5% were treated with supplemental oxygen. The mean PA:A ratio was 0.83±0.12. There were no statistically significant differences in baseline demographics between the entire cohort and the sensitivity analysis cohort (Table 1).
Table 1.
Patient demographics
| Demographics | Entire cohort n=88 | CT scan and echocardiogram performed within 50 days of each other n=43 |
|---|---|---|
| Age, years | 72.9±10.4 | 72.2±8.8 |
| Male (%) | 50 | 51.2 |
| Race (%) | ||
| Caucasian | 72.1 | 66.7 |
| Black | 22.1 | 26.2 |
| Asian | 1.1 | |
| Hispanic | 4.7 | 7.1 |
| Body mass index, kg/m2 | 28.1±6.7 | 28.1±7.2 |
| Cardiac function (%) | ||
| LV ejection fraction | 62.7±5.5 | 63.0±5.3 |
| Lung function (% predicted) | ||
| FVC | 75.6±12.8 | 76.7±13.8 |
| FEV1 | 65.0±11.9 | 64.9±12.2 |
| FEV1/FVC ratio | 0.63±0.05 | 0.63±0.05 |
| Total lung capacity (n=85) | 98.2±14.6 | 98.5±14.4 |
| Residual volume (n=85) | 122.4±31.5 | 122.3±31.2 |
| DLCO (n=84) | 54.6±16.6 | 52.2±14.3 |
| Degree of obstruction; n (%) | ||
| Mild | 25 (28.4) | 13 (30.2) |
| Moderate | 29 (33.0) | 12 (27.9) |
| Moderately severe | 34 (38.6) | 18 (41.9) |
| Oxygenation | ||
| Resting oxygen saturation (%) | 96.1±1.8 | 96.2±1.9 |
| Oxygen needs (% subjects) | ||
| Room air | 97.5 | 97.4 |
| 2 liters per minute | 2.5 | 2.6 |
| PA:A ratio (cm) | 0.83±0.12 | 0.83±0.13 |
| Time between echocardiogram and CT scan (days) | 95.0±422.2 | 5.9±23.4 |
| Time between PFT and CT scan (days) | 423.7±819.2 | 208.3±618.4 |
Notes: Data presented as mean ± standard deviation unless otherwise specified.
Abbreviations: CT, computed tomography; LV, left ventricular; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; DLCO, diffusion capacity for carbon monoxide; PFT, pulmonary function testing; PA:A, pulmonary artery: aorta.
Association of PA:A ratio with cardiac structure and systolic function
We found no association between the PA:A ratio and LV end diastolic volume index, left atrial volume index, or markers of LV hypertrophy including LV posterior wall thickness and LV mass index, in either the entire cohort or the sensitivity analysis subgroup. Furthermore, no association was noted with either LV ejection fraction or echocardiographic estimate of CO in either group. In contrast, we found there was a positive association between PA:A ratio and RV end diastolic and systolic area, but not with right atrial area. The strongest association was noted with RV wall thickness, such that every one unit change in the PA:A ratio was associated with a 0.38 cm change in RV wall thickness. We assessed three markers of RV function and their association with PA:A ratio. No association was noted with TAPSE or lateral systolic longitudinal velocity on tissue Doppler (S’ lateral), however the RV fractional area change was inversely associated with PA:A ratio in the entire cohort (Table 2). In the entire cohort the associations between PA:A ratio and RV end diastolic and systolic area, RV wall thickness, and RV fractional area change were maintained on multivariable analysis when adjusting for age, sex, race, FEV1, and diastolic function. In the sensitivity analysis the associations with RV area were maintained however, although the effect size stayed the same, RV wall thickness lost statistical significance (Table 3).
Table 2.
Univariate associations of PA:A ratio and echocardiogram parameters
| Echocardiogram parameter | Entire cohort n=88
|
CT scan and echocardiogram performed within 50 days of each other n=43
|
||
|---|---|---|---|---|
| r | P-value | r | P-value | |
| Left heart chamber size | ||||
| LV end diastolic volume index | 0.06 | 0.60 | 0.20 | 0.19 |
| Left atrial area (4 chamber view) | 0.07 | 0.53 | 0.09 | 0.54 |
| Left atrial volume index | 0.02 | 0.87 | −0.06 | 0.69 |
| Left heart hypertrophy | ||||
| LV posterior wall thickness | −0.04 | 0.71 | −0.12 | 0.46 |
| Septal wall thickness | −0.03 | 0.77 | −0.17 | 0.28 |
| LV mass index | 0.05 | 0.67 | 0.01 | 0.93 |
| Left heart function | ||||
| LV ejection fraction | −0.12 | 0.27 | −0.19 | 0.23 |
| Cardiac output | 0.13 | 0.22 | −0.01 | 0.95 |
| Right heart chamber size | ||||
| RV end diastolic area | 0.19 | 0.08 | 0.39 | 0.009 |
| RV end systolic area | 0.25 | 0.02 | 0.36 | 0.02 |
| Right atrial area | 0.05 | 0.67 | 0.21 | 0.18 |
| Right heart hypertrophy | ||||
| RV wall thickness | 0.38 | 0.001 | 0.34 | 0.04 |
| Right heart function | ||||
| TAPSE | −0.07 | 0.50 | −0.04 | 0.77 |
| S’ lateral | −0.09 | 0.45 | −0.17 | 0.31 |
| RV fractional area change | −0.20 | 0.05 | −0.12 | 0.43 |
| Diastology | ||||
| e’ septal | −0.17 | 0.15 | −0.04 | 0.81 |
| e’ lateral | −0.13 | 0.26 | −0.11 | 0.53 |
| a’ septal | 0.09 | 0.45 | −0.01 | 0.94 |
| a’ lateral | 0.03 | 0.82 | −0.14 | 0.43 |
| E velocity | 0.09 | 0.43 | 0.31 | 0.05 |
| A velocity | 0.14 | 0.24 | 0.21 | 0.22 |
| E/A ratio | 0.003 | 0.98 | 0.07 | 0.65 |
| E deceleration time | −0.08 | 0.47 | −0.08 | 0.61 |
| E/e’ septal | 0.18 | 0.13 | 0.28 | 0.09 |
| E/e’ lateral | 0.21 | 0.07 | 0.33 | 0.04 |
Notes: E/A ratio indicates ratio of early mitral inflow velocity to late mitral inflow velocity; E/e’ septal indicates ratio of early mitral inflow velocity to tissue Doppler early septal diastolic longitudinal velocity. E/e’ lateral indicates ratio of the early mitral inflow velocity to tissue Doppler early lateral diastolic longitudinal velocity. Bold figures represent statistically significant findings (P<0.05).
Abbreviations: CT, computed tomography; LV, left ventricular; RV, right ventricular; TAPSE, tricuspid annular plane systolic excursion; PA:A, pulmonary artery: aorta; e’ septal, tissue doppler early septal diastolic longitudinal velocity; e’ lateral, tissue doppler early lateral diastolic longitudinal velocity; a’ septal, tissue doppler late septal diastolic peak velocity; a’ lateral, tissue doppler late lateral diastolic peak velocity; E velocity, early mitral inflow velocity; A velocity, late mitral inflow velocity.
Table 3.
Multivariate association of PA:A ratio and echocardiogram parameters
| Echo parameter | Entire cohort* n=88 |
CT scan and echocardiogram performed within 50 days of each other* n=43 |
||
|---|---|---|---|---|
| β | P-value | β | P-value | |
| RV end diastolic area | 0.009 | 0.01 | 0.013 | 0.01 |
| RV end systolic area | 0.015 | 0.003 | 0.017 | 0.04 |
| RV wall thickness | 0.56 | 0.002 | 0.56 | 0.11 |
| RV fractional area change | −0.49 | 0.05 | −0.25 | 0.7 |
| E/e’ lateral | 0.006 | 0.09 | 0.01 | 0.03 |
Notes: E/e’ lateral indicates ratio of the early mitral inflow velocity to tissue Doppler early lateral diastolic longitudinal velocity.
Covariates: age, sex, race, FEV1, diastolic dysfunction.
Abbreviations: CT, computed tomography; RV, right ventricular; FEV1, forced expiratory volume in 1 second; PA:A, pulmonary artery:aorta.
Association of PA:A ratio and diastolic dysfunction
We were interested in our cohort of patients with preserved LV systolic function to determine what role diastolic dysfunction might play in PA:A ratio changes. In the sensitivity analysis, looking at the shorter time points between echocardiogram and CT scan, a positive association was noted between PA:A ratio and E/e’ lateral, this association was not maintained in the entire cohort (Table 2). On multivariable analysis again in the subgroup analysis E/e’ lateral was associated with PA:A ratio independent of age, sex, race, FEV1, and diastolic dysfunction, this association was not statistically significant in the entire cohort (Table 3).
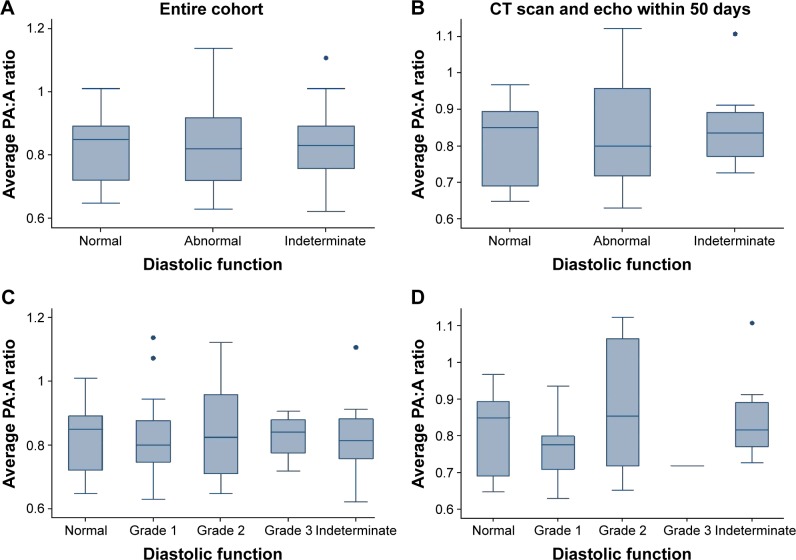
Using a standard composite definition of diastolic dysfunction, we found 19.3% (17/88) of our subjects had normal diastolic function, 56.8% (50/88) had some degree of diastolic dysfunction, and 23.9% (21/88) had indeterminate diastolic function. Of the subjects with diastolic dysfunction 34.0% (17/50) had grade 1 dysfunction, 58.0% (29/50) had grade 2 dysfunction, and 8.0% (4/50) had grade 3 dysfunction. There was no significant difference in PA:A ratio between those with normal, abnormal, or indeterminate diastolic dysfunctions (Figure 1A and B), nor among the different degrees of diastolic dysfunction (Figure 1C and D).
Association of PA:A ratio with hemodynamic measurements
We assessed noninvasive hemodynamic measurements and their relation to PA:A ratio from echocardiograms. Pulmonary artery systolic pressure (PASP) was measurable in 71.6% (63/88) of subjects (Figure 2). On univariate analysis there was an association between PASP estimate from echocardiogram and PA:A ratio. This association was maintained on multivariable modeling when adjusting for age, sex, race, lung function, and degree of diastolic dysfunction (Table 4). We also assessed if interventricular septal flattening, suggestive of increased right heart pressures, was associated with PA:A ratio and noted that the presence of septal flattening on echocardiogram was associated with a larger PA:A ratio compared to those without septal flattening; no septal flattening; 0.81±0.12 versus septal flattening; 0.90±0.13, P=0.02.
Figure 2.
The PA:A ratio across categories of diastolic dysfunction.
Notes: (A) The PA:A ratio compared across normal, abnormal, and indeterminate diastolic functions. (B) The PA:A ratio compared across normal, abnormal, and indeterminate diastolic functions in the subgroup of patients with CT scan and echocardiogram within 50 days of each other. (C) The PA:A ratio compared across severity of diastolic dysfunction. (D) The PA:A ratio compared across severity of diastolic dysfunction in the subgroup of patients with CT scan and echocardiogram within 50 days of each other.
Abbreviations: CT, computed tomography; echo, echocardiogram; PA:A, pulmonary artery: aorta.
Table 4.
Association of PA:A ratio and hemodynamics (entire cohort; n=88)
| Hemodynamics | Univariate
|
Multivariate*
|
||
|---|---|---|---|---|
| r | P-value | β | P-value | |
| PASP (echo) | 0.55 | <0.0001 | 0.004 | <0.001 |
| PASP (cath) | 0.62 | 0.006 | 0.004 | 0.04 |
| Pulmonary artery diastolic pressure | 0.50 | 0.04 | 0.007 | 0.13 |
| Mean pulmonary artery pressure | 0.56 | 0.01 | 0.005 | 0.07 |
| Pulmonary arterial wedge pressure | 0.17 | 0.5 | ||
| Pulmonary vascular resistance | 0.46 | 0.056 | 0.018 | 0.33 |
| PAD-wedge gradient | 0.47 | 0.05 | 0.006 | 0.25 |
| Cardiac output | 0.12 | 0.6 | ||
Note:
Covariates: age, sex, race, FEV1, diastolic dysfunction.
Abbreviations: Cath, catheterization; FEV1, forced expiratory volume in 1 second; PASP, pulmonary artery systolic pressure; echo, echocardiogram; PA:A, pulmonary artery: aorta; PAD, pulmonary artery diastolic.
Invasive hemodynamic measurements were available in 18 of the 88 subjects who had undergone right heart catheterization (Figure 2). No association was noted between PA:A ratio and the pulmonary arterial wedge pressure or cardiac outputs. There was a correlation between PA:A ratio and PA pressures, as well as with the PA diastolic pressure to pulmonary arterial wedge pressure gradient. The association with invasive PA systolic pressure was maintained on multivariable analysis when adjusting for age, sex, race, lung function, and degree of diastolic dysfunction (Table 4).
Discussion
The interaction between the heart and the lungs plays a key role in the morbidity and mortality seen in patients with COPD.30 Although COPD exacerbations are frequently associated with bronchitic symptoms, they can be difficult to distinguish from cardiovascular events such as heart failure exacerbations.31 The PA:A ratio is a reproducible and easily obtained measure, and in patients with clinically stable COPD is associated with risk of future COPD exacerbation.19 It is however unclear what drives the enlargement of the PA, and therefore why it would be associated with exacerbation risk. Several mechanisms have been proposed, including primary pulmonary vascular changes, parenchymal changes of the lung, and cardiovascular disease leading to elevated left heart filling pressures.32 Understanding the mechanisms that drive increases in the PA:A ratio is key to optimizing its use as a predictive tool in patients with COPD. In this article we attempted to explore the associations between the PA:A ratio and cardiac structure and function, to better understand the etiology of an increased PA:A ratio in patients with COPD. We found that the PA:A ratio is associated with right, but not left heart structural changes or left heart systolic function. Although no association was noted between PA:A ratio and overt clinical diastolic dysfunction, an association was noted between PA:A ratio and E/e’ lateral, a marker of increased left heart filling pressures.
PH is a common complication that develops in patients with COPD with known effects on both morbidity and mortality.14 It is most commonly recognized in advanced COPD, but there are increasing data to suggest this complication also occurs in patients with less severe airways disease.18,33 The mechanisms for the development of PH, like those thought to be related to enlargement of the PA:A ratio, are varied and include primary vascular changes due to hypoxic vasoconstriction, inflammation, and systolic and diastolic LV dysfunctions. Recently, Iyer et al showed that in a cohort of patients with advanced COPD being evaluated for lung transplant, an increased PA:A ratio was associated with invasive hemodynamic measurement of the mean pulmonary pressures independent of BMI, sex, oxygen saturation, and comorbid conditions such as sleep apnea and heart failure.20 The authors concluded that an elevated PA:A ratio is predictive of resting PH in advanced COPD. Our data suggest that in COPD patients with milder lung disease, PA:A ratio is also associated with pulmonary vascular changes and not overt LV dysfunction, despite the relatively high frequency of diastolic dysfunction in our clinical population. We identified that the PA:A ratio is associated with right heart structural changes and decrement in RV function that is seen with PH. Furthermore, these associations between PA:A ratio and right heart changes occur independent of systolic and diastolic heart functions, suggesting this may be reflective of primary pulmonary vascular changes.
It is interesting to note that although we did not see an association with overt diastolic dysfunction, there was an association between PA:A ratio and E/e’ lateral, a marker of LV stiffness, suggesting a link to left heart filling pressures. The association between PA:A ratio and E/e’ lateral raises several possibilities. An enlarged PA:A ratio could be a marker of either early diastolic dysfunction in some COPD patients, or perhaps indicates a unique subgroup of patients with diastolic dysfunction that results in both increased left heart filling pressures and pulmonary vascular changes. In the limited subgroup of patients in our cohort who underwent invasive hemodynamic measurements, the PA:A ratio was associated with PA pressures, including the PA diastolic pressure to pulmonary arterial wedge pressure gradient (PAD-wedge gradient), which has been shown to be a marker of pulmonary vascular disease in patients with diastolic dysfunction.34–36 Given the relatively large proportion of subjects in our cohort with diastolic dysfunction, this raises the question if PA:A ratio may be a marker of “reactive” or “out of proportion” PH in COPD patients with diastolic dysfunction, and it in fact identifies a subset of patients who are particularly sensitive to the hemodynamic shifts that occur with increased stress and inflammation, that occur around the time of exacerbation.
Our study has several limitations. It relies on single center experience and retrospective review of clinical data. The modest sample size may play a role in why, when evaluating markers of RV function, only the RV fractional area change showed a significant negative association, while other markers of RV function (TAPSE and S’ lateral) trended toward a negative association but were not statistically significant. The small number of patients undergoing invasive hemodynamic pressure measurements limits our ability to draw firm conclusions related to the association of the PA:A ratio and hemodynamic changes.
Conclusion
In conclusion, in patients with mild-to-moderate COPD the PA:A ratio is associated with RV hypertrophy, RV enlargement, and decreased RV function independent of systolic or diastolic left heart function. These changes to the PA seem to reflect primary pulmonary vascular changes and may be predictive of mild or early PH in COPD. These findings could offer a potential explanation for the increased exacerbation risk associated with an elevated PA:A ratio, as the presence of PH has been associated with increased risk of hospitalization and increased mortality.
Footnotes
Disclosure
The authors report the following conflicts of interest related to grant funding, advisory boards or speaker fees: MJC reports financial activities with Actelion Pharmaceuticals, Gilead Health Sciences, United Therapeutics, Bayer. RK reports financial activities with AstraZeneca, Boehringer Ingelheim, Forest Laboratories, Aptus Health, Spiration, BTG/PneumRX, Caremark, GlaxoSmithKline. RK, MJC: NIH R01 HL122477; MJC: 1 K12 HL 083790-01A1, SPB: K23 (K23HL133438). The authors report no other conflicts of interest in this work.
References
- 1.Minino AM, Xu J, Kochanek KD. Deaths: Preliminary Data for 2008. National Vital Statistics Reports. 2. Vol. 59. Hyattsville, MD: National Center for Health Statistics; 2010. pp. 1–52. [PubMed] [Google Scholar]
- 2.Lung function testing: selection of reference values and interpretative strategies. American Thoracic Society. Am Rev Respir Dis. 1991;144(5):1202–1218. doi: 10.1164/ajrccm/144.5.1202. No authors listed. [DOI] [PubMed] [Google Scholar]
- 3.Ma J, Ward EM, Siegel RL, Jemal A. Temporal trends in mortality in the United States, 1969–2013. JAMA. 2015;314(16):1731–1739. doi: 10.1001/jama.2015.12319. [DOI] [PubMed] [Google Scholar]
- 4.Mannino DM, Holguin F, Pavlin BI, Ferdinands JM. Risk factors for prevalence of and mortality related to restriction on spirometry: findings from the First National Health and Nutrition Examination Survey and follow-up. Int J Tuberc Lung Dis. 2005;9(6):613–621. [PubMed] [Google Scholar]
- 5.Duprez DA, Hearst MO, Lutsey PL, et al. Associations among lung function, arterial elasticity, and circulating endothelial and inflammation markers: the multiethnic study of atherosclerosis. Hypertension. 2013;61(2):542–548. doi: 10.1161/HYPERTENSIONAHA.111.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ford ES, Wheaton AG, Mannino DM, Presley-Cantrell L, Li C, Croft JB. Elevated cardiovascular risk among adults with obstructive and restrictive airway functioning in the United States: a cross-sectional study of the National Health and Nutrition Examination Survey from 2007–2010. Respir Res. 2012;13:115. doi: 10.1186/1465-9921-13-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curkendall SM, DeLuise C, Jones JK, et al. Cardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada cardiovascular disease in COPD patients. Ann Epidemiol. 2006;16(1):63–70. doi: 10.1016/j.annepidem.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest. 2005;127(6):1952–1959. doi: 10.1378/chest.127.6.1952. [DOI] [PubMed] [Google Scholar]
- 9.Hole DJ, Watt GC, Davey-Smith G, Hart CL, Gillis CR, Hawthorne VM. Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study. BMJ. 1996;313(7059):711–715. doi: 10.1136/bmj.313.7059.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schunemann HJ, Dorn J, Grant BJ, Winkelstein W, Jr, Trevisan M. Pulmonary function is a long-term predictor of mortality in the general population: 29-year follow-up of the Buffalo Health Study. Chest. 2000;118(3):656–664. doi: 10.1378/chest.118.3.656. [DOI] [PubMed] [Google Scholar]
- 11.Marcun R, Sustic A, Brguljan PM, et al. Cardiac biomarkers predict outcome after hospitalisation for an acute exacerbation of chronic obstructive pulmonary disease. Int J Cardiol. 2012;161(3):156–159. doi: 10.1016/j.ijcard.2012.05.044. [DOI] [PubMed] [Google Scholar]
- 12.Patel AR, Kowlessar BS, Donaldson GC, et al. Cardiovascular risk, myocardial injury, and exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;188(9):1091–1099. doi: 10.1164/rccm.201306-1170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhatt SP, Dransfield MT. Chronic obstructive pulmonary disease and cardiovascular disease. Transl Res. 2013;162(4):237–251. doi: 10.1016/j.trsl.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Cuttica MJ, Kalhan R, Shlobin OA, et al. Categorization and impact of pulmonary hypertension in patients with advanced COPD. Respir Med. 2010;104(12):1877–1882. doi: 10.1016/j.rmed.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Sims MW, Margolis DJ, Localio AR, Panettieri RA, Kawut SM, Christie JD. Impact of pulmonary artery pressure on exercise function in severe COPD. Chest. 2009;136(2):412–419. doi: 10.1378/chest.08-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thabut G, Dauriat G, Stern JB, et al. Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest. 2005;127(5):1531–1536. doi: 10.1378/chest.127.5.1531. [DOI] [PubMed] [Google Scholar]
- 17.Scharf SM, Iqbal M, Keller C, et al. Hemodynamic characterization of patients with severe emphysema. Am J Respir Crit Care Med. 2002;166(3):314–322. doi: 10.1164/rccm.2107027. [DOI] [PubMed] [Google Scholar]
- 18.Santos S, Peinado VI, Ramirez J, et al. Characterization of pulmonary vascular remodelling in smokers and patients with mild COPD. Eur Respir J. 2002;19(4):632–638. doi: 10.1183/09031936.02.00245902. [DOI] [PubMed] [Google Scholar]
- 19.Wells JM, Washko GR, Han MK, et al. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med. 2012;367(10):913–921. doi: 10.1056/NEJMoa1203830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iyer AS, Wells JM, Vishin S, Bhatt SP, Wille KM, Dransfield MT. CT scan-measured pulmonary artery to aorta ratio and echocardiography for detecting pulmonary hypertension in severe COPD. Chest. 2014;145(4):824–832. doi: 10.1378/chest.13-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 22.Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26(3):511–522. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 23.Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26(4):720–735. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 24.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 25.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 26.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 28.Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10(2):165–193. doi: 10.1093/ejechocard/jep007. [DOI] [PubMed] [Google Scholar]
- 29.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289(2):194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 30.Han MK, McLaughlin VV, Criner GJ, Martinez FJ. Pulmonary diseases and the heart. Circulation. 2007;116:2992–3005. doi: 10.1161/CIRCULATIONAHA.106.685206. [DOI] [PubMed] [Google Scholar]
- 31.Zvezdin B, Milutinov S, Kojicic M, et al. A postmortem analysis of major causes of early death in patients hospitalized with COPD exacerbation. Chest. 2009;136(2):376–380. doi: 10.1378/chest.08-2918. [DOI] [PubMed] [Google Scholar]
- 32.Nakanishi R, Rana JS, Shalev A, et al. Mortality risk as a function of the ratio of pulmonary trunk to ascending aorta diameter in patients with suspected coronary artery disease. Am J Cardiol. 2013;111(9):1259–1263. doi: 10.1016/j.amjcard.2013.01.266. [DOI] [PubMed] [Google Scholar]
- 33.Cuttica MJ, Shah SJ, Rosenberg SR, et al. Right heart structural changes are independently associated with exercise capacity in non-severe COPD. PLoS One. 2011;6(12):e29069. doi: 10.1371/journal.pone.0029069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerges C, Gerges M, Lang MB, et al. Diastolic pulmonary vascular pressure gradient: a predictor of prognosis in “out-of-proportion” pulmonary hypertension. Chest. 2013;143(3):758–766. doi: 10.1378/chest.12-1653. [DOI] [PubMed] [Google Scholar]
- 35.Brittain EL, Assad TR, Hemnes AR, Newman JH. The diastolic pressure gradient does not and should not predict outcomes. JACC Heart Fail. 2015;3(10):845. doi: 10.1016/j.jchf.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 36.Tampakakis E, Leary PJ, Selby VN, et al. The diastolic pulmonary gradient does not predict survival in patients with pulmonary hypertension due to left heart disease. JACC Heart Fail. 2015;3(1):9–16. doi: 10.1016/j.jchf.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]