Abstract
Retinoic acid (RA) is the ligand for nuclear RA receptors (RARs and RXRs) and is crucial for normal epithelial cell growth and differentiation. During malignant transformation, human bronchial epithelial cells acquire a block in retinoid signaling caused in part by a transcriptional defect in RARs. Here, we show that activation of c-Jun N-terminal kinase (JNK) contributes to RAR dysfunction by phosphorylating RARα and inducing degradation through the ubiquitin-proteasomal pathway. Analysis of RARα mutants and phosphopeptide mapping revealed that RARα residues Thr181, Ser445, and Ser461 are phosphorylated by JNK. Mutation of these residues to alanines prevented efficient ubiquitination of RARα and increased the stability of the protein. We investigated the importance of RARα phosphorylation by JNK as a mediator of retinoid resistance in lung cancer. Mice that develop lung cancer from activation of a latent K-ras oncogene had high intratumoral JNK activity and low RARα levels and were resistant to treatment with an RAR ligand. JNK inhibition in a human lung cancer cell line enhanced RARα levels, ligand-induced activity of RXR-RAR dimers, and growth inhibition by RA. These findings point to JNK as a key mediator of aberrant retinoid signaling in lung cancer cells.
Retinoids (vitamin A and its retinoic acid [RA] derivatives) mediate their biologic effects through two families of nuclear receptors, the RA receptors (RARα, -β, and -γ) and the retinoid X receptors (RXRα, -β, and -γ) (50). The stereoisomers all-trans RA and 9-cis RA are ligands for RARs, whereas RXRs can bind only to 9-cis RA. These receptors function as dimers (RXR-RAR heterodimers and RXR homodimers) that bind to retinoic acid response elements (RAREs) in gene promoters (49). Because they bind to distinct RAREs, RXR-RAR heterodimers and RXR homodimers activate the transcription of distinct sets of target genes (49).
In the absence of ligand, RXR-RAR heterodimers associate with a multiprotein complex containing transcriptional corepressors that induce histone deacetylation, chromatin condensation, and transcriptional suppression (52). Ligand binding causes the receptors to dissociate from corepressors and then associate with coactivators that have histone acetyltransferase activity and induce local chromatin decondensation, recruitment of the RNA polymerase II holoenzyme, and activation of target gene transcription (51, 70). Other members of the nuclear receptor family, including those activated by vitamin D, thyroid hormone, estrogen, and progesterone, share this mechanism of activation. In addition to activating these nuclear receptors, ligand also mediates receptor destruction, in that ligand binding causes receptor ubiquitination and subsequently proteolysis through the proteasomal complex (14). Thus, the ligand tightly regulates nuclear receptor signaling through its effects on receptor function and abundance.
Ubiquitination of nuclear receptors requires ligand binding. Receptor conformational changes induced by ligand are recognized by the ubiquitination enzymes E1, E2, and E3, which transfer the ubiquitin polypeptide to the target protein by creating an isopeptide linkage between the carboxy terminus of ubiquitin and the lysine side chains of the target proteins (39, 48, 60). In contrast to nuclear receptors, other proteins require phosphorylation before ubiquitination, which creates docking sites recognized by specific E3 ligases (33, 58, 69). Most hormone nuclear receptors are, in fact, phosphoproteins, and phosphorylation is a potent regulator of nuclear receptor function. Depending on the receptor, phosphorylation regulates receptor DNA binding, dimerization, coactivator recruitment, and transactivation (7, 31, 72). For example, RAR phosphorylation at the N-terminal transactivation function (AF-1) domain augments ligand-induced transactivation (62), and RXR phosphorylation inhibits its transactivation properties (68).
Nuclear receptors are substrates of a variety of kinases. RAR is a substrate for protein kinase A, cyclin-dependent kinase 7, and p38 (19, 62, 63). Retinoid signaling is inhibited by peptide growth factors and cellular stress (3, 43, 44, 53), suggesting that kinases activated by these stimuli also modulate retinoid receptor function. Stress induced by UV radiation, heat, osmotic shock, and inflammatory cytokines activates kinase signaling cascades that involve small G proteins (e.g., cdc42, Rac, and Rho), mitogen-activated protein (MAP) kinase kinase kinases (MEKK1 to -4), MAP kinase kinases (MKK4, -6, and -7), and MAP kinases (extracellular signal-regulated kinase [ERK], c-Jun N-terminal kinase [JNK], and p38) (13). Substrate specificity of these kinases partially overlaps; for example, p38 is a substrate of MKK3 and MKK6, and JNK is a substrate of MKK4 and MKK7 (36). JNK and p38 phosphorylate several transcription factors, including ATF2, c-Jun, Elk-1, and nuclear receptors (79). RXR is a substrate of JNK and ERK (1, 43, 68). Whereas phosphorylation by ERK suppresses RXR transactivation, JNK-induced phosphorylation has no detectable functional consequences on RXR.
Human bronchial epithelial (HBE) cells require RA for normal cellular growth and differentiation. However, sensitivity to the biological effects of RA is lost with malignant transformation, a point illustrated by the finding that RA treatment induces proliferative arrest of HBE cells but not non-small-cell lung cancer (NSCLC) cells (18). Retinoid resistance in NSCLC cells results, in part, from a defect in RAR function (57), but the basis of this transcriptional defect has not been identified. Upstream activators of JNK, including receptor tyrosine kinases and Ras, are constitutively activated in NSCLC cells (56). Moreover, JNK activity is increased in certain NSCLC cell lines (40, 41) and in a murine model (K-rasLA1) in which NSCLC develops through somatic activation of the K-ras oncogene (42).
In seeking to better define the mechanism by which stress inhibits retinoid signaling, we found that UV radiation inhibited transactivation of RAR but not RXR; it induced RAR phosphorylation and degradation of RAR through the ubiquitin-proteasome pathway; and it phosphorylated RAR by activating JNK. RAR phosphorylation by JNK was required for UV-induced RAR degradation. Further, we investigated the importance of JNK pathway in the retinoid resistance commonly found in lung cancer cells. Findings from studies using in vitro and in vivo models of lung cancer supported the hypothesis that JNK regulates RARα protein levels and contributes to alterations in retinoid signaling that occur during the process of malignant transformation.
MATERIALS AND METHODS
Reagents.
The JNK inhibitor SP600125 (6) was provided by Berndt Stein (Celgene Corporation, San Diego, Calif.). We purchased LY294002 and SB203580 (Calbiochem, San Diego, Calif.); His-ubiquitin, MG132, all-trans RA, 9-cis RA, E-4-[2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-2-naphthalenyl)-1-propenyl]benzoic acid (TTNPB), ATP, triphosphate 5′-adenylic acid (AMP-PNP), phorbol myristate acetate (PMA), insulin-like growth factor 1, creatine kinase, and phosphocreatine (Sigma-Aldrich, St. Louis, Mo.); lactacystin and Z-VAD-FMK (Biomol, Plymouth Meeting, Pa.); [γ-32P]ATP, [32P]orthophosphate, and [35S]methionine (ICN Biomedicals, Inc., Costa Mesa, Calif.); polyclonal antibodies against human RARα, RARβ, RARγ, RXRα, MKK4, JNK, and poly-ADP ribose polymerase (Santa Cruz Biotechnology, Santa Cruz, Calif.); U0126 and polyclonal antibodies against human phospho-JNK (Thr183/Tyr185), phospho-c-Jun (Ser63), c-Jun, phospho-Erk (Thr202/Tyr204), Erk, phospho-Akt (Ser473), and Akt (Cell Signaling Technology, Beverly, Mass.); monoclonal antibodies against Flag epitope and actin (Sigma-Aldrich); anti-HA antibody (Roche Molecular Biochemicals, Indianapolis, Ind.); and purified recombinant glutathione S-transferase (GST)-ATF2, GST-c-Jun, and HSP27 (Upstate Biotechnology, Lake Placid, N.Y.).
cDNA constructs.
Adenovirus (Ad) expressing constitutively active mutant MKK4 (ED) was kindly provided by J. Kyriakis (Molecular Cardiology Research Institute, Boston, Mass.). Constitutively active mutant MEKK1Δ, which lacks amino acids (aa) 1 to 351, has been described elsewhere (54). The HA-ubiquitin plasmid was a gift from E. Yeh (M. D. Anderson Cancer Center). Flag-tagged RARα was constructed by cloning the RARα cDNA into pCDNA 6 vector (Invitrogen Life Technologies, Carlsbad, Calif.), and the corresponding mutations (T181A, S445A, and S461A) were introduced by performing site-directed mutagenesis with the Quick-Change Mutagenesis kit (Stratagene, La Jolla, Calif.). GAL-hybrid plasmids were constructed by cloning RARα and RXRα cDNAs downstream of the GAL4-DNA binding domain in the pFA-CMV vector (Stratagene). To construct GST-tagged RARα, RARα was subcloned into the pGEX4T1 vector (Amersham-Pharmacia Biotech UK, Ltd., Buckinghamshire, United Kingdom). GST-tagged deletion mutants of RARα (spanning aa 1 to 187, 82 to 167, and 186 to 462) were constructed by PCR amplification and insertion into pGEX4T1.
Cell culture and UV treatment.
HeLa, COS-1, 3T3, and 293 cells were maintained in Dulbecco's modified Eagle medium containing 10% fetal calf serum and antibiotics (100 μg of penicillin/ml and 100 μg of streptomycin/ml). NSCLC cells were maintained in RPMI 1640 containing 10% fetal calf serum and antibiotics (penicillin and streptomycin). BEAS-2B cells, which are simian virus 40 T-antigen-immortalized HBE cells (61), were maintained in serum-free keratinocyte medium containing epidermal growth factor and bovine pituitary extracts (Invitrogen). For UV treatment, cells were serum starved overnight and UV irradiated (60 J/M2) with a UV cross-linker (Stratagene), which emits radiation at 254 nm. Cells were harvested at different time points, and nuclear and cytosolic fractions were prepared as described previously (67) and subjected to Western blotting.
Purification of GST-tagged proteins.
GST-tagged RAR proteins were expressed and purified from the BL21 bacterial strain as described previously (43). The integrity of purified proteins was assayed by Coomassie staining and Western blotting with anti-RARα antibodies.
In vivo labeling, kinase assays, and phosphopeptide mapping.
For in vivo labeling experiments, Lipofectamine 2000 (Invitrogen) was used to cotransfect HeLa cells with plasmids expressing JNK1 and Flag-tagged wild-type or mutant RARα. After 24 h, cells were serum starved overnight in phosphate-free medium and then incubated in the same medium for 4 h in the presence of 250 μCi of [32P]orthophosphate/ml. In some experiments, cells were exposed to UV radiation 1 h before harvesting. Cells were lysed in radioimmunoprecipitation assay buffer containing 100 nM okadaic acid, 1 mM orthovanadate, and protease inhibitor cocktail (Sigma-Aldrich). Flag-tagged proteins were immunoprecipitated with anti-Flag antibodies, and immunoprecipitates were subjected to electrophoresis and autoradiography.
For in vitro kinase assays, activated JNK and p38 were immunopurified from intact cells as described previously (41). Immunopurified or recombinant active kinases were incubated with 1 μg of substrate proteins in buffer containing 25 mM HEPES, 25 mM MgCl2, 25 mM β-glycerophosphate, 20 μM ATP, 0.5 mM dithiothreitol, and 10 μCi of [γ-32P]ATP for 30 min at 37°C. Labeled proteins were later separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and autoradiographed. For phosphopeptide mapping, 5 μg of recombinant GST-RARα and mutant GST-RARα (aa 1 to 187) was subjected to in vitro kinase assays with purified active JNK1. Phosphoamino acid analysis, limited tryptic digestion, separation of phosphopeptides, and manual Edman degradation were carried out as described previously (32).
Pulse-chase experiments.
HeLa cells were cotransfected with JNK1 and Flag-tagged wild-type or mutant RARα constructs. After 24 h, cells were serum starved overnight and then incubated in methionine-free medium containing 200 μCi [35S]methionine/ml for 90 min. At the start of the chase, cells were washed with phosphate-buffered saline, treated or not treated with UV radiation (60 J/M2), and then chased with medium containing an excess of cold methionine. Cells were then lysed in radioimmunoprecipitation assay buffer, and RARα was immunoprecipitated with Flag antibody, followed by SDS-PAGE and autoradiography.
In vivo and in vitro ubiquitination assays.
HeLa cells were cotransfected with Flag-tagged RARα and HA-ubiquitin expression constructs. After 48 h, cells were incubated for 1 h with 50 μM MG132 and then exposed to UV radiation. Cell pellets were dissolved in 100 μl of denaturing buffer (50 mM Tris, 1% SDS, 5 mM dithiothreitol) and boiled for 10 min. Lysates were diluted to 1 ml by phosphate-buffered saline, and ubiquitin conjugates were immunoprecipitated with anti-Flag antibody and subjected to Western blotting with anti-RARα and anti-HA antibodies.
In vitro ubiquitination assays were a modified version of methods previously described (12). Briefly, 100 ng of GST or GST-RARα was phosphorylated in vitro by incubation for 20 min at 37°C with purified active JNK1 in ubiquitination assay buffer (50 mM Tris, 10 mM ATP, 2.5 mM MgCl2, 10 mM creatine phosphate, and 3.5 U of creatine phosphokinase). The phosphorylated recombinant proteins were then ubiquitinated by the addition of S-100 HeLa fraction (20 μl) (16), His-ubiquitin (15 μg), ubiquitin aldehyde (3 μM), okadaic acid (2 μM), and MG132 (10 μM). The mixture was incubated at 37°C for 90 min. RAR-ubiquitin conjugates were purified with Ni2+-agarose beads and subjected to electrophoresis and Western blotting with anti-RARα antibodies.
RT-PCR.
For reverse transcriptase PCR (RT-PCR) analysis, HeLa and Calu-6 cells were serum starved overnight, treated or not treated with UV radiation, and then incubated with 1 μM RA for 6 h as indicated. Cells were harvested, and total RNA was extracted with an RNA isolation kit (QIAGEN, Inc., Valencia, Calif.). RT reactions were performed with 3 μg of total cellular RNA with oligo(dT) primers. One-tenth of the RT reaction mixture was used as a template for PCR to detect RARα, RARβ, and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) transcripts with specific primers and PCR conditions as previously described (71). The PCR cycle was stopped during the linear phase of the PCR.
Luciferase assays.
The DR5TK-luc and G5E1b-luc (RARE and a GAL4 response element, respectively) reporter constructs have been described elsewhere (43, 45). FuGENE (Roche) was used to transiently transfect COS-1, HeLa, and H322 cells (2 × 104) with 100 ng of DR5-TK-luc, 200 ng of β-galactosidase (β-Gal) plasmid driven by β-actin promoter (to monitor relative transfection efficiency), and, when indicated, 100 to 300 ng of MEKK1Δ plasmid. The total amount of plasmid per well was equilibrated (1 μg per well) with empty vector. After 24 h, cells were treated for 15 h with or without 1 μM RA. In the case of H322 cells, after transfection the cells were incubated for 15 h with 1 μM SP600125 alone, 1 μM RA alone, the combination, or medium alone.
For assays with GAL-hybrid constructs, HeLa cells (4 × 104) were cotransfected with 100 ng of G5E1b-luc, 200 ng of β-Gal plasmid, and 100 ng of plasmids expressing GAL-RARα or GAL-RXRα. After 36 h, cells were UV irradiated (60 J/M2). Six hours later, cells were treated for 6 h with or without 1 μM 9-cis RA.
Cells were lysed in assay buffer (100 mM potassium phosphate, 0.2% Triton X-100) and assayed for luciferase and β-Gal activities with a dual-light system (Applied Biosystems, Bedford, Mass.). Luciferase activities were corrected for differences in transfection efficiency and expressed as the means and standard deviations from three identical wells.
Cell proliferation assays.
H322 cells (3 × 103) were seeded in 96-well plates. After 24 h, cells were treated for 4 days with 1 μM RA, 5 μM SP600125, the combination, or medium alone. Relative cell density was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays as described elsewhere (40).
Animal experiments.
K-rasLA1 mice (27) (12 to 16 weeks of age) were treated daily for 28 days with 3 μg of TTNPB/kg of body weight (80) or with vehicle alone (10 mice per group) by gastric gavage (81). Tumors were identified and measured before and after treatment by microcomputed tomographic imaging (10). Tumors were also counted on the surface of the lungs at the time of necropsy. In a separate group of K-rasLA1 mice and wild-type littermates, whole-lung tissue samples were prepared and subjected to Western and RNA in situ analysis as previously described (42).
RESULTS
UV irradiation inhibits retinoid signaling through RAR.
We began investigating the effect of cellular stress on retinoid signaling with HeLa cells because they express functional RAR and RXR proteins that transcriptionally activate RAREs in response to ligand (22). We treated HeLa cells with low-dose UV radiation (60 J/m2), which activates JNK, p38, and their downstream kinases (37). UV radiation inhibited RA-induced transactivation of a DR5 response element (Fig. 1A). Consistent with this finding, UV treatment inhibited RA-induced transcription of the RARβ gene (Fig. 1B), which contains a DR5 response element in its gene promoter (15). We next investigated which components of the RXR/RAR complex (RAR, RXR, or both) are targeted by stress pathways. HeLa cells were cotransfected with a reporter containing the GAL4 response element and an expression construct containing the GAL4 DNA binding domain fused to RARα or RXRα, treated with 9-cis RA (a ligand for RARs and RXRs), and subjected to UV radiation (Fig. 1C). Relative to the effect of vehicle, treatment with 9-cis RA transactivated GAL-RAR 20 fold (P = 0.045; Mann-Whitney test) and GAL-RXR 2 fold (P < 0.001; Mann-Whitney test). Relative to the effect of ligand alone, treatment with 9-cis RA plus UV irradiation decreased GAL-RAR transactivation 3.6 fold (P = 0.045) and increased GAL-RXR transactivation 1.3 fold (P < 0.001). Given that UV inhibited ligand-induced transactivation of RXR-RAR heterodimers (as shown by DR5 luciferase activity and expression of an RXR-RAR target gene), the RAR effect appears to be dominant. Together, these findings indicate that stress inhibits retinoid signaling through RAR.
FIG. 1.
UV irradiation inhibits retinoid signaling through RAR. (A) UV irradiation inhibits ligand-induced RARE activation. HeLa cells were transiently cotransfected with DR5TK-luc reporters and the β-Gal expression plasmid. After 24 h, the cells were serum starved overnight and then treated (+) or not treated (−) with UV radiation. Cells were treated or not treated with 1 μM RA and subjected to luciferase assays. Luciferase activity was normalized to other wells by β-Gal values and expressed as the means and standard deviations from three identical wells. (B) UV radiation inhibits ligand-induced RAR target gene expression. Calu-6 cells were treated (+) or not treated (−) with UV radiation and then treated or not treated with 1 μM RA. Total RNA was isolated and analyzed for RARβ and GAPDH transcripts by semiquantitative RT-PCR. (C) UV inhibits transcriptional activation of RAR but not RXR. HeLa cells were transiently cotransfected with G5E1b-luc reporter construct, β-Gal plasmid, and GAL-RARα or GAL-RXRα. Cells were treated (+) or not treated (−) with UV, treated or not treated with 1 μM 9-cis RA (a ligand for both RAR and RXR), and subjected to luciferase assays as described above. The experiment was performed three times, and the results of one experiment are illustrated. Results are means ± standard deviations from three identical wells for GAL-RAR and nine identical wells for GAL-RXR.
Stress-induced RARα protein loss is blocked by proteasome inhibitors.
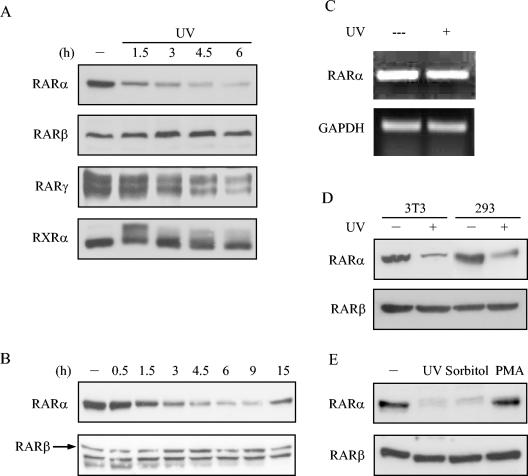
One mechanism by which stress regulates the function of certain target proteins, including p21, cdc10-dependent transcript 1, and RNA polymerase II, is through degradation by the ubiquitin-proteasome pathway (4, 5, 25). Hypothesizing that stress activates RAR degradation, we examined RARα protein levels in HeLa cells after UV irradiation. Western blots of nuclear fractions revealed a drastic reduction in protein levels of RARα, a lesser reduction in RARγ, and no reduction in RARβ or RXRα (Fig. 2A). This reduction in RARα was first detected at 90 min, reached maximal levels at 4.5 h, and returned to baseline levels at 15 h (Fig. 2B). To examine whether this loss of RARα in the nuclear fraction resulted from shuttling of proteins to cytoplasm, we examined RARα levels in whole-cell extracts and in cytosolic fractions. UV irradiation did not lead to appreciable changes in cytosolic RARα levels (data not shown), suggesting that this loss of protein was not due to nuclear export. RT-PCR analysis revealed that UV irradiation did not alter mRNA levels of RARα (Fig. 2C), suggesting that loss of RARα protein was mediated posttranscriptionally. UV irradiation decreased RARα protein levels in 293, 3T3 (Fig. 2D), and COS-1 cells (data not shown), supporting a role for stress pathways in RAR regulation in a variety of cell types. In addition to UV irradiation, RARα protein levels also decreased in response to treatment with sorbitol but not with PMA (Fig. 2E), supporting a specific role for stress pathways in the regulation of RARα levels.
FIG. 2.
Effects of stress on RAR levels. HeLa cells (A and B), 3T3 cells (D), and 293 cells (D) were treated (+) or not treated (−) with UV radiation, lysed at the indicated times (A and B) for preparation of nuclear fractions, and subjected to Western blotting with antibodies to the indicated proteins. (C) UV radiation does not alter RARα mRNA levels. HeLa cells were treated (+) or not treated (−) with UV radiation, and total RNA was isolated and analyzed for RARα and GAPDH transcripts by semiquantitative RT-PCR. (E) HeLa cells were treated (+) or not treated (−) with UV radiation, sorbitol (400 mM), or PMA (500 nM) for 4.5 h and subjected to Western blotting. Because its levels were not measurably changed by UV radiation, RARβ was used as a control in these experiments. Note that for the data shown in panel B, Western blotting with anti-RARβ antibodies gave rise to a lower-molecular-weight, nonspecific band that closely migrated with RARβ.
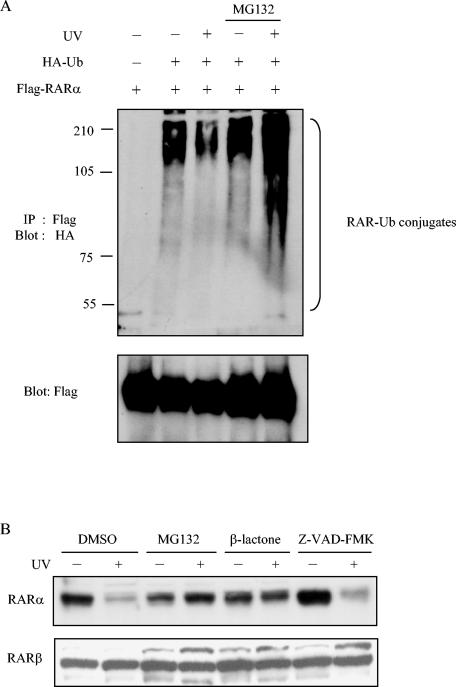
Next, to examine whether UV irradiation reduced RARα protein levels via ubiquitin-proteasome-dependent mechanisms, we transfected HeLa cells with Flag-tagged RARα along with hemagglutinin (HA)-tagged ubiquitin. Cells were treated or not treated with proteasomal inhibitor MG132, and extracts were prepared after exposure to UV radiation. RARα was immunoprecipitated with anti-Flag antibodies and immunoblotted with anti-HA antibodies. High-molecular-weight bands representing ubiquitinated forms of RARα increased in samples exposed to UV radiation in the presence of proteasomal inhibitor (Fig. 3A, lane 5). This UV-dependent loss of RARα was blocked by the 26S proteasome inhibitors MG132 and β-lactone but not by the caspase inhibitor Z-VAD-FMK (Fig. 3B), indicating that RARα loss required activation of the proteasome pathway.
FIG. 3.
Loss of RARα protein by UV radiation is blocked by proteasome inhibitors. (A) In vivo ubiquitination assays were performed with HeLa cells transiently cotransfected with Flag-tagged RARα and HA-tagged ubiquitin plasmids. After transfection, cells were preincubated with or without 50 μM MG132 for 1 h and then treated (+) or not treated (−) with UV radiation. Cells were lysed at the indicated times and subjected to immunoprecipitation (IP) and Western blotting (blot) with the indicated antibodies to detect RAR-ubiquitin conjugates and, as a control, Flag-tagged RAR. (B) HeLa cells were pretreated for 1 h with vehicle (dimethyl sulfoxide [DMSO]), proteasome inhibitors MG132 (50 μM) or lactacystin-β-lactone (10 μM), and caspase inhibitor Z-VAD-FMK (50 μM) as a control and then treated (+) or not treated (−) with UV. Cells were lysed 4.5 h later and analyzed by Western blotting.
Stress inhibits RAR through JNK.
We next investigated the specific kinases involved in the suppressive effect of stress on retinoid signaling. Transfection of COS-1 cells with a constitutively active mutant form of MEKK-1 (MEKK1Δ), an activator of stress kinases (36), inhibited basal and ligand-induced transactivation of a reporter containing a DR5 response element (Fig. 4A). The suppression of ligand-induced DR5 transactivation by MEKK1Δ greatly exceeded its effect on basal activity of TK-LUC and DR5TK-LUC, indicating that the transcriptional suppression by MEKK1Δ was primarily RAR specific. MEKK1 is known to mediate the effects of stress, in part through MKK4 and MKK7, which phosphorylate JNK and p38 (83). UV irradiation of HeLa cells activated JNK and p38 but not ERK or AKT (Fig. 4B), supporting the possibility that JNK and/or p38 contributes to RARα degradation by stress.
FIG. 4.
Inhibition of RAR function by stress signals is mediated through JNK. (A) MEKK-1 activation inhibits ligand-induced RARE activation. COS-1 cells were transiently cotransfected with expression plasmids containing β-Gal, MEKK1Δ, or empty vector (−), and reporter constructs (DR5TK-luc or TK-luc control). After 36 h, cells were treated or not treated with 1 μM RA and subjected to luciferase assays. After correction for differences in transfection efficiency with β-Gal values, luciferase values were expressed as means ± standard deviations from three identical wells. (B) UV radiation activates JNK and p38 but not AKT or ERK. HeLa cells were serum starved overnight and treated (+) or not treated (−) with UV, insulin-like growth factor 1 (100 ng/ml), or PMA (500 nM). Lysates were subjected to Western blotting with antibodies to the indicated proteins. (C and D) MKK4 activation decreases RARα levels by activating JNK. HeLa cells (106) were treated with medium alone or incubated with Ad expressing constitutively active mutant MKK4 (Ad-MKK4) or control empty vector (Ad-EV) (200 particles/cell). After 24 h, cells were lysed, and lysates were subjected to Western analysis of MKK4 and actin (C) or RARα and β (D) and in vitro kinase assays (C). For the kinase assays, JNK and p38 were immunoprecipitated from the lysates and subjected to immune complex kinase assays with GST-c-Jun as a JNK substrate and GST-ATF2 as a p38 substrate. (E and F) UV-induced RARα loss is blocked by inhibition of JNK but not PI3K or ERK. HeLa cells were treated (+) or not treated (−) with LY294002 (50 μM), U0126 (10 μM), or SP600125 (30 μM); subjected to UV treatment; and lysed. The lysates were subjected to Western blot analysis (RARα, RARβ, P-c-Jun, c-Jun, and poly-ADP ribose polymerase as a loading control) and MAPKAP-K2 in vitro kinase assays. For the kinase assays, lysates were immunoprecipitated to isolate MAPKAP-K2, a p38 substrate, which was subjected to in vitro kinase assays with HSP27 as a substrate.
To investigate the role of stress kinases, we transfected HeLa cells with an Ad expressing constitutively active mutant MKK4 (Ad-MKK4) and found that Ad-MKK4 was expressed at high levels and induced robust activation of JNK but not p38 (Fig. 4C). Ad-MKK4 transfection inhibited RARα protein levels (Fig. 4D). To further investigate the role of JNK, we examined the effect of SP600125, a JNK inhibitor (6), on UV-induced RAR degradation. Treatment with SP600125 blocked UV-induced activation of JNK but not that of p38 (Fig. 4E), demonstrating the relative specificity of SP600125 for JNK. SP600125 partially blocked the decrease in RAR protein levels induced by UV (Fig. 4E), but inhibitors of MEK-1 (U0126) and phosphatidylinositol 3-kinase (LY294002) had no appreciable effect (Fig. 4F). Together, these findings support a specific role for JNK in the regulation of RAR stability.
RAR is a JNK substrate.
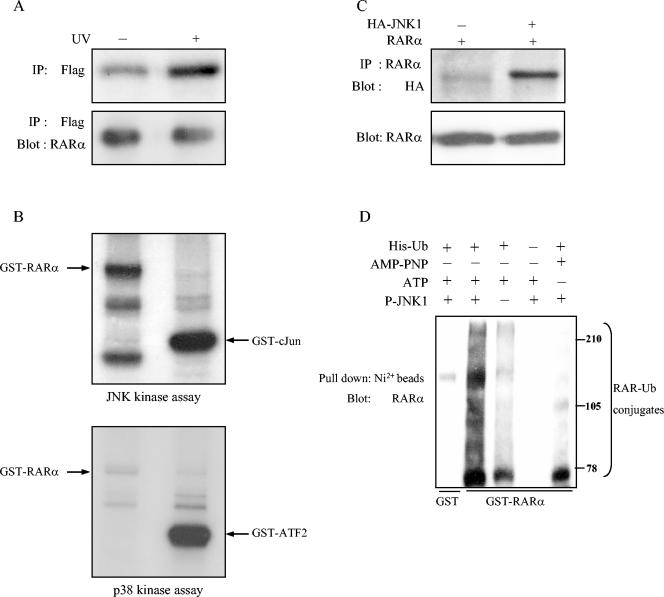
Given our finding that JNK regulates RAR stability, we hypothesized that RAR is a stress kinase substrate. To investigate this possibility, we performed in vivo labeling experiments to examine whether UV radiation induces RAR phosphorylation. HeLa cells were cotransfected with Flag-tagged RARα and JNK1, labeled with [32P]orthophosphate, stimulated with UV irradiation or not, and subjected to immunoprecipitation with anti-Flag antibodies. UV irradiation led to increased RARα phosphorylation (Fig. 5A), providing in vivo evidence that RAR is phosphorylated in response to stress.
FIG. 5.
JNK phosphorylates RARα and promotes ubiquitination. (A) JNK phosphorylates RARα in intact cells. HeLa cells were transiently cotransfected with expression vectors containing Flag-tagged RARα and JNK1, labeled with [32P]orthophosphate, treated (+) or not treated (−) with UV radiation, and lysed. RARα was immunoprecipitated (IP) from cell lysates with anti-Flag antibodies, and the immunoprecipitate was visualized by autoradiography (top) or subjected to Western blotting with anti-RARα antibodies (bottom). (B) JNK phosphorylates RARα in vitro. JNK and p38 kinases were immunoprecipitated from HeLa cells after UV irradiation to activate stress pathways and subjected to in vitro kinase assays with GST-RARα, GST-c-Jun, and GST-ATF2 as substrates. GST-c-Jun and GST-ATF2 were positive controls for JNK and p38, respectively. (C) RARα and JNK1 physically interact in vivo. COS-1 cells were transiently cotransfected with expression vectors containing RARα and HA-tagged JNK1 or empty vector (−) and lysed, and the lysates were subjected to immunoprecipitation with RARα antibodies, followed by Western blotting with anti-HA and RAR antibodies. (D) RARα degradation is JNK dependent. GST or GST-RARα was incubated with (lanes 1, 2, 4, and 5) or without (lane 3) active JNK1. Afterwards, the mixture was subjected to an ubiquitination reaction in the presence of an S-100 fraction of HeLa extract, His-ubiquitin (lanes 1, 2, 3, and 5), and ATP (lanes 1 to 4). In lane 5, ATP was replaced by AMP-PNP, which supports ubiquitination but not kinase reactions.
RARα tagged with GST subjected to in vitro kinase reactions showed that activated JNK, but not p38, phosphorylated RARα (Fig. 5B). Consistent with its role as a direct substrate, RARα physically interacted with JNK in COS-1 cells (Fig. 5C). To examine whether ubiquitination of RAR is stimulated by its phosphorylation by JNK, we subjected GST-RAR to an in vitro kinase reaction with JNK and then to an in vitro ubiquitination reaction in the presence of an S-100 fraction of HeLa cell extracts, which contain ubiquitin ligases E1, E2, and E3. JNK-phosphorylated RAR exhibited more high-molecular-weight (ubiquitinated) forms than did untreated RAR (Fig. 5D, lanes 2 and 3). Multiubiquitinated forms of RARα were reduced when His-ubiquitin or ATP was not included in the reaction mixtures (Fig. 5D, lanes 4 and 5), indicating that the higher-molecular-weight bands contain ubiquitin and that their formation depended on ATP. Notably, the ATP-absent condition (Fig. 5D, lane 5) included the nucleotide triphosphate AMP-PNP, which can support ubiquitin ligase but not JNK activity (24)—an indication that JNK activity is required for increased ubiquitination of RAR.
Identification of residues on RARα phosphorylated by JNK.
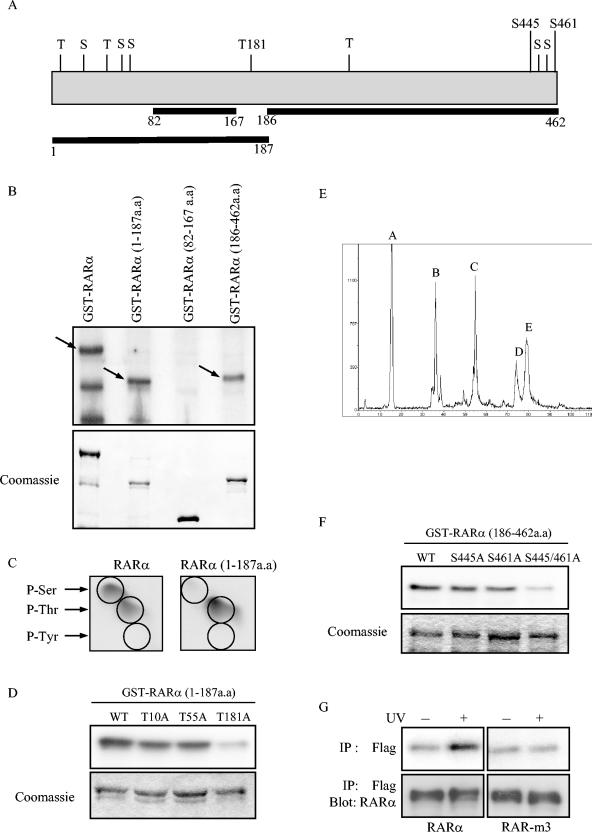
Primary amino acid sequence analysis of RARα revealed 11 putative JNK phosphorylation sites (consensus S/TP) (Fig. 6A). To identify the regions phosphorylated by JNK, we constructed several GST-tagged RARα deletion mutants for expression as recombinant proteins and subjected them to in vitro kinase reactions with active JNK1. JNK1 efficiently phosphorylated the fragments spanning aa 1 to 187 and aa 186 to 462 of RARα (Fig. 6B). Phosphoamino acid analysis of wild-type RARα revealed phosphoserine and phosphothreonine residues (Fig. 6C), a finding consistent with the Ser/Thr-specific kinase activity of JNK (29).
FIG. 6.
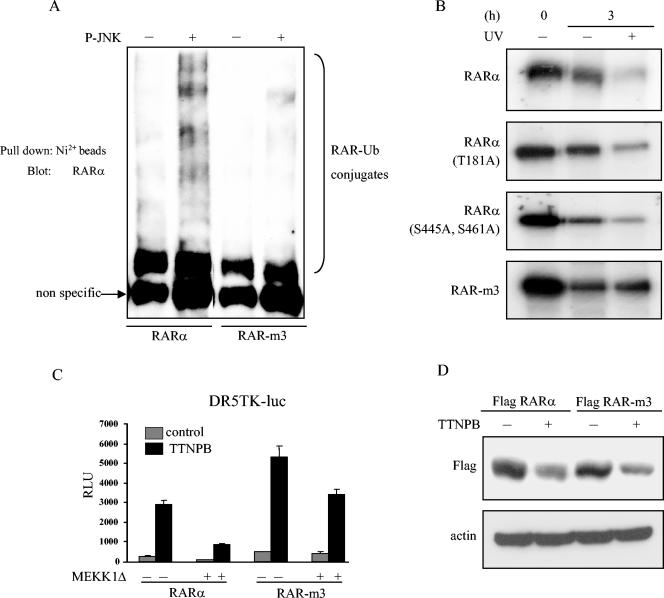
Identification of JNK phosphorylation sites on RARα. (A) Schematic representation of RARα depicting serines (S) and threonines (T), considered potential JNK phosphorylation sites. Three sites that were selected for mutagenesis (T181, S445, and S461) are indicated, as are amino acid coordinates and areas chosen for deletion constructs. (B) Localization of JNK phosphorylation sites. JNK in vitro kinase reactions wereperformed with recombinant wild-type GST-RARα and deletion mutants as substrates. Phosphorylated proteins were resolved by SDS-PAGE, followed by autoradiography (top). Phosphorylated bands of the appropriate molecular weights are indicated with arrows. Smaller bands are proteolytic fragments. Gel loading was illustrated by Coomassie staining (bottom). (C) JNK increases phosphoserine (P-Ser) and phosphothreonine (P-Thr) but not phosphotyrosine (P-Tyr). JNK in vitro kinase reactions were performed with GST-tagged wild-type RARα and RARα (aa 1 to 187) as substrates, and the reaction mixtures were subjected to phosphoamino acid analysis. (D) T181 is a JNK phosphorylation site. In vitro JNK kinase reactions were performed with recombinant GST-tagged wild-type RARα (aa 1 to 187) and the indicated mutants as substrates. Gel loading was illustrated by Coomassie staining. (E) Phosphopeptides were examined by reverse-phase HPLC analysis. In vitro kinase reactions were performed with active JNK and GST-RARα (aa 186 to 462) as substrate. The labeled peptides were separated by SDS-PAGE, and the corresponding phosphorylated bands were excised and subjected to limited trypsin digestion. The phosphopeptides were separated by reverse-phase HPLC and detected by an online radioactivity detector. The phosphopeptide peaks are illustrated. (F) S445 and S461 are JNK phosphorylation sites. In vitro JNK kinase assays were performed with GST-RARα (aa 186 to 462) and the indicated mutants as substrates. (G) UV does not phosphorylate RAR-m3 in intact cells. HeLa cells were transiently transfected with wild-type Flag-RARα or Flag-RAR-m3, labeled with [32P]orthophosphate, and lysed. RARα was immunoprecipitated from cell lysates with anti-Flag antibodies, and the immunoprecipitate was visualized by autoradiography (top) or subjected to Western blotting (IP:Blot) with anti-RARα antibodies (bottom).
Phosphoamino acid analysis of the fragment spanning aa 1 to 187 of RARα demonstrated phosphothreonine but no phosphoserine (Fig. 6C), which allowed us to exclude three of the six potential JNK phosphorylation sites in that region. Because no trypsin digestion sites were present in that region for phosphopeptide mapping, we performed site-directed mutagenesis (Thr to Ala) at all three threonine residues (Thr-10, Thr-55, and Thr-181). These mutants were then expressed as recombinant proteins containing single mutations and subjected to in vitro kinase assays with active JNK. JNK phosphorylated RARα (spanning aa 1 to 187) mutated at Thr-10 or Thr-55 as efficiently as it did the wild-type protein (Fig. 6D). However, mutation of Thr-181 abolished RARα (aa 1 to 187) phosphorylation by JNK (Fig. 6D).
Given that RARα (aa 1 to 187) phosphorylation by JNK produced only phosphothreonine, we inferred that JNK phosphorylates both serines and threonines in the C-terminal region (aa 186 to 462). To identify these residues, we generated tryptic phosphopeptides of RARα (aa 186 to 462) and separated them on a C18 reverse-phase high-performance liquid chromatography (HPLC) column (Fig. 6E). Each peak was subjected to manual Edman degradation. Peaks A and B released in cycles 7 and 13, respectively, corresponding to Ser-461 and Ser-445. Site-directed mutagenesis (Ser to Ala) at both of these sites was sufficient to abrogate phosphorylation of RARα (aa 186 to 462) (Fig. 6F).
Thus, in vitro analysis demonstrated that JNK phosphorylated RARα at Thr-181, Ser-445, and Ser-461. To examine the importance of these residues in UV-induced RARα phosphorylation in intact cells, we performed 32P-labeling experiments in HeLa cells transfected with wild-type or mutant RARα (T181A, S445A, S461A, or RAR-m3). Mutation at these sites completely blocked UV-induced RARα phosphorylation (Fig. 6G), supporting the hypothesis that these residues are the phosphorylation sites of JNK in vivo.
We next examined the importance of these JNK phosphorylation sites in RARα ubiquitination and degradation. In vitro ubiquitination assays revealed that the RAR-m3 mutant was not ubiquitinated as efficiently as wild-type RARα in the presence of JNK (Fig. 7A). In contrast, RARα mutated at one or two sites produced increased amounts of ubiqutin conjugates in the presence of JNK1 (data not shown). Pulse-chase analysis demonstrated that UV radiation did not affect the half-life of RAR-m3, whereas the wild-type, single, or doubly mutated forms of RARα underwent degradation in response to UV (Fig. 7B). These findings indicate that phosphorylation at one or more sites is sufficient for stress-induced ubiquitination and degradation of RARα. In an examination of the importance of RARα phosphorylation in stress-induced suppression of RARE activation by ligand, we found that transfection of COS-1 cells with RAR-m3 partially blocked MEKK1Δ-induced suppression of RARE activation (Fig. 7C), suggesting that RAR phosphorylation contributes to stress-induced RARE transcriptional suppression but that additional mechanisms also contribute.
FIG. 7.
RAR-m3 is protected from JNK-mediated ubiquitination and degradation. (A) Mutant RARα is resistant to JNK-mediated ubiquitination. Wild-type RARα and RAR-m3 were subjected to kinase reactions with (+) or without (−) active JNK, followed by in vitro ubiquitination reactions. (B) Mutant RARα is resistant to UV-induced degradation. Pulse-chase experiments were carried out to measure the stability of wild-type and mutant RARα. HeLa cells were cotransfected with expression vectors containing JNK1 and wild-type or mutant RARα and labeled with [35S]methionine. Transfectants were treated or not treated with UV radiation at the start of the chase (t = 0). After 3 h, cells were lysed, and RARα was immunoprecipitated with anti-Flag antibodies and visualized by autoradiography. (C) Transfection of mutant RARα partially abrogates stress kinase-induced suppression of RARE activity. COS-1 cells were cotransfected with DR5TK-luc reporters and expression vectors containing MEKK1Δ, β-Gal, and wild-type RARα or RAR-m3. Transfectants were treated or not treated with 1 μM TTNPB and subjected to luciferase assays. Results were corrected for differences in transfection efficiency and expressed as the means ± standard deviations from three identical wells. (D) Mutant RARα undergoes degradation after ligand binding. HeLa cells were transiently transfected with expression vectors containing wild-type RARα or RAR-m3. After 24 h, cells were treated (+) or not treated (−) with TTNPB (10−6 M) and lysed, after which the lysates were subjected to Western blot analysis with anti-Flag antibodies.
Ligand induces RARα degradation through a mechanism different from that of phosphorylation.
Ligand binding induces degradation of nuclear hormone receptors, including RARs, through the ubiquitin-proteasome pathway (35, 60). Conformational changes induced by ligand binding or the creation of docking sites by phosphorylation are required for E3 ubiquitin ligases to bind to the target protein (38, 60). Therefore, we hypothesized that phosphorylation and ligand binding induce RARα degradation through a common mechanism. To test this, we examined whether RAR-m3 is resistant to degradation induced by ligand binding. We transfected HeLa cells with Flag-tagged wild-type RARα or RAR-m3 and examined the levels of these receptors after treating the cells with the synthetic retinoid TTNPB, an RAR-specific ligand (80). Ligand treatment resulted in a similar decrease in the levels of both wild-type RARα and RAR-m3 (Fig. 7D). On the basis of these findings, we propose that phosphorylation by JNK and ligand binding induce RARα degradation through distinct mechanisms.
Role of JNK in retinoid resistance.
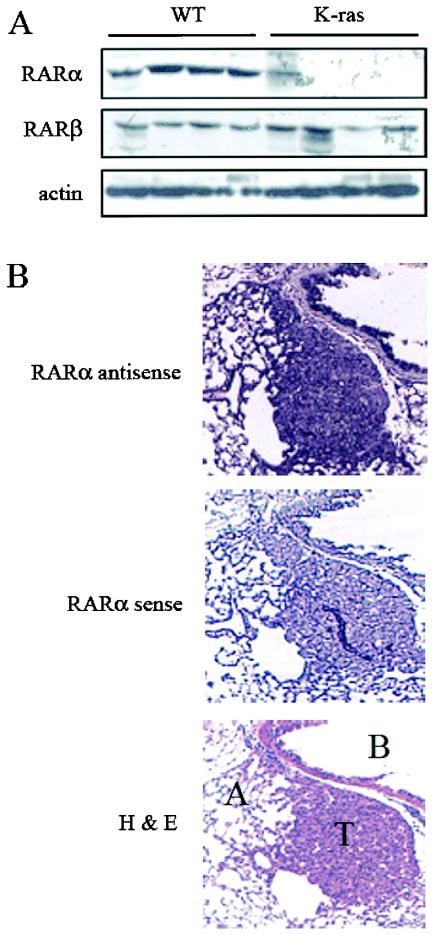
We hypothesized that JNK activation contributes to retinoid resistance in NSCLC cells. To test this question, we used two model systems, K-rasLA1 mice and human NSCLC cell lines. We previously showed that JNK is activated in lung tumors of K-rasLA1 mice (42). Treatment of K-rasLA1 mice with TTNPB had no detectable effect on lung tumor size or number (data not shown), indicating that these tumors are retinoid resistant. Lung tumors from K-rasLA1 mice expressed lower levels of RARα than did normal lung tissues from wild-type littermates (Fig. 8A). RARα mRNA levels were similar in tumors and adjacent normal lung (Fig. 8B), indicating that the reduction in RARα was not the result of decreased gene transcription. Thus, mice that develop lung cancer from activation of a latent K-ras oncogene had high intratumoral JNK activity and a posttranscriptional reduction in RARα levels and were resistant to treatment with an RAR ligand.
FIG. 8.
Loss of RARα in lung tumors from K-rasLA1 mice. (A) RAR protein levels in lung tissues from K-rasLA1 mice and wild-type (WT) littermates. Lung tissue extracts were analyzed by Western blotting with antibodies to RARα, RARβ, and actin. (B) RARα mRNA levels in tumors and adjacent normal lung tissues from K-rasLA1 mice. Lung tissues were subjected to RNA in situ studies (RARα) with antisense and sense probes to RARα. Tumor (T), alveoli (A), and bronchial epithelium (B) are indicated on an adjacent tissue section stained with hematoxylin and eosin (H&E). Hybridization of lung tissues with a sense RARα probe demonstrated no detectable stain demonstrating specificity of binding.
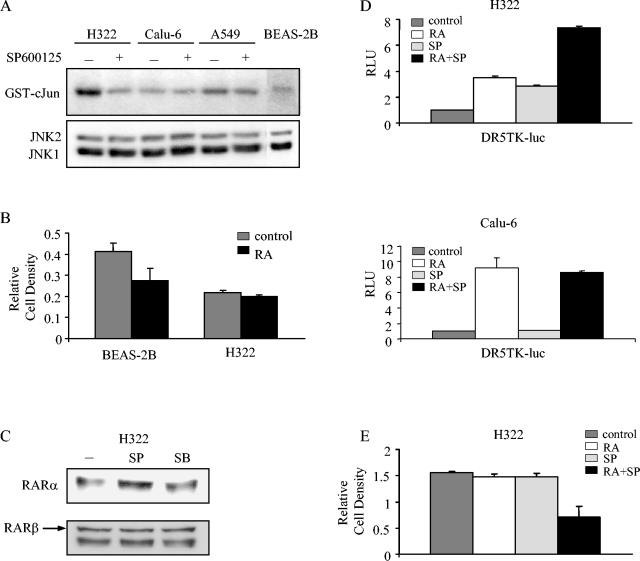
Among human NSCLC cell lines, JNK1 and JNK2 were expressed at similar levels, but JNK activity varied, with the highest basal JNK activity found in H322 cells (Fig. 9A). Relative to an immortalized HBE cell line (BEAS-2B), H322 cells were resistant to the growth inhibitory effects of RA (Fig. 9B), a finding consistent with a previous report of an RAR transcriptional defect in H322 cells (57). We then examined the dependence of RARα levels on JNK activity. Basal RARα levels in H322 cells increased after treatment with the JNK inhibitor SP600125 but not with the p38 inhibitor SB203580 (Fig. 9C). We next investigated whether JNK inhibition was sufficient to enhance ligand-induced activation of a DR5 response element. SP600125 increased ligand-induced DR5 activity by twofold, relative to the effect of ligand alone in H322 cells, but not in Calu-6 cells (Fig. 9D). Further, combined treatment with SP600125 and RA was sufficient to reduce cell proliferation by 50%, whereas treatment with either agent alone had no discernible effect (Fig. 9E). These findings support the hypothesis that JNK contributes to retinoid resistance in a subset of NSCLC cells.
FIG. 9.
Role of JNK in retinoid resistance in lung cancer. (A) JNK levels and activity in NSCLC and HBE cells. NSCLC cell lines and the simian virus 40-immortalized HBE cell line BEAS2B were treated (+) or not treated (−) with SP600125 (15 μM) for 16 h. JNK was immunoprecipitated from cell lysates and subjected to immune complex kinase assays with GST-c-Jun as a substrate. The same lysates were analyzed for JNK protein levels by Western blotting (bottom). (B) Effect of RA on BEAS-2B and H322 cell growth. BEAS2B and H322 cells were treated for 6 days with medium alone or 1 μM RA and subjected to MTT assays. Results are expressed as the means ± standard deviations from three identical wells. (C) Effect of JNK and p38 inhibitors on RAR levels. H322 cells were treated for 16 h with medium alone, the JNK inhibitor SP600125 (SP) (15 μM), or the p38 inhibitor SB203580 (SB) (10 μM) and lysed, after which the cell lysates were subjected to Western blotting. (D) Effect of RA and SP600125 on RARE activity. H322 and Calu-6 cells were transiently cotransfected with β-Gal vector and DR5TK-luc reporter constructs. Transfectants were treated for 16 h with medium alone, RA (1 μM), SP600125 (1 μM), or both and subjected to luciferase assays. Values were corrected for differences in transfection efficiency and expressed as the means ± standard deviations from three identical wells. (E) Effect of RA and SP600125 on H322 cell growth. H322 cells were treated for 4 days with medium alone, RA (1 μM), SP600125 (5 μM), or both, and cell density was measured by MTT assays. Values were expressed as the means ± standard deviations from three identical wells.
DISCUSSION
Retinoid receptors are transcriptionally activated by ligand binding. However, receptor activation is blocked by cellular stresses; for example, UV irradiation inhibits ligand-induced transcription of RXR-RAR target genes (76), and peptide growth factors decrease retinoid-induced expression of RXR-RAR target genes and block the biologic effects of retinoids on cells (3, 53). We previously demonstrated that RXR is phosphorylated in response to cellular stress (43), but findings presented here and by others (1) suggest that stress-induced RXR phosphorylation does not measurably alter RXR function or stability. This was evident in Fig. 2A, as the mobility of RXRα protein was reduced in response to UV treatment, probably due to phosphorylation, but there was no change in protein levels. Thus, the mechanisms by which stress inhibits retinoid receptor function have not been fully defined. Here, we present the first evidence that RARα is a cellular target of JNK and that RAR phosphorylation is a crucial mediator of the effects of JNK activation on retinoid signaling.
Cellular stresses activate the stress-activated protein kinases, which include the MAP kinases JNK1 to -3 and p38/HOG-1 (66). These MAP kinases are activated directly by MKK4 and MKK6 through dual phosphorylation at Thr-183 and Tyr-185 (46). p42 and p44 MAP kinases are activated by receptor tyrosine kinases through Ras/Raf/MKK1 signaling pathways (8). MAP kinases (p42, p44, p38, and JNKs) phosphorylate certain nuclear hormone receptors, including glucocorticoid receptor, estrogen receptor, peroxisomal proliferator-activated receptor λ, RAR, RXR, and steroidogenic factor 1 (1, 9, 19, 20, 26, 31, 43, 64, 72). Here, we have extended these findings by providing the first evidence that RAR is a stress kinase substrate. The three JNK phosphorylation sites we identified on RARα are conserved across mammalian species. Two of the JNK sites on RARα, T181 and S445, are conserved on RARγ but not RARβ, and RARβ was conspicuously resistant to degradation by UV radiation, providing evidence that the presence of these sites correlates with protein degradation by UV radiation. Our findings differed from those of Wang and colleagues (76), who showed that UV radiation induced loss of RAR protein by decreasing mRNA levels rather than inducing proteolysis. This difference may reflect the much higher UV dose administered in their study or biological differences between the cell types in the two studies. Together, these findings demonstrate that MAP kinases phophorylate a wide range of nuclear receptors, raising the possibility that MAP kinases are crucial regulators of nuclear receptor function. Indeed, phosphorylation induces profound changes in nuclear receptor properties, including alterations in DNA binding, ligand binding, and transcriptional activation, in a receptor-specific fashion (7, 31, 72).
We found that stress induced RARα proteolysis through the ubiquitin-proteasome pathway. UV treatment did not degrade an RARα mutant lacking JNK phosphorylation sites (RAR-m3), indicating that phosphorylation by JNK was necessary for RAR degradation. Notably, RARα degradation by UV was ligand independent. In vitro assays revealed that JNK could efficiently phosphorylate both liganded and unliganded RARα (data not shown), suggesting that conformation changes due to ligand binding did not sterically hinder RARα phosphorylation by JNK. Heretofore, nuclear receptor degradation had been observed only in the presence of ligand (23, 39, 48). RXR degrades when heterodimerized with RAR or thyroid receptor, presumably through conformational changes in response to ligand binding by RAR or thyroid receptor (60). In the case of RARγ, ligand-induced receptor degradation is activated by p38, which phosphorylates RARγ (19). Further, the 26S proteasomal subunit SUG1 associates with the C terminus of RARγ and contributes to ligand-induced degradation (19, 74). In light of these findings, we tested the hypothesis that cellular stress and ligand binding induce RARα degradation through a common pathway. We found that RAR-m3 and wild-type RARα were degraded to a similar extent in response to ligand binding, arguing against this hypothesis. Additional studies will be needed to understand why our findings do not support the RARγ model (19). Potentially contributing to this difference, the RARγ sites phosphorylated by p38 (serines 66 and 68) differed from the RARα sites phosphorylated in response to UV treatment. As a consequence, stress and ligand may differ with respect to the specific F-box proteins and proteasomal complexes that are required for ubiquitination and proteolysis of these RAR family members. Supporting this hypothesis, we observed that the kinetics of RARα degradation differed in response to phosphorylation and ligand binding.
In UV-treated skin, RARγ expression decreases coincidentally with suppression of ligand-induced RAR target gene expression (76). We observed a similar temporal linkage between RARα loss and suppression of retinoid signaling by UV treatment. In testing the hypothesis that RARα degradation is sufficient to suppress ligand-induced RARE activation, we found that transfection of RAR-m3 only partially blocked suppression of RARE activity by activated MEKK-1, arguing against the hypothesis that RARα phosphorylation is sufficient. Thus, we conclude that UV-induced suppression of retinoid signaling requires phosphorylation of RARα and other substrates, including, possibly, RARγ, RAR transcriptional coactivators (which are kinase substrates), and components of the transcription initiation complex (2, 11, 77). These substrates may be phosphorylated by JNK or by other stress kinases activated by UV. The complexity of UV effects stands in contrast to what seems to be a simpler mechanism in H322 NSCLC cells, in which JNK inhibition alone was sufficient to enhance RARα levels and potentiate ligand-induced RARE activation. Thus, we conclude that mechanisms of retinoid resistance in response to UV treatment differ from those found in NSCLC cells.
Investigations into the basis of retinoid resistance in NSCLC have revealed retinoid receptor functional defects. RAR and RXR family members expressed in NSCLC cells show no evidence of genetic mutations (17, 18). Transfection of wild-type RARs and RXRs does not restore ligand-inducible transcriptional activity (85), suggesting that the defect is extrinsic to the receptor complex. Although biochemical evidence suggests either a loss of RAR transcriptional coactivators or the presence of transcriptional suppressors (57), the precise nature of this defect has not been fully defined. We sought to examine the role of JNK in RAR dysfunction in NSCLC cells. MAP kinases are activated in NSCLC cells as a consequence of K-ras gene mutations, which are found in 30% of lung adenocarcinomas, and overexpression of receptor tyrosine kinases such as ErbB1 and ErbB2, which occurs in approximately 50% of NSCLC cells (30, 55, 65). We previously showed that retinoid treatment suppresses JNK activity in NSCLC cells by increasing the expression of MAP kinase phosphatase-1 and that increased MAP kinase phosphatase-1 expression correlates with enhanced RAR transcriptional activation by ligand (41).
On the basis of these findings, we hypothesized that JNK phosphorylates RARs, inhibiting ligand-induced transcriptional activation of the receptors and contributing to retinoid receptor dysfunction in NSCLC cells. Findings reported here in two models, a panel of NSCLC cell lines and K-rasLA1 mice, support this hypothesis and raise the possibility that JNK contributes to retinoid resistance through phosphorylation and degradation of RAR, which is the focus of ongoing studies. However, our findings in H322 cells have not excluded the possibility that JNK has substrates other than RAR that contribute to retinoid refractoriness. Previous studies indicate that retinoid resistance in NSCLC is a complex, multifactorial process. Two orphan nuclear receptors, COUP-TF and TR3 (also known as Nurr77), regulate retinoid sensitivity through COUP-TF/TR3 interactions, which inhibit RAR target gene expression, and through COUP-TF binding to DNA promoter elements in RAR target genes, which enhances RAR target gene expression (47, 82). Further, RAR target genes are silenced in NSCLC cells epigenetically through methylation of CpG islands in DNA promoters (73). We observed increased RARβ mRNA levels with RA treatment in Calu-6 cells but not in HeLa or COS-1 cells (data not shown), indicating that cell type-dependent mechanisms also regulate RARβ expression. Thus, during the process of malignant transformation, HBE cells acquire multiple biochemical and genetic changes that cause them to escape the normal growth inhibitory effects of retinoids.
These findings have implications from the standpoint of lung tumorigenesis. Retinoids are crucial for normal embryogenesis and the maintenance of epithelial cell morphology and function (49). Retinoid signaling is blocked by peptide growth factors and cellular stress, which contribute to lung tumorigenesis (3, 53). Conversely, retinoids suppress activation of kinase pathways by peptide growth factors (28, 44, 59, 84). Thus, mutual antagonism between growth stimulatory and inhibitory pathways creates a balance that is critical to maintaining normal cellular behavior. Signaling pathways required for normal development and cellular function are often suppressed or mutated in cancer (21). Consistent with this theme, expression of RARs is suppressed in NSCLC cells, and reintroduction of RARs inhibits the growth of lung cancer cells (75, 78). Further, TR3 is a kinase substrate that enhances the mitogenic effects of peptide growth factors in NSCLC cells (34). From these findings, kinase pathways contribute to lung tumorigenesis, in part, through their effects on nuclear receptors, including RARs.
Acknowledgments
This work was supported in part by NIH grants R01CA80686 and U01CA84306.
REFERENCES
- 1.Adam-Stitah, S., L. Penna, P. Chambon, and C. Rochette-Egly. 1999. Hyperphosphorylation of the retinoid X receptor alpha by activated c-Jun NH2-terminal kinases. J. Biol. Chem. 274:18932-18941. [DOI] [PubMed] [Google Scholar]
- 2.Ait-Si-Ali, S., S. Ramirez, F. X. Barre, F. Dkhissi, L. Magnaghi-Jaulin, J. A. Girault, P. Robin, M. Knibiehler, L. L. Pritchard, B. Ducommun, D. Trouche, and A. Harel-Bellan. 1998. Histone acetyltransferase activity of CBP is controlled by cycle-dependent kinases and oncoprotein E1A. Nature 396:184-186. [DOI] [PubMed] [Google Scholar]
- 3.Andreatta-Van Leyen, S., J. R. Hembree, and R. L. Eckert. 1994. Regulation of insulin-like growth factor 1 binding protein 3 levels by epidermal growth factor and retinoic acid in cervical epithelial cells. J. Cell Physiol. 160:265-274. [DOI] [PubMed] [Google Scholar]
- 4.Beaudenon, S. L., M. R. Huacani, G. Wang, D. P. McDonnell, and J. M. Huibregtse. 1999. Rsp5 ubiquitin-protein ligase mediates DNA damage-induced degradation of the large subunit of RNA polymerase II in Saccharomyces cerevisiae. Mol. Cell. Biol. 19:6972-6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bendjennat, M., J. Boulaire, T. Jascur, H. Brickner, V. Barbier, A. Sarasin, A. Fotedar, and R. Fotedar. 2003. UV irradiation triggers ubiquitin-dependent degradation of p21(WAF1) to promote DNA repair. Cell 114:599-610. [DOI] [PubMed] [Google Scholar]
- 6.Bennett, B. L., D. T. Sasaki, B. W. Murray, E. C. O'Leary, S. T. Sakata, W. Xu, J. C. Leisten, A. Motiwala, S. Pierce, Y. Satoh, S. S. Bhagwat, A. M. Manning, and D. W. Anderson. 2001. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc. Natl. Acad. Sci. USA 98:13681-13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhat, M. K., K. Ashizawa, and S. Y. Cheng. 1994. Phosphorylation enhances the target gene sequence-dependent dimerization of thyroid hormone receptor with retinoid X receptor. Proc. Natl. Acad. Sci. USA 91:7927-7931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boulton, T. G., S. H. Nye, D. J. Robbins, N. Y. Ip, E. Radziejewska, S. D. Morgenbesser, R. A. DePinho, N. Panayotatos, M. H. Cobb, and G. D. Yancopoulos. 1991. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell 65:663-675. [DOI] [PubMed] [Google Scholar]
- 9.Camp, H. S., S. R. Tafuri, and T. Leff. 1999. c-Jun N-terminal kinase phosphorylates peroxisome proliferator-activated receptor-gamma1 and negatively regulates its transcriptional activity. Endocrinology 140:392-397. [DOI] [PubMed] [Google Scholar]
- 10.Cavanaugh, D., E. Johnson, R. E. Price, J. Kurie, E. L. Travis, and D. D. Cody. 2004. In vivo respiratory-gated micro-CT imaging in small-animal oncology models. Mol. Imaging 3:55-62. [DOI] [PubMed] [Google Scholar]
- 11.Chawla, S., G. E. Hardingham, D. R. Quinn, and H. Bading. 1998. CBP: a signal-regulated transcriptional coactivator controlled by nuclear calcium and CaM kinase IV. Science 281:1505-1509. [DOI] [PubMed] [Google Scholar]
- 12.Chen, Z., J. Hagler, V. J. Palombella, F. Melandri, D. Scherer, D. Ballard, and T. Maniatis. 1995. Signal-induced site-specific phosphorylation targets I kappa B alpha to the ubiquitin-proteasome pathway. Genes Dev. 9:1586-1597. [DOI] [PubMed] [Google Scholar]
- 13.Davis, R. J. 2000. Signal transduction by the JNK group of MAP kinases. Cell 103:239-252. [DOI] [PubMed] [Google Scholar]
- 14.Dennis, A. P., R. U. Haq, and Z. Nawaz. 2001. Importance of the regulation of nuclear receptor degradation. Front. Biosci. 6:D954-D959. [DOI] [PubMed] [Google Scholar]
- 15.Dey, A., S. Minucci, and K. Ozato. 1994. Ligand-dependent occupancy of the retinoic acid receptor beta 2 promoter in vivo. Mol. Cell. Biol. 14:8191-8201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dignam, J. D., R. M. Lebovitz, and R. G. Roeder. 1983. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 11:1475-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gebert, J. F., N. Moghal, J. V. Frangioni, D. J. Sugarbaker, and B. G. Neel. 1991. High frequency of retinoic acid receptor beta abnormalities in human lung cancer. Oncogene 6:1859-1868. [PubMed] [Google Scholar]
- 18.Geradts, J., J. Y. Chen, E. K. Russell, J. R. Yankaskas, L. Nieves, and J. D. Minna. 1993. Human lung cancer cell lines exhibit resistance to retinoic acid treatment. Cell Growth Differ. 4:799-809. [PubMed] [Google Scholar]
- 19.Gianni, M., A. Bauer, E. Garattini, P. Chambon, and C. Rochette-Egly. 2002. Phosphorylation by p38MAPK and recruitment of SUG-1 are required for RA-induced RAR gamma degradation and transactivation. EMBO J. 21:3760-3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammer, G. D., I. Krylova, Y. Zhang, B. D. Darimont, K. Simpson, N. L. Weigel, and H. A. Ingraham. 1999. Phosphorylation of the nuclear receptor SF-1 modulates cofactor recruitment: integration of hormone signaling in reproduction and stress. Mol. Cell 3:521-526. [DOI] [PubMed] [Google Scholar]
- 21.Hanahan, D., and R. A. Weinberg. 2000. The hallmarks of cancer. Cell 100:57-70. [DOI] [PubMed] [Google Scholar]
- 22.Hauksdottir, H., B. Farboud, and M. L. Privalsky. 2003. Retinoic acid receptors beta and gamma do not repress, but instead activate target gene transcription in both the absence and presence of hormone ligand. Mol. Endocrinol. 17:373-385. [DOI] [PubMed] [Google Scholar]
- 23.Hauser, S., G. Adelmant, P. Sarraf, H. M. Wright, E. Mueller, and B. M. Spiegelman. 2000. Degradation of the peroxisome proliferator-activated receptor gamma is linked to ligand-dependent activation. J. Biol. Chem. 275:18527-18533. [DOI] [PubMed] [Google Scholar]
- 24.Hershko, A., and A. Ciechanover. 1998. The ubiquitin system. Annu. Rev. Biochem. 67:425-479. [DOI] [PubMed] [Google Scholar]
- 25.Higa, L. A., I. S. Mihaylov, D. P. Banks, J. Zheng, and H. Zhang. 2003. Radiation-mediated proteolysis of CDT1 by CUL4-ROC1 and CSN complexes constitutes a new checkpoint. Nat. Cell Biol. 5:1008-1015. [DOI] [PubMed] [Google Scholar]
- 26.Hu, E., J. B. Kim, P. Sarraf, and B. M. Spiegelman. 1996. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARγ. Science 274:2100-2103. [DOI] [PubMed] [Google Scholar]
- 27.Johnson, L., K. Mercer, D. Greenbaum, R. T. Bronson, D. Crowley, D. A. Tuveson, and T. Jacks. 2001. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature 410:1111-1116. [DOI] [PubMed] [Google Scholar]
- 28.Kamei, Y., L. Xu, T. Heinzel, J. Torchia, R. Kurokawa, B. Gloss, S. C. Lin, R. A. Heyman, D. W. Rose, C. K. Glass, and M. G. Rosenfeld. 1996. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell 85:403-414. [DOI] [PubMed] [Google Scholar]
- 29.Karin, M. 1995. The regulation of AP-1 activity by mitogen-activated protein kinases. J. Biol. Chem. 270:16483-16486. [DOI] [PubMed] [Google Scholar]
- 30.Kasprzyk, P. G., T. L. Sullivan, J. D. Hunt, C. T. Gubish, C. A. Scoppa, M. Oelkuct, R. Bird, P. H. Fischer, J. M. Siegfried, and C. R. King. 1996. Activity of anti-erbB-2 recombinant toxin OLX-209 on lung cancer cell lines in the absence of erbB-2 gene amplification. Clin. Cancer Res. 2:75-80. [PubMed] [Google Scholar]
- 31.Kato, S., H. Endoh, Y. Masuhiro, T. Kitamoto, S. Uchiyama, H. Sasaki, S. Masushige, Y. Gotoh, E. Nishida, H. Kawashima, et al. 1995. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science 270:1491-1494. [DOI] [PubMed] [Google Scholar]
- 32.Knotts, T. A., R. S. Orkiszewski, R. G. Cook, D. P. Edwards, and N. L. Weigel. 2001. Identification of a phosphorylation site in the hinge region of the human progesterone receptor and additional amino-terminal phosphorylation sites. J. Biol. Chem. 276:8475-8483. [DOI] [PubMed] [Google Scholar]
- 33.Koepp, D. M., L. K. Schaefer, X. Ye, K. Keyomarsi, C. Chu, J. W. Harper, and S. J. Elledge. 2001. Phosphorylation-dependent ubiquitination of cyclin E by the SCFFbw7 ubiquitin ligase. Science 294:173-177. [DOI] [PubMed] [Google Scholar]
- 34.Kolluri, S. K., N. Bruey-Sedano, X. Cao, B. Lin, F. Lin, Y. H. Han, M. I. Dawson, and X. K. Zhang. 2003. Mitogenic effect of orphan receptor TR3 and its regulation by MEKK1 in lung cancer cells. Mol. Cell. Biol. 23:8651-8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kopf, E., J. L. Plassat, V. Vivat, H. de The, P. Chambon, and C. Rochette-Egly. 2000. Dimerization with retinoid X receptors and phosphorylation modulate the retinoic acid-induced degradation of retinoic acid receptors alpha and gamma through the ubiquitin-proteasome pathway. J. Biol. Chem. 275:33280-33288. [DOI] [PubMed] [Google Scholar]
- 36.Kyriakis, J. M., and J. Avruch. 2001. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 81:807-869. [DOI] [PubMed] [Google Scholar]
- 37.Kyriakis, J. M., and J. Avruch. 1996. Sounding the alarm: protein kinase cascades activated by stress and inflammation. J. Biol. Chem. 271:24313-24316. [DOI] [PubMed] [Google Scholar]
- 38.Laney, J. D., and M. Hochstrasser. 1999. Substrate targeting in the ubiquitin system. Cell 97:427-430. [DOI] [PubMed] [Google Scholar]
- 39.Lange, C. A., T. Shen, and K. B. Horwitz. 2000. Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteasome. Proc. Natl. Acad. Sci. USA 97:1032-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee, H. Y., H. Srinivas, D. Xia, Y. Lu, R. Superty, R. LaPushin, C. Gomez-Manzano, A. M. Gal, G. L. Walsh, T. Force, K. Ueki, G. B. Mills, and J. M. Kurie. 2003. Evidence that phosphatidylinositol 3-kinase- and mitogen-activated protein kinase kinase-4/c-Jun NH2-terminal kinase-dependent pathways cooperate to maintain lung cancer cell survival. J. Biol. Chem. 278:23630-23638. [DOI] [PubMed] [Google Scholar]
- 41.Lee, H. Y., N. Sueoka, W. K. Hong, D. J. Mangelsdorf, F. X. Claret, and J. M. Kurie. 1999. All-trans-retinoic acid inhibits Jun N-terminal kinase by increasing dual-specificity phosphatase activity. Mol. Cell. Biol. 19:1973-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee, H. Y., Y. A. Suh, J. I. Lee, K. A. Hassan, L. Mao, T. Force, B. E. Gilbert, T. Jacks, and J. M. Kurie. 2002. Inhibition of oncogenic K-ras signaling by aerosolized gene delivery in a mouse model of human lung cancer. Clin. Cancer Res. 8:2970-2975. [PubMed] [Google Scholar]
- 43.Lee, H. Y., Y. A. Suh, M. J. Robinson, J. L. Clifford, W. K. Hong, J. R. Woodgett, M. H. Cobb, D. J. Mangelsdorf, and J. M. Kurie. 2000. Stress pathway activation induces phosphorylation of retinoid X receptor. J. Biol. Chem. 275:32193-32199. [DOI] [PubMed] [Google Scholar]
- 44.Lee, H. Y., G. L. Walsh, M. I. Dawson, W. K. Hong, and J. M. Kurie. 1998. All-trans-retinoic acid inhibits Jun N-terminal kinase-dependent signaling pathways. J. Biol. Chem. 273:7066-7071. [DOI] [PubMed] [Google Scholar]
- 45.Lillie, J. W., and M. R. Green. 1989. Transcription activation by the adenovirus E1a protein. Nature 338:39-44. [DOI] [PubMed] [Google Scholar]
- 46.Lin, A., A. Minden, H. Martinetto, F. X. Claret, C. Lange-Carter, F. Mercurio, G. L. Johnson, and M. Karin. 1995. Identification of a dual specificity kinase that activates the Jun kinases and p38-Mpk2. Science 268:286-290. [DOI] [PubMed] [Google Scholar]
- 47.Lin, B., G. Q. Chen, D. Xiao, S. K. Kolluri, X. Cao, H. Su, and X. K. Zhang. 2000. Orphan receptor COUP-TF is required for induction of retinoic acid receptor beta, growth inhibition, and apoptosis by retinoic acid in cancer cells. Mol. Cell. Biol. 20:957-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lonard, D. M., Z. Nawaz, C. L. Smith, and B. W. O'Malley. 2000. The 26S proteasome is required for estrogen receptor-alpha and coactivator turnover and for efficient estrogen receptor-alpha transactivation. Mol. Cell 5:939-948. [DOI] [PubMed] [Google Scholar]
- 49.Mangelsdorf, D. J., and R. M. Evans. 1995. The RXR heterodimers and orphan receptors. Cell 83:841-850. [DOI] [PubMed] [Google Scholar]
- 50.Mangelsdorf, D. J., C. Thummel, M. Beato, P. Herrlich, G. Schutz, K. Umesono, B. Blumberg, P. Kastner, M. Mark, P. Chambon, et al. 1995. The nuclear receptor superfamily: the second decade. Cell 83:835-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McKenna, N. J., R. B. Lanz, and B. W. O'Malley. 1999. Nuclear receptor coregulators: cellular and molecular biology. Endocr. Rev. 20:321-344. [DOI] [PubMed] [Google Scholar]
- 52.McKenna, N. J., J. Xu, Z. Nawaz, S. Y. Tsai, M. J. Tsai, and B. W. O'Malley. 1999. Nuclear receptor coactivators: multiple enzymes, multiple complexes, multiple functions. J. Steroid Biochem. Mol. Biol. 69:3-12. [DOI] [PubMed] [Google Scholar]
- 53.Miller, L. A., L. Z. Cheng, and R. Wu. 1993. Inhibition of epidermal growth factor-like growth factor secretion in tracheobronchial epithelial cells by vitamin A. Cancer Res. 53:2527-2533. [PubMed] [Google Scholar]
- 54.Minden, A., A. Lin, M. McMahon, C. Lange-Carter, B. Derijard, R. J. Davis, G. L. Johnson, and M. Karin. 1994. Differential activation of ERK and JNK mitogen-activated protein kinases by Raf-1 and MEKK. Science 266:1719-1723. [DOI] [PubMed] [Google Scholar]
- 55.Mitsudomi, T., S. M. Steinberg, H. K. Oie, J. L. Mulshine, R. Phelps, J. Viallet, H. Pass, J. D. Minna, and A. F. Gazdar. 1991. ras gene mutations in non-small cell lung cancers are associated with shortened survival irrespective of treatment intent. Cancer Res. 51:4999-5002. [PubMed] [Google Scholar]
- 56.Mitsudomi, T., J. Viallet, J. L. Mulshine, R. I. Linnoila, J. D. Minna, and A. F. Gazdar. 1991. Mutations of ras genes distinguish a subset of non-small-cell lung cancer cell lines from small-cell lung cancer cell lines. Oncogene 6:1353-1362. [PubMed] [Google Scholar]
- 57.Moghal, N., and B. G. Neel. 1995. Evidence for impaired retinoic acid receptor-thyroid hormone receptor AF-2 cofactor activity in human lung cancer. Mol. Cell. Biol. 15:3945-3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Montagnoli, A., F. Fiore, E. Eytan, A. C. Carrano, G. F. Draetta, A. Hershko, and M. Pagano. 1999. Ubiquitination of p27 is regulated by Cdk-dependent phosphorylation and trimeric complex formation. Genes Dev. 13:1181-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nicholson, R. C., S. Mader, S. Nagpal, M. Leid, C. Rochette-Egly, and P. Chambon. 1990. Negative regulation of the rat stromelysin gene promoter by retinoic acid is mediated by an AP1 binding site. EMBO J. 9:4443-4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Osburn, D. L., G. Shao, H. M. Seidel, and I. G. Schulman. 2001. Ligand-dependent degradation of retinoid X receptors does not require transcriptional activity or coactivator interactions. Mol. Cell. Biol. 21:4909-4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reddel, R. R., Y. Ke, B. I. Gerwin, M. G. McMenamin, J. F. Lechner, R. T. Su, D. E. Brash, J. B. Park, J. S. Rhim, and C. C. Harris. 1988. Transformation of human bronchial epithelial cells by infection with SV40 or adenovirus-12 SV40 hybrid virus, or transfection via strontium phosphate coprecipitation with a plasmid containing SV40 early region genes. Cancer Res. 48:1904-1909. [PubMed] [Google Scholar]
- 62.Rochette-Egly, C., S. Adam, M. Rossignol, J. M. Egly, and P. Chambon. 1997. Stimulation of RAR alpha activation function AF-1 through binding to the general transcription factor TFIIH and phosphorylation by CDK7. Cell 90:97-107. [DOI] [PubMed] [Google Scholar]
- 63.Rochette-Egly, C., M. Oulad-Abdelghani, A. Staub, V. Pfister, I. Scheuer, P. Chambon, and M. P. Gaub. 1995. Phosphorylation of the retinoic acid receptor-alpha by protein kinase A. Mol. Endocrinol. 9:860-871. [DOI] [PubMed] [Google Scholar]
- 64.Rogatsky, I., S. K. Logan, and M. J. Garabedian. 1998. Antagonism of glucocorticoid receptor transcriptional activation by the c-Jun N-terminal kinase. Proc. Natl. Acad. Sci. USA 95:2050-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rusch, V., D. Klimstra, I. Linkov, and E. Dmitrovsky. 1995. Aberrant expression of p53 or the epidermal growth factor receptor is frequent in early bronchial neoplasia and coexpression precedes squamous cell carcinoma development. Cancer Res. 55:1365-1372. [PubMed] [Google Scholar]
- 66.Schaeffer, H. J., and M. J. Weber. 1999. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol. Cell. Biol. 19:2435-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schreiber, E., P. Matthias, M. M. Muller, and W. Schaffner. 1989. Rapid detection of octamer binding proteins with ‘mini-extracts’, prepared from a small number of cells. Nucleic Acids Res. 17:6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Solomon, C., J. H. White, and R. Kremer. 1999. Mitogen-activated protein kinase inhibits 1,25-dihydroxyvitamin D3-dependent signal transduction by phosphorylating human retinoid X receptor alpha. J. Clin. Investig. 103:1729-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spencer, E., J. Jiang, and Z. J. Chen. 1999. Signal-induced ubiquitination of IkappaBalpha by the F-box protein Slimb/beta-TrCP. Genes Dev. 13:284-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Steinmetz, A. C., J. P. Renaud, and D. Moras. 2001. Binding of ligands and activation of transcription by nuclear receptors. Annu. Rev. Biophys. Biomol. Struct. 30:329-359. [DOI] [PubMed] [Google Scholar]
- 71.Suh, Y. A., H. Y. Lee, A. Virmani, J. Wong, K. K. Mann, W. H. Miller, Jr., A. Gazdar, and J. M. Kurie. 2002. Loss of retinoic acid receptor beta gene expression is linked to aberrant histone H3 acetylation in lung cancer cell lines. Cancer Res. 62:3945-3949. [PubMed] [Google Scholar]
- 72.Tremblay, A., G. B. Tremblay, F. Labrie, and V. Giguere. 1999. Ligand-independent recruitment of SRC-1 to estrogen receptor beta through phosphorylation of activation function AF-1. Mol. Cell 3:513-519. [DOI] [PubMed] [Google Scholar]
- 73.Virmani, A. K., A. Rathi, S. Zochbauer-Muller, N. Sacchi, Y. Fukuyama, D. Bryant, A. Maitra, S. Heda, K. M. Fong, F. Thunnissen, J. D. Minna, and A. F. Gazdar. 2000. Promoter methylation and silencing of the retinoic acid receptor-beta gene in lung carcinomas. J. Natl. Cancer Inst. 92:1303-1307. [DOI] [PubMed] [Google Scholar]
- 74.vom Baur, E., C. Zechel, D. Heery, M. J. Heine, J. M. Garnier, V. Vivat, B. Le Douarin, H. Gronemeyer, P. Chambon, and R. Losson. 1996. Differential ligand-dependent interactions between the AF-2 activating domain of nuclear receptors and the putative transcriptional intermediary factors mSUG1 and TIF1. EMBO J. 15:110-124. [PMC free article] [PubMed] [Google Scholar]
- 75.Wan, H., W. K. Hong, and R. Lotan. 2001. Increased retinoic acid responsiveness in lung carcinoma cells that are nonresponsive despite the presence of endogenous retinoic acid receptor (RAR) beta by expression of exogenous retinoid receptors retinoid X receptor alpha, RAR alpha, and RAR gamma. Cancer Res. 61:556-564. [PubMed] [Google Scholar]
- 76.Wang, Z., M. Boudjelal, S. Kang, J. J. Voorhees, and G. J. Fisher. 1999. Ultraviolet irradiation of human skin causes functional vitamin A deficiency, preventable by all-trans retinoic acid pre-treatment. Nat. Med. 5:418-422. [DOI] [PubMed] [Google Scholar]
- 77.Wang, Z., D. W. Rose, O. Hermanson, F. Liu, T. Herman, W. Wu, D. Szeto, A. Gleiberman, A. Krones, K. Pratt, R. Rosenfeld, C. K. Glass, and M. G. Rosenfeld. 2000. Regulation of somatic growth by the p160 coactivator p/CIP. Proc. Natl. Acad. Sci. USA 97:13549-13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weber, E., R. K. Ravi, E. S. Knudsen, J. R. Williams, L. E. Dillehay, B. D. Nelkin, G. P. Kalemkerian, J. R. Feramisco, and M. Mabry. 1999. Retinoic acid-mediated growth inhibition of small cell lung cancer cells is associated with reduced myc and increased p27Kip1 expression. Int. J. Cancer 80:935-943. [DOI] [PubMed] [Google Scholar]
- 79.Whitmarsh, A. J., and R. J. Davis. 1996. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J. Mol. Med. 74:589-607. [DOI] [PubMed] [Google Scholar]
- 80.Willhite, C. C., M. I. Dawson, and U. Reichert. 1996. Receptor-selective retinoid agonists and teratogenic activity. Drug Metab. Rev. 28:105-119. [DOI] [PubMed] [Google Scholar]
- 81.Wu, K., H. T. Kim, J. L. Rodriquez, S. G. Hilsenbeck, S. K. Mohsin, X. C. Xu, W. W. Lamph, J. G. Kuhn, J. E. Green, and P. H. Brown. 2002. Suppression of mammary tumorigenesis in transgenic mice by the RXR-selective retinoid, LGD1069. Cancer Epidemiol. Biomarkers Prev. 11:467-474. [PubMed] [Google Scholar]
- 82.Wu, Q., Y. Li, R. Liu, A. Agadir, M. O. Lee, Y. Liu, and X. Zhang. 1997. Modulation of retinoic acid sensitivity in lung cancer cells through dynamic balance of orphan receptors nur77 and COUP-TF and their heterodimerization. EMBO J. 16:1656-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xia, Y., Z. Wu, B. Su, B. Murray, and M. Karin. 1998. JNKK1 organizes a MAP kinase module through specific and sequential interactions with upstream and downstream components mediated by its amino-terminal extension. Genes Dev. 12:3369-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang-Yen, H. F., X. K. Zhang, G. Graupner, M. Tzukerman, B. Sakamoto, M. Karin, and M. Pfahl. 1991. Antagonism between retinoic acid receptors and AP-1: implications for tumor promotion and inflammation. New Biol. 3:1206-1219. [PubMed] [Google Scholar]
- 85.Zhang, X. K., Y. Liu, M. O. Lee, and M. Pfahl. 1994. A specific defect in the retinoic acid response associated with human lung cancer cell lines. Cancer Res. 54:5663-5669. [PubMed] [Google Scholar]