Abstract
Polymers provide a versatile platform for mimicking various aspects of physiological extracellular matrix properties such as chemical composition, rigidity, and topography for use in cell and tissue engineering applications. In this review, we provide a brief overview of patterning methods of various polymers with a particular focus on biocompatibility and processability. The materials highlighted here are widely used polymers including thermally curable polydimethyl siloxane, ultraviolet-curable polyurethane acrylate and polyethylene glycol, thermo-sensitive poly(N-isopropylacrylamide) and thermoplastic and conductive polymers. We also discuss how micro- and nanofabricated polymeric substrates of tunable elastic modulus can be used to engineer cell and tissue structure and function. Such synergistic effect of topography and rigidity of polymers may be able to contribute to constructing more physiologically relevant microenvironment.
Keywords: Patterning, Biocompatible polymers, Topography, Rigidity, Cell–biomaterial interface
INTRODUCTION
The rapid evolution of cell and tissue engineering has necessitated the use of various materials such as ceramics, metals and polymers as tissue engineering scaffolds for specific cell types. It has been widely recognized that polymers possess a number of advantages as tissue engineering scaffolds in terms of biocompatibility, transparency, and processability. For example, ceramics (e.g., oxides and nitrides) are bioinert with high elastic modulus but their use is limited due to inherent brittleness and opaqueness. Metals also present high stiffness and resilience, but some are susceptible to corrosion. Moreover, both materials lack bioactivity and thus researchers have increasingly employed polymers as materials which can suitably reproduce the physiological extracellular matrix (ECM) environment with the added benefit of increased cell adhesion and biocompatibility.
The design and preparation of biomimetic polymer scaffold in terms of physical, chemical and biological similarity to native ECM plays a critical role in constructing optimal microenvironments for cells and tissues. For the last few decades, many characteristics of ECM microenvironments have been replicated by using various methods in terms of rigidity,73,115 chemical concentration,84 shear stress56 and micro/nanotopography.24,62 Among these characteristics, the rigidity and topography of biomaterials has been of major interest for mechanotransduction of cell responses.74,150
The elasticity of materials and its importance in the biomedical sciences have long been of interest to researchers. Elastic materials such as polyacrylamide allow for an elastic substrate whose modulus spans several orders of magnitude, similar to that of human tissues.115 The most striking demonstration was reported by Engler et al.,27 showing that the differentiation of mesenchymal stem cells (MSCs) was directly correlated with the stiffness of the substratum. This seminal work, together with a series of following studies, reveals that elasticity could be a key factor in controlling various responses of cells. Despite the potential of the findings, the materials used in the differentiation studies were too soft to emulate the full spectrum of material rigidity found in the human tissue. Furthermore, the integration of micro- and nanostructures for tissue engineering scaffolds has been a challenge due to poor processability of the polyacrylamide, limiting the widespread fabrication of well-organized in vivo like structures for tendons, ligaments, collagen fibers in brain and muscle fibers.171
In the cellular mechanotransduction studies, polyacrylamide or gelatin gels were widely used since these materials have shown the ability to control biochemistry and mechanics independently.115 For example, the elastic moduli of polyacrylamide and gelatin gels can be controlled in the range of 150 Pa–150 kPa29,80,108,115 and 1–100 kPa,45,130 respectively. Despite these biocompatibility and tunable elastic moduli, such low mechanical properties render them too soft to fabricate micro- or nanoscale structures with high fidelity. Specifically, when fabricating micro and nanostructures in softer materials, a rounding of corners or shrinkage of height usually occurs due the lack of material rigidity, resulting in a loss of pattern fidelity. The mechanical properties of the material also affect the resolution of the feature sizes that can be fabricated. It is noted in this regard that, to construct well-defined microscale structures with high complexity mimicking that of in vivo tissues, at least few hundreds kPa of elastic modulus is required.39 Consequently, more rigid polymeric materials have been introduced that can be structured with heat, ultraviolet (UV) or solvents. These materials are capable of creating well-defined micro and nanopatterns with smaller feature sizes than those of softer materials. For example, polydimethyl siloxane (PDMS), a well-known silicon elastomer used in soft lithography, has elastic modulus in the range of 0.6–3.5 MPa,17,110,144 which allows a patterning resolution down to few hundreds of nanometers with moderate fidelity.66,67 This relatively low elastic modulus still limits the application into well-defined cell and tissue scaffolds with small scale (down to ~100 nm), as the human tissue microenvironment in which cells reside in consists of various sizes of well-organized matrix structure ranging from 50 nm to sub-microns.28,160 For this reason, a range of other polymeric materials are required for engineering a more relevant in vitro microenvironment.
In this review, we address the patterning methods and material properties needed, with a particular focus on mechanical properties and biocompatibility to fabricate well-organized, topographically patterned cell culture substrates. The polymers covered in this review could be classified into four categories: thermo-curable, UV-curable, thermoplastic and conducting polymers. Furthermore, the materials will be compared in terms of elastic modulus, tunable mechanical properties and pattering methods to overcome inherent patterning limitations of each polymer.
CURRENT ISSUES IN CONSTRUCTING BIOMIMETIC POLYMER SCAFFOLDS
As mentioned earlier, the elastic modulus of a material plays an important role in regulating cellular behavior as seen from directed differentiation of stem cells into various cell types in accordance to differential rigidity.27,135 In Fig. 1, the elastic moduli of tissues in human body as well as various synthetic biomaterials used in cell and tissue engineering are summarized. Human tissues have their own rigidity based on specific cell types and structural organization,73 ranging from few kPa to few tens of GPa (arterial wall,1,114,143 brain,34,82,120,152 breast,76,136 cancellous bone,1,105,133 cartilage,114,143 cornea,163 cortical bone,1,133 heart,36,156 kidney,26 liver,94,165 prostate,76 saphenous vein,1 skin114,143 and tendon/ligament60,114,143). The liver and breast display very low elastic modulus around ~1 kPa, which is similar to the modulus of polyacrylamide (150 Pa–150 kPa) or gelatin (1–100 kPa). On the other hand, the cortical and cancellous bones have very high rigidity of around ~10 GPa, which corresponds to poly(methyl methacrylate) (PMMA) (2–4 GPa).
FIGURE 1.
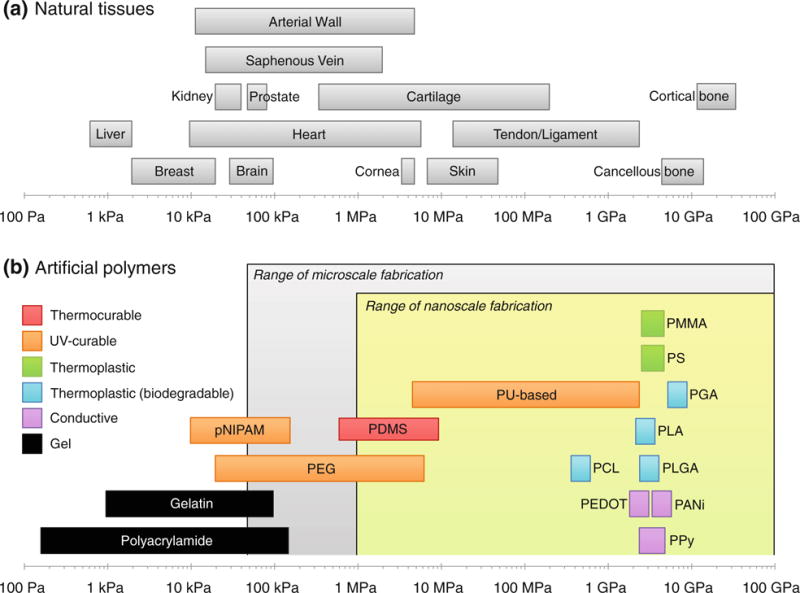
Mechanical properties of natural tissues and synthetic polymers. (a) Range of the elastic modulus of various tissues in human body. Modified from Nemir and West.115 (b) The same of various biocompatible polymers used for in vitro studies with respect to patterning resolution and mechanical properties.
In order to construct physically similar microenvironment in vitro, one must consider appropriate mechanical properties of materials used. For example, although natural polymers such as collagen, gelatin, alginate and agarose gels have relatively high biocompatibility for implantation into human body and similar elastic moduli of physiological soft tissues, they are too compliant to construct micro and nanostructures with high fidelity. In the case of large scale structures such as microvilli of gastrointestinal tract epithelium (size ~500 μm),149 the aforementioned materials can provide physiologically relevant structures with low patterning resolution. In the case of mimicking nanoscale features such as matrix fibers in myocardium,70 however, such low modulus of the materials can pose a potential problem in constructing smaller pattern sizes with diameters of a few hundreds of nanometers. For this reason, it would be beneficial to recognize the limitations of each material in terms of structuring capability, and find alternative methods for the creation of physiologically relevant micro and nanostructures.
For the last decade, the effects of rigidity and topography in cell and tissue engineering have been explored independently. Rigidity has demonstrated direct differentiation potential for stem cells with response to diverse stiffness of surfaces.27 Similarly, topography has also affected stem cell differentiation as seen from the differentiation of stem cells into osteoblasts with topography and dimensionality similar to that of real tissues.23 Although both cases have revealed the differentiation capability of each physical cue, the real tissues in vivo have well-organized texture and topography as well as specific rigidity. For example, the brain has collagen fibers with an elastic modulus range of 20–100 kPa and with fibrils of a diameter of 200–500 nm.41,152 For this reason, the construction of topographically patterned substrate with proper rigidity is of great importance to investigate synergistic effects on cell behavior and function.
Recently, several studies have been reported in the context of synergetic role of rigidity and topography. These studies demonstrated different cell migration, spreading, alignment,15,145,159 and shape,117,123,173 as compared to that in the presence of single physical cue of rigidity or topography. Despite the capability of tuning rigidity and topography in the studies, the chemistry of materials was usually heterogeneous; the combined effects from the chemistry and mechanical cues were not decoupled. To investigate such synergistic effect more systematically, the chemical consistency is required with tunable modulus, while incorporating high biocompatibility or bioinertness into the patterned polymer scaffold. Such combinations of appropriate environmental cues potentially provide a new direction for increasingly advanced and sophisticated in vitro tissue engineering platforms.
CLASSIFICATION OF PATTERNING METHODS FOR TOPOGRAPHICALLY DEFINED POLYMER SCAFFOLD
Patterning methods for synthetic polymers can be classified into two categories: template-free and template-assisted methods. Each category is further classified based on the patterning principle. In Fig. 2, three representative methods that have been frequently used to form a topographically defined substrate are included in each category: (i) electrospinning, self-assembly, and wrinkle/crack formation for the template-free method (or bottom-up method) and (ii) photolithography, electrochemical deposition, soft lithography, and nanoimprint lithography (NIL) for the template-assisted method (or top-down method). Here, photolithography is included without detailed descriptions for its excellent maturity and popularity in patterning fields. Also, chemical patterning such as microcontact printing is not included in soft lithography as it does not create a surface topography. Therefore, referring to soft lithography, mold-based approaches are only considered such as replica molding (RM), soft molding (SoMo), and capillary force lithography (CFL). A number of extensive reviews are available for the details of each patterning technique.24,62,68,73,74,134,167,171
FIGURE 2.
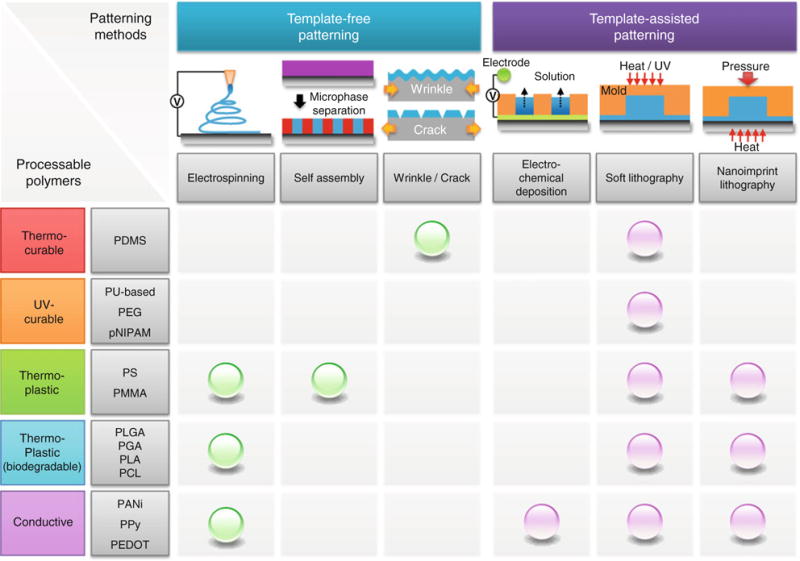
Classification of patterning methods with template-free and template-assisted principles and their availability with the existing various synthetic polymers.
Figure 2 summarizes the existing patterning methods available for each synthetic polymer. It is noted that each polymer could be used in single or multiple patterning methods depending on its properties. For example, UV-curable polymers such as polyurethane acrylate (PUA), polyethylene glycol (PEG) acrylate, Norland Optical Adhesive (NOA), poly(N-isopropyl acrylamide) (pNIPAM) have mostly been used in soft lithography in the form of RM and CFL, while thermoplastic polymers such as polymethyl methacrylate (PMMA) and polystyrene (PS) being used in multiple methods from electrospinning to NIL. It is therefore important to recognize the limitation, properties, and uses of each material in various patterning methods.
In Fig. 3, representative template-free patterning methods are displayed with various polymers, which include electrospinning, microphase separation of block copolymer, and PDMS stretching for reconfigurable wrinkles and cracks. These bottom-up patterning methods are capable of creating well-ordered surface textures in a simple and cost-effective manner for various cell and tissue engineering applications. In the first example, the electrospinning of PLA fibers was used to investigate the effect of alignment and orientation of fibers in wound healing. It was observed that different wound healing speed was observed presumably due to the contact guided growth following the fibers (Fig. 3a). In the second example, self-assembly of PS-b-PMMA block copolymer via microphase separation was used to assess different actin filament expression on various topographically patterned surfaces (Fig. 3b). In the third example, wrinkles or cracks were formed on rigid film supported on soft PDMS substrate. On a wrinkled substrate (PDMS), cardiac-like cellular morphology was generated (Fig. 3c, (i)–(iii)), while on a cracked channel elongated cellular shape was formed upon cyclic stretching (Fig. 3c, (iv)–(v)).
FIGURE 3.
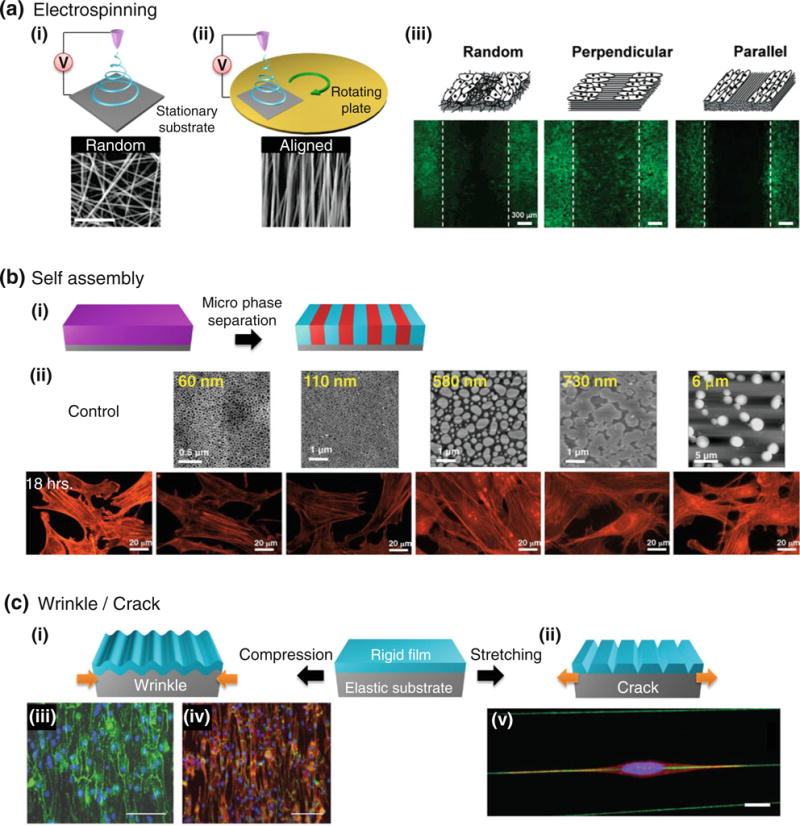
Template-free patterning methods and their applications. (a) Electrospinning of PLA fibers on stationary or rotating substrate, in which random (i) or aligned (ii) fibers can be formed. (iii) Depending on the alignment and orientation of fibers, wound healing speed was different over 48 h time span (actin filaments: green, nuclei: blue). For example, the wound healing was the fastest on perpendicularly ordered fiber matrix. Reprinted with permission from Patel et al.125 (b) Self assembly of PS-b-PMMA block copolymer. (i) With neutral interfaces between film-air and film-substrate, vertically aligned nano- to microscale patterns can be fabricated by self assembly of nanoscopic polymer domains. (ii) On topographically defined surfaces, the degree of actin stress fiber formation (fibroblast) was observed to decrease as the feature size increased. Reprinted with permission from Tsai et al.158 (c) Wrinkle and crack formation via compression (wrinkle) (i) or stretching (crack) (ii) of surface modified PDMS. (iii, iv) On wrinkled substrate, neonatal cardiac cells showed alignment. Connexin-43 (green) in (ii) and N-Cadherin (green) and actin (red) in (iii). Scale bars indicate 100 μm. Reprinted with permission from Luna et al.99 (v) An elongated myoblast cell on a crack stained with actin (red), nucleus (blue) and crack with collagen (green). Reprinted with permission from Zhu et al.177
Similarly, template-assisted patterning methods are briefly summarized in Fig. 4 along with their exemplary applications. In the template-assisted methods, polymer thin films are typically processed into desired shapes by applying a variety of external stimuli such as oxidation, pressure, heat, and UV irradiation. In the first example, patterns of electrochemically deposited conducting polymer (PPy) were used to apply an electrical stimulus to the cultured cells. With contact guidance by PLA:PLGA fibers, the patterned surfaces induced highly oriented undifferentiated myoblasts (Fig. 4a). In the second example, the most well-established soft lithography was illustrated in the form of replica molding. This method is a simple and low-expertise route to 2D or 3D topographically patterned surfaces with thermo-curable materials. Here, an array of high aspect-ratio micropillars was used to measure traction forces exerted by the cultured cells (Fig. 4b). In the third example, NIL is presented with PMMA polymer, which was used to investigate the role of pattern ordering on osteogenic differentiation (Fig. 4c).
FIGURE 4.
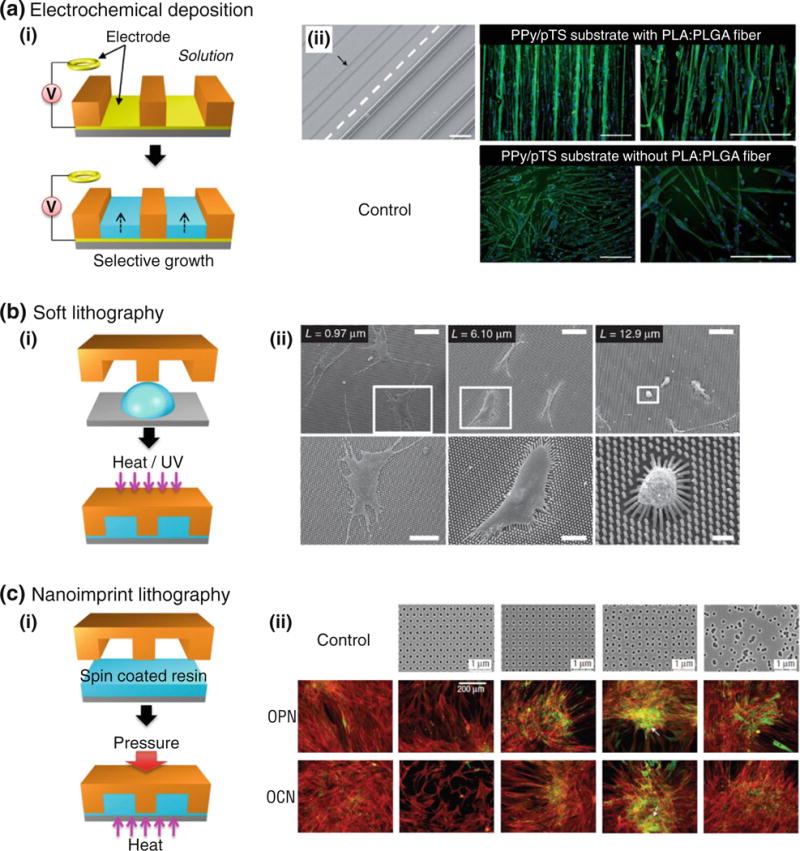
Template-assisted patterning methods and their applications. (a) (i) Schematic illustration of electrochemical deposition. (ii) SEM image of patterned surface and fluorescent images of skeletal muscle cells that adhered and proliferated for 2 days and finally differentiated into myotubes at day 4 (arrow indicates delaminated points of PLA:PLGA fiber). Immunofluorescence images of differentiated, desmin (green)-expressing myotubes on PPy/pTS substrate with (top panel) and without (bottom panel) PLA:PLGA fiber arrays. Cell nuclei are shown in blue. Scale bars are 200 μm. Reprinted with permission from Razal et al.131 (b) (i) Schematic illustration of soft lithography in the form of UV-assisted capillary force lithography (CFL). (ii) SEM images of hMSCs cultured on various micropost arrays. Bottom panel is an enlarged view from each top panel. Scale bars, 100 μm (top panel), 50 μm (first column, bottom), 30 μm (second column, bottom) and 10 μm (third column, bottom). Reprinted with permission from Fu et al.31 (c) (i) Schematic illustration of nanoimprint lithography. (ii) Effect of ordering on human mesenchymal stem cell differentiation. Top panel: SEM images of PMMA hole arrays with various orderings (diameter: 120 nm, depth: 100 nm, average center-to-center spacing: 300 nm). These arrays include hexagonal, square, displaced square with random displacement. Middle and bottom panels: Immunostaining images of osteopontin (OPN) and osteocalcin (OCN) (actin: red, OPN/OCN: green). Reprinted with permission from Dalby et al.23
SYNTHETIC POLYMERS AND THEIR PROPERTIES/USES IN PATTERNING METHODS
In this section, various synthetic polymers will be described with a particular focus on their properties in terms of biocompatibility and processability and their uses in various patterning methods. Their elastic moduli, patterning limit, and available patterning methods are summarized in Table 1.
TABLE 1.
Summary of material properties of various polymeric biomaterials used in cell and tissue engineering.
| Category | Material | Elastic modulus | Patterning limit | Patterning method | References (modulus) |
|---|---|---|---|---|---|
| Thermo-curable polymer | Polydimethyl siloxane (PDMS) | 0.6–3.5 MPa | 500–800 nm | Wrinkle/crack | 4,72,106,110 |
| h-PDMS | 9 MPa | <100 nm | Soft lithography | 17,118 | |
| UV curable polymer | Polyurethane (PU) | 6 MPa–2.5 GPa | <100 nm | Soft lithography | 16,116,174 |
| Polyethylene glycol (PEG) | 500 kPa–1.6 GPa | <50 nm | 3,10,14,88 | ||
| Poly(N-isopropylacrylamide) (pNIPAM) | 9.8–170 kPa | <1 μm | 151 | ||
| Thermoplastic | Poly(methyl methacrylate) (PMMA) | 2–3.5 GPa | ~10 nm | Electrospinning Soft lithography |
5,53,176 |
| Polystyrene (PS) | 3–3.5 GPa | <70 nm | Nanoimprint lithography Self assembly |
5,176 | |
| Thermoplastic (biodegradable) | Polylactic-co-glycolic acid (PLGA) | 2 GPa | <100 nm | Electrospinning Soft lithography |
6,42,107 |
| Polyglycolic acid (PGA) | 7 GPa | Nanoimprint lithography | 6,42,107 | ||
| Polylactic acid (PLA) | 2.7 GPa | 2,42,107 | |||
| Polycaprolactone (PCL) | 400 MPa | 42,107 | |||
| Conducting polymer | Polyaniline (PANi) | 2–4 GPa | <100 nm | Electrospinning | 32 |
| Polypyrrole (PPy) | 1.2–3.7 GPa | Electrochemical deposition | 113 | ||
| Poly(3,4-ethylenedioxythiophene) (PEDOT) | 1.1–2.2 GPa | Soft lithography Nanoimprint lithography |
79 |
Thermally Curable Polymer
Polydimethyl Siloxane (PDMS)
PDMS is one of the three primary reference biomaterials chosen by National Heart, Lung and Blood Institute (NHLBI) with the two other polymers of low-density polyethylene (LDPE) and fluorinated ethylene propylene (FEP).9 According to the references on hemocompatibility, biocompatibility, inflammatory behavior in vivo studies, PDMS causes only mild inflammatory reaction when implanted without irritating the skin, and induces no adverse effect on animal models such as rabbits and mice.9 Additionally, a recently reported dry adhesive skin patch made of PDMS pillars demonstrated negligible skin irritation.77
PDMS was first introduced by Whiteside’s group in the early 1990s in the form of soft lithography in order to massively produce micro- and submicron-scale structures.129,167 Traditionally, micropatterning utilized inorganic hard materials in photolithography at the expense of higher costs and laborious fabrication processes. Since the introduction of PDMS in micropatterning, one can directly fabricate various two-dimensional or three-dimensional patterns in a cost-effective and low-expertise fashion, which has dramatically improved the patterning capability in a typical laboratory setup. Microstructures of PDMS are made by mixing the prepolymer and cross-linker with an appropriate ratio (usually 10:1), followed by backfilling into a pre-patterned master and curing at 60–70 °C in an oven for an hour or two.129 Depending on the amount of curing agent and curing time, PDMS has tunable elastic modulus in the range of 0.6–3.5 MPa.4,72,106,110 As a result, when the pattern scale is smaller than 1 μm, the resolution decreases significantly.39 Although the patterning ability of PDMS is limited to 500–800 nm, the resolution can be further enhanced by increasing the ratio of cross-linker or adding a hard modulator (hard PDMS, E = ~9 MPa), which allows for sub-100-nm pattern resolution.17,118
One important application of micropatterned PDMS involved the use of microscale PDMS pillars for measuring traction force of cells via observing the bending of structures.31 In this study, the rigidity of pillars was modulated by varying the aspect ratio of microposts with the identical PDMS materials. Then, the amount of deflection or bending of the micropillars was easily measured when a shear force is applied to the top, where the bending was related to the magnitude of the applied shear force.
One of the important characteristics of PDMS is its high elongation at break (~160%).17 This property enables an application of cyclic stretching (stretching and releasing) onto single or multiple cells (colonies) with desired tensions and frequency. Since some human tissues such as muscle,102 heart,12 cartilage,8 ligament and tendon164 are inherently exposed to mechanical loads, it is potentially beneficial to observe the effect of external forces onto the mechano-sensitive cells. When a uniaxial stress was applied to mimic uniaxial stretching in vivo, some cells have shown elongation and orientation to the direction of stretching.13,93 With this stretching-induced alignment, the aligned ligament cells showed more efficient calcium wave propagation compared to the randomly oriented cells,59 and human patellar tendon fibroblasts (HPTFs) expressed more α-smooth muscle actin protein according to the alignment angle.164
In order to create self-organized micropatterns of PDMS, alternative methods such as wrinkle and crack formation have been used in some applications. Wrinkling is a mechanical instability occurring on a thin, stiff film adhered onto an elastomeric substrate. When an elastomeric substrate is treated with oxygen plasma or UV/Ozone, or deposited with metal layer or diamond-like carbon upon stretching, a multi-layered structure is formed with a thin stiff film on a soft substrate. Upon releasing, the stiff surface is buckled while the underlying substrate is relaxed, resulting in a spontaneous formation of well-ordered wrinkles.170 The wavelength and amplitude of wrinkles can be modulated by adjusting the thickness and modulus of thin film35 as well as the alignment via controlling the direction of mechanical strain.170 The wrinkled pattern has been used as a scaffold for heart cells, in which a certain degree of alignment and protein localization of mouse and human cardiomyocytes were observed.99
Cracking is a result of mechanical fracture occurring on a thin film adhered onto an elastomeric substrate. Similar to wrinkle patterning, PDMS surface treated with oxygen plasma or UV/Ozone can give rise to cracks upon stretching in response to the applied mechanical strain. In the case of oxygen plasma treatment, few hundreds of nanometer scale cracks are generated50,109 whereas UV/Ozone treatment induces few micron-range cracks.69 The crack formation and propagation has been studied mainly from a mechanics aspect, so that an application to tissue engineering has been rarely reported. With reconfigurable cracks, cellular elongation of mouse myoblasts was demonstrated upon cyclic stretching.177
UV-Curable Polymers
The major advantage of UV-curable polymers is a short-processing time by using UV-exposure (λ = 250– 400 nm) of few tens of seconds. In most UV-curable polymers, incorporated or trapped oxygen retards cross-linking by radical scavenging in the course of photo-crosslinking.58 To prevent such inhibition effects upon curing, a flexible and transparent support such as polyethylene terephthalate (PET) or polycarbonate (PC) sheet, or other engineering plastics can be used as a blanket or backing support of polymer structures.18 Therefore, either free-standing structures or structures supported on a backing support can be fabricated. Although the process of UV-curable patterning is simple and well-established, cell and tissue engineering applications could be restricted due to significant auto-fluorescence of plastic films, limiting the imaging of tissues.128 Thus, for biomedical research, a few hundreds of nanoscale patterns on cover glass are recommended to reduce auto-fluorescence.
Polyurethane (PU)-Based Materials
PU is a versatile UV-curable polymer whose chemical structure can be readily modified. Commercially available PU-based polymers include polyurethane acrylate (PUA, Minuta Tech. Inc., Korea) and NOA (Norland Optical Adhesive, NY, USA). PUA is a UV-curable polymer that was first introduced in 2004.18 Similar to other UV-curable materials, PUA can be cross-linked in tens of seconds upon UV-exposure, resulting in a transparent and flexible thin polymer structure with or without a backing plane. Since PUA has several notable characteristics such as transparency for optical imaging, chemical stability for long-term cell culture and tunable surface energy for easy molding, it has been successfully utilized as a cell culture platform either in single cell studies83,121,122 or various tissue engineering applications for diverse cell types such as human embryonic stem cells (hESCs),86 human mesenchymal stem cells (hMSCs),175 fibroblasts,63,71 cancer cells,78 neurons.55
The most distinguished characteristic of PUA is that its modulus can be tuned between 20 and 320 MPa, by adjusting the amount of soft and hard modulators.16,174 By utilizing the modulus-tunability and patterning method of CFL, the effect of rigidity has been investigated with the identical patterns without losing chemical consistency.174 Also, various multiscale, hierarchical structures can be fabricated with the aid of partial curing kinetics, which would be useful to recapitulate complex, hierarchically organized structures.57
NOA is the brand name of polyurethane-based UV-curable adhesive which is commercially available. Due to high transparency and simple curing process, it is usually used as an optical adhesive in fixing glass lenses38 or for micro lens arrays.22 As compared to PUA or other UV-curable polymers, the curing process of NOA is not affected by the presence of oxygen. For this reason, it can be cured even in an open environment without the use of a transparent blanket. Furthermore, NOAs adhesion properties onto glass substrate are moderate to good, thus not requiring any type of pretreatment or an adhesion promoter. When using NOA as a patterning material, a flexible mold with low surface energy is needed. Although the detailed chemical structures and additives are not known, NOA shows a wide range of elastic modulus (6 MPa–2.5 GPa, available from the provider’s website).116 As a cell culture scaffold, sub-100-nm NOA patterns have been used for culturing endothelial cells,95 fibroblasts,104 human embryonic stem cells,111,112 and breast cancer cells101 without significant adverse effects.
Polyethylene Glycol (PEG) Acrylate
PEG is a Food and Drug Administration (FDA)-approved UV-curable hydrogel that has been frequently used for drug delivery and tissue engineering.161 Due to its minimized adverse effects, it is widely used for tissue implantation surgery.46 Furthermore, it can also be used as a material to prevent cell adhesions for microchips.64 Similar to PUA, PEG acrylate patterns are fabricated onto glass substrate in the form of a thin, structured film with UV-exposure of few tens of seconds. However, as PEG is a hydrogel, it has swelling problems upon exposure to water or media. As such, the patterns are easily delaminated from the substrate.132 To prevent delamination, the substrate surface can be treated with an adhesion promoter (phosphoric acrylate or acrylic acid dissolved in propylene glycol monomethyl ether acetate (PGMEA), 10 vol.%).
PEG and its related hydrogels have a broad range of modulus tunability depending on their modified chemical structures. Basic PEG has a simple chain structure with a relatively low elastic modulus of ~500 Pa.3,88 This elastic material is suitable as a model matrix for measuring traction force of cells in a real time manner since it is easily deformed by the morphological change of cells.88 Thus, the pure PEG could not be used for constructing well-defined micro- or nanoscale patterns. With the addition of acrylate group at both ends of the polymer chain then elevates its modulus to three orders of magnitude (~500 kPa),10 allowing for the fabrication of submicron to few hundreds of nanometer structures without losing its biocompatibility.44,54 Further modification could be achieved by terminating the polymer chain with methacrylate group, resulting in the increase of modulus up to 1.6 GPa.14 With PEG dimethacrylate (PEG-DMA), nanopillars of high aspect ratio (diameter of 750 nm, height of 7 μm) have been successfully fabricated without collapse even in the presence of capillary force.14 With PEG diacrylate (PEG-DA), 50-nm-wide nanogrooves have been fabricated with high fidelity to be used as a nanopatterned scaffold for rat cardiomyocytes.65,70
Due to its biologically inert properties, PEG is also widely used for fabricating an anti-adhesion surface for cells. For example, microscale PEG patterns have been fabricated to obtain single or multiple cell aggregates.61 On non-adherent PEG substrates, co-culture of heterogeneous cells such as hepatocytes and fibroblasts87 and differentiation of mesenchymal stem cells within confined geometry127 have also been demonstrated.
Poly(N-isopropyl acrylamide) (pNIPAM)
Poly(N-isopropyl acrylamide) (pNIPAM or pNIPAAm) is a thermo-responsive polymer which can expand or shrink upon a thermal stimulus. One of the distinctive characteristics of pNIPAM is the ability to change phases in the physiologically relevant temperature range. Namely, the polymer has a lower critical solution temperature (LCST) of ~32 °C which is around the body temperature.20,139 Above the LCST, it shows a relatively hydrophobic surface, which is related to the packed conformation. In sharp contrast, below the LCST, it demonstrates a hydrophilic surface due to swelling by hydration.20,47
In addition to the tunable hydrophobicity, the material shows a dramatic difference in the mechanical property. For example, at 25 °C, its elastic modulus is ~9.8 kPa due to swelling, while at 40 °C its elastic modulus is around ~170 kPa due to dehydration.151 By utilizing this modulus tunability along with shape deformation, Khademhosseini and coworkers153,154 have recently demonstrated the use of pNIPAM as an active mold for patterning hydrogels and as microwells for forming and retrieving cell aggregates. Some studies have also demonstrated the patterning of pNIPAM surface with e-beam lithography. In the presence of the fabricated microgrooves of pNIPAM, a cell sheet with aligned cell morphology was obtained.51 With the help of the cell detachment characteristic above a certain temperature, selective cell removal and subsequent co-culture experiments were also presented.168,169
The cytotoxicity of pNIPAM has been studied in a number of drug delivery and tissue engineering applications. When the material was used as a drug delivery vehicle (eye drop) for glaucoma therapy, no difference of cell death rate was observed compared to PBS (phosphate buffered saline).48,162 When it was used as an embolic material, in vivo injection test showed no acute toxicity in mice below the dose of 250 mg kg−1.103 It was also used as a three-dimensional cell scaffold, presenting no significant problems with the exception of minor inflammation after the injection.119 Furthermore, many in vitro cell culture results and in vivo transplantation from pNIPAM plate to human body have shown no distinct adverse effects. Therefore, it can be seen that pNIPAM is a biocompatible material and possesses great potential in cell and tissue engineering applications.
Thermoplastic Polymers
A thermoplastic polymer becomes liquefied or molten upon heating above the melting temperature (Tm). The material also becomes plastic above the glass transition temperature (Tg), allowing for further modification such as drawing, bending and molding at an elevated temperature.146 Since these materials are solids at room temperature, heat or solvent treatment can be used to make fine structures.
Conventional Thermoplastics (PMMA/PS)
The biocompatibility of PMMA can be evaluated from the implantation studies in vivo. For many years, various transplantable parts made of PMMA were implanted into human bodies such as porous membranes onto human kidneys124 and intraocular lenses.98 In these reports, PMMA demonstrated long-term stability and reasonable performance without appreciable adverse effects. In contrast, PS is known to have high cellular adhesion properties but to cause strong inflammatory response upon implantation. Due to this undesirable effect, PS is usually used as a control to decide relative biocompatibility of other materials or compare relative cell affinities between materials.11,97
The most well-known fabrication method for thermoplastic polymers is Nanoimprint lithography (NIL, also known as hot embossing), which requires heat above Tg (PMMA: 85 to 165 °C, PS: 95 °C) and high pressure.43 Since the materials used in NIL usually have high elastic modulus on the order of GPa, they can represent high pattern resolution down to ~10 nm.89,166 An alternative method for patterning thermoplastic polymers utilizes reduced viscosity of the materials via temperature rise or solvent treatment. For example, a thermoplastic polymer layer can be patterned by placing a patterned PDMS mold followed by temperature rise above Tg, leaving behind a negative replica of the mold by capillary action (capillary force lithography, CFL).147,148 Similarly, a solvent-laden polymer film directly fills into the cavity of PDMS mold by capillary action, which can be termed soft molding (SoMo).134 In this way, various micro- or nanopatterns of thermoplastic polymers such as PMMA, PS and PLGA have been constructed with high pattern fidelity.33,84,85 It is noted in this regard that PMMA and PS have relatively high elastic modulus on the order of several GPa, capable of rendering several tens of nanometer patterns with high physical integrity.
In addition to the above template-assisted methods, a template-free method is possible with thermoplastic polymers. One such technique is electrospinning, in which a jet of liquid-phase polymer is ejected from a cone or nozzle, drawn by a controlled electric field, and finally stacked on ground-state plate. It is known that few tens of nanometer to few micrometer fibril structures can be constructed in the electrospinning. For more sophisticated, mesh-like structures, precise control of the electric field is required.41 Also, by adjusting the composition of solution or melt, the diameter and chemical distribution of the fibers could be modulated.155,172 An alternative template-free method is block copolymer lithography (BCL),100 where two nanophase polymer domains are self-assembled into various morphologies such as spherical, hexagonal or lamellar lattice structure. Such a periodic, ordered structure showed increased cell spreading area with the decrease of domain size,158 and more actin filament formation at smaller feature scale.157
Biodegradable Thermoplastics (PLGA/PGA/PLA/PCL)
Biodegradable polymers, more specifically synthetic biodegradable polymers, refer to the polymers that lose their initial integrity within the body tissues over time.30 These biodegradable polymers include polylactic-co-glycolic acid (PLGA), polyglycolic acid (PGA), polylactide (PLA) and polycaprolactone (PCL).42 Due to their biocompatibility, biodegradability and high rigidity, these synthetic polymers have been widely used for human therapy such as absorbable sutures as well as fixation units for medical surgeries.107
A family of PGA, PLA and their copolymers (PLGA) are FDA-approved due to their biocompatibility upon implantation which has allowed clinical applications. However, some side effects have also been recognized such as production of acid and release of small particles upon degradation.42 PLGA is a quickly biodegradable copolymer which can tune its properties by varying the relative molar ratio between lactic acid and glycolic acid (50:50, 65:35, 75:25, 85:15 are commercially available). By adjusting the lactoyl content from 50 to 85%, the degradation time can be controlled from 1 to 2 months (50% of lactoyl content) to 5–6 months (85% of lactoyl content) while retaining their elastic modulus at ~2 GPa.6,42,107 In the cases of PGA, PLA, and PCL, their degradation times are relatively long compared to that of PLGA (PGA: 6–12 months, PLA: >24 months and PCL: >24 months).
Although their chemical structures are slightly different, the fabrication techniques could be identical. For example, a thin film of these biodegradable polymers can be dissolved in a wide range of common solvents such as chloroform, toluene, tetrahydrofuran, acetone and ethyl acetate and spin-coated to be used in a simple molding technique (e.g., SoMo). Alternatively, a thin film can be thermally imprinted above the polymer’s Tg (PGA: 35–40 °C, PLA: 60–65 °C, PLGA: 40–60 °C, PCL: 265 to 260 °C), which is relatively low as compared to PMMA and PS.107 Furthermore, these biodegradable polymers have high elastic modulus (PGA: 7.0 GPa, PLA: 2.7 GPa, PLGA: 2 GPa and PCL: 0.4 GPa), which allows for the fabrication of few tens of nanometer patterns with the existing template-assisted methods.
For template-less structuring with biodegradable polymers, electrospinning is also widely used to prepare fibril structures. It was observed that the ordering and scale of fibers are important for the contact guidance-induced elongation, morphogenesis and migration of cells.125,172 In particular, cells with inherent anisotropic organization in vivo such as skeletal muscle tissue, ligaments, articular cartilage and blood vessels showed high sensitivity to the alignment of fibers.96 Additionally, bioactive molecules such as growth factors and specific signaling molecules play crucial roles in stem cell differentiation and homing of cells to the specific repair site. Further information on the electrospinning of biopolymers and their applications can be found elsewhere.96,138,155
Conducting Polymers
Certain tissues such as cardiac or nerve tissues convey their signals to adjacent cells by conducting electric pulses named ‘action potentials’.7,142 Action potential generation is involved in many crucial physiological processes, including the beating of heart at a desired frequency in a synchronized fashion.141 Since the transfer of signals is important for observing active cellular functions, the introduction of conducting materials into tissue engineering is required for certain cell types. For instance, when studying neurogenesis or cardiogenesis from stem cells, the cellular functions of differentiated cells can be judged by whether they have similar functions or electrical signals to real tissues.
For many years, conducting polymers such as polyaniline (PANi),90 polypyrrole (PPy),37 poly(3,4-ethylenedioxythiophene) (PEDOT)52 or mixtures of conducting polymers have been used to address this issue. For example, cells cultured on an electroactive surface showed enhanced neuronal differentiation,75 promoted nerve regeneration,137 and significant increase in neurite lengths.140 However, these results were obtained from the cells cultured on smooth surface without the incorporation of topographical effects. Recently, topographically modified electroactive surfaces have been introduced as a cell culturing platform to support growth of excitable tissue cells. There are a number of available patterning methods such as CFL,52 NIL49 or lift-off25 for patterning conducting polymers. Since most of the conducting polymers are rigid (elastic modulus; PANi: 2–4 GPa,32 PPy: 1.2–3.7 GPa,113 PEDOT: 1.1–2.2 GPa79) they can form tens of nanometer scale features with high fidelity. It is worthwhile noting that due to their low breaking stress, the patterned thin films need to be handled with care.91 Furthermore, precise patterning techniques are yet to come for highly controlled active structures.92 In several studies, the researchers have employed an electrochemical deposition (or electro polymerization) in order to apply an electrical potential during the cell culture on conducting substrates.126,131,140
Concerning the neuron culture, the biocompatibility of conducting polymers can be determined from an efficacy test by counting percentage of cells bearing neurites or measuring the length of neurite compared to the control surfaces. There are three factors which can influence toxicity: unreacted monomers, motility and toxicity of dopant ions, and residual solvents.40 According to the Material Safety Data Sheets (MSDS) available in Sigma-Aldrich, monomers show higher toxicity than dopants, but both of the components are slightly to moderately toxic.40 Moreover, Schmidt et al.140 demonstrated less adverse tissue response of PPy as compared to PLGA upon animal implantation. From this study, it can be assumed that conducting polymers such as PPy and PEDOT have relatively good biological performance. Furthermore, biocompatibility can be enhanced by adding bioactive factors such as laminin peptide21 and hyaluronic acid (HA)19 or coating biocompatible polymers such as PLGA onto the polymer surface.81 It seems that further studies need to be performed to find an optimal condition between biocompatibility and mechanical, electrical and biological properties.
CONCLUSIONS
In this review, we have described material properties and patterning techniques of various polymers toward topographically defined substrates in cell and tissue engineering applications. As motivated by the pioneering work by Engler et al., there are increasing demands on topographically patterned substrate with tunable modulus in order to investigate synergistic role of rigidity and topography in mechanotransduction of cells.
Here, the patterning methods were classified into two categories of template-free (or bottom-up) and template-assisted methods (or top-down). Then, the existing synthetic biocompatible polymers were described in the order of thermo-curable, UV-curable, thermoplastic and conducting polymers with particular emphasis on biocompatibility and processability. It has been shown that each biocompatible polymer is suited to specific patterning methods depending on its materials properties.
Based on the information provided in this review, an appropriate combination of material and patterning method should be chosen to create diverse and robust cell culture platforms with in vivo like cellular microenvironment. Such more physiologically relevant microenvironments may be able to significantly advance tissue engineering research while providing insight into the effects of topography and rigidity in synergy on cell behavior and function.
Acknowledgments
This work was supported by National Research Foundation Grant funded by the Korean Government (NRF-2011-220-D00035), WCU (World Class University) program (R31-2008-000-10083-0) and Basic Science Research Program (2010-0027955). D. H. Kim thanks Department of Bioengineering at the University of Washington for the new faculty startup fund. D. H. Kim is also supported by a Perkins Coie Award for Discovery. A. Jiao was supported by NIH training Grant T32-EB001650-07.
ABBREVIATIONS
Cells and related terms
- ECM
extracellular matrix
- MSCs
mesenchymal stem cells
- hMSCs
human mesenchymal stem cells
- hESCs
human embryonic stem cells
- HPTFs
human patellar tendon fibroblasts
- PBS
phosphate buffered saline
- OPN
osteopontin
- OCN
osteocalcin
Polymers
- PDMS
polydimethyl siloxane
- LDPE
low-density polyethylene
- FEP
fluorinated ethylene propylene
- PET
polyethylene terephthalate
- PC
polycarbonate
- PU
polyurethane
- PUA
polyurethane acrylate
- NOA
Norland Optical Adhesive
- PEG
polyethylene glycol
- PEG-DMA
polyethylene glycol dimethacrylate
- PEG-DA
polyethylene glycol diacrylate
- PGMEA
propylene glycol monomethyl ether acetate
- pNIPAM
poly(N-isopropylacrylamide)
- LCST
lower critical solution temperature
- PMMA
poly(methyl methacrylate)
- PS
polystyrene
- PLGA
poly(lactic-co-glycolic acid)
- PGA
polyglycolic acid
- PLA
polylactide
- PCL
polycaprolactone
- PANi
polyaniline
- PPy
polypyrrole
- PEDOT
poly(3,4-ehtylenedioxythiophene)
- PTS
paratoluenesulfonate
- HA
hyaluronic acid
Lithography
- UV
ultraviolet
- NIL
nanoimprint lithography
- RM
replica molding
- SoMo
soft molding
- CFL
capillary force lithography
- BCL
block copolymer lithography
Others
- NHLBI
National Heart, Lung and Blood Institute
- FDA
Food and Drug Administration
- MSDS
Material Safety Data Sheets
Footnotes
Associate Editor Michael Shuler oversaw the review of this article.
References
- 1.Abe H, Hayashi K, Sato M. Data Book on Mechanical Properties of Living Cells, Tissues, and Organs. Tokyo: Springer; 1996. [Google Scholar]
- 2.Agrawal CM, Haas KF, Leopold DA, Clark HG. Evaluation of poly(L-lactic acid) as a material for intravascular polymeric stents. Biomaterials. 1992;13:176–182. doi: 10.1016/0142-9612(92)90068-y. [DOI] [PubMed] [Google Scholar]
- 3.Almany L, Seliktar D. Biosynthetic hydrogel scaffolds made from fibrinogen and polyethylene glycol for 3D cell cultures. Biomaterials. 2005;26:2467–2477. doi: 10.1016/j.biomaterials.2004.06.047. [DOI] [PubMed] [Google Scholar]
- 4.Armani D, Liu C, Aluru N. Re-configurable fluid circuits by PDMS elastomer micromachining. Presented at Micro Electro Mechanical Systems, 1999. MEMS ‘99. Twelfth IEEE International Conference on; 17–21 Jan 1999; pp. 222–227. [Google Scholar]
- 5.Ashby MF. Materials Selection in Mechanical Design. Burlington, MA: Butterworth-Heinemann; 2011. [Google Scholar]
- 6.ASM International. Materials and Coatings for Medical Devices: Cardiovascular. Materials Park, OH: ASM International; 2009. [Google Scholar]
- 7.Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci. 2007;8:451–465. doi: 10.1038/nrn2148. [DOI] [PubMed] [Google Scholar]
- 8.Beaupre GS, Stevens SS, Carter DR. Mechanobiology in the development, maintenance, and degeneration of articular cartilage. J Rehabil Res Dev. 2000;37:145–151. [PubMed] [Google Scholar]
- 9.Belanger MC, Marois Y. Hemocompatibility, biocompatibility, inflammatory and in vivo studies of primary reference materials low-density polyethylene and polydimethylsiloxane: a review. J Biomed Mater Res. 2001;58:467–477. doi: 10.1002/jbm.1043. [DOI] [PubMed] [Google Scholar]
- 10.Bikram M, Fouletier-Dilling C, Hipp JA, Gannon F, Davis AR, Olmsted-Davis EA, West JL. Endochondral bone formation from hydrogel carriers loaded with BMP2-transduced cells. Ann Biomed Eng. 2007;35:796–807. doi: 10.1007/s10439-007-9263-4. [DOI] [PubMed] [Google Scholar]
- 11.Bratlie KM, Dang TT, Lyle S, Nahrendorf M, Weissleder R, Langer R, Anderson DG. Rapid biocompatibility analysis of materials via in vivo fluorescence imaging of mouse models. PLoS ONE. 2010;5:e10032. doi: 10.1371/journal.pone.0010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camelliti P, Borg TK, Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc Res. 2005;65:40–51. doi: 10.1016/j.cardiores.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 13.Campbell BH, Clark WW, Wang JHC. A multi-station culture force monitor system to study cellular contractility. J Biomech. 2003;36:137–140. doi: 10.1016/s0021-9290(02)00325-1. [DOI] [PubMed] [Google Scholar]
- 14.Chandra D, Taylor JA, Yang S. Replica molding of high-aspect-ratio (sub-)micron hydrogel pillar arrays and their stability in air and solvents. Soft Matter. 2008;4:979–984. doi: 10.1039/b717711a. [DOI] [PubMed] [Google Scholar]
- 15.Chen CC, Hsieh PCH, Wang GM, Chen WC, Yeh ML. The influence of surface morphology and rigidity of the substrata on cell motility. Mater Lett. 2009;63:1872–1875. [Google Scholar]
- 16.Choi SJ, Kim HN, Bae WG, Suh KY. Modulus- and surface energy-tunable ultraviolet-curable polyurethane acrylate: properties and applications. J Mater Chem. 2011;21:14325–14335. [Google Scholar]
- 17.Choi KM, Rogers JA. A photocurable poly(dimethylsiloxane) chemistry designed for soft lithographic molding and printing in the nanometer regime. J Am Chem Soc. 2003;125:4060–4061. doi: 10.1021/ja029973k. [DOI] [PubMed] [Google Scholar]
- 18.Choi SJ, Yoo PJ, Baek SJ, Kim TW, Lee HH. An ultraviolet-curable mold for sub-100-nm lithography. J Am Chem Soc. 2004;126:7744–7745. doi: 10.1021/ja048972k. [DOI] [PubMed] [Google Scholar]
- 19.Collier JH, Camp JP, Hudson TW, Schmidt CE. Synthesis and characterization of polypyrrole-hyaluronic acid composite biomaterials for tissue engineering applications. J Biomed Mater Res. 2000;50:574–584. doi: 10.1002/(sici)1097-4636(20000615)50:4<574::aid-jbm13>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 20.Cooperstein MA, Canavan HE. Biological cell detachment from poly(N-isopropyl acrylamide) and its applications. Langmuir. 2010;26:7695–7707. doi: 10.1021/la902587p. [DOI] [PubMed] [Google Scholar]
- 21.Cui XY, Martin DC. Electrochemical deposition and characterization of poly(3,4-ethylenedioxythiophene) on neural microelectrode arrays. Sens Actuators B-Chem. 2003;89:92–102. [Google Scholar]
- 22.Dal Zilio S, Tvingstedt K, Inganas O, Tormen M. Fabrication of a light trapping system for organic solar cells. Microelectron Eng. 2009;86:1150–1154. [Google Scholar]
- 23.Dalby MJ, Gadegaard N, Tare R, Andar A, Riehle MO, Herzyk P, Wilkinson CDW, Oreffo ROC. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater. 2007;6:997–1003. doi: 10.1038/nmat2013. [DOI] [PubMed] [Google Scholar]
- 24.Dolatshahi-Pirouz A, Nikkhah M, Kolind K, Dokmeci MR, Khademhosseini A. Micro- and nanoengineering approaches to control stem cell–biomaterial interactions. J Funct Biomater. 2011;2:88–106. doi: 10.3390/jfb2030088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong B, Lu N, Zelsmann M, Kehagias N, Fuchs H, Torres CMS, Chi LF. Fabrication of high-density large-area conducting-polymer nanostructures. Adv Funct Mater. 2006;16:1937–1942. [Google Scholar]
- 26.Emelianov SY, Erkamp RQ, Lubinski MA, Skovoroda AR, O’Donnell M. Non-linear tissue elasticity: adaptive elasticity imaging for large deformations. Presented at Ultrasonics Symposium, 1998. Proceedings, 1998 IEEE; 1998. pp. 1753–1756. [Google Scholar]
- 27.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 28.Fisher OZ, Khademhosseini A, Langer R, Peppas NA. Bioinspired materials for controlling stem cell fate. Accounts Chem Res. 2010;43:419–428. doi: 10.1021/ar900226q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flanagan LA, Ju YE, Marg B, Osterfield M, Janmey PA. Neurite branching on deformable substrates. Neuroreport. 2002;13:2411–2415. doi: 10.1097/01.wnr.0000048003.96487.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freed LE, Vunjaknovakovic G, Biron RJ, Eagles DB, Lesnoy DC, Barlow SK, Langer R. Biodegradable polymer scaffolds for tissue engineering. Nat Biotechnol. 1994;12:689–693. doi: 10.1038/nbt0794-689. [DOI] [PubMed] [Google Scholar]
- 31.Fu JP, Wang YK, Yang MT, Desai RA, Yu XA, Liu ZJ, Chen CS. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat Methods. 2010;7:733–739. doi: 10.1038/nmeth.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gajendran P, Saraswathi R. Polyaniline–carbon nanotube composites. Pure Appl Chem. 2008;80:2377–2395. [Google Scholar]
- 33.Gates BD, Xu QB, Love JC, Wolfe DB, Whitesides GM. Unconventional nanofabrication. Annu Rev Mater Res. 2004;34:339–372. [Google Scholar]
- 34.Gefen A, Margulies SS. Are in vivo and in situ brain tissues mechanically similar? J Biomech. 2004;37:1339–1352. doi: 10.1016/j.jbiomech.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 35.Genzer J, Groenewold J. Soft matter with hard skin: from skin wrinkles to templating and material characterization. Soft Matter. 2006;2:310–323. doi: 10.1039/b516741h. [DOI] [PubMed] [Google Scholar]
- 36.Ghista D, Vayo W, Sandler H. Elastic modulus of the human intact left ventricle—determination and physiological interpretation. Med Biol Eng Comput. 1975;13:151–161. doi: 10.1007/BF02477722. [DOI] [PubMed] [Google Scholar]
- 37.Gilmore KJ, Kita M, Han Y, Gelmi A, Higgins MJ, Moulton SE, Clark GM, Kapsa R, Wallace GG. Skeletal muscle cell proliferation and differentiation on polypyrrole substrates doped with extracellular matrix components. Biomaterials. 2009;30:5292–5304. doi: 10.1016/j.biomaterials.2009.06.059. [DOI] [PubMed] [Google Scholar]
- 38.Goldenberg LM, Gritsai Y, Sakhno O, Kulikovska O, Stumpe J. All-optical fabrication of 2D and 3D photonic structures using a single polymer phase mask. J Opt. 2010;12:015103. [Google Scholar]
- 39.Gordan OD, Persson BNJ, Cesa CM, Mayer D, Hoffmann B, Dieluweit S, Merkel R. On pattern transfer in replica molding. Langmuir. 2008;24:6636–6639. doi: 10.1021/la800728x. [DOI] [PubMed] [Google Scholar]
- 40.Green RA, Lovell NH, Wallace GG, Poole-Warren LA. Conducting polymers for neural interfaces: challenges in developing an effective long-term implant. Biomaterials. 2008;29:3393–3399. doi: 10.1016/j.biomaterials.2008.04.047. [DOI] [PubMed] [Google Scholar]
- 41.Greiner A, Wendorff JH. Electrospinning: a fascinating method for the preparation of ultrathin fibres. Angew Chem Int Edit. 2007;46:5670–5703. doi: 10.1002/anie.200604646. [DOI] [PubMed] [Google Scholar]
- 42.Gunatillake PA, Adhikari R. Biodegradable synthetic polymers for tissue engineering. Eur Cell Mater. 2003;5:1–16. doi: 10.22203/ecm.v005a01. [DOI] [PubMed] [Google Scholar]
- 43.Guo LJ. Nanoimprint lithography: methods and material requirements. Adv Mater. 2007;19:495–513. [Google Scholar]
- 44.Hahn MS, Taite LJ, Moon JJ, Rowland MC, Ruffino KA, West JL. Photolithographic patterning of polyethylene glycol hydrogels. Biomaterials. 2006;27:2519–2524. doi: 10.1016/j.biomaterials.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 45.Hall TJ, Bilgen M, Insana MF, Krouskop TA. Phantom materials for elastography. IEEE Trans Ultrason Ferr. 1997;44:1355–1365. [Google Scholar]
- 46.Heo YJ, Shibata H, Okitsu T, Kawanishi T, Takeuchi S. Long-term in vivo glucose monitoring using fluorescent hydrogel fibers. Proc Natl Acad Sci USA. 2011;108:13399–13403. doi: 10.1073/pnas.1104954108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirose M, Kwon OH, Yamato M, Kikuchi A, Okano T. Creation of designed shape cell sheets that are noninvasively harvested and moved onto another surface. Biomacromolecules. 2000;1:377–381. doi: 10.1021/bm0002961. [DOI] [PubMed] [Google Scholar]
- 48.Hsiue GH, Chang RW, Wang CH, Lee SH. Development of in situ thermosensitive drug vehicles for glaucoma therapy. Biomaterials. 2003;24:2423–2430. doi: 10.1016/s0142-9612(03)00035-8. [DOI] [PubMed] [Google Scholar]
- 49.Huang CY, Dong B, Lu N, Yang BJ, Gao LG, Tian L, Qi DP, Wu Q, Chi LF. A strategy for patterning conducting polymers using nanoimprint lithography and isotropic plasma etching. Small. 2009;5:583–586. doi: 10.1002/smll.200801197. [DOI] [PubMed] [Google Scholar]
- 50.Huh D, Mills KL, Zhu XY, Burns MA, Thouless MD, Takayama S. Tuneable elastomeric nanochannels for nanofluidic manipulation. Nat Mater. 2007;6:424–428. doi: 10.1038/nmat1907. [DOI] [PubMed] [Google Scholar]
- 51.Idota N, Tsukahara T, Sato K, Okano T, Kitamori T. The use of electron beam lithographic graft-polymerization on thermoresponsive polymers for regulating the directionality of cell attachment and detachment. Biomaterials. 2009;30:2095–2101. doi: 10.1016/j.biomaterials.2008.12.058. [DOI] [PubMed] [Google Scholar]
- 52.Im SG, Yoo PJ, Hammond PT, Gleason KK. Grafted conducting polymer films for nano-patterning onto various organic and inorganic substrates by oxidative chemical vapor deposition. Adv Mater. 2007;19:2863–2867. [Google Scholar]
- 53.Ishiyama C, Higo Y. Effects of humidity on Young’s modulus in poly(methyl methacrylate) J Polym Sci Polym Phys. 2002;40:460–465. [Google Scholar]
- 54.Jang JH, Dendukuri D, Hatton TA, Thomas EL, Doyle PS. A route to three-dimensional structures in a microfluidic device: stop-flow interference lithography. Angew Chem Int Ed. 2007;46:9027–9031. doi: 10.1002/anie.200703525. [DOI] [PubMed] [Google Scholar]
- 55.Jang KJ, Kim MS, Feltrin D, Jeon NL, Suh KY, Pertz O. Two distinct filopodia populations at the growth cone allow to sense nanotopographical extracellular matrix cues to guide neurite outgrowth. PLoS ONE. 2010;5:e15966. doi: 10.1371/journal.pone.0015966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Janmey PA, McCulloch CA. Cell mechanics: integrating cell responses to mechanical stimuli. Annu Rev Biomed Eng. 2007;9:1–34. doi: 10.1146/annurev.bioeng.9.060906.151927. [DOI] [PubMed] [Google Scholar]
- 57.Jeong HE, Lee JK, Kim HN, Moon SH, Suh KY. A nontransferring dry adhesive with hierarchical polymer nanohairs. Proc Natl Acad Sci USA. 2009;106:5639–5644. doi: 10.1073/pnas.0900323106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jeong HE, Suh KY. On the role of oxygen in fabricating microfluidic channels with ultraviolet curable materials. Lab Chip. 2008;8:1787–1792. doi: 10.1039/b810348h. [DOI] [PubMed] [Google Scholar]
- 59.Jones BF, Wall ME, Carroll RL, Washburn S, Banes AJ. Ligament cells stretch-adapted on a micro-grooved substrate increase intercellular communication in response to a mechanical stimulus. J Biomech. 2005;38:1653–1664. doi: 10.1016/j.jbiomech.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 60.Kadler KE, Hulmes DJS, Hojima Y, Prockop DJ. Assembly of type-I collagen fibrils denovo by the specific enzymatic cleavage of pC collagen—the fibrils formed at about 37-degrees-C are similar in diameter, roundness, and apparent flexibility to the collagen fibrils seen in connective-tissue. Ann N Y Acad Sci. 1990;580:214–224. doi: 10.1111/j.1749-6632.1990.tb17930.x. [DOI] [PubMed] [Google Scholar]
- 61.Khademhosseini A, Jon S, Suh KY, Tran TNT, Eng G, Yeh J, Seong J, Langer R. Direct patterning of protein- and cell-resistant polymeric monolayers and microstructures. Adv Mater. 2003;15:1995–2000. [Google Scholar]
- 62.Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Microscale technologies for tissue engineering and biology. Proc Natl Acad Sci USA. 2006;103:2480–2487. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim DH, Han K, Gupta K, Kwon KW, Suh KY, Levchenko A. Mechanosensitivity of fibroblast cell shape and movement to anisotropic substratum topography gradients. Biomaterials. 2009;30:5433–5444. doi: 10.1016/j.biomaterials.2009.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim P, Jeong HE, Khademhosseini A, Suh KY. Fabrication of non-biofouling polyethylene glycol micro- and nanochannels by ultraviolet-assisted irreversible sealing. Lab Chip. 2006;6:1432–1437. doi: 10.1039/b610503c. [DOI] [PubMed] [Google Scholar]
- 65.Kim P, Kim HY, Kim JK, Reiter G, Suh KY. Multi-curvature liquid meniscus in a nanochannel: evidence of interplay between intermolecular and surface forces. Lab Chip. 2009;9:3255–3260. doi: 10.1039/b911271e. [DOI] [PubMed] [Google Scholar]
- 66.Kim P, Kwak R, Lee SH, Suh KY. Solvent-assisted decal transfer lithography by oxygen-plasma bonding and anisotropic swelling. Adv Mater. 2010;22:2426–2429. doi: 10.1002/adma.200903440. [DOI] [PubMed] [Google Scholar]
- 67.Kim YS, Lee HH, Hammond PT. High density nanostructure transfer in soft molding using polyurethane acrylate molds and polyelectrolyte multilayers. Nanotechnology. 2003;14:1140–1144. [Google Scholar]
- 68.Kim DH, Lee H, Lee YK, Nam JM, Levchenko A. Biomimetic nanopatterns as enabling tools for analysis and control of live cells. Adv Mater. 2010;22:4551–4566. doi: 10.1002/adma.201000468. [DOI] [PubMed] [Google Scholar]
- 69.Kim HN, Lee SH, Suh KY. Controlled mechanical fracture for fabricating microchannels with various size gradients. Lab Chip. 2011;11:717–722. doi: 10.1039/c0lc00277a. [DOI] [PubMed] [Google Scholar]
- 70.Kim DH, Lipke EA, Kim P, Cheong R, Thompson S, Delannoy M, Suh KY, Tung L, Levchenko A. Nanoscale cues regulate the structure and function of macroscopic cardiac tissue constructs. Proc Natl Acad Sci USA. 2010;107:565–570. doi: 10.1073/pnas.0906504107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim DH, Seo CH, Han K, Kwon KW, Levchenko A, Suh KY. Guided cell migration on micro-textured substrates with variable local density and anisotropy. Adv Funct Mater. 2009;19:1579–1586. doi: 10.1002/adfm.200990041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim KH, Song NY, Choo BK, Pribat D, Jang J, Park KC. Mechanical characteristics of the hard-polydimethylsiloxane for smart lithography. Presented at EKC2008 Proceedings of the EU-Korea Conference on Science and Technology; 2008. pp. 229–237. [Google Scholar]
- 73.Kim DH, Wong PK, Park J, Levchenko A, Sun Y. Microengineered platforms for cell mechanobiology. Annu Rev Biomed Eng. 2009;11:203–233. doi: 10.1146/annurev-bioeng-061008-124915. [DOI] [PubMed] [Google Scholar]
- 74.Kotov NA, Winter JO, Clements IP, Jan E, Timko BP, Campidelli S, Pathak S, Mazzatenta A, Lieber CM, Prato M, Bellamkonda RV, Silva GA, Kam NWS, Patolsky F, Ballerini L. Nanomaterials for neural interfaces. Adv Mater. 2009;21:3970–4004. [Google Scholar]
- 75.Kotwal A, Schmidt CE. Electrical stimulation alters protein adsorption and nerve cell interactions with electrically conducting biomaterials. Biomaterials. 2001;22:1055–1064. doi: 10.1016/s0142-9612(00)00344-6. [DOI] [PubMed] [Google Scholar]
- 76.Krouskop TA, Wheeler TM, Kallel F, Garra BS, Hall T. Elastic moduli of breast and prostate tissues under compression. Ultrason Imaging. 1998;20:260–274. doi: 10.1177/016173469802000403. [DOI] [PubMed] [Google Scholar]
- 77.Kwak MK, Jeong HE, Suh KY. Rational design and enhanced biocompatibility of a dry adhesive medical skin patch. Adv Mater. 2011;23:3949–3953. doi: 10.1002/adma.201101694. [DOI] [PubMed] [Google Scholar]
- 78.Kwon KW, Choi SS, Lee SH, Kim B, Lee SN, Park MC, Kim P, Hwang SY, Suh KY. Label-free, microfluidic separation and enrichment of human breast cancer cells by adhesion difference. Lab Chip. 2007;7:1461–1468. doi: 10.1039/b710054j. [DOI] [PubMed] [Google Scholar]
- 79.Lang U, Naujoks N, Dual J. Mechanical characterization of PEDOT:PSS thin films. Synth Met. 2009;159:473–479. [Google Scholar]
- 80.Leach JB, Brown XQ, Jacot JG, DiMilla PA, Wong JY. Neurite outgrowth and branching of PC12 cells on very soft substrates sharply decreases below a threshold of substrate rigidity. J Neural Eng. 2007;4:26–34. doi: 10.1088/1741-2560/4/2/003. [DOI] [PubMed] [Google Scholar]
- 81.Lee JY, Bashur CA, Goldstein AS, Schmidt CE. Polypyrrole-coated electrospun PLGA nanofibers for neural tissue applications. Biomaterials. 2009;30:4325–4335. doi: 10.1016/j.biomaterials.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee H, Bellamkonda RV, Sun W, Levenston ME. Biomechanical analysis of silicon microelectrode-induced strain in the brain. J Neural Eng. 2005;2:81–89. doi: 10.1088/1741-2560/2/4/003. [DOI] [PubMed] [Google Scholar]
- 83.Lee SH, Jeong HE, Park MC, Hur JY, Cho HS, Park SH, Suh KY. Fabrication of hollow polymeric microstructures for shear-protecting cell containers. Adv Mater. 2008;20:788–792. [Google Scholar]
- 84.Lee SH, Kang DH, Kim HN, Suh KY. Use of directly molded poly(methyl methacrylate) channels for microfluidic applications. Lab Chip. 2010;10:3300–3306. doi: 10.1039/c0lc00127a. [DOI] [PubMed] [Google Scholar]
- 85.Lee SH, Kim HN, Kwak RK, Suh KY. Effects of mold rising angle and polymer concentration in solvent-assisted molding. Langmuir. 2009;25:12024–12029. doi: 10.1021/la903236d. [DOI] [PubMed] [Google Scholar]
- 86.Lee MR, Kwon KW, Jung H, Kim HN, Suh KY, Kim K, Kim KS. Direct differentiation of human embryonic stem cells into selective neurons on nanoscale ridge/groove pattern arrays. Biomaterials. 2010;31:4360–4366. doi: 10.1016/j.biomaterials.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 87.Lee JY, Shah SS, Yan J, Howland MC, Parikh AN, Pan TR, Revzin A. Integrating sensing hydrogel microstructures into micropatterned hepatocellular cocultures. Langmuir. 2009;25:3880–3886. doi: 10.1021/la803635r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Legant WR, Miller JS, Blakely BL, Cohen DM, Genin GM, Chen CS. Measurement of mechanical tractions exerted by cells in three-dimensional matrices. Nat Methods. 2010;7:969–971. doi: 10.1038/nmeth.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li ZW, Gu YN, Wang L, Ge HX, Wu W, Xia QF, Yuan CS, Chen Y, Cui B, Williams RS. Hybrid nanoimprint-soft lithography with sub-15 nm resolution. Nano Lett. 2009;9:2306–2310. doi: 10.1021/nl9004892. [DOI] [PubMed] [Google Scholar]
- 90.Li MY, Guo Y, Wei Y, MacDiarmid AG, Lelkes PI. Electrospinning polyaniline-contained gelatin nanofibers for tissue engineering applications. Biomaterials. 2006;27:2705–2715. doi: 10.1016/j.biomaterials.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 91.Li SL, Macosko CW, White HS. Electrochemical processing of conducting polymer fibers. Science. 1993;259:957–960. [Google Scholar]
- 92.Li G, Shrotriya V, Yao Y, Huang JS, Yang Y. Manipulating regioregular poly(3-hexylthiophene): [6,6]-phenyl-C-61-butyric acid methyl ester blends—route towards high efficiency polymer solar cells. J Mater Chem. 2007;17:3126–3140. [Google Scholar]
- 93.Li ZZ, Yang GG, Khan M, Stone D, Woo SLY, Wang JHC. Inflammatory response of human tendon fibroblasts to cyclic mechanical stretching. Am J Sports Med. 2004;32:435–440. doi: 10.1177/0095399703258680. [DOI] [PubMed] [Google Scholar]
- 94.Li PC, Yeh WC, Jeng YM, Hsu HC, Kuo PL, Li ML, Yang PM, Lee PH. Elastic modulus measurements of human liver and correlation with pathology. Ultrasound Med Biol. 2002;28:467–474. doi: 10.1016/s0301-5629(02)00489-1. [DOI] [PubMed] [Google Scholar]
- 95.Liliensiek SJ, Wood JA, Yong JA, Auerbach R, Nealey PF, Murphy CJ. Modulation of human vascular endothelial cell behaviors by nanotopographic cues. Biomaterials. 2010;31:5418–5426. doi: 10.1016/j.biomaterials.2010.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lim SH, Mao HQ. Electrospun scaffolds for stem cell engineering. Adv Drug Deliver Rev. 2009;61:1084–1096. doi: 10.1016/j.addr.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 97.Liu WE, Ma ML, Bratlie KM, Dang TT, Langer R, Anderson DG. Real-time in vivo detection of biomaterial-induced reactive oxygen species. Biomaterials. 2011;32:1796–1801. doi: 10.1016/j.biomaterials.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lloyd AW, Faragher RGA, Denyer SP. Ocular biomaterials and implants. Biomaterials. 2001;22:769–785. doi: 10.1016/s0142-9612(00)00237-4. [DOI] [PubMed] [Google Scholar]
- 99.Luna JI, Ciriza J, Garcia-Ojeda ME, Kong M, Herren A, Lieu DK, Li RA, Fowlkes CC, Khine M, McCloskey KE. Multiscale biomimetic topography for the alignment of neonatal and embryonic stem cell-derived heart cells. Tissue Eng Part C Methods. 2011;17:579–588. doi: 10.1089/ten.TEC.2010.0410. [DOI] [PubMed] [Google Scholar]
- 100.Marencic AP, Register RA. Controlling order in block copolymer thin films for nanopatterning applications. Annu Rev Chem Biomol. 2010;1:277–297. doi: 10.1146/annurev-chembioeng-073009-101007. [DOI] [PubMed] [Google Scholar]
- 101.Markovitz-Bishitz Y, Tauber Y, Afrimzon E, Zurgil N, Sobolev M, Shafran Y, Deutsch A, Howitz S, Deutsch M. A polymer microstructure array for the formation, culturing, and high throughput drug screening of breast cancer spheroids. Biomaterials. 2010;31:8436–8444. doi: 10.1016/j.biomaterials.2010.07.050. [DOI] [PubMed] [Google Scholar]
- 102.Mathews MB. Connective tissue. Macromolecular structure and evolution. Mol Biol Biochem Biophys. 1975;19:1–318. [PubMed] [Google Scholar]
- 103.Matsumaru Y, Hyodo A, Nose T, Ito S, Hirano T, Ohashi S. Application of thermosensitive polymers as a new embolic material for intravascular neurosurgery. J Biomater Sci Polym Edn. 1996;7:795–804. doi: 10.1163/156856296x00138. [DOI] [PubMed] [Google Scholar]
- 104.Meng J, Kong H, Han ZZ, Wang CY, Zhu GJ, Xie SS, Xu HY. Enhancement of nanofibrous scaffold of multiwalled carbon nanotubes/polyurethane composite to the fibroblasts growth and biosynthesis. J Biomed Mater Res A. 2009;88A:105–116. doi: 10.1002/jbm.a.31862. [DOI] [PubMed] [Google Scholar]
- 105.Mente PL, Lewis JL. Experimental-method for the measurement of the elastic-modulus of trabecular bone tissue. J Orthop Res. 1989;7:456–461. doi: 10.1002/jor.1100070320. [DOI] [PubMed] [Google Scholar]
- 106.Michel B, Schmid H. Siloxane polymers for high-resolution, high-accuracy soft lithography. Macromolecules. 2000;33:3042–3049. [Google Scholar]
- 107.Middleton JC, Tipton AJ. Synthetic biodegradable polymers as orthopedic devices. Biomaterials. 2000;21:2335–2346. doi: 10.1016/s0142-9612(00)00101-0. [DOI] [PubMed] [Google Scholar]
- 108.Mih JD, Tschumperlin DJ. Lung fibroblast behavior is tuned by substrate stiffness. Proc Am Thorac Soc. 2008;5:364–365. [Google Scholar]
- 109.Mills KL, Huh D, Takayama S, Thouless MD. Instantaneous fabrication of arrays of normally closed, adjustable, and reversible nanochannels by tunnel cracking. Lab Chip. 2010;10:1627–1630. doi: 10.1039/c000863j. [DOI] [PubMed] [Google Scholar]
- 110.Mills KL, Zhu XY, Takayama SC, Thouless MD. The mechanical properties of a surface-modified layer on polydimethylsiloxane. J Mater Res. 2008;23:37–48. doi: 10.1557/JMR.2008.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mohr JC, de Pablo JJ, Palecek SP. 3-D microwell culture of human embryonic stem cells. Biomaterials. 2006;27:6032–6042. doi: 10.1016/j.biomaterials.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 112.Mohr JC, Zhang JH, Azarin SM, Soerens AG, de Pablo JJ, Thomson JA, Lyons GE, Palecek SP, Kamp TJ. The microwell control of embryoid body size in order to regulate cardiac differentiation of human embryonic stem cells. Biomaterials. 2010;31:1885–1893. doi: 10.1016/j.biomaterials.2009.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Murray P, Spinks GM, Wallace GG, Burford RP. In situ mechanical properties of tosylate doped (pTS) polypyrrole. Synth Met. 1997;84:847–848. [Google Scholar]
- 114.Nahum AM, Melvin J. Accidental Injury: Biomechanics and Prevention. New York: Springer; 2002. [Google Scholar]
- 115.Nemir S, West JL. Synthetic materials in the study of cell response to substrate rigidity. Ann Biomed Eng. 2010;38:2–20. doi: 10.1007/s10439-009-9811-1. [DOI] [PubMed] [Google Scholar]
- 116.Norland Products Corporation Database. http://www.norlandprod.com/adhesiveindex2.html.
- 117.Ochsner M, Textor M, Vogel V, Smith ML. Dimensionality controls cytoskeleton assembly and metabolism of fibroblast cells in response to rigidity and shape. PLoS ONE. 2010;5:e9445. doi: 10.1371/journal.pone.0009445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Odom TW, Love JC, Wolfe DB, Paul KE, Whitesides GM. Improved pattern transfer in soft lithography using composite stamps. Langmuir. 2002;18:5314–5320. [Google Scholar]
- 119.Ohya S, Nakayama Y, Matsuda T. In vivo evaluation of poly(N-isopropylacrylamide) (PNIPAM)-grafted gelatin as an in situ-formable scaffold. J Artif Organs. 2004;7:181–186. doi: 10.1007/s10047-004-0265-9. [DOI] [PubMed] [Google Scholar]
- 120.Ommaya AK. Mechanical properties of tissues of the nervous system. J Biomech. 1968;1:127–138. doi: 10.1016/0021-9290(68)90015-8. [DOI] [PubMed] [Google Scholar]
- 121.Park MC, Hur JY, Cho HS, Park SH, Suh KY. High-throughput single-cell quantification using simple microwell-based cell docking and programmable time-course live-cell imaging. Lab Chip. 2011;11:79–86. doi: 10.1039/c0lc00114g. [DOI] [PubMed] [Google Scholar]
- 122.Park MC, Hur JY, Kwon KW, Park SH, Suh KY. Pumpless, selective docking of yeast cells inside a microfluidic channel induced by receding meniscus. Lab Chip. 2006;6:988–994. doi: 10.1039/b602961b. [DOI] [PubMed] [Google Scholar]
- 123.Park J, Kim HN, Kim DH, Levchenko A, Suh KY. Quantitative analysis of the combined effect of substrate rigidity and topographic guidance on cell morphology. IEEE Trans Nanobiosci. 2011 doi: 10.1109/TNB.2011.2165728. [DOI] [PubMed] [Google Scholar]
- 124.Pascual M, Swinford RD, TolkoffRubin N. Acute renal failure: role of dialysis membrane biocompatibility. Annu Rev Med. 1997;48:467–476. doi: 10.1146/annurev.med.48.1.467. [DOI] [PubMed] [Google Scholar]
- 125.Patel S, Kurpinski K, Quigley R, Gao HF, Hsiao BS, Poo MM, Li S. Bioactive nanofibers: synergistic effects of nanotopography and chemical signaling on cell guidance. Nano Lett. 2007;7:2122–2128. doi: 10.1021/nl071182z. [DOI] [PubMed] [Google Scholar]
- 126.Pedrotty DM, Koh J, Davis BH, Taylor DA, Wolf P, Niklason LE. Engineering skeletal myoblasts: roles of three-dimensional culture and electrical stimulation. Am J Physiol Heart C. 2005;288:H1620–H1626. doi: 10.1152/ajpheart.00610.2003. [DOI] [PubMed] [Google Scholar]
- 127.Peng R, Yao X, Ding J. Effect of cell anisotropy on differentiation of stem cells on micropatterned surfaces through the controlled single cell adhesion. Biomaterials. 2011;32:8048–8057. doi: 10.1016/j.biomaterials.2011.07.035. [DOI] [PubMed] [Google Scholar]
- 128.Piruska A, Nikcevic I, Lee SH, Ahn C, Heineman WR, Limbach PA, Seliskar CJ. The autofluorescence of plastic materials and chips measured under laser irradiation. Lab Chip. 2005;5:1348–1354. doi: 10.1039/b508288a. [DOI] [PubMed] [Google Scholar]
- 129.Qin D, Xia YN, Whitesides GM. Soft lithography for micro- and nanoscale patterning. Nat Protoc. 2010;5:491–502. doi: 10.1038/nprot.2009.234. [DOI] [PubMed] [Google Scholar]
- 130.Radmacher M, Domke J. Measuring the elastic properties of thin polymer films with the atomic force microscope. Langmuir. 1998;14:3320–3325. [Google Scholar]
- 131.Razal JM, Kita M, Quigley AF, Kennedy E, Moulton SE, Kapsa RMI, Clark GM, Wallace CG. Wet-spun biodegradable fibers on conducting platforms: novel architectures for muscle regeneration. Adv Funct Mater. 2009;19:3381–3388. [Google Scholar]
- 132.Revzin A, Russell RJ, Yadavalli VK, Koh WG, Deister C, Hile DD, Mellott MB, Pishko MV. Fabrication of poly(ethylene glycol) hydrogel microstructures using photolithography. Langmuir. 2001;17:5440–5447. doi: 10.1021/la010075w. [DOI] [PubMed] [Google Scholar]
- 133.Rho JY, Ashman RB, Turner CH. Young’s modulus of trabecular and cortical bone material: ultrasonic and microtensile measurements. J Biomech. 1993;26:111–119. doi: 10.1016/0021-9290(93)90042-d. [DOI] [PubMed] [Google Scholar]
- 134.Rogers JA, Lee HH. Unconventional Nanopatterning Techniques and Applications. Hoboken, NJ: Wiley; 2009. [Google Scholar]
- 135.Saha K, Keung AJ, Irwin EF, Li Y, Little L, Schaffer DV, Healy KE. Substrate modulus directs neural stem cell behavior. Biophys J. 2008;95:4426–4438. doi: 10.1529/biophysj.108.132217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Samani A, Zubovits J, Plewes D. Elastic moduli of normal and pathological human breast tissues: an inversion-technique-based investigation of 169 samples. Phys Med Biol. 2007;52:1565–1576. doi: 10.1088/0031-9155/52/6/002. [DOI] [PubMed] [Google Scholar]
- 137.Sanghvi AB, Miller KPH, Belcher AM, Schmidt CE. Biomaterials functionalization using a novel peptide that selectively binds to a conducting polymer. Nat Mater. 2005;4:496–502. doi: 10.1038/nmat1397. [DOI] [PubMed] [Google Scholar]
- 138.Schiffman JD, Schauer CL. A review: electrospinning of biopolymer nanofibers and their applications. Polym Rev. 2008;48:317–352. [Google Scholar]
- 139.Schild HG. Poly(N-isopropylacrylamide)—experiment, theory and application. Prog Polym Sci. 1992;17:163–249. [Google Scholar]
- 140.Schmidt CE, Shastri VR, Vacanti JP, Langer R. Stimulation of neurite outgrowth using an electrically conducting polymer. Proc Natl Acad Sci USA. 1997;94:8948–8953. doi: 10.1073/pnas.94.17.8948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shimizu T, Yamato M, Akutsu T, Shibata T, Isoi Y, Kikuchi A, Umezu M, Okano T. Electrically communicating three-dimensional cardiac tissue mimic fabricated by layered cultured cardiomyocyte sheets. J Biomed Mater Res. 2002;60:110–117. doi: 10.1002/jbm.1284. [DOI] [PubMed] [Google Scholar]
- 142.Silva JR, Pan H, Wu D, Nekouzadeh A, Decker KF, Cui JM, Baker NA, Sept D, Rudy Y. A multiscale model linking ion-channel molecular dynamics and electrostatics to the cardiac action potential. Proc Natl Acad Sci USA. 2009;106:11102–11106. doi: 10.1073/pnas.0904505106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Silver FH, Kato YP, Ohno M, Wasserman AJ. Analysis of mammalian connective-tissue—relationship between hierarchical structures and mechanical-properties. J Long-Term Eff Med. 1992;2:165–198. [PubMed] [Google Scholar]
- 144.Sitti M, Fearing RS. Synthetic gecko foot-hair micro/nano-structures as dry adhesives. J Adhes Sci Technol. 2003;17:1055–1073. [Google Scholar]
- 145.Sochol RD, Higa AT, Janairo RRR, Li S, Lin LW. Unidirectional mechanical cellular stimuli via micropost array gradients. Soft Matter. 2011;7:4606–4609. [Google Scholar]
- 146.Stevens MP. Polymer Chemistry: An Introduction. New York: Oxford University Press; 1999. [Google Scholar]
- 147.Suh KY, Kim YS, Lee HH. Capillary force lithography. Adv Mater. 2001;13:1386–1389. [Google Scholar]
- 148.Suh KY, Park MC, Kim P. Capillary force lithography: a versatile tool for structured biomaterials interface towards cell and tissue engineering. Adv Funct Mater. 2009;19:2699–2712. [Google Scholar]
- 149.Sung JH, Yu JJ, Luo D, Shuler ML, March JC. Microscale 3-D hydrogel scaffold for biomimetic gastrointestinal (GI) tract model. Lab Chip. 2011;11:389–392. doi: 10.1039/c0lc00273a. [DOI] [PubMed] [Google Scholar]
- 150.Tabesh H, Amoabediny G, Nik NS, Heydari M, Yosefifard M, Siadat SOR, Mottaghy K. The role of biodegradable engineered scaffolds seeded with Schwann cells for spinal cord regeneration. Neurochem Int. 2009;54:73–83. doi: 10.1016/j.neuint.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 151.Takigawa T, Yamawaki T, Takahashi K, Masuda T. Change in Young’s modulus of poly(N-isopropylacrylamide) gels byume phase transition. Polym Gels Netw. 1997;5:585–589. [Google Scholar]
- 152.Taylor Z, Miller K. Reassessment of brain elasticity for analysis of biomechanisms of hydrocephalus. J Biomech. 2004;37:1263–1269. doi: 10.1016/j.jbiomech.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 153.Tekin H, Anaya M, Brigham MD, Nauman C, Langer R, Khademhosseini A. Stimuli-responsive microwells for formation and retrieval of cell aggregates. Lab Chip. 2010;10:2411–2418. doi: 10.1039/c004732e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tekin H, Tsinman T, Sanchez JG, Jones BJ, Camci-Unal G, Nichol JW, Langer R, Khademhosseini A. Responsive micromolds for sequential patterning of hydrogel microstructures. J Am Chem Soc. 2011;133:12944–12947. doi: 10.1021/ja204266a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Teo WE, Ramakrishna S. A review on electrospinning design and nanofibre assemblies. Nanotechnology. 2006;17:R89–R106. doi: 10.1088/0957-4484/17/14/R01. [DOI] [PubMed] [Google Scholar]
- 156.Thubrikar M, Piepgrass WC, Bosher LP, Nolan SP. The elastic modulus of canine aortic valve leaflets in vivo and in vitro. Circ Res. 1980;47:792–800. doi: 10.1161/01.res.47.5.792. [DOI] [PubMed] [Google Scholar]
- 157.Tsai IY, Kimura M, Russell TP. Fabrication of a gradient heterogeneous surface using homopolymers and diblock copolymers. Langmuir. 2004;20:5952–5957. doi: 10.1021/la049957w. [DOI] [PubMed] [Google Scholar]
- 158.Tsai IY, Kimura M, Stockton R, Green JA, Puig R, Jacobson B, Russell TP. Fibroblast adhesion to micro- and nano-heterogeneous topography using diblock copolymers and homopolymers. J Biomed Mater Res A. 2004;71A:462–469. doi: 10.1002/jbm.a.30183. [DOI] [PubMed] [Google Scholar]
- 159.Tzvetkova-Chevolleau T, Stephanou A, Fuard D, Ohayon J, Schiavone P, Tracqui P. The motility of normal and cancer cells in response to the combined influence of the substrate rigidity and anisotropic microstructure. Biomaterials. 2008;29:1541–1551. doi: 10.1016/j.biomaterials.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 160.Vasita R, Katti DS. Nanofibers and their applications in tissue engineering. Int J Nanomed. 2006;1:15–30. doi: 10.2147/nano.2006.1.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Veronese FM, Pasut G. PEGylation, successful approach to drug delivery. Drug Discov Today. 2005;10:1451–1458. doi: 10.1016/S1359-6446(05)03575-0. [DOI] [PubMed] [Google Scholar]
- 162.Vihola H, Laukkanen A, Valtola L, Tenhu H, Hirvonen J. Cytotoxicity of thermosensitive polymers poly(N-isopropylacrylamide), poly(N-vinylcaprolactam) and amphiphilically modified poly(N-vinylcaprolactam) Biomaterials. 2005;26:3055–3064. doi: 10.1016/j.biomaterials.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 163.Wang H, Prendiville PL, McDonnell PJ, Chang WV. An ultrasonic technique for the measurement of the elastic moduli of human cornea. J Biomech. 1996;29:1633–1636. [PubMed] [Google Scholar]
- 164.Wang JHC, Yang GG, Li ZZ. Controlling cell responses to cyclic mechanical stretching. Ann Biomed Eng. 2005;33:337–342. doi: 10.1007/s10439-005-1736-8. [DOI] [PubMed] [Google Scholar]
- 165.Wen-Chun Y, Yung-Ming J, Hey-Chi H, Po-Ling K, Meng-Lin L, Pei-Ming Y, Huang LPo, Pai-Chi L. Young’s modulus measurements of human liver and correlation with pathological findings. Presented at Ultrasonics Symposium, 2001 IEEE; 2001. pp. 1233–1236. [Google Scholar]
- 166.Wu W, Tong WM, Bartman J, Chen YF, Walmsley R, Yu ZN, Xia QF, Park I, Picciotto C, Gao J, Wang SY, Morecroft D, Yang J, Berggren KK, Williams RS. Sub-10 nm nanoimprint lithography by wafer bowing. Nano Lett. 2008;8:3865–3869. doi: 10.1021/nl802295n. [DOI] [PubMed] [Google Scholar]
- 167.Xia YN, Whitesides GM. Soft lithography. Annu Rev Mater Sci. 1998;28:153–184. [Google Scholar]
- 168.Yamato M, Konno C, Utsumi M, Kikuchi A, Okano T. Thermally responsive polymer-grafted surfaces facilitate patterned cell seeding and co-culture. Biomaterials. 2002;23:561–567. doi: 10.1016/s0142-9612(01)00138-7. [DOI] [PubMed] [Google Scholar]
- 169.Yamato M, Kwon OH, Hirose M, Kikuchi A, Okano T. Novel patterned cell coculture utilizing thermally responsive grafted polymer surfaces. J Biomed Mater Res. 2001;55:137–140. doi: 10.1002/1097-4636(200104)55:1<137::aid-jbm180>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 170.Yang S, Khare K, Lin PC. Harnessing surface wrinkle patterns in soft matter. Adv Funct Mater. 2010;20:2550–2564. [Google Scholar]
- 171.Yang Y, Leong KW. Nanoscale surfacing for regenerative medicine. Wires Nanomed Nanobiotechnol. 2010;2:478–495. doi: 10.1002/wnan.74. [DOI] [PubMed] [Google Scholar]
- 172.Yang F, Murugan R, Wang S, Ramakrishna S. Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials. 2005;26:2603–2610. doi: 10.1016/j.biomaterials.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 173.Yim EKF, Darling EM, Kulangara K, Guilak F, Leong KW. Nanotopography-induced changes in focal adhesions, cytoskeletal organization, and mechanical properties of human mesenchymal stem cells. Biomaterials. 2010;31:1299–1306. doi: 10.1016/j.biomaterials.2009.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yoo PJ, Choi SJ, Kim JH, Suh D, Baek SJ, Kim TW, Lee HH. Unconventional patterning with a modulus-tunable mold: from imprinting to microcontact printing. Chem Mater. 2004;16:5000–5005. [Google Scholar]
- 175.You MH, Kwak MK, Kim DH, Kim K, Levchenko A, Kim DY, Suh KY. Synergistically enhanced osteogenic differentiation of human mesenchymal stem cells by culture on nanostructured surfaces with induction media. Biomacromolecules. 2010;11:1856–1862. doi: 10.1021/bm100374n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Young RJ. Introduction to Polymers. London: Chapman & Hall; 1981. [Google Scholar]
- 177.Zhu XY, Mills KL, Peters PR, Bahng JH, Liu EH, Shim J, Naruse K, Csete ME, Thouless MD, Takayama S. Fabrication of reconfigurable protein matrices by cracking. Nat Mater. 2005;4:403–406. doi: 10.1038/nmat1365. [DOI] [PubMed] [Google Scholar]


