Abstract
Precision medicine is defined by the National Institute of Health’s Precision Medicine Initiative Working Group as an approach to disease treatment that takes into account individual variability in genes, environment, and lifestyle. There has been increased interest in applying the concept of precision medicine to idiopathic pulmonary fibrosis, in particular to search for genetic and molecular biomarker-based profiles (so called endotypes) that identify mechanistically distinct disease subgroups. The relevance of precision medicine to idiopathic pulmonary fibrosis is yet to be established, but we believe that it holds great promise to provide targeted and highly effective therapies to patients. In this manuscript, we describe the field’s nascent efforts in genetic/molecular endotype identification and how environmental and behavioral subgroups may also be relevant to disease management.
Keywords: idiopathic pulmonary fibrosis, stratified medicine, precision medicine, endotypes, biological markers
Idiopathic pulmonary fibrosis (IPF) is a progressive fibrosing interstitial lung disease of older adults, affecting an estimated 60 to 100,000 Americans (1, 2). The pathobiology of IPF remains incompletely understood, but recent evidence has highlighted the central importance of the alveolar epithelial cell, cellular senescence, and the recapitulation of developmental pathways (3, 4). Recently, two medications, nintedanib and pirfenidone, have been approved for the treatment of IPF on the basis of reductions in the rate of physiologic decline, but the disease still progresses in treated patients and there remains no cure (5, 6).
In 2011, the evidence-based guidelines for the management of IPF from the American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/Latin American Thoracic Association reinforced the central role of radiology and pathology in disease diagnosis. As defined by the guideline panel, the diagnosis of IPF requires the identification of usual interstitial pneumonia pattern on chest high-resolution computed tomography (HRCT) or a combination of HRCT and surgical lung biopsy features that support the presence of usual interstitial pneumonia pattern. The diagnosis further requires a multidisciplinary discussion involving clinicians, radiologists, and pathologists to ensure that the clinical presentation is consistent with an idiopathic condition. This descriptive approach to IPF diagnosis has served the field well by identifying patients who share a progressive clinical phenotype. It remains unknown, however, whether IPF has distinct, clinically relevant subgroups defined by differences in genetics, molecular mechanisms, environmental factors, or patient behaviors.
Precision medicine, as defined by the National Institutes of Health’s Precision Medicine Initiative Working Group, is an approach to disease treatment and prevention that seeks to maximize therapeutic effectiveness by taking into account these individual genetic, molecular, environmental, and lifestyle differences (7). A recent editorial suggested we are at an inflection point in the growth of medical science between a steady but slowing contribution from traditional approaches to clinical investigation (e.g., observation and descriptive categorization) and a new and accelerating “precision medicine”–based contribution driven by advances in molecular genomics, computational speed, and bioinformatics (8). This is an exciting and potentially revolutionary moment in medicine. In this article, we describe the nascent efforts at precision medicine in the field of IPF, with particular focus on possible endotype identification and how environmental and behavioral subgroups may also be relevant to disease management.
The Promise of Precision Medicine
To date, most approaches to precision medicine have focused on the search for distinct genetic and/or molecular disease subgroups. This approach has garnered increasing attention in the pulmonary community, given its revolutionary impact on the fields of lung cancer, cystic fibrosis, and asthma (9). The term “endotype” was coined by Dr. Gary Anderson to refer to these molecularly defined subgroups, and many investigators now believe that IPF likely contains distinct endotypes that may be identified through biomarker profiles (10–12).
The field of oncology is being transformed by precision medicine, with many novel therapies being developed that target specific disease endotypes. Examples include metastatic breast cancer, where tumors expressing human epidermal growth factor receptor 2 (EGFR2) have been shown to benefit from the EGFR2 monoclonal antibody trastuzumab (13), and non–small cell lung cancer, where tumors with mutations in EGFR (14) demonstrate benefit from tyrosine kinase inhibitors such as erlotinib, gefitinib, and afatinib (15–18). In recognition of the impact precision medicine has had on drug development in oncology, the National Institutes of Health, in collaboration with academia and industry, has established a Lung Master Protocol (Lung MAP), which assigns subjects to clinical trials of endotype-directed therapies on the basis of their genetic and molecular profiles (19). This promises to greatly increase the efficiency of clinical drug development.
Asthma provides another example of the potential impact of endotypes. Asthma, like IPF, was historically defined clinically (20, 21) and was broadly characterized as an inflammatory airways disease (21, 22). In 2009, the concept of “Th2-high” and “Th2-low” asthma endotypes was advanced (23), and clinical trials of lebrikizumab, a monoclonal antibody against IL-13, were stratified by Th2-high and -low endotypes (24, 25). Lebrikizumab appeared more effective in patients with the Th2-high endotype. Tralokinumab, another anti–IL-13 monoclonal antibody, also appears more effective in patients with evidence of up-regulated IL-13 (26).
In 2015, the U.S. government launched the Precision Medicine Initiative with a mission to “enable a new era of medicine through research, technology, and policies that empower patients, researchers, and providers to work together toward development of individualized treatments” (27). The $215 million investment initially targeted the oncology field, but the longer-term goal is to promote precision medicine–based investigation across a wide range of disease. In the United Kingdom, there has been a similar commitment to creating more precise treatments through the Medical Research Council’s Stratified Medicine Initiative and through key partnerships between industry and the UK’s National Health Service (28). These investments highlight the promise of precision medicine to revolutionize many other fields of medicine in the decades to come.
Precision Medicine and IPF
Precision medicine–based research is just beginning in IPF, and its relevance remains to be determined. The examples of oncology and asthma, where clinically relevant endotypes appear driven by a single genetic or mechanistic difference, may not be as germane to conditions like IPF that presumably have multiple, overlapping pathobiological pathways. However, like asthma, IPF is a clinically heterogeneous disease, and it seems logical that this clinical heterogeneity reflects differences in pathobiologically relevant genetic/molecular, environmental, and behavioral factors that could be targeted using a precision medicine–based approach.
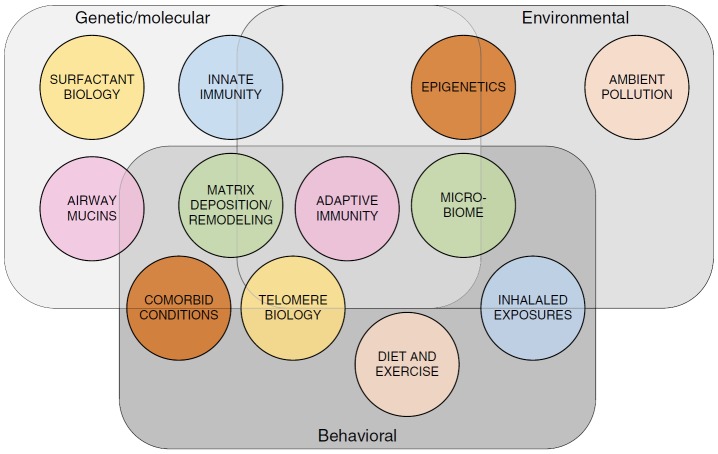
In the following sections, we use the categories of genetic, molecular, environmental, and behavioral to review the published literature in IPF to date and identify potential areas for future precision medicine–based research (Figure 1). In reality, these areas surely overlap (e.g., gene–environment interactions), and it seems likely that patients with IPF will have several distinct mechanisms important to their individual disease pathobiology. If correct, highly specific “precision medicine–based” therapies may require combination to be effective.
Figure 1.
Precision medicine and idiopathic pulmonary fibrosis (IPF). The three categories of “genetics/molecular markers,” “environment,” and “behavior” constitute the foundational elements of potential precision medicine–based subgroups in IPF and are represented here by overlapping rectangles. The overlying circles represent selected precision medicine–based concepts influenced by the three foundational categories that may prove relevant to precision medicine–based care in IPF.
Genetic and Molecular Endotypes
Genetic and molecular endotypes suggested by the literature to date can be roughly organized into the mechanistic categories of epithelial cell dysfunction and senescence, aberrant innate and adaptive immunity, and abnormal lung remodeling. Future research into these potential endotypes should include racially and ethnically diverse populations of patients that allow for a more extensive exploration of this area.
Epithelial Cell Dysfunction and Senescence
Mutations within genes encoding surfactant proteins (SP-A and SP-D) have been described in familial IPF (29–31), and levels of surfactant protein levels are elevated in patients with IPF and associated with poor prognosis (32–37). Polymorphisms in the promoter region of the gene encoding mucin 5B (MUC5B) have been associated with IPF (38–40) and predict a distinct clinical course (41). Mutations in telomerase reverse transcriptase and telomerase RNA component have been described in patients with IPF (42), and IPF is associated with short telomeres in both peripheral blood mononuclear cells and alveolar epithelial cells. The extent of telomere shortening appears to predict survival (43, 44). These findings suggest that a predisposition of epithelial cells to injury combined with impaired cellular renewal characteristic of telomere dysfunction may lead to the interstitial changes typical of IPF.
Aberrant Innate and Adaptive Immunity
Although the lack of benefit from immune-modulatory therapies (most recently prednisone, azathioprine, and acetylcysteine) has led some to conclude that inflammation and immune activation are not relevant in IPF (45), genetic and molecular studies suggest a more complex picture. For example, lung fibroblasts from patients with IPF demonstrate activation of toll-like receptor (TLR) 3, and single-nucleotide polymorphisms (SNPs) within the TLR-3 gene are associated with a faster rate of IPF progression (46). In addition, SNPs within the toll-interacting protein (TOLLIP) gene have been associated with IPF susceptibility and clinical course (47). Elevated levels of circulating chemokine ligand 18 (CCL18) (48), autoantibodies against heat shock protein-70 (49), and decreased expression of genes associated with T-cell costimulatory pathways are likewise predictive of IPF progression (50). Collectively, these findings suggest that aberrations in innate and adaptive immunity may indeed represent important mechanisms in IPF, perhaps defining immune-based endotypes. More targeted immune-targeted therapies may prove important in such patients.
Abnormal Lung Remodeling
Matrix metalloproteinase (MMP)-7, a protease overexpressed by alveolar epithelial cells in IPF (51), is up-regulated in both peripheral blood and bronchoalveolar lavage fluid of patients with IPF (51, 52). MMP-7 levels have been associated with HRCT abnormalities in at-risk patients and poor prognosis in patients with established disease (41, 52–55). Elevated levels of both the matrix-associated lysyl oxidase-like 2 (LOXL2) (56) and integrin αvβ6 (57) are also present in subgroups of patients with IPF who have a more rapid disease progression, and they represent possible treatment targets. Markers of MMP activity, a subset of protein fragments generated by extracellular matrix turnover, have been associated with disease progression as well as survival (58). These findings suggest that aberrant matrix remodeling may be an important driver of disease progression in selected patients.
Environmental and Behavioral Factors
Essentially unstudied is the potential contribution of environment (both intra- and extrapatient) to the pathobiology of IPF. The intrapatient environment is impacted by diverse patient-specific factors such as chronic microaspiration (secondary to gastroesophageal reflux) (59) and the lung’s microbial population (the so-called “lung microbiome”). There is evidence to suggest that microaspiration may contribute to disease progression and acute exacerbation, and the presence of Streptococcus and Staphylococcus strains as well as overall bacterial load seems predictive of more rapid disease progression (60, 61). Two planned or recently started trials (CleanUP-IPF [Co-trimoxazole and Proton Pump Inhibition Using Pragmatic Design in Idiopathic Pulmonary Fibrosis] and EME-TIPAC [The Efficacy and Mechanism Evaluation of Treating Idiopathic Pulmonary Fibrosis with the Addition of Co-trimoxazole]) are building on this concept to look at the use of cotrimoxazole or doxycycline in carefully characterized patients with IPF.
Epigenetic modification of the genome by environmental factors (e.g., air pollution, infection) represents another important potential contributor to IPF pathobiology. DNA methylation, histone modification, and noncoding microRNA (miRNA) all represent epigenetic factors that have been explored in IPF. Progressive remodeling within IPF lungs may be due in part to particular methylation profiles (62). Histone modification has been shown to lead to resistance from apoptosis in IPF-derived fibroblasts (63, 64).
Behavioral contributors to IPF pathobiology have also been little studied. It is clear from epidemiological studies that cigarette smoking is strongly associated with IPF, and it is possible that this is a central pathobiological driver of disease in some patients. Describing the contribution of cigarette smoke to the development and progression of IPF should be a top priority. Other behavioral factors such as diet and exercise (or lack thereof) could contribute to disease progression and are modifiable with targeted therapeutic interventions.
Examples of Precision Medicine–based Investigation in IPF
We highlight below several recently completed or ongoing clinical studies relevant to the discussion of precision medicine–based investigation. Some of these studies represent attempts at endotype-driven approaches to early-phase clinical trials, and others provide examples of where endotype-driven design might prove relevant. These studies do not prove the value of precision medicine in IPF; instead, these trials are presented as examples for future sponsors and clinical investigators to consider when developing the next generation of trials and weighing the pros and cons of precision medicine–based design.
TOLLIP, MUC5B, and the Response to N-Acetylcysteine
As discussed, SNPs within TOLLIP and MUC5B have been associated with risk of IPF (38–40, 46, 47). A recent retrospective review of data from several completed clinical trials in IPF explored a possible pharmacogenomic relationship between these SNPs and response to N-acetylcysteine (NAC) (65). Interestingly, a specific SNP (rs3750920) within TOLLIP was associated with a reduction in the risk of disease progression, hospitalization, transplant, or death in those receiving NAC therapy. Patients homozygous for the risk allele appeared to benefit from NAC therapy, whereas those with the alternate genotype appeared to be harmed by NAC therapy. It was hypothesized that SNP-driven differences in TOLLIP-mediated TLR signaling could lead to an oxidant-driven disease endotype in which NAC therapy would be particularly beneficial (65). Although exploratory, this early look at the potential interaction between genetics and therapeutic efficacy could explain discordant clinical trial results regarding the efficacy of NAC therapy in IPF (45, 66) and should prompt future clinical trials to include pharmacogenomic analyses in their study design.
Oral Immunotherapy with Type V Collagen
Circulating autoantibodies against type V collagen are detectable in ∼40% of patients with IPF (67). Injury to the lung may expose type V collagen to immune cells and autoimmune-mediated injury (68, 69). On the basis of this hypothesis, a phase I clinical trial of oral immunotherapy with bovine type V collagen was performed in patients with IPF with circulating anti–type V collagen antibodies (70). Increasing doses of purified oral type V collagen were safe and led to a dose-dependent stabilization in circulating MMP7 levels and decreases in C1q binding to anti–type V collagen antibodies, suggesting mechanistic proof of concept (70).
Autoantibody Reduction Therapy
A subgroup of patients with IPF has circulating autoantibodies against epithelial cells (HEp-2 cells) and heat shock protein-70 that may contribute to parenchymal injury and progressive fibroproliferation (49, 71). These observations have prompted a phase II clinical trial of rituximab (a chimeric monoclonal antibody against CD20) in IPF that is currently enrolling patients (www.clinicaltrials.gov, NCT01969409). In this trial, only patients with IPF with measurable circulating autoantibodies against HEp-2 cells are enrolled, and the primary endpoint is change in HEp-2 antibody titers.
Laparoscopic Antireflux Surgery
As discussed earlier, gastroesophageal reflux is prevalent in IPF (59) and may contribute to disease progression. Retrospective analyses of observational and clinical trial cohorts have investigated whether the treatment of abnormal gastroesophageal reflux with antacid therapy or antireflux surgery may lead to slowing of disease progression (72–74). Unlike antacid therapy, antireflux surgery addresses gastroesophageal reflux directly by eliminating or reducing the frequency of reflux events. The relevance of nonacid gastroesophageal reflux (and therefore of surgical rather than medical management) is another unknown. To test the hypothesis that the subgroup of patients with IPF with abnormal gastroesophageal reflux will benefit from surgical correction of both acid and nonacid reflux, a phase II clinical trial of laparoscopic antireflux surgery in IPF is currently underway (www.clinicaltrials.gov, NCT01982968), with the primary endpoint being change in FVC.
Matrix-directed Therapy
Elevated levels of circulating LOXL2 (a regulator of collagen crosslinking) have been found in a subgroup of patients with IPF, and it is hypothesized that these patients might be responsive to LOXL2-directed therapy. An industry-sponsored phase II study of simtuzumab, an anti-LOXL2 monoclonal antibody (www.clinicaltrials.gov, NCT01769196) was recently stopped early for lack of efficacy. Enrollment was open to a wide spectrum of patients with IPF, and no requirement for elevated LOXL2 was included. The study includes a plan to assess treatment response on high versus low serum levels of LOXL2, which, if suggestive, could provide additional impetus to sponsors and clinical trialists to adopt precision medicine–based enrollment criteria.
Conclusions
We believe precision medicine represents the new frontier for IPF clinical research, one that provides an opportunity to explore a more mechanistic, pathobiological, environmental, and behavioral approach to disease classification and treatment (8, 75). If successful, precision medicine–based investigations will identify IPF subpopulations that share pathobiological pathways and target those pathways with novel mechanism-specific drugs. We believe that scientists, investigators, sponsors, patients, and regulators should work together to develop and fund innovative, meritorious precision medicine–based research in IPF. To start the process, we suggest the IPF community consider holding an “IPF Precision Medicine Summit” to discuss the promise and risks of precision medicine–based research and to develop a roadmap for the next 5 to 10 years of investigation. It is undeniable that precision medicine holds great promise in IPF and provides the possibility of transformative change in disease diagnosis and management that will lead to better and longer lives for patients.
Footnotes
Author Contributions: R.B., F.J.M., and H.R.C.: conception and design of research; R.B., L.R., and H.R.C.: prepared figures and tables; all authors drafted the manuscript, edited and revised the manuscript, and approved the final version of the manuscript.
CME will be available for this article at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201601-0169CI on March 18, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Ley B, Collard HR. Epidemiology of idiopathic pulmonary fibrosis. Clin Epidemiol. 2013;5:483–492. doi: 10.2147/CLEP.S54815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 3.King TE, Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949–1961. doi: 10.1016/S0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- 4.Wolters PJ, Collard HR, Jones KD. Pathogenesis of idiopathic pulmonary fibrosis. Annu Rev Pathol. 2014;9:157–179. doi: 10.1146/annurev-pathol-012513-104706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King TE, Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, Gorina E, Hopkins PM, Kardatzke D, Lancaster L, et al. ASCEND Study Group. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 6.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y, et al. INPULSIS Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 7.Hudson K, Lifton R, Patrick-Lake B, Burchard EG, Coles T, Collins R, Conrad A, Desmond-Hellmann S, Dishman E, Giusti K, et al. National Institutes of HealthThe precision medicine initiative cohort program: building a research foundation for 21st century medicine. 2015. [accessed 2015 Jul 15]. Available from: http://www.nih.gov/precisionmedicine/09172015-pmi-working-group-report.pdf
- 8.Hawgood S, Hook-Barnard IG, O’Brien TC, Yamamoto KR. Precision medicine: beyond the inflection point. Sci Transl Med. 2015;7:300ps17. doi: 10.1126/scitranslmed.aaa9970. [DOI] [PubMed] [Google Scholar]
- 9.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kropski JA, Lawson WE, Blackwell TS. Personalizing therapy in idiopathic pulmonary fibrosis: a glimpse of the future? Am J Respir Crit Care Med. 2015;192:1409–1411. doi: 10.1164/rccm.201509-1789ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spagnolo P, Tzouvelekis A, Maher TM. Personalized medicine in idiopathic pulmonary fibrosis: facts and promises. Curr Opin Pulm Med. 2015;21:470–478. doi: 10.1097/MCP.0000000000000187. [DOI] [PubMed] [Google Scholar]
- 12.Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet. 2008;372:1107–1119. doi: 10.1016/S0140-6736(08)61452-X. [DOI] [PubMed] [Google Scholar]
- 13.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 14.Aggarwal C. Targeted therapy for lung cancer: present and future. Ann Palliat Med. 2014;3:229–235. doi: 10.3978/j.issn.2224-5820.2014.06.01. [DOI] [PubMed] [Google Scholar]
- 15.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 16.Leighl NB. Treatment paradigms for patients with metastatic non-small-cell lung cancer: first-, second-, and third-line. Curr Oncol. 2012;19:S52–S58. doi: 10.3747/co.19.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 18.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, et al. Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 19.Herbst RS, Gandara DR, Hirsch FR, Redman MW, LeBlanc M, Mack PC, Schwartz LH, Vokes E, Ramalingam SS, Bradley JD, et al. Lung Master Protocol (Lung-MAP): a biomarker-driven protocol for accelerating development of therapies for squamous cell lung cancer: SWOG S1400. Clin Cancer Res. 2015;21:1514–1524. doi: 10.1158/1078-0432.CCR-13-3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agache IO. From phenotypes to endotypes to asthma treatment. Curr Opin Allergy Clin Immunol. 2013;13:249–256. doi: 10.1097/ACI.0b013e32836093dd. [DOI] [PubMed] [Google Scholar]
- 21.Wenzel SE. Asthma: defining of the persistent adult phenotypes. Lancet. 2006;368:804–813. doi: 10.1016/S0140-6736(06)69290-8. [DOI] [PubMed] [Google Scholar]
- 22.Bhakta NR, Woodruff PG. Human asthma phenotypes: from the clinic, to cytokines, and back again. Immunol Rev. 2011;242:220–232. doi: 10.1111/j.1600-065X.2011.01032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, Koth LL, Arron JR, Fahy JV. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180:388–395. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, Harris JM, Scheerens H, Wu LC, Su Z, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365:1088–1098. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]
- 25.Hanania NA, Noonan M, Corren J, Korenblat P, Zheng Y, Fischer SK, Cheu M, Putnam WS, Murray E, Scheerens H, et al. Lebrikizumab in moderate-to-severe asthma: pooled data from two randomised placebo-controlled studies. Thorax. 2015;70:748–756. doi: 10.1136/thoraxjnl-2014-206719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piper E, Brightling C, Niven R, Oh C, Faggioni R, Poon K, She D, Kell C, May RD, Geba GP, et al. A phase II placebo-controlled study of tralokinumab in moderate-to-severe asthma. Eur Respir J. 2013;41:330–338. doi: 10.1183/09031936.00223411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The White House. Precision medicine initiative 2015. [accessed 2015 Jul 25]Available from: https://www.whitehouse.gov/precision-medicine
- 28.Medical Research Council. Our research: background 2015. [accessed 2015 Aug 4]Available from: http://www.mrc.ac.uk/research/initiatives/stratified-medicine/background/
- 29.Thomas AQ, Lane K, Phillips J, III, Prince M, Markin C, Speer M, Schwartz DA, Gaddipati R, Marney A, Johnson J, et al. Heterozygosity for a surfactant protein C gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred. Am J Respir Crit Care Med. 2002;165:1322–1328. doi: 10.1164/rccm.200112-123OC. [DOI] [PubMed] [Google Scholar]
- 30.Nogee LM, Dunbar AE, III, Wert SE, Askin F, Hamvas A, Whitsett JA. A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N Engl J Med. 2001;344:573–579. doi: 10.1056/NEJM200102223440805. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Kuan PJ, Xing C, Cronkhite JT, Torres F, Rosenblatt RL, DiMaio JM, Kinch LN, Grishin NV, Garcia CK. Genetic defects in surfactant protein A2 are associated with pulmonary fibrosis and lung cancer. Am J Hum Genet. 2009;84:52–59. doi: 10.1016/j.ajhg.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohnishi H, Yokoyama A, Kondo K, Hamada H, Abe M, Nishimura K, Hiwada K, Kohno N. Comparative study of KL-6, surfactant protein-A, surfactant protein-D, and monocyte chemoattractant protein-1 as serum markers for interstitial lung diseases. Am J Respir Crit Care Med. 2002;165:378–381. doi: 10.1164/ajrccm.165.3.2107134. [DOI] [PubMed] [Google Scholar]
- 33.Barlo NP, van Moorsel CH, Ruven HJ, Zanen P, van den Bosch JM, Grutters JC. Surfactant protein-D predicts survival in patients with idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 2009;26:155–161. [PubMed] [Google Scholar]
- 34.Greene KE, King TE, Jr, Kuroki Y, Bucher-Bartelson B, Hunninghake GW, Newman LS, Nagae H, Mason RJ. Serum surfactant proteins-A and -D as biomarkers in idiopathic pulmonary fibrosis. Eur Respir J. 2002;19:439–446. doi: 10.1183/09031936.02.00081102. [DOI] [PubMed] [Google Scholar]
- 35.Collard HR, Calfee CS, Wolters PJ, Song JW, Hong SB, Brady S, Ishizaka A, Jones KD, King TE, Jr, Matthay MA, et al. Plasma biomarker profiles in acute exacerbation of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2010;299:L3–L7. doi: 10.1152/ajplung.90637.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kinder BW, Brown KK, McCormack FX, Ix JH, Kervitsky A, Schwarz MI, King TE., Jr Serum surfactant protein-A is a strong predictor of early mortality in idiopathic pulmonary fibrosis. Chest. 2009;135:1557–1563. doi: 10.1378/chest.08-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takahashi H, Fujishima T, Koba H, Murakami S, Kurokawa K, Shibuya Y, Shiratori M, Kuroki Y, Abe S. Serum surfactant proteins A and D as prognostic factors in idiopathic pulmonary fibrosis and their relationship to disease extent. Am J Respir Crit Care Med. 2000;162:1109–1114. doi: 10.1164/ajrccm.162.3.9910080. [DOI] [PubMed] [Google Scholar]
- 38.Borie R, Crestani B, Dieude P, Nunes H, Allanore Y, Kannengiesser C, Airo P, Matucci-Cerinic M, Wallaert B, Israel-Biet D, et al. The MUC5B variant is associated with idiopathic pulmonary fibrosis but not with systemic sclerosis interstitial lung disease in the European Caucasian population. Plos One. 2013;8:e70621. doi: 10.1371/journal.pone.0070621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, Fingerlin TE, Zhang W, Gudmundsson G, Groshong SD, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med. 2011;364:1503–1512. doi: 10.1056/NEJMoa1013660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Noth I, Garcia JG, Kaminski N. A variant in the promoter of MUC5B and idiopathic pulmonary fibrosis. N Engl J Med. 2011;364:1576–1577. doi: 10.1056/NEJMc1013504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peljto AL, Zhang Y, Fingerlin TE, Ma SF, Garcia JG, Richards TJ, Silveira LJ, Lindell KO, Steele MP, Loyd JE, et al. Association between the MUC5B promoter polymorphism and survival in patients with idiopathic pulmonary fibrosis. JAMA. 2013;309:2232–2239. doi: 10.1001/jama.2013.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, Lawson WE, Xie M, Vulto I, Phillips JA, III, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356:1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 43.Stuart BD, Lee JS, Kozlitina J, Noth I, Devine MS, Glazer CS, Torres F, Kaza V, Girod CE, Jones KD, et al. Effect of telomere length on survival in patients with idiopathic pulmonary fibrosis: an observational cohort study with independent validation. Lancet Respir Med. 2014;2:557–565. doi: 10.1016/S2213-2600(14)70124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cronkhite JT, Xing C, Raghu G, Chin KM, Torres F, Rosenblatt RL, Garcia CK. Telomere shortening in familial and sporadic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178:729–737. doi: 10.1164/rccm.200804-550OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raghu G, Anstrom KJ, King TE, Jr, Lasky JA, Martinez FJ Idiopathic Pulmonary Fibrosis Clinical Research Network. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med. 2012;366:1968–1977. doi: 10.1056/NEJMoa1113354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Dwyer DN, Armstrong ME, Trujillo G, Cooke G, Keane MP, Fallon PG, Simpson AJ, Millar AB, McGrath EE, Whyte MK, et al. The Toll-like receptor 3 L412F polymorphism and disease progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2013;188:1442–1450. doi: 10.1164/rccm.201304-0760OC. [DOI] [PubMed] [Google Scholar]
- 47.Noth I, Zhang Y, Ma SF, Flores C, Barber M, Huang Y, Broderick SM, Wade MS, Hysi P, Scuirba J, et al. Genetic variants associated with idiopathic pulmonary fibrosis susceptibility and mortality: a genome-wide association study. Lancet Respir Med. 2013;1:309–317. doi: 10.1016/S2213-2600(13)70045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prasse A, Probst C, Bargagli E, Zissel G, Toews GB, Flaherty KR, Olschewski M, Rottoli P, Müller-Quernheim J. Serum CC-chemokine ligand 18 concentration predicts outcome in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:717–723. doi: 10.1164/rccm.200808-1201OC. [DOI] [PubMed] [Google Scholar]
- 49.Kahloon RA, Xue J, Bhargava A, Csizmadia E, Otterbein L, Kass DJ, Bon J, Soejima M, Levesque MC, Lindell KO, et al. Patients with idiopathic pulmonary fibrosis with antibodies to heat shock protein 70 have poor prognoses. Am J Respir Crit Care Med. 2013;187:768–775. doi: 10.1164/rccm.201203-0506OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herazo-Maya JD, Noth I, Duncan SR, Kim S, Ma SF, Tseng GC, Feingold E, Juan-Guardela BM, Richards TJ, Lussier Y, et al. Peripheral blood mononuclear cell gene expression profiles predict poor outcome in idiopathic pulmonary fibrosis. Sci Transl Med. 2013;5:205ra136. doi: 10.1126/scitranslmed.3005964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zuo F, Kaminski N, Eugui E, Allard J, Yakhini Z, Ben-Dor A, Lollini L, Morris D, Kim Y, DeLustro B, et al. Gene expression analysis reveals matrilysin as a key regulator of pulmonary fibrosis in mice and humans. Proc Natl Acad Sci USA. 2002;99:6292–6297. doi: 10.1073/pnas.092134099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosas IO, Richards TJ, Konishi K, Zhang Y, Gibson K, Lokshin AE, Lindell KO, Cisneros J, Macdonald SD, Pardo A, et al. MMP1 and MMP7 as potential peripheral blood biomarkers in idiopathic pulmonary fibrosis. Plos Med. 2008;5:e93. doi: 10.1371/journal.pmed.0050093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Richards TJ, Kaminski N, Baribaud F, Flavin S, Brodmerkel C, Horowitz D, Li K, Choi J, Vuga LJ, Lindell KO, et al. Peripheral blood proteins predict mortality in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;185:67–76. doi: 10.1164/rccm.201101-0058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Song JW, Do KH, Jang SJ, Colby TV, Han S, Kim DS. Blood biomarkers MMP-7 and SP-A: predictors of outcome in idiopathic pulmonary fibrosis. Chest. 2013;143:1422–1429. doi: 10.1378/chest.11-2735. [DOI] [PubMed] [Google Scholar]
- 55.Kropski JA, Pritchett JM, Zoz DF, Crossno PF, Markin C, Garnett ET, Degryse AL, Mitchell DB, Polosukhin VV, Rickman OB, et al. Extensive phenotyping of individuals at risk for familial interstitial pneumonia reveals clues to the pathogenesis of interstitial lung disease. Am J Respir Crit Care Med. 2015;191:417–426. doi: 10.1164/rccm.201406-1162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chien JW, Richards TJ, Gibson KF, Zhang Y, Lindell KO, Shao L, Lyman SK, Adamkewicz JI, Smith V, Kaminski N, et al. Serum lysyl oxidase-like 2 levels and idiopathic pulmonary fibrosis disease progression. Eur Respir J. 2014;43:1430–1438. doi: 10.1183/09031936.00141013. [DOI] [PubMed] [Google Scholar]
- 57.Saini G, Porte J, Weinreb PH, Violette SM, Wallace WA, McKeever TM, Jenkins G. αvβ6 integrin may be a potential prognostic biomarker in interstitial lung disease. Eur Respir J. 2015;46:486–494. doi: 10.1183/09031936.00210414. [DOI] [PubMed] [Google Scholar]
- 58.Jenkins RG, Simpson JK, Saini G, Bentley JH, Russell AM, Braybrooke R, Molyneaux PL, McKeever TM, Wells AU, Flynn A, et al. Longitudinal change in collagen degradation biomarkers in idiopathic pulmonary fibrosis: an analysis from the prospective, multicentre PROFILE study. Lancet Respir Med. 2015;3:462–472. doi: 10.1016/S2213-2600(15)00048-X. [DOI] [PubMed] [Google Scholar]
- 59.Raghu G, Freudenberger TD, Yang S, Curtis JR, Spada C, Hayes J, Sillery JK, Pope CE, II, Pellegrini CA. High prevalence of abnormal acid gastro-oesophageal reflux in idiopathic pulmonary fibrosis. Eur Respir J. 2006;27:136–142. doi: 10.1183/09031936.06.00037005. [DOI] [PubMed] [Google Scholar]
- 60.Han MK, Zhou Y, Murray S, Tayob N, Noth I, Lama VN, Moore BB, White ES, Flaherty KR, Huffnagle GB, et al. COMET Investigators. Lung microbiome and disease progression in idiopathic pulmonary fibrosis: an analysis of the COMET study. Lancet Respir Med. 2014;2:548–556. doi: 10.1016/S2213-2600(14)70069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Molyneaux PL, Cox MJ, Willis-Owen SA, Mallia P, Russell KE, Russell AM, Murphy E, Johnston SL, Schwartz DA, Wells AU, et al. The role of bacteria in the pathogenesis and progression of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2014;190:906–913. doi: 10.1164/rccm.201403-0541OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang IV, Pedersen BS, Rabinovich E, Hennessy CE, Davidson EJ, Murphy E, Guardela BJ, Tedrow JR, Zhang Y, Singh MK, et al. Relationship of DNA methylation and gene expression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2014;190:1263–1272. doi: 10.1164/rccm.201408-1452OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Coward WR, Watts K, Feghali-Bostwick CA, Knox A, Pang L. Defective histone acetylation is responsible for the diminished expression of cyclooxygenase 2 in idiopathic pulmonary fibrosis. Mol Cell Biol. 2009;29:4325–4339. doi: 10.1128/MCB.01776-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang SK, Scruggs AM, Donaghy J, Horowitz JC, Zaslona Z, Przybranowski S, White ES, Peters-Golden M. Histone modifications are responsible for decreased Fas expression and apoptosis resistance in fibrotic lung fibroblasts. Cell Death Dis. 2013;4:e621. doi: 10.1038/cddis.2013.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oldham JM, Ma SF, Martinez FJ, Anstrom KJ, Raghu G, Schwartz DA, Valenzi E, Witt L, Lee C, Vij R, et al. IPFnet Investigators. TOLLIP, MUC5B, and the response to N-acetylcysteine among individuals with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2015;192:1475–1482. doi: 10.1164/rccm.201505-1010OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Demedts M, Behr J, Buhl R, Costabel U, Dekhuijzen R, Jansen HM, MacNee W, Thomeer M, Wallaert B, Laurent F, et al. IFIGENIA Study Group. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005;353:2229–2242. doi: 10.1056/NEJMoa042976. [DOI] [PubMed] [Google Scholar]
- 67.Vittal R, Mickler EA, Fisher AJ, Zhang C, Rothhaar K, Gu H, Brown KM, Emtiazdjoo A, Lott JM, Frye SB, et al. Type V collagen induced tolerance suppresses collagen deposition, TGF-β and associated transcripts in pulmonary fibrosis. Plos One. 2013;8:e76451. doi: 10.1371/journal.pone.0076451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sumpter TL, Wilkes DS. Role of autoimmunity in organ allograft rejection: a focus on immunity to type V collagen in the pathogenesis of lung transplant rejection. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1129–L1139. doi: 10.1152/ajplung.00330.2003. [DOI] [PubMed] [Google Scholar]
- 69.Burlingham WJ, Love RB, Jankowska-Gan E, Haynes LD, Xu Q, Bobadilla JL, Meyer KC, Hayney MS, Braun RK, Greenspan DS, et al. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007;117:3498–3506. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilkes DS, Chew T, Flaherty KR, Frye S, Gibson KF, Kaminski N, Klemsz MJ, Lange W, Noth I, Rothhaar K. Oral immunotherapy with type V collagen in idiopathic pulmonary fibrosis. Eur Respir J. 2015;45:1393–1402. doi: 10.1183/09031936.00105314. [DOI] [PubMed] [Google Scholar]
- 71.Magro CM, Waldman WJ, Knight DA, Allen JN, Nadasdy T, Frambach GE, Ross P, Marsh CB. Idiopathic pulmonary fibrosis related to endothelial injury and antiendothelial cell antibodies. Hum Immunol. 2006;67:284–297. doi: 10.1016/j.humimm.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 72.Lee JS, Collard HR, Anstrom KJ, Martinez FJ, Noth I, Roberts RS, Yow E, Raghu G IPFnet Investigators. Anti-acid treatment and disease progression in idiopathic pulmonary fibrosis: an analysis of data from three randomised controlled trials. Lancet Respir Med. 2013;1:369–376. doi: 10.1016/S2213-2600(13)70105-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee JS, Ryu JH, Elicker BM, Lydell CP, Jones KD, Wolters PJ, King TE, Jr, Collard HR. Gastroesophageal reflux therapy is associated with longer survival in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184:1390–1394. doi: 10.1164/rccm.201101-0138OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kreuter M Wuyts W Renzoni E Koschel D, Maher TM, Kolb M, Weycker D, Spagnolo P, Kirchgaessler KU, Herth FJ et al. . Antacid therapy and disease outcomes in idiopathic pulmonary fibrosis: a pooled analysis Lancet Respir Med[online ahead of print] 2016 Mar 31; DOI: 10.1016/S2213-2600(16)00067-9 [DOI] [PubMed] [Google Scholar]
- 75.Britten N, Pope C, Halford S, Richeldi L. What if we made stratified medicine work for patients? Lancet Respir Med. 2016;4:8–10. doi: 10.1016/S2213-2600(15)00499-3. [DOI] [PubMed] [Google Scholar]