Abstract
In this study, polyethylene glycol (PEG)ylated 10-hydroxycamptothecin (mPEG1000-HCPT) was synthesized and used as a stabilizer to prepare 10-hydroxycamptothecin (HCPT) nanosuspensions for their in vitro and in vivo antitumor investigation. The resultant HCPT nanosuspensions (HCPT-NSps) had a very high drug payload of 94.90% (w/w) and a mean particle size of 92.90±0.20 nm with narrow size distribution (polydispersity index of 0.16±0.01). HCPT-NSps could be lyophilized without the need of the addition of any cryoprotectant and then be reconstituted into nanosuspensions of a similar size by direct resuspension in water. HCPT was in crystalline form in HCPT-NSps. Using mPEG1000-HCPT as stabilizer, insoluble camptothecin and 7-ethyl-10-hydroxycamptothecin could also be easily made into nanosuspensions with similar features such as high drug payload, small particle size, and cryoprotectant-free freeze drying. The 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide assay indicated that the HCPT-NSps had a significantly higher cytotoxicity than HCPT injections, with 3.77 times lower IC50 value against HepG2 cells and 14.1 times lower IC50 value against MCF-7 cells. An in vivo study in H22 tumor-bearing mice after intravenous injection of HCPT-NSps demonstrated that HCPT-NSps significantly improved the antitumor efficacy compared to the commercially available HCPT injections (86.38% vs 34.97%) at the same dose of 5 mg/kg. Even at 1/4 of the dose, HCPT-NSps could also achieve a similar antitumor efficacy to that of HCPT injections. mPEG1000-HCPT may be a highly efficient stabilizer able to provide camptothecin-based drugs, and probably other antitumor agents containing aromatic structure, with unique nanosuspensions or nanocrystals for improved in vivo therapeutic efficacy.
Keywords: 10-hydroxycamptothecin, nanosuspensions, stabilizer, antitumor efficacy
Introduction
Camptothecin (CPT) and related analogs are promising antitumor agents that target the nuclear enzyme topoisomerase I, inhibit the relegation of the cleaved DNA strand, and lead to the death of tumor cells. 10-Hydroxycamptothecin (HCPT), a natural CPT analog that is more potent and less toxic, has recently undergone extensive evaluation worldwide. However, its poor water solubility and the inner instability induced by opening the labile lactone ring at physiological pH prevent HCPT from having an effective clinical application.1 Nanoscale drug-delivery systems are widely used to solve such problems, including polymeric micelles,2–4 nanoemulsions,5 and nanoparticles.6–10 However, their drug-loading content as reported so far is unsatisfactory (most <15%, seldom >25%), and there are other limitations such as burst release and adverse effects induced by excipients.
Nanosuspensions are nanosized drug-delivery systems consisting essentially of pure drug nanoparticles with minimal surfactants and/or polymeric materials for stabilization.11–14 The large specific surface areas and high drug payload make nanosuspensions one of the most effective methods to resolve the dissolution problem for insoluble drugs. However, this advantage of high drug payload has been offset to some extent because nearly all nanosuspensions have to be lyophilized to improve their storage stability, resulting in the need for various protectants added to the nanosuspensions and great decrease of the actual drug payload in the final product. The drug-loading contents of HCPT nanosuspensions (HCPT-NSps) that have been reported thus far have not been greater than 60%, and the insufficient effectiveness of the existing stabilizers is believed to be one of the main reasons.
Inspired by the well-known fact that a compound can be easily dissolved in a solvent of similar polarity, we spectulate that nanosuspensions may be compatible with or well stabilized by amphiphilic stabilizers whose hydrophobic block is similar in polarity and/or structure to the encapsulated hydrophobic drugs. This result may occur because the hydrophobic part of this kind of stabilizer may firmly bind to the surface of the core drug crystals during preparation. The greater the similarity, the better the compatibility or stabilizing effect. It can be easily deduced that the best stabilizing effect may be achieved when the polyethylene glycol (PEG)ylated conjugate of the hydrophobic drug itself was used as stabilizer.
In this report, PEGylated 10-hydroxycamptothecin (mPEG1000-HCPT) was designed and synthesized to prepare HCPT-NSps with a high drug payload and good in vitro and in vivo behaviors. The resultant HCPT-NSps achieved a very high drug payload of 94.90% (w/w) that could be directly lyophilized without the need of addition of any cryoprotectant and then be easily reconstituted into nanosuspensions of similar size. The obtained HCPT-NSps exhibited sustained drug release and superior in vivo antitumor efficacy.
For hydrophobic drugs of small molecules, PEGylation is an efficient way to improve their solubility, increase their plasma half-life and, in most cases, enhance their therapeutic efficacy.15–17 PEGylated CPT18–20 or mPEG1000-HCPT21 have been synthesized and evaluated in vitro and in vivo. So far, only a few studies have used a PEGylated drug as carrier to further encapsulate drugs, but the drug payload was limited (<20%) in the resultant liposome-like structure.22 To the best of our knowledge, this report is the first to use a PEGylated drug as a stabilizer for the nanosuspension preparation of the drug itself. The results of this study should also provide new insight into the preparation of nanosuspensions and the delivery of small molecular hydrophobic drugs to tumor sites for antitumor effect enhancement and toxicity reduction.
Material and methods
Materials
α-Methoxy-ω-carboxylic acid poly(ethyleneglycol) (mPEG1000-COOH) (molecular weight [MW] 1 kDa) was purchased from Biomatrik Co. Ltd., Zhejiang, People’s Republic of China. HCPT, 7-ethyl-10-hydroxycamptothecin, and CPT (>99.5% purity) were obtained from Aladdin Co. Ltd., Shanghai, People’s Republic of China. HCPT injections (hydroxycamptothecin sodium salt solution) were supplied by Shenghe Pharm. Ltd. (Szechwan, People’s Republic of China). Dimethyl formamide (DMF, ACS grade) and acetonitrile (high performance liquid chromatography [HPLC] grade) were provided by Fisher Scientific (Pittsburgh, PA, USA). GPT/GOT/Cr/BUN kits were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, People’s Republic of China). Deionized water was used in the experiment, and all of the other reagents were of analytical grade.
Animals and tumor cells
ICR male mice (20±1 g) were purchased from the Vital River Laboratory Animal Technology Co., Ltd, Beijing, People’s Republic of China. The animals were provided with standard diet ad libitum and were acclimatized for at least 1 week before experimentation. All of the animal experiments were approved by the Ethics Committee of Institute of Medicinal Plant Development (IMPLAD) and performed in accordance with the Regulations for Animal Experiments and Guidelines for Ethics as defined by the Institute of Medicinal Plant Development (Beijing, People’s Republic of China). The cells that were used in the experiment were bought from Cell Culture Center, Institute of Basic Medical Sciences, the Chinese Academy of Medical Science (Beijing, People’s Republic of China) and were grown in Roswell Park Memorial Institute-1640 medium (Gibco, St Louis, MO, USA) with 10% fetal calf serum (Gibco), 100 units/mL streptomycin and penicillin G at 37°C in 5% CO2.
Synthesis and characterization of mPEG1000-HCPT
mPEG1000-HCPT was synthesized according to the method described by Hong et al.21 Briefly, 1-ethyl-3,(3-di-methylaminopropyl carbodiimide) hydrochloride (0.14 g, 0.40 mmol) and 1,4-(dimethylamino) pyridine (6.60 mg, 0.05 mmol) were added to a solution of HCPT (0.15 g, 0.40 mmol) and mPEG1000-COOH (0.20 mg, 0.20 mmol) in 30 mL of anhydrous N,N-dimethyl formamide at 0°C under an argon atmosphere. The mixture was stirred in an ice bath for 4 h and then at room temperature for 24 h. The reaction mixture was poured into 200 mL of 5% NaHCO3 to remove excess HCPT, extracted by dichloromethane (3×200 mL), and washed with 5% NaHCO3 (3×300 mL), water (2×300 mL), 0.1 mol/L HCl (2×300 mL), and saturated aqueous NaCl (2×300 mL) in turn. The organic phase was dried over anhydrous Mg2SO4, filtered, concentrated under reduced pressure, and recrystallized from ether to obtain mPEG1000-HCPT conjugate as a white solid. A 300-MHz proton nuclear magnetic resonance (NMR) spectrometer (Bruker, Karlsruhe, Baden-Wuerttemberg, Germany; Mercury plus 300 MHz spectrometer) was used for 1H NMR measurements in dimethyl sulfoxide (DMSO) to determine the chemical structure and composition of mPEG1000-HCPT. 1H NMR (300 MHz, DMSO): 1H NMR (300 MHz, DMSO) δ=8.69 (s, 1H, H-7), 8.22 (d, 1H, H-12), 7.90 (d, 1H, H-11), 7.65 (s, 1H, H-7), 7.39 (s, 1H, H-14), 5.42 (d, 2H, H-17), 5.26 (s, 2H, H-5), 3.23–3.83 (m, 122H, –OCH2CH2), 2.92 (t, 2H, CH2CO), 1.87 (m, 2H, H-19), 0.88 (m, 3H, H-18).
Autoflex (Bruker, Karlsruhe, Baden-Wuerttemberg, Germany) was used to perform matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS). A nitrogen laser emitting at 355 nm and a saturated solution of α-cyano-4-hydroxycinnamic acid in tetrahydrofuran were used as the matrix for the experiment. Mass spectra were acquired in positive reflector mode with an acceleration voltage of 20 kV.
The product purity was determined by HPLC assay. The product was dissolved in methanol as a stock solution. The stock solution was divided into two parts. One part was hydrolyzed in 1 mol/L HCl at 50°C for 4 h to release HCPT from mPEG1000-HCPT and then analyzed by HPLC to determine the total HCPT in the product. The other part was directly detected by HPLC to measure the free HCPT in the product.
Preparation and characterization of HCPT-NSps
Preparation of HCPT-NSps
HCPT-NSps were prepared by a combination of precipitation and high-pressure homogenization. Briefly, 50 mg of HCPT and 2.5 mg of mPEG1000-HCPT were dissolved in 0.5 mL of DMF. The resulting organic solution was slowly injected into 5 mL of distilled water under ultrasonication at 4°C (bath sonicator, 250 W, 40 HZ). The mixture was then centrifuged at 13,000 rpm for 20 min. Then, the collected sediment was redispersed in 5 mL of distilled water under ultrasonication (bath sonicator, 250 W, 40 Hz) at 4°C for 2 min, followed by homogenization using a JN-3000 PLUS homogenizer (NBIO Inc., Guangzhou, Guangdong province, People’s Republic of China; the optimal homogenization conditions were 2,000 bar, 4°C and 12 cycles). Drug payload or drug loading content is calculated by the following equation: (weight of the drug in nanoparticles/weight of the nanoparticles) ×100%. In the experiment, the weight of 1 mL of the lyophilized nanosuspensions was measured, and 1 mL of methanol was added to disintegrate the nanosuspensions and release encapsulated HCPT, which was then analyzed by HPLC to determine the actual drug payload.
Particle size analysis and zeta potential (ZP) measurement
The mean particle size and polydispersity index (PDI) of the HCPT-NSps were determined by dynamic light scattering (DLS) with a Malvern Nano-ZS Zetasizer (Malvern Instruments, Malvern, UK). The ZP was also measured with a Malvern Nano-ZS Zetasizer. Prior to the measurement, the samples were diluted with distilled water to a suitable scattering intensity. Both particle size and zeta potential were determined in triplicate for a single batch of nanosuspensions, and each measurement was performed with 12 runs (10 s duration between adjacent runs) at 25°C.
Transmission electron microscopy (TEM)
A morphological examination of HCPT-NSps was conducted using JEM-1400 TEM (JEM1400; JEOL Ltd., Akishima, Tokyo, Japan). One drop of HCPT-NSps was placed on a 300-mesh copper grid, air-dried, and stained with phosphotungstic acid solution (1% w/v).
X-ray diffraction (XRD) measurement
The HCPT bulk powders, lyophilized HCPT-NSps, and physical mixture of HCPT and mPEG1000-HCPT were, respectively, evaluated by an X-ray diffractometer (DX-2700; FangYuan Instrument co., Ltd, Dandong, Liaoning Provinve, People’s Republic of China) using Cu-k-alpha radiation (1.5406 Å, 40 kV, 100 mA) with a 0.01° step size and a radiation range of 3°–80° of 2θ.
For XRD assay, the physical mixture was made just by mixing mPEG1000-HCPT powder and HCPT powder together (in the same weight ratio as in HCPT-NSps) in a small vial followed by vortexing for 1 min.
Lyophilization and stability test
To enhance the chemical and physical stability, HCPT-NSps were lyophilized for storage and subsequent use. Briefly, HCPT-NSps were rapidly cooled to −80°C for 12 h and then freeze-dried in an LGJ-10B freeze dryer (Sihuan Laboratory Instruments Ltd, Chengdu, Sichuan Province, People’s Republic of China) under vacuum (pressure <10 pa) for 24 h. No cryoprotectant addition was needed for this procedure.
To study their stability in 5% glucose solution, HCPT-NSps were mixed with the same volume of 10% glucose solution, kept at 37°C, followed by size determination at different time intervals using a Malvern Nano-ZS Zetasizer (Malvern Instruments). For stability test in plasma, HCPT-NSps in 5% glucose (10 mg/mL nanosuspensions) were mixed with four times volume of blank plasma and incubated at 37°C for 12 h, followed by size determination at different time intervals. For hemolysis test, HCPT-NSps in 5% glucose were mixed with the same volume of 4% RBC and incubated at 37°C for 12 h. The mixture was centrifuged at 3,000 rpm for 10 min and the optical density of supernatant at 570 nm was measured using a microplate reader with 5% glucose solution as negative control and distilled water as blank control. The hemolysis rate (%) was calculated by the following equation: = (1 − ODexperimental group/ODblank group) ×100.
Preparation of nanosuspensions for other drugs using mPEG1000-HCPT
Aliquots of 50 mg of 7-ethyl-10-hydroxycamptothecin or CPT and 2.5 mg of mPEG1000-HCPT were dissolved in 0.5 mL of DMF. The organic solution obtained was slowly injected into 5 mL of distilled water under ultrasonication at 4°C. The mixture was then centrifuged at 13,000 rpm for 20 min. Then, the collected sediment was redispersed in 5 mL of distilled water under ultrasonication (bath sonicator, 250 W, 40 Hz) at 4°C for 2 min, followed by homogenization using a JN-3000 PLUS homogenizer (NBIO Inc., Guangzhou, Guangdong province, People’s Republic of China; the optimal homog-enization conditions were 2,000 bar, 4°C, and 12 cycles).
Both the drug payload and particle size of 7-ethyl-10-hydroxycamptothecin or CPT nanosuspensions were determined by the HCPT nanosuspension method.
The 7-ethyl-10-hydroxycamptothecin or CPT nanosuspensions were rapidly cooled to −80°C for 12 h, freeze-dried in an LGJ-10B freeze-dryer (Sihuan Laboratory Instruments Ltd, Chengdu, Sichuan Province, People’s Republic of China) under vacuum (pressure <10 pa) for 24 h and then redispersed in distilled water for storage for 0, 20, 40, 80, and 120 days. No cryoprotectant addition was needed for this procedure.
HPLC analysis
The concentrations of HCPT and mPEG1000-HCPT were analyzed by HPLC (Ultimate 3000; DIONEX, Sunnyvale, CA, USA) using a C18 column (5 μm, 250×4.6 mm, Waters Symmetry, Milford, MA, USA). The purity of mPEG1000-HCPT and HCPT was assessed by HPLC chromatography using UV detection at 368 nm with a mobile phase of acetonitrile/aqueous triethylamine-acetate buffer (35/65, v/v, pH 5.5) at a flow rate of 0.55 mL/min. In order to make HPLC analysis for the in vitro drug release, the detection wavelength was set at 368 nm (UV detector; DIONEX), and the mobile phase was a 28:72 (v/v) mixture of acetonitrile and ammonium acetate (0.075 mol/L, pH 6.4) with a flow rate of 0.8 mL/min at 30°C.
In vitro drug release study
The in vitro release of HCPT from the nanosuspensions was evaluated using dialysis bag diffusion method with HCPT injections and coarse HCPT suspensions as control. The coarse HCPT suspensions were made by directly dispersing HCPT bulk powder in phosphate-buffered saline (PBS) and by adjusting the final concentration to 0.2 mg/mL HCPT. Commercially available HCPT injections were diluted with PBS to a concentration of 0.2 mg/mL HCPT; HCPT-NSps were also diluted with PBS to a concentration of 0.2 mg/mL HCPT. Here PBS means phosphate buffered solution (50 mM, pH 7.4) with no addition of sodium chloride. Then, 1 mL of HCPT-NSps or HCPT injections containing 200 μg of HCPT was placed into a pre-swelled dialysis bag (MWCO =3,500 Da) and immersed into 100 mL of 0.1 mol/L PBS (pH 7.4) at 37°C with gentle agitation (100 rpm). Periodically, 1 mL of the release medium was withdrawn for HPLC analysis, and then 1 mL fresh PBS was added to the system. All of the assays were performed in triplicate.
Cytotoxicity assay and cellular uptake
The in vitro cytotoxicity was evaluated by 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) colorimetric assay. In brief, HepG2 cells or MCF-7 cells in the logarithm growth period were seeded into a 96-well tissue culture plate at a density of 4×104 cells/well for 24 h in a humidified atmosphere of 5% CO2 at 37°C. The medium was then replaced with 200 μL HCPT-NSps or HCPT injections, and the plates were incubated for 24 h. Then, 20 μL of 5 mg/mL MTT in PBS was added to each well and the plate was incubated for 4 h. The medium was then replaced with 200 μL of DMSO, and the OD at 570 nm was measured using a microplate reader (BioTek, Winooski, VT, USA).
The cellular uptake was performed using HepG2 cells by visualization with fluorescence microscopy. Briefly, the cells at logarithmic phase were added to 24-well plates (2×105 cells/well) and incubated at 37°C to allow cell attachment. After 24 h of incubation, 1 mL HCPT injections or HCPT-NSps of different concentrations (5 μg/mL, 25 μg/mL, or 50 μg/mL, diluted with media without FBS) were added in the wells, and the plates were incubated for 4 h in 5% CO2 at 37°C. Then the colloidal dispersion was removed after gentle aspiration, and the cells were rinsed four times with pre-warmed PBS (pH 7.4). Cell fixation was achieved by dipping in 4% formaldehyde solution for 20 min at room temperature. Visualization study was conducted using Delta Vision Microscopy Imaging Systems (GE Healthcare Life Sciences, Marlborough, MA, USA). The uptake of HCPT by HepG2 cells was observed in DAPI Channel (λex=390 nm and λem =435 nm). The transmittance was 10% while the exposure time was 0.08 s.
In vivo antitumor effect
To determine the therapeutic advantage of HCPT-NSps, the in vivo antitumor effect was investigated in H22 tumor-bearing mice. Six groups were used after subcutaneously implanting H22 cells into the armpits of the mice (eight mice in each group). The blank control group received 0.2 mL of normal saline, the stabilizer group was administered with mPEG1000-HCPT solution in normal saline (with the same concentration as in HCPT-NSps), and the positive control group received 5 mg/kg of HCPT injections, all administrated by intravenous injection through the tail vein. The three tested groups were intravenously administrated with HCPT-NSps at 1.25 mg/kg, 2.5 mg/kg, and 5 mg/kg, respectively, every 3 days for 7 days. The body weights of the mice and the tumor volumes were monitored throughout the experiment. After treatment, the mice were sacrificed; the tumors were removed and weighed. The tumor inhibitory rate (TIR) was calculated as follows: TIR (%) = (1 − average tumor weight of the treated group/average tumor weight of the blank group) ×100.
At the end of the in vivo antitumor experiment, the tumors, livers, and kidneys were collected from the sacrificed mice. Dissected tissues were fixed in 10% phosphate buffered formaldehyde and subsequently embedded in paraffin. The samples were cut into 5 μm thick sections, mounted on glass slides, and deparaffinized. The slides were stained with Prussian blue with hematoxylin and eosin (H&E) counterstaining. Femurs were fixed with 70% ethanol and embedded in methylmetacrylate. The plastic-embedded, demineralized bone samples were cut into 5 μm thick sections and mounted on glass slides. Bone samples were stained with Prussian blue or Prussian blue with H&E counterstaining.
Results and discussion
Synthesis of mPEG1000-HCPT conjugate
Because free HCPT is relatively easy to separate from mPEG1000-HCPT conjugate, excess HCPT was used in the reaction to consume as much mPEG1000-COOH as possible. There are two hydroxyl groups in the structure of HCPT: one aromatic (10-OH) and one aliphatic (20-OH). Although the 20-OH could be grafted by PEG derivatives,18–23 its reactivity is much less than 10-OH due to the steric hindrance.24
The HCPT spot by TLC plate was yellow at 365 nm. According to the research by He et al,25 if the PEG conjugation site was at 10-OH, the resultant PEGylated HCPT would present a blue spot by TLC at 365 nm. The same phenomenon was observed in our study, demonstrating that our PEGylation site was also at 10-OH. However, when acylated at 20-OH (aliphatic hydroxyl), there was no effect on the aromatic system of HCPT, and the TLC spot of the resultant conjugate remained unchanged at 365 nm.
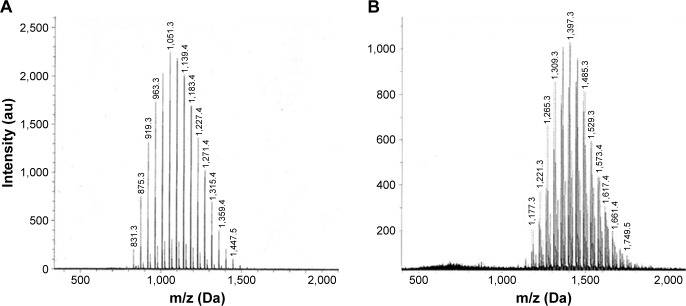
Figure 1 shows the MALDI-TOF mass spectra of mPEG1000-COOH and the synthesized mPEG1000-HCPT, which gave a convenient confirmation of the formation of the polydisperse polymer conjugates.21 Because the mw of HCPT is 364 Da, if the synthesis of mPEG1000-HCPT is successful, the MW of the resultant mPEG1000-HCPT conjugate should theoretically increase by 346 (364−18) compared to the MW of mPEG1000-COOH. As seen in Figure 1, a comparison of MALDI spectra of mPEG1000-COOH (average MW =1,051.3 Da) with mPEG1000-HCPT (average MW =1,397.3 Da) revealed a shift of just 346 Da; this demonstrates that the synthesis of mPEG1000-HCPT was successful.
Figure 1.
The MALDI-TOF-MS spectra of mPEG1000-COOH (A) and mPEG1000-HCPT (B).
Notes: The average molecular weight of mPEG1000-COOH was shown to be 1,051.3 Dalton, while the average molecular weight of mPEG1000-HCPT was 1,397.3 Dalton, with the subtraction of 346 Dalton.
Abbreviations: HCPT, 10-hydroxycamptothecin; MALDI-TOF-MS, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry; PEG, polyethylene glycol.
The final product mPEG1000-HCPT conjugate showed a single oval spot on TLC. HPLC chromatograms demonstrated that the obtained mPEG1000-HCPT conjugate was a single peak (Figure S1B, the retention time [RT] being 13.2 min) with no free HCPT left (the RT being 9.50 min, as seen in Figure S1A). The hydrolysis of mPEG1000-HCPT in combination with HPLC detection further demonstrated the success of the synthesis of this goal conjugate. After hydrolysis in 1 mol/L HCl at 50°C, mPEG1000-HCPT could slowly release free HCPT, whose HPLC chromatogram contained the peaks of both free HCPT and mPEG1000-HCPT (Figure S1C). After 4 h of hydrolysis, the peak for mPEG1000-HCPT completely disappeared, and HPLC could detect only free HCPT in the hydrolysis solution (Figure S1D). The HPLC analysis confirmed that the purity of mPEG1000-HCPT was greater than 99.57% and the yield of the mPEG1000-HCPT was calculated to be 72.56%.
Preparation and characterization of HCPT-NSps
Characteristic particle size analysis and zeta potential measurement
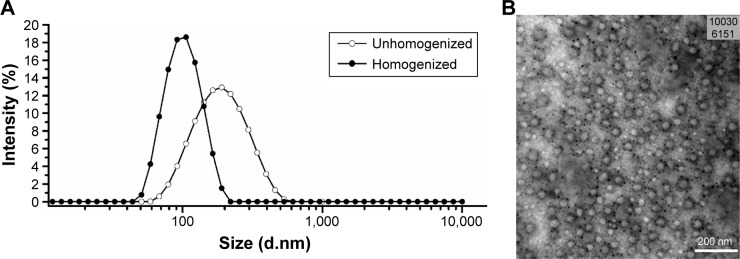
Figure 2A shows the mean particle size and PDI of the HCPT-NSps. The mean particle size and the PDI of the homogenized nanosuspensions were 92.90 nm and 0.158 using mPEG1000-HCPT as a stabilizer. Low PDI indicates that the distribution of the particle size was very narrow. In our study, the mean ZP of the resultant HCPT-NSps was −32.5 mV, which shows the good physical stability of HCPT-NSps.26,27
Figure 2.
Characterization of HCPT-NSps.
Notes: (A) The particle size distribution of unhomogenized and homogenized HCPT-NSps determined by dynamic light scattering. The average particle size of unhomogenized HCPT-NSps was 170.15±0.3 nm with PDI value being 0.14±0.01, while the homogenized nanosuspensions had smaller size of 92.90±0.2 nm and a little higher PDI value of 0.16±0.01. (B) The micrograph of HCPT-NSps observed by transmission electron microscopy. The small dark punctated spots observed are due to phosphotungstic acid staining.
Abbreviations: HCPT, 10-hydroxycamptothecin; NSps, nanosuspensions; PDI, polydispersity index.
In our preliminary study, in addition to mPEG1000-HCPT, mPEG2000-HCPT conjugate was also synthesized and used as a stabilizer to prepare HCPT-NSps, targeting smaller size and better stability. However, the HCPT-NSps obtained displayed only a small increase in particle size and worse stability (increased size) when stored at 4°C and room temperature. It seems longer PEG segment may not bring more beneficial effect on HCPT-NSps. The reason may be that, like other surfactants, a stabilizer needs comparable size of hydrophobic and hydrophilic segments to achieve good stabilizing effect. Since HCPT is a small molecule with MW of only 364 Da, the MW of PEG segment of mPEG1000-HCPT should not be too big for good amphipathy and using as a stabilizer. Thus, mPEG1000-HCPT was selected as a stabilizer to prepare HCPT nanosuspensions.
Drug-loading content of HCPT-NSps
In our experiment, for 50 mg of HCPT, 2.5 mg of mPEG1000-HCPT was used to prepare HCPT-NSps, and no other excipients were used; thus, the theoretical drug payload was equal to 50/(50+2.5) ×100% =95.23% (w/w). The actual drug payload as determined by HPLC was identical (94.90%). To the best of our knowledge, such a high drug payload has seldom been reported for lyophilized nanosuspensions due to the insufficient effectiveness of the existing stabilizer. The so-far reported drug-loading contents for HCPT-NSps are seldom greater than 60%.15–17 To the best of our knowledge, this is the first report to use a PEGylated HCPT as a stabilizer for the preparation of HCPT-NSps. Due to the total similarity of the drug to the hydrophobic part of the amphiphilic stabilizer, mPEG1000-HCPT may firmly bind to the surface of the core drug crystals with the mPEG1000 chain extending outward to form a hydrophilic layer (shell), thereby possessing high stabilizing efficiency and much less stabilizer consumption.
Morphology study
Figure 2B shows a TEM micrograph of the HCPT-NSps. The TEM micrograph indicated that the nanosuspensions were smaller than observed by DLS examination due to particle shrinkage caused by the drying process,28 and all the nanosuspensions had a near-spherical shape with minor deformation. In the micrograph, small dark punctated spots that were formed due to phosphotungstic acid staining were observed.
XRD measurement
Figure 3 shows the XRD patterns for the HCPT bulk powders, physical mixture, and lyophilized nanosuspensions. The diffraction patterns of the HCPT bulk powders and physical mixture showed characteristic high-energy diffraction peaks at 6.9°, 9.34°, 11.63°, 13.85°, and 25.61°, indicating the crystalline structure of HCPT. For the lyophilized nanosuspensions, the diffraction pattern was consistent with that of the bulk powders, suggesting that HCPT also existed in crystalline form in the HCPT-NSps obtained, and this result was different from those found in the literature.21,26 This result is possibly due to the high structural similarity of the hydrophobic segment of the stabilizer to the aromatic structure of HCPT.
Figure 3.
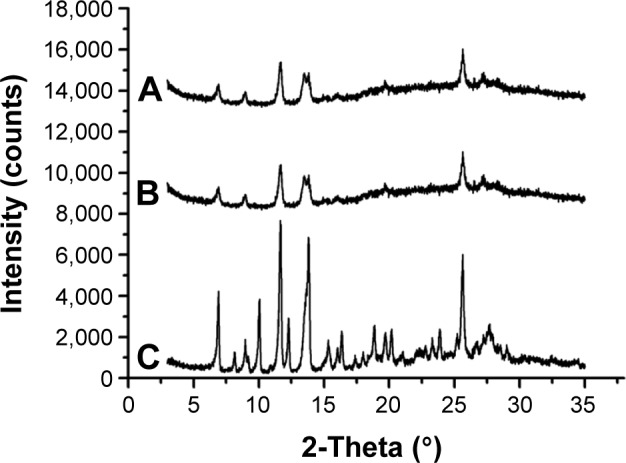
The XRD patterns of HCPT-NSps, physical mixture, and HCPT bulk powders.
Notes: (A) HCPT-NSps, (B) physical mixture of HCPT bulk powder and mPEG1000-HCPT in the same weight ratio as in HCPT-NSps, and (C) HCPT bulk powders.
Abbreviations: XRD, X-ray diffraction; HCPT, 10-hydroxycamptothecin; NSps, nanosuspensions; PEG, polyethylene glycol.
Because a very limited amount of stabilizer was used in the preparation, the structure of the HCPT-NSps in this study is close to that of nanocrystals. Nearly all the reported nanocrystals presented with a regular crystalline shape or irregular fragment due to powerful grinding or homogenization of the original crystal drugs by top-down method; however, the HCPT-NSps had a uniform near-spherical shape. The drug in nanosuspensions that were prepared by solvent precipitation method was basically in an amorphous state;26,28 this study presents an unusual case.
Lyophilization and stability test
Lyophilization is one of the most effective ways to improve the storage stability of liposomes, nanoparticles, and nanosuspensions. In most cases, a considerable number of cryoprotectants are required to maintain the structure of the nanoparticles and avoid adhesion or aggregation during lyophilization.21,26,28 Thus, the lyophilized powder particles become nanoparticles upon being resuspended in water. However, addition of various cryoprotectants will further decrease the actual drug-loading content of the nanoparticles in the final product and causes inconvenience, especially when a large clinical dose is required. Thanks to the novel stabilizer mPEG1000-HCPT that was designed and synthesized in this study, the obtained HCPT-NSps could be directly lyophilized without any cryoprotectant and can then form nanosuspensions of similar particle size (93.81 nm vs 92.90 nm) and PDI (0.086 vs 0.158) just by resuspending in water. This was achieved when a very small amount of mPEG1000-HCPT (as low as 5% of drug weight) was used. Even at such a low amount, the resultant HCPT-NSps could be stored at 4°C for at least 60 days retaining nearly the same size (Table 1). This might be due to the fact that the hydrophobic moiety of the amphiphilic stabilizer mPEG1000-HCPT is bound so firmly to the drug nanocrystal core during nanosuspension preparation that the integrity of the HCPT-NSps remains unchanged during cryoprotectant-free lyophilization. Then, upon reconstitution with water, PEG chains act as steric stabilizers and they can be quickly hydrated and form nanosuspensions of similar size. The results of this study will provide new insight into the preparation of nanosuspensions with a very high drug payload and cryoprotectant-free lyophilization.
Table 1.
Change in particle size of HCPT-NSps after storage at 4°C
| Time (day) | 0 | 10 | 20 | 40 | 60 |
|---|---|---|---|---|---|
| Size (nm) | 92.90±0.2 | 93.61±0.3 | 94.01±0.2 | 93.40±0.4 | 94.03±0.3 |
| PDI | 0.16±0.01 | 0.19±0.01 | 0.19±0.03 | 0.17±0.03 | 0.18±0.01 |
Notes: All values are presented as mean ± SD; n=3.
Abbreviations: HCPT, 10-hydroxycamptothecin; NSps, nanosuspensions; SD, standard deviation; PDI, polydispersity index.
The stability of the HCPT-NSps in plasma, 5% glucose solution and hemolysis test was studied to examine the suitability of HCPT-NSps for intravenous injection. In the plasma and 5% glucose solution, the mean particle size and PDI did not significantly change with respect to incubation time (96.11 nm and 0.114 respectively after 8h). In the hemolysis test, after incubation with 2% RBC at a concentration of 10 mg/mL of HCPT, HCPT-NSps showed negligible hemolytic effect, with a hemolysis rate of only 4.0%, indicating that the resultant nanosuspensions were suitable for intravenous administration.
Preparation of nanosuspensions for other drugs using mPEG1000-HCPT
Figures S2 and S3 show the mean particle size and PDI of the nanosuspensions of both drugs. The mean particle size of the homogenized CPT and 7-ethyl-10-hydroxycamptothecin nanosuspensions were 120.9 nm and 133.2 nm, respectively, with their PDI being 0.130 and 0.136, respectively. The actual drug payload of CPT and 7-ethyl-10-hydroxycamptothecin nanosuspensions was determined by HPLC to be 91.23% and 90.09%, respectively.
The obtained CPT and 7-ethyl-10-hydroxycamptothecin nanosuspensions could be directly lyophilized without any cryoprotectant and then reconstituted into nanosuspensions of similar particle size just by resuspending in water (137.1 nm and 133.2 nm, respectively, Tables S1 and S2). Storage stability test demonstrated that the reconstituted nanosuspensions were very stable with nearly no increase in particle size when stored at 4°C for 180 days (Tables S1 and S2).
In conclusion, using mPEG1000-HCPT as stabilizers, CPT and 7-ethyl-10-hydroxycamptothecin nanosuspensions were also successfully prepared with tiny particle size, narrow PDI and a high drug loading of more than 90%. In addition, these two nanosuspensions could be kept stable during lyophilization without adding any cryoprotectant. This might be because 7-ethyl-10-hydroxycamptothecin, CPT, and HCPT have the same basic structure, all belonging to a derivative of CPT; in addition, in the preparation and lyophilization processes, the hydrophobic moiety of mPEG1000-HCPT may firmly bind to the surface of the core drug crystals, thus resulting in an increased stabilizing efficiency. Such results indicate the feasibility and efficiency of using mPEG-drug to prepare nanosuspensions of the drug itself and its derivatives.
In vitro drug release study
The in vitro drug release behavior of the HCPT-NSps was compared to that of the HCPT injections and HCPT bulk powders, and the typical cumulative dissolution profiles are shown in Figure 4. There was no obvious burst release from HCPT-NSps, and the maximum drug release (98.6%) was reached at 24 h. In comparison, 93.0% of the drug was released quickly from HCPT injections within 2 h, and only 39.5% of the drug was released from the HCPT bulk powders within 24 h. To obtain the release kinetics, these data were fitted into first-order, zero-order, and Higuchi equations with R2=0.9578, 0.8134, and 0.9816, respectively, indicating that HCPT release from nanosuspensions nearly followed Higuchi kinetics. The slow drug release from HCPT-NSps helped guarantee the accumulation of more encapsulated drugs at tumor site via EPR effect.
Figure 4.
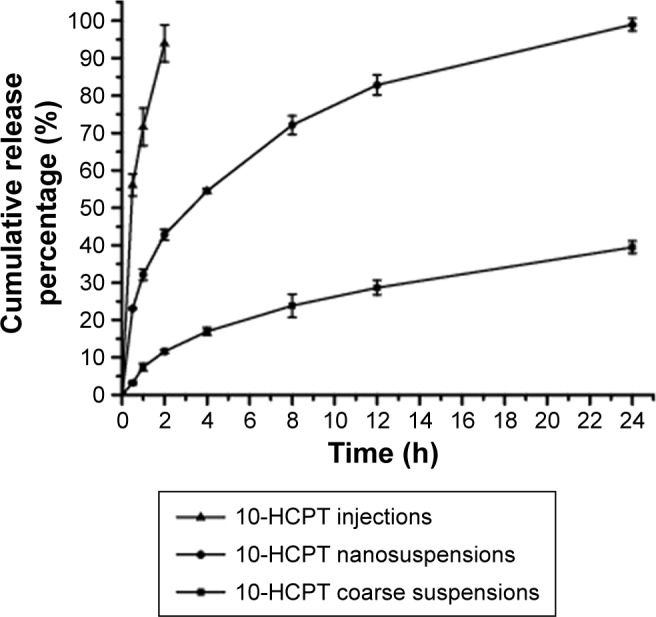
In vitro drug release profiles of 10-HCPT injections, 10-HCPT nanosuspensions, and 10-HCPT coarse suspensions in dialysis tubing (MWCO: 8,000–14,000 Da) at 37°C against PBS (pH 7.4) (mean ± SD, n=3).
Abbreviations: HCPT, 10-hydroxycamptothecin; NSps, nanosuspensions; PBS, phosphate buffered solution; SD, standard deviation.
Cytotoxicity assay and cellular uptake
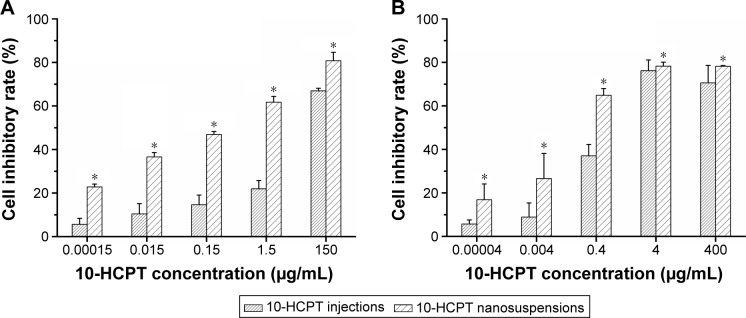
Figure 5 shows the cytotoxicity against the HepG2 and MCF-7 cells by HCPT-NSps at various concentrations using HCPT injections as a control. The result indicated that both HCPT-NSps and HCPT injections were cytotoxic against HepG2 and MCF-7 cells in the tested concentration range. However, HCPT-NSps exhibited a much stronger tumor cell growth inhibition than HCPT injections at all concentrations (Figure 5), whether it was against HepG2 cells (IC50 values: 0.1593 μg/mL vs 0.6000 μg/mL) or MCF-7 cells (IC50 values: 0.316 μg/mL vs 4.44 μg/mL). The main reasons behind the enhanced tumor inhibitory activity of HCPT-NSps are as follows: 1) only 8.9% of HCPT in the basic HCPT injections was in lactone form with the rest being inactive carboxylate form as actually determined,29 while nearly 100% of the HCPT was in lactone form in HCPT-NSps; 2) although the carboxylate form of HCPT in the injections could be partly transformed into the lactone form in the culture medium during the experiment and the released drug from HCPT-NSps could be partly transformed into the carboxylate under the same condition, HCPT-NSps could still maintain higher concentration of lactone form of HCPT than the injections due to slow drug release and the protective effect on encapsulated drugs; 3) in addition to releasing the drug in the culture medium, HCPT-NSps may be transported directly into tumor cells through endocytosis, resulting in a higher drug concentration in tumor cells.
Figure 5.
The growth inhibition of HCPT-NSps and HCPT injections against HepG2 and MCF-7 cells.
Notes: (A) The growth inhibition of HCPT-NSps and HCPT injections against HepG2 cells (IC50 value: 0.1593 μg/mL vs 0.6000 μg/mL). (B) The growth inhibition against MCF-7 cells (IC50 value: 0.316 μg/mL vs 4.44 μg/mL). All values are presented as mean ± SD; n=5, *P<0.01 vs HCPT injections.
Abbreviations: HCPT, 10-hydroxycamptothecin; NSps, nanosuspensions; SD, standard deviation.
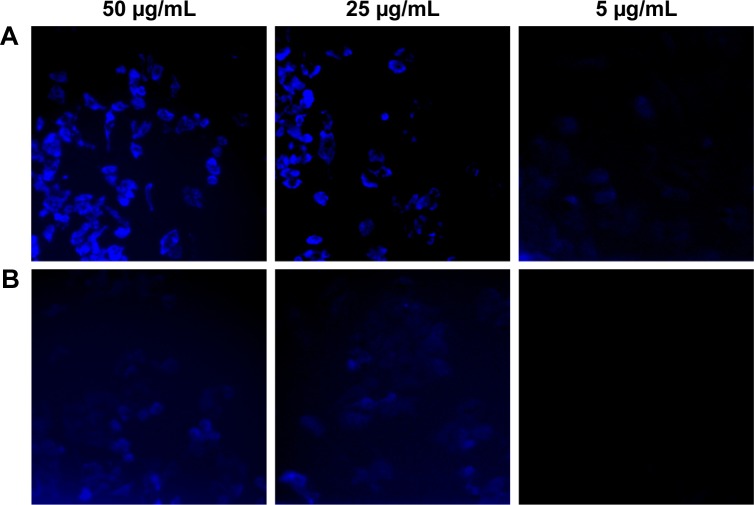
Since HepG2 cell line was more sensitive to HCPT-NSps and HCPT injections in MTT assay, this cell line was selected for subsequent cellular uptake studies. Both carboxylate form and lactone form of HCPT have fluorescence of similar strength, which can be observed through DAPI channel. As intuitively observed in Figure 6, drug uptake by HepG2 cells was dependent on concentration for both HCPT injections and HCPT-NSps. But, at each time interval and each concentration, the uptake of HCPT-NSps was much higher than that of HCPT injections, which shows that nanosuspensions did have superiority over injections in this regard.
Figure 6.
The fluorescence microscopy images showing cellular uptake of HCPT-NSps and HCPT injections by HepG2 cells.
Notes: Cellular uptake of (A) HCPT-NSps and (B) HCPT injections. HepG2 cells were incubated with HCPT-NSps and HCPT injections, at 37°C for 4 h. HCPT-NSps displayed more cellular uptake than HCPT injections at all the three concentrations.
Abbreviations: HCPT, 10-hydroxycamptothecin; NSps, nanosuspensions.
In vivo antitumor effect
Table 2 lists the tumor weight and tumor inhibitory rate against H22 tumors for all the groups. The blank control group induced an average tumor weight of 1.014 g. At the same dose of 5 mg/kg, HCPT-NSps-injected group showed a mean tumor weight of only 0.138 g, which is significantly smaller than that of the HCPT injections group (0.660 g, P<0.01). The inhibition rate of HCPT-NSps was 86.38%, which was 2.47-fold that of the same dose of HCPT injections (34.97%). Even at half of the dose (2.5 mg/kg), HCPT-NSps demonstrated better antitumor therapeutic efficacy than HCPT injections (70.31% vs 34.97%, P<0.01). Further study indicated that, using HCPT-NSps as antitumor agents, the required dose could be decreased to 1/4 (1.25 mg/kg) with a similar therapeutic efficacy to that of HCPT injections (43.42% vs 34.97%). Piloerection and diminished vigor were observed in the HCPT-injected mice. In contrast, mice treated with HCPT-NSps, even at higher doses, remained vigorous and appeared healthy throughout the experiment.
Table 2.
The in vivo antitumor effects of different formulations of HCPT on H22 tumor-bearing mice
| Group | Dose (mg/kg) | Tumor weight (g) | Inhibition rate (%) |
|---|---|---|---|
| Blank control | 1.014±0.177 | ||
| Stabilizer | 1.138±0.108 | ||
| HCPT injection | 5.0 | 0.660±0.072# | 34.97% |
| HCPT-NSps | 5.0 | 0.138±0.019##,* | 86.38% |
| HCPT-NSps | 2.5 | 0.301±0.025##,* | 70.31% |
| HCPT-NSps | 1.25 | 0.574±0.051## | 43.42% |
Notes: All values are presented as mean ± SD; n=3,
P<0.01 vs HCPT injections,
P<0.05 vs blank control,
P<0.01 vs blank control.
Abbreviations: HCPT, 10-hydroxycamptothecin; NSps, nanosuspensions; SD, standard deviation.
Figure 7 shows the relative change in the body weight of mice over time during the in vivo experiment. It was clear that mice in the normal saline group gained body weight quickly throughout the experiment. Differently, mice in the HCPT injections group experienced a short time of body weight decrease after dosing, followed by a slow body weight increase. But the mice in the group injected with 5 mg/kg of HCPT-NSps maintained a relatively steady increase in body weight. At the end of the trial, the relative body weight of mice treated with normal saline, 5 mg/kg HCPT injections, and 5 mg/kg HCPT-NSps were 1.27, 1.21, and 1.22 fold, which indicates that 5 mg/kg dose was acceptable for both HCPT injections and HCPT-NSps as it does not cause any significant damage.
Figure 7.
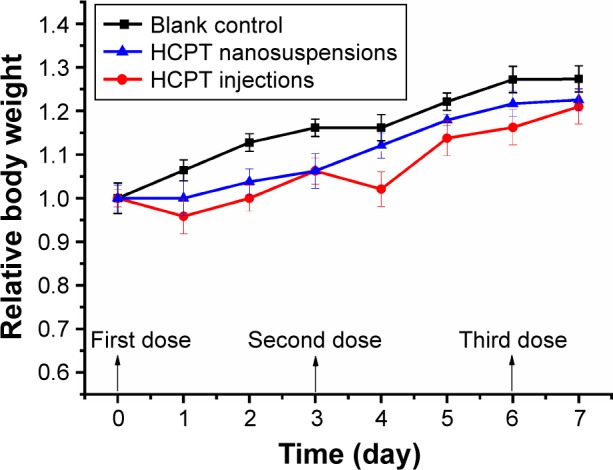
Relative body weight of mice after intravenous administration of blank control, HCPT, and HCPT-NSps in H22 tumor-bearing mice at a concentration of 5 mg/kg body weight.
Note: All values are presented as mean ± SD; n=8.
Abbreviations: HCPT, 10-hydroxycamptothecin; NSps, nanosuspensions; SD, standard deviation.
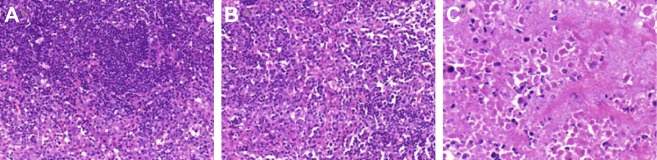
Pathological examinations of tumors exhibited morphological changes in tumors after treatment (Figure 8). The cells in the normal saline group were intact, with rich cell membranes, cytoplasm, and nuclei in pathological fissile phase (Figure 8A), while the cells in the injected groups were injured to some extent; the fissile phase was shorter and the cell membrane was slightly damaged (Figure 8B). More importantly, in the HCPT-NSps-injected group (Figure 8C), the cell morphology changed significantly, with only a few normal residual cells, and there were masses of necrotic cells in the tumors with obvious pyknosis, sparse organelles in the cytoplasm, and disrupted membranes.
Figure 8.
H&E staining of tumor paraffin sections post-HCPT injections.
Notes: (A) Blank control, (B) HCPT injections (5 mg/kg), and (C) HCPT-NSps (5 mg/kg). Normal saline-injected tumors were used as control and no pathological changes were observed. Tumor cells subjected to HCPT injections were slightly damaged, but no significant fission changes were observed. However, massive cellular death was detected after treatment with HCPT-NSps, and only a small population of tumor cells was normal. Magnification of all the pictures is 400×.
Abbreviations: HCPT, 10-hydroxycamptothecin; NSps, nanosuspensions; H&E staining, hematoxylin and eosin staining.
As the main toxicity of HCPT is the bone marrow suppression, this study examined the morphological changes of marrow after treatment. As shown in Figure S4, in the HCPT-injected group, the total number of bone marrow cells, erythroid and myeloid hematopoietic cells, and megakaryocytes was significantly reduced, and the proportion of myeloid hematopoietic cells at various stages was abnormal, indicating that the sodium salt of the carboxylate significantly inhibited the bone marrow. However, no abnormal bone marrow cells were evidently observed in the HCPT-NSps group, the erythroid and myeloid hematopoietic cells appeared normal, the number of megakaryocytes slightly decreased, and the ratio between the various stages of the cell showed no abnormalities, with no obvious bone marrow suppression.
Pathological examinations of liver and kidney (Figure S4) exhibited insignificant difference between HCPT injections group and HCPT-NSps group. In comparison with normal saline, neither HCPT-NSps nor HCPT injections at 5 mg/kg caused evident damage to liver and kidney.
Conclusion
In this study, some fundamental work has been done on HCPT-NSps that are stabilized by mPEG1000-HCPT. This novel stabilizer provides HCPT-NSps with beneficial features including, 1) easy preparation, 2) tiny particle size (92.90 nm), 3) high drug payload (94.90%), 4) freeze drying without protectants, 5) effective protection of HCPT in lactone form by encapsulation and sustained drug release, and 6) significantly improved anticancer efficacy in vivo. Using mPEG1000-HCPT as a stabilizer, 7-ethyl-10-hydroxycamptothecin nanosuspensions and CPT nanosuspensions were also successfully prepared with a similar high drug payload, small size, and unusual feature of cryoprotectant-free freeze drying. There are always some difficulties in converting some hydrophobic drugs into nanosuspensions with existing pharmaceutical adjuvants. This study provided a potential solution for these drugs, that is, using mPEG-drug conjugate as stabilizer and finding a suitable solvent to dissolve both the insoluble drug and the mPEG-drug conjugate, then the nanosuspensions may be easily prepared for these drugs via anti-solvent nanoprecipitation method. The nanosuspensions thus obtained may have a very high drug payload and may also be featured with other pharmaceutically and therapeutically beneficial in vivo behaviors.
Supplementary materials
The HPLC chromatograms of HCPT and mPEG1000-HCPT during acid hydrolysis.
Notes: (A) HCPT, (B) mPEG1000-HCPT before hydrolysis, (C) mPEG1000-HCPT after hydrolysis in 1 mol/L HCl at 50°C for 2 h, and (D) mPEG1000-HCPT after hydrolysis in 1 mol/L HCl at 50°C for 4 h.
Abbreviations: HCPT, 10-Hydroxycamptothecin; PEG, polyethylene glycol; min, minute; WVL, wavelength.
The particle size of 7-ethyl-10-hydroxycamptothecin nanosuspensions before and after homogenization.
The particle size of camptothecin nanosuspensions before and after homogenization.
Photomicrographs (original magnification 40×) of pathological sections (H&E stains) from liver, kidney, and bone marrow.
Notes: (A) liver, (B) kidney, and (C) bone marrow. Both HCPT and HCPT-NSps were injected at a dosage of 5 mg/kg. It was demonstrated that neither HCPT-NSps nor HCPT injections (5 mg/kg) caused significant damage to liver and kidney. Mice subjected to HCPT-NSps did not show pathological change in bone marrow, while mice subjected to HCPT injections did.
Abbreviations: HCPT, 10-Hydroxycamptothecin; NSps, nanosuspensions; H&E, hematoxylin and eosin; inj, injections.
Table S1.
Change in particle size of 7-ethyl-10-hydroxycamptothecin nanosuspensions reconstituted after storage in the form of lyophilized powder at different times (mean ± SD, n=3)
| Time (day) | 0 | 20 | 40 | 80 | 120 | 180 |
|---|---|---|---|---|---|---|
| Size (nm) | 137.1±0.4 | 139.0±0.4 | 134.5±0.2 | 137.0±0.4 | 135.1±0.5 | 137.4±0.4 |
| PDI | 0.14±0.04 | 0.18±0.02 | 0.17±0.03 | 0.11±0.02 | 0.19±0.04 | 0.19±0.02 |
Notes: All values are presented as mean ± SD; n=3.
Abbreviations: SD, standard deviation; PDI, polydispersity index.
Table S2.
Change in particle size of camptothecin nanosuspensions reconstituted after storage in the form of lyophilized powder at different times
| Time (day) | 0 | 20 | 40 | 80 | 120 | 180 |
|---|---|---|---|---|---|---|
| Size (nm) | 121.5±0.2 | 122.2±0.3 | 121.3±0.3 | 123.9±0.4 | 122.7±0.5 | 125.9±0.4 |
| PDI | 0.14±0.02 | 0.18±0.01 | 0.12±0.01 | 0.09±0.03 | 0.13±0.01 | 0.19±0.02 |
Notes: All values are presented as mean ± SD; n=3.
Abbreviations: SD, standard deviation; PDI, polydispersity index.
Acknowledgments
This work was financially supported by the NSFC-Guangdong Joint Foundation (U1401223), CAMS Innovation Fund for Medical Sciences (CIFMS, No 2016-I2M-1-012), and Beijing Natural Science Foundation (7152099).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Zhang L, Hu Y, Jiang X, Yang C, Lu W, Yang YH. Camptothecin derivative-loaded poly(caprolactone-co-lactide)-b-PEG-b-poly(caprolactone-co-lactide) nanoparticles and their biodistribution in mice. J Control Release. 2004;96(1):135–148. doi: 10.1016/j.jconrel.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Yang X, Li L, Wang Y, Tan Y. Preparation, pharmacokinetics and tissue distribution of micelles made of reverse thermo-responsive polymers. Int J Pharm. 2009;370(1–2):210–215. doi: 10.1016/j.ijpharm.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 3.Zhou YY, Du YZ, Wang L, Yuan H, Zhou JP, Hu FQ. Preparation and pharmacodynamics of stearic acid and poly (lactic-co-glycolic acid) grafted chitosan oligosaccharide micelles for 10-hydroxycamptothecin. Int J Pharm. 2010;393(1–2):143–151. doi: 10.1016/j.ijpharm.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 4.Zhu H, Cao J, Cui S, Qian Z, Gu Y. Enhanced tumor targeting and antitumor efficacy via hydroxycamptothecin-encapsulated folate-modified N-succinyl-N′-octyl chitosan micelles. J Pharm Sci. 2013;102(4):1318–1332. doi: 10.1002/jps.23470. [DOI] [PubMed] [Google Scholar]
- 5.Zhao YX, Liu DX, Liang WQ, Ye ZW. In-vivo pharmacokinetics, tissue distribution and anti-tumor effect of hydroxycamptothecin delivered in oil-in-water submicron emulsions. J Pharm Pharmacol. 2012;64(6):783–791. doi: 10.1111/j.2042-7158.2012.01484.x. [DOI] [PubMed] [Google Scholar]
- 6.Yang L, Cui F, Cun D, Tao A, Shi K, Lin W. Preparation, characterization and biodistribution of the lactone form of HCPT-loaded BSA nanopar-ticles. Int J Pharm. 2007;340(1–2):163–172. doi: 10.1016/j.ijpharm.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 7.Zhang H, Hollis CP, Zhang Q, Li T. Preparation and antitumor study of camptothecin nanocrystals. Int J Pharm. 2011;415(1–2):293–300. doi: 10.1016/j.ijpharm.2011.05.075. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Wei P, Li J, Li L. Pharmacokinetic analysis and optimization of hydroxycamptothecin-loaded nanoparticles for liver targeting. Drug Dev Ind Pharm. 2012;38(7):837–847. doi: 10.3109/03639045.2011.630393. [DOI] [PubMed] [Google Scholar]
- 9.Qu JB, Shao HH, Jing GL, Huang F. PEG-chitosan-coated iron oxide nanoparticles with high saturated magnetization as carriers of 10-hydroxycamptothecin: preparation, characterization and cytotoxicity studies. Colloids Surf B Biointerfaces. 2013;102:37–44. doi: 10.1016/j.colsurfb.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Zu Y, Meng L, Zhao X, et al. Preparation of 10-hydroxycamptothecin-loaded glycyrrhizic acid-conjugated bovine serum albumin nanoparticles for hepatocellular carcinoma-targeted drug delivery. Int J Nanomedicine. 2013;8:1207–1222. doi: 10.2147/IJN.S40493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patravale VB, Date AA, Kulkarni RM. Nanosuspensions: a promising drug delivery strategy. J Pharm Pharmcol. 2004;56:827–840. doi: 10.1211/0022357023691. [DOI] [PubMed] [Google Scholar]
- 12.Kakran M, Shegokar R, Sahoo NG, Shaal LA, Li L, Müller RH. Fabrication of quercetin nanocrystals: comparison of different methods. Eur J Pharm Biopharm. 2012;80(1):113–121. doi: 10.1016/j.ejpb.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Leone F, Cavalli R. Drug nanosuspensions: a ZIP tool between traditional and innovative pharmaceutical formulations. Expert Opin Drug Deliv. 2015;12(10):1607–1625. doi: 10.1517/17425247.2015.1043886. [DOI] [PubMed] [Google Scholar]
- 14.Yang L, Jiang J, Hong J, et al. High drug payload 10-hydroxycamptothecin nanosuspensions stabilized by cholesterol-PEG: in vitro and in vivo investigation. J Biomed Nanotechnol. 2015;11(4):711–721. doi: 10.1166/jbn.2015.2050. [DOI] [PubMed] [Google Scholar]
- 15.Pasut G, Veronese FM. PEG conjugates in clinical development or use as anticancer agents: an overview. Adv Drug Deliv Rev. 2009;60:1177–1188. doi: 10.1016/j.addr.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Knop K, Hoogenboom R, Fischer D, Schubert US. Poly (ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew Chem Int Ed. 2010;49(36):6288–6308. doi: 10.1002/anie.200902672. [DOI] [PubMed] [Google Scholar]
- 17.Shen J, Zheng D, Zhao Z, et al. Synthesis, characterization, in vitro and in vivo evaluation of PEGylated oridonin conjugates. Int J Pharm. 2013;456(1):80–86. doi: 10.1016/j.ijpharm.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Conover CD, Greenwald RB, Pendri A, Shum KL. Camptothecin delivery systems: the utility of amino acid spacers for the conjugation of camptothecin with polyethylene glycol to create prodrugs. Anticancer Drug Des. 1999;14(6):499–506. [PubMed] [Google Scholar]
- 19.Fleming AB, Haverstick K, Saltzman WM. In vitro cytotoxicity and in vivo distribution after direct delivery of PEG-camptothecin conjugates to the rat brain. Bioconjug Chem. 2004;15(6):1364–1375. doi: 10.1021/bc034180o. [DOI] [PubMed] [Google Scholar]
- 20.Yu D, Peng P, Dharap SS, et al. Antitumor activity of poly(ethylene glycol)–camptothecin conjugate: the inhibition of tumor growth in vivo. J Control Release. 2005;110(1):90–102. doi: 10.1016/j.jconrel.2005.09.050. [DOI] [PubMed] [Google Scholar]
- 21.Hong M, Zhu S, Jiang Y, et al. Novel anti-tumor strategy: PEG-hydroxycamptothecin conjugate loaded transferrin-PEG-nanoparticles. J Control Release. 2010;141(1):22–29. doi: 10.1016/j.jconrel.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 22.Shen Y, Jin E, Zhang B, et al. Prodrugs forming high drug loading multifunctional nanocapsules for intracellular cancer drug delivery. J Am Chem Soc. 2010;132(12):4259–4265. doi: 10.1021/ja909475m. [DOI] [PubMed] [Google Scholar]
- 23.Greenwald RB, Choe YH, Wu D. Selective phenolic acylation of 10-hydroxycamptothecin using poly (ethylene glycol) carboxylic acid. Bioorg Med Chem Lett. 2003;13(3):577–580. doi: 10.1016/s0960-894x(02)00926-5. [DOI] [PubMed] [Google Scholar]
- 24.Li YF, Zhang R. Reversed-phase high-performance liquid chromatography method for the simultaneous quantitation of the lactone and carboxylate forms of the novel natural product anticancer agent 10-hydroxycamptothecin in biological fluids and tissues. J Chromatogr B Biomed Appl. 1996;686(2):257–265. doi: 10.1016/s0378-4347(96)00222-8. [DOI] [PubMed] [Google Scholar]
- 25.He X, Lu W, Jiang X, Cai J, Zhang X, Ding J. Synthesis and biological evaluation of bis and monocarbonate prodrugs of 10-hydroxycamptothecins. Bioorg Med Chem. 2004;12(15):4003–4008. doi: 10.1016/j.bmc.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Pu X, Sun J, Wang Y, et al. Development of a chemically stable 10-hydroxycamptothecin nanosuspensions. Int J Pharm. 2009;379(1):167–173. doi: 10.1016/j.ijpharm.2009.05.062. [DOI] [PubMed] [Google Scholar]
- 27.Zhao YX, Hua HY, Chang M, Liu WH, Zhao Y, Liu HM. Preparation and cytotoxic activity of hydroxycamptothecin nanosuspensions. Int J Pharm. 2010;392(1–2):64–71. doi: 10.1016/j.ijpharm.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 28.Han M, Liu X, Guo Y, Wang Y, Wang X. Preparation, characterization, biodistribution and antitumor efficacy of hydroxycamptothecin nanosuspensions. Int J Pharm. 2013;455(1–2):85–92. doi: 10.1016/j.ijpharm.2013.07.056. [DOI] [PubMed] [Google Scholar]
- 29.Hertzberg RP, Caranfa MJ, Holden KG, et al. Modification of the hydroxylactone ring of camptothecin: inhibition of mammalian topoi-somerase I and biological activity. J Med Chem. 1989;32:715–720. doi: 10.1021/jm00123a038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The HPLC chromatograms of HCPT and mPEG1000-HCPT during acid hydrolysis.
Notes: (A) HCPT, (B) mPEG1000-HCPT before hydrolysis, (C) mPEG1000-HCPT after hydrolysis in 1 mol/L HCl at 50°C for 2 h, and (D) mPEG1000-HCPT after hydrolysis in 1 mol/L HCl at 50°C for 4 h.
Abbreviations: HCPT, 10-Hydroxycamptothecin; PEG, polyethylene glycol; min, minute; WVL, wavelength.
The particle size of 7-ethyl-10-hydroxycamptothecin nanosuspensions before and after homogenization.
The particle size of camptothecin nanosuspensions before and after homogenization.
Photomicrographs (original magnification 40×) of pathological sections (H&E stains) from liver, kidney, and bone marrow.
Notes: (A) liver, (B) kidney, and (C) bone marrow. Both HCPT and HCPT-NSps were injected at a dosage of 5 mg/kg. It was demonstrated that neither HCPT-NSps nor HCPT injections (5 mg/kg) caused significant damage to liver and kidney. Mice subjected to HCPT-NSps did not show pathological change in bone marrow, while mice subjected to HCPT injections did.
Abbreviations: HCPT, 10-Hydroxycamptothecin; NSps, nanosuspensions; H&E, hematoxylin and eosin; inj, injections.
Table S1.
Change in particle size of 7-ethyl-10-hydroxycamptothecin nanosuspensions reconstituted after storage in the form of lyophilized powder at different times (mean ± SD, n=3)
| Time (day) | 0 | 20 | 40 | 80 | 120 | 180 |
|---|---|---|---|---|---|---|
| Size (nm) | 137.1±0.4 | 139.0±0.4 | 134.5±0.2 | 137.0±0.4 | 135.1±0.5 | 137.4±0.4 |
| PDI | 0.14±0.04 | 0.18±0.02 | 0.17±0.03 | 0.11±0.02 | 0.19±0.04 | 0.19±0.02 |
Notes: All values are presented as mean ± SD; n=3.
Abbreviations: SD, standard deviation; PDI, polydispersity index.
Table S2.
Change in particle size of camptothecin nanosuspensions reconstituted after storage in the form of lyophilized powder at different times
| Time (day) | 0 | 20 | 40 | 80 | 120 | 180 |
|---|---|---|---|---|---|---|
| Size (nm) | 121.5±0.2 | 122.2±0.3 | 121.3±0.3 | 123.9±0.4 | 122.7±0.5 | 125.9±0.4 |
| PDI | 0.14±0.02 | 0.18±0.01 | 0.12±0.01 | 0.09±0.03 | 0.13±0.01 | 0.19±0.02 |
Notes: All values are presented as mean ± SD; n=3.
Abbreviations: SD, standard deviation; PDI, polydispersity index.