Abstract
GLUT4 is necessary for acute insulin- and contraction-induced skeletal muscle glucose uptake, but its role in chronic muscle loading (overload)-induced glucose uptake is unknown. Our goal was to determine whether GLUT4 is required for overload-induced glucose uptake. Overload was induced in mouse plantaris muscle by unilateral synergist ablation. After 5 days, muscle weights and ex vivo [3H]-2-deoxy-d-glucose uptake were assessed. Overload-induced muscle glucose uptake and hypertrophic growth were not impaired in muscle-specific GLUT4 knockout mice, demonstrating that GLUT4 is not necessary for these processes. To assess which transporters mediate overload-induced glucose uptake, chemical inhibitors were used. The facilitative GLUT inhibitor cytochalasin B, but not the sodium-dependent glucose cotransport inhibitor phloridzin, prevented overload-induced uptake demonstrating that GLUTs mediate this effect. To assess which GLUT, hexose competition experiments were performed. Overload-induced [3H]-2-deoxy-d-glucose uptake was not inhibited by d-fructose, demonstrating that the fructose-transporting GLUT2, GLUT5, GLUT8, and GLUT12 do not mediate this effect. To assess additional GLUTs, immunoblots were performed. Overload increased GLUT1, GLUT3, GLUT6, and GLUT10 protein levels twofold to fivefold. Collectively, these results demonstrate that GLUT4 is not necessary for overload-induced muscle glucose uptake or hypertrophic growth and suggest that GLUT1, GLUT3, GLUT6, and/or GLUT10 mediate overload-induced glucose uptake.
Introduction
Lifestyle modification, including an increase in physical activity, has been demonstrated to reduce the incidence of type 2 diabetes (1). Resistance exercise training has been specifically recommended due to its ability to reduce fasted blood glucose levels (2,3), fasted blood insulin levels (3), and hemoglobin A1c levels (2–4), as well as to increase whole-body glucose disposal (2,5), skeletal muscle mass (2–4), and muscle glucose uptake (5). Given the importance of muscle in maintaining systemic glucose homeostasis, understanding how resistance training alters muscle glucose metabolism may lead to new treatments for type 2 diabetes.
Resistance exercise training is defined as repeated muscle contraction against a load (2–5). In rodents, chronic muscle loading (overload) can be achieved via surgical ablation of synergist muscles or tendons (6–9), and studies have shown that it rapidly and consistently induces adaptations in skeletal muscle similar to resistance training, such as muscle hypertrophy (6–9). In both insulin-sensitive and insulin-resistant mouse muscle, 3–4 days of overload is sufficient to increase muscle mass (30–40%) (6–8) and glucose uptake (∼80%) (6), suggesting that resistance training and overload may use the same cellular mechanisms to regulate muscle metabolism. Unfortunately, these mechanisms are not well understood.
GLUT4 is considered to be the main GLUT in skeletal muscle as a number of studies have demonstrated a positive association between GLUT4 protein levels and muscle glucose uptake. In mouse skeletal muscle, overexpression of GLUT4 increased basal (20–300%), insulin-induced (60–200%), and contraction-induced (35%) muscle glucose uptake (10,11). In contrast, muscle-specific loss of GLUT4 decreased basal glucose uptake (70–80%) and completely prevented insulin- and contraction-induced muscle glucose uptake (11–13). Thus, these findings demonstrate that GLUT4 plays an essential role in mediating skeletal muscle glucose uptake in response to short-term stimulation.
In contrast to short-term stimulation, the role of GLUT4 in mediating muscle glucose uptake in response to long-term stimulation, such as resistance training or muscle overload, is less clear. Studies conducted in humans and rodents have demonstrated an increase (5,9,14–18) as well as no change (16,18–20) in skeletal muscle GLUT4 protein levels in response to resistance training or muscle overload. In addition, previous work in rodent muscle has demonstrated a dissociation between resistance training–induced increases in muscle glucose uptake and GLUT4 protein levels (18), suggesting that another GLUT may be involved. Thus, the role of GLUT4 in mediating resistance training/loading–induced increases in muscle glucose uptake is not clear. Therefore, the objective of this study was to determine whether GLUT4 expression is necessary for the long-term adaptation of overload to stimulate glucose uptake in skeletal muscle.
Research Design and Methods
Materials
Plasmids containing mouse GLUT1 (catalog #MR207871), GLUT3 (catalog #MR2097915), GLUT6 (catalog #MR219710), and GLUT10 (catalog #MR227535) and an HEK293 cell GLUT10 overexpression lysate (catalog #LY410718) were purchased from OriGene Technologies. 2-Deoxy-d-glucose (catalog #D8375), d-mannitol (catalog #M4125), phloridzin dihydrate (catalog #P3449), cytochalasin B (catalog #C6762), l-glucose (catalog #G5500), d-glucose (catalog #G8270), d-fructose (catalog #F0127), d-galactose (catalog #G0750), and d-xylose (catalog #X3833) were purchased from Sigma-Aldrich. 2-[1,2-3H(N)]-Deoxy-d-glucose (catalog #NET549001MC) and d-[1-14C]-mannitol (catalog #NEC314250UC) were purchased from PerkinElmer.
Animals
All procedures were performed in accordance with the East Carolina University Institutional Animal Care and Use Committee and the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Mice were housed in cages at 21–22°C with a 12 h light/dark cycle and fed a standard chow diet (Prolab RMH 3000, catalog #5P00; PMI Nutrition International). Food and water were available ad libitum.
Female CD-1 mice (7–8 weeks old) were obtained from Charles River Laboratories. GLUT4 LoxP mice were generated as previously described (21) and obtained from Dr. Barbara B. Kahn (Beth Israel Deaconess Medical Center). GLUT4 LoxP mice were bred with muscle creatine kinase Cre recombinase transgenic mice (MCK-Cre+; C57BL/6J strain; The Jackson Laboratory) to generate the following mice: wild-type (WT), GLUT4 LoxP+/−, LoxP+/+ (control), MCK-Cre+ (control), muscle-specific GLUT4 heterozygous (mGLUT4 HET) and muscle-specific GLUT4 knockout (mGLUT4 KO). For these studies, both male and female mGLUT4 KO, mGLUT4 HET, and their WT/control littermates (11–12 weeks old) were used.
Body Composition
Mice were weighed, and body composition was assessed using an EchoMRI Model 700 Body Composition Analyzer prior to any surgical procedure.
Transfection of Mouse Muscle Using In Vivo Electroporation
In vivo muscle gene transfer/electroporation was performed using methods described by Hinkley et al. (22). Five days post-transfection, muscles were excised, frozen in liquid nitrogen, and processed for immunoblot analyses.
Unilateral Synergist Ablation Surgery
Muscle overload was induced via unilateral ablation of synergist muscles using methods described by Ferey et al. (7). Mice were anesthetized with isoflurane (2–3%). For plantaris muscle overload, the distal two-thirds of the gastrocnemius and soleus muscles were ablated. For soleus muscle overload, the distal two-thirds of the gastrocnemius muscle was ablated. A sham surgery was performed on the contralateral leg. After 1, 3, or 5 days (as indicated in the figure legends), mice were fasted overnight, anesthetized with pentobarbital sodium (90–100 mg/kg body weight) for 40 min or isoflurane (2–3%) for 3–5 min, and euthanized by cervical dislocation. Muscles were excised, weighed, and then used to assess [3H]-2-deoxy-d-glucose uptake or processed for immunoblot analyses.
Ex Vivo Muscle [3H]-2-Deoxy-d-Glucose Uptake
Ex vivo muscle [3H]-2-deoxy-d-glucose uptake was assessed using methods adapted from Hinkley et al. (22). Muscles were preincubated in continuously oxygenated 37°C Krebs-Ringer bicarbonate buffer (KRBB) composed of the following (in mmol/L): 117 NaCl, 4.7 KCl, 2.5 CaCl2 ⋅ 2H2O, 1.2 KH2PO4, 1.2 MgSO4 ⋅ 7H2O, and 24.6 NaHCO3, pH 7.5, supplemented with 2 mmol/L pyruvate. For glucose uptake, muscles were incubated in KRBB supplemented with 1.5 μCi/mL [3H]-2-deoxy-d-glucose, 1 mmol/L 2-deoxy-d-glucose, 0.45 μCi/mL [14C]-mannitol, and 7 mmol/L mannitol, unless otherwise indicated. All radioactive incubations were conducted at 30°C for 10 min. Cytochalasin B and phloridzin were added into buffers as described in the figure legends.
Hexose competition experiments were performed using methods adapted from Ryder et al. (23). Muscles were preincubated in KRBB plus pyruvate and then incubated in 90% KRBB supplemented with 1.5 μCi/mL [3H]-2-deoxy-d-glucose, 1 mmol/L 2-deoxy-d-glucose, 0.45 μCi/mL [14C]-mannitol, and 35 mmol/L d-fructose, d-galactose, d-glucose, d-xylose, or l-glucose. The osmolarity of the radioactive solution was kept at ∼310 mOsm by the 10% dilution of the KRBB and the removal of nonradiolabeled mannitol.
After radioactive incubations, muscles were frozen in liquid nitrogen, weighed, and solubilized in 1 mol/L NaOH at 80°C for 15 min. Solubilized muscles were neutralized with 1 mol/L HCl. Nonsoluble particulates were precipitated by centrifugation at 10,000g for 1 min. Aliquots were removed for scintillation counting of the [3H] and [14C] labels, and the extracellular and intracellular spaces calculated to determine [3H]-2-deoxy-d-glucose uptake.
Immunoblot Analyses
Immunoblot analyses were performed using standard methods as previously described by Hinkley et al. (22) and Ferey et al. (7). Frozen muscles were homogenized in buffer containing the following (in mmol/L): 20 Tris-HCl, pH 7.5, 5 EDTA, 10 Na4P2O7, 100 NaF, 2 NaVO4, 0.01 leupeptin, 3 benzamidine, 1 phenylmethylsulfonylfluoride, 1% Tergitol, and 10 μg/mL aprotinin. Samples were rotated end over end at 4°C for 60 min and centrifuged at 14,000g for 30 min. Lysate protein concentrations were determined via the Bradford method. Lysates (80 μg) were subjected to SDS-PAGE, and proteins were transferred onto nitrocellulose membranes. Blocking, primary, and secondary antibody conditions were as described in Table 1. Horseradish peroxide–conjugated secondary antibodies were detected using chemiluminescence reagents (PerkinElmer). Densitometric analysis of immunoblots was performed using Image Lab Software (Bio-Rad).
Table 1.
Immunoblotting conditions
| Antigen | Blocking | 1° Antibody | 2° Antibody |
|---|---|---|---|
| GLUT1 | 5% Nonfat dry milk | 1:4,000 in 5% BSA (catalog #07-1401; Millipore) | 1:2,000 Rabbit-HRP (catalog #PI31460; Thermo Fisher Scientific) |
| GLUT3 | 5% Nonfat dry milk | 1:5,000 in 5% BSA (catalog #AB1344; Millipore) | 1:2,000 Rabbit-HRP (catalog #PI31460; Thermo Fisher Scientific) |
| GLUT4 | 5% Nonfat dry milk | 1:2,000 in 5% BSA (catalog #07-1404; Millipore) | 1:2,000 Rabbit-HRP (catalog #PI31460; Thermo Fisher Scientific) |
| GLUT6 | 5% BSA | 1:500 in 5% BSA (catalog #118025; Abcam) | 1:5,000 Mouse-HRP (catalog #12-349; Millipore) |
| GLUT10 | 5% Nonfat dry milk | 1:500 in 5% BSA (catalog #sc-21635; Santa Cruz Biotechnology) | 1:5,000 Goat-HRP (catalog #V8051; Promega) |
Antibodies and immunoblotting conditions used in the assessment of GLUT isoform protein levels. HRP, horseradish peroxidase.
Statistical Analysis
Data are presented as the mean ± SEM. Statistical significance was defined as P < 0.05 and was determined by Student t tests, one-way ANOVA, or two-way ANOVA and Student-Newman-Keuls post hoc analysis. The number of mice or muscles used to determine statistical significance is indicated in the text or figure legends.
Results
Overload-Induced Muscle Hypertrophy Is Not Impaired in mGLUT4 KO Mice
Previous work demonstrated that muscle-specific loss of GLUT4 led to slight (10–15%) or nonsignificant alterations in mouse body weight (11,12,24), and this small difference in phenotype was attributed to the heterogeneity of the background strain (i.e., mixture of C57BL6, 129, and FVB). Consistent with those findings, at the current level of backcross to the C57BL6 strain neither female or male mGLUT4 KO mice exhibited a significant difference in body weight, fat mass, or lean mass compared with WT/controls (Table 2 and Supplementary Table 1).
Table 2.
mGLUT4 KO mice do not exhibit impairments in skeletal muscle growth
| Genotype | WT/CON | mGLUT4 HET | mGLUT4 KO |
|---|---|---|---|
| Presurgery body weight | |||
| Fed state (g) | 22.6 ± 0.5 | 23.9 ± 0.7 | 21.3 ± 0.6b |
| Body composition | |||
| Fat mass (g) | 3.1 ± 0.3 | 3.9 ± 0.4 | 3.1 ± 0.3 |
| Lean mass (g) | 16.8 ± 0.3 | 17.5 ± 0.4 | 15.9 ± 0.4 |
| Fat mass (%) | 14.2 ± 1.0 | 16.1 ± 1.4 | 14.2 ± 1.3 |
| Lean mass (%) | 74.6 ± 1.1 | 73.5 ± 1.4 | 74.6 ± 1.2 |
| Pre–tissue collection body weight | |||
| Fasted state (g) | 20.5 ± 0.4 | 21.3 ± 0.5 | 19.0 ± 0.6b |
| Plantaris muscle weight | |||
| Sham (mg) | 12.5 ± 0.3 | 12.4 ± 0.3 | 12.5 ± 0.4 |
| Overload (mg) | 18.8 ± 0.4a | 18.6 ± 0.7a | 17.5 ± 0.8a |
| Plantaris muscle weight/body | |||
| Sham (mg/g) | 0.61 ± 0.02 | 0.59 ± 0.02 | 0.66 ± 0.02 |
| Overload (mg/g) | 0.93 ± 0.04a | 0.89 ± 0.02a | 0.93 ± 0.05a |
| Percentage change in muscle weight (%) | 50.9 ± 4.0 | 50.9 ± 4.6 | 40.1 ± 4.9 |
Body weight and body composition were examined in female WT/control (WT/CON: GLUT4 LoxP+/−, GLUT4 LoxP+/+, and Cre+), mGLUT4 HET, and mGLUT4 KO mice at 11–12 weeks old. Plantaris muscle hypertrophy was induced by unilateral synergist ablation of the distal two-thirds of the gastrocnemius and soleus muscles. The contralateral limb was sham operated and served as the control. After 5 days, plantaris muscles were excised and weighed. Statistical significance was defined as P < 0.05 and denoted as follows: avs. sham-operated controls, bvs. mGLUT4 HET mice. N = 8–15 mice or muscles/group.
To determine whether GLUT4 was necessary for overload-induced muscle hypertrophy, unilateral synergist ablation of the distal two-thirds of the gastrocnemius and soleus muscles was performed to induce plantaris overload. As shown in Table 2, after 5 days overload-induced muscle hypertrophy was not impaired in female mGLUT4 HET or mGLUT4 KO mice compared with WT/controls. Similar results were obtained in male mice (Supplementary Table 1). Thus, GLUT4 is not necessary for overload-induced muscle growth in either sex.
Overload-Induced Muscle Glucose Uptake Is Not Impaired in mGLUT4 KO Mice
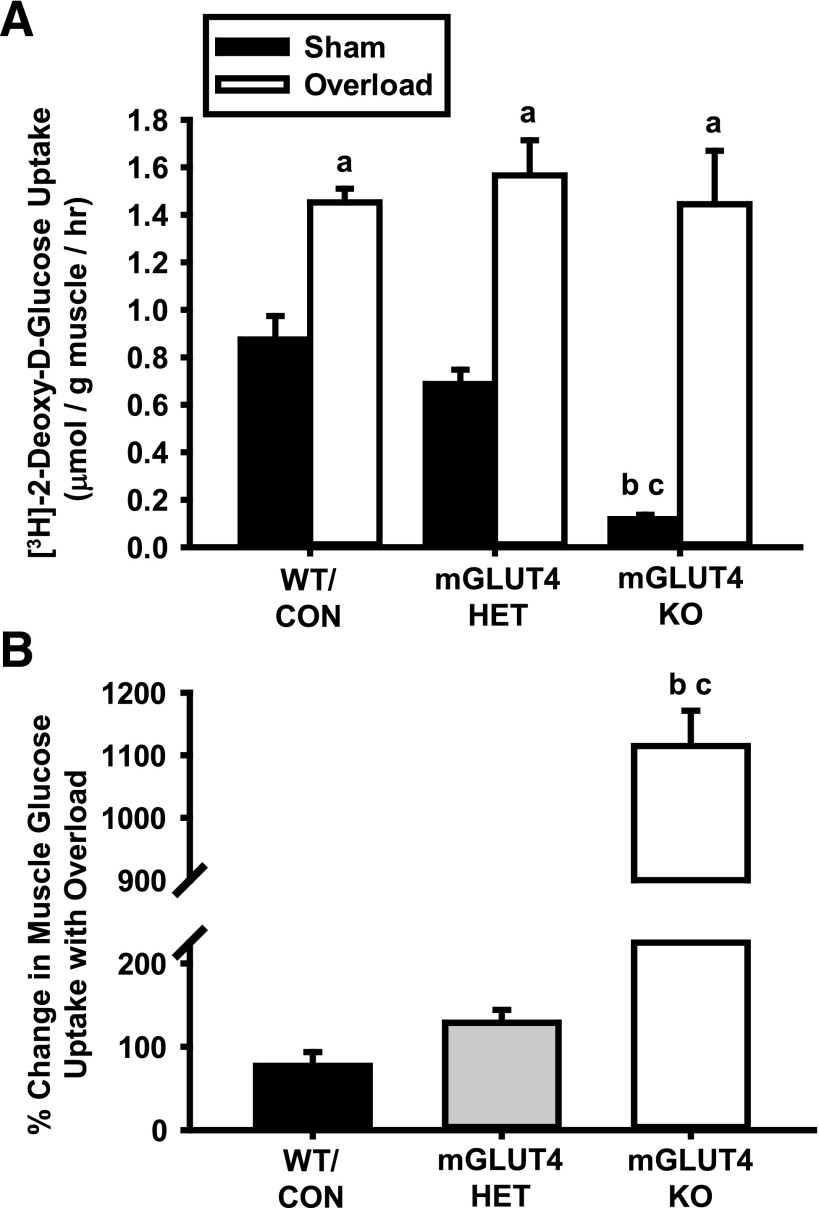
To determine whether GLUT4 was necessary for overload-induced muscle glucose uptake, muscles were incubated in buffer containing [3H]-2-deoxy-d-glucose. Female mGLUT4 KO mice had significantly lower basal muscle glucose uptake compared with both WT/control and mGLUT4 HET mice, but in response to overload all three groups increased glucose uptake to the same level (Fig. 1A). Thus, the percentage change in muscle glucose uptake in response to overload was enhanced in the mGLUT4 KO mice compared with WT/control and mGLUT4 HET mice (Fig. 1B). Similar results were obtained in male mice (Supplementary Fig. 1A and B). Thus, GLUT4 is not necessary for overload-induced muscle glucose uptake in either sex.
Figure 1.
Muscle-specific loss of GLUT4 does not impair overload-induced skeletal muscle glucose uptake. At 11–12 weeks old, female WT/control (WT/CON), mGLUT4 HET, and mGLUT4 KO mice underwent unilateral synergist ablation surgery to induce plantaris muscle hypertrophy. After 5 days, muscles were excised and [3H]-2-deoxy-d-glucose uptake was assessed ex vivo. A: Rate of muscle glucose uptake. B: Percentage change in glucose uptake relative to the contralateral, sham-operated control muscle. Statistical significance was defined as P < 0.05 and denoted as follows: a, vs. sham-operated control mice; b, vs. WT/CON mice; and c, vs. mGLUT4 HET mice. N = 4–8 muscles/group.
Overload Stimulates Muscle Glucose Uptake via a GLUT and Not a Sodium-Dependent Glucose Cotransporter
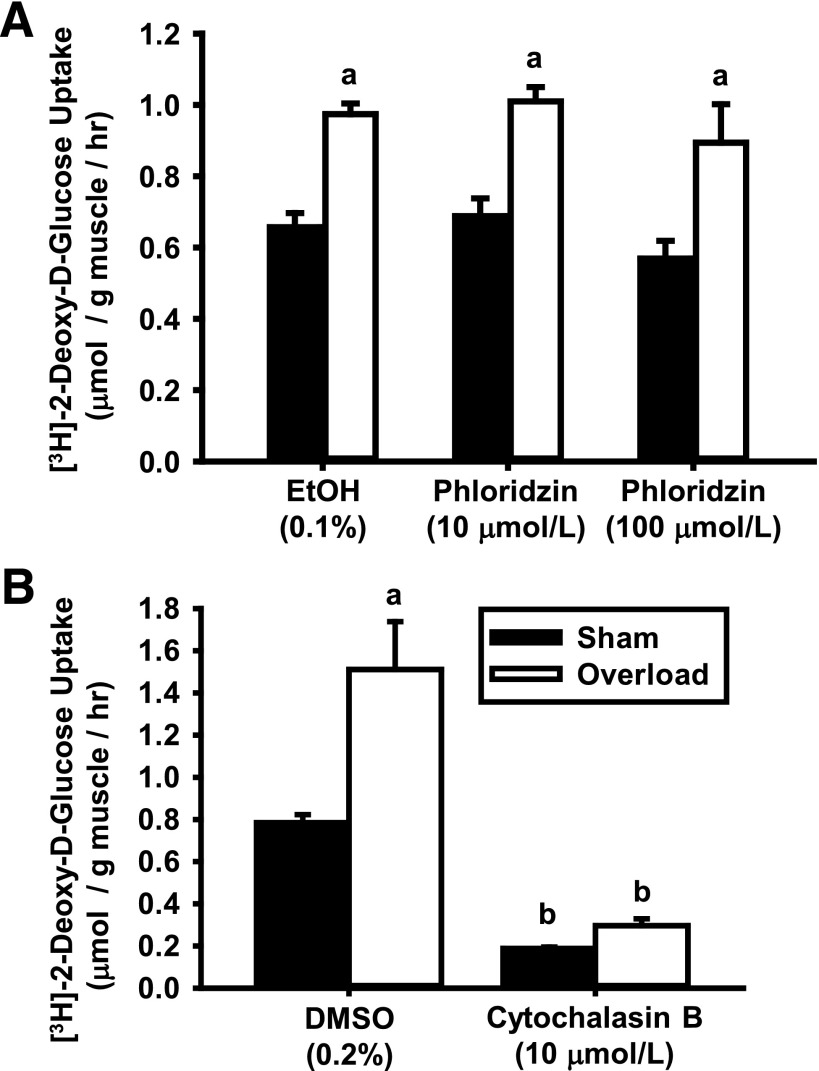
There are two main glucose transporter families in mammalian cells: the solute carrier family 2, which consists of facilitated GLUTs; and the solute carrier family 5, which consists of sodium-dependent glucose cotransporters (SGLTs). To determine whether overload uses SGLTs or GLUTs to increase glucose uptake, isolated muscles were treated in the short term with the chemical SGLT inhibitor phloridzin or the GLUT inhibitor cytochalasin B, and [3H]-2-deoxy-d-glucose uptake was assessed. As shown in Fig. 2, while phloridzin did not alter glucose uptake (Fig. 2A), cytochalasin B lowered basal and overload-induced glucose uptake compared with vehicle (Fig. 2B). Since the overload-induced increase in muscle mass was the same among the groups (Supplementary Tables 2 and 3), these results indicate that GLUTs mediate overload-induced glucose uptake.
Figure 2.
Overload increases skeletal muscle glucose uptake via a facilitated GLUT and not an SGLT. WT CD-1 female mice underwent unilateral synergist ablation surgery to induce plantaris muscle hypertrophy. After 5 days, muscles were excised and preincubated in KRBB for 30 min. A: For SGLT inhibition, muscles were incubated with phloridzin (10 or 100 μmol/L) or its vehicle (0.1% EtOH) for 60 min prior to the assessment of [3H]-2-deoxy-d-glucose uptake for 10 min. B: For GLUT inhibition, muscles were incubated in KRBB for 60 min and then simultaneously incubated with [3H]-2-deoxy-d-glucose and either cytochalasin B (10 μmol/L) or its vehicle (0.2% DMSO) for 10 min. The entire muscle incubation period for both inhibitor studies was 100 min. Statistical significance was defined as P < 0.05 and denoted as follows: a, vs. sham; b, vs. vehicle (either DMSO or EtOH). N = 4–6 muscles/group.
GLUT7, GLUT9, GLUT11, GLUT13/H+/myo-Inositol Transporter, and GLUT14 Cannot Mediate Overload-Induced [3H]-2-Deoxy-d-Glucose Uptake in Mouse Skeletal Muscle
There are 14 members of the GLUT family found in mammalian cells (GLUT1 to GLUT14). To narrow down the list of GLUT isoforms involved in overload-induced glucose uptake, the isoforms were screened for the following characteristics: 1) presence in the mouse genome, 2) ability to transport 2-deoxy-d-glucose, and 3) inhibition by cytochalasin B. Importantly for this study, previous work has demonstrated that the mouse genome does not possess GLUT11 or GLUT14 (25,26), that neither GLUT7 nor GLUT13 (also known as the H+/myo-inositol transporter) can transport 2-deoxy-d-glucose (27,28), and that [3H]-2-deoxy-d-glucose transport via GLUT9 is not inhibited by cytochalasin B (29). Collectively, these findings demonstrate that GLUT7, GLUT9, GLUT11, GLUT13/H+/myo-inositol transporter, and GLUT14 cannot be part of the mechanism by which overload mediates [3H]-2-deoxy-d-glucose uptake in mouse muscle.
Overload-Induced Glucose Uptake Is Not Mediated via a Fructose-Transporting GLUT
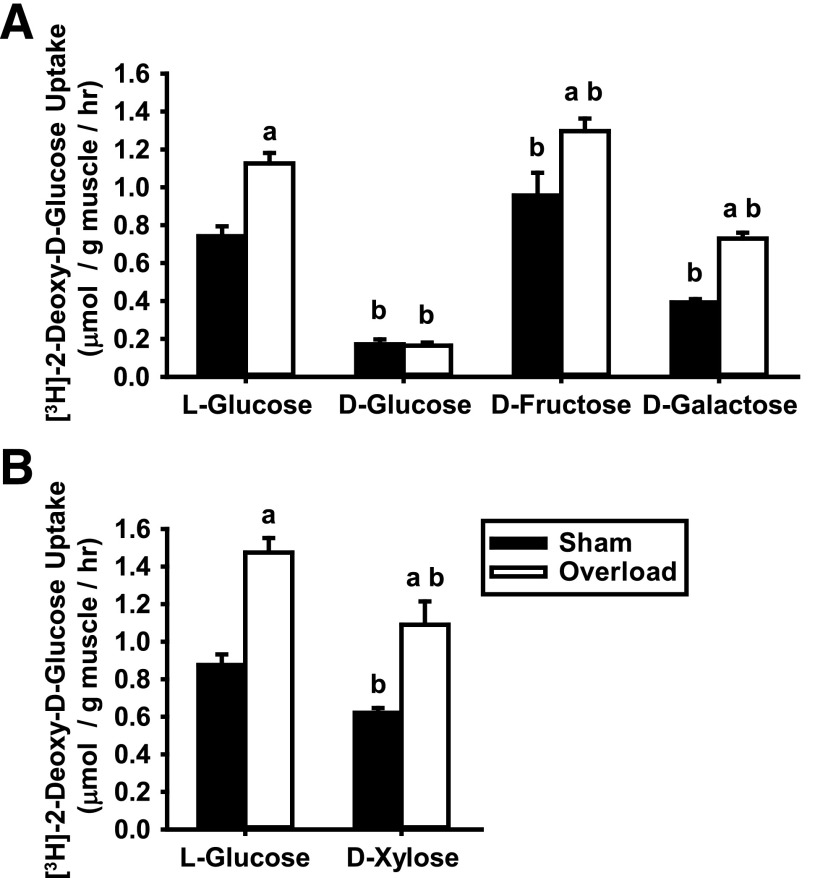
Hexose competition experiments have been performed in Xenopus oocytes expressing individual GLUT isoforms (27,30–35), and these studies have demonstrated considerable substrate transport heterogeneity among the GLUTs. For example, GLUT2, GLUT5, GLUT8, and GLUT12 have a higher affinity for transporting d-fructose over 2-deoxy-d-glucose (30–33), while GLUT1, GLUT3, and GLUT10 have a higher affinity for transporting d-galactose (30,34,35), and GLUT3 has a higher affinity for d-xylose (30,35). To further narrow down the list of GLUTs involved in overload-induced glucose uptake, experiments were performed to assess the ability of different hexoses to compete against [3H]-2-deoxy-d-glucose for transport into muscle cells. For all experiments, overload-induced increases in muscle mass were not significantly different (Supplementary Tables 4A and B). As shown in Fig. 3A, d-glucose completely blocked [3H]-2-deoxy-d-glucose uptake in both the sham and overload-stimulated muscles compared with l-glucose controls. In contrast, d-fructose did not impair either sham or overload-induced [3H]-2-deoxy-d-glucose uptake (Fig. 3A), demonstrating that the fructose-transporting GLUT2, GLUT5, GLUT8, and GLUT12 are not involved. d-galactose and d-xylose inhibited both sham and overload-induced [3H]-2-deoxy-d-glucose uptake by ∼40–50% (Fig. 3A and B), suggesting a possible role for GLUT1, GLUT3, and/or GLUT10. To date, no studies have examined GLUT6 substrate transport preference.
Figure 3.
Overload-induced increases in skeletal muscle glucose uptake involve a GLUT that can transport d-glucose, d-galactose, and d-xylose but not l-glucose or d-fructose. WT CD-1 female mice (6–8 weeks of age) underwent unilateral synergist ablation surgery to induce plantaris muscle hypertrophy. After 5 days, muscles were excised and preincubated in KRBB for 90 min, and [3H]-2-deoxy-d-glucose uptake was assessed ex vivo for 10 min in the presence of different hexoses as follows: 35 mmol/L l-glucose, d-glucose, d-fructose, and d-galactose (A) or 35 mmol/L l-glucose or d-xylose (B). Statistical significance was defined as P < 0.05 and denoted as follows: a, vs. sham; b, vs. l-glucose. N = 4–6 muscles/group.
Overload Increases GLUT1, GLUT3, GLUT6, and GLUT10 Protein Levels in Mouse Muscle
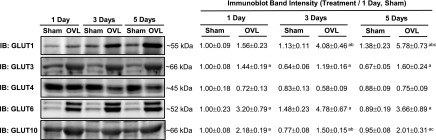
The results of the hexose competition experiments suggested that overload uses GLUT1, GLUT3, GLUT6, and/or GLUT10 to increase muscle glucose uptake. To assess the potential involvement of these GLUTs, immunoblots were performed in muscles 1, 3, and 5 days after overload. Commercially available GLUT antibodies were validated for the ability to detect their specified protein using GLUT isoform overexpression samples and/or tissues known to express that GLUT isoform (Supplementary Fig. 2). Muscle weights time-dependently increased after overload (Supplementary Table 5). Overload did not significantly alter GLUT4 protein levels (Fig. 4). In contrast, overload significantly increased GLUT1, GLUT3, GLUT6, and GLUT10 protein levels (Fig. 4), suggesting that any or all of these GLUTs could be involved in overload-induced muscle glucose uptake.
Figure 4.
Effect of overload (OVL) on muscle GLUT isoform protein levels. Female WT mice underwent unilateral synergist ablation surgery to induce plantaris muscle hypertrophy. After 1, 3, or 5 days, muscles were excised and processed to assess the protein expression of GLUT isoforms by immunoblot (IB) analysis. Representative blots and quantification are provided above. Statistical significance was defined as P < 0.05 and denoted as follows: a, vs. sham; b, vs. 1 day; c, vs. 3 days. N = 6–7 muscles/group.
Overload-Induced GLUT1 and GLUT6 Protein Levels Are Altered in mGLUT4 KO Mice
To determine whether the overload-induced increase in GLUT1, GLUT3, GLUT6, or GLUT10 protein levels was enhanced by the loss of GLUT4, immunoblots were performed in muscles from the mGLUT4 HET and mGLUT4 KO mice. Immunoblot results for female mice are provided in Fig. 5, whereas results for male mice are provided in Supplementary Fig. 3. A direct comparison of GLUT immunoblot data between male and female mice is provided in Supplementary Fig. 4. As shown in Fig. 5, the overload-induced increases in GLUT3 and GLUT10 protein levels were not altered by the loss of GLUT4. In contrast, although the overload-induced increase in GLUT1 trended toward being higher in the mGLUT4 KO mice (P = 0.13), the overload-induced increase in GLUT6 was significantly impaired (Fig. 5). In addition, GLUT6 protein levels were lower in the sham muscles from the mGLUT4 KO mice compared with WT/control mice (P = 0.05).
Figure 5.
Effects of muscle-specific loss of GLUT4 on overload (OVL)-induced changes in GLUT isoform protein levels. At 11–12 weeks old, female WT/control (WT/CON), mGLUT4 HET, and mGLUT4 KO mice underwent unilateral synergist ablation surgery to induce plantaris muscle hypertrophy. After 5 days, muscles were excised and processed to assess GLUT isoform protein levels by immunoblot (IB) analysis. Representative blots and quantification are provided above. Statistical significance was defined as P < 0.05 and denoted as follows: a, vs. sham; b, vs. WT/CON; c, vs. mGLUT4 HET; d, genotype main effect vs. WT/CON; e, genotype main effect vs. mGLUT4 HET. N = 5–7 muscles/group.
Discussion
The data presented in this study are the first to demonstrate that GLUT4 is not necessary for overload to stimulate glucose uptake or hypertrophic growth in skeletal muscle. In addition, these studies demonstrate that overload-induced muscle glucose uptake is mediated via a facilitated GLUT with a hexose transport preference of d-glucose > d-galactose ≈ d-xylose > 2-deoxy-d-glucose and not d-fructose. Immunoblot analyses found that muscle GLUT1, GLUT3, GLUT6, and GLUT10 protein levels are increased by overload, suggesting that one or more of these transporters is responsible for overload-induced glucose uptake.
Five days of overload increased glucose uptake ∼80% in plantaris muscles from both male and female WT/control mice (11–12 weeks old) (Figs. 1–3 and Supplementary Fig. 1) and ∼90% in soleus muscle from female mice (8–9 weeks old) (Supplementary Fig. 4). These findings, along with previous work that showed that 4 days of overload increased soleus muscle glucose uptake ∼80% in male mice that were 25–30 weeks old (6), demonstrate that overload can stimulate muscle glucose uptake irrespective of fiber type, sex, or age.
In this study, muscle GLUT4 protein levels were not significantly changed by overload (Fig. 4). Although this is in contrast with work that demonstrated an ∼70% increase in GLUT4 protein levels after overload (9), there are a number of important differences between these studies that could account for the observed inconsistency in findings, including species examined (rat vs. mouse), age of animals (6 vs. 2–3 months old), duration of overload (120 vs. 5 days), type of surgery (bilateral vs. unilateral), and muscle examined (medial gastrocnemius vs. plantaris). Consistent with no change in GLUT4 protein levels, overload-induced muscle glucose uptake was not impaired in either the male or female mGLUT4 KO mice (Fig. 1 and Supplementary Fig. 1). This finding is in agreement with work in rat hind limb muscle that demonstrated a disconnect between resistance training–induced increases in muscle glucose uptake and GLUT4 protein levels (18) and work in human muscle that demonstrated no change in GLUT4 protein after resistance training in healthy subjects (4,5). Thus, collectively these results suggest that GLUT4 does not regulate loading-induced glucose uptake in healthy muscle. However, overload and resistance training are not identical stimuli and elicit some differential adaptations in muscle (e.g., change in fiber type), so future studies will need to examine the necessity of GLUT4 in other models that induce hypertrophy (e.g., weighted ladder climbing).
Treatment of mouse plantaris muscles with the SGLT inhibitor phloridzin (10 or 100 μmol/L) had no significant effect on basal or overload-induced glucose uptake (Fig. 2A). These findings are in contrast with previous work in mouse soleus in which a 30-min preincubation with phloridzin (5 mmol/L) impaired basal and overload-induced glucose uptake 70–75% (6). Since the current study allowed a longer time (60 min) for phloridzin diffusion to the innermost muscle fibers, the discrepancy between these findings is most likely due to inhibitor dose. Phloridzin has an IC50 of ∼0.760 μmol/L for SGLT1 (36), ∼0.066 μmol/L for SGLT2 (36), ∼360 μmol/L for GLUT1 (37), and ∼140 μmol/L for GLUT4 (37). Thus, the decreased glucose uptake observed with 5 mmol/L phloridzin was most likely due to nonspecific inhibition of GLUTs and not to a muscle fiber type–specific difference in inhibitor action.
d-Fructose had no effect on either sham or overload-induced [3H]-2-deoxy-d-glucose uptake (Fig. 3A). These results are consistent with those found in mouse soleus muscle demonstrating no inhibition of basal or insulin-induced [3H]-2-deoxy-d-glucose uptake by d-fructose (23) and indicate that the GLUTs capable of transporting fructose (i.e., GLUT2, GLUT5, GLUT8, GLUT9, and GLUT12) do not functionally contribute to basal, insulin-induced, or overload-induced muscle glucose uptake. d-Galactose and d-xylose both partially impaired basal and overload-induced [3H]-2-deoxy-d-glucose uptake (Fig. 3A and B). Since previous work has shown that d-galactose is a competitive inhibitor of [3H]-2-deoxy-d-glucose uptake via GLUT1, GLUT3, and GLUT10 (30,34) and d-xylose is a competitive inhibitor of [3H]-2-deoxy-d-glucose uptake via GLUT3 (30), these results suggest that GLUT1, GLUT3, and/or GLUT10 mediate overload-induced muscle glucose uptake.
The mouse GLUT1 gene encodes a 492-amino acid protein with a predicted molecular weight of ∼54 kDa, and in this study GLUT1 was detected at ∼55 kDa in mouse muscle (Supplementary Fig. 2A). Overload time-dependently increased muscle GLUT1 protein levels up to ∼480% in WT mice (Fig. 4), and overload-induced increases in GLUT1 protein were elevated ∼50% in mGLUT4 KO mice compared with WT/control mice (Fig. 5). These data are consistent with those in cardiac muscle, in which pressure overload time-dependently increased GLUT1 protein up to ∼200% by 4 days (38), and in cardiac-specific GLUT4 KO mice, which exhibited ∼200% increases in cardiac GLUT1 protein levels (21). At 5 days postoverload, GLUT1 protein was increased ∼480% (Fig. 4), but muscle glucose uptake was only increased ∼80% (Figs. 1–3), suggesting that at least some of the GLUT1 proteins were not actively transporting glucose. These findings are consistent with those in muscle-specific GLUT1 overexpression mice in which >40-fold increases in GLUT1 protein only increased basal muscle glucose uptake approximately ninefold (39). Thus, future studies will need to assess the activity of muscle GLUT1 in order to determine a role for this protein in overload-induced muscle glucose uptake.
The mouse GLUT3 gene encodes a 493-amino acid protein with a predicted molecular weight of ∼54 kDa, and in this study GLUT3 was detected at ∼66 kDa in mouse sciatic nerve and muscle (Supplementary Fig. 2B). These data are consistent with those from studies detecting GLUT3 in mouse neurons (mRNA and protein) (40) and C2C12 muscle cells (mRNA) (41) but are in contrast with work that failed to detect GLUT3 protein in mouse soleus (23). The reason underlying this inconsistency in the predicted versus observed molecular weight of GLUT3, as well as the discrepancy in detection of GLUT3 protein in mouse muscle, is unclear. However, since [3H]-2-deoxy-d-glucose uptake via GLUT3 can be competitively inhibited by d-xylose (30) and muscle [3H]-2-deoxy-d-glucose uptake was impaired by d-xylose in this study (Fig. 3B), these results support the conclusion that GLUT3 protein is present in mouse plantaris muscle and suggest that GLUT3 expression in rodent muscle may be fiber type specific. Overload time-dependently increased GLUT3 protein levels up to ∼140% in WT mice (Fig. 4). Since overload stimulates muscle satellite cell recruitment and myocyte fusion (42) and GLUT3 mRNA levels transiently increase during myocyte fusion (43), these findings suggest that overload-induced increases in GLUT3 protein levels may be due to an increase in muscle satellite cells. Overload-induced increases in GLUT3 protein levels were not impaired in mGLUT4 KO mice (Fig. 5), suggesting that GLUT3 could regulate overload-induced glucose uptake. Consistent with this hypothesis, work in Xenopus oocytes has shown that GLUT3 exhibits a fivefold or greater capacity to transport glucose than GLUT1 or GLUT4 (35). Thus, even a small increase in GLUT3 protein levels could have a significant impact on muscle glucose uptake.
The mouse GLUT6 gene encodes a 497-amino acid protein with a predicted molecular weight of ∼55 kDa, and in this study GLUT6 protein was detected at ∼52 kDa in mouse brain and muscle (Supplementary Fig. 2D). These data are consistent with those in human tissues in which GLUT6 mRNA was strongly detected in brain with lower transcript levels found in muscle (44). Overload increased muscle GLUT6 protein levels ∼200–300% in WT/control and mGLUT4 KO mice (Figs. 4 and 5), suggesting that GLUT6 could mediate overload-induced glucose uptake. In support of this hypothesis, studies have demonstrated that GLUT6 transports d-glucose within a physiological range (1–5 mmol/L) (44) and that GLUT6 knockdown impaired [3H]-2-deoxy-d-glucose uptake in malignant human endometrial cells (45). However, even though studies in 3T3L1 adipocytes demonstrated that mutation of an N-terminal di-leucine motif in GLUT6 caused its relocalization to the cell surface (46), to date no studies have demonstrated GLUT6 translocation in response to any stimulus in any cell type (46). Thus, future studies will need to determine whether overload increases cell surface GLUT6 protein levels. Interestingly, GLUT6 protein levels were decreased ∼40–50% in the sham and overload-stimulated muscles from the female and male mGLUT4 KO mice (Fig. 5 and Supplementary Figs. 3 and 4), suggesting that GLUT4 and GLUT6 may interact to coordinately regulate glucose uptake. Future studies will need to determine the relationship between GLUT4 and GLUT6 in skeletal muscle.
The mouse GLUT10 gene encodes a 536-amino acid protein with a predicted molecular weight of ∼57 kDa, and in this study GLUT10 was detected at ∼66 kDa in mouse muscle (Supplementary Fig. 2C). Although the reason behind the discrepancy in the predicted versus observed molecular weight of GLUT10 is unclear, the current finding is consistent with previous work that reported GLUT10 mRNA in C2C12 cells (41) and mouse muscle (47). Overload increased GLUT10 protein levels 100–150% in muscles from WT/control, mGLUT4 HET, and mGLUT4 KO mice (Figs. 4 and 5), suggesting that GLUT10 could regulate overload-induced glucose uptake. However, in 3T3L1 adipocytes GLUT10 is localized to the Golgi and mitochondria and not to the cell surface (48). Thus, even though GLUT10 has the lowest Km value for 2-deoxy-d-glucose transport of all of the overload-regulated GLUTs (i.e., Km ∼0.3 mmol/L) (34), without localization to the cell surface, GLUT10 could not be involved in overload-induced muscle [3H]-2-deoxy-d-glucose uptake. Future studies will need to determine the intracellular localization of GLUT10 in muscle.
A direct comparison of the GLUT immunoblot data from the male and female mice revealed a sex difference in the expression of GLUT4, GLUT6, and GLUT10 (Supplementary Fig. 5). Although the physiological significance of these findings is presently unclear, it may explain the different rates of muscle glucose uptake observed between the sexes (e.g., basal muscle glucose uptake rates were 0.88 ± 0.10 μmol/g/h in WT/control female mice [Fig. 1] and 0.59 ± 0.04 μmol/g/h in male mice [Supplementary Fig. 1]).
In conclusion, the results from this study demonstrated that GLUT4 is not required for overload-induced increases in skeletal muscle glucose uptake or hypertrophic growth and suggest that GLUT1, GLUT3, GLUT6, and/or GLUT10 may play a key role in the regulation of overload-induced glucose uptake. Future studies are currently underway to identify the GLUTs that are responsible for this effect and to determine whether they also play a key role in the beneficial effects on whole-body glucose metabolism elicited by resistance exercise training in individuals with type 2 diabetes.
Supplementary Material
Article Information
Acknowledgments. The authors thank the following individuals for their thoughtful contributions to the validation of the GLUT antibodies used in this study: Patrick R. Davis, Jeremie L.A. Ferey, and Joshua C.M. Ferey of the Departments of Kinesiology, Biochemistry & Molecular Biology, and Physiology, Brody School of Medicine, East Carolina Diabetes and Obesity Institute, East Carolina University.
Funding. This project was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases grant R00-AR-056298 (to C.A.W.) and National Institute of Diabetes and Digestive and Kidney Diseases grants R37-DK-43051 (to B.B.K.) and R01-DK-103562 (to C.A.W.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. S.L.M. and C.A.W. participated in the design/conception of the work and data collection/generation, analysis, and/or interpretation and drafted, reviewed, and/or edited the manuscript. D.L.S. and B.B.K. participated in data collection/generation, analysis, and/or interpretation and drafted, reviewed, and/or edited the manuscript. C.A.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 15th Biennial Muscle Biology Conference, Advances in Skeletal Muscle Biology in Health and Disease, University of Florida, Gainesville, FL, 8–10 March 2017.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db16-1075/-/DC1.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the National Institute of Diabetes and Digestive and Kidney Diseases, or the National Institutes of Health.
References
- 1.Diabetes Prevention Program (DPP) Research Group The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care 2002;25:2165–2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bacchi E, Negri C, Zanolin ME, et al. Metabolic effects of aerobic training and resistance training in type 2 diabetic subjects: a randomized controlled trial (the RAED2 study). Diabetes Care 2012;35:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldi JC, Snowling N. Resistance training improves glycaemic control in obese type 2 diabetic men. Int J Sports Med 2003;24:419–423 [DOI] [PubMed] [Google Scholar]
- 4.Castaneda C, Layne JE, Munoz-Orians L, et al. A randomized controlled trial of resistance exercise training to improve glycemic control in older adults with type 2 diabetes. Diabetes Care 2002;25:2335–2341 [DOI] [PubMed] [Google Scholar]
- 5.Holten MK, Zacho M, Gaster M, Juel C, Wojtaszewski JF, Dela F. Strength training increases insulin-mediated glucose uptake, GLUT4 content, and insulin signaling in skeletal muscle in patients with type 2 diabetes. Diabetes 2004;53:294–305 [DOI] [PubMed] [Google Scholar]
- 6.Augert G, Van de Werve G, Le Marchand-Brustel Y. Effect of work-induced hypertrophy on muscle glucose metabolism in lean and obese mice. Diabetologia 1985;28:295–301 [DOI] [PubMed] [Google Scholar]
- 7.Ferey JL, Brault JJ, Smith CA, Witczak CA. Constitutive activation of CaMKKα signaling is sufficient but not necessary for mTORC1 activation and growth in mouse skeletal muscle. Am J Physiol Endocrinol Metab 2014;307:E686–E694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sitnick M, Bodine SC, Rutledge JC. Chronic high fat feeding attenuates load-induced hypertrophy in mice. J Physiol 2009;587:5753–5765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larkin LM, Kuzon WM Jr., Halter JB. Synergist muscle ablation and recovery from nerve-repair grafting: contractile and metabolic function. J Appl Physiol (1985) 2000;89:1469–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen PA, Gulve EA, Marshall BA, et al. Skeletal muscle glucose transport and metabolism are enhanced in transgenic mice overexpressing the Glut4 glucose transporter. J Biol Chem 1995;270:1679–1684 [DOI] [PubMed] [Google Scholar]
- 11.Kim JK, Zisman A, Fillmore JJ, et al. Glucose toxicity and the development of diabetes in mice with muscle-specific inactivation of GLUT4. J Clin Invest 2001;108:153–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zisman A, Peroni OD, Abel ED, et al. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat Med 2000;6:924–928 [DOI] [PubMed] [Google Scholar]
- 13.Kim YB, Peroni OD, Aschenbach WG, et al. Muscle-specific deletion of the Glut4 glucose transporter alters multiple regulatory steps in glycogen metabolism. Mol Cell Biol 2005;25:9713–9723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JY, Choi MJ, So B, Kim HJ, Seong JK, Song W. The preventive effects of 8 weeks of resistance training on glucose tolerance and muscle fiber type composition in Zucker rats. Diabetes Metab J 2015;39:424–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krüger K, Gessner DK, Seimetz M, et al. Functional and muscular adaptations in an experimental model for isometric strength training in mice. PLoS One 2013;8:e79069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall KE, McDonald MW, Grisé KN, Campos OA, Noble EG, Melling CW. The role of resistance and aerobic exercise training on insulin sensitivity measures in STZ-induced Type 1 diabetic rodents. Metabolism 2013;62:1485–1494 [DOI] [PubMed] [Google Scholar]
- 17.Gjøvaag TF, Dahl HA. Effect of training with different mechanical loadings on MyHC and GLUT4 changes. Med Sci Sports Exerc 2009;41:129–136 [DOI] [PubMed] [Google Scholar]
- 18.Yaspelkis BB 3rd, Singh MK, Trevino B, Krisan AD, Collins DE. Resistance training increases glucose uptake and transport in rat skeletal muscle. Acta Physiol Scand 2002;175:315–323 [DOI] [PubMed] [Google Scholar]
- 19.Rowlands DS, Page RA, Sukala WR, et al. Multi-omic integrated networks connect DNA methylation and miRNA with skeletal muscle plasticity to chronic exercise in Type 2 diabetic obesity. Physiol Genomics 2014;46:747–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castaneda F, Layne JE, Castaneda C. Skeletal muscle sodium glucose co-transporters in older adults with type 2 diabetes undergoing resistance training. Int J Med Sci 2006;3:84–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abel ED, Kaulbach HC, Tian R, et al. Cardiac hypertrophy with preserved contractile function after selective deletion of GLUT4 from the heart. J Clin Invest 1999;104:1703–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hinkley JM, Ferey JL, Brault JJ, Smith CA, Gilliam LAA, Witczak CA. Constitutively active CaMKKα stimulates skeletal muscle glucose uptake in insulin-resistant mice in vivo. Diabetes 2014;63:142–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryder JW, Kawano Y, Chibalin AV, et al. In vitro analysis of the glucose-transport system in GLUT4-null skeletal muscle. Biochem J 1999;342:321–328 [PMC free article] [PubMed] [Google Scholar]
- 24.Carvalho E, Kotani K, Peroni OD, Kahn BB. Adipose-specific overexpression of GLUT4 reverses insulin resistance and diabetes in mice lacking GLUT4 selectively in muscle. Am J Physiol Endocrinol Metab 2005;289:E551–E561 [DOI] [PubMed] [Google Scholar]
- 25.Scheepers A, Schmidt S, Manolescu A, et al. Characterization of the human SLC2A11 (GLUT11) gene: alternative promoter usage, function, expression, and subcellular distribution of three isoforms, and lack of mouse orthologue. Mol Membr Biol 2005;22:339–351 [DOI] [PubMed] [Google Scholar]
- 26.Wu X, Freeze HH. GLUT14, a duplicon of GLUT3, is specifically expressed in testis as alternative splice forms. Genomics 2002;80:553–557 [DOI] [PubMed] [Google Scholar]
- 27.Li Q, Manolescu A, Ritzel M, et al. Cloning and functional characterization of the human GLUT7 isoform SLC2A7 from the small intestine. Am J Physiol Gastrointest Liver Physiol 2004;287:G236–G242 [DOI] [PubMed] [Google Scholar]
- 28.Uldry M, Ibberson M, Horisberger JD, Chatton JY, Riederer BM, Thorens B. Identification of a mammalian H(+)-myo-inositol symporter expressed predominantly in the brain. EMBO J 2001;20:4467–4477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Augustin R, Carayannopoulos MO, Dowd LO, Phay JE, Moley JF, Moley KH. Identification and characterization of human glucose transporter-like protein-9 (GLUT9): alternative splicing alters trafficking. J Biol Chem 2004;279:16229–16236 [DOI] [PubMed] [Google Scholar]
- 30.Gould GW, Thomas HM, Jess TJ, Bell GI. Expression of human glucose transporters in Xenopus oocytes: kinetic characterization and substrate specificities of the erythrocyte, liver, and brain isoforms. Biochemistry 1991;30:5139–5145 [DOI] [PubMed] [Google Scholar]
- 31.Rand EB, Depaoli AM, Davidson NO, Bell GI, Burant CF. Sequence, tissue distribution, and functional characterization of the rat fructose transporter GLUT5. Am J Physiol 1993;264:G1169–G1176 [DOI] [PubMed] [Google Scholar]
- 32.Ibberson M, Uldry M, Thorens B. GLUTX1, a novel mammalian glucose transporter expressed in the central nervous system and insulin-sensitive tissues. J Biol Chem 2000;275:4607–4612 [DOI] [PubMed] [Google Scholar]
- 33.Rogers S, Chandler JD, Clarke AL, Petrou S, Best JD. Glucose transporter GLUT12-functional characterization in Xenopus laevis oocytes. Biochem Biophys Res Commun 2003;308:422–426 [DOI] [PubMed] [Google Scholar]
- 34.Dawson PA, Mychaleckyj JC, Fossey SC, Mihic SJ, Craddock AL, Bowden DW. Sequence and functional analysis of GLUT10: a glucose transporter in the type 2 diabetes-linked region of chromosome 20q12-13.1. Mol Genet Metab 2001;74:186–199 [DOI] [PubMed] [Google Scholar]
- 35.Burant CF, Bell GI. Mammalian facilitative glucose transporters: evidence for similar substrate recognition sites in functionally monomeric proteins. Biochemistry 1992;31:10414–10420 [DOI] [PubMed] [Google Scholar]
- 36.Oguma T, Nakayama K, Kuriyama C, et al. Intestinal sodium glucose cotransporter 1 inhibition enhances glucagon-like peptide-1 secretion in normal and diabetic rodents. J Pharmacol Exp Ther 2015;354:279–289 [DOI] [PubMed] [Google Scholar]
- 37.Kasahara T, Kasahara M. Characterization of rat Glut4 glucose transporter expressed in the yeast Saccharomyces cerevisiae: comparison with Glut1 glucose transporter. Biochim Biophys Acta 1997;1324:111–119 [DOI] [PubMed] [Google Scholar]
- 38.Morissette MR, Howes AL, Zhang T, Heller Brown J. Upregulation of GLUT1 expression is necessary for hypertrophy and survival of neonatal rat cardiomyocytes. J Mol Cell Cardiol 2003;35:1217–1227 [DOI] [PubMed] [Google Scholar]
- 39.Hansen PA, Wang W, Marshall BA, Holloszy JO, Mueckler M. Dissociation of GLUT4 translocation and insulin-stimulated glucose transport in transgenic mice overexpressing GLUT1 in skeletal muscle. J Biol Chem 1998;273:18173–18179 [DOI] [PubMed] [Google Scholar]
- 40.Nagamatsu S, Kornhauser JM, Burant CF, Seino S, Mayo KE, Bell GI. Glucose transporter expression in brain. cDNA sequence of mouse GLUT3, the brain facilitative glucose transporter isoform, and identification of sites of expression by in situ hybridization. J Biol Chem 1992;267:467–472 [PubMed] [Google Scholar]
- 41.Janot M, Audfray A, Loriol C, Germot A, Maftah A, Dupuy F. Glycogenome expression dynamics during mouse C2C12 myoblast differentiation suggests a sequential reorganization of membrane glycoconjugates. BMC Genomics 2009;10:483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fujimaki S, Machida M, Wakabayashi T, Asashima M, Takemasa T, Kuwabara T. Functional overload enhances satellite cell properties in skeletal muscle. Stem Cells Int 2016;2016:7619418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guillet-Deniau I, Leturque A, Girard J. Expression and cellular localization of glucose transporters (GLUT1, GLUT3, GLUT4) during differentiation of myogenic cells isolated from rat foetuses. J Cell Sci 1994;107:487–496 [PubMed] [Google Scholar]
- 44.Doege H, Bocianski A, Joost HG, Schürmann A. Activity and genomic organization of human glucose transporter 9 (GLUT9), a novel member of the family of sugar-transport facilitators predominantly expressed in brain and leucocytes. Biochem J 2000;350:771–776 [PMC free article] [PubMed] [Google Scholar]
- 45.Byrne FL, Poon IK, Modesitt SC, et al. Metabolic vulnerabilities in endometrial cancer. Cancer Res 2014;74:5832–5845 [DOI] [PubMed] [Google Scholar]
- 46.Lisinski I, Schürmann A, Joost HG, Cushman SW, Al-Hasani H. Targeting of GLUT6 (formerly GLUT9) and GLUT8 in rat adipose cells. Biochem J 2001;358:517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wood IS, Hunter L, Trayhurn P. Expression of Class III facilitative glucose transporter genes (GLUT-10 and GLUT-12) in mouse and human adipose tissues. Biochem Biophys Res Commun 2003;308:43–49 [DOI] [PubMed] [Google Scholar]
- 48.Lee YC, Huang HY, Chang CJ, Cheng CH, Chen YT. Mitochondrial GLUT10 facilitates dehydroascorbic acid import and protects cells against oxidative stress: mechanistic insight into arterial tortuosity syndrome. Hum Mol Genet 2010;19:3721–3733 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.