Abstract
Hepatitis C virus (HCV) infection is a major public health burden in Egypt, where it bears the highest prevalence rate in the world. Estimates for prevalence are based upon data reported from the 2008 and 2015 Egypt Demographic Health Surveys. In this review, we demonstrate the prevalence results of both surveys and analyze the difference in the results. The overall HCV prevalence is estimated to be declining. However, the clinical impact of chronic HCV infection is expected to grow considerably. A mathematical model shows that by increasing the rate of treatment, the expected number of patients will decline significantly in 2030. The current and expected future burden of chronic HCV infection to the Egyptian economy, including direct and indirect costs due to disability and loss of lives, has been estimated and discussed in this review. The economic burden will continue to grow, but a model shows that the introduction of highly effective therapies will result in a significant reduction in the cumulative total economic burden of HCV by 2030. In recognition of the HCV tremendous health and economic burden, the Egyptian government established the National Committee for Control of Viral Hepatitis to implement an integrated nationwide strategy to provide patient care and ensure global treatment access. This review illustrates the epidemiological and disease burden aspects of HCV in Egypt in addition to introducing the national plan and program for managing HCV, which has been successful so far in treating a large number of patients, with the aim of achieving disease control and eventual elimination in Egypt.
Keywords: hepatitis C in Egypt, prevalence, HCV burden, treatment strategy
Video abstract
Prevalence
The prevalence of hepatitis C virus (HCV) infection in Egypt is the highest in the world.1 This became apparent early on, soon after the discovery of HCV. Seroprevalence among Egyptian blood donors in the Kingdom of Saudi Arabia was found to be much higher than that in blood donors from all other nationalities.2 Since then, it became apparent that HCV infection was widespread among Egyptians and that it was the main cause of liver disease in the country. Until the HCV epidemic became apparent, schistosomiasis was the most important public health problem in Egypt.3 In 1918, Christopherson made the discovery that injections with the antimony salt, tartar emetic, could induce a cure.4 Mass treatment of the parasite was then introduced, and from the 1950s to the 1980s, nationwide mass anti-schistosomal therapy with a series of intravenous injections of tartar emetic was adopted by the Egyptian Ministry of Health (MOH) with the advice and support of the World Health Organization (WHO).5,6 More than 2 million injections were given annually to an average of 250,000 patients. Over the 18 years of treatment, 36 million injections were administered to >6 million people, almost all with unsterilized and shared syringes and needles. This represents the largest ever iatrogenic spread of blood-borne infection.6
Several national studies over time showed very high seroprevalence,7–10 and a large study in the Nile Delta in 1996 found a seroprevalence of 24% and viremic prevalence of 15% among 3,999 examined adults and children, with seroprevalence in adults >40%.11
The Demographic Health Survey (DHS) of 200812 showed a national seroprevalence of 14.7% among those aged between 15 and 59 years, with viremic prevalence of 9.7% in this age group that increased with age and was higher in males than in females in all age groups studied (Figure 1).
Figure 1.
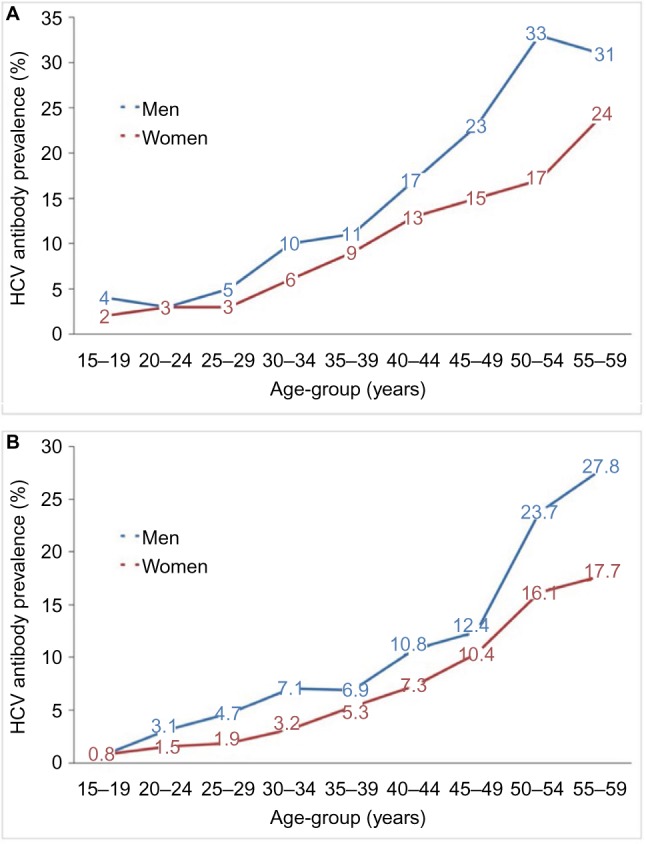
Percent of men and women with hepatitis C antibody by age in Egypt in (A) 200812 and (B) 2015.15
Abbreviation: HCV, hepatitis C virus.
In a modeling study, to estimate the prevalence in those aged <15 years in 2008, an exponential decline in viremic prevalence was trended, and prevalence in those aged >59 years was set by the authors to be equal to that in those aged 59 years. Using this model, it was estimated that the national seroprevalence in 2008 was 12.5% and the viremic prevalence was 8.5%, and that 6.3 (5.7–7.0) million people were living with HCV infection.13
A mathematical model was used to estimate the 2014 prevalence. Assuming that 65,000 patients were treated annually with pegylated (PEG) interferon and ribavirin (RBV) with a sustained virologic response (SVR) rate of ~50%, that 32,000 patients were cured, that an estimated 150,000 new infections occur annually leading to 100,000 chronic HCV infections and that 150,000 persons with HCV die (120,000 of causes other than liver disease and 30,000 of HCV-related complications), seroprevalence was modeled to 10.6% and viremic prevalence to 7.3% in 2014.13,14
Decreasing prevalence
The DHS of 201515 included the age groups 1–59 years. The seroprevalence in the age groups 15–59 years was 10% (compared to 14.7% in the 2008 DHS), and the prevalence in the group aged <15 years was 0.4%, which brought the total seroprevalence in those aged <60 years to 6.3% and the viremic prevalence to 4.4% (7% in the age groups 15–59 years and 0.2% in those aged <15 years).
Kandeel et al16 analyzed the prevalence data in the 2015 DHS and concluded that the significantly lower prevalence in those aged 15–19 years compared to the 2008 data points to a significant decrease in new infections in the age groups that were not represented in the previous DHS. They also highlighted the fact that the lower prevalence in the 2015 DHS is partly attributed to the aging of the group with the highest prevalence and their shift outside the age range of 1–59 years in the 2015 DHS. These were the age groups with the highest prevalence in the 2008 study and who mainly received anti-schistosomal injections in the 1950s and 1960s.
Still ongoing transmission
Although it appears to indicate that HCV infection is going away, however, this is not totally true. Table 1 shows the total national data in the 2008 DHS with modeling of the prevalence in the age groups <15 years and keeping the prevalence in those aged >59 years equal to that in those 55–59 years old and the 2015 DHS data for the whole population with keeping the prevalence in those aged >59 years equal to the prevalence in those aged 55–59 years in the 2008 DHS. If those aged >59 years are added to the 2015 DHS data, the viremic prevalence in the total population would be 6.2%, and a total number of 5,600,000 patients would be living with HCV infection in Egypt in 2016 compared to 5,825,000 in 2008. Although viremic infections in those aged <25 years decreased from 805,000 to 300,000 cases (a reduction of 505,000 cases), the total prevalence in the population decreased by 325,000 cases only. This points to an ongoing infection in the older age groups.
Table 1.
Age-specific HCV antibody and RNA prevalence in Egypt in 2008 and 2015
| Age group (years) | 2008 Population47 | HCV Ab, % | HCV RNA, % | HCV RNA, n | 2015 Population48 | HCV Ab, % | HCV RNA, % | HCV RNA, n |
|---|---|---|---|---|---|---|---|---|
| <5 | 7,718,920 | 2.1a | 1.0a | 74,133 | 10,073,000 | 0.4 | 0.2 | 20,146 |
| 5–9 | 7,644,227 | 2.6a | 1.4a | 104,879 | 9,352,000 | 0.3 | 0.25 | 23,380 |
| 10–14 | 7,718,49 | 3.3a | 2.0a | 151,282 | 8,386,000 | 0.7 | 0.3 | 25,158 |
| 15–19 | 8,539,832 | 4.1 | 2.8 | 239,115 | 8,597,000 | 1.0 | 0.8 | 68,776 |
| 20–24 | 7,873,192 | 4.9 | 3.0 | 236,196 | 9,150,000 | 3.2 | 2.2 | 201,300 |
| 25–29 | 6,391,623 | 6.1 | 3.9 | 249,273 | 8,606,000 | 4.4 | 3.0 | 258,180 |
| 30–34 | 4,733,495 | 11.8 | 8.3 | 392,880 | 6,898,000 | 7.1 | 4.9 | 338,002 |
| 35–39 | 4,656,897 | 13.8 | 9.9 | 461,033 | 5,412,000 | 8.2 | 6.0 | 324,720 |
| 40–44 | 4,092,499 | 23.0 | 15.0 | 613,875 | 4,857,000 | 11.6 | 9.0 | 437,130 |
| 45–49 | 3,674,382 | 28.6 | 18.9 | 694,458 | 4,458,000 | 16.3 | 11.3 | 503,754 |
| 50–54 | 3,061,286 | 38.3 | 25.3 | 774,505 | 3,870,000 | 27.9 | 19.9 | 770,130 |
| 55–59 | 2,265,429 | 39.4 | 27.4 | 620,728 | 3,161,000 | 33.9 | 22.1 | 698,581 |
| 60–64 | 1,705,502 | 39.4b | 27.4b | 467,308b | 2,317,000 | 39.4c | 27.4c | 634,858c |
| 65–69 | 1,193,600 | 39.4b | 27.4b | 327,046b | 1,630,000 | 39.4c | 27.4c | 446,620c |
| 70–74 | 789,892 | 39.4b | 27.4b | 216,430b | 1,075,000 | 39.4c | 27.4c | 294,550c |
| >75 | 738,764 | 39.4b | 27.4b | 202,421b | 1,120,000 | 39.4c | 27.4c | 306,880c |
| Total | 72,798,031 | 12.01b | 8b | 5,825,563b | 88,962,000 | 8.6c | 6c | 5,352,165c |
By 2015, the age groups 15–45 years in the 2008 DHS have aged 7 years and moved to the groups aged 10 years in the 2015 DHS. The prevalence in these groups 7 years later has slightly increased, and the increase in prevalence in these age groups points to still ongoing transmission among the adult population. The increase in the number of HCV-infected patients in this age group (now aged 25–59 years) indicates an ongoing transmission in this age group at a rate of 1.4/1000 patient-years (95% confidence interval [95% CI]: 1.1/1000–1.8/1000 patient-years) (Table 2).
Table 2.
HCV viremic prevalence in 2008 and 2015 in adults aged 15–50 years in 2008
| 2008
|
2015
|
Difference | ||||
|---|---|---|---|---|---|---|
| Age group (years) | HCV RNA, % | HCV RNA, n | Age group (years) | HCV RNA, % | HCV RNA, n | |
| 15–19 | 2.8 | 239,115 | 25–29 | 3.0 | 258,180 | 19,065 |
| 20–24 | 3.0 | 236,196 | 30–34 | 4.9 | 338,002 | 101,806 |
| 25–29 | 3.9 | 249,273 | 35–39 | 6.0 | 324,720 | 75,447 |
| 30–34 | 8.3 | 392,880 | 40–44 | 9.0 | 437,130 | 44,250 |
| 35–39 | 9.9 | 461,033 | 45–49 | 11.3 | 503,754 | 42,681 |
| 40–44 | 15.0 | 613,875 | 50–54 | 19.9 | 770,130 | 156,255 |
| 45–49 | 18.9 | 694,458 | 55–59 | 22.1 | 698,581 | 4,123 |
The 2015 DHS included tests for anti- hepatitis B core antibody (anti-HB) and hepatitis B surface antigen (HBsAg) in addition to testing for HCV. The age prevalence of anti-HBc (indicating exposure to hepatitis B virus [HBV] infection) mirrors the age prevalence of anti-HCV in both males and females (Figure 2), indicating parenteral exposure to both viruses and pointing out to ongoing iatrogenic exposure even in population who were not exposed to parenteral anti-schistosomal therapy (those aged <40 years).
Figure 2.
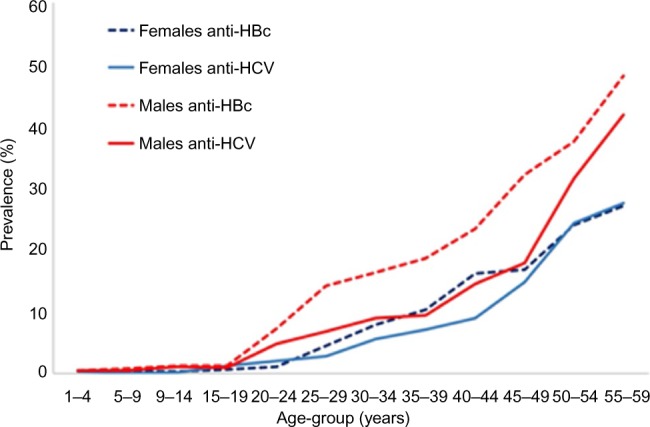
The age prevalence of anti-HBc and anti-HCV Abs in males and females.15
Abbreviations: HCV, hepatitis C virus; Abs, antibodies; HBc, Hepatitis B core antibody.
Impact of HCV infection
Chronic infection with HCV is the leading cause of end-stage liver disease, hepatocellular carcinoma (HCC) and liver-related death in Egypt. HCV causes chronic hepatitis in 60%–80% of the patients, and 10%–20% of those patients develop cirrhosis over 20–30 years of HCV infection. About 1%–5% of the patients with liver cirrhosis may develop liver cancer and 3%–6% may decompensate during the following 20–30 years. The risk of death in the following year after an episode of decompensation is between 15% and 20%.17
HCV-associated disease is one of the leading causes of HCC and the main indications for liver transplantation.18 In Egypt, a systematic review including 13 studies on 2,386 patients estimated the annual rates of death/transplantation, decompensation and HCC in patients with compensated HCV cirrhosis to be 4.58%, 6.37% and 3.36%, respectively.19 A single-center prospective study of 1,286 Egyptian patients with HCV cirrhosis estimated the annual incidence of HCC with 5.3%.20
In addition, HCV infection increases the risk of type 2 diabetes mellitus and is associated with a number of extra-hepatic manifestations (arthralgia, cryoglobulinemia, skin manifestations, sicca syndrome and thyroid disorders)21 and increases the risk for circulatory diseases, kidney diseases, renal failure, cancers of the esophagus, prostate and thyroid22 and all-cause mortality.23 Overall, 15%–35% of patients with chronic HCV have circulating cryoglobulins, and 5%–25% of them will develop clinical consequences including mixed essential cryoglobulinemia, systemic vasculitis, peripheral neuropathy and membranoproliferative glomerulonephritis.24
The incremental prevalence of diabetes attributable to HCV came from previously published literatures.25,26 There were an estimated 580,000 cases of HCV-attributable diabetes in 2013 (9.7% of the viremic population). The risk of developing a non-Hodgkin’s B-cell lymphoma is increased in patients with chronic HCV, as a consequence of long-term B-cell stimulation. The age-standardized incidence rates of non-Hodgkin’s lymphoma (NHL) in Egypt is 14.2/100,000,27 representing ~12,500 new cases annually. The proportion caused by HCV infection is 42% or an estimated 4,930 incident NHL cases annually. Achieving SVR reduces the incidence of lymphoma, decreases the risk of type 2 diabetes and its associated complications and improves patients’ overall quality of life.28
Definitely HCV has a major impact on the quality of life. Individuals with HCV may experience chronic fatigue, depression, fibromyalgia and anxiety resulting in lower quality of life.29
In Egypt in 2014, there were an estimated 125,000 viremic individuals being newly diagnosed each year: 10% of those with chronic hepatitis, 30% of those with compensated cirrhosis, while the majority (60%) were diagnosed with decompensated cirrhosis or HCC.14,30 The number of patients in different disease stages, including chronic hepatitis (METAVIR stages F0–F3), compensated cirrhosis (F4), decompensated cirrhosis, HCC and liver transplant, is estimated in Table 3.29
Table 3.
Estimate of HCV disease burden in 2013 and 2030, in Egypt
| 2013 Estimate | 2030 Estimate | Change from 2013, % | |
|---|---|---|---|
| Total number of infected individuals | 6,000,000 | 280,000 | −95 |
| Number of compensated cirrhosis | 630,000 | 76,000 | −88 |
| Number of decompensated cirrhosis | 138,000 | 17,000 | −87 |
| Number of HCC | 16,000 | 2,400 | −85 |
| Number of HCV-related mortality | 33,000 | 7,500 | −77 |
Despite this tremendous burden, most patients remain undiagnosed and therefore not appropriately managed. The number of patients needed to be treated annually to reach disease elimination by 2030 was calculated in a modeling study14,29 and was estimated to be 350,000 patients a year, with treatments that are 90% effective. To achieve this, the number of annually diagnosed new patients must exceed 350,000 a year. If this is coupled with a decrease in the incidence of new cases by >20% annually, the burden of HCV-related disease in 2030 would decrease as shown in Table 3.14
Another study31 estimated the current and the future burden of HCV in Egypt by applying different treatment scenarios regarding treatment rate and treatment efficacy using Markov model to follow up HCV-infected cohort over time among different age groups using Egyptian DHS 2008 data. Patients with cirrhosis are expected to increase from 750,000 cases in 2015 to peak to 925,000 cases by 2022 and then slightly decrease to reach 800,000 cases by 2030. By increasing the rate of treatment to 8% (treating 300,000–450,000 patients), the expected total viremic HCV cases will reach 1,000,000 cases by 2030, liver-related deaths will be <15,000 deaths and the number of patients with cirrhosis will decline by 87% to reach ~100,000 cases by 2030.
Economic burden
The total burden of chronic HCV infection to the Egyptian economy, including direct costs (HCV-related health care costs) and indirect costs due to disability (the value of lost productivity among chronically infected individuals) and due to loss of life, has been evaluated.30 Direct costs were calculated for HCV-infected individuals who were diagnosed and under care. Indirect costs, years of life lost due to disability (YLD) and years of life lost due to premature death (YLL) were estimated using disability templates from the WHO32 and calculated to determine cumulative disability-adjusted life years (DALYs). This was used to calculate the total economic value of lost productivity using Egyptian estimates for the value of a statistical life year.30
The total economic burden of HCV in Egypt in 2015 was estimated at US$3.81 billion, equivalent to 1.4% of total Gross Domestic Product (GDP),33 relatively as large as the cost of diabetes in the US.34 Direct health care costs of HCV-related disease exceed $700 million annually and consume ~4.0% of the total health expenditure in Egypt, indicating that HCV and its related complications are a substantial health and economic burden.35
About 70% of the HCV prevalent population in 2015 was born between 1945 and 1975, and thus, the disease burden related to HCV and associated costs will continue to grow as the number of individuals experiencing advanced liver disease and HCC increases, although the overall HCV prevalence is declining. Indirect costs account for two-thirds of total costs associated with HCV in a US study.36 In Egypt, the estimated cumulative direct costs range from 26% (for historical treatment of PEG) to 33% (for recent treatment, despite the reduced cost of direct-acting antivirals [DAAs] and the availability of generic drugs) of total costs.30 Estimated indirect costs accounted for at least 80% of total costs for most disease stages, and estimated lifetime DALYs ranged from 5.7 for chronic hepatitis to 25.9 for HCC. Estimated YLLs ranged from 1.1 (chronic hepatitis) to 24.7 (HCC).
Despite the fact that HCV infection rates are decreasing, the clinical and economic impact of chronic HCV infection is expected to grow considerably. Infected population are expected to progress to more advanced stages of liver disease, despite the decline in the total number of infected individuals, and thus, a peak in cases of HCC, decompensated cirrhosis, liver-related deaths and compensated cirrhosis is expected. Older individuals were prone to have more advanced liver disease and associated liver-related deaths and higher all-cause mortality rate.37
Estes et al30 evaluated the impact of adopting highly effective therapy for HCV and estimated the national economic burden of hepatitis C infection in 2015 and modeled future costs comparing to the historical case had DAAs not been adopted for HCV treatment by the national treatment program. Future disease progression was based on previously published work,37 and costs were projected from 2015 to 2030 based on historical therapy of PEG and RBV. The impact of changing treatment to the currently used DAAs with greater efficacy and the additive effect of increasing the treated population and reducing new infections were considered in terms of future economic burden and compared with the historical therapeutic regimen.
Table 4 shows the cumulative DALYs and direct, indirect and total costs between 2013 and 2030. Had DAAs not been used, the cumulative total economic burden of HCV disease between 2013 and 2030 was estimated at $89.1 billion. The introduction of highly effective therapies with scaling-up of treatment to >350,000 patients a year will result in a significant decrease in DALYs, direct and indirect costs and a reduction in the cumulative total economic burden of HCV by 35.4% to $57.6 billion.30
Table 4.
Estimate of cumulative direct and indirect costs of HCV in different treatment scenarios between 2013 and 2030 in Egypt
| Base case | Increased efficacy and treatment | Percent change | |
|---|---|---|---|
| Cumulative DALY 2013–2030 | 7,875,440 | 4,923,210 | −37.5% |
| Cumulative direct costs 2013–2030 (US$) | 23,244,377,860 | 18,632,607,710 | −19.8% |
| Cumulative indirect costs 2013–2030 (US$) | 65,822,552,110 | 38,929,874,750 | −40.9% |
| Cumulative total costs 2013–2030 (US$) | 89,066,929,970 | 57,562,482,460 | −35.4% |
Note: Data from Estes et al.30
Abbreviations: HCV, hepatitis C virus; DALY, disability-adjusted life year.
The model by Shelbaya et al31 showed that the estimated cost of applying 1% treatment rate will be $600 million in 2015 and then decline to $500 million by 2030, where all viremic HCV cases will decline by 15% to reach ~5.5 million cases. Adopting 5% treatment rate will decrease the cost from $900 million in 2015 to $550 million by 2030 as this policy will lead to decrease all viremic cases by 61% to reach 2.5 million cases. Applying 8% treatment rate will increase the cost of treatment to $1.3 billion in the first year of treatment and then decrease gradually to reach $580 million by 2030, leading to decrease in all viremia cases by 84.6% to reach 1 million cases by 2030.
Mankoula developed a Markov model representing the progression among HCV-infected cohort within different age groups from 2015 till 2025. The burden of hepatitis C was estimated, and the direct and indirect health care cost of the proportion of members who go through each stage of the disease and its complications was calculated. Under the current management strategy of treating 125,000 patients/year with DAA, it is estimated that chronic active HCV patients will show minimal decrease to reach ~4.1 million cases, with $23.3 billion estimated as a direct cost, and the total costs are $48.3 billion between 2015 and 2025.38
Increasing the treatment rate to reach 1 million patients annually for 5 years in addition to decreasing the annual incidence in the coming 10 years will drop HCV cases to ~636,000 by 2025, with the direct costs estimated to be only $16.2 billion and total costs are estimated to be $34.2 billion between 2015 and 2025, which is 29.2% lower than the current management scenario.38
Treatment strategy and outcome
The national treatment strategy for control of HCV infection in Egypt39 was set by the National Committee for Control of Viral Hepatitis (NCCVH) which was established by the MoH in 2006, in response to the magnitude of the HCV problem and burden of disease in Egypt.40 This committee had an advisory board of volunteer hepatology and epidemiology professors and included international experts from Pasteur Institute, Paris, and the University of California, San Francisco.
The objectives of the NCCVH were assessment of HCV disease burden, establishing the infrastructure for a national treatment program and setting a national strategy for control of viral hepatitis that was first published in 2007. The primary policy was to provide antiviral medications to all patients at either reduced cost or totally free (on the expense of the state).
The role of NCCVH was to set action plans, issue treatment protocols and practice guidelines and establish and oversee the specialized viral hepatitis treatment centers. The committee also led negotiations with the pharmaceutical manufacturers to reduce the prices of antiviral medications for the treatment program.
The geographical distribution of treatment centers was planned so that any patient would be within 50 km to a nearby center. Treatment centers are operated through a well-trained team of specialized hepatologists and infectious disease specialists. Until 2017, >54 centers were established providing care to >800,000 chronic patients. A dedicated intranet network (the National Network for Treatment Centers [NNTC]) was established, and users in all centers were interconnected to a central database where baseline and follow-up patient data were recorded.
In 2014, negotiations between the NCCVH and Gilead Sciences, the US manufacturer of sofosbuvir (SOF) (Sovaldi®), led to an agreement to provide the drug for Egyptian patients treated through the national program at the reduced price of $300 per bottle (compared to $28,000 per bottle in the US market).41 The deal with Gilead Sciences was the base for setting the cost of introducing other DAAs in Egypt. Similar negotiations with the other DAA manufacturers led to equivalent reduction of prices: simeprevir (SMV) (Janssen) and daclatasvir (DCV) (Bristol-Meyers-Squibb) at $250, paretaprevir–ombitasvir (AbbVie) and SOF–ledipasvir (Gilead Sciences) for the equivalent of $300. Local production of generic DAAs was encouraged, and the cost of treatment has reduced further.
With the introduction of genotype-4-effective DAAs to Egypt, the main problem was organizing and streamlining patient visits to the then available 26 specialized centers. The estimated number of patients who were waiting for new treatments was >750,000: ~150,000 patients who failed previous standard of care therapy with interferon (IFN) and RBV, 300,000–500,000 patients who were estimated to have been “not fit” for IFN therapy, in addition to ~150,000 who were previously diagnosed and preferred not to be treated with IFN and to wait the new medications. This necessitated a novel administrative solution that was innovated for the first time in the health care setting in Egypt. A specially designed web-based online registration system (www.nccvh.org.eg) was created in order to register patients with HCV and schedule appointments for the first visit at the treatment centers. The centers’ capacity set the daily workload and appointments, and patients were referred to the nearest treatment center to their residence.
With the first week after launching the site, >300,000 patients registered and received appointments online. This created a further problem, as the waiting times exceeded 6 months in some centers (according to the prevalence in the area and the capacity of the center). The management of this was through opening of more centers in the crowded areas to help ease the wait list, and by mid-2016, the wait time for the first appointment was reduced to within a week of registration in all centers.
The main source of funding for this program was through “the governmental support funds” directed to those who are not covered by health insurance. These funds cover treatment and all pre- and posttreatment investigation for >90% of the patients.
Patients visit treatment centers monthly for clinical and laboratory follow-up and receive the monthly supply of medication, and an SVR is assessed at 12 weeks after the end of treatment. Patients with cirrhosis are offered ultrasonography and alpha fetoprotein every 6 months as surveillance for HCC.
To enhance patients’ compliance with follow-up to assess SVR, a “certificate of cure from hepatitis C” was issued for patients who test negative for HCV RNA 12 weeks after the end of treatment.
The national goal of the HCV management program in Egypt was to reduce the prevalence <2% within 10 years and to near elimination of the disease (prevalence <1%) by 2030. Modeling studies before the start of the DAA treatment program showed that Egypt needs to scale up treatment to 350,000 patients a year by medications that are associated with >90% cure rate to achieve these ambitious goals.14,30,37 This scale up required governmental and societal commitments and further reduction in cost.
Initially, treatment priority was given to those with advanced fibrosis, and this was in line with most international recommendations.42,43 SOF, PEG and RBV were the only available medications initially, and with the availability of results of SOF-based therapy for HCV genotype 4 from clinical trials,44 treatment was initially provided for patients with F3 and F4 fibrosis. Although the SVR rates in patients who received SOF–RBV for 24 weeks was significantly lower than in those who received IFN in addition to SOF–RBV (76% vs 90%), no other alternatives were available for IFN-ineligible patients for the first 6 months of the program.
SMV, which became available during the next 6 months, was used in combination with SOF for the treatment of ~100,000 patients. The guidelines were modified based on the results of a local clinical trial45 to include SOF–SMV for 12 weeks in patients with or without Child-Pugh A cirrhosis. When the results of SOF–RBV for 24 weeks were published,46 the regimen was replaced by SOF–SMV. This was followed by the availability of locally produced generic SOF in the following 6 months and generic DCV 3 months later. Accordingly, the national guidelines were modified to be exclusively IFN free, by giving SOF–DCV with or without RBV for 12 weeks to all patients. The main rationale behind this was cost, as the cost of the generic combination of SOF–DCV was 20% of the reduced cost of SOF–PEG–RBV or SOF–SMV. The reduction in prices continued and is now at US$27 for a 4-week supply of generic SOF–DCV combination, which has been exclusively used for all patients in the program since early 2016. The high response rates with the locally produced generics support the use of low-cost generics in similar programs in limited resource settings.
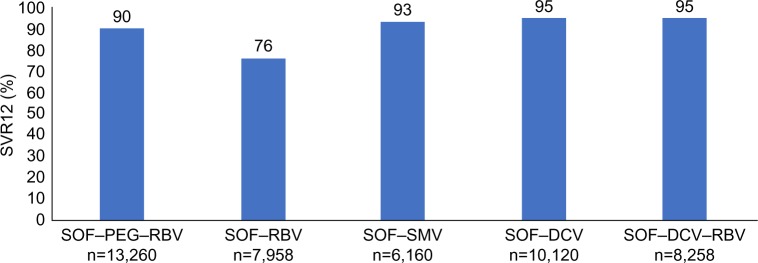
Table 5 and Figure 3 show the results of SVR12 with the different treatments used in the national program.
Table 5.
Outcome of therapy with different DAA regimens in Egypt in 2015–2016
| SOF–PEG–RBV 12 weeks | SOF–RBV 24 weeks | SOF–SMV 12 weeks | SOF–DCV 12 weeks | SOF–DCV–RBV 12 weeks | |
|---|---|---|---|---|---|
| Number | 13,260 | 7,958 | 6,160 | 10,120 | 8,258 |
| SVR12, n (%) | 11,907 (90) | 6,040 (76) | 5,739 (93) | 9,653 (95) | 7,820 (95) |
| Nonresponse, n (%) | 904 (7) | 934 (12) | 292 (5) | 359 (3.5) | 349 (4) |
| Relapse, n (%) | 450 (3) | 984 (12) | 129 (2) | 108 (1) | 89 (1) |
Figure 3.
Real-life SVR12 results with different DAA treatment regimens in the Egyptian national program.
Note: El Raziky et al and Doss et al.45,46
Abbreviations: SVR, sustained virologic response; DAA, direct-acting antivirals; SOF, sofosbuvir; PEG, pegylated interferon; RBV, ribavirin; SMV, simeprevir; DCV, daclatasvir.
Conclusion
The large national treatment program to treat patients with HCV infection was feasible and manageable. Scaling up of the treatment program was possible with the availability of more medications, with more affordability through both allocating more resources and decreasing costs, with the decision to treat all stages of fibrosis and with removing the requirement of strict fibrosis assessment.
Each country’s availability of resources, availability of medication and expected number of patients will determine the initial treatment and prioritization strategies. In the Egyptian program, rapid adaptation to changes in availability and modification of the guidelines were a key to the success in including and managing this huge number of patients in this short time. With limited resources and limited availability of drugs, choosing liver fibrosis as a priority parameter for mass treatment projects was the best recourse for national program in order to prioritize patients in whom therapy is urgently needed.
The treatment of hundreds of thousands of patients with >90% SVR rates in a short time will hopefully lead to achieving the target of HCV disease control and eventual elimination in Egypt. How far will successful therapy change the epidemiology of the disease remains to be seen.
Footnotes
Disclosure
Imam Waked received grants/research supports or speaker’s honoraria from Abbvie, Gilead Sciences, Janssen, Marcyrl, Mylan, Onxio, Pharco, and Roche. The authors report no other conflicts of interest in this work.
References
- 1.Blach S, Zeuzem S, Manns M, et al. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2016;2(3):161–176. doi: 10.1016/S2468-1253(16)30181-9. [DOI] [PubMed] [Google Scholar]
- 2.Saeed AA, al-Admawi AM, al-Rasheed A, et al. Hepatitis C virus infection in Egyptian volunteer blood donors in Riyadh. Lancet. 1991;338(8764):459–460. doi: 10.1016/0140-6736(91)91094-b. [DOI] [PubMed] [Google Scholar]
- 3.Strickland G. Liver disease in Egypt: hepatitis C superseded schistosomiasis as a result of iatrogenic and biological factors. Hepatology. 2006;43(5):915–922. doi: 10.1002/hep.21173. [DOI] [PubMed] [Google Scholar]
- 4.Elgharably A, Gomaa AI, Crossey MME, Norsworthy PJ, Waked I, Taylor-Robinson SD. Hepatitis C in Egypt – past, present, and future. Int J Gen Med. 2017;10:1–6. doi: 10.2147/IJGM.S119301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rao MR, Naficy AB, Darwish MA, et al. Further evidence for association of hepatitis C infection with parenteral schistosomiasis treatment in Egypt. BMC Infect Dis. 2002;2:29. doi: 10.1186/1471-2334-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frank C, Mohamed MK, Strickland GT, et al. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet. 2000;355(9207):887–891. doi: 10.1016/s0140-6736(99)06527-7. [DOI] [PubMed] [Google Scholar]
- 7.Kamel MA, Ghaffar YA, Wasef MA, Wright M, Clark L, Miller FD. High HCV prevalence in Egyptian blood donors. Lancet. 1992;340(8816):427. doi: 10.1016/0140-6736(92)91508-6. [DOI] [PubMed] [Google Scholar]
- 8.Mohamoud Y, Mumtaz G, Riome S, Miller D, Raddad L. The epidemiology of hepatitis C virus in Egypt: a systematic review and data synthesis. BMC Infect Dis. 2013;13:288. doi: 10.1186/1471-2334-13-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darwish MA, Raouf TA, Rushdy P, Constantine NT, Rao MR, Edelman R. Risk factors associated with a high seroprevalence of hepatitis C virus infection in Egyptian blood donors. Am J Trop Med Hyg. 1993;49(4):440–447. doi: 10.4269/ajtmh.1993.49.440. [DOI] [PubMed] [Google Scholar]
- 10.Bassily S, Hyams KC, Fouad RA, Samaan MD, Hibbs RG. A high risk of hepatitis C infection among Egyptian blood donors: the role of parenteral drug abuse. Am J Trop Med Hyg. 1995;52(6):503–505. doi: 10.4269/ajtmh.1995.52.503. [DOI] [PubMed] [Google Scholar]
- 11.Abdel-Aziz F, Habib M, Mohamed MK, et al. Hepatitis C virus (HCV) infection in a community in the Nile Delta: population description and HCV prevalence. Hepatology. 2000;32(1):111–115. doi: 10.1053/jhep.2000.8438. [DOI] [PubMed] [Google Scholar]
- 12.El-Zanaty F, Way A. Egypt Demographic and Health Survey 2008. Cairo: Ministry of Health, El-Zanaty and Associates and Macro International; 2009. p. 431. [Google Scholar]
- 13.Razavi H, Waked I, Sarrazin C, et al. The present and future disease burden of hepatitis C virus with today’s treatment paradigm. J Viral Hepat. 2014;21(suppl 1):34–59. doi: 10.1111/jvh.12248. [DOI] [PubMed] [Google Scholar]
- 14.Waked I, Doss W, El-Sayed M, et al. The current and future disease burden of chronic hepatitis C virus infection in Egypt. Arab J Gastroenterol. 2014;15(2):45–52. doi: 10.1016/j.ajg.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Ministry of Health and Population [Egypt], El-Zanaty and Associates [Egypt], ICF International . Egypt Health Issues Survey 2015. Cairo, Rockville, MD: Ministry of Health and Population, ICF International; 2015. [Google Scholar]
- 16.Kandeel A, Genedy M, El-Refai S, Funk A, Fontanet A, Talaat M. The prevalence of HCV infection in Egypt 2015: implications for future policy on prevention and treatment. Liver Int. 2017;37:45–53. doi: 10.1111/liv.13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61(1):S58–S68. doi: 10.1016/j.jhep.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Sanyal AJ. The Institute of Medicine report on viral hepatitis: a call to action. Hepatology. 2010;51(3):727–728. doi: 10.1002/hep.23583. [DOI] [PubMed] [Google Scholar]
- 19.Alazawi W, Cunningham M, Dearden J, Foster GR. Systematic review: outcome of compensated cirrhosis due to chronic hepatitis C infection. Aliment Pharmacol Ther. 2010;32(3):344–355. doi: 10.1111/j.1365-2036.2010.04370.x. [DOI] [PubMed] [Google Scholar]
- 20.Eltabbakh M, Zaghla H, Abdel-Razek W, et al. Utility and cost effectiveness of screening for hepatocellular carcinoma in a resource limited setting. Med Oncol. 2015;32(4):432–437. doi: 10.1007/s12032-014-0432-7. [DOI] [PubMed] [Google Scholar]
- 21.Maasoumy B, Wedemeyer H. Natural history of acute and chronic hepatitis C. Best Pract Res Clin Gastroenterol. 2012;26(4):401–412. doi: 10.1016/j.bpg.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 22.Lee M-H, Yang HI, Lu SN, et al; R. E.V.E.A.L.-HCV Study Group. Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: a community-based long-term prospective study. J Infect Dis. 2012;206(4):469–477. doi: 10.1093/infdis/jis385. [DOI] [PubMed] [Google Scholar]
- 23.van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308(24):2584–2593. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 24.Adinolfi LE, Restivo L, Guerrera B, et al. Chronic HCV infection is a risk factor of ischemic stroke. Atherosclerosis. 2013;231(1):22–26. doi: 10.1016/j.atherosclerosis.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Elhawary EI, Mahmoud GF, El-Daly MA, Mekky FA, Esmat GG, Abdel-Hamid M. Association of HCV with diabetes mellitus: an Egyptian case-control study. Virology. 2011;8(1):367. doi: 10.1186/1743-422X-8-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White DL, Ratziu V, El-Serag HB. Hepatitis C infection and risk of diabetes: a systematic review and meta-analysis. J Hepatol. 2008;49(5):831–844. doi: 10.1016/j.jhep.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soliman A, Boffetta P. Chapter 14: lymphoma and leukemia. In: Freedman LS, Edwards BK, Ries LAG, Young JL, editors. Cancer Incidence in Four Member Countries (Cyprus, Egypt, Israel, and Jordan) of the Middle East Cancer Consortium (MECC) Compared with US SEER. Bethesda, MD: National Cancer Institute; 2017. 2006. [Accessed April 13, 2017]. pp. 131–140. NIH Pub. No. 06-5873. Available from: http://seer.cancer.gov/archive/publications/mecc/mecc_monograph.pdf. [Google Scholar]
- 28.Hsu YC, Lin JT, Ho HJ, et al. Antiviral treatment for hepatitis C virus infection is associated with improved renal and cardiovascular outcomes in diabetic patients. Hepatology. 2014;59(4):1293–1302. doi: 10.1002/hep.26892. [DOI] [PubMed] [Google Scholar]
- 29.Ibrahim EM, Madian A. Impact of hepatitis C on health-related quality of life in Egypt. J Am Sci. 2011;7(11):430–439. [Google Scholar]
- 30.Estes C, Abdel-Kareem M, Abdel-Razek W, et al. Economic burden of hepatitis C in Egypt: the future impact of highly effective therapies. Aliment Pharmacol Ther. 2015;42(6):696–706. doi: 10.1111/apt.13316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shelbaya A, Kuznik A, Salem M, Mankola W, Sadik K. P1265: estimating the epidemiologic and economic impact of different treatment rates for hepatitis C virus (HCV) in Egypt. J Hepatol. 2015;62:S832–S833. [Google Scholar]
- 32.World Health Organization [webpage on the Internet] Disability Adjusted Life Years (DALY) [Accessed April 13, 2017]. Available at: http://www.who.int/healthinfo/global_burden_disease/metrics_daly/en.
- 33.Central Agency for Public Mobilization and Statistics, CAPMAS [webpage on the Internet] Statistical Yearbook – National Accounts: Expenditure on GDP at Market 12/13–14/15. [Accessed April 13, 2017]. Available from: http://www.capmas.gov.eg/Pages/StaticPages.aspx?page_id=5034.
- 34.American Diabetes Association Economic costs of diabetes in the US in 2012. Diabetes Care. 2013;36(4):1033. doi: 10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Bank [webpage on the Internet] Health Expenditure, Total (% of GDP) [Accessed April 13, 2017]. Available from: http://data.worldbank.org/indicator/SH.XPD.TOTL.ZS.
- 36.Wong JB, McQuillan GM, McHutchison JG, Poynard T. Estimating future hepatitis C morbidity, mortality, and costs in the United States. Am J Public Health. 2000;90(10):1562–1569. doi: 10.2105/ajph.90.10.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wedemeyer H, Duberg AS, Buti M, et al. Strategies to manage hepatitis C virus (HCV) disease burden. J Viral Hepat. 2014;21(suppl 1):60–89. doi: 10.1111/jvh.12249. [DOI] [PubMed] [Google Scholar]
- 38.Mankoula W. Estimating Economic and Epidemiological Burden of Hepatitis C in Egypt, 2015–2025. [Accessed January 18, 2016]. webpage on the Internet. Available from: http://dar.aucegypt.edu/handle/10526/4590.
- 39.El-Akel W, El-Sayed MH, El Kassas M, et al. National treatment programme of hepatitis C in Egypt: hepatitis C virus model of care. J Viral Hepat. 2017;24(4):262–267. doi: 10.1111/jvh.12668. [DOI] [PubMed] [Google Scholar]
- 40.Doss W, Mohamed MK, Esmat G, et al. Egyptian National Control Strategy for Viral Hepatitis 2008–2012. Arab Republic of Egypt, Ministry of Health and Population, National Committee for the Control of Viral Hepatitis; 2008. [Accessed December 10, 2016]. Available from: http://www.hepnile.org/images/stories/doc/NSP_10_April_2008_final2.pdf. [Google Scholar]
- 41.Donald GM. Curing Hepatitis C, in an Experiment the Size of Egypt. The New York Times; 2015. [Accessed April 13, 2017]. webpage on the Internet. Available from: http://www.nytimes.com/2015/12/16/health/hepatitis-c-treatment-egypt.html. [Google Scholar]
- 42.AASLD [homepage on the Internet] AASLD/IDSA Guidelines. [Accessed December 10, 2016]. Available from: http://www.hcvguidelines.org.
- 43.European Association for the Study of the Liver EASL recommendations on treatment of hepatitis C 2016. J Hepatol. 2017;66(1):153–194. doi: 10.1016/j.jhep.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 44.Doss W, Shiha G, Hassany M, et al. Sofosbuvir plus ribavirin for treating Egyptian patients with hepatitis C genotype 4. J Hepatol. 2015;63(3):581–585. doi: 10.1016/j.jhep.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 45.El Raziky M, Gamil M, Ashour MK, et al. Simeprevir plus sofosbuvir for 8 or 12 weeks in treatment-naïve and -experienced hepatitis C virus genotype 4 patients with or without cirrhosis. J Viral Hepat. 2017;24(2):102–110. doi: 10.1111/jvh.12625. [DOI] [PubMed] [Google Scholar]
- 46.Doss W, Esmat G, El-Serafy M, et al. Real-Life Results of Sofosbuvir Based Therapy for Egyptian Patients with Hepatitis C and Advanced Fibrosis-Cirrhosis. Barcelona: EASL; 2016. [Google Scholar]
- 47.Central Agency for Public Mobilization Statistics, CAPMAS Estimates of Midyear Population by Age Groups. 2006–2012. [Accessed April 13, 2017]. Available from: http://www.capmas.gov.eg/Pages/Publications.aspx?page_id=5104&Year=16539.
- 48.Central Agency for Public Mobilization and Statistics, CAPMAS [webpage on the Internet] Estimates of Midyear Population by Age Groups. 2006–2015. [Accessed April 13, 2017]. Available from: http://www.capmas.gov.eg/Pages/StaticPages.aspx?page_id=5034.