Abstract
Objective
Postoperative nausea and vomiting (PONV) is commonly observed after eye enucleation and orbital hydroxyapatite implant surgery. This prospective, randomized, double-blind trial was conducted to investigate the hypothesis that compared with monotherapy using a higher dose of palonosetron, using a lower dose of palonosetron in combination with droperidol could reduce the incidence of PONV and achieve similar prophylaxis against PONV after the aforementioned surgery.
Patients and methods
A total of 129 patients who were in the American Society of Anesthesiologists Classes I and II, aged between 18 and 70 years, and scheduled for eye enucleation and orbital hydroxyapatite implant surgery, were enrolled in this study. They were randomized into three groups: Group P2.5 (2.5 μg/kg palonosetron), Group P7.5 (7.5 μg/kg palonosetron), and Group P+D (2.5 μg/kg palonosetron and 15 μg/kg droperidol). Patients received the different antiemetic regimens intravenously 5 min before surgery. The severity of nausea and vomiting and the complete response (CR) rate during a 72-h postoperative period were assessed.
Results
All patients completed the trial. The nausea score of Group P2.5 was significantly higher than those of the other two groups at 0–4 h and 24–48 h (P<0.05). Vomiting scores among all groups were similar during all intervals (P>0.05). Compared with Group P2.5, the CR rate was significantly improved at all intervals in Group P+D, except at 4–72 h, and was also elevated at 24–72 h in Group P7.5 (P<0.05). Fewer patients in Group P2.5 did not experience any nausea or vomiting throughout the study (49%) compared with those in Group P7.5 (67%) and Group P+D (81%; P<0.01).
Conclusion
Combining low-dose palonosetron with droperidol potentiated prophylaxis for PONV and achieved a similar prophylactic effect as that with a higher dose of palonosetron.
Keywords: eye enucleation, orbital hydroxyapatite implant, palonosetron, PONV
Introduction
Postoperative nausea and vomiting (PONV) is a common perioperative complication. Nausea and vomiting can result in a series of adverse events, such as wound dehiscence, postoperative bleeding, and reflux and aspiration.1,2 A review has suggested that multiple risk factors such as age, female gender, obesity, nonsmoking status, history of motion sickness, inhalational anesthetics, duration of surgery, and anesthesia contribute to the incidence of PONV.3 A previous study has demonstrated that orbital hydroxyapatite implant surgery itself is one of the risk factors for PONV.4 Approximately 75% of patients undergoing this surgery suffer from PONV when no anti-emetics are used.4 Therefore, antiemetic intervention is necessary in this type of surgery. There are various types of antiemetics, which act on different receptors that play an important role in PONV, such as cholinergic (muscarinic), dopaminergic (D2), histaminergic (H1), and serotonergic (5-hydroxytryptamine type 3 [5-HT3]) receptors.3 Palonosetron, a second-generation 5-HT3 receptor antagonist, is used to prevent PONV in our clinical practice, and the normal dose is 2.5 μg/kg; however, a considerable proportion of patients still suffer from PONV at this dose. Therefore, the dose was increased to 7.5 μg/kg, as it was hypothesized that this higher dose could further decrease the rate of PONV, given that the efficacy of palonosetron in PONV prophylaxis is regarded as dose dependent.5 However, since palonosetron is quite expensive (~438 RMB/0.25 mg), a higher dose of palonosetron can result in an increased economic burden for patients. It has previously been suggested that a combination of two different antiemetics is more effective than monotherapy in preventing PONV.6,7 Therefore, it was hypothesized that a combination therapy could achieve equivalent efficacy and cost less compared with monotherapy using a higher dose of palonosetron. The efficacy of a combination of a 5-HT3 receptor antagonist and droperidol, a butyrophenone dopamine receptor antagonist, for prophylaxis against PONV has been reported previously.6,8 However, the 5-HT3 receptor antagonists involved in these studies were all first-generation agents. In comparison, palonosetron is characterized by a higher receptor affinity and a significantly longer half-life.9 Additionally, because of its long half-life and increased affinity for the receptor, it can provide efficacy against PONV for 72 h.10 As for the safety of palonosetron, Eisenberg et al11 studied the use of palonosetron in a range of 0.3–90 μg/kg and found that all doses were well tolerated. Droperidol has been used to prevent PONV for many years but with undesirable consequences such as arrhythmia, sedation, and extrapyramidal symptoms.12 A meta-analysis13 recommended that the dose of droperidol should not exceed 15 μg/kg, as the adverse consequences appear to be dose dependent. Therefore, this prospective, randomized, double-blind controlled trial was conducted to investigate the hypothesis that, compared with monotherapy using a higher dose of palonosetron, using a lower dose of palonosetron in combination with droperidol could reduce the incidence of PONV and achieve similar prophylaxis against PONV.
Patients and methods
Patients and study design
This trial was approved by the ethics committee of the The Eye, Ear, Nose and Throat Hospital of Fudan University and registered on the following website: Chinese Clinical Trial Registry (registration number: ChiCTR-IPR-15005852).
Patients, aged 18–70 years, categorized as American Society of Anesthesiologists Classes I and II, who were scheduled for elective eye enucleation and orbital hydroxyapatite implant surgery, were enrolled in the trial from January 20, 2015, to June 30, 2015. The exclusion criteria were as follows: a previous history of gastrointestinal disease and liver and kidney diseases, an allergy to 5-HT3 receptor antagonists and droperidol, and an intake of antiemetics 24 h preoperatively. All patients provided informed consents to participate in the current study.
Blinding and randomization
The current study used a randomized, double-blind, positive-controlled method for patients, researchers, and staff in charge of data collection. The data were assessed by the researchers when they were unaware of treatment allocation. The patients were randomly assigned into one of three groups (Group P2.5: received 2.5 μg/kg palonosetron; Group P7.5: received 7.5 μg/kg palonosetron; Group P+D: received 2.5 μg/kg palonosetron and 15 μg/kg droperidol; n=43 for each group) using a computer-generated random number list in a block of three kept in sealed envelopes. As the orbital hydroxyapatite implant surgery itself is an independent risk factor for PONV,4 no blank control group was used in the current study. The envelope was opened by a nurse anesthetist not involved in the study, who also prepared the palonosetron (Ousai [palonosetron hydrochloride injection]; specification: 5 mL, 0.25 mg; Qilu Pharmaceutical Co., Ltd., Hainan, China) and droperidol treatments (droperidol injection; specification: 2 mL, 5 mg; Xudong Haipu Pharmaceutical Co., Ltd., Shanghai, China). This nurse anesthetist diluted different doses of palonosetron into 10 mL aliquots and droperidol into 5 mL aliquots using 0.9% saline and also 5 mL 0.9% saline aliquots for Group P7.5 and Group P2.5 as a placebo treatment. Group P2.5 received 2.5 μg/kg palonosetron (10 mL) and 0.9% saline (5 mL), Group P7.5 received 7.5 μg/kg palonosetron (10 mL) and 0.9% saline (5 mL), and Group P+D received 2.5 μg/kg palonosetron (10 mL) and 15 μg/kg droperidol (5 mL).
Anesthesia
The anesthesiologist injected palonosetron or a combination of palonosetron and droperidol via the median cubital vein between 10 s and 5 min before anesthesia induction. Propofol (2–3 mg/kg), rocuronium (0.6 mg/kg), and sufentanil (0.3 μg/kg) were used for anesthesia induction upon intubation using an appropriately sized laryngeal mask. Sevoflurane inhalation was used during the surgery for anesthetic maintenance. The end-expiratory concentration of sevoflurane was adjusted to ~1 minimum alveolar concentration (MAC). Sufentanil was administered intermittently with a total dose of 0.7 μg/kg. Parecoxib was administered for analgesia 10 min before the end of surgery. The inhalation of sevoflurane ceased at the end of surgery. After 1 mg/kg propofol was administered intravenously, the patients were transferred to the postoperative recovery room, and no antagonist was administered. The laryngeal mask was removed once tidal volume was ≥8 mL/kg and PetCO2 was ≤50 mmHg. The patients were transferred to the ward when they were conscious. Rescue treatment and a metoclopramide 150 μg/kg intramuscular injection were administered when vomiting occurred ≥5 times/day.
Observation items and evaluation of efficacy
Another anesthesiologist blinded to the anesthesia process and grouping information followed up and recorded the data. The primary outcomes were the severity of nausea and vomiting and the complete response (CR) rates at 4, 24, 48, and 72 h after surgery. The rescue measures taken, postoperative pain, and side effects in the three groups were also documented. Since patients with eye enucleation and orbital hydroxyapatite implant surgery are generally discharged 72 h after the surgery, they could be observed in the hospital for 72 h postoperatively.
The primary outcome measured in this study was the CR rate. CR was defined as no occurrence of nausea and vomiting, and the CR rate was calculated as the number of patients with CR/total number of qualified patients. The main efficacy end points for the CR rate were the rates at 0–24, 24–72, and 0–72 h after the surgery. As the postoperative fasting time was 4 h and CR values before and after eating could be studied, the efficacy end points also included secondary end points such as CR at 0–4 and 4–72 h after the surgery.
Secondary outcomes, such as nausea and vomiting severity, postoperative pain, recovery time, adverse events, and the total cost for the antiemetic intervention of each patient, were also appraised.
Nausea was defined as a subjectively unpleasant sensation with the urge to vomit.12 Nausea severity was evaluated using a 4-score grading method: 0 (no nausea), 1 (mild nausea, no influence on eating and daily life), 2 (moderate nausea, having an influence on eating and daily life), and 3 (severe nausea, unable to eat, and need to stay in bed).
Forceful expulsion of gastric contents out of the mouth was defined as vomiting,12 and this also included retching, which meant labored, spasmodic, rhythmic contraction of the respiratory muscles without expulsion of the gastric contents.11 Vomiting severity was evaluated using a 4-score grading method: 0 (no vomiting), 1 (1–2 times/day), 2 (3–5 times/day), and 3 (>5 times/day).
Postoperative pain was assessed using a visual analog scale (VAS) with options from 0 to 10 at 4, 24, 48, and 72 h postoperatively, and if the VAS score exceeded 4, patients would receive intravenous tramadol (50 mg) once. The recovery time, defined as the duration between the moment when the surgery was completed and the moment when the patient could follow verbal commands and open their eye, was evaluated. Potential adverse events related to the study drugs, such as headache, constipation, arrhythmia, and extrapyramidal symptoms, were also recorded. The total cost of the antiemetic intervention for each patient was also analyzed.
Statistical analysis
Almost 50% of patients who received monotherapy of 2.5 μg/kg palonosetron experienced PONV at 0–72 h after surgery. It was presumed that a combination of 2.5 μg/kg palonosetron and droperidol could produce similar efficacy in preventing PONV as that with a higher dose of palonosetron (7.5 μg/kg) that reduced the rate of PONV to 20%. Therefore, a sample size of 34 patients in each group was calculated at α=0.05 and β=0.2 using sample size software (NCSS-PASS, Kaysville, UT, USA). The sample size in each group was expanded to 43 patients (25%) to compensate for losses to follow-up.
GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA) was used to perform data analysis. Continuous data with a normal distribution are presented as mean ± standard deviation. One-factor analysis of variance was used to analyze the data among the groups, followed by the Student–Newman–Keuls method for the post hoc pairwise test. Continuous data without a normal distribution were presented as median (interquartile range). The Kruskal–Wallis test was used to analyze the data among the groups followed by the Dunn’s test for a post hoc pairwise test. Nominal data are presented as the number of patients. Chi-square and Fisher’s exact tests were applied to compare the data among the groups, followed by partitions of the chi-square test for the post hoc pairwise test. A P-value of <0.05 was regarded as statistically significant. The type-I error of multiple comparisons of continuous data without a normal distribution and nominal data was corrected using the Holm–Bonferroni method. To indicate a statistical difference, a minimum of three two-sided P-values should not be >0.0167 (0.05/3). If this was achieved, the second smallest P-value should not surpass 0.025 (0.05/2) so as to be statistically significant. If this requirement was also met, the significant threshold for the third P-value was 0.05. The sequential procedure was aborted when the P-value was more than the threshold.
Results
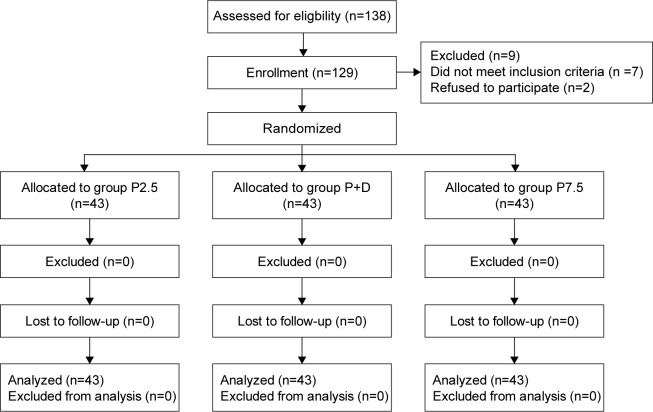
A total of 129 patients were enrolled in the study, and all patients completed this clinical trial (Figure 1). The baseline characteristics, such as age, height, body weight, anesthesia time, and Apfel’s simplified risk score for PONV (female gender, nonsmoking status, prior history of motion sickness or PONV, postoperative opioids),14 of the patients were balanced among all the three groups (P>0.05; Table 1).
Figure 1.
Flow diagram of the study.
Notes: Group P2.5: 2.5 μg/kg palonosetron; Group P7.5: 7.5 μg/kg palonosetron; and Group P+D: 2.5 μg/kg palonosetron and 15 μg/kg droperidol.
Table 1.
Baseline characteristics of the patients
| Group P2.5 (n=43) |
Group P7.5 (n=43) |
Group P+D (n=43) |
P-value | |
|---|---|---|---|---|
| Age (years), mean ± standard deviation | 40.60±13.08 | 44.93±14.01 | 42.56±13.48 | 0.3351 |
| Gender (male/female), n | 29/14 | 29/14 | 30/13 | 1 |
| Height (cm), mean ± standard deviation | 167.09±7.89 | 166.74±7.00 | 168.21±7.33 | 0.6335 |
| Body weight (kg), mean ± standard deviation | 61.96±12.07 | 64.28±8.70 | 69.12±14.20 | 0.5698 |
| Duration of anesthesia (min), mean ± standard deviation | 73.30±19.07 | 69.12±14.20 | 77.23±22.97 | 0.1473 |
| Apfel’s simplified risk score, median (IQR) | 1 (0–2) | 0 (0–2) | 1 (0–2) | 0.1858 |
Notes: Group P2.5: 2.5 μg/kg palonosetron; Group P7.5: 7.5 μg/kg palonosetron; and Group P+D: 2.5 μg/kg palonosetron and 15 μg/kg droperidol.
Three patients in Group P2.5 and one patient in Group P+D experienced delayed vomiting (vomiting after 24 h) once. Only one patient in Group P2.5 suffered from vomiting between 48 and 72 h postoperatively, and the rest of the patients experienced vomiting between 24 and 48 h after surgery. One patient in each group experienced vomiting for >5 times/day, and the vomiting did not recur after administering 150 mg/kg metoclopramide intramuscularly. No significant difference was found between the vomiting scores of each group. A significant difference in the nausea score was observed at 0–4 h (P=0.0007) and 24–48 h (P=0.0002) between each group. The nausea severity in Group P7.5 and Group P+D was significantly lower than that in Group P2.5 at 0–4 h (PP2.5–P7.5=0.0159, PP2.5–P+D=0.0003) and 24–48 h (PP2.5–P7.5=0.0032, PP2.5–P+D=0.0032), while the nausea score between Group P7.5 and Group P+D was similar (P=0.3580). No significant difference in the vomiting score was observed at any other intervals, between the three groups (Table 2). The CR rate of Group P2.5 was significantly lower than that of Group P+D at all time intervals, except for 4–72 h, and that of Group P7.5 at 24–72 h (Table 3). The recovery time of Group P2.5, Group P7.5, and Group P+D was 33.32±8.36 min, 30.00±7.01 min, and 29.67±8.69 min, respectively. No significant difference was found between the three groups, in terms of recovery time (P=0.076).
Table 2.
Nausea and vomiting score of patients in the three groups
| Time after surgery | Group P2.5 (n=43) |
Group P7.5 (n=43) |
Group P+D (n=43) |
|---|---|---|---|
| 0–4 h | |||
| Nausea, n (%) | 21 (48.8) | 11 (25.6) | 6 (14.0) |
| Nausea score | 0 (0–1)* | 0 (0–0.5) | 0 (0–0) |
| 4–24 h | |||
| Nausea, n (%) | 16 (37.2) | 14 (32.6) | 7 (16.3) |
| Nausea score | 0 (0–1) | 0 (0–1) | 0 (0–0) |
| 0–24 h | |||
| Vomiting, n (%) | 11 (25.6) | 7 (16.3) | 6 (14.0) |
| Vomiting score | 0 (0–0.5) | 0 (0–0) | 0 (0–0) |
| 24–48 h | |||
| Nausea, n (%) | 14 (32.6) | 0 (0) | 0 (0) |
| Nausea score | 0 (0–0)* | 0 (0–0) | 0 (0–0) |
| Vomiting, n (%) | 2 (4.7) | 0 (0) | 1 (2.3) |
| Vomiting score | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| 48–72 h | |||
| Nausea, n (%) | 2 (4.7) | 0 (0) | 0 (0) |
| Nausea score | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Vomiting, n (%) | 1 (2.3) | 0 (0) | 0 (0) |
| Vomiting score | 0 (0–0) | 0 (0–0) | 0 (0–0) |
Notes: Group P2.5: 2.5 μg/kg palonosetron; Group P7.5: 7.5 μg/kg palonosetron; and Group P+D: 2.5 μg/kg palonosetron and 15 μg/kg droperidol. Data concerning nausea and vomiting scores are expressed as median (interquartile range) for nausea/vomiting score and number (percentage of patients) for PONV. Dunn’s test was used for post hoc pairwise comparison.
P<0.05/3 compared with Group P7.5 and Group P+D.
Abbreviation: PONV, postoperative nausea and vomiting.
Table 3.
CR rate of patients in the three groups
| Time after surgery | Group P2.5 (n=43) |
Group P7.5 (n=43) |
Group P+D (n=43) |
P-value |
|---|---|---|---|---|
| 0–24 h | 21 (49) | 29 (67) | 36 (84)* | 0.0028 |
| 24–72 h | 34 (79) | 43 (100)** | 42 (98)* | 0.0006 |
| 0–4 h | 21 (49) | 31 (72) | 37 (86)* | 0.001 |
| 4–72 h | 27 (63) | 29 (67) | 36 (84) | 0.0789 |
| 0–72 h | 21 (49) | 29 (67) | 35 (81)* | 0.0061 |
Notes: Group P2.5: 2.5 μg/kg palonosetron; Group P7.5: 7.5 μg/kg palonosetron; and Group P+D: 2.5 μg/kg palonosetron and 15 μg/kg droperidol. Data are presented as n (%) of patients. Partition of chi-square test with Bonferroni correction was used for post hoc pairwise comparisons.
P<0.05/3 compared with Group P2.5;
P<0.01 compared with Group P2.5.
Abbreviation: CR, complete response.
In the present trial, all the antiemetic regimens were well tolerated. Side effects related to palonosetron and droperidol were not observed in the three treatment groups. The VAS scores and the number of patients receiving rescue analgesia were similar among all the groups at any interval (Table 4).
Table 4.
VAS scores and number of patients receiving rescue analgesia among the three groups
| Time after surgery | Group P2.5 (n=43) |
Group P7.5 (n=43) |
Group P+D (n=43) |
P-value |
|---|---|---|---|---|
| 4 h | 2 (1.5) | 2 (2) | 2 (1) | 0.7854 |
| 24 h | 2 (1) | 2 (1) | 2 (1) | 0.3698 |
| 48 h | 0 (1) | 0 (1) | 0 (1) | 0.8936 |
| 72 h | 0 (0) | 0 (0) | 0 (0) | 0.1432 |
| Number of patients receiving rescue analgesia, n (%) | 3 (7.0) | 3 (7.0) | 1 (2.3) | 0.6980 |
Notes: Group P2.5: 2.5 μg/kg palonosetron; Group P7.5: 7.5 μg/kg palonosetron; and Group P+D: 2.5 μg/kg palonosetron and 15 μg/kg droperidol. VAS scores are expressed as median (interquartile range). Dunn’s test was used for post hoc pairwise comparisons.
Abbreviation: VAS, visual analog scale.
The total expense of the antiemetic intervention was significantly different between the three groups (Group P2.5: 438 (438–438) RMB, Group P7.5: 876 (876–876) RMB, and Group P+D: 439.92 (439.92–439.92); P<0.01). The cost in Group P7.5 was significantly higher than that in the other two groups (P<0.01), while the cost in Group P+D was also higher than that in Group P2.5 (P<0.01), although the difference between the two groups was rather small.
Discussion
Palonosetron has a higher receptor affinity (pKi =10.45) and a longer half-life (40 h) compared with the first-generation products, including granisetron and ramosetron.9 Rojas et al15 found that palonosetron was characterized by allosteric binding and positive cooperativity with the 5-HT3 receptor. They also speculated that palonosetron could prolong antagonism of the 5-HT3 receptor by promoting receptor internalization. However, Hothersall et al16 suggested that palonosetron displayed a longer inhibition through pseudo-irreversible interactions with the receptor rather than by activating internalization. Droperidol is mainly used to treat schizophrenia but also has antiemetic efficacy because of its antagonistic effects against dopamine receptors.17 The present study demonstrated that a combination of low-dose palonosetron at 2.5 μg/kg and droperidol at 15 μg/kg was therapeutically superior to monotherapy using the same dose of palonosetron for PONV prevention. The combination therapy also provided an effect comparable to that of monotherapy using high-dose palonosetron at 7.5 μg/kg, as the severity of nausea and vomiting and the CR rate between Group P7.5 and Group P+D were similar at any interval (P>0.05; Tables 2 and 3).
Some studies have demonstrated that a combination of a 5-HT3 antagonist and droperidol could provide superior efficacy in preventing PONV, compared with a single antiemetic medication alone.6,12 However, in previous studies, PONV was assessed for up to 24 h and the second-generation 5-HT3 antagonist, palonosetron, was not studied in combination with other antiemetics. The dosage chosen in previous studies was fixed and not based on body weight. However, the present study compared the efficacy of different doses of palonosetron and a combination of palonosetron with droperidol and evaluated PONV for up to 72 h. The dose of palonosetron chosen in this study was based on a previous study by Eisenberg et al11 (0.3–90 μg/kg), and the dose selected for droperidol was based on a meta-analysis that showed that low-dose droperidol (≤15 μg/kg) demonstrated antiemetic efficacy and the adverse effects were dose dependent.13
Sinclair et al18 reported that a patient’s age, previous history of PONV, smoking status, anesthesia time, and type of surgery are all independent risk factors for PONV. Therefore, the present study compared patient baseline characteristics and the duration of anesthesia. No significant difference in baseline characteristics was found among the groups (P>0.05), suggesting that the difference in efficacy was related only to the agents administered.
The present study demonstrated that higher doses of palonosetron (7.5 μg/kg) or a lower dose of palonosetron (2.5 μg/kg) in combination with droperidol significantly decreased the postoperative nausea score compared with low-dose palonosetron alone at 0–4 and 24–48 h. However, no significant difference in nausea prophylaxis was observed between the two regimens (P>0.05), indicating that nausea prophylaxis using palonosetron seems to be dose dependent and combining a lower dose of palonosetron with droperidol could achieve the same efficacy as monotherapy using a much higher dose of palonosetron. Because of the large cost difference between palonosetron and first-generation 5-HT3 antagonists, it is more cost-effective to use a combination of antiemetics to achieve a balance between benefit and cost.10
No significant difference was found in terms of vomiting score at 0–24, 24–48, and 48–72 h among the three trial groups (P>0.05). Two patients who (4.7%) received palonosetron at 2.5 μg/kg and one patient (2.3%) who received palonosetron at 2.5 μg/kg and droperidol demonstrated delayed vomiting (24 h after surgery or chemotherapy) within 24–28 h post surgery, and one patient still vomited within 48–72 h post surgery. Adding droperidol did not further improve the anti-vomiting effect. Henzi et al17 studied the efficacy and adverse events of droperidol and found that its efficacy in nausea prophylaxis was superior to its anti-vomiting efficacy. Peixoto et al19 also found that droperidol was unable to significantly reduce the incidence of postoperative vomiting compared with placebo. On the contrary, Apfel et al20 concluded that droperidol prevented postoperative nausea and postoperative vomiting equally well.
In the present study, palonosetron at 2.5 μg/kg could significantly reduce the incidence of PONV after enucleation and orbital hydroxyapatite implant surgery to 51% compared with no intervention (75%, as mentioned earlier; P=0.0004). Therefore, all three regimens could provide effective prophylaxis against PONV in this type of surgery. While adding droperidol could significantly potentiate the efficacy of the treatment, the CR rate of Group P7.5 at 24–72 h was significantly higher than that of Group P2.5, implying that the efficacy enhancement was dose dependent.
Adverse events caused by droperidol, including extrapyramidal reaction, sedation, headache, and QT prolongation, were not observed in the current study, and adding droperidol did not cause a prolonged recovery time. Schaub et al13 concluded that receiving a low dose of droperidol (>15 μg/kg) would not remarkably change the incidence of sedation or dizziness compared with placebo. Eberhart et al21 suggested that there is no increased risk of sedation with droperidol, in the lowest range of doses between 0.25 and 0.625 mg, compared with placebo. They also found that with increased dose, the differences in terms of sedation and drowsiness between droperidol and a placebo were statistically significant.
The current study did not observe side effects related to palonosetron, such as headache and constipation, and this suggests that receiving palonosetron could be safe for patients. Bicer et al10 studied the use of palonosetron (0.5, 1.0, and 1.5 μg/kg) for children undergoing strabismus surgery and did not document any clinical adverse events such as headache or constipation. The study of Eisenberg et al11 on palonosetron for chemotherapy showed that only some adverse events were considered to be associated with palonosetron and the majority of events (83.9%) were mild or moderate. Furthermore, all serious events were considered to be secondary to chemotherapy or underlying disease rather than related to palonosetron.
The CR rate at 4–72 h was similar among all groups. Adding a dose of palonosetron alone or in combination with droperidol did not appear to further reduce the incidence of PONV in patients after taking food. The reason for this is not known.
Postoperative pain was evaluated at different intervals, as pain has been suggested to be a risk factor for PONV.22,23 No significant difference in the intensity of pain was found in the current trial, so the effect of pain on PONV appears to be negligible. However, PONV could also affect postoperative pain. In a study conducted by Matsota et al,6 the pain score of patients receiving a combination of antiemetics was significantly lower than that of patients receiving monotherapy, which was ascribed to a significantly lower rate of vomiting so that fewer patients suffered from abdominal pain and cramps. Therefore, pain and PONV can interact with each other, and the interaction is subtle.
The current study did not investigate the efficacy of a combination of multiple doses of droperidol. The study of Henzi et al17 showed that the anti-vomiting effect, rather than the anti-nausea effect, of droperidol in adults was dose dependent.
Conclusion
All three regimens were effective in decreasing the incidence of PONV. Combining low-dose palonosetron with droperidol potentiated the efficacy of PONV prophylaxis and achieved a similar prophylactic effect as that with a higher dose of palonosetron, without increasing the incidence of adverse events. Considering the high cost of palonosetron, a combination of low-dose palonosetron with 15 μg/kg droperidol should be considered for PONV prophylaxis.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Myles PS, Williams DL, Hendrata M, Anderson H, Weeks AM. Patient satisfaction after anaesthesia and surgery: results of a prospective survey of 10,811 patients. Br J Anaesth. 2000;84(1):6–10. doi: 10.1093/oxfordjournals.bja.a013383. [DOI] [PubMed] [Google Scholar]
- 2.Gan TJ. Risk factors for postoperative nausea and vomiting. Anesth Analg. 2006;102(6):1884–1898. doi: 10.1213/01.ANE.0000219597.16143.4D. [DOI] [PubMed] [Google Scholar]
- 3.Chatterjee S, Rudra A, Sengupta S. Current concepts in the management of postoperative nausea and vomiting. Anesthesiol Res Pract. 2011;2011:748031. doi: 10.1155/2011/748031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waterman H, Slater R, Leatherbarrow B, et al. Postoperative nausea and vomiting following orbital hydroxyapatite implant surgery. Eur J Anaesth. 1998;15(5):590–594. doi: 10.1046/j.1365-2346.1998.00365.x. [DOI] [PubMed] [Google Scholar]
- 5.Kovac AL, Eberhart L, Kotarski J, Clerici G, Apfel C, Palonosetron 04-07 Study Group A randomized, double-blind study to evaluate the efficacy and safety of three different doses of palonosetron versus placebo in preventing postoperative nausea and vomiting over a 72-hour period. Anesth Analg. 2008;107(2):439–444. doi: 10.1213/ane.0b013e31817abcd3. [DOI] [PubMed] [Google Scholar]
- 6.Matsota P, Angelidi M, Pandazi A, et al. Ondansetron-droperidol combination vs. Ondansetron or droperidol monotherapy in the prevention of postoperative nausea and vomiting. Arch Med Sci. 2015;11(2):362–370. doi: 10.5114/aoms.2015.50968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryoo SH, Yoo JH, Kim MG, Lee KH, Kim SI. The effect of combination treatment using palonosetron and dexamethasone for the prevention of postoperative nausea and vomiting versus dexamethasone alone in women receiving intravenous patient-controlled analgesia. Korean J Anesthesiol. 2015;68(3):267–273. doi: 10.4097/kjae.2015.68.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Awad IT, Murphy D, Stack D, Swanton BJ, Meeke RI, Shorten GD. A comparison of the effects of droperidol and the combination of droperidol and ondansetron on postoperative nausea and vomiting for patients undergoing laparoscopic cholecystectomy. J Clin Anesth. 2002;14(7):481–485. doi: 10.1016/s0952-8180(02)00394-x. [DOI] [PubMed] [Google Scholar]
- 9.Stoltz R, Cyong JC, Shah A, Parisi S. Pharmacokinetic and safety evaluation of palonosetron, a 5-hydroxytryptamine-3 – receptor antagonist, in U.S. and Japanese health subjects. J Clin Pharmacol. 2004;44(5):520–531. doi: 10.1177/0091270004264641. [DOI] [PubMed] [Google Scholar]
- 10.Bicer C, Aksu R, Ulgey A, et al. Different doses of palonosetron for the prevention of postoperative nausea and vomiting in children undergoing strabismus surgery. Drugs R D. 2011;11(1):29–36. doi: 10.2165/11586940-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisenberg P, MacKintosh FR, Ritch P, Cornett PA, Macciocchi A. Efficacy, safety and pharmacokinetics of palonosetron in patients receiving highly emetogenic cisplatin-based chemotherapy: a dose-ranging clinical study. Ann Oncol. 2004;15(2):330–334. doi: 10.1093/annonc/mdh047. [DOI] [PubMed] [Google Scholar]
- 12.Fujii Y, Toyooka H, Tanaka H. A granisetron-droperidol combination prevents postoperative vomiting in children. Anesth Analg. 1998;87(4):761–765. doi: 10.1097/00000539-199810000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Schaub I, Lysakowscki C, Elia N, et al. Low-dose droperidol (≤1mg or 15 μg kg−1) for the prevention of postoperative nausea and vomiting in adults: quantitative systematic review of randomised controlled trials. Eur J Anaesthesiol. 2012;29(6):286–294. doi: 10.1097/EJA.0b013e328352813f. [DOI] [PubMed] [Google Scholar]
- 14.Apfel CC, Läärä E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology. 1999;91(3):693–700. doi: 10.1097/00000542-199909000-00022. [DOI] [PubMed] [Google Scholar]
- 15.Rojas C, Thomas AG, Alt J, et al. Palonosetron triggers 5-HT(3) receptor internalization and causes prolonged inhibition of receptor function. Eur J Pharmacol. 2010;626(2–3):193–199. doi: 10.1016/j.ejphar.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Hothersall JD, Moffat C, Connolly CN. Prolonged inhibition of 5-HT3 receptors by palonosetron results from surface receptor inhibition rather than inducing receptor internalization. Br J Pharmacol. 2013;169(6):1252–1262. doi: 10.1111/bph.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henzi I, Sonderegger J, Tramer MR. Efficacy, dose-response, and adverse effects of droperidol for prevention of postoperative nausea and vomiting. Can J Anaesth. 2000;47(6):531–551. doi: 10.1007/BF03018945. [DOI] [PubMed] [Google Scholar]
- 18.Sinclair DR, Chung F, Mezei G. Can postoperative nausea and vomiting be predicted? Anesthesiology. 1999;91(1):109–118. doi: 10.1097/00000542-199907000-00018. [DOI] [PubMed] [Google Scholar]
- 19.Peixoto AJ, Celich MF, Zardo L, Peixoto Filho AJ. Ondansetron or droperidol for prophylaxis of nausea and vomiting after intrathecal morphine. Eur J Anaesthesiol. 2006;23(8):670–675. doi: 10.1017/S0265021506000482. [DOI] [PubMed] [Google Scholar]
- 20.Apfel CC, Cakmakkaya OS, Frings G, et al. Droperidol has comparable clinical efficacy against both nausea and vomiting. Br J Anaesth. 2009;103(3):359–363. doi: 10.1093/bja/aep177. [DOI] [PubMed] [Google Scholar]
- 21.Eberhart LH, Morin AM, Bothner U, et al. Droperidol and 5-HT3-receptor antagonists, alone or combination, for prophylaxis of postoperative nausea and vomiting. A meta-analysis of randomised controlled trials. Acta Anaesthesiol Scand. 2000;44(10):1252–1257. doi: 10.1034/j.1399-6576.2000.441011.x. [DOI] [PubMed] [Google Scholar]
- 22.Kenny GN. Risk factors for postoperative nausea and vomiting. Anaesthesia. 1994;49(suppl):6–10. doi: 10.1111/j.1365-2044.1994.tb03576.x. [DOI] [PubMed] [Google Scholar]
- 23.Joo J, Park SH, Park HJ, et al. Ramosetron versus ondansetron for postoperative nausea and vomiting in strabismus surgery patients. BMC Anesthesiol. 2016;16(1):41. doi: 10.1186/s12871-016-0210-5. [DOI] [PMC free article] [PubMed] [Google Scholar]