Abstract
Angiogenesis is defined as the formation of new blood vessels from pre-existing vessels, and has been characterized as an essential process for tumor cell proliferation and viability. This has led to the development of pharmacological agents for anti-angiogenesis to disrupt the vascular supply and starve tumor of nutrients and oxygen, primarily through blockade of VEGF/VEGFR signaling. This effort has resulted in 11 anti-VEGF drugs approved for certain advanced cancers, alone or in combination with chemotherapy or other targeted therapies. But this success had only limited impact on overall survival of cancer patients, and rarely resulted in durable responses. Given the recent success of immunotherapies, combinations of anti-angiogenics with immune checkpoint blockers have become an attractive strategy. However, implementing such combinations will require a better mechanistic understanding of their interaction. Due to overexpression of pro-angiogenic factors in tumors, their vasculature is often tortuous and disorganized, with excessively branched leaky vessels. This enhances vascular permeability, which in turn is associated with high interstitial fluid pressure, and a reduction in blood perfusion and oxygenation. Judicious dosing of anti-angiogenic treatment can transiently normalize the tumor vasculature by decreasing vascular permeability and improving tumor perfusion and blood flow, and synergize with immunotherapy in this time-window. However, anti-angiogenics may excessively prune tumor vessels in a dose and time-dependent manner, which induces hypoxia and immunosuppression, including increased expression of the immune checkpoint programmed death receptor ligand (PD-L1). This review focuses on revisiting the concept of anti-angiogenesis in combination with immunotherapy as a strategy for cancer treatment.
Keywords: Anti-angiogenesis, immunotherapy, VEGF, vascular normalization, hypoxia, immunosuppression
Introduction
Angiogenesis, the formation of new blood vessels from pre-existing blood vessels via a process called sprouting, is one of the hallmarks of cancer. Angiogenesis may occur under physiological conditions, such as during embryonic development or during wound healing in adults. In cancer progression, pathological angiogenesis is driven by the overexpression of pro-angiogenic factors. This creates a local imbalance between pro-angiogenic and anti-angiogenic factors, which leads to recruitment of a new vascular supply. Unlike wound healing, where angiogenesis undergoes a resolution phase, tumor angiogenesis continues abnormally in growing tumors, as they require vascular supply to provide essential nutrients and oxygen to proliferating cancer cells [1,2]. Angiogenesis is often triggered by low oxygenation concentrations in tissues (hypoxia), which leads to expression of multiple growth factors via the hypoxia-induced factors (HIFs) by cancer cells and stromal cell recruited to tumors (fibroblasts, macrophages) [3–8].
The late Dr. Judah Folkman proposed the concept that solid tumors require pathological angiogenesis for their growth [9]. This concept was supported by many reports published over the last half of century [10–13]. There is an important distinction between physiological and pathological angiogenesis in that the latter leads to a vasculature with abnormal structure and function. Solid tumor vessels are often tortuous and disorganized, and excessively leaky. This enhances vascular permeability, which in turn is associated with high interstitial fluid pressure, and a reduction in blood perfusion and oxygenation [14–16]. While tumors have the ability to grow and progress despite the inefficient blood supply and hypoxia, delivery of drugs and their efficacy is reduced [17–20]. During physiological angiogenesis, pericyte recruitment plays an essential role in the maintenance of the structure and function of vasculature. However, pericyte coverage is often lacking or abnormal (loose) in the tumor vasculature. This feature contributes to vascular permeability to fluids and metastatic cancer cells [21–23].
Disruption of the vascular supply by blocking the pro-angiogenic factors or inhibiting activity of their cognate receptors with pharmacological agents has been pursued initially to promote tumor starvation, trigger cell death, increase the vulnerability to the exposure to the standard care of treatment, with the goal of increasing overall survival (OS), progression-free survival (PFS) or regression of tumors [10,12,24]. However, pruning of vessels after anti-angiogenic treatments was shown to increase hypoxia, which in turn promoted the rapid tumor progression via multiple mechanisms, including increased migration, inflammation, stem-like cell phenotype, among others [25,26]. Moreover, hypoxic tumors are more resistant to the standard cytotoxic treatments, including chemotherapeutic agents and or radiation therapy [27,28].
Dr. Rakesh K. Jain introduced the concept that the appropriate dose of anti-angiogenic treatment can lead to a normalization of the tumor vasculature, by reducing vascular permeability and interstitial fluid pressure, and improving blood flow and tumor perfusion. The normalized tumor vasculature can reduce tissue hypoxia, enhance the delivery of cytotoxic agents and of oxygen for radiation therapy, but also anti-tumor immunity [15,25,26,29]. Preclinical and clinical studies supported the hypothesis that anti-angiogenic therapy can normalize the tumor vasculature, at least transiently. In addition, vascular normalization was associated with improved survival in brain, breast, colorectal and lung cancer patients treated with cytotoxics [30–34].
Moving forward, these insights may be useful in designing approaches to more substantially improve OS in cancer patients, for example by combining anti-angiogenic agents with immunotherapy. This review focuses on this potential new avenue in cancer therapy.
Tumor angiogenesis and VEGF
The vascular endothelial growth factor (VEGF) family of growth factors and their receptors – VEGFR-1, VEGFR-2, VEGFR-3, neuropilin (NRP)-1, and NRP-2 – play intricate roles in initiating and promoting tumor angiogenesis [35–37]. The VEGF family consists of VEGF-A, VEGF-B, VEGF-C, VEGF-D and placental growth factors (PlGF1–4) [38,39]. They have been linked with tumor angiogenesis (VEGF-A, PlGF), maintenance of new blood vessels (VEGF-B), lymphangiogenesis and angiogenesis (VEGF-C/D), vascular permeability (VEGF-A/C), chemotaxis (VEGF-A, PlGF), migration (VEGF-A, PlGF), differentiation (VEGF-D) and survival (VEGF-A/B/C, PlGF). VEGF-A (referred to as VEGF), initially discovered as the vascular permeability factor (VPF), is a critical mediator of tumor angiogenesis in many solid tumors [11,16,40]. There are two major isoforms of VEGF, a soluble (VEGF121) and an insoluble form (VEGF165). VEGF binds to VEGFR-1 with higher affinity than to VEGFR-2, but VEGFR-1 has lower tyrosine receptor kinase activity compared to VEGFR-2. In fact, VEGFR-1 is able to sequester VEGF and thereby preventing the tyrosine kinase activation of VEGFR-2. The role of VEGFR-1 activity is less well characterized compared to VEGFR-2. VEGF165 binds to the VEGFR-2 co-receptor NRP-1 and induce endothelial cell migration. NRP-1 serves a co-receptor also for class 3 semaphorin (SEM3A) to control vascular permeability and vessel maturation during angiogenesis. VEGFR-3 and NRP-2 receptors are thought to predominately regulate lymphangiogenesis induced by their ligands VEGF-C and VEGF-D. Another form of VEGFR-1 is truncated, referred to as soluble VEGFR-1 (sVEGFR-1 or sFLT1), and functions as an endogenous inhibitor of angiogenesis by sequestering/blocking VEGF and PlGF [13,35,36,41–43].
Tumor angiogenesis can be initiated also independently of VEGF-related pathways. The angiopoietin (Ang)/Tie-2 (Tek) pathway is another player in tumor angiogenesis. Ang-1, which binds to the Tie-2 receptor, can promote tumor angiogenesis as well as pericyte coverage maturation of the vascular network. Endothelial cells secrete Ang-2, which binds to Tie-2 to increase tumor angiogenic activity by destabilizing the vessels in the presence of VEGF, but it may decrease endothelial cell survival in the absence of VEGF. Thus, the balance between Ang-1 and Ang-2 plays an important role in tumor angiogenesis [44–50].
VEGF-induced angiogenesis is mediated in part through the expression of adhesion molecules such as the α6β1 and α6β4 integrins, which regulate the attachment of endothelial cells to the extracellular matrix and thereby promoting migration and survival of the tumor vasculature. Other integrins (for example αvβ3, αvβ5 and α5β1) have also been shown to mediate angiogenesis [51–53]. Other mediators of tumor angiogenesis include basic fibroblast growth factor (bFGF/FGF-2), platelet-derived growth factor-B (PDGF-B), stromal cell-derived factor (SDF)-1α, stem cell factor (SCF), interleukin (IL)-1, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, endothelin-1 (Et-A, Et-B), vashohibin-1/2, thrombospondin (TSP), and transforming growth factor (TGF)-β and their receptors [18,36]. Other important receptors in angiogenesis include Delta-like 4 ligand (DLL4), Notch4, and Ephrin B2, which can interact with the VEGF pathway in the modulation of tumor angiogenesis [54,55]. Intracellular factors such as Srs can increase VEGF-A mRNA transcription and synthesis, as well as the secretion of VEGF. Activation of Src enhances activity of its downstream target, focal adhesion kinase (Fak) and paxillin, which in turn induces VEGF mediated tumor vascular permeability and migration. Because Src and Fak increase vascular permeability and migration, they can also play critical roles in the metastatic cascade [56–59]. Other pro-angiogenic factors include midkine transcript 2 (MDK), endoglin (ENG), follistatin (FST), angiogenic factor G path and FHA domains 1 (AGGF1). So far, 28 pro-angiogenic factors/genes have been discovered to regulate VEGF, or independently mediate tumor angiogenesis [17,18].
Anti-angiogenic therapy for solid tumors: Anti-VEGF agents
VEGF is overexpressed in a vast majority of solid tumors and is widely considered to be a key player in mediating tumor angiogenesis [35,36]. For this reason, over the past decades, the development of anti-angiogenic treatment was predominately focused on the development of VEGF inhibitors. Preclinical evidence provided that monotherapy of blocking VEGF reduced microvascular density, inhibited tumor growth in subcutaneous human xenografts of many cancer types. Anti-angiogenic treatment even resulted into a marked decrease of metastasis and in particular in colorectal cancer preclinical models [60,61]. Dr. Napoleone Ferrara (Genentech) designed and developed the first anti-angiogenic inhibitor (bevacizumab), a recombinant humanized monoclonal antibody that blocks VEGF-A [10,62]. Intravenous administration of bevacizumab depletes VEGF in the bloodstream, which inhibits the interaction between VEGF and VEGF receptors [63]. In an initial phase III clinical trial, bevacizumab did not improve OS when combined with chemotherapy in breast cancer [64]. However, several subsequent trials of bevacizumab with chemotherapy increased OS and/or PFS in metastatic colorectal cancer (first and second line), as well as lung cancer, ovarian cancer, endometrial cancer, mesothelioma and cervical cancers [30,65–75]. A combination of bevacizumab with interferon alpha (IFN-α) immunotherapy prolonged PFS and is now a standard of care in metastatic renal cell carcinoma [76]. In the majority of cancers (melanoma, breast, pancreatic and prostate cancer), bevacizumab failed to increase survival when combined with chemotherapy [77–79]. A VEGF receptor chimera, VEGF-Trap or aflibercept, was developed as a blocker of multiple VEGF family members, VEGF, VEGF-B and PlGF, and more broadly inhibit VEGFR-2 kinase activity. Aflibercept showed increased OS and PFS in a randomized phase III clinical trial when used in combination with chemotherapy as second line treatment in metastatic colorectal cancer patients [80] (Table 1).
Table 1.
FDA approved anti-angiogenic compounds for solid tumors. * Not licensed yet
| Molecular target | Therapy | Malignancy | Improvement in (months) | |
|---|---|---|---|---|
| PFS | OS | |||
| VEGF-A | Bevacizumab | Glioblastoma | N/A | 0.5 |
|
| ||||
| VEGF-A | Bevacizumab + Fluoropyrimidine based chemotherapy | Metastatic colorectal cancer | 1.7 | 1.4 |
|
| ||||
| VEGF-A | Bevacizumab + FOLFOX4 | Metastatic colorectal cancer | 2.6 | 2.1 |
|
| ||||
| VEGF-A | Bevacizumab + Irinotecan + fluorouracil + leucovorin | Metastatic colorectal cancer | 4.4 | 4.7 |
|
| ||||
| VEGF-A | Bevacizumab + Paclitaxel or Cisplatin, Topotecan | Recurrent metastatic cervical cancer | 2.3 | 3.7 |
|
| ||||
| VEGF-A | Bevacizumab + Carboplatin + Paclitaxel | Recurrent metastatic non-squamous NSCLC | 1.7 | 2 |
|
| ||||
| VEGF-A | Bevacizumab + IFN-α | Advanced renal cell carcinoma | 4.8 | NS |
|
| ||||
| VEGF-A | Bevacizumab + pemetrexed + cisplatin | Pleural mesothelioma | 2.7 | |
|
| ||||
| VEGF-A | Bevacizumab + Paclitaxel or Pegylated liposomal Doxorubicin or Topotecan | Peritoneal cancer or recurrent epithelial Ovarian cancer fallopian tube cancer | 3.8 | 5.3 |
|
| ||||
| VEGF-A | Bevacizumab + Paclitaxel | Metastatic HER2- inflammatory breast cancer | 1.7 | 7.9 |
|
| ||||
| VEGF-A, VEGF-B, PlGF | Aflibercept + FOLFIRI | Metastatic colorectal cancer | 2.2 | 1.4 |
|
| ||||
| VEGFR-2 | Ramucirumab | Gastro-esophageal junction adenocarcinoma or Gastric adenocarcinoma | 0.8 | 1.4 |
|
| ||||
| VEGFR-2 | Ramucirumab + Paclitaxel | Advanced gastro-esophageal junction adenocarcinoma or gastric adenocarcinoma | 1.5 | 2.2 |
|
| ||||
| VEGFR-2 | Ramucirumab + Docetaxel | Metastatic NSCLC | N/A | 1.4 |
|
| ||||
| VEGFR-2 | Ramucirumab + FOLFIRI | Metastatic colorectal carcinoma | 1.2 | 1.6 |
|
| ||||
| VEGFR-1–2, PDGFR-α/β | Sunitinib | Pancreatic neuroendocrine tumors | 4.8 | N/A |
|
| ||||
| VEGFR-1–2, PDGFR-α/β | Sunitinib | Gastrointestinal stromal tumors | 4.5 | NS |
|
| ||||
| VEGFR-1–2, PDGFR-α/β | Sunitinib + IFN-α | Advanced clear cell carcinoma Renal cancer | 25.3 | N/A |
|
| ||||
| VEGFR-1–3, PDGFR-α, FGFR1–4 | Lenvatinib | Recurrent or metastatic radioactive iodine refractory differentiated thyroid carcinoma | 14.7 | N/A |
|
| ||||
| VEGFR-1–3, PDGFR-α, FGFR1–4 | Lenvatinib | Advanced hepatocellular carcinoma | * | * |
|
| ||||
| VEGFR-1–3, PDGFR-α, FGFR1–4 | Lenvatinib + Everolimus | Advanced renal cell carcinoma | 9.1 | NS |
|
| ||||
| VEGFR-2 | Vandetanib | Advanced differentiated thyroid carcinoma | 6.2 | N/A |
|
| ||||
| VEGFR-2, PDGFRβ | Sorafenib | Advanced hepatocellular Carcinoma | NS | 2.8 |
|
| ||||
| VEGFR-2, PDGFRβ | Sorafenib | Advanced renal cell carcinoma | 2.7 | NS |
|
| ||||
| VEGFR-2, PDGFRβ | Sorafenib | Recurrent or metastatic differentiated thyroid cancer | 5.0 | N/A |
|
| ||||
| VEGFR-1–3, PDGFRβ, FGFR-1–2 | Pazopanib hydrochloride | Advanced soft tissue carcinoma | 6.2 | N/A |
|
| ||||
| VEGFR-1–3, PDGFRβ, FGFR-1–2 | Pazopanib hydrochloride | Advanced renal cell carcinoma | 5.0 | N/A |
|
| ||||
| VEGFR-1–3, PDGFRβ | Axitinib | Advanced renal cell carcinoma | 6.2 | N/A |
|
| ||||
| VEGFR-1–3, PDGFRβ, FGFR-1–2 | Regorafenib | Advanced gastrointestinal stromal tumors | 2.2 | 1.4 |
|
| ||||
| VEGFR-1–3, PDGFRβ, FGFR-1–2 | Regorafenib | Chemo-refractory metastatic colorectal cancer | 0.2 | 1.4 |
|
| ||||
| VEGFR-1–3, PDGFRβ, FGFR-1–2 | Regorafenib* | Refractory hepatocellular carcinoma | 7.2 | NS |
|
| ||||
| VEGFR-1–3 | Cediranib + carboplatin or cisplatin | Ovarian cancer | 1.1 | ** |
|
| ||||
| VEGFR-2, Tie2 | Cabozantinib | Refractory advanced renal carcinoma | 3.6 | 4.9 |
|
| ||||
| VEGFR-2, Tie2 | Cabozantinib | Metastatic medullary thyroid cancer | 7.2 | NS |
|
| ||||
| VEGFR-2, Tie2 | Cabozantinib | Pancreatic neuroendocrine tumors | ** | ** |
Current list of FDA approved anti-angiogenic compounds for solid tumors.
Significantly improved progression free-survival and overall survival, but not licensed yet for FDA approval,
Clinical trail is still recruiting patients and data needs to be fully analyzed, but the result is promising and therefore is likely to be FDA approved
Anti-angiogenic therapy for solid tumors: Anti-VEGFR agents
Others pursued the development of angiogenesis inhibitors that target the VEGF receptor tyrosine kinase activity. Tyrosine kinase inhibitors (TKI) and monoclonal antibodies were developed to inhibit VEGF receptors and their downstream targets, in order to suppress endothelial proliferation and disrupt the vascular supply of nutrients and oxygen. Multiple TKIs have been approved as monotherapy for cancer based on improvement of OS or PFS in phase III trials. These include (i) sorafenib in advanced renal cell carcinoma, metastatic differentiated thyroid cancer and unresectable hepatocellular carcinoma, (ii) sunitinib in advanced renal cell carcinoma, gastrointestinal stromal tumors and pancreatic neuroendocrine tumors, (iii) axitinib in advanced renal cell carcinoma, (iv) regorafenib in chemo-refractory metastatic colorectal cancer, unresectable hepatocellular carcinoma and GIST, (v) pazopanib in metastatic soft tissue carcinoma and advanced renal cell carcinoma, (vi) vandetanib in unresectable locally advanced or metastatic medullary thyroid carcinoma, (vii) cabozantinib in refractory advanced renal carcinoma, metastatic medullary thyroid cancer, pancreatic neuroendocrine tumors, (viii) lenvatinib in locally recurrent or metastatic, progressive, radioactive iodine-refractory differentiated thyroid carcinoma, unresectable hepatocellular carcinoma, and renal cell carcinoma (with everolimus) [81–94]. Ramucirumab, a monoclonal antibody against VEGFR-2, was shown to increase survival when combined with chemotherapy in gastro-esophageal junction adenocarcinoma or gastric adenocarcinoma, metastatic non-small cell lung carcinoma and colorectal cancers [95–97]. Strikingly, monotherapy increased survival in metastatic gastro-esophageal junction adenocarcinoma or gastric adenocarcinoma. The reasons for this activity remain unknown but may be highly relevant for combinations with immunotherapy in this disease.
Cediranib (a pan-VEGFR inhibitor) prolonged PFS when combined with platinum-based chemotherapy in relapsed platinum-sensitive ovarian cancer; the OS endpoint is currently being analyzed for this clinical trial [98] (Table 1). Similar to other anti-VEGF drugs, this agent failed as monotherapy or with chemotherapy in multiple other solid tumors [79,99–101].
In summary, TKIs and antibodies targeting VEGFR-2 demonstrated efficacy (increased PFS and OS) when used alone or with chemotherapy or other molecularly targeted agents in certain cancers. While several other phase III clinical trials are still ongoing (Table 2), the urgent question emerging from these studies is what mediates resistance to this treatment modality, which typically prolongs survival by 2–3 months on average. The concept that resistance to anti-VEGF therapy is mediated by pro-angiogenic factors other than VEGF has been proposed based on preclinical studies, but is yet to be translated into a survival benefit in the clinic.
Table 2.
Selected ongoing phase III clinical trails involving anti-angiogenic inhibitors
| Anti-Angiogenic target | Therapy | Malignancy | Trails |
|---|---|---|---|
| VEGFR-2 PDGFRβ |
Sorafenib + Stereotactic body therapy | Liver cancer | NCT01730937 |
| VEGFR-2 | Ramucirumab + Erlotinib | Advanced NSCLC | NCT02411448 |
| VEGFR-1–3 PDGFRβ |
Tivozanib | Refractory Advanced Renal Carcinoma | NCT02627962 |
| VEGFR-1–3, PDGFRβ | Cediranib maleate + Olaparinib + Standard chemotherapy |
Refractory Ovarian, Fallopian tube Peritoneal cancer |
NCT02502266 |
Current list of phase III angiogenesis inhibitors tested in solid tumors.
Clinical development of other anti-angiogenic agents in phase III trials
Most preclinical and clinical research was dedicated to the drug development for VEGF and VEGFR inhibition. However, other pro-angiogenic factors are also involved in regulating tumor angiogenesis. Anti-angiogenic agents are currently in clinical development for these targets, and some have reached phase III clinical trial testing. Preclinical studies revealed that cilengitide, an inhibitor for αvβ3, αvβ5 and α5β1 integrins, reduced vascular density, vascular permeability and increased survival in a model of orthotopically-implanted glioblastoma in rats [102]. Cilengitide induced tumor cell apoptosis, promoted endothelial cell detachment, disassembly of the cytoskeleton and inhibited SRC/FAK/AKT pathway [103]. However, a phase III clinical trial showed that the combination of temozolomide, radiotherapy, and cilengitide failed to extend PFS or OS in newly diagnosed patients with methylated O6-methylguanine-DNA methyltransferase (MGMT, a biomarker of response to temozolomide) glioblastoma [104].
Targeting of Ang-1/2 and Tie2 receptor axis with trebananib, a peptide-Fc fusion protein, reduced endothelial cell proliferation, angiogenesis and growth of ovarian xenografts tumors in preclinical studies [105,106]. Although trebananib increased PFS (median 7.2 vs. 5.4 months), it failed to improve the OS in a phase III clinical trial in recurrent ovarian cancer patients [107].
Inhibition of FGFR-1–4, PDGFRβ, and VEGFR-1–3 with dovitinib demonstrated anti-tumor activity in xenografts models of renal cell carcinoma, but dovitinib failed in a phase III trail to prolong OS and PFS in renal cell carcinoma [108,109]. A preclinical study showed that treatment with brivanib a FGFR-1, VEGFR-1–2 inhibitor reduced proliferation, microvascular density and growth of tumors in hepatocellular carcinoma models in mice. However, brivanib failed to demonstrate efficacy in phases III trials in advanced hepatocellular carcinoma patients [110–113].
Mechanisms of resistance to anti-angiogenics
Activation of alternative pro-angiogenic signaling pathways
Anti-VEGF/VEGFR therapy is currently a standard of care in multiple solid tumors. However, even in these patients, tumors are inherently resistant or develop adaptive resistance to VEGF/VEGFR inhibition. This is can be mediated through many different mechanisms, but is mainly thought to be governed by activation of alternative angiogenic pathways that promote tumor angiogenesis in a VEGF-independent manner, as seen in preclinical models [114,115]. Some clinical correlative studies support this hypothesis. For example, treatment with anti-VEGF agents has been shown to increase VEGF-C, VEGF-D, PlGF, SDF1-α, and other factors in the blood circulation of cancer patients [61,116]. The roles of these and other pro-angiogenic factors (PDGF, Ang-2, bFGF, endoglin, TGF-β etc.) remains to be established in cancer patients [101,117].
Another approach is to target the hypoxia inducible factor (HIF)-1α, which is activated/stabilized in many cancers and is upstream of multiple pro-angiogenic genes (VEGF family members, PDGF, Ang-2, etc.) [118,119]. Since HIF-1α is induced by hypoxia, which may be aggravated after anti-angiogenic therapy, this factor may be involved in treatment resistance. Indeed, HIF1-α was upregulated in bevacizumab resistance metastatic colorectal cancer and might be a potential biomarker of anti-VEGF therapy resistance [120].
Anti-angiogenic resistance mediated by stromal cells
Anti-angiogenic adaptive resistance might be also mediated by the recruitment of local and distal (bone marrow derived cells or BMDCs) stromal cells [3,8,114,121,122]. Anti-angiogenic therapies can upregulate SDF1-α, CXCR4, G-CSF, and PlGF expression, leading to increased recruitment of pro-angiogenic bone marrow cells and cancer associated fibroblasts (CAF) [123,124].
Excessive pruning of vessels after anti-VEGF treatment increases intratumoral hypoxia in tumors, which in turn upregulates SDF1-α/CXCR4 axis and HIF-1-α that regulates both VEGF-dependent and VEGF-independent angiogenesis. These factors promote the recruitment of BMDCs such as M2 (pro-tumor)-tumor associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs) and regulatory T-cells (Tregs). All these immune populations can promote angiogenesis, tumor growth, epithelial-to-mesenchymal transition (EMT) and metastasis, but also mediate immune suppression [78,124–128].
Adaptive resistance to anti-angiogenic therapy may also be mediated by the increased infiltration and activation of CAFs, which produce PDGF, SDF1-α, IGF, TGF-β, MMPs, IL-6, Ang-2 and other growth factors that promote tumor invasion, angiogenesis, immunosuppression, growth and metastasis [117]. The presence of CAFs in solid tumors correlates with worse outcome in patients. The role of CAFs in anti-angiogenic resistance is poorly defined and needs a better characterization [117]. Recently, we have shown that blocking the SDF1-α/CXCR4 pathway with the FDA approved drug, AMD3100 in hepatocellular carcinoma may overcome sorafenib resistance mediated in part by CAFs (activated hepatic stellate cells) [124].
Anti-angiogenesis resistance due to alternative modes of tumor vascularization
Angiogenesis is known to be an essential hallmark for solid tumors, but other modes of tumor new vessel formation exist, and have been linked with intrinsic or adaptive resistance to anti-angiogenic treatment [126]. Numerous reports in preclinical studies demonstrated that cancer cells are able to use co-option of the existing vasculature for nutritional and oxygen supply [129]. Vascular co-option was first observed in glioblastoma, but also was later reported in other types of solid tumors [130]. This phenomenon has been also linked with hypoxia. Hypoxic cancer cells can co-opt the normal blood vessels and migrate within the host solid tumor organ. Hypoxia is considered to play a crucial factor in vascular co-option by promoting an aggressive behavior of the cancer cells, by promoting EMT, tumor invasiveness and metastasis. Vascular co-option has been also reported in, (primary and metastatic) liver and lung tumor and it has been proposed as a resistance mechanism to anti-angiogenic treatment with bevacizumab in colorectal cancer and sorafenib in hepatocellular carcinoma [131–133]. However, the specific molecular mechanisms leading to vascular co-option that distinguish it from angiogenesis remain partly elusive. Other types of neovascularization involve the direct participation of malignant cells, such as vasculogenic mimicry (when cancer cells line microvascular channels) or vasculogenesis from cancer stem cell differentiation to endothelial cells (reported in glioblastoma [134–138]. Vessel intussusception (splitting angiogenesis) is another mode of new vessel formation by splitting of one vessel into two or more vessels, and has been observed in solid tumors [139–141]. Finally, vasculogenesis based on bone marrow-derived precursors of endothelial cells has been studied extensively, but its involvement in tumor angiogenesis and treatment resistance remains controversial [142,143].
Revisiting anti-angiogenesis
There is a very large body of literature demonstrating the efficacy of anti-angiogenic treatments alone or with other therapies in preclinical models of primary and metastatic tumors. In the clinic, anti-angiogenesis using multiple drugs has become a standard and widely used treatment modality for cancers such as renal, lung, liver, or colorectal cancer. However, addressing the problem of treatment resistance remains an unmet need, as anti-angiogenics have not made a similar impact in other cancers, despite clear evidence (based on pharmacodynamic markers) that treatment had a biologic effect (for example, decrease in vascular permeability in brain tumors). Clearly, new strategies involving the use of anti-angiogenic treatment with novel interventions that could have durable benefits, such as immunotherapy, have become particularly attractive.
Reprogramming the tumor vasculature by normalization to enhance anti-tumor immunity
As discussed above, anti-VEGF therapies could lead to hypoxia and acidosis in cancer, in a time and dose-dependent manner due to excessive pruning of tumor vessels [144,145] (Figure 1). In addition to affecting the cancer cells, and the delivery and efficacy of anti-cancer agents, hypoxia and acidosis severely compromise the functionality of immune effector cells, while leading to preferential recruitment of pro-tumor immune cells (Tregs, MDSCs, M2-type TAMs) [15,29,146,147]. Preclinical studies and clinical evidence indicate that judicious dosing of anti-angiogenic treatment can normalize tumor vasculature, at least transiently [30–33,148,149]. Anti-VEGF therapies can normalize the tumor vasculature by increasing the pericyte coverage that improves vessel stabilization, and by reducing vascular permeability/leakiness [21,150]. These changes also inhibit intravasation of metastatic seeking cancer cells. Functionally, vascular normalization reduces hypoxia, and increases the delivery and efficacy of cytotoxic agents [25,33,151]. But normalization of the tumor vasculature can also reduce immunosuppression exerted by Tregs and regulatory B-cells, and promote anti-tumor immunity by enhancing the uptake of antigen presentation in dendritic cells, M1-associated macrophages and activation of cytotoxic CD8+ T-cells [15]. This has been demonstrated in a preclinical model of breast cancer using various doses of anti-mouse VEGFR-2 antibody (DC101 ImClone/Eli Lilly). When used at lower doses, DC101 decreased hypoxia and cancer cell proliferation [152]. Clinical studies have not been usually designed to compare various doses of anti-angiogenics, but when they did they showed a difference or an advantage for the lower dose of the anti-angiogenic agent (such as the use of 5 mg versus 10 mg of bevacizumab plus fluorouracil with leucovorin in metastatic colorectal [30]. The effect of dose titration of anti-angiogenics on the immune microenvironment of human cancer remains unknown. However, based on preclinical evidence, clinical trials of anti-angiogenics with immunotherapy should take into account the appropriate dosing of anti-angiogenic agents and their timing/scheduling with immunotherapy that would ensure synergy (Figure 1).
Figure 1. Potential Effects of Antiangiogenic Therapy on Tumor Vascular Function and Impact on Anti-tumor Immunity.
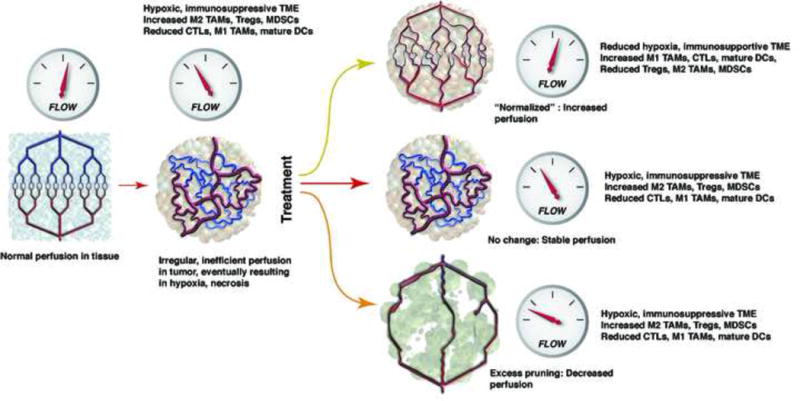
Blood vasculature is normal tissues is maintained by a balanced expression of pro- and anti-angiogenic molecules, which ensures a normal structure and function (blood flow, left). The normal architecture and function is lost in cancer, due to abnormal expression of pro-angiogenic factors by malignant and stromal cells. This leads to inefficient blood perfusion, hypoxia, and an immunosuppressive tumor microenvironment (TME). This is characterized by increased infiltration by T regulatory cells (Tregs), myeloid suppressor cells (MDSCs), and pro-tumor (M2-type) tumor-associated macrophages (TAMs), and reduced infiltration by cytotoxic T cells (CTLs) and mature dendritic cells (DCs)/antigen presenting cells. Antiangiogenic therapy may have different effects on tumor vasculature, depending on the dose, timing and type of antiangiogenic agent. Treatment may result in: 1) a transiently normalized vasculature, which leads to reductions in hypoxia and reprogramming of the immune TME toward an immunosupportive one, with predominance of anti-tumor (M1-type) TAMs, CTLs and mature DCs, 2) no change, or 3) excessive pruning of the vasculature, with further decreases in blood flow and increases in hypoxia. These effects may be critically important for combination of antiangiogenics with immunotherapies (See Ref. 200). Illustration courtesy of Dr. Lance L. Munn (MGH Boston).
Cancer immunotherapy
The immune system is highly effective in mediating immune response against foreign antigens, but malignant cancer can evade immune-surveillance via multiple mechanisms of immunosuppression. Tregs are potent suppressors of immune effector cells. Indeed, whereas Foxp3+ Tregs are often increased only low numbers of cytotoxic CD8+ T cells are infiltrating in solid tumors. High numbers of Tregs in tumors contribute to the evasion of tumor antigen recognition by APC and conventional T-cells and therefore prevent cytotoxic CD8+ T-cell activity [153,154]. Conventional T-cells are characterized by expression of the αβ T-cell receptor and a co-receptor (CD4 or CD8). Tregs regulate immunosuppression through dendritic cells, NK cells, macrophages, B-cells and CD4+ and CD-8+ T-cells via cell-cell contact and humoral actions. The balance between Treg and CD8+ T-cells is thought to control the balance between active anti-tumor responses and immunosuppression.
Over the last decade, cancer immunotherapy has emerged as a breakthrough for various types of cancer, including NSCLC, urothelial carcinoma, melanoma and head and neck carcinoma [75,155–163] (Table 3). Multiple preclinical and clinical studies revealed that several solid tumors overexpress immunoregulatory proteins (called immune checkpoint molecules), which correlates with worse prognosis in survival of patients. The immune checkpoint programmed death-1 (PD-1) is a checkpoint molecule that is expressed on the extracellular surface of natural killer T-cells (NKT), B-cells, dendritic cells, monocyte/macrophages, CD4+ and CD8+ T-cells [164,165]. PD-1 interacts with its ligand-programmed death-ligand-1 (PD-L1) – present on immune cells as well as on certain types of cancer and stromal cells – to inhibit activation, proliferation and survival of T-cells. The PD-1 receptor also interacts with PD-L2, a ligand selectively expressed on certain types of macrophages and tolerogenic dendritic cells, whose functionality is poorly defined. Under physiological conditions PD-1, PD-L1 and PD-L2 play important roles in the activation of T-cells during peripheral tolerance, which involves macrophages and tolerogenic dendritic cells that inhibit auto reactive T-cells to cause immune tissue damage and preventing autoimmunity. The activation of PD-1/PD-L1 pathway may result in the induction of T-cells mediated anergy, apoptosis and “exhaustion” to initiate T-cell suppression [165,166]. Solid tumors are capable to “hijack” the PD-1/PD-L1 axis to induce immune suppression and to escape T-cell receptor recognition. The activation of PD-1/PD-L1 pathway regulates CD4+ T-cells differentiation into Treg expressing Foxp3 and thereby initiating immune suppression. The expression of PD-L1 by cancer cells may limit the attack of conventional cytotoxic CD8+ T-cells and prevent tumor lysis. The regulation of PD-1 and PD-L1 expression in tumor-infiltrating immune cells and cancer cells is incompletely understood, but it has been linked with oncogenic mutations, hypoxia and sustaining immune suppression. Blocking PD-1/PD-L1 axis has been shown to lead to a reversion of an immunosuppression phenotype and activation of the adaptive immune system. Inhibition of the PD-1/PD-L1 pathway triggers tumor antigen recognition, proliferation, infiltration, and activation of cytotoxic CD8+ T-cells [154,167,168]. Most importantly, blockade with monoclonal antibodies against PD-1 (nivolumab, pembrolizumab) or PD-L1 (atezolizumab) in phase III clinical trails has proven successful in inducing durable responses and increasing survival various types of cancer, which led to their approval by the US FDA [155–160,163,169] (Table 3).
Table 3.
FDA approved cancer immunotherapies for solid tumors
| Anti-tumor Immunity target | Therapy | Malignancy | Improvement in (months) | |
|---|---|---|---|---|
| PFS | OS | |||
| PD-1 | Nivolumab | Advanced squamous cell- NSCLC | 0.7 | 3.3 |
|
| ||||
| PD-1 | Nivolumab | Advanced none-squamous NSCLC | NS | 2.9 |
|
| ||||
| PD-1 | Nivolumab | Recurrent or metastatic HNSCC | N/A | 2.4 |
|
| ||||
| PD-1 | Nivolumab | Advanced renal Cell Carcinoma | NS | 5.4 |
|
| ||||
| PD-1 | Nivolumab | Metastatic urothelial Carcinoma | N/A | 8.7 |
|
| ||||
| PD-1 | Nivolumab | Metastatic melanoma | 2.9 | >60 |
|
| ||||
| PD-1 | Pembrolizumab | Metastatic non-squamous NSCLC | 4.3* | Ongoing* |
|
| ||||
| PD-1 | Pembrolizumab | Recurrent or metastatic HNSCC | Ongoing* | Ongoing* |
|
| ||||
| PD-1 | Pembrolizumab | Unresectable or metastatic melanoma | 1.4* | Ongoing* |
|
| ||||
| PD-L1 | Atezolizumab | Metastatic NSCLC | N/A | 4.2 2.9 |
|
| ||||
| PD-L1 | Atezolizumab | Metastatic Urothelial Carcinoma | NS | 3.5 |
|
| ||||
| CTLA-4 | Ipilimumab | Metastatic melanoma | NS | 3.6 2.1 |
|
| ||||
| PD-1 + CTLA-4 | Nivolumab + Ipilimumab | Metastatic melanoma | 8.6 | N/A |
|
| ||||
| Anti-tumor vaccine Oncolytic virus | Talimogene Laherparepvec (T-Vec) | Recurrent unresectable Melanoma | NS | 4.4 |
|
| ||||
| Dendritic cell based anti-tumor vaccine | Sipuleucel-T (Provenge) | Hormone refractory prostate cancer | 5.1 | 4.5 |
Current list of FDA approved cancer immunotherapies for solid tumors.
Clinical trail is still recruiting patients and data needs to be fully analyzed, but the result is promising when compared to standard care of treatment.
Tregs constitutively express other immunosuppressive molecules, including cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), which is an exclusive extracellular surface protein with strong co-inhibitory capabilities to control immune suppression. The T-cell receptor can be stimulated to mediate immunosuppression by CTLA-4 or be activated through co-stimulatory signaling of CD28 in conventional T-cells. It has been proposed that CTLA-4 binds with high affinity or can downregulate co-stimulatory molecules CD80/CD86 ligands on APC by outcompeting them, to prevent the co-stimulation of conventional T-cells. In order to sustain Treg mediated immunosuppression, the expression of CTLA-4 and co-immune modulating stimulatory signals, including LFA-1 is required. Tumors frequently contain CTLA-4-expressing T-cells, which inhibit tumor antigen T cell receptor recognition and reduce the proliferation, infiltration, activation and tumor cell eradication by CD8+ T-cells. CTLA-4 blockade with a humanized monoclonal antibody as monotherapy or combined with anti-PD-1 antibodies increased survival in advanced melanoma [170–174] (Table 3). It is thought that blockade of CTLA-4 releases “the break” of the immunosuppressive function and reverses into a strongly enhanced tumor antigen recognition paired with anti-tumor immunity controlled by CD8+ T-cells [175]. However, the molecular pathways for the exact regulation of T-cell immune suppression by CTLA-4 signaling remain incompletely characterized.
Foreign antigens are normally recognized by APCs and presented via major histocompatibility-II (MHC-II), which initiates antigen processing to smaller peptides that is presented on the surface with an MHC-II molecule for antigen presentation and for T-cell receptor recognition, which triggers T-cell [176] proliferation and activation [176,177]. However, APCs are unable to recognize tumor antigens lacking MHC-II molecules and to recruit T-cells for activation of the adaptive immune system [178]. Sipuleucel-T is a cancer therapeutic vaccine that targets patient specific tumor epitopes. The patients APCs are exposed to antigen prostatic acid phosphatase (95% expressed in prostate cancer), fusion protein and granulocyte-macrophage colony stimulation factor (GM-CSF) for the maturation of the APCs [179–181]. The patient’s matured and exposed APCs with tumor antigen recognition are then reinfused in the patients. It is postulated that these APCs carrying tumor antigens are directly recognized by T-cells, which is followed up with an expansion and activation of CD4+ helper and CD8+ T-cells. But the exact mechanism of action of sipuleucel-T is elusive [182,183]. Sipuleucel-T is FDA approved based on an increased OS by 3 months in hormone refractory prostate cancer patients [162,184] (Table 3).
Overall, cancer immunotherapy has evolved as a success in several types of advanced cancer and in particular blockade of CTLA-4, PD-1/PD-L1 or their combination resulted in durable responses in many patients [174]. This has lead to a tremendous interest in the field, with hundreds of clinical trials of immune checkpoint blockers and/or vaccination strategies.
Combining immunotherapy with anti-angiogenic treatment for cancer
Despite these advances, immunotherapy is effective in a subset of cancer patients (up to approximately 20% in most cancers). It is thought that these are the cases where there is a pre-existing immune response against the tumors. In all the other cases, tumors rapidly develop resistance to treatment. Less is known about the resistance to treatment of cancer immunotherapies, but there are hints provided by preclinical studies that upregulation of immune suppressive molecules, including indoleamine 2-3-dioxygenase (IDO), TIM3, glucocorticoid-induced TNF receptor (GITR), lymphocyte-activation gene 3 (LAG3), IL-10, IL-35, TGF-β might contribute to the resistance to treatment [185].
There are intricate relationships between angiogenesis and immunity in tumors. Vascular endothelium plays a barrier function and has an important role in activation of immunity by increasing the expression of endothelial cell adhesion molecules that directly interact with macrophages, NK cells, granulocytes, B and T-cells for antigen recognition, rolling, adhesion and extravasation during immune responses. In tumors, vascular endothelial often have abnormal expression of adhesion molecules, including CD34, intracellular cell adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1). Downregulation of these adhesion molecules is in part mediated by angiogenic factors, including VEGF. VEGF also inhibits the maturation of dendritic cells, which suppresses immune activation [147,186–188].
As discussed above, potent anti-angiogenic treatment can promote tumor vessel pruning and hypoxia. Hypoxia increases the expression of SDF1-α and CCL28, which initiates a state of tolerance by recruiting Tregs, MDSCs and M2-type TAMs to induce an immunosuppressive tumor microenvironment [6,189,190]. Intratumoral hypoxia also compromises APC functionality and the stimulation of T-cell responses. For example, sunitinib elevated intratumoral infiltration of CD4+ and CD8+ T-cells, but also increased intratumoral infiltration of Foxp3+ Tregs.
Testing of combinations of anti-angiogenic treatment with immunotherapy has reached phase III development, and includes trials in hepatocellular carcinoma, advanced renal cell carcinoma, NSCLC and ovarian cancer patients (Table 4).
Table 4.
Selected ongoing phase III clinical trails involving anti-angiogenic inhibitors combined with cancer immunotherapy
| Anti-Angiogenic target | Anti-tumor immunity target | Combination drugs | Malignancy | Trails |
|---|---|---|---|---|
| VEGF-A | PD-L1 | Bevacizumab +Atezolizumab | Advanced renal cell carcinoma | NCT02420821 |
| VEGFR-1–3, PDGFRβ, | PD-L1 | Axitinib + Avelumab | Advanced renal cell carcinoma | NCT02684006 |
| VEGF-A | PD-L1 | Bevacizumab+ MPDL3280A + Carboplatin + Paclitaxel | Stage IV NSCLC | NCT02366143 |
| VEGF-A | PD-L1 | Bevacizumab+ Atezolizumab+ Pegylated Liposomal Doxorubicin Hydrochloride | Recurrent Ovarian, Fallopian tube peritoneal cancer | NCT02839707 |
| VEGFR-2, PDGFRβ, | GM-CSF (virus based vaccine) | Sorafenib +Pexa Vec | Hepatocellular carcinoma | NCT02562755 |
List of phase III clinical trails of combining anti-angiogenic treatment with cancer immunotherapies in solid tumors.
Interactions between anti-angiogenic treatment and immunotherapy
Preclinical and clinical studies have shown that CTLA-4 blockade plus sunitinib reduced Tregs, increased CD8+ T-cells and inhibited the growth of renal cell carcinoma. Interestingly, anti-angiogenic treatment with sorafenib, sunitinib or bevacizumab increased PD-L1 expression [124,147,191]. PD-L1 is considered to be a target of HIF1-α and the elevation in its expression may be a consequence of anti-angiogenic treatment-induced hypoxia [192]. But other studies have shown that anti-angiogenic treatment could elevate the expression of PD-L1 independently of hypoxia or HIF1-α. In addition, VEGF could increase PD-1 expression on T-cells and mediate “exhaustion” of CD8+ T-cells in tumors [154,164,193]. Our previous studies have shown that standard (high doses) of sorafenib modestly delayed liver tumor growth, but also induced hypoxia. As a result, there was an activation of the SDF1-α/CXCR4 pathway after sorafenib treatment, increased tumor infiltration by Tregs, MDSCs, and M2-type TAMs, and increased expression of EMT genes and PD-L1 in the cancer cells. This mediated treatment resistance, as inhibition of SDF1-α/CXCR4 axis plus sorafenib prevented these immunosuppressive effects and significantly delayed primary and metastatic tumor growth. However, the reduced immunosuppression did not result in increased intratumoral infiltration by CD8+ T-cells. It was only when blockade of anti-PD-1 was added to sorafenib and CXCR inhibition that we found increased intratumoral infiltration and activation of CD8+ T-cells [124]. In another study, we demonstrated that a high dose of VEGFR-2 blocker led to hypoxia and immunosuppression mediated by Tregs and M2-type TAMs in breast cancer. In contrast, a lower dose of the same VEGFR-2 blocker increased perfusion, infiltration of CD8+ T-cells and polarized M2-type TAMs towards the M1-type TAMs. In addition, combination of low dose VEGFR-2 blockade with a cancer vaccine increased IFN-γ+CD8+T-cells and decreased Tregs, and led to a prolongation of overall survival in spontaneous MMTV-PyVT breast cancer in mice [15,152]. In murine models of colorectal and melanoma, combining VEGF inhibition with a GM-CSF-secreting cancer vaccine reduced Tregs, elevated CD8+ T-cells and increased survival [194]. Finally, a preclinical study of anti-angiogenic treatment with endostatin, pigment epithelium-derived factor, IL-12 and GM-CSF decreased PD-1 and CTLA-4 expression and delayed tumor growth in a hepatoma model [186,187,195].
Initial clinical data also support a potentially synergistic interaction between these treatment modalities. A phase I clinical trial of dual blockade of CTLA-4 (ipilimumab) and VEGF (bevacizumab) showed increased tumor antigen recognition, tumor-associated endothelial activation and infiltration of T-cells in melanomas [196]. Another clinical study revealed that atezolizumab (an anti-PD-L1 antibody) combined with bevacizumab increased the number of intratumoral CD8+ T-cells and antigen-specific T-cell migration [197]. Over one hundred phase I/II clinical trials are currently testing combinations of anti-angiogenics with immunotherapy.
Conclusions
Clinical development of anti-angiogenic therapy was a success and translated into increased OS and PFS in many cancers. This is in line with the concept that tumor angiogenesis is a hallmark of cancer. However, the benefits are limited and there is no method of selection of patients likely to respond to this therapy [198,199]. Moreover, treatment resistance to anti-angiogenic drugs may be mediated by increased hypoxia, which can also aggravate immunosuppression. This is critically important for optimally combining anti-angiogenic drugs with immunotherapy, which is the most promising strategy currently [24,29,51,53,200–203]. Understanding the interaction between these two therapeutic interventions, especially as it relates to proper reprogramming of the tumor immune microenvironment to enhance anti-tumor immunity, will be critical for the success of this combination strategy for cancer treatment.
Acknowledgments
We would like to thank Nisha Gupta, Echoe Bouta and Patrycja Nowak-Sliwinka for helpful comments and Lance L. Munn for providing the illustration. We apologize to authors whose original work we could not cite due to limitations on the number of references.
Funding:
RRR received funding from Dutch Cancer Society (KWF), Stichting Nijbakker-Morra, Stichting Bekker-La-Bastide Fonds, Dittmer Fonds, VU Fondsendesk. DGD’s work was supported through NIH grants P01-CA080124, R01-CA159258, R21-CA139168 and Proton Beam/Federal Share Program, and the American Cancer Society grant 120733-RSG-11-073-01-TBG.
Footnotes
Conflicts of interest: DGD receives research funding from Merrimack, Leap Tx, Bristol-Meyers-Squibb and Bayer.
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegue E, Song H, Vandenberg S, Johnson RS, Werb Z, Bergers G. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13(3):206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dulloo I, Phang BH, Othman R, Tan SY, Vijayaraghavan A, Goh LK, Martin-Lopez M, Marques MM, Li CW, Wang de Y, Marin MC, Xian W, McKeon F, Sabapathy K. Hypoxia-inducible TAp73 supports tumorigenesis by regulating the angiogenic transcriptome. Nat Cell Biol. 2015;17(4):511–523. doi: 10.1038/ncb3130. [DOI] [PubMed] [Google Scholar]
- 5.Gupta N, Duda DG. Role of stromal cell-derived factor 1alpha pathway in bone metastatic prostate cancer. J Biomed Res. 2016;30(3):181–185. doi: 10.7555/JBR.30.20150114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petit I, Jin D, Rafii S. The SDF-1-CXCR4 signaling pathway: a molecular hub modulating neo-angiogenesis. Trends Immunol. 2007;28(7):299–307. doi: 10.1016/j.it.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salceda S, Caro J. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem. 1997;272(36):22642–22647. doi: 10.1074/jbc.272.36.22642. [DOI] [PubMed] [Google Scholar]
- 8.Shojaei F, Wu X, Malik AK, Zhong C, Baldwin ME, Schanz S, Fuh G, Gerber HP, Ferrara N. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol. 2007;25(8):911–920. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- 9.Folkman J, Merler E, Abernathy C, Williams G. Isolation of a tumor factor responsible for angiogenesis. J Exp Med. 1971;133(2):275–288. doi: 10.1084/jem.133.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438(7070):967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 11.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1(1):27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 12.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6(4):273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 13.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358(19):2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65(1–2):271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 15.Huang Y, Goel S, Duda DG, Fukumura D, Jain RK. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res. 2013;73(10):2943–2948. doi: 10.1158/0008-5472.CAN-12-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20(21):4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 17.Bartels K, Grenz A, Eltzschig HK. Hypoxia and inflammation are two sides of the same coin. Proc Natl Acad Sci U S A. 2013;110(46):18351–18352. doi: 10.1073/pnas.1318345110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krock BL, Skuli N, Simon MC. Hypoxia-induced angiogenesis: good and evil. Genes Cancer. 2011;2(12):1117–1133. doi: 10.1177/1947601911423654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9(6):685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 20.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86(3):353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 21.Cooke VG, LeBleu VS, Keskin D, Khan Z, O’;Connell JT, Teng Y, Duncan MB, Xie L, Maeda G, Vong S, Sugimoto H, Rocha RM, Damascena A, Brentani RR, Kalluri R. Pericyte depletion results in hypoxia-associated epithelial-to-mesenchymal transition and metastasis mediated by met signaling pathway. Cancer Cell. 2012;21(1):66–81. doi: 10.1016/j.ccr.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen XL, Nam JO, Jean C, Lawson C, Walsh CT, Goka E, Lim ST, Tomar A, Tancioni I, Uryu S, Guan JL, Acevedo LM, Weis SM, Cheresh DA, Schlaepfer DD. VEGF-induced vascular permeability is mediated by FAK. Dev Cell. 2012;22(1):146–157. doi: 10.1016/j.devcel.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jain RK, Booth MF. What brings pericytes to tumor vessels? J Clin Invest. 2003;112(8):1134–1136. doi: 10.1172/JCI20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu AX, Duda DG, Sahani DV, Jain RK. HCC and angiogenesis: possible targets and future directions. Nat Rev Clin Oncol. 2011;8(5):292–301. doi: 10.1038/nrclinonc.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 26.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001;7(9):987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 27.Jain RK, Duda DG, Willett CG, Sahani DV, Zhu AX, Loeffler JS, Batchelor TT, Sorensen AG. Biomarkers of response and resistance to antiangiogenic therapy. Nat Rev Clin Oncol. 2009;6(6):327–338. doi: 10.1038/nrclinonc.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11(6):393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 29.Jain RK. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J Clin Oncol. 2013;31(17):2205–2218. doi: 10.1200/JCO.2012.46.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, Griffing S, Bergsland E. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21(1):60–65. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 31.Heist RS, Duda DG, Sahani DV, Ancukiewicz M, Fidias P, Sequist LV, Temel JS, Shaw AT, Pennell NA, Neal JW, Gandhi L, Lynch TJ, Engelman JA, Jain RK. Improved tumor vascularization after anti-VEGF therapy with carboplatin and nab-paclitaxel associates with survival in lung cancer. Proc Natl Acad Sci U S A. 2015;112(5):1547–1552. doi: 10.1073/pnas.1424024112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, Kozak KR, Cahill DP, Chen PJ, Zhu M, Ancukiewicz M, Mrugala MM, Plotkin S, Drappatz J, Louis DN, Ivy P, Scadden DT, Benner T, Loeffler JS, Wen PY, Jain RK. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11(1):83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tolaney SM, Boucher Y, Duda DG, Martin JD, Seano G, Ancukiewicz M, Barry WT, Goel S, Lahdenrata J, Isakoff SJ, Yeh ED, Jain SR, Golshan M, Brock J, Snuderl M, Winer EP, Krop IE, Jain RK. Role of vascular density and normalization in response to neoadjuvant bevacizumab and chemotherapy in breast cancer patients. Proc Natl Acad Sci U S A. 2015;112(46):14325–14330. doi: 10.1073/pnas.1518808112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cameron D, Brown J, Dent R, Jackisch C, Mackey J, Pivot X, Steger GG, Suter TM, Toi M, Parmar M, Laeufle R, Im YH, Romieu G, Harvey V, Lipatov O, Pienkowski T, Cottu P, Chan A, Im SA, Hall PS, Bubuteishvili-Pacaud L, Henschel V, Deurloo RJ, Pallaud C, Bell R. Adjuvant bevacizumab-containing therapy in triple-negative breast cancer (BEATRICE): primary results of a randomised, phase 3 trial. Lancet Oncol. 2013;14(10):933–942. doi: 10.1016/S1470-2045(13)70335-8. [DOI] [PubMed] [Google Scholar]
- 35.Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002;2(10):795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- 36.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 37.Staton CA, Kumar I, Reed MW, Brown NJ. Neuropilins in physiological and pathological angiogenesis. J Pathol. 2007;212(3):237–248. doi: 10.1002/path.2182. [DOI] [PubMed] [Google Scholar]
- 38.Hattori K, Heissig B, Wu Y, Dias S, Tejada R, Ferris B, Hicklin DJ, Zhu Z, Bohlen P, Witte L, Hendrikx J, Hackett NR, Crystal RG, Moore MA, Werb Z, Lyden D, Rafii S. Placental growth factor reconstitutes hematopoiesis by recruiting VEGFR1(+) stem cells from bone-marrow microenvironment. Nat Med. 2002;8(8):841–849. doi: 10.1038/nm740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Snuderl M, Batista A, Kirkpatrick ND, Ruiz de Almodovar C, Riedemann L, Walsh EC, Anolik R, Huang Y, Martin JD, Kamoun W, Knevels E, Schmidt T, Farrar CT, Vakoc BJ, Mohan N, Chung E, Roberge S, Peterson T, Bais C, Zhelyazkova BH, Yip S, Hasselblatt M, Rossig C, Niemeyer E, Ferrara N, Klagsbrun M, Duda DG, Fukumura D, Xu L, Carmeliet P, Jain RK. Targeting placental growth factor/neuropilin 1 pathway inhibits growth and spread of medulloblastoma. Cell. 2013;152(5):1065–1076. doi: 10.1016/j.cell.2013.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jain RK, Xu L. alphaPlGF: a new kid on the antiangiogenesis block. Cell. 2007;131(3):443–445. doi: 10.1016/j.cell.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 41.Lampropoulou A, Ruhrberg C. Neuropilin regulation of angiogenesis. Biochem Soc Trans. 2014;42(6):1623–1628. doi: 10.1042/BST20140244. [DOI] [PubMed] [Google Scholar]
- 42.Wu FT, Stefanini MO, Mac Gabhann F, Kontos CD, Annex BH, Popel AS. A systems biology perspective on sVEGFR1: its biological function, pathogenic role and therapeutic use. J Cell Mol Med. 2010;14(3):528–552. doi: 10.1111/j.1582-4934.2009.00941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dawson MR, Duda DG, Fukumura D, Jain RK. VEGFR1-activity-independent metastasis formation. Nature. 2009;461(7262):E4. doi: 10.1038/nature08254. discussion E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277(5322):55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 45.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87(7):1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 46.Udani V, Santarelli J, Yung Y, Cheshier S, Andrews A, Kasad Z, Tse V. Differential expression of angiopoietin-1 and angiopoietin-2 may enhance recruitment of bone-marrow-derived endothelial precursor cells into brain tumors. Neurol Res. 2005;27(8):801–806. doi: 10.1179/016164105X49319. [DOI] [PubMed] [Google Scholar]
- 47.Goel S, Gupta N, Walcott BP, Snuderl M, Kesler CT, Kirkpatrick ND, Heishi T, Huang Y, Martin JD, Ager E, Samuel R, Wang S, Yazbek J, Vakoc BJ, Peterson RT, Padera TP, Duda DG, Fukumura D, Jain RK. Effects of vascular-endothelial protein tyrosine phosphatase inhibition on breast cancer vasculature and metastatic progression. J Natl Cancer Inst. 2013;105(16):1188–1201. doi: 10.1093/jnci/djt164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gu J, Yamamoto H, Ogawa M, Ngan CY, Danno K, Hemmi H, Kyo N, Takemasa I, Ikeda M, Sekimoto M, Monden M. Hypoxia-induced up-regulation of angiopoietin-2 in colorectal cancer. Oncol Rep. 2006;15(4):779–783. [PubMed] [Google Scholar]
- 49.Rigamonti N, De Palma M. A role for angiopoietin-2 in organ-specific metastasis. Cell Rep. 2013;4(4):621–623. doi: 10.1016/j.celrep.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 50.Kloepper J, Riedemann L, Amoozgar Z, Seano G, Susek K, Yu V, Dalvie N, Amelung RL, Datta M, Song JW, Askoxylakis V, Taylor JW, Lu-Emerson C, Batista A, Kirkpatrick ND, Jung K, Snuderl M, Muzikansky A, Stubenrauch KG, Krieter O, Wakimoto H, Xu L, Munn LL, Duda DG, Fukumura D, Batchelor TT, Jain RK. Ang-2/VEGF bispecific antibody reprograms macrophages and resident microglia to anti-tumor phenotype and prolongs glioblastoma survival. Proc Natl Acad Sci U S A. 2016;113(16):4476–4481. doi: 10.1073/pnas.1525360113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, Jain RK. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91(3):1071–1121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reynolds AR, Hart IR, Watson AR, Welti JC, Silva RG, Robinson SD, Da Violante G, Gourlaouen M, Salih M, Jones MC, Jones DT, Saunders G, Kostourou V, Perron-Sierra F, Norman JC, Tucker GC, Hodivala-Dilke KM. Stimulation of tumor growth and angiogenesis by low concentrations of RGD-mimetic integrin inhibitors. Nat Med. 2009;15(4):392–400. doi: 10.1038/nm.1941. [DOI] [PubMed] [Google Scholar]
- 53.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, Wiegand SJ. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci U S A. 2007;104(9):3219–3224. doi: 10.1073/pnas.0611206104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe ML, Kalen M, Gerhardt H, Betsholtz C. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445(7129):776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 56.Hiratsuka S, Goel S, Kamoun WS, Maru Y, Fukumura D, Duda DG, Jain RK. Endothelial focal adhesion kinase mediates cancer cell homing to discrete regions of the lungs via E-selectin up-regulation. Proc Natl Acad Sci U S A. 2011;108(9):3725–3730. doi: 10.1073/pnas.1100446108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eliceiri BP, Puente XS, Hood JD, Stupack DG, Schlaepfer DD, Huang XZ, Sheppard D, Cheresh DA. Src-mediated coupling of focal adhesion kinase to integrin alpha(v)beta5 in vascular endothelial growth factor signaling. J Cell Biol. 2002;157(1):149–160. doi: 10.1083/jcb.200109079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lietha D, Cai X, Ceccarelli DF, Li Y, Schaller MD, Eck MJ. Structural basis for the autoinhibition of focal adhesion kinase. Cell. 2007;129(6):1177–1187. doi: 10.1016/j.cell.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Canel M, Serrels A, Miller D, Timpson P, Serrels B, Frame MC, Brunton VG. Quantitative in vivo imaging of the effects of inhibiting integrin signaling via Src and FAK on cancer cell movement: effects on E-cadherin dynamics. Cancer Res. 2010;70(22):9413–9422. doi: 10.1158/0008-5472.CAN-10-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380(6573):439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 61.Lieu CH, Tran H, Jiang ZQ, Mao M, Overman MJ, Lin E, Eng C, Morris J, Ellis L, Heymach JV, Kopetz S. The association of alternate VEGF ligands with resistance to anti-VEGF therapy in metastatic colorectal cancer. PLoS One. 2013;8(10):e77117. doi: 10.1371/journal.pone.0077117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ferrara N, Hillan KJ, Novotny W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun. 2005;333(2):328–335. doi: 10.1016/j.bbrc.2005.05.132. [DOI] [PubMed] [Google Scholar]
- 63.Willett CG, Duda DG, di Tomaso E, Boucher Y, Ancukiewicz M, Sahani DV, Lahdenranta J, Chung DC, Fischman AJ, Lauwers GY, Shellito P, Czito BG, Wong TZ, Paulson E, Poleski M, Vujaskovic Z, Bentley R, Chen HX, Clark JW, Jain RK. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer: a multidisciplinary phase II study. J Clin Oncol. 2009;27(18):3020–3026. doi: 10.1200/JCO.2008.21.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miller KD, Chap LI, Holmes FA, Cobleigh MA, Marcom PK, Fehrenbacher L, Dickler M, Overmoyer BA, Reimann JD, Sing AP, Langmuir V, Rugo HS. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23(4):792–799. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 65.de Gramont A, Van Cutsem E, Schmoll HJ, Tabernero J, Clarke S, Moore MJ, Cunningham D, Cartwright TH, Hecht JR, Rivera F, Im SA, Bodoky G, Salazar R, Maindrault-Goebel F, Shacham-Shmueli E, Bajetta E, Makrutzki M, Shang A, Andre T, Hoff PM. Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT): a phase 3 randomised controlled trial. Lancet Oncol. 2012;13(12):1225–1233. doi: 10.1016/S1470-2045(12)70509-0. [DOI] [PubMed] [Google Scholar]
- 66.Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, Sovak MA, Yi J, Nycum LR. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30(17):2039–2045. doi: 10.1200/JCO.2012.42.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, Mannel RS, Homesley HD, Fowler J, Greer BE, Boente M, Birrer MJ, Liang SX, Gynecologic Oncology G Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365(26):2473–2483. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 68.Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson AB, 3rd, Eastern Cooperative Oncology Group Study E Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25(12):1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 69.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 70.Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, Carey MS, Beale P, Cervantes A, Kurzeder C, du Bois A, Sehouli J, Kimmig R, Stahle A, Collinson F, Essapen S, Gourley C, Lortholary A, Selle F, Mirza MR, Leminen A, Plante M, Stark D, Qian W, Parmar MK, Oza AM, Investigators I A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365(26):2484–2496. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 71.Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, Sorio R, Vergote I, Witteveen P, Bamias A, Pereira D, Wimberger P, Oaknin A, Mirza MR, Follana P, Bollag D, Ray-Coquard I. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32(13):1302–1308. doi: 10.1200/JCO.2013.51.4489. [DOI] [PubMed] [Google Scholar]
- 72.Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzen F, Cassidy J. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26(12):2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 73.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 74.Tewari KS, Sill MW, Long HJ, 3rd, Penson RT, Huang H, Ramondetta LM, Landrum LM, Oaknin A, Reid TJ, Leitao MM, Michael HE, Monk BJ. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370(8):734–743. doi: 10.1056/NEJMoa1309748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zalcman G, Mazieres J, Margery J, Greillier L, Audigier-Valette C, Moro-Sibilot D, Molinier O, Corre R, Monnet I, Gounant V, Riviere F, Janicot H, Gervais R, Locher C, Milleron B, Tran Q, Lebitasy MP, Morin F, Creveuil C, Parienti JJ, Scherpereel A, French Cooperative Thoracic I Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet. 2016;387(10026):1405–1414. doi: 10.1016/S0140-6736(15)01238-6. [DOI] [PubMed] [Google Scholar]
- 76.Rini BI, Bellmunt J, Clancy J, Wang K, Niethammer AG, Hariharan S, Escudier B. Randomized phase III trial of temsirolimus and bevacizumab versus interferon alfa and bevacizumab in metastatic renal cell carcinoma: INTORACT trial. J Clin Oncol. 2014;32(8):752–759. doi: 10.1200/JCO.2013.50.5305. [DOI] [PubMed] [Google Scholar]
- 77.Corrie PG, Marshall A, Dunn JA, Middleton MR, Nathan PD, Gore M, Davidson N, Nicholson S, Kelly CG, Marples M, Danson SJ, Marshall E, Houston SJ, Board RE, Waterston AM, Nobes JP, Harries M, Kumar S, Young G, Lorigan P. Adjuvant bevacizumab in patients with melanoma at high risk of recurrence (AVAST-M): preplanned interim results from a multicentre, open-label, randomised controlled phase 3 study. Lancet Oncol. 2014;15(6):620–630. doi: 10.1016/S1470-2045(14)70110-X. [DOI] [PubMed] [Google Scholar]
- 78.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8(4):299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 79.Jayson GC, Kerbel R, Ellis LM, Harris AL. Antiangiogenic therapy in oncology: current status and future directions. Lancet. 2016;388(10043):518–529. doi: 10.1016/S0140-6736(15)01088-0. [DOI] [PubMed] [Google Scholar]
- 80.Van Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausova J, Macarulla T, Ruff P, van Hazel GA, Moiseyenko V, Ferry D, McKendrick J, Polikoff J, Tellier A, Castan R, Allegra C. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30(28):3499–3506. doi: 10.1200/JCO.2012.42.8201. [DOI] [PubMed] [Google Scholar]
- 81.Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, Michaelson MD, Gorbunova VA, Gore ME, Rusakov IG, Negrier S, Ou YC, Castellano D, Lim HY, Uemura H, Tarazi J, Cella D, Chen C, Rosbrook B, Kim S, Motzer RJ. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378(9807):1931–1939. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 82.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 83.Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, de la Fouchardiere C, Pacini F, Paschke R, Shong YK, Sherman SI, Smit JW, Chung J, Kappeler C, Pena C, Molnar I, Schlumberger MJ, investigators D Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384(9940):319–328. doi: 10.1016/S0140-6736(14)60421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Demetri GD, Reichardt P, Kang YK, Blay JY, Rutkowski P, Gelderblom H, Hohenberger P, Leahy M, von Mehren M, Joensuu H, Badalamenti G, Blackstein M, Le Cesne A, Schoffski P, Maki RG, Bauer S, Nguyen BB, Xu J, Nishida T, Chung J, Kappeler C, Kuss I, Laurent D, Casali PG, investigators Gs Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):295–302. doi: 10.1016/S0140-6736(12)61857-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.George S, Wang Q, Heinrich MC, Corless CL, Zhu M, Butrynski JE, Morgan JA, Wagner AJ, Choy E, Tap WD, Yap JT, Van den Abbeele AD, Manola JB, Solomon SM, Fletcher JA, von Mehren M, Demetri GD. Efficacy and safety of regorafenib in patients with metastatic and/or unresectable GI stromal tumor after failure of imatinib and sunitinib: a multicenter phase II trial. J Clin Oncol. 2012;30(19):2401–2407. doi: 10.1200/JCO.2011.39.9394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G, Investigators R Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 87.Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouche O, Mineur L, Barone C, Adenis A, Tabernero J, Yoshino T, Lenz HJ, Goldberg RM, Sargent DJ, Cihon F, Cupit L, Wagner A, Laurent D, Group CS Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 88.Wells SA, Jr, Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, Baudin E, Elisei R, Jarzab B, Vasselli JR, Read J, Langmuir P, Ryan AJ, Schlumberger MJ. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2012;30(2):134–141. doi: 10.1200/JCO.2011.35.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bible KC, Suman VJ, Molina JR, Smallridge RC, Maples WJ, Menefee ME, Rubin J, Sideras K, Morris JC, 3rd, McIver B, Burton JK, Webster KP, Bieber C, Traynor AM, Flynn PJ, Goh BC, Tang H, Ivy SP, Erlichman C, Endocrine Malignancies Disease Oriented G, Mayo Clinic Cancer C, Mayo Phase C Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: results of a phase 2 consortium study. Lancet Oncol. 2010;11(10):962–972. doi: 10.1016/S1470-2045(10)70203-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Motzer RJ, Hutson TE, McCann L, Deen K, Choueiri TK. Overall survival in renal-cell carcinoma with pazopanib versus sunitinib. N Engl J Med. 2014;370(18):1769–1770. doi: 10.1056/NEJMc1400731. [DOI] [PubMed] [Google Scholar]
- 91.Motzer RJ, Hutson TE, Glen H, Michaelson MD, Molina A, Eisen T, Jassem J, Zolnierek J, Maroto JP, Mellado B, Melichar B, Tomasek J, Kremer A, Kim HJ, Wood K, Dutcus C, Larkin J. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 2015;16(15):1473–1482. doi: 10.1016/S1470-2045(15)00290-9. [DOI] [PubMed] [Google Scholar]
- 92.Choueiri TK, Escudier B, Powles T, Mainwaring PN, Rini BI, Donskov F, Hammers H, Hutson TE, Lee JL, Peltola K, Roth BJ, Bjarnason GA, Geczi L, Keam B, Maroto P, Heng DY, Schmidinger M, Kantoff PW, Borgman-Hagey A, Hessel C, Scheffold C, Schwab GM, Tannir NM, Motzer RJ, Investigators M Cabozantinib versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373(19):1814–1823. doi: 10.1056/NEJMoa1510016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Elisei R, Schlumberger MJ, Muller SP, Schoffski P, Brose MS, Shah MH, Licitra L, Jarzab B, Medvedev V, Kreissl MC, Niederle B, Cohen EE, Wirth LJ, Ali H, Hessel C, Yaron Y, Ball D, Nelkin B, Sherman SI. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013;31(29):3639–3646. doi: 10.1200/JCO.2012.48.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kurzrock R, Sherman SI, Ball DW, Forastiere AA, Cohen RB, Mehra R, Pfister DG, Cohen EE, Janisch L, Nauling F, Hong DS, Ng CS, Ye L, Gagel RF, Frye J, Muller T, Ratain MJ, Salgia R. Activity of XL184 (Cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol. 2011;29(19):2660–2666. doi: 10.1200/JCO.2010.32.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, dos Santos LV, Aprile G, Ferry DR, Melichar B, Tehfe M, Topuzov E, Zalcberg JR, Chau I, Campbell W, Sivanandan C, Pikiel J, Koshiji M, Hsu Y, Liepa AM, Gao L, Schwartz JD, Tabernero J, Investigators RT Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383(9911):31–39. doi: 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 96.Garon EB, Ciuleanu TE, Arrieta O, Prabhash K, Syrigos KN, Goksel T, Park K, Gorbunova V, Kowalyszyn RD, Pikiel J, Czyzewicz G, Orlov SV, Lewanski CR, Thomas M, Bidoli P, Dakhil S, Gans S, Kim JH, Grigorescu A, Karaseva N, Reck M, Cappuzzo F, Alexandris E, Sashegyi A, Yurasov S, Perol M. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384(9944):665–673. doi: 10.1016/S0140-6736(14)60845-X. [DOI] [PubMed] [Google Scholar]
- 97.Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, Cunningham D, Rougier P, Komatsu Y, Ajani J, Emig M, Carlesi R, Ferry D, Chandrawansa K, Schwartz JD, Ohtsu A, Group RS Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15(11):1224–1235. doi: 10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- 98.Ledermann JA, Embleton AC, Raja F, Perren TJ, Jayson GC, Rustin GJ, Kaye SB, Hirte H, Eisenhauer E, Vaughan M, Friedlander M, Gonzalez-Martin A, Stark D, Clark E, Farrelly L, Swart AM, Cook A, Kaplan RS, Parmar MK, collaborators I Cediranib in patients with relapsed platinum-sensitive ovarian cancer (ICON6): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;387(10023):1066–1074. doi: 10.1016/S0140-6736(15)01167-8. [DOI] [PubMed] [Google Scholar]
- 99.Schmoll HJ, Cunningham D, Sobrero A, Karapetis CS, Rougier P, Koski SL, Kocakova I, Bondarenko I, Bodoky G, Mainwaring P, Salazar R, Barker P, Mookerjee B, Robertson J, Van Cutsem E. Cediranib with mFOLFOX6 versus bevacizumab with mFOLFOX6 as first-line treatment for patients with advanced colorectal cancer: a double-blind, randomized phase III study (HORIZON III) J Clin Oncol. 2012;30(29):3588–3595. doi: 10.1200/JCO.2012.42.5355. [DOI] [PubMed] [Google Scholar]
- 100.Schmidt C. Cediranib aims for a comeback. J Natl Cancer Inst. 2015;107(3) doi: 10.1093/jnci/djv068. [DOI] [PubMed] [Google Scholar]
- 101.Vasudev NS, Reynolds AR. Anti-angiogenic therapy for cancer: current progress, unresolved questions and future directions. Angiogenesis. 2014;17(3):471–494. doi: 10.1007/s10456-014-9420-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mas-Moruno C, Rechenmacher F, Kessler H. Cilengitide: the first anti-angiogenic small molecule drug candidate design, synthesis and clinical evaluation. Anticancer Agents Med Chem. 2010;10(10):753–768. doi: 10.2174/187152010794728639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Oliveira-Ferrer L, Hauschild J, Fiedler W, Bokemeyer C, Nippgen J, Celik I, Schuch G. Cilengitide induces cellular detachment and apoptosis in endothelial and glioma cells mediated by inhibition of FAK/src/AKT pathway. J Exp Clin Cancer Res. 2008;27:86. doi: 10.1186/1756-9966-27-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stupp R, Hegi ME, Gorlia T, Erridge SC, Perry J, Hong YK, Aldape KD, Lhermitte B, Pietsch T, Grujicic D, Steinbach JP, Wick W, Tarnawski R, Nam DH, Hau P, Weyerbrock A, Taphoorn MJ, Shen CC, Rao N, Thurzo L, Herrlinger U, Gupta T, Kortmann RD, Adamska K, McBain C, Brandes AA, Tonn JC, Schnell O, Wiegel T, Kim CY, Nabors LB, Reardon DA, van den Bent MJ, Hicking C, Markivskyy A, Picard M, Weller M, European Organisation for R, Treatment of C, Canadian Brain Tumor C, team Cs Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1100–1108. doi: 10.1016/S1470-2045(14)70379-1. [DOI] [PubMed] [Google Scholar]
- 105.Neal J, Wakelee H. AMG-386, a selective angiopoietin-1/-2-neutralizing peptibody for the potential treatment of cancer. Curr Opin Mol Ther. 2010;12(4):487–495. [PubMed] [Google Scholar]
- 106.Coxon A, Bready J, Min H, Kaufman S, Leal J, Yu D, Lee TA, Sun JR, Estrada J, Bolon B, McCabe J, Wang L, Rex K, Caenepeel S, Hughes P, Cordover D, Kim H, Han SJ, Michaels ML, Hsu E, Shimamoto G, Cattley R, Hurh E, Nguyen L, Wang SX, Ndifor A, Hayward IJ, Falcon BL, McDonald DM, Li L, Boone T, Kendall R, Radinsky R, Oliner JD. Context-dependent role of angiopoietin-1 inhibition in the suppression of angiogenesis and tumor growth: implications for AMG 386, an angiopoietin-1/2-neutralizing peptibody. Mol Cancer Ther. 2010;9(10):2641–2651. doi: 10.1158/1535-7163.MCT-10-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Monk BJ, Poveda A, Vergote I, Raspagliesi F, Fujiwara K, Bae DS, Oaknin A, Ray-Coquard I, Provencher DM, Karlan BY, Lhomme C, Richardson G, Rincon DG, Coleman RL, Herzog TJ, Marth C, Brize A, Fabbro M, Redondo A, Bamias A, Tassoudji M, Navale L, Warner DJ, Oza AM. Anti-angiopoietin therapy with trebananib for recurrent ovarian cancer (TRINOVA-1): a randomised, multicentre, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2014;15(8):799–808. doi: 10.1016/S1470-2045(14)70244-X. [DOI] [PubMed] [Google Scholar]
- 108.Motzer RJ, Porta C, Vogelzang NJ, Sternberg CN, Szczylik C, Zolnierek J, Kollmannsberger C, Rha SY, Bjarnason GA, Melichar B, De Giorgi U, Grunwald V, Davis ID, Lee JL, Esteban E, Urbanowitz G, Cai C, Squires M, Marker M, Shi MM, Escudier B. Dovitinib versus sorafenib for third-line targeted treatment of patients with metastatic renal cell carcinoma: an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15(3):286–296. doi: 10.1016/S1470-2045(14)70030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sivanand S, Pena-Llopis S, Zhao H, Kucejova B, Spence P, Pavia-Jimenez A, Yamasaki T, McBride DJ, Gillen J, Wolff NC, Morlock L, Lotan Y, Raj GV, Sagalowsky A, Margulis V, Cadeddu JA, Ross MT, Bentley DR, Kabbani W, Xie XJ, Kapur P, Williams NS, Brugarolas J. A validated tumorgraft model reveals activity of dovitinib against renal cell carcinoma. Sci Transl Med. 2012;4(137):137ra175. doi: 10.1126/scitranslmed.3003643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Huynh H, Ngo VC, Fargnoli J, Ayers M, Soo KC, Koong HN, Thng CH, Ong HS, Chung A, Chow P, Pollock P, Byron S, Tran E. Brivanib alaninate, a dual inhibitor of vascular endothelial growth factor receptor and fibroblast growth factor receptor tyrosine kinases, induces growth inhibition in mouse models of human hepatocellular carcinoma. Clin Cancer Res. 2008;14(19):6146–6153. doi: 10.1158/1078-0432.CCR-08-0509. [DOI] [PubMed] [Google Scholar]
- 111.Johnson PJ, Qin S, Park JW, Poon RT, Raoul JL, Philip PA, Hsu CH, Hu TH, Heo J, Xu J, Lu L, Chao Y, Boucher E, Han KH, Paik SW, Robles-Avina J, Kudo M, Yan L, Sobhonslidsuk A, Komov D, Decaens T, Tak WY, Jeng LB, Liu D, Ezzeddine R, Walters I, Cheng AL. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol. 2013;31(28):3517–3524. doi: 10.1200/JCO.2012.48.4410. [DOI] [PubMed] [Google Scholar]
- 112.Kudo M, Han G, Finn RS, Poon RT, Blanc JF, Yan L, Yang J, Lu L, Tak WY, Yu X, Lee JH, Lin SM, Wu C, Tanwandee T, Shao G, Walters IB, Dela Cruz C, Poulart V, Wang JH. Brivanib as adjuvant therapy to transarterial chemoembolization in patients with hepatocellular carcinoma: A randomized phase III trial. Hepatology. 2014;60(5):1697–1707. doi: 10.1002/hep.27290. [DOI] [PubMed] [Google Scholar]
- 113.Llovet JM, Decaens T, Raoul JL, Boucher E, Kudo M, Chang C, Kang YK, Assenat E, Lim HY, Boige V, Mathurin P, Fartoux L, Lin DY, Bruix J, Poon RT, Sherman M, Blanc JF, Finn RS, Tak WY, Chao Y, Ezzeddine R, Liu D, Walters I, Park JW. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J Clin Oncol. 2013;31(28):3509–3516. doi: 10.1200/JCO.2012.47.3009. [DOI] [PubMed] [Google Scholar]
- 114.van Beijnum JR, Nowak-Sliwinska P, Huijbers EJ, Thijssen VL, Griffioen AW. The great escape; the hallmarks of resistance to antiangiogenic therapy. Pharmacol Rev. 2015;67(2):441–461. doi: 10.1124/pr.114.010215. [DOI] [PubMed] [Google Scholar]
- 115.Gotink KJ, Broxterman HJ, Labots M, de Haas RR, Dekker H, Honeywell RJ, Rudek MA, Beerepoot LV, Musters RJ, Jansen G, Griffioen AW, Assaraf YG, Pili R, Peters GJ, Verheul HM. Lysosomal sequestration of sunitinib: a novel mechanism of drug resistance. Clin Cancer Res. 2011;17(23):7337–7346. doi: 10.1158/1078-0432.CCR-11-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xu L, Duda DG, di Tomaso E, Ancukiewicz M, Chung DC, Lauwers GY, Samuel R, Shellito P, Czito BG, Lin PC, Poleski M, Bentley R, Clark JW, Willett CG, Jain RK. Direct evidence that bevacizumab, an anti-VEGF antibody, up-regulates SDF1alpha, CXCR4, CXCL6, and neuropilin 1 in tumors from patients with rectal cancer. Cancer Res. 2009;69(20):7905–7910. doi: 10.1158/0008-5472.CAN-09-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Clarke JM, Hurwitz HI. Understanding and targeting resistance to anti-angiogenic therapies. J Gastrointest Oncol. 2013;4(3):253–263. doi: 10.3978/j.issn.2078-6891.2013.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mahon PC, Hirota K, Semenza GL. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001;15(20):2675–2686. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tang N, Wang L, Esko J, Giordano FJ, Huang Y, Gerber HP, Ferrara N, Johnson RS. Loss of HIF-1alpha in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer Cell. 2004;6(5):485–495. doi: 10.1016/j.ccr.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 120.Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59(22):5830–5835. [PubMed] [Google Scholar]
- 121.Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP, Lin PC. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6(4):409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 122.Huijbers EJ, van Beijnum JR, Thijssen VL, Sabrkhany S, Nowak-Sliwinska P, Griffioen AW. Role of the tumor stroma in resistance to anti-angiogenic therapy. Drug Resist Updat. 2016;25:26–37. doi: 10.1016/j.drup.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 123.Hiratsuka S, Duda DG, Huang Y, Goel S, Sugiyama T, Nagasawa T, Fukumura D, Jain RK. C-X-C receptor type 4 promotes metastasis by activating p38 mitogen-activated protein kinase in myeloid differentiation antigen (Gr-1)-positive cells. Proc Natl Acad Sci U S A. 2011;108(1):302–307. doi: 10.1073/pnas.1016917108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen Y, Ramjiawan RR, Reiberger T, Ng MR, Hato T, Huang Y, Ochiai H, Kitahara S, Unan EC, Reddy TP, Fan C, Huang P, Bardeesy N, Zhu AX, Jain RK, Duda DG. CXCR4 inhibition in tumor microenvironment facilitates anti-programmed death receptor-1 immunotherapy in sorafenib-treated hepatocellular carcinoma in mice. Hepatology. 2015;61(5):1591–1602. doi: 10.1002/hep.27665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Loges S, Schmidt T, Carmeliet P. Mechanisms of resistance to anti-angiogenic therapy and development of third-generation anti-angiogenic drug candidates. Genes Cancer. 2010;1(1):12–25. doi: 10.1177/1947601909356574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8(8):592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Reiberger T, Chen Y, Ramjiawan RR, Hato T, Fan C, Samuel R, Roberge S, Huang P, Lauwers GY, Zhu AX, Bardeesy N, Jain RK, Duda DG. An orthotopic mouse model of hepatocellular carcinoma with underlying liver cirrhosis. Nat Protoc. 2015;10(8):1264–1274. doi: 10.1038/nprot.2015.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dirkx AE, Oude Egbrink MG, Wagstaff J, Griffioen AW. Monocyte/macrophage infiltration in tumors: modulators of angiogenesis. J Leukoc Biol. 2006;80(6):1183–1196. doi: 10.1189/jlb.0905495. [DOI] [PubMed] [Google Scholar]
- 129.Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, Yancopoulos GD, Wiegand SJ. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284(5422):1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 130.Rubenstein JL, Kim J, Ozawa T, Zhang M, Westphal M, Deen DF, Shuman MA. Anti-VEGF antibody treatment of glioblastoma prolongs survival but results in increased vascular cooption. Neoplasia. 2000;2(4):306–314. doi: 10.1038/sj.neo.7900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kuczynski EA, Yin M, Bar-Zion A, Lee CR, Butz H, Man S, Daley F, Vermeulen PB, Yousef GM, Foster FS, Reynolds AR, Kerbel RS. Co-option of Liver Vessels and Not Sprouting Angiogenesis Drives Acquired Sorafenib Resistance in Hepatocellular Carcinoma. J Natl Cancer Inst. 2016;108(8) doi: 10.1093/jnci/djw030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Frentzas S, Simoneau E, Bridgeman VL, Vermeulen PB, Foo S, Kostaras E, Nathan MR, Wotherspoon A, Gao ZH, Shi Y, Van den Eynden G, Daley F, Peckitt C, Tan X, Salman A, Lazaris A, Gazinska P, Berg TJ, Eltahir Z, Ritsma L, van Rheenen J, Khashper A, Brown G, Nystrom H, Sund M, Van Laere S, Loyer E, Dirix L, Cunningham D, Metrakos P, Reynolds AR. Vessel co-option mediates resistance to anti-angiogenic therapy in liver metastases. Nat Med. 2016;22(11):1294–1302. doi: 10.1038/nm.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jeong HS, Jones D, Liao S, Wattson DA, Cui CH, Duda DG, Willett CG, Jain RK, Padera TP. Investigation of the Lack of Angiogenesis in the Formation of Lymph Node Metastases. J Natl Cancer Inst. 2015;107(9) doi: 10.1093/jnci/djv155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sun B, Zhang D, Zhang S, Zhang W, Guo H, Zhao X. Hypoxia influences vasculogenic mimicry channel formation and tumor invasion-related protein expression in melanoma. Cancer Lett. 2007;249(2):188–197. doi: 10.1016/j.canlet.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 135.Sun B, Zhang S, Zhang D, Du J, Guo H, Zhao X, Zhang W, Hao X. Vasculogenic mimicry is associated with high tumor grade, invasion and metastasis, and short survival in patients with hepatocellular carcinoma. Oncol Rep. 2006;16(4):693–698. [PubMed] [Google Scholar]
- 136.Chen Y, Jing Z, Luo C, Zhuang M, Xia J, Chen Z, Wang Y. Vasculogenic mimicry-potential target for glioblastoma therapy: an in vitro and in vivo study. Med Oncol. 2012;29(1):324–331. doi: 10.1007/s12032-010-9765-z. [DOI] [PubMed] [Google Scholar]
- 137.van der Schaft DW, Hillen F, Pauwels P, Kirschmann DA, Castermans K, Egbrink MG, Tran MG, Sciot R, Hauben E, Hogendoorn PC, Delattre O, Maxwell PH, Hendrix MJ, Griffioen AW. Tumor cell plasticity in Ewing sarcoma, an alternative circulatory system stimulated by hypoxia. Cancer Res. 2005;65(24):11520–11528. doi: 10.1158/0008-5472.CAN-05-2468. [DOI] [PubMed] [Google Scholar]
- 138.van der Schaft DW, Seftor RE, Seftor EA, Hess AR, Gruman LM, Kirschmann DA, Yokoyama Y, Griffioen AW, Hendrix MJ. Effects of angiogenesis inhibitors on vascular network formation by human endothelial and melanoma cells. J Natl Cancer Inst. 2004;96(19):1473–1477. doi: 10.1093/jnci/djh267. [DOI] [PubMed] [Google Scholar]
- 139.Patan S, Munn LL, Jain RK. Intussusceptive microvascular growth in a human colon adenocarcinoma xenograft: a novel mechanism of tumor angiogenesis. Microvasc Res. 1996;51(2):260–272. doi: 10.1006/mvre.1996.0025. [DOI] [PubMed] [Google Scholar]
- 140.Djonov VG, Kurz H, Burri PH. Optimality in the developing vascular system: branching remodeling by means of intussusception as an efficient adaptation mechanism. Dev Dyn. 2002;224(4):391–402. doi: 10.1002/dvdy.10119. [DOI] [PubMed] [Google Scholar]
- 141.Hlushchuk R, Riesterer O, Baum O, Wood J, Gruber G, Pruschy M, Djonov V. Tumor recovery by angiogenic switch from sprouting to intussusceptive angiogenesis after treatment with PTK787/ZK222584 or ionizing radiation. Am J Pathol. 2008;173(4):1173–1185. doi: 10.2353/ajpath.2008.071131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kioi M, Vogel H, Schultz G, Hoffman RM, Harsh GR, Brown JM. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest. 2010;120(3):694–705. doi: 10.1172/JCI40283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jain RK, Duda DG. Role of bone marrow-derived cells in tumor angiogenesis and treatment. Cancer Cell. 2003;3(6):515–516. doi: 10.1016/s1535-6108(03)00138-7. [DOI] [PubMed] [Google Scholar]
- 144.Kodack DP, Chung E, Yamashita H, Incio J, Duyverman AM, Song Y, Farrar CT, Huang Y, Ager E, Kamoun W, Goel S, Snuderl M, Lussiez A, Hiddingh L, Mahmood S, Tannous BA, Eichler AF, Fukumura D, Engelman JA, Jain RK. Combined targeting of HER2 and VEGFR2 for effective treatment of HER2-amplified breast cancer brain metastases. Proc Natl Acad Sci U S A. 2012;109(45):E3119–3127. doi: 10.1073/pnas.1216078109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Erber R, Thurnher A, Katsen AD, Groth G, Kerger H, Hammes HP, Menger MD, Ullrich A, Vajkoczy P. Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J. 2004;18(2):338–340. doi: 10.1096/fj.03-0271fje. [DOI] [PubMed] [Google Scholar]
- 146.Motz GT, Coukos G. The parallel lives of angiogenesis and immunosuppression: cancer and other tales. Nat Rev Immunol. 2011;11(10):702–711. doi: 10.1038/nri3064. [DOI] [PubMed] [Google Scholar]
- 147.Liu XD, Hoang A, Zhou L, Kalra S, Yetil A, Sun M, Ding Z, Zhang X, Bai S, German P, Tamboli P, Rao P, Karam JA, Wood C, Matin S, Zurita A, Bex A, Griffioen AW, Gao J, Sharma P, Tannir N, Sircar K, Jonasch E. Resistance to Antiangiogenic Therapy Is Associated with an Immunosuppressive Tumor Microenvironment in Metastatic Renal Cell Carcinoma. Cancer Immunol Res. 2015;3(9):1017–1029. doi: 10.1158/2326-6066.CIR-14-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Batchelor TT, Gerstner ER, Emblem KE, Duda DG, Kalpathy-Cramer J, Snuderl M, Ancukiewicz M, Polaskova P, Pinho MC, Jennings D, Plotkin SR, Chi AS, Eichler AF, Dietrich J, Hochberg FH, Lu-Emerson C, Iafrate AJ, Ivy SP, Rosen BR, Loeffler JS, Wen PY, Sorensen AG, Jain RK. Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc Natl Acad Sci U S A. 2013;110(47):19059–19064. doi: 10.1073/pnas.1318022110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dings RP, Vang KB, Castermans K, Popescu F, Zhang Y, Oude Egbrink MG, Mescher MF, Farrar MA, Griffioen AW, Mayo KH. Enhancement of T-cell-mediated antitumor response: angiostatic adjuvant to immunotherapy against cancer. Clin Cancer Res. 2011;17(10):3134–3145. doi: 10.1158/1078-0432.CCR-10-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Meng MB, Zaorsky NG, Deng L, Wang HH, Chao J, Zhao LJ, Yuan ZY, Ping W. Pericytes: a double-edged sword in cancer therapy. Future Oncol. 2015;11(1):169–179. doi: 10.2217/fon.14.123. [DOI] [PubMed] [Google Scholar]
- 151.Winkler F, Kozin SV, Tong RT, Chae SS, Booth MF, Garkavtsev I, Xu L, Hicklin DJ, Fukumura D, di Tomaso E, Munn LL, Jain RK. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6(6):553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 152.Huang Y, Yuan J, Righi E, Kamoun WS, Ancukiewicz M, Nezivar J, Santosuosso M, Martin JD, Martin MR, Vianello F, Leblanc P, Munn LL, Huang P, Duda DG, Fukumura D, Jain RK, Poznansky MC. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci U S A. 2012;109(43):17561–17566. doi: 10.1073/pnas.1215397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pacholczyk R, Kern J. The T-cell receptor repertoire of regulatory T cells. Immunology. 2008;125(4):450–458. doi: 10.1111/j.1365-2567.2008.02992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206(13):3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhaufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crino L, Blumenschein GR, Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Aren Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, Castellano D, Choueiri TK, Gurney H, Donskov F, Bono P, Wagstaff J, Gauler TC, Ueda T, Tomita Y, Schutz FA, Kollmannsberger C, Larkin J, Ravaud A, Simon JS, Xu LA, Waxman IM, Sharma P, CheckMate I. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373(19):1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, Savage KJ, Hernberg MM, Lebbe C, Charles J, Mihalcioiu C, Chiarion-Sileni V, Mauch C, Cognetti F, Arance A, Schmidt H, Schadendorf D, Gogas H, Lundgren-Eriksson L, Horak C, Sharkey B, Waxman IM, Atkinson V, Ascierto PA. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 159.Ferris RL, Blumenschein G, Jr, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, Worden F, Saba NF, Iglesias Docampo LC, Haddad R, Rordorf T, Kiyota N, Tahara M, Monga M, Lynch M, Geese WJ, Kopit J, Shaw JW, Gillison ML. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med. 2016;375(19):1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, Plimack ER, Vaena D, Grimm MO, Bracarda S, Arranz JA, Pal S, Ohyama C, Saci A, Qu X, Lambert A, Krishnan S, Azrilevich A, Galsky MD. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017 doi: 10.1016/S1470-2045(17)30065-7. [DOI] [PubMed] [Google Scholar]
- 161.Yuan J, Gnjatic S, Li H, Powel S, Gallardo HF, Ritter E, Ku GY, Jungbluth AA, Segal NH, Rasalan TS, Manukian G, Xu Y, Roman RA, Terzulli SL, Heywood M, Pogoriler E, Ritter G, Old LJ, Allison JP, Wolchok JD. CTLA-4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit. Proc Natl Acad Sci U S A. 2008;105(51):20410–20415. doi: 10.1073/pnas.0810114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Anassi E, Ndefo UA. Sipuleucel-T (provenge) injection: the first immunotherapy agent (vaccine) for hormone-refractory prostate cancer. P T. 2011;36(4):197–202. [PMC free article] [PubMed] [Google Scholar]
- 163.Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, Dawson N, O’;Donnell PH, Balmanoukian A, Loriot Y, Srinivas S, Retz MM, Grivas P, Joseph RW, Galsky MD, Fleming MT, Petrylak DP, Perez-Gracia JL, Burris HA, Castellano D, Canil C, Bellmunt J, Bajorin D, Nickles D, Bourgon R, Frampton GM, Cui N, Mariathasan S, Abidoye O, Fine GD, Dreicer R. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chen J, Jiang CC, Jin L, Zhang XD. Regulation of PD-L1: a novel role of pro-survival signalling in cancer. Ann Oncol. 2016;27(3):409–416. doi: 10.1093/annonc/mdv615. [DOI] [PubMed] [Google Scholar]
- 166.Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying Cancers Based on T-cell Infiltration and PD-L1. Cancer Res. 2015;75(11):2139–2145. doi: 10.1158/0008-5472.CAN-15-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Dolan DE, Gupta S. PD-1 pathway inhibitors: changing the landscape of cancer immunotherapy. Cancer Control. 2014;21(3):231–237. doi: 10.1177/107327481402100308. [DOI] [PubMed] [Google Scholar]
- 168.Balar AV, Weber JS. PD-1 and PD-L1 antibodies in cancer: current status and future directions. Cancer Immunol Immunother. 2017 doi: 10.1007/s00262-017-1954-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chow LQ, Haddad R, Gupta S, Mahipal A, Mehra R, Tahara M, Berger R, Eder JP, Burtness B, Lee SH, Keam B, Kang H, Muro K, Weiss J, Geva R, Lin CC, Chung HC, Meister A, Dolled-Filhart M, Pathiraja K, Cheng JD, Seiwert TY. Antitumor Activity of Pembrolizumab in Biomarker-Unselected Patients With Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma: Results From the Phase Ib KEYNOTE-012 Expansion Cohort. J Clin Oncol. 2016 doi: 10.1200/JCO.2016.68.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Weber JS, O’Day S, Urba W, Powderly J, Nichol G, Yellin M, Snively J, Hersh E. Phase I/II study of ipilimumab for patients with metastatic melanoma. J Clin Oncol. 2008;26(36):5950–5956. doi: 10.1200/JCO.2008.16.1927. [DOI] [PubMed] [Google Scholar]
- 171.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, Waterfield W, Schadendorf D, Smylie M, Guthrie T, Jr, Grob JJ, Chesney J, Chin K, Chen K, Hoos A, O’Day SJ, Lebbe C. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11(2):155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 173.Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, Davidson N, Richards J, Maio M, Hauschild A, Miller WH, Jr, Gascon P, Lotem M, Harmankaya K, Ibrahim R, Francis S, Chen TT, Humphrey R, Hoos A, Wolchok JD. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 174.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Grosso JF, Jure-Kunkel MN. CTLA-4 blockade in tumor models: an overview of preclinical and translational research. Cancer Immun. 2013;13:5. [PMC free article] [PubMed] [Google Scholar]
- 176.Garrido F, Aptsiauri N, Doorduijn EM, Garcia Lora AM, van Hall T. The urgent need to recover MHC class I in cancers for effective immunotherapy. Curr Opin Immunol. 2016;39:44–51. doi: 10.1016/j.coi.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Thibodeau J, Bourgeois-Daigneault MC, Lapointe R. Targeting the MHC Class II antigen presentation pathway in cancer immunotherapy. Oncoimmunology. 2012;1(6):908–916. doi: 10.4161/onci.21205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Fruci D, Benevolo M, Cifaldi L, Lorenzi S, Lo Monaco E, Tremante E, Giacomini P. Major histocompatibility complex class i and tumour immuno-evasion: how to fool T cells and natural killer cells at one time. Curr Oncol. 2012;19(1):39–41. doi: 10.3747/co.19.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hammerstrom AE, Cauley DH, Atkinson BJ, Sharma P. Cancer immunotherapy: sipuleucel-T and beyond. Pharmacotherapy. 2011;31(8):813–828. doi: 10.1592/phco.31.8.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gilbert SC. T-cell-inducing vaccines – what’s the future. Immunology. 2012;135(1):19–26. doi: 10.1111/j.1365-2567.2011.03517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Goyvaerts C, Breckpot K. Pros and Cons of Antigen-Presenting Cell Targeted Tumor Vaccines. J Immunol Res. 2015;2015:785634. doi: 10.1155/2015/785634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Small EJ, Fratesi P, Reese DM, Strang G, Laus R, Peshwa MV, Valone FH. Immunotherapy of hormone-refractory prostate cancer with antigen-loaded dendritic cells. J Clin Oncol. 2000;18(23):3894–3903. doi: 10.1200/JCO.2000.18.23.3894. [DOI] [PubMed] [Google Scholar]
- 183.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12(4):265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, Verjee SS, Jones LA, Hershberg RM. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24(19):3089–3094. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 185.Pitt JM, Vetizou M, Daillere R, Roberti MP, Yamazaki T, Routy B, Lepage P, Boneca IG, Chamaillard M, Kroemer G, Zitvogel L. Resistance Mechanisms to Immune-Checkpoint Blockade in Cancer: Tumor-Intrinsic and -Extrinsic Factors. Immunity. 2016;44(6):1255–1269. doi: 10.1016/j.immuni.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 186.Hellwig SM, Damen CA, van Adrichem NP, Blijham GH, Groenewegen G, Griffioen AW. Endothelial CD34 is suppressed in human malignancies: role of angiogenic factors. Cancer Lett. 1997;120(2):203–211. doi: 10.1016/s0304-3835(97)00310-8. [DOI] [PubMed] [Google Scholar]
- 187.Griffioen AW, Damen CA, Blijham GH, Groenewegen G. Endoglin/CD 105 may not be an optimal tumor endothelial treatment target. Breast Cancer Res Treat. 1996;39(2):239–242. doi: 10.1007/BF01806191. [DOI] [PubMed] [Google Scholar]
- 188.Griffioen AW, Damen CA, Martinotti S, Blijham GH, Groenewegen G. Endothelial intercellular adhesion molecule-1 expression is suppressed in human malignancies: the role of angiogenic factors. Cancer Res. 1996;56(5):1111–1117. [PubMed] [Google Scholar]
- 189.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10(8):858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 190.Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, Gimotty PA, Gilks CB, Lal P, Zhang L, Coukos G. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475(7355):226–230. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 191.Chen Y, Liu YC, Sung YC, Ramjiawan RR, Lin TT, Chang CC, Jeng KS, Chang CF, Liu CH, Gao DY, Hsu FF, Duyverman AM, Kitahara S, Huang P, Dima S, Popescu I, Flaherty KT, Zhu AX, Bardeesy N, Jain RK, Benes CH, Duda DG. Overcoming sorafenib evasion in hepatocellular carcinoma using CXCR4-targeted nanoparticles to co-deliver MEK-inhibitors. Sci Rep. 2017;7:44123. doi: 10.1038/srep44123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211(5):781–790. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hato T, Goyal L, Greten TF, Duda DG, Zhu AX. Immune checkpoint blockade in hepatocellular carcinoma: current progress and future directions. Hepatology. 2014;60(5):1776–1782. doi: 10.1002/hep.27246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Li B, Lalani AS, Harding TC, Luan B, Koprivnikar K, Huan Tu G, Prell R, VanRoey MJ, Simmons AD, Jooss K. Vascular endothelial growth factor blockade reduces intratumoral regulatory T cells and enhances the efficacy of a GM-CSF-secreting cancer immunotherapy. Clin Cancer Res. 2006;12(22):6808–6816. doi: 10.1158/1078-0432.CCR-06-1558. [DOI] [PubMed] [Google Scholar]
- 195.Huang KW, Wu HL, Lin HL, Liang PC, Chen PJ, Chen SH, Lee HI, Su PY, Wu WH, Lee PH, Hwang LH, Chen DS. Combining antiangiogenic therapy with immunotherapy exerts better therapeutical effects on large tumors in a woodchuck hepatoma model. Proc Natl Acad Sci U S A. 2010;107(33):14769–14774. doi: 10.1073/pnas.1009534107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hodi FS, Lawrence D, Lezcano C, Wu X, Zhou J, Sasada T, Zeng W, Giobbie-Hurder A, Atkins MB, Ibrahim N, Friedlander P, Flaherty KT, Murphy GF, Rodig S, Velazquez EF, Mihm MC, Jr, Russell S, DiPiro PJ, Yap JT, Ramaiya N, Van den Abbeele AD, Gargano M, McDermott D. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol Res. 2014;2(7):632–642. doi: 10.1158/2326-6066.CIR-14-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wallin JJ, Bendell JC, Funke R, Sznol M, Korski K, Jones S, Hernandez G, Mier J, He X, Hodi FS, Denker M, Leveque V, Canamero M, Babitski G, Koeppen H, Ziai J, Sharma N, Gaire F, Chen DS, Waterkamp D, Hegde PS, McDermott DF. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat Commun. 2016;7:12624. doi: 10.1038/ncomms12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Harrison RK. Phase II and phase III failures: 2013–2015. Nat Rev Drug Discov. 2016;15(12):817–818. doi: 10.1038/nrd.2016.184. [DOI] [PubMed] [Google Scholar]
- 199.Seruga B, Ocana A, Amir E, Tannock IF. Failures in Phase III: Causes and Consequences. Clin Cancer Res. 2015;21(20):4552–4560. doi: 10.1158/1078-0432.CCR-15-0124. [DOI] [PubMed] [Google Scholar]
- 200.Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26(5):605–622. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Jain RK, Martin JD, Stylianopoulos T. The role of mechanical forces in tumor growth and therapy. Annu Rev Biomed Eng. 2014;16:321–346. doi: 10.1146/annurev-bioeng-071813-105259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Weiss A, Nowak-Sliwinska P. Current Trends in Multidrug Optimization. J Lab Autom. 2016 doi: 10.1177/2472630316682338. 2472630316682338. [DOI] [PubMed] [Google Scholar]
- 203.Nowak-Sliwinska P, Weiss A, Ding X, Dyson PJ, van den Bergh H, Griffioen AW, Ho CM. Optimization of drug combinations using Feedback System Control. Nat Protoc. 2016;11(2):302–315. doi: 10.1038/nprot.2016.017. [DOI] [PubMed] [Google Scholar]


