Abstract
Rationale: Plasma-detectable biomarkers that rapidly and accurately diagnose bacterial infections in children with suspected pneumonia could reduce the morbidity of respiratory disease and decrease the unnecessary use of antibiotic therapy.
Objectives: Using 56 markers measured in a multiplexed immunoassay, we sought to identify proteins and protein combinations that could discriminate bacterial from viral or malarial diagnoses.
Methods: We selected 80 patients with clinically diagnosed pneumonia (as defined by the World Health Organization) who also met criteria for bacterial, viral, or malarial infection based on clinical, radiographic, and laboratory results. Ten healthy community control subjects were enrolled to assess marker reliability. Patients were subdivided into two sets: one for identifying potential markers and another for validating them.
Measurements and Main Results: Three proteins (haptoglobin, tumor necrosis factor receptor 2 or IL-10, and tissue inhibitor of metalloproteinases 1) were identified that, when combined through a classification tree signature, accurately classified patients into bacterial, malarial, and viral etiologies and misclassified only one patient with bacterial pneumonia from the validation set. The overall sensitivity and specificity of this signature for the bacterial diagnosis were 96 and 86%, respectively. Alternative combinations of markers with comparable accuracy were selected by support vector machine and regression models and included haptoglobin, IL-10, and creatine kinase–MB.
Conclusions: Combinations of plasma proteins accurately identified children with a respiratory syndrome who were likely to have bacterial infections and who would benefit from antibiotic therapy. When used in conjunction with malaria diagnostic tests, they may improve diagnostic specificity and simplify treatment decisions for clinicians.
Keywords: biomarkers, pneumonia, immunoassay, bacteria
At a Glance Commentary
Scientific Knowledge on the Subject
Current approaches for distinguishing bacterial from viral or parasitic causes of acute respiratory symptoms are unreliable and inaccurate. Proteins including haptoglobin, lipocalin-2, and procalcitonin were found to have diagnostic value. However, no single biomarker accurately distinguishes between viral, bacterial, and malarial etiologies in settings where all may be involved.
What This Study Adds to the Field
We identified accurate biomarker signatures that could differentiate bacterial from viral and malarial causes of respiratory symptoms in febrile children. We propose combining these markers to produce a more accurate test suitable for development as a point-of-care diagnostic.
Febrile acute respiratory distress is a leading cause of pediatric hospital admissions and is associated with significant childhood morbidity. The etiology is usually bacterial or viral infection, but in malaria-endemic areas, malarial infection can produce a similar syndrome (1). Rapid and accurate determination of the etiology could guide therapy, ensuring that children with respiratory symptoms and associated bacterial infection receive prompt antibiotic therapy, and limiting overuse of antibiotics in patients with viral and malarial infections (2), which might improve outcomes and slow the development of antibiotic resistance. Particularly in resource-constrained settings, where the three etiologies of this clinical syndrome are common, accurate diagnoses are challenging (3, 4).
Symptoms caused by bacterial, viral, and malarial infections usually overlap (5) and etiologic diagnosis is based chiefly on clinical and radiographic findings, which can be inaccurate, poorly reproducible (6), generally unavailable in resource-challenged settings, and may not reliably distinguish viral from bacterial etiologies or predict outcomes (7). Radiographic evidence of pneumonia has been used as an endpoint in studies of pneumococcal vaccine, but chest X-rays, particularly in resource-limited settings, have moderate reliability (7–16) and may result in both false positive and false negative diagnoses of bacterial pneumonia. Laboratory tests for viruses and bacteria have low sensitivity or specificity and are not readily available. Blood or pleural fluid cultures are highly specific for the diagnosis of bacterial infection but have low sensitivity (17) and require specialized laboratory facilities. Blood culture results become available only 24 hours or more after blood collection, and results can be inconclusive due to contamination or low-volume samples (18). Polymerase chain reaction tests for bacteria and virus also yield delayed results, require specialized resources, and may have low sensitivity and/or specificity (6, 19, 20), decreasing the usefulness of these tests for decisions regarding treatment. Furthermore, in children, sputum samples are difficult to collect and antigen detection in the urine has limited usefulness (21). Although malaria tests can detect the parasite in blood, malaria may coexist with other infections while not causing the respiratory syndrome (22, 23), resulting in children with bacterial pneumonia being sent home with antimalarial drugs but not antibiotics (24).
To develop an assay that distinguishes bacterial from nonbacterial infections and could facilitate treatment decisions, we explored candidate proteins in a multiplexed immunoassay to identify markers or combinations of markers that could be developed into immunochromatographic tests and then validated in subsequent cohorts. Immunochromatographic tests are used for rapid and inexpensive diagnosis of many diseases, including malaria. Although association of several markers with the three etiologies has been shown, no individual marker has diagnostic value (25–38). Haptoglobin and lipocalin-2 were found to accurately distinguish probable bacterial pneumonia from malaria (39).
Some of the results of this study have been previously reported in the form of an abstract (40).
Methods
Study Population and Procedures
Children less than 10 years old presenting to the outpatient clinic of the Manhiça District Hospital (Manhiça, Mozambique) with fever on admission (>37.5°C axillary temperature) or a history of fever in the preceding 24 hours, symptoms of World Health Organization–defined clinical pneumonia (increased respiratory rate and cough or difficult breathing) (41), and fulfilling criteria for hospital admission between January 2010 and November 2012 were assessed for recruitment. Exclusion criteria included use of antimalarial drugs in the preceding 2 weeks, established or likely diagnosis of tuberculosis (history of cough lasting more than 2 wk or history of direct contact with a patient with tuberculosis —27 patients), participation in conflicting studies, and, to exclude likely cases of Pneumocystis jirovecii, oxyhemoglobin saturation of 85% or less (40 patients). Exclusion of suspected infections with these pathogens aimed to improve the specificity of case definitions, recognizing that their exclusion might also have excluded true cases of bacterial or viral infection. Informed consent was sought from parent/guardians. Before the initiation of treatment, a nasopharyngeal aspirate was taken to determine respiratory viral infection and venous blood was collected for blood culture and human immunodeficiency virus (HIV) testing, malaria diagnosis (by microscopy), and multiplexed immunoassay. A chest X-ray was obtained from all children and read by two experienced clinicians according to the procedure proposed by Cherian and colleagues (8). For multiplexed immunoassays, plasma was extracted from 2 ml of the blood sample within 75 minutes of collection. All laboratory tests were processed blinded to diagnosis and clinical progression. Further details of all procedures are presented in the online supplement.
Healthy community control subjects matched to cases by sex, age, and neighborhood were identified through a demographic surveillance system. Control children were examined and excluded if they presented with fever or respiratory symptoms or if they had taken any medication in the previous 30 days. Blood was collected for full blood cell count, detection of malarial parasites, and multiplexed immunoassay. If positive for malaria, children were excluded from this healthy control group.
This study was approved by the Mozambique National Bioethics Committee and the institutional review boards of the Broad Institute (Cambridge, MA) and the Barcelona Center for International Health Research (Barcelona, Spain).
Etiologic Diagnosis Classification
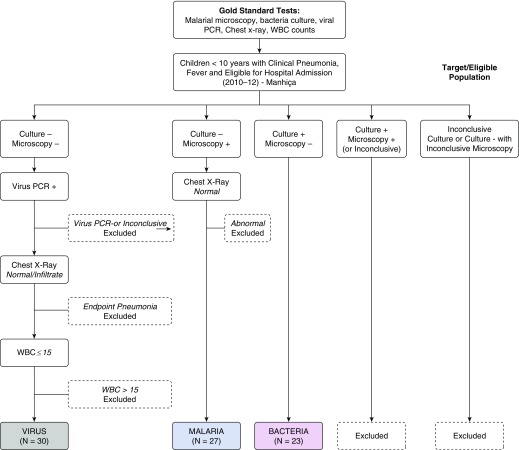
To be classified as a bacterial case, pathogenic bacteria had to be isolated from blood or pleural effusion in the absence of malarial parasites (Figure 1). Patients were classified as having viral pneumonia if there were no detectable malarial parasites; they produced a negative blood culture; they had a normal chest X-ray or infiltrates other than lobar consolidation and without pleural effusion; they had fewer than 15 × 109 leukocytes/L in the peripheral blood; and nasopharyngeal aspirate polymerase chain reaction indicated the presence of respiratory viral nucleic acids. Patients were classified as having malaria if the blood culture was negative, the chest X-ray was normal, and there were malarial parasites present in a thick smear according to the following age-related threshold: more than 0 asexual parasites detected in children aged less than 1 year and more than 2,500 asexual parasites/μl of blood in children over 1 year of age (42). Clinical examination and collection of samples for multiplexed immunoassay were performed on admission.
Figure 1.
Patient classification into three etiologic groups. Inconclusive tests included those that were contaminated (bacteria), those that were missing results, and those that did not meet the parasitemia threshold (microscopy). Chest X-ray results were classified as (1) normal, (2) infiltrates other than end-point pneumonia, and (3) lobar consolidation or pleural effusion (endpoint pneumonia). PCR = polymerase chain reaction; WBC = white blood cell.
Multiplexed Immunoassay
The 99 cytokines, chemokines, other proteins, and metabolites available in the Myriad Rules Based Medicine (Myriad RBM, Austin, TX) multiplexed immunoassay (HumanMAP v. 1.6, HumanMAP v. 2.0, and HumanMAP v. 3.0) were measured in approximately 100 μl of plasma. Forty-three markers were excluded from this work because they could not be quantified in more than 30% of samples or were not measured for all patients.
Statistical Analysis
Immunoassay markers with 30% or less of samples below the lower limit of quantification were imputed by sampling from a uniform distribution ranging from 0.01 to the minimal value of the marker. The majority of markers had a negligible amount of samples imputed (see Table E1 in the online supplement). For all marker analyses, the study population was randomly divided into a training set containing two-thirds of the patients to explore markers and to fit and select models; and a validation set containing one-third of the patients to assess and confirm all candidate markers and models. With this division, the expected overestimated accuracy in the training set could be appropriately verified in the validation set. Assessment of the accuracy of each marker to classify subjects into each of the three groups was evaluated using the area under the receiver operating characteristic curve (AUC-ROC). Statistical significance was based on Q values and not P values. Q values were estimated for all P values to adjust for multiple testing and were considered significant to the level at which one or fewer false positive tests could be present among all significant tests. In the validation set, we repeated all analyses and estimated accuracy based on cutoff values determined in the training set by using Youden criteria modified to penalize missing a bacterial case by a cost factor of 2 (43).
We sought biomarker signatures for all 56 proteins (and/or clinical features) through classification tree, multinomial logistic regression with elastic net, and support vector machine models. All analyses were conducted with the R package version 2.15.3 (R Project for Statistical Computing, Vienna, Austria). Additional details are presented in the online supplement.
Results
Study Population
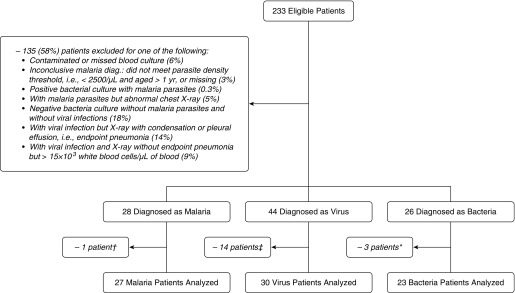
A total of 233 patients with febrile respiratory distress were enrolled, but 135 did not meet criteria for any of the three etiologic groups (Figures 1 and 2 and the online supplement). Of the 98 patients classified into one of the three etiologies, 80 patients (27 malarial, 30 viral, and 23 bacterial), all hospitalized, were analyzed. Patients in the three etiologic groups were comparable regarding most demographic and clinical characteristics (Table 1). In general, malarial etiology patients had the lowest frequency of respiratory symptoms and platelet counts. Viral etiology patients were the least anemic and had the highest monocyte and lymphocyte counts. Bacterial etiology patients had the highest leukocyte and neutrophil counts. Coinfections with virus were common in malarial etiology patients (63%) and bacterial etiology patients (52%). HIV coinfection was more common among bacterial etiology patients (39%) than among malarial etiology (7%) and viral etiology (13%) patients. The most common viral infections were rhinovirus (40%), respiratory syncytial virus (29%), and adenovirus (24%). The most common bacterial infection was Streptococcus pneumoniae (52%).
Figure 2.
Target and study population. †Subject excluded because of insufficient sample collection. ‡Subjects excluded because of insufficient sample collection and feasibility considerations. *Subjects excluded because of insufficient sample collection and delays in sample shipment. diag. = diagnosis.
Table 1.
Comparison of All Studied Patients with a Definitive Etiologic Diagnosis of Malarial, Viral, or Bacterial Febrile Clinical Pneumonia*
| Patient Characteristic | Clinical Pneumonia Etiology |
P Value§ | ||||
|---|---|---|---|---|---|---|
| Malarial† (n = 27) | Viral (n = 30) | Bacterial‡ (n = 23) | ||||
| Demographic | ||||||
| Female sex, n (%) | 15 (56) | 13 (43) | 13 (57) | 0.55 | ||
| Age (mo), median (IQR) | 18 (10–37) | 13 (5–22) | 13 (6–27) | 0.40 | ||
| Weight (kg), mean ± SD | 10.0 ± 4.3 | 8.8 ± 3.5 | 9.4 ± 4.0 | 0.50 | ||
| Clinical history and examination on admission |
||||||
| History of antibiotic therapy, n (%) | 0 | 0 | 1 (4) | 0.29|| | ||
| Temperature (°C), mean ± SD | 38.4 ± 1.4 | 37.8 ± 1.1 | 38.3 ± 1.1 | 0.13 | ||
| Hyperpyrexia (temperature ≥ 39°C), n (%) | 13 (48) | 4 (13) | 9 (39) | 0.01 | ||
| Oxygen saturation (%), mean ± SD | 96.7 ± 2.1 | 94.9 ± 3.4 | 95.8 ± 2.2 | 0.04 | ||
| Respiratory rate, mean ± SD | 54 ± 9 | 58 ± 9 | 59 ± 17 | 0.23 | ||
| Chest in-drawing, n (%) | 7 (26) | 26 (87) | 23 (83) | <0.0001 | ||
| Nasal flaring, n (%) | 4 (15) | 19 (63) | 16 (70) | <0.0001 | ||
| Crackles, n (%) | 9 (33) | 20 (67) | 15 (65) | 0.02 | ||
| Wheezing, n (%) | 3 (11) | 9 (30) | 4 (17) | 0.22|| | ||
| Rhonchi, n (%) | 1 (4) | 13 (43) | 6 (26) | 0.002|| | ||
| Capillary refill (s), mean ± SD | 2.2 ± 1.1 | 1.7 ± 0.5 | 2.0 ± 0.6 | 0.07 | ||
| Nutritional and anemic status | ||||||
| WAZ, mean ± SD | −1.89 ± 1.86 | −1.69 ± 1.35 | −1.33 ± 1.53 | 0.46 | ||
| MUAC (cm), mean ± SD | 13.9 ± 1.9 | 13.6 ± 1.4 | 13.5 ± 1.8 | 0.68 | ||
| Anemia status, n (%)¶ | 0.006|| | |||||
| No anemia (Hct, >33%) | 1 (4) | 5 (17) | 2 (9) | |||
| Mild anemia (Hct, 25–33%) | 9 (33) | 19 (63) | 8 (35) | |||
| Moderate anemia (Hct, 15–25%) | 13 (48) | 5 (17) | 13 (57) | |||
| Severe anemia (Hct, ≤15%) | 4 (15) | 1 (3) | 0 | |||
| Hematocrit (%), mean ± SD | 23 ± 7 | 29 ± 6 | 25 ± 5 | 0.0007 | ||
| Infection and inflammation–associated laboratory
markers |
||||||
| HIV status positive, n (%) | 2 (7) | 4 (13) | 9 (39) | 0.02|| | ||
| Viral coinfections, n (%) | 17 (63) | — | 12 (52) | 0.45 | ||
| Number of virus in infections, n (%)** | 0.88 | |||||
| −1 | 10 (37) | 19 (63) | 8 (35) | |||
| ≥2 | 7 (26) | 11 (37) | 4 (17) | |||
| WBC count (109/L), mean ± SD | 14 ± 9 | 10 ± 3 | 20 ± 13 | 0.0003 | ||
| Leukocytosis (>20 × 109/L), n (%)¶ | 5 (19) | 0 | 9 (39) | <0.0001|| | ||
| Neutrophils, median (IQR) | 4.6 (3.6–5.4) | 4.1 (2.2–5.6) | 7.8 (5.0–11.6) | 0.008 | ||
| Neutrophil %, median (IQR) | 56 (39–61) | 38 (29–46) | 58 (41–54) | 0.002 | ||
| Monocytes, median (IQR) | 0.80 (0.38–1.70) | 1.25 (0.94–1.40) | 1.39 (0.67–1.67) | 0.18 | ||
| Monocyte %, median (IQR) | 8.2 (5.8–13.0) | 11.9 (8.8–17.0) | 6.7 (5.8–8.8) | 0.01 | ||
| Lymphocytes, median (IQR) | 2.8 (1.9–5.3) | 4.8 (3.3–5.7) | 4.6 (3.3–5.3) | 0.19 | ||
| Lymphocyte %, median (IQR) | 37 (28–46) | 43 (40–57) | 32 (22–35) | 0.007 | ||
| Platelets (1012/L), median (IQR) | 139 (63–227) | 339 (266–477) | 386 (184–453) | <0.0001 | ||
| X-ray results, n (%) | <0.001|| | |||||
| Normal | 27 (100) | 17 (57) | 5 (26)†† | |||
| Other infiltrates | 0 | 13 (43) | 1 (5)†† | |||
| Consolidation or effusion | 0 | 0 | 13 (68) | |||
| Hospitalization outcomes | ||||||
| Hospitalization duration (d), median (IQR)‡‡ | 3.8 (2.0–4.9) | 3.8 (2.0–5.0) | 4.0 (1.0–4.5) | 0.85 | ||
| In-hospital deaths, n (%) | 0 | 1 (3) | 2 (9) | 0.17 | ||
Definition of abbreviations: Hct = hematocrit; IQR = interquartile range; MUAC = middle upper arm circumference; WAZ = weight-for-age z-score; WBC = white blood cell.
Percentages vary with the number of subjects with available information to the corresponding variable.
Median (IQR) parasite density was 134,766 (1,480–274,032) in the malaria group (the only with any parasitemia).
The following bacteria were detected in culture: Streptococcus pneumoniae (13 patients), Staphylococcus aureus (2 patients), Neisseria meningitidis (1 patient), Pseudomonas sp. (1 patient), Haemophilus influenzae (1 patient), Acinetobacter (1 patient), Salmonella (2 patients), Streptococcus viridans (1 patient), other gram-negative bacillus (1 patient). Salmonella has been detected as an etiologic agent of pneumonia in Mozambique (51, 52).
P values for categorical variables were estimated by Pearson chi-square test, unless indicated otherwise; P values for continuous variables were estimated by Kruskal–Wallis test when reporting medians and interquartile ranges, and by analysis of variance when reporting means and standard deviations.
Fisher exact test was used to determine P value.
Cutoff values to define anemia and leukocytosis were based on Quintó and colleagues (53) and Behrman and colleagues (54).
Estimated on the basis of patients with any infection. Viral infections were caused by rhinovirus (23 patients), respiratory syncytial virus (17 patients), adenovirus (14 patients), parainfluenza virus (8 patients), metapneumovirus (7 patients), bocavirus (6 patients), influenza virus (4 patients), and enterovirus (3 patients).
Patients with normal chest X-ray had the following pathogens: S. pneumoniae (2 patients), S. aureus (1 patient), N. meningitidis (1 patient), Pseudomonas sp. (1 patient). The patients with infiltrates other than consolidation and effusion had S. pneumoniae.
Results excluding or including subjects who died were comparable.
Levels of Markers in the Three Diagnostic Groups
To investigate whether any single marker could discriminate the three etiologies, we explored accuracy and cutoff values for the markers in the training set and confirmed accuracy in the validation set. In the 52 patients included in the training set (17 malarial, 20 viral, and 15 bacterial infections), only haptoglobin accurately differentiated the three diagnostic groups with AUC-ROC greater than 0.85 (Table 2 and Tables E3 and E4). The specific virus involved in viral infections did not substantially impact levels of markers, and the distribution of selected bacterial markers in patients with and without viral coinfection was similar (Figure E1). Variability in the virus group was the lowest of all etiologic groups (Figure E2).
Table 2.
Plasma Proteins Differentially Detected in Any of the Three Two-Group Comparisons in the Training Set of Patients* as Based on Area under the Receiver Operating Characteristic Curve ≥ 0.85†
| Protein Marker | AUC-ROC‡ |
||
|---|---|---|---|
| Bacteria vs. Virus | Bacteria vs. Malaria | Virus vs. Malaria | |
| CK-MB, ng/ml | 0.92 | 0.82 | 0.55 |
| EN-RAGE, ng/ml | 0.91 | 0.80 | 0.55 |
| MMP9, ng/ml | 0.87 | 0.74 | 0.57 |
| TBG, µg/ml | 0.90 | 0.74 | 0.74 |
| AAT, mg/ml | 0.99 | 0.75 | 0.89 |
| CRP, µg/ml | 0.98 | 0.59 | 0.96 |
| IL-6, pg/ml | 0.93 | 0.57 | 0.91 |
| TIMP-1, ng/ml | 0.92 | 0.60 | 0.96 |
| vWF, µg/ml | 0.91 | 0.60 | 0.89 |
| IL-10, pg/ml | 0.54 | 0.92 | 0.97 |
| RANTES, ng/ml | 0.56 | 0.88 | 0.88 |
| SAP, µg/ml | 0.72 | 0.89 | 0.81 |
| TNF receptor-2, ng/ml | 0.71 | 0.88 | 0.98 |
| CD40 antigen, ng/ml | 0.74 | 0.66 | 0.93 |
| Ferritin, ng/ml | 0.83 | 0.74 | 0.94 |
| IL-16, pg/ml | 0.82 | 0.66 | 0.94 |
| IL-18, pg/ml | 0.68 | 0.73 | 0.92 |
| MIP-1β, pg/ml | 0.70 | 0.80 | 0.97 |
| MPO, ng/ml | 0.82 | 0.75 | 0.98 |
| TNF-α, pg/ml | 0.61 | 0.79 | 0.95 |
| VCAM-1, ng/ml | 0.54 | 0.84 | 0.99 |
| Haptoglobin, mg/ml | 0.86 | 0.98 | 0.86 |
Definition of abbreviations: AAT = alpha-1 antitrypsin; AUC-ROC = area under the receiver operating characteristic curve; CK = creatine kinase; CRP = C-reactive protein; EN-RAGE = extracellular newly identified RAGE (receptor for advanced glycation endproducts)-binding protein (synonymous with S100A12 protein); MIP-1β = macrophage inflammatory protein 1β; MMP9 = matrix metalloproteinase 9; MPO = myeloperoxidase; RANTES = T-cell specific protein RANTES (regulated upon activation, normal T-cell expressed and secreted); SAP = serum amyloid P-component; TBG = thyroxine-binding globulin; TIMP-1 = tissue inhibitor of metalloproteinases 1; TNF = tumor necrosis factor; VCAM-1 = vascular cell adhesion molecule 1; vWF = von Willebrand factor.
Sample sizes were as follows: 17 patients with malarial pneumonia, 20 with viral pneumonia, and 15 with bacterial pneumonia.
Markers are clustered by the groups they differentially detected.
All areas under the receiver operating characteristic curve equal to or exceeding 0.80 were statistically significant as based on a cutoff of Q value of DeLong’s test (comparing with a 0.50 null value) and t tests.
The distribution of most selected markers in the validation set was similar to the distribution of the corresponding marker in the training set (Figure E2). Overall, the best single marker for bacterial etiology as opposed to malarial or viral etiologies was haptoglobin (cutoff, 0.995) with a sensitivity of 96% (95% confidence interval [CI]: 80, 100%) but with a limited specificity of 68% (95% CI: 55, 80%) (Table E5).
Impact of Coinfection on Markers
Because HIV coinfections were more prevalent in the bacterial etiology group than in the other two etiologic groups, we repeated analyses in patients free of HIV infection. In the training set, AUC-ROCs of markers presented in Table 2 were comparable to those of patients who were HIV-negative (Table E6). In the validation set, the risks of misclassifying HIV-positive and HIV-negative patients into any of the three etiologic groups were comparable.
Healthy Community Control Subjects
Distributions of several relevant markers in control subjects were distinct from distributions in patients (Figure E2 and Table E7), and had low variability.
Biomarker Signatures
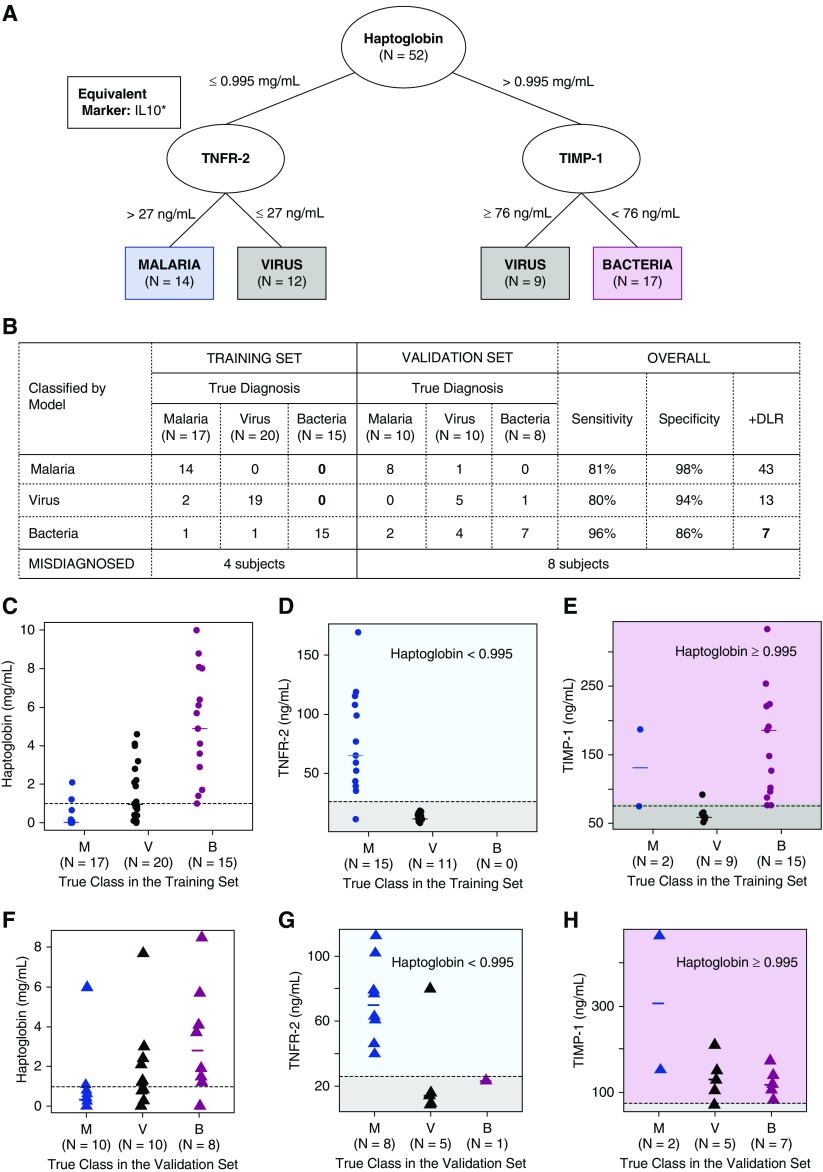
We further assessed whether combinations of markers could increase the accuracy of determining viral and malarial etiologies, while still accurately classifying bacterial etiology. Using a classification tree model, a signature was selected in which tumor necrosis factor (TNF) receptor 2 (or IL-10) and tissue inhibitor of metalloproteinases 1 (TIMP-1) were added to haptoglobin and patients determined by haptoglobin as having bacterial and nonbacterial etiologies were subclassified into viral and malarial etiologies (Figure 3). This signature correctly classified 15 of 15 bacterial etiology patients in the training set and 7 of 8 in the validation set. The overall sensitivity of this signature to diagnose bacterial etiology was 96% (95% CI: 78, 99%); the specificity was 86% (95% CI: 74, 94%); and the positive diagnostic likelihood ratio was 6.8 (95% CI: 3.8, 14.6). Overall, approximately 19% of malarial etiology patients and 20% of viral etiology patients were misclassified into one of the three diagnoses.
Figure 3.
Biomarker signature for febrile clinical pneumonia selected through a classification tree. Misclassification of a true bacteria patient was penalized more than misclassification of a true virus or malaria patient (detail in online supplement). (A) Classification tree with markers that discriminated groups (in ovals) and specific groups to which patients were assigned (in rectangles) by each branch. In the box, IL-10 that achieved optimality criteria equivalent to the criteria of TNFR2. (B) Misclassification frequencies of the signature in the training and validation sets with sensitivities, specificities, and positive diagnostic likelihood ratio (+DLR) to diagnose each pathogen (vs. any other). C–E correspond to patients in the training set; F–H correspond to patients in the validation set. (C and F) Distribution of haptoglobin with the corresponding cutoff value selected by the tree (dashed line) in M, V, and B patients. (D and G) Distribution of TNFR2 with cutoff value selected by the tree (dashed line) in patients in the left branch of the tree (haptoglobin ≤ 0.995). (E and H) Distribution of TIMP-1 with cutoff value selected by the tree (dashed line) in patients in the right branch of the tree (haptoglobin > 0.995). *Signature replacing TNFR2 by IL-10 (cutoff value ≥ 131.5) misclassified the same number of subjects in the training and validation set. Biomarker signature replacing TNFR2 by TNF-α (cutoff value ≥ 37.5), MPO (cutoff value ≥ 898.5), and MIP-1β (cutoff value ≥ 446) performed exactly like the tree with TNFR2 in the training set but misclassified one additional malaria subject in the validation set as a virus case. B = bacteria; M = malaria; MIP-1β = macrophage inflammatory protein 1β; MPO = myeloperoxidase; TIMP-1 = tissue inhibitor of metalloproteinases 1; TNF-α = tumor necrosis factor-α; TNFR2 = tumor necrosis factor receptor 2; V = virus.
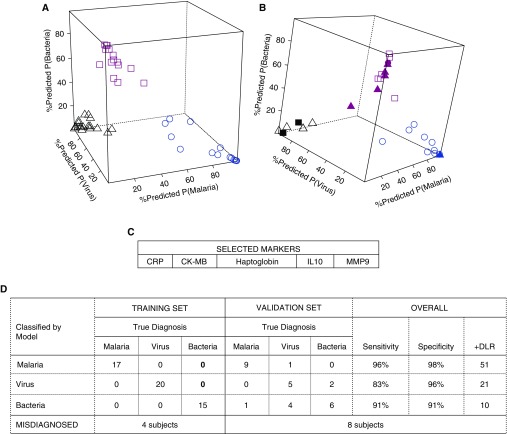
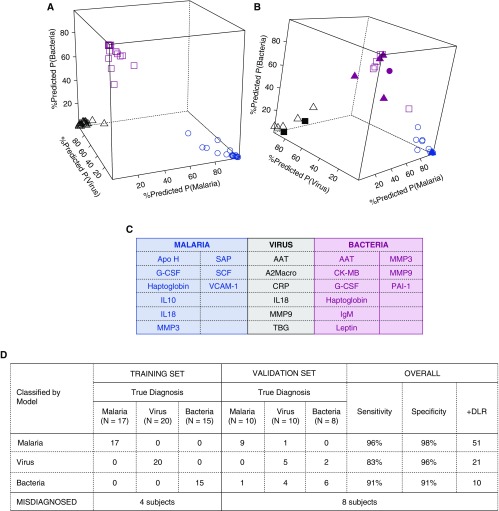
Two additional biomarker signatures derived with support vector machine (SVM) and regression models attained comparable accuracy in the training and validation sets (Figures 4 and 5), although the SVM was more parsimonious and included only five markers. The sensitivity and specificity of these signatures to diagnose bacterial etiology were 91% (95% CI: 72, 99%) and 91% (95% CI: 81, 97%), respectively; and the positive diagnostic likelihood ratio was 10.4 (95% CI: 4.8, 29.1). In the validation set, except for one malarial etiology patient and one bacterial etiology patient, the classification tree, regression, and SVM signatures misclassified the same patients (Table E8).
Figure 4.
Biomarker signature for febrile clinical pneumonia selected by support vector machine (SVM) models. Predicted probabilities of diagnoses of malaria, virus, or bacteria in the training set (A) in which all patients were correctly classified, and in the validation set (B), in which some patients were misclassified. Blue, black, and pink represent malaria-, virus-, and bacteria-assigned diagnosis, respectively. Open shapes represent patients with matched true and assigned diagnosis (circles, malaria; triangles, virus; squares, bacteria). Solid shapes represent patients misclassified; for example, solid black squares are patients who were assigned a diagnosis of virus but were true bacteria patients, solid pink triangles are patients who were assigned a diagnosis of bacteria but were true virus patients, and solid pink circles are patients who were assigned a bacteria diagnosis but were true malaria patients. In C, markers selected in the SVM biomarker signature are listed and in D, training and validation errors. Overall, this signature was an excellent classifier for malaria, good for bacteria, and poorer classifier for virus patients. +DLR = positive diagnostic likelihood ratio; CK = creatine kinase; CRP = C-reactive protein; MMP9 = matrix metalloproteinase 9.
Figure 5.
Biomarker signature for febrile clinical pneumonia selected by multinomial logistic regression models (fitted via elastic net penalty). Predicted probabilities of diagnoses of malaria, virus, or bacteria in the training set (A) in which all patients were correctly classified, and in the validation set (B), in which some patients were misclassified. Blue, black, and pink represent malaria-, virus-, and bacteria-assigned diagnosis, respectively. Open shapes represent patients with matched true and assigned diagnosis (circles, malaria; triangles, virus; squares, bacteria). Solid shapes represent patients misclassified; for example, solid black squares are patients who were assigned a diagnosis of virus but were true bacteria patients, solid pink triangles are patients who were assigned a diagnosis of bacteria but were true virus patients, and solid pink circles are patients who were assigned a bacteria diagnosis but were true malaria patients. In C, markers selected in the support vector machine biomarker signature are listed and in D, training and validation errors. Overall, this signature was an excellent classifier for malaria, good for bacteria, and poorer classifier for virus patients. +DLR = positive diagnostic likelihood ratio; A2Macro = α2-macroglobulin; AAT = alpha-1 antitrypsin; Apo H = apolipoprotein H; CK-MB = creatine kinase–MB; CRP = C-reactive protein; G-CSF = granulocyte colony-stimulating factor; MMP3 = matrix metalloproteinase 3; MMP9 = matrix metalloproteinase 9; PAI-1 = plasminogen activator inhibitor 1; SAP = serum amyloid P-component; SCF = stem cell factor; TBG = thyroxine-binding globulin; VCAM-1 = vascular cell adhesion molecule 1.
All three signatures did not misclassify as bacterial (or malarial or viral) any health community control children.
Clinical Findings in Biomarker Signatures
When clinical features and plasma proteins were modeled together, no clinical marker was selected. Moreover, the accuracy of classification tree signatures based only on clinical and laboratory findings to detect bacterial infection was inferior (sensitivity < 45% and specificity < 68% in all models; online supplement and Figure E3) to the accuracy of classification trees based on plasma proteins.
Discussion
Several plasma proteins accurately classified malarial, viral, and bacterial etiologies in children with febrile clinical pneumonia. Haptoglobin alone distinguished bacterial from viral and malarial respiratory disease with high sensitivity but low specificity and did not distinguish between malarial and viral etiologies. When biomarker signatures identified by classification tree combined haptoglobin with TNF receptor 2 (or IL-10) and TIMP-1, distinction between the three etiologies was possible. We identified two alternative biomarker signatures with good accuracy for bacterial etiologies based on SVM and regression models. Both the classification tree and SVM signatures included a small set of markers, had high sensitivities for identifying bacterial infection (96% for classification tree and 91% for SVM), resulted in few misclassifications of viral or malarial as bacterial etiology patients (14% for classification tree and 9% for SVM), and outperformed clinical and laboratory markers. Although misclassification of patients in the viral etiology group was higher, the proposed signatures performed as well as other existing approaches for viral diagnoses. The variability of signature markers in healthy community control subjects was low, suggesting they were reliable markers. The identified signatures were not impacted by HIV coinfection, an important consideration in sub-Saharan Africa.
The different concentration of plasma proteins associated with the three etiologies may be attributable to the cells involved in the immune response to each pathogen. Increased haptoglobin is associated with bacterial pneumonia (39, 44–47), probably because of the role of neutrophils in clearance of bacterial infections in the lungs. The persistence of haptoglobin increases in bacterial pneumonia may also explain the usefulness of this marker for identifying bacterial infections (46). Other cytokines included in the signatures proposed here have also been associated with the three infections (45, 48–50).
We relied on blood cultures to ascertain a diagnosis of bacterial infection and on stringent criteria to classify patients, minimizing the impact of misclassification biases on our results. The goal was to be able to ascertain that a patient with clinical pneumonia had associated bacterial infection requiring antibiotic therapy, even if the primary cause of the pneumonia was not bacterial. At present, there is no sensitive and specific gold standard for associated viral or bacterial infection in patients with clinical pneumonia. The World Health Organization has proposed the use of radiographic endpoint pneumonia (pleural effusion or lobar consolidated infiltrates) to diagnose bacterial pneumonia in studies of the pneumococcal vaccine, despite the limited inter- and intrarater reliability of chest X-rays (7–16). This low to moderate reliability may preclude the use of chest X-ray as a gold standard in studies designed to discover biomarkers that will minimize the risk of overlooking bacterial infections when treating patients in a clinic. In our study, the sensitivity of radiographic end-point pneumonia to detect bacterial infection was poor, with 32% of patients with febrile clinical pneumonia and associated bacterial disease presenting without radiographic end-point pneumonia, despite the fact that radiographic tests were adequate and were reviewed by two experienced clinicians. Among patients with no evidence of bacterial infection, 33% of patients with malarial infections and 30% with viral infections had radiographic endpoint pneumonia.
The criteria used in this study to assign patients to diagnostic groups aimed to obtain an accurate diagnosis. However, these criteria resulted in excluding a large number of patients with viral etiologies who had high leukocyte counts. Consequently, potential biases could have occurred if, for example, the levels of selected markers were associated with disease severity. Not excluding patients with high leukocyte counts, however, could have misclassified undetected bacterial etiology patients as viral. Further studies could elucidate the importance of selection bias in our estimates.
More definitive conclusions about the proposed biomarkers will depend on confirmatory studies with alternative gold standards, larger sample sizes, and in different populations to estimate accuracy and reliability and to establish the generalizability of these markers. Factors such as disease severity, prior antibiotic treatment, and length of illness (26–29) could have affected marker levels in this study. Studies with longitudinal sampling could also clarify the progression of marker levels with disease evolution and their prognostic accuracy. Viral coinfections and the specific virus, other than HIV, did not appear to impact markers in the present study. Although patients had several different viruses, levels of markers for all these viruses were similar and variability of markers in this group was the lowest.
Optimal treatment of patients requires prompt etiologic diagnosis of the clinical pneumonia. In malaria endemic areas, the diagnostic dilemma is more challenging because malaria may produce a respiratory distress syndrome clinically indistinguishable from that resulting from bacterial infections. On the other hand, bacterial or viral pneumonias in patients with malarial infections are common, and withholding of antibiotic therapy from patients with bacterial infections may occur. Conversely, antibiotic therapy is frequently overprescribed as a result of suspected bacterial infections. A point-of-care test that would rule out bacterial infection would allow better management of antibiotic therapy in resource-limited and resource-replete areas. The biomarker signature proposed in this study could be incorporated into a future rapid diagnostic test to rule out diagnoses of bacterial infection. Treatment decisions based on these signatures would still result in the unnecessary prescription of antibiotics to patients with viral etiologies but would substantially reduce unnecessary use of antibiotics while ensuring that patients with bacterial infections receive antibiotics.
Acknowledgments
Acknowledgment
The authors are indebted to the children and mothers participating in the study. In addition, the authors thank Terrie Taylor, Carmen Mejia, Shannon Power, and David Wypij for assistance with this manuscript.
Footnotes
Supported by the Bill and Melinda Gates Foundation (grant OPP50092). Q.B. has a fellowship from the program Miguel Servet of the ISCIII (Plan Nacional de I+D+I 2008–2011, grant CP11/00269). L.M. has a fellowship from the program Río Hortega of the ISCIII (CM13/00260). The funders had no role in the study design, collection, analysis, interpretation of data, writing of the manuscript, or in the decision to submit the manuscript for publication.
Author Contributions: C.V., Q.B., R.C.W., R.A., D.A.M., M.A.G., M.L., P.L.A., S.A.C., D.F.W., and J.P.M. conceptualized and designed the study. S.A., K.D.A., R.A., L.M., and M.L. performed the research. C.V. and Q.B. prepared the study master database. C.V. and Y.T. analyzed the data. C.V., Y.T., and Q.B. drafted the initial manuscript. C.V., Q.B., R.A., M.L., K.D.A., D.A.M., M.A.G., D.F.W., P.L.A., S.A.C., and R.C.W. contributed with data collection instruments. C.V., Q.B., R.C.W., R.A., D.A.M., M.A.G., M.L., D.F.W., J.P.M., J.H., K.P., and Y.T. contributed with interpretation of data. S.A., K.D.A., and M.L. coordinated and supervised sample and data collection at the study site, contributing with data acquisition; they also supported data management activities. J.H. and J.S. contributed with analytical tools and provided key intellectual contribution with the choice of analytical approach. R.C.W., R.A., D.A.M., M.A.G., M.L., D.F.W., and J.P.M. revised the manuscript critically for important intellectual content; all authors reviewed and approved the final manuscript as submitted.
This article has an online supplement, which is accessible from this issue’s table of contents online at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201506-1100OC on October 15, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Nair H, Simões EA, Rudan I, Gessner BD, Azziz-Baumgartner E, Zhang JS, Feikin DR, Mackenzie GA, Moïsi JC, Roca A, et al. Severe Acute Lower Respiratory Infections Working Group. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet. 2013;381:1380–1390. doi: 10.1016/S0140-6736(12)61901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donnelly JP, Baddley JW, Wang HE. Antibiotic utilization for acute respiratory tract infections in U.S. emergency departments. Antimicrob Agents Chemother. 2014;58:1451–1457. doi: 10.1128/AAC.02039-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reyburn H, Mwakasungula E, Chonya S, Mtei F, Bygbjerg I, Poulsen A, Olomi R. Clinical assessment and treatment in paediatric wards in the north-east of the United Republic of Tanzania. Bull World Health Organ. 2008;86:132–139. doi: 10.2471/BLT.07.041723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.English M, Esamai F, Wasunna A, Were F, Ogutu B, Wamae A, Snow RW, Peshu N. Assessment of inpatient paediatric care in first referral level hospitals in 13 districts in Kenya. Lancet. 2004;363:1948–1953. doi: 10.1016/S0140-6736(04)16408-8. [DOI] [PubMed] [Google Scholar]
- 5.Bassat Q, Machevo S, O’Callaghan-Gordo C, Sigaúque B, Morais L, Díez-Padrisa N, Ribó JL, Mandomando I, Nhampossa T, Ayala E, et al. Distinguishing malaria from severe pneumonia among hospitalized children who fulfilled integrated management of childhood illness criteria for both diseases: a hospital-based study in Mozambique. Am J Trop Med Hyg. 2011;85:626–634. doi: 10.4269/ajtmh.2011.11-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramirez M, Melo-Cristino J. Expanding the diagnosis of pediatric bacteremic pneumococcal pneumonia from blood cultures to molecular methods: advantages and caveats. Clin Infect Dis. 2010;51:1050–1052. doi: 10.1086/656580. [DOI] [PubMed] [Google Scholar]
- 7.Bradley JS, Byington CL, Shah SS, Alverson B, Carter ER, Harrison C, Kaplan SL, Mace SE, McCracken GH, Jr, Moore MR, et al. Pediatric Infectious Diseases Society; Infectious Diseases Society of America. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53:e25–e76. doi: 10.1093/cid/cir531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cherian T, Mulholland EK, Carlin JB, Ostensen H, Amin R, de Campo M, Greenberg D, Lagos R, Lucero M, Madhi SA, et al. Standardized interpretation of paediatric chest radiographs for the diagnosis of pneumonia in epidemiological studies. Bull World Health Organ. 2005;83:353–359. [PMC free article] [PubMed] [Google Scholar]
- 9.Edwards M, Lawson Z, Morris S, Evans A, Harrison S, Isaac R, Crocker J, Powell C. The presence of radiological features on chest radiographs: how well do clinicians agree? Clin Radiol. 2012;67:664–668. doi: 10.1016/j.crad.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Bloomfield FH, Teele RL, Voss M, Knight DB, Harding JE. Inter- and intra-observer variability in the assessment of atelectasis and consolidation in neonatal chest radiographs. Pediatr Radiol. 1999;29:459–462. doi: 10.1007/s002470050617. [DOI] [PubMed] [Google Scholar]
- 11.Davies HD, Wang EE, Manson D, Babyn P, Shuckett B. Reliability of the chest radiograph in the diagnosis of lower respiratory infections in young children. Pediatr Infect Dis J. 1996;15:600–604. doi: 10.1097/00006454-199607000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Albaum MN, Hill LC, Murphy M, Li YH, Fuhrman CR, Britton CA, Kapoor WN, Fine MJ PORT Investigators. Interobserver reliability of the chest radiograph in community-acquired pneumonia. Chest. 1996;110:343–350. doi: 10.1378/chest.110.2.343. [DOI] [PubMed] [Google Scholar]
- 13.Hagaman JT, Rouan GW, Shipley RT, Panos RJ. Admission chest radiograph lacks sensitivity in the diagnosis of community-acquired pneumonia. Am J Med Sci. 2009;337:236–240. doi: 10.1097/MAJ.0b013e31818ad805. [DOI] [PubMed] [Google Scholar]
- 14.Johnson J, Kline JA. Intraobserver and interobserver agreement of the interpretation of pediatric chest radiographs. Emerg Radiol. 2010;17:285–290. doi: 10.1007/s10140-009-0854-2. [DOI] [PubMed] [Google Scholar]
- 15.Loeb MB, Carusone SB, Marrie TJ, Brazil K, Krueger P, Lohfeld L, Simor AE, Walter SD. Interobserver reliability of radiologists’ interpretations of mobile chest radiographs for nursing home–acquired pneumonia. J Am Med Dir Assoc. 2006;7:416–419. doi: 10.1016/j.jamda.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 16.O’Grady KA, Torzillo PJ, Ruben AR, Taylor-Thomson D, Valery PC, Chang AB. Identification of radiological alveolar pneumonia in children with high rates of hospitalized respiratory infections: comparison of WHO-defined and pediatric pulmonologist diagnosis in the clinical context. Pediatr Pulmonol. 2012;47:386–392. doi: 10.1002/ppul.21551. [DOI] [PubMed] [Google Scholar]
- 17.Murdoch DR, O’Brien KL, Scott JA, Karron RA, Bhat N, Driscoll AJ, Knoll MD, Levine OS. Breathing new life into pneumonia diagnostics. J Clin Microbiol. 2009;47:3405–3408. doi: 10.1128/JCM.01685-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonsalves WI, Cornish N, Moore M, Chen A, Varman M. Effects of volume and site of blood draw on blood culture results. J Clin Microbiol. 2009;47:3482–3485. doi: 10.1128/JCM.02107-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Avni T, Mansur N, Leibovici L, Paul M. PCR using blood for diagnosis of invasive pneumococcal disease: systematic review and meta-analysis. J Clin Microbiol. 2010;48:489–496. doi: 10.1128/JCM.01636-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Resti M, Moriondo M, Cortimiglia M, Indolfi G, Canessa C, Becciolini L, Bartolini E, de Benedictis FM, de Martino M, Azzari C. Italian Group for the Study of Invasive Pneumococcal Disease. Community-acquired bacteremic pneumococcal pneumonia in children: diagnosis and serotyping by real-time polymerase chain reaction using blood samples. Clin Infect Dis. 2010;51:1042–1049. doi: 10.1086/656579. [DOI] [PubMed] [Google Scholar]
- 21.Charkaluk ML, Kalach N, Mvogo H, Dehecq E, Magentie H, Raymond J, Gendrel D, Kremp O, Decoster A. Assessment of a rapid urinary antigen detection by an immunochromatographic test for diagnosis of pneumococcal infection in children. Diagn Microbiol Infect Dis. 2006;55:89–94. doi: 10.1016/j.diagmicrobio.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Koram KA, Molyneux ME. When is “malaria” malaria? The different burdens of malaria infection, malaria disease, and malaria-like illnesses. Am J Trop Med Hyg. 2007;77(Suppl. 6):1–5. [PubMed] [Google Scholar]
- 23.D’Acremont V, Kilowoko M, Kyungu E, Philipina S, Sangu W, Kahama-Maro J, Lengeler C, Cherpillod P, Kaiser L, Genton B. Beyond malaria—causes of fever in outpatient Tanzanian children. N Engl J Med. 2014;370:809–817. doi: 10.1056/NEJMoa1214482. [DOI] [PubMed] [Google Scholar]
- 24.Gwer S, Newton CR, Berkley JA. Over-diagnosis and co-morbidity of severe malaria in African children: a guide for clinicians. Am J Trop Med Hyg. 2007;77(Suppl. 6):6–13. [PMC free article] [PubMed] [Google Scholar]
- 25.Maniaci V, Dauber A, Weiss S, Nylen E, Becker KL, Bachur R. Procalcitonin in young febrile infants for the detection of serious bacterial infections. Pediatrics. 2008;122:701–710. doi: 10.1542/peds.2007-3503. [DOI] [PubMed] [Google Scholar]
- 26.Calbo E, Alsina M, Rodríguez-Carballeira M, Lite J, Garau J. The impact of time on the systemic inflammatory response in pneumococcal pneumonia. Eur Respir J. 2010;35:614–618. doi: 10.1183/09031936.00052709. [DOI] [PubMed] [Google Scholar]
- 27.Ramírez P, Ferrer M, Martí V, Reyes S, Martínez R, Menéndez R, Ewig S, Torres A. Inflammatory biomarkers and prediction for intensive care unit admission in severe community-acquired pneumonia. Crit Care Med. 2011;39:2211–2217. doi: 10.1097/CCM.0b013e3182257445. [DOI] [PubMed] [Google Scholar]
- 28.Martínez R, Menéndez R, Reyes S, Polverino E, Cillóniz C, Martínez A, Esquinas C, Filella X, Ramírez P, Torres A. Factors associated with inflammatory cytokine patterns in community-acquired pneumonia. Eur Respir J. 2011;37:393–399. doi: 10.1183/09031936.00040710. [DOI] [PubMed] [Google Scholar]
- 29.Zobel K, Martus P, Pletz MW, Ewig S, Prediger M, Welte T, Bühling F CAPNETZ Study Group. Interleukin 6, lipopolysaccharide-binding protein and interleukin 10 in the prediction of risk and etiologic patterns in patients with community-acquired pneumonia: results from the German Competence Network CAPNETZ. BMC Pulm Med. 2012;12:6. doi: 10.1186/1471-2466-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haran JP, Buglione-Corbett R, Lu S. Cytokine markers as predictors of type of respiratory infection in patients during the influenza season. Am J Emerg Med. 2013;31:816–821. doi: 10.1016/j.ajem.2013.01.030. [DOI] [PubMed] [Google Scholar]
- 31.Christ-Crain M, Jaccard-Stolz D, Bingisser R, Gencay MM, Huber PR, Tamm M, Müller B. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomised, single-blinded intervention trial. Lancet. 2004;363:600–607. doi: 10.1016/S0140-6736(04)15591-8. [DOI] [PubMed] [Google Scholar]
- 32.Krüger S, Ewig S, Giersdorf S, Hartmann O, Suttorp N, Welte T German Competence Network for the Study of Community Acquired Pneumonia (CAPNETZ) Study Group. Cardiovascular and inflammatory biomarkers to predict short- and long-term survival in community-acquired pneumonia: results from the German Competence Network, CAPNETZ. Am J Respir Crit Care Med. 2010;182:1426–1434. doi: 10.1164/rccm.201003-0415OC. [DOI] [PubMed] [Google Scholar]
- 33.Krüger S, Ewig S, Marre R, Papassotiriou J, Richter K, von Baum H, Suttorp N, Welte T CAPNETZ Study Group. Procalcitonin predicts patients at low risk of death from community-acquired pneumonia across all CRB-65 classes. Eur Respir J. 2008;31:349–355. doi: 10.1183/09031936.00054507. [DOI] [PubMed] [Google Scholar]
- 34.Waterer GW, Rello J, Wunderink RG. Management of community-acquired pneumonia in adults. Am J Respir Crit Care Med. 2011;183:157–164. doi: 10.1164/rccm.201002-0272CI. [DOI] [PubMed] [Google Scholar]
- 35.Don M, Valent F, Korppi M, Falleti E, De Candia A, Fasoli L, Tenore A, Canciani M. Efficacy of serum procalcitonin in evaluating severity of community-acquired pneumonia in childhood. Scand J Infect Dis. 2007;39:129–137. doi: 10.1080/00365540600951283. [DOI] [PubMed] [Google Scholar]
- 36.Heiskanen-Kosma T, Korppi M. Serum C-reactive protein cannot differentiate bacterial and viral aetiology of community-acquired pneumonia in children in primary healthcare settings. Scand J Infect Dis. 2000;32:399–402. doi: 10.1080/003655400750044971. [DOI] [PubMed] [Google Scholar]
- 37.Korppi M, Remes S, Heiskanen-Kosma T. Serum procalcitonin concentrations in bacterial pneumonia in children: a negative result in primary healthcare settings. Pediatr Pulmonol. 2003;35:56–61. doi: 10.1002/ppul.10201. [DOI] [PubMed] [Google Scholar]
- 38.Thayyil S, Shenoy M, Hamaluba M, Gupta A, Frater J, Verber IG. Is procalcitonin useful in early diagnosis of serious bacterial infections in children? Acta Paediatr. 2005;94:155–158. doi: 10.1111/j.1651-2227.2005.tb01883.x. [DOI] [PubMed] [Google Scholar]
- 39.Huang H, Ideh RC, Gitau E, Thézénas ML, Jallow M, Ebruke B, Chimah O, Oluwalana C, Karanja H, Mackenzie G, et al. Discovery and validation of biomarkers to guide clinical management of pneumonia in African children. Clin Infect Dis. 2014;58:1707–1715. doi: 10.1093/cid/ciu202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valim C, Tan Y, Ahmad R, Lanaspa M, Acácio S, Gillette M, Madrid L, Almendinger K, Milner D, Pellé K, Harezlak J, et al. Biomarkers diagnose the pathogen of febrile respiratory distress [abstract 50] Am J Trop Med Hyg. 2014;91(Suppl. 5):15–16. [Google Scholar]
- 41.Mulholland EK, Simoes EA, Costales MO, McGrath EJ, Manalac EM, Gove S. Standardized diagnosis of pneumonia in developing countries. Pediatr Infect Dis J. 1992;11:77–81. doi: 10.1097/00006454-199202000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, Milman J, Mandomando I, Spiessens B, Guinovart C, Espasa M, et al. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet. 2004;364:1411–1420. doi: 10.1016/S0140-6736(04)17223-1. [DOI] [PubMed] [Google Scholar]
- 43.Perkins NJ, Schisterman EF. The Youden Index and the optimal cut-point corrected for measurement error. Biom J. 2005;47:428–441. doi: 10.1002/bimj.200410133. [DOI] [PubMed] [Google Scholar]
- 44.Tsai MH, Lin TY, Hsieh SY, Chiu CY, Chiu CH, Huang YC. Comparative proteomic studies of plasma from children with pneumococcal pneumonia. Scand J Infect Dis. 2009;41:416–424. doi: 10.1080/00365540902936909. [DOI] [PubMed] [Google Scholar]
- 45.Pors SE, Chadfield MS, Sørensen DB, Offenberg H, Heegaard PM, Bisgaard M, Jensen HE. Pathology, tissue metalloproteinase transcription and haptoglobin responses in mice after experimental challenge with different isolates of Pasteurella multocida obtained from cases of porcine pneumonia. J Comp Pathol. 2011;145:251–260. doi: 10.1016/j.jcpa.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 46.Pomorska-Mól M, Markowska-Daniel I, Kwit K, Stępniewska K, Pejsak Z. C-reactive protein, haptoglobin, serum amyloid A and pig major acute phase protein response in pigs simultaneously infected with H1N1 swine influenza virus and Pasteurella multocida. BMC Vet Res. 2013;9:14. doi: 10.1186/1746-6148-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Theilgaard-Mönch K, Jacobsen LC, Nielsen MJ, Rasmussen T, Udby L, Gharib M, Arkwright PD, Gombart AF, Calafat J, Moestrup SK, et al. Haptoglobin is synthesized during granulocyte differentiation, stored in specific granules, and released by neutrophils in response to activation. Blood. 2006;108:353–361. doi: 10.1182/blood-2005-09-3890. [DOI] [PubMed] [Google Scholar]
- 48.Takashima K, Tateda K, Matsumoto T, Iizawa Y, Nakao M, Yamaguchi K. Role of tumor necrosis factor α in pathogenesis of pneumococcal pneumonia in mice. Infect Immun. 1997;65:257–260. doi: 10.1128/iai.65.1.257-260.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grau GE, Taylor TE, Molyneux ME, Wirima JJ, Vassalli P, Hommel M, Lambert PH. Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med. 1989;320:1586–1591. doi: 10.1056/NEJM198906153202404. [DOI] [PubMed] [Google Scholar]
- 50.Boeuf PS, Loizon S, Awandare GA, Tetteh JK, Addae MM, Adjei GO, Goka B, Kurtzhals JA, Puijalon O, Hviid L, et al. Insights into deregulated TNF and IL-10 production in malaria: implications for understanding severe malarial anaemia. Malar J. 2012;11:253. doi: 10.1186/1475-2875-11-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sigaúque B, Roca A, Bassat Q, Morais L, Quintó L, Berenguera A, Machevo S, Bardaji A, Corachan M, Ribó J, et al. Severe pneumonia in Mozambican young children: clinical and radiological characteristics and risk factors. J Trop Pediatr. 2009;55:379–387. doi: 10.1093/tropej/fmp030. [DOI] [PubMed] [Google Scholar]
- 52.Mandomando I, Macete E, Sigaúque B, Morais L, Quintó L, Sacarlal J, Espasa M, Vallès X, Bassat Q, Aide P, et al. Invasive non-typhoidal Salmonella in Mozambican children. Trop Med Int Health. 2009;14:1467–1474. doi: 10.1111/j.1365-3156.2009.02399.x. [DOI] [PubMed] [Google Scholar]
- 53.Quintó L, Aponte JJ, Menéndez C, Sacarlal J, Aide P, Espasa M, Mandomando I, Guinovart C, Macete E, Hirt R, et al. Relationship between haemoglobin and haematocrit in the definition of anaemia. Trop Med Int Health. 2006;11:1295–1302. doi: 10.1111/j.1365-3156.2006.01679.x. [DOI] [PubMed] [Google Scholar]
- 54.Behrman RE, Kliegman R, Jenson HB. Philadelphia: WB Saunders; 2000. Nelson textbook of pediatrics. [Google Scholar]