Abstract
BRCA2 is a tumor suppressor gene that is linked to hereditary breast and ovarian cancer. Although the Brca2 protein participates in homologous DNA recombination (HR), its precise role remains unclear. From chicken DT40 cells, we generated BRCA2 gene-deficient cells which harbor a truncation at the 3′ end of the BRC3 repeat (brca2tr). Comparison of the characteristics of brca2tr cells with those of other HR-deficient DT40 clones revealed marked similarities with rad51 paralog mutants (rad51b, rad51c, rad51d, xrcc2, or xrcc3 cells). The phenotypic similarities include a shift from HR-mediated diversification to single-nucleotide substitutions in the immunoglobulin variable gene segment and the partial reversion of this shift by overexpression of Rad51. Although recent evidence supports at least Xrcc3 and Rad51C playing a role late in HR, our data suggest that Brca2 and the Rad51 paralogs may also contribute to HR at the same early step, with their loss resulting in the stimulation of an alternative, error-prone repair pathway.
Individuals carrying germ line mutations of the BRCA2 gene have a high risk of developing breast and ovarian cancer (40, 45, 76). The Brca2 protein acts as a tumor suppressor, and its loss results in genome instability (1, 30, 35). Mice carrying a deletion of all eight BRC repeats die during early embryonic development, while mice carrying a truncation at the 3′ end of the third BRC repeat (brca2tr mice) are partially viable (10, 13, 29, 35). However, interpretation of their phenotype may be hampered by additional mutations acquired during the derivation of the line as a result of the genomic instability imposed by impaired Brca2 function.
While yeast Rad51, Rad52, and Rad54 contribute to homologous DNA recombination (HR) to similar extents, vertebrate Rad51 plays a dominant role in HR (25, 48, 57). HR is initiated by polymerization of Rad51 on single-stranded DNA at sites of DNA damage, leading to formation of nucleoprotein filaments (3, 33, 50, 72). The filaments undergo homologous pairing and strand exchange with other intact homologous DNA to form the Holliday junction recombination intermediates, followed by resolution of the two homologous DNAs. It has been suggested that Brca2 may regulate the Rad51 recombinase (30, 36, 67, 70), because the brca2 mutation impairs ionizing radiation (IR)-induced subnuclear Rad51 focus formation (20, 58, 61, 73), which is believed to represent the polymerization of Rad51 at sites of DNA damage (16, 27, 39, 55). However, the biological significance of impaired Rad51 focus formation has not been clarified, because brca2tr cells exhibit only marginal IR sensitivity and thus retain a substantial fraction of the Rad51-dependent capability for repair of IR-induced double strand breaks (DSBs). Furthermore, in mammalian cells, there is neither a detailed phenotypic assay that can address the role of each molecule in the HR reaction nor a collection of HR mutant cell lines with which each phenotype can be accurately compared. Therefore, reverse genetic studies of mammalian cells have given little insight into the role of Brca2 in HR.
Defective Rad51 focus formation has also been reported in mutants carrying mutations of the RAD51 paralog genes (52, 53), i.e., RAD51B, RAD51C, RAD51D, XRCC2, and XRCC3, which share 20 to 30% amino acid sequence identity with each other and with RAD51. These mutants exhibit similar phenotypes with respect to their response to DNA damage. This defect is partially corrected by overexpression of Rad51, mirroring the finding with yeast that overexpression of Rad51 suppresses the DNA damage sensitivity of rad55 and rad57 mutants (22). These findings are most easily explained in terms of the paralogs playing an early role in the initiation of the recombination reaction by promoting the loading of Rad51 onto single-stranded DNA (52, 53). However, recent biochemical as well as genetic data suggest that some of the Rad51 paralogs may participate in HR at a later step. Brenneman et al. reported on the HR products recovered from the xrcc3 mutant hamster cell line irs1-SF, where HR is stimulated by site-specific endonuclease cleavage in an artificial construct (7). They displayed radically altered spectra, i.e., increased frequencies of discontinuous tracts and frequent local rearrangements associated with HR, suggesting that Xrcc3 must play a role in a later step of HR. Since then, accumulating evidence has suggested a role for the Rad51 paralogs in Holliday junction resolution. Purified Rad51B has been shown to bind Holliday structures preferentially (68, 69), and the Xrcc2/Rad51D complex can stimulate the resolution of Holliday structures by the Bloom helicase (6). Most recently, Rad51C has been shown to be a key component of a human nuclear extract fraction capable of branch migration and resolution of Holliday junctions in vitro (28). Therefore, to reevaluate the role of the Rad51 paralogs, we here analyzed diversification of immunoglobulin (Ig) genes in all rad51 paralog mutants as well as brca2 mutant cells.
DT40 cells provide a unique opportunity for dissecting the mechanism of HR by comparison of the phenotypes of a range of HR mutants (49). Ig diversification in the chicken is achieved predominantly by gene conversion, in which segments from upstream pseudogenes are copied into the expressed Ig V segment, leading to formation of a variety of HR products. Gene conversion is occasionally accompanied by nontemplated point mutations. As with Ig somatic mutation in mice and humans (34), both gene conversion and point mutation in DT40 cells are dependent on activation-induced deaminase (AID) (2, 17). AID acts to deaminate cytidines within the Ig V regions. The resulting uracil is then excised by uracil DNA glycosylase to generate an abasic site and probably single-strand gaps, which may lead to gene conversion and somatic mutation (12, 32).
Here, we analyzed the phenotype of brca2tr cells, focusing on the diversification of the variable segment of the Ig light-chain (Igλ) gene (8). The data showed that brca2tr and all rad51 paralog mutant cells exhibited similar phenotypes, with a shift from predominant gene conversion to the same pattern of extensive hypermutation. This shift was partially reverted by overexpression of Rad51. These results suggest that Brca2 and the Rad51 paralogs act at an early step of HR, which in DT40 masks any later role. In this early role, the relative activities of these molecules could determine the choice between HR and the other error-prone repair pathways.
MATERIALS AND METHODS
Construction of the BRCA2 conditional null and BRC truncation-targeting vectors.
To construct the BRCA2 conditional null targeting vector, ∼2.6-, ∼3.6-, and ∼3.1-kb fragments at the BRCA2 locus (54) were amplified by LA Taq DNA polymerase (Takara, Kyoto, Japan) from DT40 genomic DNA by using primer pairs 5′-CGGGGTACCTCTATTTGAAGCAGCAGTAGC-3′ and 5′-CGCGGATCCACCATGGAGAGCTTCGTCTTT-3′, 5′-GGTGCTAATTGCTGCTAATTGAGGAGGCAA-3′ and 5′-CCTGAACACAGGAAGTTCTGTACTAACATG-3′, and 5′-CCGCTCGAGAAGATGCTGGCCTCATCCATT-3′ and 5′-TGCTCTAGAGCAGCAGTGTAGTTGTGGCAG-3′. The identity of genomic DNA fragments was confirmed by base sequencing. The ∼3.6-kb fragment was cloned into the TOPO pCR-XL cloning vector (Invitrogen, Carlsbad, Calif.) to make the pMY297 vector. The loxP adaptor fragment was constructed by annealing oligonucleotides 5′-GGCCGCATAACTCGTATAATGTATGCTATACGAAGTTATC-3′ and 5′-TCGAGATAACTTCGTATAGCATACATTATACGAAGTTATGC-3′ and then inserted into the NotI-XhoI sites in the multicloning site of pMY297 vector. The following fragments were inserted into the multicloning site of the resulting vector: the ∼3.1-kb fragment at the XhoI-XbaI sites, the ∼2.6-kb fragment at the KpnI-BamHI sites, and the blasticidin-S (bsr) selection marker cassette at the BamHI site.
To construct the BRCA2BRC truncation-targeting vectors, the ∼1.1- and ∼9.2-kb fragments at the BRCA2 locus were amplified from DT40 genomic DNA. The ∼1.1-kb fragment was amplified with the primer pair 5′-AATGCGGCCGCACTATTCTCCTTCCACACTGACAC-3′ and 5′-GAATGCGGCCGCGGGTAGCCAGTTTGAATTCACAC-3′ and Pyrobest DNA polymerase (Takara) to avoid mutations. The ∼9.2-kb fragment was amplified with the primer pair 5′-GGGCTACTAACTTATCTGGTGATACAAGAG-3′ and 5′-GTACTTCTCTGCAATGTGTGACTGTTGCTC-3′ and then cloned into the TOPO pCR-XL cloning vector (Invitrogen) to make the topo/9.2-kb vector. The identity of genomic DNA fragments was confirmed by sequencing. The ∼1.1-kb fragment and the histidinol-D drug resistance cassette were inserted into NotI and EcoRV sites, respectively, in the multicloning site of the topo/9.2-kb vector.
DNA transfections and generation of brca2tr cells.
The BRCA2 conditional null and BRCA2BRC truncation-targeting vectors were linearized with KpnI and BamHI, respectively. To generate heterozygous BRCA2 conditional null mutant cells (BRCA2+/con), the BRCA2 conditional null targeting vectors was transfected into wild-type cells (BRCA2+/+) (Fig. 1A). The genomic DNA of transfectants was digested with EcoRI, and gene-targeting events were confirmed by Southern blot analysis with probe 1, which was amplified from genomic DNA with the primer pair 5′-AAAGAGCTAAGAGTTGTGATGCATCACATG-3′ and 5′-TCTCTGTGGTGTTCTGCAAGGATTCCTTAC-3′. The expected sizes of bands detected by probe 1 were 6.9 kb from the wild-type allele and 4.2 kb from the targeted allele.
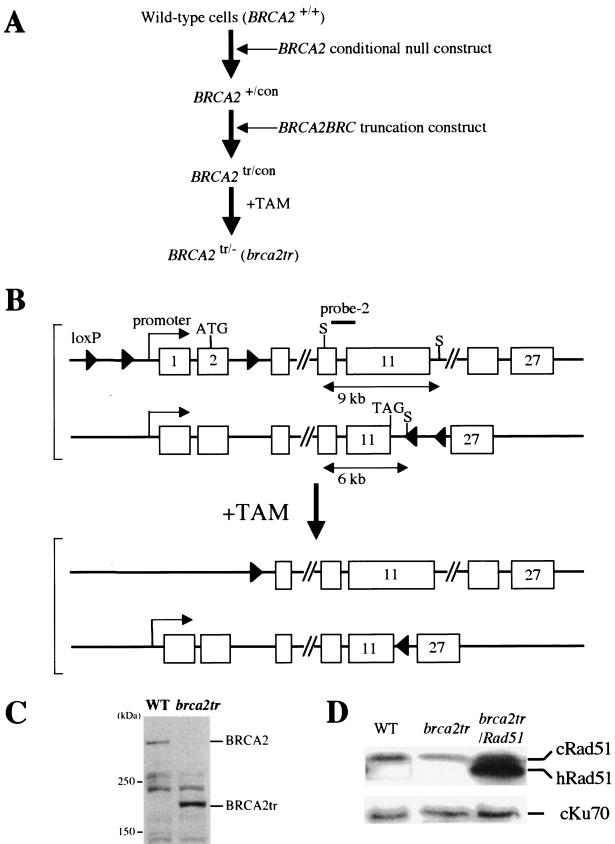
FIG.1.
Gene targeting of the BRCA2 loci. (A) Experimental strategy. (B) Schematic representation of part of the BRCA2 locus, the configuration of the targeted alleles, and the gene-disrupted allele after treatment with TAM. The BRCA2 conditional null construct, which contains a bsr selection marker gene flanked by two loxP signals (ploxPbsr) in the upstream of the BRCA2 locus and the third loxP signal in intron 2, was used to target the BRCA2 locus. The promoter and exons 1 and 2, which are flanked by loxP sites, would be deleted upon exposure of the cells to TAM. Knock-in of the BRC truncation construct inserts a termination codon in the 5′ end of the BRC4 repeat, resulting in deletion of nucleotides 4369 to 9955 of the coding sequences of the chicken BRCA2 gene (54). Of note, since BRC3 sequences are not conserved in the chicken BRCA2 gene, the brca2tr cells would have only two functional BRC repeats, BRC1 and BRC2. S indicates relevant SacI sites. The open boxes and arrowheads represent the exons and loxP signals, respectively. (C) Western blot analysis of whole-cell extracts of cell lines with the indicated genotype. Each lane contains 200 μg of protein. The wild-type and brca2tr mutant cells show 340- and 160-kDa bands, respectively. (D) Western blot analysis of gene-disrupted clones that carry the human Rad51 transgene. Human Rad51 migrates slightly faster than the chicken counterpart in sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis. The same membrane was probed with the anti-chicken Ku70 antiserum as a control. WT, wild type.
To generate BRCA2 truncated mutant cells, the BRCA2BRC truncation-targeting vector was transfected into BRCA2+/con cells (BRCA2tr/con). The genomic DNA of transfectants was digested with SacI, and the gene targeting was confirmed by Southern blot analysis with probe 2, which was amplified with the primer pair 5′-TTGAGAAATGCACAGGAGCTCTCAAGTGCC-3′ and 5′-GGCTAGGTCAGCAGCTCGACTGTTCAC-3′. The expected sizes of bands were 9 kb from the BRCA2 conditional null allele and 6 kb from the BRC truncated allele in BRCA2tr/con cells (Fig. 1B). To conditionally inactivate the BRCA2 gene, BRCA2tr/con cells were treated with 200 nM 4-hydroxytamoxifen (TAM) for 3 days. To confirm the conditional null deletion, genomic DNA was isolated from clones derived from the TAM treated cells, digested with EcoRI, and subjected to Southern blot analysis with probe 1. The expected sizes of bands were 6.9 kb from the BRCA2 truncated allele and ∼10 kb from the null allele that lost the promoter and the initiation codon.
Rad51 focus formation assay.
Cells were harvested at 2 h after gamma irradiation. Cytospin slides were prepared by using Cytospin 3 (Shandon, Pittsburgh, Pa.). Staining and visualization of Rad51 foci were performed as previously described (63) with an anti-Rad51 rabbit polyclonal antibody (EMD Biosciences, Darmstadt, Germany).
Measurement of targeted integration frequencies.
To analyze targeted integration events at the Ovalbumin (9) and RAD54 (5) loci, targeted construct DNAs were transfected into cells, and Southern blot analysis was performed following selection of clones resistant to the appropriate antibiotics.
Western analysis.
A glutathione S-transferase fusion protein containing amino acids 1 to 203 of chicken BRCA2 was purified and sent to MBL Co., Ltd. (Nagano, Japan) for raising of antibodies in rabbits. The polyclonal antibodies were purified by affinity chromatography. Extracts of DT40 cells were prepared in ice-cold lysis buffer (50 mM Tris-HCl [pH 7.5], 120 mM NaCl, 0.5% Nonidet P-40, 1 mM phenylmethylsulfonyl fluoride with protease inhibitor cocktail [Nakarai Tesque, Kyoto, Japan]). The protein concentration was measured with a protein assay kit (Bio-Rad, Hercules, Calif.). Protein (200 μg) was fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (NuPage 3 to 8% Tris-acetate gel; Invitrogen) and transferred to nitrocellulose membranes (Protran BA85; Schleicher & Schuell, Dassel, Germany). The membranes were blocked with SuperBlock blocking buffer (Pierce, Rockford, Ill.) and incubated with the affinity-purified anti-BRCA2 antibody described above. Western blots were developed with ImmunoStar reagents (Wako, Osaka, Japan).
Analysis of the rate of sIgM gain and loss.
The frequency of generation of surface IgM (sIgM) loss variants as well as sIgM gain revertants was monitored by flow cytometric analysis of cells that had been expanded for 3 weeks after subcloning and then stained with fluorescein isothiocyanate-conjugated goat anti-chicken IgM (Bethyl, Montgomery, Tex.). In each analysis, the abundance of sIgM-negative cells was determined as the percentage of live cells (as judged by propidium iodide uptake and scatter gating) whose fluorescein isothiocyanate fluorescence fell at least eightfold below that of the sIgM-positive peak.
Nucleotide sequence analysis of rearranged Vλ segments derived from sIgM gain and loss fractions.
Fractions of three independent sIgM loss variants were sorted from each genotype with a FACSCalibur. Fractions of two independent sIgM gain revertants were also obtained from each genotype by using MACS separation columns (Miltenyi Biotec, Bergisch Gladbach, Germany). Genomic DNA was amplified by PCR with Pyrobest DNA polymerase (30 cycles of 94°C for 30 s, 60°C for 1 min, and 72°C for 1 min). The rearranged Vλ was amplified by using the CVLF6 (5′-CAGGAGCTCGCGGGGCCGTCACTGATTGCCG-3′) and CVLR3 (5′-GCGCAAGCTTCCCCAGCCTGCCGCCAAGTCCAAG-3′) primers, as previously described (44). After purification with a QIAquick gel extraction kit (Qiagen, Hilden, Germany), PCR products were cloned into the TOPO pCR2.1 cloning vector (Invitrogen) and sequenced with the M13 forward (−20) or reverse primer and an ABI PRISM 3100 sequencer (Applied Biosystems). Sequence alignment with GENETYX-MAC (Software Development, Tokyo, Japan) allowed identification of changes from the consensus sequences in each clone. The frequencies of hypermutation and gene conversion were determined as previously described (44).
RESULTS
Conditional generation of brca2tr cells and their proliferative properties.
To generate brca2tr cells, we used the strategy shown in Fig. 1A. Since the brca2tr mutation is associated with genome instability, as observed in mammalian brca2 mutant cells (23, 24, 59, 60, 71), we generated conditionally Brca2-deficient DT40 clones and analyzed their phenotypes immediately after conditional inactivation of the endogenous BRCA2 gene (19). We made compound heterozygous mutant cells, in which one allele of BRCA2 is modified to express a BRC-truncated Brca2 protein, while the other allele can be conditionally inactivated (Fig. 1B). To make the latter allele, we employed the TAM-inducible Cre-loxP system (14, 74, 75) and inserted the loxP signals into the endogenous BRCA2 allele. Cre-mediated deletion of the BRCA2 allele was verified by Western blot analysis (Fig. 1C). This also showed that the truncated Brca2 protein was expressed in the brca2tr cells at the same level as intact Brca2 protein in wild-type cells.
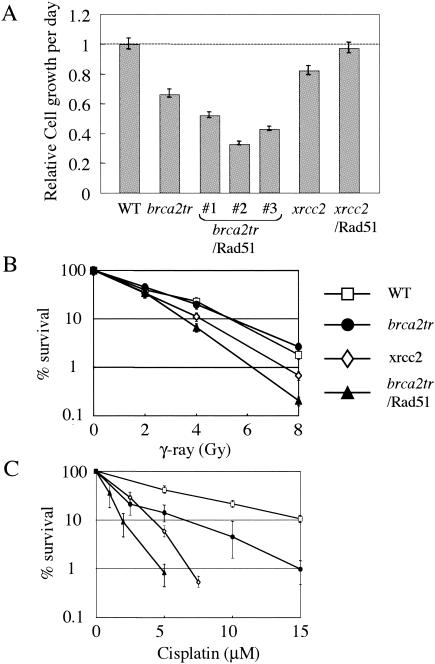
The phenotypes of brca2tr and xrcc2 cells are displayed in Fig. 2. The proliferative properties of brca2tr cells were monitored by growth curves and by cell cycle analysis. The growth rate of brca2tr cells was significantly lower than that of wild-type cells (Fig. 2A). A pulse-chase experiment with bromodeoxyuridine showed that the length of a single cell cycle is comparable for wild-type and brca2tr cells (data not shown). On the other hand, a higher proportion of dead cells was seen in brca2tr (20 to 30%) as well as xrcc2 (data not shown) cultures, which may explain their lower growth rates.
FIG. 2.
Growth rate and viability of brca2tr cells. (A) The relative rate of cell growth per day is plotted for the indicated genotypes. (B and C) Sensitivity of wild-type and brca2tr cells to DNA-damaging agents. The fractions of surviving colonies after the indicated treatment of cells compared with untreated controls of the same genotype are shown on the y axis on a logarithmic scale. (B) Gamma rays; (C) cisplatin. The plating efficiency was 20 to 30% for brca2tr cells and 100% for wild-type cells. The dose of 137Cs gamma rays and concentration of cisplatin are displayed on the x axis on a linear scale in each graph. Data shown are representative of those from at least three independent experiments. WT, wild type. Error bars indicate standard deviations.
To assess the biologically relevant DNA repair capacity of each mutant, we performed colony survival assays following exposure of cells to DNA-damaging agents. brca2tr cells showed no significant increase in sensitivity to IR (Fig. 2B), although they were sensitive to killing by cisplatin (cis-diaminedichloroplatinum II) (Fig. 2C). Likewise, deletion of Xrcc2 had a more pronounced effect on cisplatin sensitivity than on IR sensitivity. We believe that this reflects a more important role for the Rad51 paralogs and Brca2 in the HR-dependent release of the replication block than in repairing induced DSBs.
Defective homologous DNA recombination in brca2tr cells.
To evaluate the HR capability of brca2tr cells, we measured gene-targeting efficiency at two loci. The ratio of targeted to random integration was substantially reduced in brca2tr cells (Table 1). To directly test whether brca2tr cells are defective for HR-mediated repair of DSBs, we used the artificial recombination substrate SCneo (15) (see Table S1 in the supplemental material). We detected on average ∼210-fold fewer G418-resistant colonies after transfection of the I-SceI plasmid into brca2tr cells compared to Brca2+ cells. Taking the lower plating efficiency of brca2tr cells (3.1% of that of Brca2+ cells) into account, HR-medicated repair in brca2tr cells was ∼6.5-fold less efficient than that in Brca2+ cells Thus, both gene targeting and HR-mediated DSB repair were significantly reduced in brca2tr cells. We also analyzed Rad51 focus formation at 3 h after exposure to IR (Fig. 3). We found that the focus formation was severely impaired in brca2tr cells, as previously observed in mammalian brca2 mutants (23, 71, 73) and every Rad51 paralog gene-disrupted DT40 clone (52, 53). Thus, despite the severe defect in IR-induced Rad51 focus formation, these mutations had little impact on IR sensitivity (Fig. 2B).
TABLE 1.
Targeted integration frequenciesa
| Genotype | No. of target clones/no. of drug-resistant clones (% frequency) at targeted locus:
|
|
|---|---|---|
| Ovalbumin | RAD54 | |
| Wild type | 36/42 (85.7) | 32/71 (45.1) |
| brca2tr | 0/42 (0) | 4/42 (9.52) |
Wild-type and brca2tr cells were transfected with targeting constructs of the indicated loci.
FIG. 3.
Immunofluorescence visualization of Rad51 subnuclear foci. Wild-type (A and B), brca2tr (C and D), and xrcc2 (E and F) cells were analyzed at 3 h after treatment with 2 Gy of IR. (G) Average number of Rad51 foci per cell at 3 h after treatment with 2 Gy of IR. At least 100 morphologically intact cells were analyzed. WT, wild type. Error bars indicate standard deviations.
We previously showed that Rad51 overexpression partially suppresses the phenotypes of all rad51 paralog mutants, including the sensitivity to killing by cisplatin (52, 53). We therefore examined the effect of Rad51 overexpression on brca2tr cells (brca2tr/Rad51) (Fig. 1D). Unexpectedly, overexpression of Rad51 had an apparently toxic effect on brca2tr cells, manifested by further decreases in the growth rates of all the three brca2tr/Rad51 clones analyzed (Fig. 2A) as well as by increased sensitivity to IR (Fig. 2B) and cisplatin (Fig. 2C). This toxicity might be caused by retention of Rad51 in the cytoplasm, as previously reported for a rodent brca2-deficient cell line (24). Thus, despite strong parallels between the phenotypes of brca2tr and xrcc2 cells, the effect of Rad51 overexpression was not the same in the two mutants. This may reflect differential roles of the two proteins in facilitating the action of Rad51.
brca2tr cells and all rad51 paralog mutants display extensive accumulation of single-nucleotide substitutions in the Ig V segment.
Ig gene conversion events in wild-type cells occasionally introduce frameshift mutations in the Ig V segments, leading to loss of sIgM expression (8). sIgM loss variants can also be generated by point mutations that create nonsense codons or disrupt the pairing of the heavy and light chains. To estimate the rate of Ig gene diversification, we measured the sIgM-negative fraction in 36 sIgM-positive subclones at 3 weeks after subcloning, as described previously (44). The sIgM-negative fraction was on average 2.3-fold higher in brca2tr cells than in wild-type cells (Fig. 4A), despite a defect in HR in the mutant. To investigate the cause of the increased sIgM loss rate in brca2tr cells, we determined nucleotide sequences of Ig Vλ from sIgM-negative variants. Observed mutations were classified as being attributable to gene conversion templated by upstream V pseudogenes or to nontemplated point mutation (hypermutation) or as falling into an ambiguous category. In wild-type cells, a majority of the changes in selected sIgM-negative variants exhibited gene conversion events (Fig. 4B and C), as previously reported (8). In marked contrast, we found that sIgM-negative brca2tr cells showed a significant change in the pattern of mutations: 65% of the analyzed sequences carried nontemplated point mutations, while only 4.2% of the events were attributable to gene conversion (Fig. 4C). Presumably, the other ∼30% sIgM-negative cells carried inactivating mutations in the Ig heavy-chain gene (44). These observations as well as the reduced Ig gene conversion rate (Fig. 5A) suggest that defective Brca2 results in a shift in Ig V diversification from gene conversion to hypermutation.
FIG.4.
Analysis of Vλ sequences cloned from sIgM loss variants. (A) Fluctuation analysis of the generation frequency of sIgM loss variants. The abundance of sIgM loss variants was determined in several parallel cultures derived from sIgM-positive single cells after clonal expansion (3 weeks); median percentages are noted above each data set and are indicated by the dashed lines. WT, wild type. (B) Comparison of Vλ sequences from sIgM loss cells sorted from parental sIgM-positive clones of wild-type or brca2tr cells. Each horizontal line represents the rearranged Vλ1/Jλ (402 bp). Point mutations (lollipop shape) and gene conversion tracts (horizontal bars above lines) are indicated. (C) Proportions of Vλ sequences carrying different number of point mutations (PM), gene conversions (GC), or mutations of ambiguous origin (Amb) among sorted sIgM-negative populations. Segment sizes are proportional to the number of sequences carrying the number of mutations indicated around the peripheries of the pie charts. The total number of Vλ sequences analyzed is indicated in the centers of the charts, with the data compiled from analysis of three brca2tr cells. (D) Nucleotide substitution preferences deduced from point mutations in sequences from brca2tr cells. All of the V sequences having more than two point mutations showed different patterns, while 12 of the 27 analyzed V sequences having a single-base substitution showed identical events. Thus, most of the mutations may represent independent events rather than clonal expansion. (E) Ig diversification patterns in wild-type cells and rad51c, rad51d, and brca2tr mutants. The frequencies of gene conversion tracts and nontemplated point mutations per mutated sequence in sorted sIgM-negative cells are plotted. The ratio of point mutations per gene conversion is shown to the right of each bar. The mutation data are derived from 105 WT sequences from 5 subclones, 38 each of rad51c and rad51d sequences from two subclones, and 40 brca2tr sequences from three subclones. Ambiguous mutations (44) were excluded from this analysis. Data indicated by asterisks are from reference 44.
FIG. 5.
Fluctuation analysis of the generation frequency of sIgM gain revertants. (A) The abundance of sIgM gain variants was determined in several parallel cultures derived from sIgM-negative single cells after clonal expansion (3 weeks); median percentages are noted above each data set and are indicated by the dashed lines. (B) Gene conversion tract spectra, showing the average tract length in the rearranged Vλ segment from the sIgM-positive revertants. The black bars and open boxes represent the gene conversion tract spectra and one-base deletions, respectively. WT, wild type.
The nucleotide sequence analysis of Ig Vλ in brca2tr cells revealed that they constitutively performed transversion-favored dC/dG hypermutation at a high frequency (Fig. 4D). This finding is in agreement with that for xrcc2, xrcc3, and rad51b cells (44). Of note, the patterns of hypermutation are strikingly similar in brca2tr cells and these rad51 paralog mutant cells. This similarity led us to investigate the rate of Ig gene conversion and hypermutation in the other rad51 paralog mutants, rad51c and rad51d cells. To this end, we determined the nucleotide sequences of the Ig Vλ segments of sIgM-negative fractions of each genotype. The data also showed a shift in the pattern of Ig V diversification from gene conversion to hypermutation (Fig. 4E). In summary, brca2tr cells and all five rad51 paralog mutants exhibit a similar shift from gene conversion to point mutation at the Ig locus.
brca2tr and xrcc2 cells can undergo Ig gene conversion with high accuracy.
To assess the accuracy of gene conversion events, we analyzed larger numbers of Ig gene conversion events by employing another method, i.e., monitoring the gain of sIgM expression from sIgM-negative cells (sIgM gain) that carried a specific frameshift mutation in the Vλ segment (8). For this assay, we made brca2tr and xrcc2 cells from wild-type DT40 cells carrying the defined frameshift mutation in the Vλ segment. In wild-type cells, sIgM gain is caused mainly by elimination of the frame shift mutation through superimposed gene conversion events. To assess the rate of gene conversion at the Igλ locus, we measured the percentage of sIgM gain fractions for 36 subclones each derived from brca2tr, xrcc2, and wild-type genotypes at 3 weeks after subcloning. Compared with wild-type cells, the brca2tr and xrcc2 clones exhibited 3.3- and 4.4-fold decreases in sIgM gain revertant cells, respectively (Fig. 5A). There are two potential reasons for the reduced kinetics of sIgM gain in the mutants: the HR frequency is reduced, or HR occurs at a similar frequency but its accuracy is reduced, leading to accumulation of missense and frameshift mutations. To distinguish between these possibilities, we determined nucleotide sequences in the Vλ segments from a large number of sIgM gain revertants.
In xrcc2 and wild-type cells, the frameshift was replaced by pseudo-V sequences in all sequences recovered from the sIgM gain revertants (Fig. 5B). In contrast, in brca2tr cells, 86.5% of the sequences carried a one-base deletion that was not associated with gene conversion (see Fig. S1 in the supplemental material), whereas only 10.8% showed superimposed gene conversion events. The frequent detection of this one-base deletion is unlikely to be an artifact of clonal expansion, since the percentages of sIgM gain revertants showed little variation (Fig. 5A), and base sequence analysis of two subclones showed consistent data. This deletion was at an RGY(W) motif, a hypermutation hot spot (4, 43), and was never associated with detectable gene conversion events, suggesting that it reflects a putative AID-dependent DNA damage site, which may eventually be repaired by another, unknown error-prone repair pathway. We determined the nucleotide sequences of 37 and 92 Vλ segments from brca2tr and xrcc2 cells, respectively, and found no gene conversion events that included base substitutions or local rearrangements at or near gene conversion tracts. These observations indicate that in brca2tr and xrcc2 cells, HR efficiency is significantly reduced, whereas once HR is initiated, it is carried out accurately.
Rad51 overexpression partially suppresses a defective Ig V diversification phenotype of the rad51 paralog and brca2tr mutants.
An increased rate of hypermutation in the Ig V segment and a defect in Rad51 focus formation are always found together in all examined HR-deficient gene-disrupted DT40 clones, such as in brca2tr cells and all rad51 paralog mutant clones. Thus, the close correlation led us to postulate a causal relationship between these two phenotypes, with hypermutation resulting from defective Rad51 recruitment or assembly. To address this possibility, we investigated the effect of Rad51 overexpression on Ig V diversification in xrcc2 and brca2tr cells. As expected, Rad51 overexpression in xrcc2 cells (xrcc2/Rad51) elevated the rate of sIgM gain (Fig. 5A), while it decreased the number of sIgM loss events (see Fig. S2 in the supplemental material). Interestingly, brca2tr/Rad51 cells also showed the same pattern of sIgM gain and loss (Fig. 5A; see Fig. S2 in the supplemental material). Nucleotide sequence analysis of Ig Vλ confirmed that the sIgM gain was indeed caused by gene conversion in both brca2tr/Rad51 and xrcc2/Rad51 cells (Fig. 5B). Of note, this seems to be inconsistent with the finding that Rad51 sensitized brca2tr cells, but not xrcc2 cells, to killing by IR and cisplatin (Fig. 2B and C). Such sensitization might be explained by retention of Rad51 in the cytoplasm of brca2tr cells (24) rather than decreased efficiency of DNA repair in brca2tr/Rad51 cells. The cytoplasmic stress could reduce colony survival in the presence of genotoxic stress. An interesting precedent for this phenomenon is that phosphatidylinositol 3-kinase or protein kinase C-mediated signal also increases cellular sensitivity to cisplatin (51, 62).
We therefore conclude that Rad51 overexpression at least partially restored the mutant phenotype of Ig V diversification in xrcc2 and brca2tr cells. The observations from wild-type, xrcc2, and brca2tr cells and from these mutants that overexpress Rad51 indicate that inefficient recruitment of Rad51 at the Ig V segment may be responsible for activation of an alternative error-prone repair pathway.
DISCUSSION
We show that brca2tr DT40 cells exhibit a phenotype similar to that of mammalian brca2 mutant cells, including elevated sensitivities to genotoxic stress, impaired HR capability, and defective Rad51 focus formation. The defective Rad51 focus formation is shared only with the five rad51 paralog mutants (52, 53) and not with any other HR-deficient DT40 mutants with mutations in the MRE11, NBS1, RAD52, RAD54, and FANCG genes (52, 56, 63-65). Interestingly, we found that all rad51 paralog mutants, but not other HR-deficient cells, also exhibited a shift in the pattern of diversification at the Ig Vλ locus from gene conversion to somatic hypermutation (Fig. 4E). In brca2tr and xrcc2 cells, this shift is largely reversed by overexpression of Rad51 (see Fig. S2 in the supplemental material). These observations support the following conclusions. First, Brca2 and the Rad51 paralogs may affect the frequency of HR but not its accuracy in Ig gene conversion. Second, hypermutation and gene conversion are two alternative pathways for Ig V diversification. Third, the activity of Brca2 and the Rad51 paralogs could affect the choice of the two alternative repair pathways. Fourth, given the role of some Rad51 paralogs in a later step of HR (6, 7, 28, 68, 69), these Rad51 paralogs may have dual functions, one at an initial step and the other possibly at a later step in HR. We will discuss each of these points in turn.
Defects in Brca2 or Rad51 paralogs do not affect the accuracy of HR at the Ig locus of DT40.
Xrcc2- and Xrcc3-deficient hamster cell lines exhibit a reduced rate of HR in response to an endonuclease-induced DSB (21, 38). Recently, Brenneman et al. reported that the HR products recovered from the xrcc3 mutant hamster cell line irs1-SF following site-specific endonuclease cleavage of a neo direct repeat construct displayed radically altered spectra, with increased gene conversion tract lengths, increased frequencies of discontinuous tracts, and frequent local rearrangements associated with HR (7). We therefore reevaluated the HR capabilities of all rad51 paralog mutants by analyzing Ig gene conversion products. Also, we newly generated xrcc2 cells carrying a defined frameshift mutation at the Ig V segment, in order to determine nucleotide sequences of a large number of Ig gene conversion products. Our data are not fully consistent with those of Brenneman et al. In agreement is the observation that gene conversion tract lengths were increased (Fig. 5B). Conceivably, a defect in Xrcc2 may destabilize homologous pairing of shorter sequences, resulting in a relative increase in longer gene conversion tracts. On the other hand, neither discontinuous tracts nor aberrant rearrangements were detectable in any of 92 gene conversion products. The discrepancy may be explained by differential contributions of Xrcc3 to early and late steps of HR in the hamster and DT40 cells, as DT40 cells exhibit an extraordinarily high gene targeting efficiency compared to any other vertebrate cells. Our results are therefore more in agreement with those for Xrcc2-deficient rodent cells (21). We conclude that the accuracy of HR is not compromised in the absence of Xrcc2.
Nucleotide sequence analysis of Ig V revealed that in brca2tr cells one-base deletions occurred frequently at an RGY(W) motif (see Fig. S1 in the supplemental material). The deletion might indicate a primary AID-dependent DNA damage site, because the motif is a hypermutation hot spot in the mammalian Ig V genes (4, 43). In brca2tr as well as xrcc2 cells, neither discontinuous tracts nor aberrant rearrangements were detectable in any of 37 sIgM gain events. These findings reveal that Brca2 and Xrcc2 regulate the efficiency, but not accuracy, of HR in Ig gene conversion.
Hypermutation and gene conversion are two alternative pathways for Ig V diversification.
Both brca2tr cells and all rad51 paralog mutants displayed the transversion-favored dC/dG hypermutation in the Vλ segment (Fig. 4D). There are two scenarios for the hypermutation. First, a defect in Brca2 and Rad51 paralogs may reduce the accuracy of HR between intragenic pseudo-V and Vλ segments, as an interesting precedent is found in Rad51 paralog-deficient budding yeast (41). The second scenario assumes that the gene conversion and hypermutation constitute two alternative pathways. To evaluate these scenarios, we analyzed the pattern of Ig V diversification in the following five genotypes: wild type, brca2tr, brca2tr/Rad51, xrcc2, and xrcc2/Rad51. We found for these genotypes that the frequencies of gene conversion and hypermutation were inversely correlated. Furthermore, no hypermutation events were associated with gene conversion events, and no detectable gene conversion tracts were accompanied by nontemplated mutations. These results strongly suggest that the observed gene conversion and hypermutation constitute two alternative pathways of Ig V diversification. Thus, in brca2tr cells and all rad51 paralog mutants, defective initiation of gene conversion may lead to activation of an error-prone pathway probably involving translesion DNA synthesis.
The activity of Brca2 and the Rad51 paralogs could affect the choice of the two alternative repair pathways.
Both gene conversion and somatic hypermutation appear to be triggered by AID-catalyzed deamination of deoxycytidine (dC) to deoxyuridine (dU) within the Ig V gene (2, 11, 17, 31, 37, 42). The resulting uracil is then excised by uracil DNA glycosylase to generate an abasic site. The abasic site can be processed and form a single-strand gap, which might be a substrate for HR. brca2tr and the rad51 paralog mutant cells showed a reduced rate of Ig gene conversion, indicating that these proteins play a role in HR particularly initiated by a single-strand gap. This notion can explain the more significant elevation of sensitivity to cross-linking agents than to IR in brca2tr and the rad51 paralog mutants, because DNA cross-linking agents, but not IR, can induce large numbers of single-strand gaps following a replication block. In conclusion, Brca2 and the Rad51 paralogs might play a more important role in HR reactions initiated by single-strand gaps than in HR-dependent DSB repair.
Both brca2tr cells and all rad51 paralog mutants exhibited an accumulation of somatic hypermutations (Fig. 4E). An arising question is why the HR-deficient DT40 mutants, for example, rad54 cells, do not display hypermutation, although their Ig gene conversion rates are also significantly reduced (5). We note that there is close correlation between defective Rad51 focus formation and a shift from Ig V gene conversion to hypermutation among the HR-deficient DT40 clones. Thus, a likely explanation is that defective recruitment of Rad51 at sites of DNA damage may be causally linked to the hypermutation phenotype, promoting the channeling of AID-dependent DNA damage via an alternate error-prone pathway. Conversely, in rad54 cells, efficient Rad51 assembly at the AID-dependent DNA damage may irreversibly inhibit such an alternative pathway, with the resulting unrepaired DNA damage eventually killing the cells because of defective HR at a later stage. Thus, Brca2 and the Rad51 paralogs could affect the choice of the two alternative repair pathways.
Possible dual function of Rad51 paralogs in homologous DNA recombination.
Recently, accumulating evidence suggests that some Rad51 paralogs may play a key role in a later step of HR (7, 28). These findings led us to reevaluate the early role of every Rad51 paralog by analyzing Ig V diversification in the rad51 paralog as well as brca2tr mutants.
We show here that hypermutation and gene conversion are two alternative pathways for Ig V diversification. Moreover, brca2tr and all rad51 paralog mutant cells exhibited a shift to Ig V hypermutation (Fig. 4E), which was closely correlated with defective Rad51 focus formation. This phenotypic similarity of these mutants indicates that all Rad51 paralogs play a key role also in an early step of HR, as suggested for Brca2 from biochemical and three-dimensional structure studies (36, 46, 67, 70). Of note, the early role and later ones, such as resolution of Holliday junctions, are not necessarily mutually exclusive. Presumably, a defect in an early step in this phenotypic assay might mask any role for Rad51 paralogs in the later steps.
Our results also have implications for the mechanisms of chromosome instability, the potential tumor suppressor activity of Brca2 and the Rad51 paralogs, and the role of these molecules in cellular tolerance to cisplatin. Large numbers of abasic sites are generated each day in the DNAs of higher eukaryotic cells (26). Replicating chromosomes are fragile, and substantial fractions of these abasic sites may block DNA replication, leading to formation of single-strand gaps (18). Likewise, cisplatin specifically kills cycling cells by interfering with DNA replication. Resulting single-strand gaps appear to be repaired by two major postreplicational repair pathways, HR and error-prone translesion DNA synthesis, which have substantially overlapping roles (47, 66). However, little is known about the choice of these postreplicational repair pathways in vivo. We have reported here that brca2tr cells exhibited not only a reduced rate of HR, as reported previously for mammalian brca2 cells (30), but also a dramatic increase in the usage of another alternative repair pathway involving hypermutation. Thus, the analysis of Ig gene conversion in brca2tr cells will shed light on the precise role for Brca2 in prevention of mutagenesis as well as in HR.
Supplementary Material
Acknowledgments
We thank Y. Sato and M. Nagao for their technical assistance. We also thank D. Bishop (University of Chicago) and M. Neuberger (MRC, Cambridge, United Kingdom.) for critical reading and discussion.
Financial support was provided in part by CREST, JST (Saitama, Japan); by a Center of Excellence grant for Scientific Research from the Ministry of Education, Culture, Sports and Technology; and by grants from The Uehara Memorial Foundation and The Naito Foundation. This work was also funded in part by grants from the Virtual Research Institute of Aging of Nippon Boehringer Ingelheim.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Abbott, D. W., M. L. Freeman, and J. T. Holt. 1998. Double-strand break repair deficiency and radiation sensitivity in BRCA2 mutant cancer cells. J. Natl. Cancer Inst. 90:978-985. [DOI] [PubMed] [Google Scholar]
- 2.Arakawa, H., J. Hauschild, and J. M. Buerstedde. 2002. Requirement of the activation-induced deaminase (AID) gene for immunoglobulin gene conversion. Science 295:1301-1306. [DOI] [PubMed] [Google Scholar]
- 3.Baumann, P., F. E. Benson, and S. C. West. 1996. Human Rad51 protein promotes ATP-dependent homologous pairing and strand transfer reactions in vitro. Cell 87:757-766. [DOI] [PubMed] [Google Scholar]
- 4.Betz, A. G., C. Rada, R. Pannell, C. Milstein, and M. S. Neuberger. 1993. Passenger transgenes reveal intrinsic specificity of the antibody hypermutation mechanism: clustering, polarity, and specific hot spots. Proc. Natl. Acad. Sci. USA 90:2385-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bezzubova, O., A. Silbergleit, Y. Yamaguchi-Iwai, S. Takeda, and J. M. Buerstedde. 1997. Reduced X-ray resistance and homologous recombination frequencies in a RAD54−/− mutant of the chicken DT40 cell line. Cell 89:185-193. [DOI] [PubMed] [Google Scholar]
- 6.Braybrooke, J. P., J. L. Li, L. Wu, F. Caple, F. E. Benson, and I. D. Hickson. 2003. Functional interaction between the Bloom's syndrome helicase and the RAD51 paralog, RAD51L3 (RAD51D). J. Biol. Chem. 278:48357-48366. [DOI] [PubMed] [Google Scholar]
- 7.Brenneman, M. A., B. M. Wagener, C. A. Miller, C. Allen, and J. A. Nickoloff. 2002. XRCC3 controls the fidelity of homologous recombination: roles for XRCC3 in late stages of recombination. Mol. Cell 10:387-395. [DOI] [PubMed] [Google Scholar]
- 8.Buerstedde, J. M., C. A. Reynaud, E. H. Humphries, W. Olson, D. L. Ewert, and J. C. Weill. 1990. Light chain gene conversion continues at high rate in an ALV-induced cell line. EMBO J. 9:921-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buerstedde, J. M., and S. Takeda. 1991. Increased ratio of targeted to random integration after transfection of chicken B cell lines. Cell 67:179-188. [DOI] [PubMed] [Google Scholar]
- 10.Connor, F., D. Bertwistle, P. J. Mee, G. M. Ross, S. Swift, E. Grigorieva, V. L. Tybulewicz, and A. Ashworth. 1997. Tumorigenesis and a DNA repair defect in mice with a truncating Brca2 mutation. Nat. Genet. 17:423-430. [DOI] [PubMed] [Google Scholar]
- 11.Di Noia, J., and M. S. Neuberger. 2002. Altering the pathway of immunoglobulin hypermutation by inhibiting uracil-DNA glycosylase. Nature 419:43-48. [DOI] [PubMed] [Google Scholar]
- 12.Di Noia, J. M., and M. S. Neuberger. 2004. Immunoglobulin gene conversion in chicken DT40 cells largely proceeds through an abasic site intermediate generated by excision of the uracil produced by AID-mediated deoxycytidine deamination. Eur. J. Immunol. 34:504-508. [DOI] [PubMed] [Google Scholar]
- 13.Friedman, L. S., F. C. Thistlethwaite, K. J. Patel, V. P. Yu, H. Lee, A. R. Venkitaraman, K. J. Abel, M. B. Carlton, S. M. Hunter, W. H. Colledge, M. J. Evans, and B. A. Ponder. 1998. Thymic lymphomas in mice with a truncating mutation in Brca2. Cancer Res. 58:1338-1343. [PubMed] [Google Scholar]
- 14.Fujimori, A., S. Tachiiri, E. Sonoda, L. H. Thompson, P. K. Dhar, M. Hiraoka, S. Takeda, Y. Zhang, M. Reth, and M. Takata. 2001. Rad52 partially substitutes for the Rad51 paralog XRCC3 in maintaining chromosomal integrity in vertebrate cells. EMBO J. 20:5513-5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukushima, T., M. Takata, C. Morrison, R. Araki, A. Fujimori, M. Abe, K. Tatsumi, M. Jasin, PK. Dhar, E. Sonoda, T. Chiba, and S. Takeda. 2001. Genetic analysis of the DNA-dependent protein kinase reveals an inhibitory role of Ku in late S-G2 phase DNA double-strand break repair. J. Biol. Chem. 276:44413-44418. [DOI] [PubMed] [Google Scholar]
- 16.Haaf, T., E. Raderschall, G. Reddy, D. C. Ward, C. M. Radding, and E. I. Golub. 1999. Sequestration of mammalian Rad51-recombination protein into micronuclei. J. Cell Biol. 144:11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris, R. S., J. E. Sale, S. K. Petersen-Mahrt, and M. S. Neuberger. 2002. AID is essential for immunoglobulin V gene conversion in a cultured B cell line. Curr. Biol. 12:435-438. [DOI] [PubMed] [Google Scholar]
- 18.Hoeijmakers, J. H. 2001. Genome maintenance mechanisms for preventing cancer. Nature 411:366-374. [DOI] [PubMed] [Google Scholar]
- 19.Hudson, D. F., C. Morrison, S. Ruchaud, and W. C. Earnshaw. 2002. Reverse genetics of essential genes in tissue-culture cells: ‘dead cells talking.’ Trends Cell Biol. 12:281-287. [DOI] [PubMed] [Google Scholar]
- 20.Jasin, M. 2002. Homologous repair of DNA damage and tumorigenesis: the BRCA connection. Oncogene 21:8981-8993. [DOI] [PubMed] [Google Scholar]
- 21.Johnson, R. D., N. Liu, and M. Jasin. 1999. Mammalian XRCC2 promotes the repair of DNA double-strand breaks by homologous recombination. Nature 401:397-399. [DOI] [PubMed] [Google Scholar]
- 22.Johnson, R. D., and L. S. Symington. 1995. Functional differences and interactions among the putative RecA homologs Rad51, Rad55, and Rad57. Mol. Cell. Biol. 15:4843-4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kraakman-van der Zwet, M., W. J. Overkamp, R. E. van Lange, J. Essers, A. van Duijn-Goedhart, I. Wiggers, S. Swaminathan, P. P. van Buul, A. Errami, R. T. Tan, N. G. Jaspers, S. K. Sharan, R. Kanaar, and M. Z. Zdzienicka. 2002. Brca2 (XRCC11) deficiency results in radioresistant DNA synthesis and a higher frequency of spontaneous deletions. Mol. Cell. Biol. 22:669-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kraakman-van der Zwet, M., W. W. Wiegant, and M. Z. Zdzienicka. 2003. Brca2 (XRCC11) deficiency results in enhanced mutagenesis. Mutagenesis 18:521-525. [DOI] [PubMed] [Google Scholar]
- 25.Lim, D. S., and P. Hasty. 1996. A mutation in mouse Rad51 results in an early embryonic lethal that is suppressed by a mutation in p53. Mol. Cell. Biol. 16:7133-7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindahl, T. 1993. Instability and decay of the primary structure of DNA. Nature 362:709-715. [DOI] [PubMed] [Google Scholar]
- 27.Liu, Y., and N. Maizels. 2000. Coordinated response of mammalian Rad51 and Rad52 to DNA damage. EMBO Rep. 1:85-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu, Y., J. Y. Masson, R. Shah, P. O'Regan, and S. C. West. 2004. RAD51C is required for Holliday junction processing in mammalian cells. Science 303:243-246. [DOI] [PubMed] [Google Scholar]
- 29.Ludwig, T., D. L. Chapman, V. E. Papaioannou, A. Efstratiadis, T. Ludwig, D. L. Chapman, and V. E. Papaioannou. 1997. Targeted mutations of breast cancer susceptibility gene homologs in mice: lethal phenotypes of Brca1, Brca2, Brca1/Brca2, Brca1/p53, and Brca2/p53 nullizygous embryos. Genes Dev. 11:1226-1241. [DOI] [PubMed] [Google Scholar]
- 30.Moynahan, M. E., A. J. Pierce, and M. Jasin. 2001. BRCA2 is required for homology-directed repair of chromosomal breaks. Mol. Cell 7:263-272. [DOI] [PubMed] [Google Scholar]
- 31.Muramatsu, M., K. Kinoshita, S. Fagarasan, S. Yamada, Y. Shinkai, and T. Honjo. 2000. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 102:553-563. [DOI] [PubMed] [Google Scholar]
- 32.Neuberger, M. S., R. S. Harris, J. Di Noia, and S. K. Petersen-Mahrt. 2003. Immunity through DNA deamination. Trends Biochem Sci. 28:305-312. [DOI] [PubMed] [Google Scholar]
- 33.Ogawa, T., X. Yu, A. Shinohara, and E. H. Egelman. 1993. Similarity of the yeast RAD51 filament to the bacterial RecA filament. Science 259:1896-1899. [DOI] [PubMed] [Google Scholar]
- 34.Okazaki, I., K. Yoshikawa, K. Kinoshita, M. Muramatsu, H. Nagaoka, and T. Honjo. 2003. Activation-induced cytidine deaminase links class switch recombination and somatic hypermutation. Ann. N. Y. Acad. Sci. 987:1-8. [DOI] [PubMed] [Google Scholar]
- 35.Patel, K. J., V. P. C. C. Yu, H. Lee, A. Corcoran, F. C. Thestlethwaite, M. J. Evans, W. H. Colledge, L. S. Friedman, B. A. J. Ponder, and A. R. Venkitaraman. 1998. Involvement of Brca2 in DNA repair. Mol. Cell 1:347-357. [DOI] [PubMed] [Google Scholar]
- 36.Pellegrini, L., D. S. Yu, T. Lo, S. Anand, M. Lee, T. L. Blundell, and A. R. Venkitaraman. 2002. Insights into DNA recombination from the structure of a RAD51-BRCA2 complex. Nature 420:287-293. [DOI] [PubMed] [Google Scholar]
- 37.Petersen-Mahrt, S. K., R. S. Harris, and M. S. Neuberger. 2002. AID mutates E. coli, suggesting a DNA deamination mechanism for antibody diversification. Nature 418:99-103. [DOI] [PubMed] [Google Scholar]
- 38.Pierce, A. J., R. D. Johnson, L. H. Thompson, and M. Jasin. 1999. XRCC3 promotes homology-directed repair of DNA damage in mammalian cells. Genes Dev. 13:2633-2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raderschall, E., E. I. Golub, and T. Haaf. 1999. Nuclear foci of mammalian recombination proteins are located at single-stranded DNA regions formed after DNA damage. Proc. Natl. Acad. Sci. USA 96:1921-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rahman, N., and M. R. Stratton. 1998. The genetics of breast cancer susceptibility. Annu. Rev. Genet. 32:95-121. [DOI] [PubMed] [Google Scholar]
- 41.Rattray, A. J., B. K. Shafer, C. B. McGill, and J. N. Strathern. 2002. The roles of REV3 and RAD57 in double-strand-break-repair-induced mutagenesis of Saccharomyces cerevisiae. Genetics 162:1063-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Revy, P., T. Muto, Y. Levy, F. Geissmann, A. Plebani, O. Sanal, N. Catalan, M. Forveille, R. Dufourcq-Labelouse, A. Gennery, I. Tezcan, F. Ersoy, H. Kayserili, A. G. Ugazio, N. Brousse, M. Muramatsu, L. D. Notarangelo, K. Kinoshita, T. Honjo, A. Fischer, and A. Durandy. 2000. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2). Cell 102:565-575. [DOI] [PubMed] [Google Scholar]
- 43.Rogozin, I. B., and N. A. Kolchanov. 1992. Somatic hypermutagenesis in immunoglobulin genes. II. Influence of neighbouring base sequences on mutagenesis. Biochim. Biophys. Acta 1171:11-18. [DOI] [PubMed] [Google Scholar]
- 44.Sale, J. E., D. M. Calandrini, M. Takata, S. Takeda, and M. S. Neuberger. 2001. Ablation of XRCC2/3 transforms immunoglobulin V gene conversion into somatic hypermutation. Nature 412:921-926. [DOI] [PubMed] [Google Scholar]
- 45.Scully, R., and D. M. Livingston. 2000. In search of the tumor-suppressor functions of BRCA1 and BRCA2. Nature 408:429-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shin, D. S., L. Pellegrini, D. S. Daniels, B. Yelent, L. Craig, D. Bates, D. S. Yu, M. K. Shivji, C. Hitomi, A. S. Arvai, N. Volkmann, H. Tsuruta, T. L. Blundell, A. R. Venkitaraman, and J. A. Tainer. 2003. Full-length archaeal Rad51 structure and mutants: mechanisms for RAD51 assembly and control by BRCA2. EMBO J. 22:4566-4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sonoda, E., T. Okada, G. Y. Zhao, S. Tateishi, K. Araki, M. Yamaizumi, T. Yagi, N. S. Verkaik, D. C. van Gent, M. Takata, and S. Takeda. 2003. Multiple roles of Rev3, the catalytic subunit of polzeta in maintaining genome stability in vertebrates. EMBO J. 22:3188-3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sonoda, E., M. S. Sasaki, J.-M. Buerstedde, O. Bezzubova, A. Shinohara, H. Ogawa, M. Takata, Y. Yamaguchi-Iwai, and S. Takeda. 1998. Rad51 deficient vertebrate cells accumulate chromosomal breaks prior to cell death. EMBO J. 17:598-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sonoda, E., M. Takata, Y. M. Yamashita, C. Morrison, and S. Takeda. 2001. Homologous DNA recombination in vertebrate cells. Proc. Natl. Acad. Sci. USA 98:8388-8394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sung, P., and D. L. Robberson. 1995. DNA strand exchange mediated by a RAD51-ssDNA nucleoprotein filament with polarity opposite to that of RecA. Cell 82:453-461. [DOI] [PubMed] [Google Scholar]
- 51.Tachiiri, S., K. Sasai, N. Oya, and M. Hiraoka. 2000. Enhanced cell killing by overexpression of dominant-negative phosphatidylinositol 3-kinase subunit, Δp85, following genotoxic stresses. Jpn. J. Cancer Res. 91:1314-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takata, M., M. S. Sasaki, E. Sonoda, T. Fukushima, C. Morrison, J. S. Albala, S. M. Swagemakers, R. Kanaar, L. H. Thompson, and S. Takeda. 2000. The Rad51 paralog Rad51B promotes homologous recombinational repair. Mol. Cell. Biol. 20:6476-6482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takata, M., M. S. Sasaki, S. Tachiiri, T. Fukushima, E. Sonoda, D. Schild, L. H. Thompson, and S. Takeda. 2001. Chromosome instability and defective recombinational repair in knockout mutants of the five Rad51 paralogs. Mol. Cell. Biol. 21:2858-2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takata, M., S. Tachiiri, A. Fujimori, L. H. Thompson, Y. Miki, M. Hiraoka, S. Takeda, and M. Yamazoe. 2002. Conserved domains in the chicken homologue of BRCA2. Oncogene 21:1130-1134. [DOI] [PubMed] [Google Scholar]
- 55.Tashiro, S., J. Walter, A. Shinohara, N. Kamada, and T. Cremer. 2000. Rad51 accumulation at sites of DNA damage and in postreplicative chromatin. J. Cell Biol. 150:283-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tauchi, H., J. Kobayashi, K. Morishima, D. C. Van Gent, T. Shiraishi, N. S. Verkaik, D. VanHeems, E. Ito, A. Nakamura, E. Sonoda, M. Takata, S. Takeda, S. Matsuura, and K. Komatsu. 2002. Nbs1 is essential for DNA repair by homologous recombination in higher vertebrate cells. Nature 420:93-98. [DOI] [PubMed] [Google Scholar]
- 57.Tsuzuki, T., Y. Fujii, K. Sakumi, Y. Tominaga, K. Nakao, M. Sekiguchi, A. Matsushiro, Y. Yoshimura, and T. Morita. 1996. Targeted disruption of the Rad51 gene leads to lethality in embryonic mice. Proc. Natl. Acad. Sci. USA 93:6236-6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tutt, A., and A. Ashworth. 2002. The relationship between the roles of BRCA genes in DNA repair and cancer predisposition. Trends Mol. Med. 8:571-576. [DOI] [PubMed] [Google Scholar]
- 59.Tutt, A., A. Gabriel, D. Bertwistle, F. Connor, H. Paterson, J. Peacock, G. Ross, and A. Ashworth. 1999. Absence of Brca2 causes genome instability by chromosome breakage and loss associated with centrosome amplification. Curr. Biol. 9:1107-1110. [DOI] [PubMed] [Google Scholar]
- 60.Tutt, A. N., C. T. van Oostrom, G. M. Ross, H. van Steeg, and A. Ashworth. 2002. Disruption of Brca2 increases the spontaneous mutation rate in vivo: synergism with ionizing radiation. EMBO Rep. 3:255-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Venkitaraman, A. R. 2002. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell 108:171-182. [DOI] [PubMed] [Google Scholar]
- 62.Warenius, H. M., L. A. Seabra, and P. Maw. 1996. Sensitivity to cis-diamminedichloroplatinum in human cancer cells is related to expression of cyclin D1 but not c-raf-1 protein. Int. J. Cancer 67:224-231. [DOI] [PubMed] [Google Scholar]
- 63.Yamaguchi-Iwai, Y., E. Sonoda, J.-M. Buerstedde, O. Bezzubova, C. Morrison, M. Takata, A. Shinohara, and S. Takeda. 1998. Homologous recombination, but not DNA repair, is reduced in vertebrate cells deficient in RAD52. Mol. Cell. Biol. 18:6430-6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamaguchi-Iwai, Y., E. Sonoda, M. S. Sasaki, C. Morrison, T. Haraguchi, Y. Hiraoka, Y. M. Yamashita, T. Yagi, M. Takata, C. Price, N. Kakazu, and S. Takeda. 1999. Mre11 is essential for the maintenance of chromosomal DNA in vertebrate cells. EMBO J. 18:6619-6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamamoto, K., M. Ishiai, N. Matsushita, H. Arakawa, J. E. Lamerdin, J. M. Buerstedde, M. Tanimoto, M. Harada, L. H. Thompson, and M. Takata. 2003. Fanconi anemia FANCG protein in mitigating radiation- and enzyme-induced DNA double-strand breaks by homologous recombination in vertebrate cells. Mol. Cell. Biol. 23:5421-5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yamashita, Y. M., T. Okada, T. Matsusaka, E. Sonoda, G. Y. Zhao, K. Araki, S. Tateishi, M. Yamaizumi, and S. Takeda. 2002. RAD18 and RAD54 cooperatively contribute to maintenance of genomic stability in vertebrate cells. EMBO J. 21:5558-5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang, H., P. D. Jeffrey, J. Miller, E. Kinnucan, Y. Sun, N. H. Thoma, N. Zheng, P. L. Chen, W. H. Lee, and N. P. Pavletich. 2002. BRCA2 function in DNA binding and recombination from a BRCA2-DSS1-ssDNA structure. Science 297:1837-1848. [DOI] [PubMed] [Google Scholar]
- 68.Yokoyama, H., H. Kurumizaka, S. Ikawa, S. Yokoyama, and T. Shibata. 2003. Holliday junction binding activity of the human Rad51B protein. J. Biol. Chem. 278:2767-2772. [DOI] [PubMed] [Google Scholar]
- 69.Yokoyama, H., N. Sarai, W. Kagawa, R. Enomoto, T. Shibata, H. Kurumizaka, and S. Yokoyama. 2004. Preferential binding to branched DNA strands and strand-annealing activity of the human Rad51B, Rad51C, Rad51D and Xrcc2 protein complex. Nucleic Acids Res. 32:2556-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu, D. S., E. Sonoda, S. Takeda, C. L. Huang, L. Pellegrini, T. L. Blundell, and A. R. Venkitaraman. 2003. Dynamic control of Rad51 recombinase by self-association and interaction with BRCA2. Mol. Cell 12:1029-1041. [DOI] [PubMed] [Google Scholar]
- 71.Yu, V. P., M. Koehler, C. Steinlein, M. Schmid, L. A. Hanakahi, A. J. van Gool, S. C. West, and A. R. Venkitaraman. 2000. Gross chromosomal rearrangements and genetic exchange between nonhomologous chromosomes following BRCA2 inactivation. Genes Dev. 14:1400-1406. [PMC free article] [PubMed] [Google Scholar]
- 72.Yu, X., S. A. Jacobs, S. C. West, T. Ogawa, and E. H. Egelman. 2001. Domain structure and dynamics in the helical filaments formed by RecA and Rad51 on DNA. Proc. Natl. Acad. Sci. USA 98:8419-8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yuan, S. S., S. Y. Lee, G. Chen, M. Song, G. E. Tomlinson, and E. Y. Lee. 1999. BRCA2 is required for ionizing radiation-induced assembly of Rad51 complex in vivo. Cancer Res. 59:3547-3551. [PubMed] [Google Scholar]
- 74.Zhang, Y., C. Riesterer, A. M. Ayrall, F. Sablitzky, T. D. Littlewood, and M. Reth. 1996. Inducible site-directed recombination in mouse embryonic stem cells. Nucleic Acids Res. 24:543-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang, Y., J. Wienands, C. Zurn, and M. Reth. 1998. Induction of the antigen receptor expression on B lymphocytes results in rapid competence for signaling of SLP-65 and Syk. EMBO J. 17:7304-7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zheng, L., S. Li, T. G. Boyer, and W. H. Lee. 2000. Lessons learned from BRCA1 and BRCA2. Oncogene 19:6159-6175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.