Abstract
Pten (phosphatase with tensin homology), a dual-specificity phosphatase, is a negative regulator of the phosphoinositide 3-kinase (PI3K)/Akt signaling pathway. Pten regulates a vast array of biological functions including growth, metabolism, and longevity. Although the PI3K/Akt pathway is a key determinant of the insulin-dependent increase in glucose uptake into muscle and adipose cells, the contribution of this pathway in muscle to whole-body glucose homeostasis is unclear. Here we show that muscle-specific deletion of Pten protected mice from insulin resistance and diabetes caused by high-fat feeding. Deletion of muscle Pten resulted in enhanced insulin-stimulated 2-deoxyglucose uptake and Akt phosphorylation in soleus but, surprisingly, not in extensor digitorum longus muscle compared to littermate controls upon high-fat feeding, and these mice were spared from developing hyperinsulinemia and islet hyperplasia. Muscle Pten may be a potential target for treatment or prevention of insulin resistance and diabetes.
Defects in insulin action in target organs, principally muscle, liver, and fat, lead to insulin resistance and type 2 diabetes (23). The contribution of insulin action in these individual target organs to whole-body glucose homeostasis is complex. Recent advances in genetic tools have allowed us to dissect mechanisms of insulin signaling in individual target tissues and have brought new insight into molecular mechanisms of insulin signaling with respect to whole-body physiology (18, 26).
The phosphoinositide 3-kinase (PI3K)/Akt pathway is well characterized as a mediator of the receptor tyrosine kinases, including the insulin receptor. Activation of this pathway promotes a vast array of cellular processes, including cellular proliferation, differentiation, and survival (reviewed in reference 34). The biological response to the activation of the PI3K pathway is highly regulated and tissue specific (1, 21). In particular, the PI3K/Akt pathway is an important effector of insulin actions mediating glucose uptake and glycogen synthesis in muscle and fat (8, 17, 20, 48) and inhibiting glycogenolysis and glucose release from the liver (8).
Pten is a potent negative modulator of the PI3K/Akt pathway (37). In Caenorhabditis elegans, its homolog DAF-18 acts in the insulin receptor-like pathway and regulates longevity and dauer larva development (13, 27, 30). In humans, Pten was first described as a tumor suppressor (24, 39). Initial mutations were described for Cowden's syndrome, where hamartomas and increased susceptibility to cancer occur. Recently, polymorphisms of PTEN have been described in association with type 2 diabetes in a Japanese cohort (16).
Null mutation of Pten in mice leads to embryonic lethality, precluding studies of disease mechanisms (41). Tissue-specific Pten knockout models have, however, brought new insight into the biological role of Pten in different tissue and cell types (1). In proliferating or tumor-prone tissues such as the liver, endometrium, skin, prostate, or breast, Pten deletion has a permissive effect on tumor development (2, 15, 38, 42). On the other hand, Pten controls cell size in highly specialized cells such as neurons (3). Pten has also been shown to modulate highly specialized functions of a given tissue, such as class switching of immunoglobulin in B cells or metabolic actions of insulin in liver (15, 40, 43). Pten reduction in liver and fat, by systemic administration of Pten antisense oligonucleotide, protected db/db mice from developing diabetes (5). Therefore, the biological role of Pten is highly dependent on the tissue type and can range from its antiproliferative effect in tumor development to modulation of a highly specialized function for a given tissue.
Skeletal muscle is a major insulin responsive tissue, and insulin resistance in muscle is a predominant early defect in the pathogenesis of type 2 diabetes (47). However, the contribution of a defect in insulin signaling in muscle towards insulin resistance and diabetes is not entirely clear. Muscle-specific insulin receptor knockout (MIRKO) mice did not appear to display a major defect in glucose homeostasis, whereas muscle-specific deletion of GLUT4 contributes to development of insulin resistance and diabetes (4, 51). Phosphatases such as protein tyrosine phosphatase 1B (PTP-1B) and SHIP2 also play a role in the development of diabetes and obesity. The extent of their effect varies depending on the specific phosphatase, from neonatal hypoglycemia to protection from obesity (7, 9). Furthermore, the specific role of these molecules in different insulin target tissues, which results in the perturbation of whole-body fuel metabolism, is not yet known.
The aim of this study was to examine the modulatory role of Pten in insulin action in skeletal muscle and its contribution to whole-body insulin resistance and diabetes. We used the Cre-loxP system, wherein Cre expression was driven by the muscle creatine kinase (MCK) promoter (4). MCK is expressed in skeletal and cardiac muscle. In cardiac muscle, Pten has been shown to play a role in cardiac hypertrophy and contractility (10). However, the role of Pten in insulin signaling in skeletal muscle has not been examined. Here we show that deletion of muscle Pten protects mice from the development of high-fat-induced insulin resistance and diabetes. This protection continues even in aged mice, without demonstrable tumor development.
MATERIALS AND METHODS
Mouse protocol.
The generation and genotyping of muscle-specific Pten knockout mice were as previously described (10). Briefly, Ptenfl/fl mice with exons 4 and 5 of Pten flanked by loxP sites by homologous recombination (41) were mated with mice carrying the Cre transgene under the control of the muscle creatine kinase promoter (mckCre) (4). Ptenfl/+ cre+ mice were intercrossed to generate Ptenfl/fl cre+ and Pten+/+ cre+ mice, referred here as mckPten−/− and mckPten+/+ mice, respectively. Because mice were maintained on a mixed 129J-C57BL/6 background, only littermates served as controls for all experiments. All mice were housed in pathogen-free facilities on a 12-h light-dark cycle and were fed ad libitum either a standard rodent chow (irradiated, 5% fat; Harlan Teklad, Indianapolis, Ind.) or, for studies involving a high-fat diet, Harlan Teklad TD 01435 special diet (45% of calories derived from fat) in accordance with the Ontario Cancer Institute Animal Care Facility Protocol. The activity of the mice was not restricted.
Metabolic studies.
Blood glucose levels were determined from tail venous blood with an automated glucose monitor (One touch II; Lifescan, Inc., Milpitas, Calif.). Insulin and glucose tolerance tests were done on animals that had been fasted overnight. Animals were injected with either 1.5 U of human regular insulin (Eli Lilly Canada Inc., Toronto, Ontario, Canada) per kg of body weight or 1 g of glucose per kg of body weight into the peritoneal cavity. Blood glucose levels were measured immediately before and 15, 30, 45, 60, and 120 min after the injection. Insulin levels were measured from tail venous blood following an overnight fast by radioimmunoassay with rat insulin as a standard (Linco Research Inc., St. Charles, Mo.).
2-Deoxy-d-glucose uptake into isolated skeletal muscles.
Intact soleus and extensor digitorum longus (EDL) muscles were incubated with or without 2 mU of insulin per ml. 2-Deoxyglucose uptake in insulin-stimulated muscles was then measured for 20 min as described previously (33).
Western blotting.
Soleus and EDL muscles were pulverized in liquid nitrogen, homogenized, and lysed as described previously (35), and 2× Laemmli sample buffer containing 7.5% β-mercaptoethanol was added to 30 μg of lysate. The samples were heated for 15 min at 65°C, resolved by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis (SDS-10% PAGE), and then immunoblotted with polyclonal anti-phospho-Akt (Thr308) and pan-Akt antibodies (1:1,000 dilution of primary antibodies; Cell Signaling Technology, Beverly, Mass.).
Immunohistochemistry and PAS staining.
Pancreatic tissue was fixed overnight in a solution of freshly prepared 4% paraformaldehyde in 0.1 M phosphate-buffered saline (pH 7.4) at 4°C. Samples were dehydrated and prepared as paraffin blocks. Seven-micrometer-thick sections of paraffin-embedded pancreatic tissue were stained with hematoxylin and eosin for histological examination. For periodic acid-Schiff (PAS) staining, slides were preheated for 5 min with 1% periodic acid in mQ-water followed by a wash step for 1 min in tap water, followed by a wash dip for 5 s in mQ-water. The slides then were treated with Schiff's reagent (Sigma) for 15 min at room temperature.
Statistical analysis.
All groups of data for 2-deoxyglucose uptake results and Western blots for total and phospho Akt were compared by using one-way analysis of variance (ANOVA) followed by Tukey or Newman-Keuls post hoc tests with the statistical software program GraphPad Prism.
RESULTS
Generation of mckPten−/− mice and metabolic assessment on chow diet..
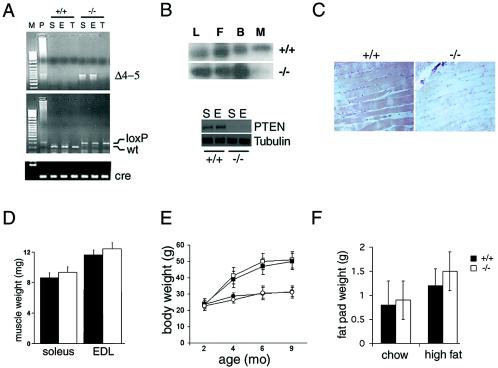
The generation of muscle-specific Pten knockout mice, referred to here as mckPten−/− mice, has been described previously (10). These mice appeared generally healthy and had a life span similar to that of their littermate controls up to 24 months. The Pten+/+ cre+ controls gave results similar to those for Pten+/+ cre− and Ptenfl/fl cre− mice upon metabolic testing. The presence of the recombined allele specifically in muscles of mckPten−/− mice in conjunction with the absence of the Pten in both soleus and EDL skeletal muscles was shown by PCR and Western blotting (Fig. 1A and B). The presence of faint residual Pten protein in mckPten−/− mice is possibly due to Pten expression in nonmyocyte cells such as endothelial cells or fibroblasts. The absence of Pten in soleus muscles of Pten−/− cre+ mice was also shown by immunohistochenistry (Fig. 1C). The histological assessment of muscle from mckPten−/− mice also showed normal morphology, with no increase in cell size or evidence of muscle tumors (Fig. 1C). Furthermore, the weights of the muscles were similar for the mckPten−/− and the mckPten+/+ mice (Fig. 1D). mckPten−/− mice gained weight at a rate similar to that for their mckPten+/+ littermate controls on normal chow diet (Fig. 1E). Similarly, the epididymal fat masses were similar in chow-fed mckPten+/+ (0.8 ± 0.4 g) and mckPten−/− (0.9 ± 0.4 g) mice (P = not significant [NS]) when they were compared at 6 months of age (Fig. 1F).
FIG. 1.
Targeted deletion of Pten in muscle and general characterization of mckPten+/+ and mckPten−/− mice. (A) PCR analysis of Cre-mediated recombination of the Pten locus (Δ4-5, top) and genotyping for the Pten-loxP allele (middle) and cre (bottom) as described previously (2). Results obtained from genomic DNAs of soleus (S) and EDL (E) muscles and tail (T) are shown. (B) Absence of Pten expression in muscle. Western blots of expression of Pten in livers (L), epididymal fat (F), brains (B), and thigh muscles (M) of 6-month-old mckPten+/+ and mckPten−/− mice are shown (top). Soleus (S) and EDL (E) muscle types show the same degree of Pten deletion (bottom). (C) Immunohistochemistry of Pten staining in soleus muscles of 6-month-old mckPten+/+ and mckPten−/− mice (magnification, ×25). (D) Similar weights of soleus and EDL muscles from 6-month-old mckPten+/+ (closed bars) and mckPten−/− (open bars) mice on a high-fat diet (n = 8 to 10 muscles per group; P = NS). (E) Similar weight gains by mckPten+/+ (closed symbols) and mckPten−/− (open symbols) mice on a chow (circles) or high-fat (squares) diet (n = 10 to 15 mice per group; P = NS). (F) Similar epididymal fat pad weights from 6-month-old mckPten+/+ and mckPten−/− mice after a chow or high-fat diet (P = NS). Error bars indicate standard errors of the means.
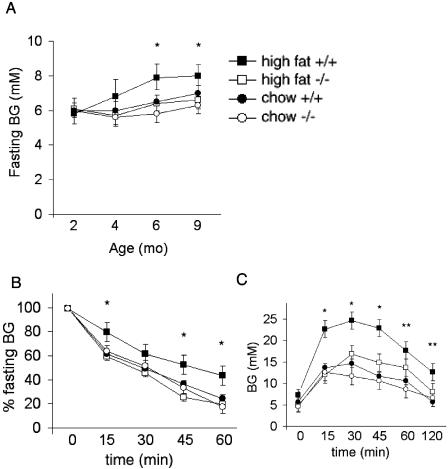
There was a trend toward lower fasting glucose levels in mckPten−/− mice; however, the difference between the chow-fed mckPten+/+ and mckPten−/− mice was not statistically different (Fig. 2A). Insulin sensitivity as measured by the insulin tolerance test in mckPten−/− mice was similar to that in littermate controls on chow diet when measured at 6 months (Fig. 2B). At that age, mckPten+/+ and mckPten−/− chow-fed mice also had similar glucose excursions when given an intraperitoneal glucose challenge (Fig. 2C). mckPten−/− mice did not develop spontaneous hypoglycemia at any time, suggesting that deletion of Pten in muscle does not give rise to potentially deleterious insulin hypersensitivity. These results show that Pten deletion in muscle does not alter insulin sensitivity or β-cell capacity when the mice are fed a normal chow diet.
FIG. 2.
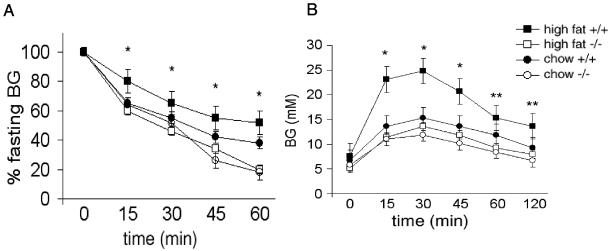
Protection from insulin resistance and diabetes in high-fat-fed mckPten−/− mice. (A) Fasting glucose levels of mckPten+/+ and mckPten−/− mice on a chow or high-fat diet. *, P < 0.05, comparing mckPten+/+ and mckPten−/− mice on a high-fat diet. (B) Insulin tolerance tests were performed on 6-month-old mckPten+/+ and mckPten−/− mice as described in Materials and Methods. *, P < 0.05, comparing mckPten+/+ and mckPten−/− mice on a high-fat diet. (C) Glucose tolerance tests were performed on 6-month-old mckPten+/+ and mckPten−/− mice on a chow or high-fat diet as described in Materials and Methods. * and **, P < 0.01 and P < 0.1, respectively, comparing mckPten+/+ and mckPten−/− mice on a high-fat diet. All results are expressed as means ± standard errors of the means from at least 10 animals of each genotype.
Protection from high-fat-induced insulin resistance and diabetes.
To test the hypothesis that Pten deletion in skeletal muscle prevents the development of insulin resistance, high-fat feeding starting at 8 weeks of age was used to induce insulin resistance. Weight gain was not significantly affected by deletion of Pten in muscle (Fig. 1E). The epididymal fat pad weights measured at 6 months of age were also similar for the mckPten+/+ (1.2 ± 0.2 g) and mckPten−/− (1.4 ± 0.4 g) mice (P = NS) (Fig. 1F). Fasting glucose levels began to rise as expected in mckPten+/+ mice on the high-fat diet. In stark contrast, mckPten−/− mice maintained of fasting glucose levels similar to those of chow-fed mice (Fig. 2A). Despite being on a high-fat diet, mckPten−/− mice maintained insulin sensitivity similar to that of chow-fed mice as measured by the insulin tolerance test (Fig. 2B). Furthermore, the mckPten−/− mice fed a high-fat diet maintained glucose levels similar to those in mice on chow diet after an intraperitoneal glucose challenge, whereas mckPten+/+ control mice developed glucose intolerance (Fig. 2C). These results show that muscle-specific deletion of Pten has a protective effect against development of insulin resistance and diabetes in mice.
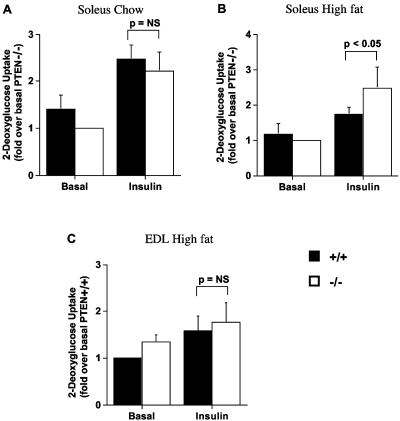
Insulin-stimulated glucose uptake into soleus muscle is improved in mckPten−/− mice fed a high-fat diet.
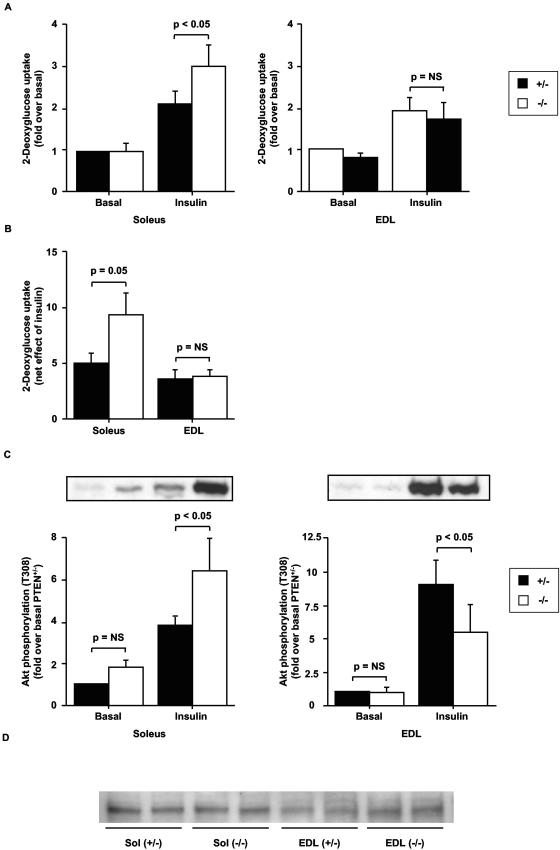
To assess whether the enhanced insulin sensitivity in the mckPten−/− mice on a high-fat diet is the result of higher glucose uptake into muscle, we measured basal and insulin-stimulated 2-deoxyglucose uptake into isolated soleus muscles of 6-month-old mckPten+/+ and mckPten−/− mice fed a high-fat or chow diets. When fed normal chow, mckPten+/+ and mckPten−/− mice had similar levels of basal (mckPten+/+, 7.3 ± 1.2 pmol/mg of muscle/20 min; mckPten−/−, 5.3 ± 0.4 pmol/mg of muscle/20 min [P = NS]) and insulin-stimulated (mckPten+/+, 13.1 ± 1.4 pmol/mg of muscle/20 min; mckPten−/−, 11.6 ± 1.6 pmol/mg of muscle/20 min [P = NS]) 2-deoxyglucose uptake into soleus muscle (Fig. 3A). When fed a high-fat diet, mckPten+/+ and mckPten−/− mice had similar levels of basal 2-deoxyglucose uptake into soleus muscle (mckPten+/+, 2.2 ± 1.1 pmol/mg of muscle/20 min; mckPten−/−, 1.9 ± 1.0 pmol/mg of muscle/20 min)). However, insulin-stimulated 2-deoxyglucose uptake was significantly elevated in soleus muscles of mckPten−/− mice (4.0 ± 1.7 pmol/mg of muscle/20 min) compared to their wild-type littermates (3.1 ± 1.6 pmol/mg of muscle/20 min) (P < 0.05 by ANOVA) (Fig. 3B). In contrast, both basal (mckPten+/+, 1.69 ± 0.84 pmol/mg of muscle/20 min; mckPten−/−, 2.03 ± 0.87 pmol/mg of muscle/20 min [P = NS]) and insulin-stimulated (mckPten+/+, 2.49 ± 1.26 pmol/mg of muscle/20 min; mckPten−/−, 2.35 ± 1.09 pmol/mg of muscle/20 min [P = NS]) glucose uptake into EDL muscle was not significantly different in high-fat-fed mckPten+/+ and mckPten−/− mice (Fig. 3C). These results suggest that the improved glucose homeostasis observed in mckPten−/−mice on a high-fat diet may be the result of increased insulin-stimulated glucose uptake primarily into slow-twitch oxidative fibers.
FIG. 3.
Enhanced insulin-stimulated 2-deoxyglucose uptake into isolated soleus muscles of high-fat-fed mckPten−/− mice. (A and B) Soleus or (C) EDL muscles isolated from 6-month-old mice fed (A) chow or (B and C) high fat were incubated with or without 2 mU of insulin per ml for 30 min. 2-Deoxyglucose uptake was measured over 20 min. Results are the means ± standard errors from four independent experiments. All values are normalized relative to the lowest 2-deoxyglucose uptake value in order to express all values as greater than one.
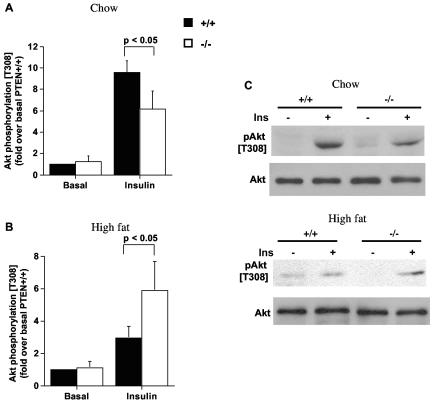
Enhanced insulin-stimulated Akt phosphorylation in soleus muscles of mckPten−/− mice fed a high-fat diet.
Since Akt is downstream of PI3K and is implicated in insulin-stimulated glucose uptake, the insulin-stimulated phosphorylation status of Akt in the soleus muscles of both groups of mice on chow or high-fat diets was determined. On chow diet, insulin-stimulated Akt phosphorylation was significantly reduced in mckPten−/− mice (6.1- ± 1.7-fold) compared to mckPten+/+ mice (9.6- ± 1.1-fold) (P < 0.05 by ANOVA) (Fig. 4A and C). As shown above, this reduction in Akt phosphorylation did not translate into a significant decrease in 2-deoxyglucose uptake during chow feeding, possibly because the residual Akt activity is sufficient to support insulin-stimulated glucose uptake (48). Consistent with the glucose uptake results for mice fed a high-fat diet, insulin-stimulated Akt phosphorylation in these mice was significantly higher in mckPten−/− mice (5.9- ± 1.8-fold) than in mckPten+/+ mice (3.0- ± 0.7-fold) (P < 0.05 by ANOVA) (Fig. 4B and C). These results indicate that impaired insulin signaling to Akt in muscles of mice fed a high-fat diet can be improved by reducing Pten expression.
FIG. 4.
Enhanced insulin-stimulated Akt phosphorylation in isolated soleus muscles of high-fat-fed mckPten−/− mice. Soleus muscles isolated from 6-month-old mice fed (A) chow or (B) high fat were incubated with or without 2 mU of insulin per ml for 10 min. (C) Lysates (30 μg) were resolved by SDS-10% PAGE and immunoblotted with anti-phospho-T308 Akt or anti-pan-Akt antibody. Representative immunoblots are shown. Immunoblots were scanned within the linear range and quantified with NIH Image J software. The quantified values represent the means ± standard errors from five independent experiments. All values are normalized relative to basal Akt phosphorylation in mckPten+/+ mice.
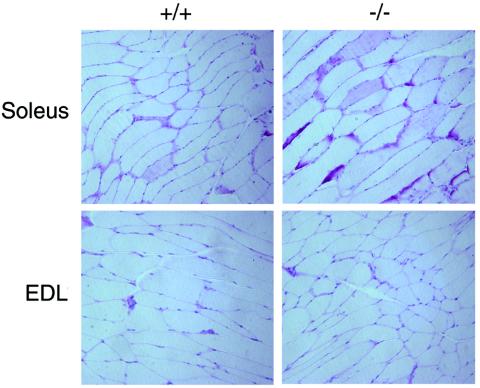
To determine whether the enhanced 2-deoxyglucose uptake and enhanced whole-body insulin sensitivity as determined by the insulin tolerance test resulted in enhanced glycogen synthesis in muscle, mice fasted overnight to achieve glycogen depletion were injected with an intraperitoneal glucose load (1g/kg), and glucose uptake was stimulated with insulin (1.5 U/kg). At 3 h after glucose and insulin injection, soleus and EDL muscles were isolated and stained for glycogen by PAS staining as described in Materials and Methods. Consistent with the findings of in vivo dynamic testing as well as in vitro results from isolated muscles, there was an enhanced glycogen presence in soleus muscles of mckPten−/− mice compared to those of mckPten+/+ mice (Fig. 5, upper row). On the other hand, there were no significant differences in the amount of glycogen present in the EDL muscles between the two genotypes following glucose and insulin injection (Fig. 5, lower row). There were no differences in the degree of PAS staining between the genotypes in both EDL and soleus muscles following an overnight fast (data not shown). These data suggest that slow-twitch muscles show enhanced glycogen synthesis upon glucose and insulin stimulation in mckPten−/− mice.
FIG. 5.
Increased glycogen as measured by PAS staining in soleus muscles of mckPten−/− mice compared to mckPten+/+ mice. There is no difference in PAS staining in EDL muscles between the two genotypes. Overnight-fasted mice (high-fat fed, 6 months old) were injected with glucose (1 g/kg) and insulin (1.5 U/kg) and sacrificed at 3 h postinjection.
Protection from diabetes development continues in aged mckPten−/− mice on a high-fat diet without adverse tumor development.
In young mice up to 6 months in age, insulin and glucose tolerance tests were not statistically different in mckPten+/− (Ptenfl/+ cre+) and mckPten−/− mice fed a high-fat diet (data not shown), whereas differences were seen between mckPten−/− and mckPten+/+ mice, suggesting a gene dosage effect. Insulin sensitivity declines with age in both rodents and humans (12, 28). Therefore, we examined whether deletion of muscle Pten continues to give a protective effect against insulin resistance in aged mice fed a high-fat diet. Furthermore, given that Pten is a well-known tumor suppressor with antiproliferative and survival properties in many tissues, we examined whether a continued metabolic benefit would exist without any deleterious effect, such as tumor development. For this purpose, we examined metabolic parameters in mckPten−/− mice on a prolonged diet of high fat up to 15 months of age. For these aged mice, we examined mckPten+/− mice rather than mckPten+/+ mice as controls to determine whether the improvement in metabolic parameters can be elicited with half a gene dosage of Pten.
The body weights of older mckPten−/− mice fed a normal chow or high-fat diet did not differ from those of mckPten+/− littermates (data not shown). Similarly after a high-fat diet, the epididymal fat mass was similar in mckPten+/− (3.3 ± 0.6 g) and mckPten−/− (3.4 ± 0.9 g) mice (P = 0.97) at 15 months of age. Despite the parallel gain in body weight and fat mass, mckPten−/− mice remained consistently more insulin sensitive than mckPten+/− mice on a prolonged high-fat diet, as measured by the insulin tolerance test (Fig. 6A). Furthermore, these older mckPten−/− mice fed a high-fat diet had lower glucose levels upon glucose challenge (Fig. 6B). In addition, the prolonged protective metabolic effect in mckPten−/− mice continued without gross or histologic development of tumors.
FIG. 6.
Continued protection from insulin resistance and diabetes in older mckPten−/− mice on prolonged high-fat feeding. (A) Insulin tolerance tests were performed on 15-month-old mckPten+/− and mckPten−/− mice on a chow or high-fat diet as described in Materials and Methods. (B) Glucose tolerance tests were performed on 15-month-old mckPten+/− and mckPten−/− mice on a chow or high-fat diet as described in Materials and Methods. * and **, P < 0.01 and P < 0.1, respectively, comparing mckPten+/− and mckPten−/− mice on a high-fat diet. All results are expressed as means ± standard errors of the means from at least 10 animals of each genotype.
To confirm that the preserved insulin sensitivity in the aged mckPten−/− mice on a high-fat diet was associated with improved glucose uptake into muscle fibers, we assessed basal and insulin-stimulated 2-deoxyglucose uptake into soleus and EDL muscles isolated from 15-month-old mckPten−/− or mckPten+/− mice fed a high-fat diet. In the slow-twitch oxidative soleus muscle, basal 2-deoxyglucose uptake was similar in mckPten+/− (4.9 ± 1.0 pmol/mg of muscle/20 min) and mckPten−/− (4.6 ± 0.6 μmol/mg of muscle/20 min) mice (Fig. 7A). However, insulin-stimulated 2-deoxyglucose uptake was significantly higher in the mckPten−/− mice (13.9 ± 2.4 μmol/mg of muscle/20 min) than in their mckPten+/− littermates (9.8 ± 1.4 pmol/mg of muscle/20 min) (P < 0.05 by ANOVA) (Fig. 7B). These findings further indicate that the improved glucose homeostasis observed in aged mckPten−/− mice results from increased insulin-mediated glucose disposal in skeletal muscles. However, similar to findings for younger mice, in the fast-twitch EDL muscle, neither basal (mckPten+/−, 3.3 ± 0.5 pmol/mg of muscle/20 min; mckPten−/−, 4.9 ± 1.3 μmol/mg of muscle/20 min [NS]) nor insulin-stimulated (mckPten+/−, 8.5 ± 1.7 pmol/mg of muscle/20 min; mckPten−/−, 6.9 ± 0.5 μmol/mg of muscle/20 min [NS]) 2-deoxyglucose uptake differed significantly between heterozygous and homozygous mice (Fig. 7A). These findings suggest that the improved glucose homeostasis observed in the mckPten−/− mice may be due to increased insulin-mediated glucose disposal preferentially into slow-twitch, oxidative fiber-containing skeletal muscles.
FIG.7.
Insulin-stimulated 2-deoxyglucose uptake into isolated soleus muscle and Akt phosphorylation in prolonged high-fat feeding. (A) Soleus (left panel) or EDL (right panel) muscles isolated from 15-month-old mice fed a high-fat diet were incubated with or without 2 mU of insulin per ml for 30 min. 2-Deoxyglucose uptake was determined over 20 min. Results are the means ± standard errors from five independent experiments. (B) Net insulin effect on 2-deoxyglucose uptake in both muscles. (C) Soleus (left panel) or EDL (right panel) muscles isolated from 15-month-old mice fed a high-fat diet were incubated with or without 2 mU of insulin per ml for 10 min. Lysates (30 μg) were resolved by SDS-10% PAGE and immunoblotted with anti-phospho-T308 Akt-antibody. Representative immunoblots are shown (upper panels). Immunoblots were scanned within the linear range and quantified with the NIH image computer software. The quantified values (lower panels) represent the means ± standard errors from five experiments. All values are expressed relative to basal Akt phosphorylation in mckPten+/− mice, which was assigned a value of 1. (D) Lysates (25 μg) of isolated muscles were resolved by SDS-10% PAGE and immunoblotted with anti-Akt. A representative immunoblot is shown.
Akt phosphorylation in soleus and EDL muscles of 15-month-old mice fed a high-fat diet was assessed. Consistent with the effect of Pten deletion in muscle on glucose uptake, insulin-stimulated Akt phosphorylation was elevated in the soleus muscles (mckPten+/−, 3.8- ± 0.4-fold; mckPten−/−, 6.4- ± 1.5-fold [P < 0.05 by ANOVA]) but not in the EDL muscles (mckPten+/−, 9.0- ± 1.7-fold; mckPten−/−, 5.4- ± 2.0-fold [P < 0.05 by ANOVA]) of mckPten−/− mice compared to their heterozygous littermates (Fig. 7C).
Protection from hyperinsulinemia and β-cell hypertrophy.
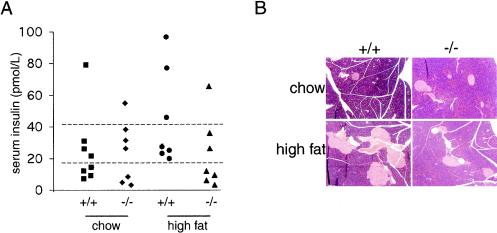
In order to prevent the progression of insulin resistance to overt diabetes, the capacity of pancreatic β cells to mount a hyperinsulinemic response to meet the increased demand for insulin is crucial. Typically, insulin resistance elicits hypertrophy or hyperplasia of β cells and increased serum insulin levels. We therefore examined whether β-cell compensation is alleviated in the more insulin-sensitive mckPten−/− mice when they are given a high-fat diet. Fasting insulin levels in the sera of 6-month-old mckPten−/− and mckPten+/+ mice fed a normal chow and a high-fat diet were measured. In keeping with the notion that preserved insulin sensitivity would spare mckPten−/− mice from mounting a hyperinsulinemic response, a higher proportion of the mckPten−/− mice had lower fasting plasma insulin levels than control littermates (Fig. 8A).
FIG. 8.
Protection from hyperinsulinemia and islet hyperplasia in high-fat-fed mckPten−/− mice. (A) Fasting plasma insulin levels. After an overnight fast, plasma insulin levels in venous blood samples from 6-month-old mckPten+/+ and mckPten−/− mice fed a chow (left two columns) or high-fat diet (right two columns) were determined. Dashed lines represent arbitrary cutoff levels for comparative analysis. (B) Sparing of islet hyperplasia in mckPten−/− mice fed a high-fat diet. Representative hematoxylin- and eosin-stained histological sections of pancreata from 6-month-old mckPten+/+ and mckPten−/− mice fed a chow or high-fat diet are shown.
Consistent with the higher serum insulin levels, pancreatic sections of mckPten+/+ mice fed a high-fat diet revealed a higher degree of islet hyperplasia and hypertrophy (Fig. 8B), suggesting β-cell neogenesis and proliferation in response to insulin resistance. In contrast, mckPten−/− mice fed a high-fat diet showed a lower number of islets that were significantly smaller, similar to islets in mice of either genotype fed a chow diet. There was no significant difference in the islet morphology between the mckPten+/+ and mckPten−/− mice on a chow diet (Fig. 8B).
These results show that Pten deletion in muscle protects against the proliferative demand on the β cells as a consequence of enhanced insulin sensitivity. This proliferative demand is an additional contributing factor to disease progression in type 2 diabetes. Pten deletion in muscle gave protection against insulin resistance and diabetes without development of muscle tumors or other deleterious effects in the high-fat and age-induced diabetes model.
DISCUSSION
The PI3K pathway plays a major role in insulin-stimulated glucose uptake (6, 14, 19, 44, 46). Previous studies using 3T3-L1 adipocytes overexpressing Pten showed a reduction in insulin-stimulated glucose uptake (29, 31). Moreover, db/db mice were protected from developing diabetes when Pten was specifically inhibited by using an antisense oligonucleotide strategy by lowering Pten levels primarily in liver and fat (5). Despite this evidence supporting the modulatory role of Pten in insulin action, the specific role of insulin signaling in muscle that contributes to development of insulin resistance and diabetes remains elusive. We report here that muscle-specific deletion of Pten prevented the development of insulin resistance and diabetes induced by feeding a high-fat diet to mice (Fig. 2 and 4). Reflecting the preserved insulin sensitivity, islet hyperplasia-hypertrophy and hyperinsulinemia were absent in mckPten−/− mice, whereas their control littermates had hypertrophic and hyperplastic islets and were hyperinsulinemic (Fig. 8).
In both age groups of high-fat-fed mice, muscle-specific deletion of Pten preserved insulin-stimulated glucose uptake in slow-twitch, oxidative soleus muscle but not in fast-twitch EDL muscle (Fig. 3 and 5). Similarly, insulin-stimulated Akt phosphorylation was enhanced in soleus but not EDL muscles from mckPten−/− mice compared to heterozygous littermates. The distinct responses might reflect the differences in fiber type composition of the two muscle types: mouse soleus muscle has a much higher content of slow-twitch, oxidative type I fibers (approximately 40 to 50%) (45), whereas mouse EDL muscle is composed mainly of fast-twitch, glycolytic type IIB fibers (36). Moreover, slow-twitch, oxidative fibers are more insulin sensitive and show higher insulin-stimulated glucose uptake than fast-twitch, glycolytic muscle fibers (25, 50). Muscles composed mainly of type I fibers are more susceptible to high-fat-feeding-induced insulin resistance than type 2 fiber-containing muscles (50; D. Konrad and A. Klip, unpublished observations). Thus, one could argue that high-fat feeding and aging affect mainly soleus muscle and not EDL muscle, and therefore, only deletion of Pten in the insulin-resistant muscle had a compensatory effect. Consistent with this tenet, insulin-stimulated PI3K activity is reduced in soleus but not in EDL muscles in high-fat-fed mice (50). In mckPten−/− mice, this defect is rescued by the absence of Pten, presumably by improved glucose uptake into oxidative muscle. Interestingly, a recent study reemphasizes the importance of reduced mitochondrial oxidative capacity in the pathogenesis of insulin resistance in elderly people (32). We report here the occurrence of insulin resistance mainly in the more oxidative soleus muscle and not in the glycolytic EDL muscle.
Even though skeletal muscle plays an important role in glucose homeostasis under physiological conditions (11), its functional contribution to insulin resistance and type 2 diabetes was recently questioned by the finding that muscle-specific deletion of the insulin receptor did not result in whole-body insulin resistance and diabetes (4). Possible explanations offered included insulin signaling via the insulin-like growth factor-1 receptor and glucose uptake by non-insulin-dependent pathways (22). On the other hand, insulin resistance in skeletal muscle is a consistent finding in type 2 diabetes patients (25). Moreover, several studies report the occurrence of insulin resistance in skeletal muscle as an early event in the development of type 2 diabetes mellitus (47). Our findings further support the concept that alterations in skeletal muscle play at least a permissive role in the development of type 2 diabetes.
It is well documented that insulin sensitivity declines with age, and several factors have been implicated in this decline (28). Here, we show that deletion of muscle Pten gives prolonged protection against insulin resistance and diabetes even in aged mice fed a prolonged high-fat diet, without the deleterious effects often expected with Pten inactivation. This result highlights the ability of Pten to potently regulate different cellular processes in a highly context-dependent manner, tailoring regulatory effects to different cell types. In muscle, a major function of Pten appears to be metabolic regulation of insulin action. In keeping with these results, polymorphism in the 5′-untranslated region of the Pten gene has been found in a diabetic cohort in Japan. This polymorphism was associated with an increased expression level of Pten and decreased phosphorylation of Akt upon stimulation with insulin in an experimental cell line model (16).
Negative regulators of insulin signaling are attractive targets for the development of new therapeutic approaches to treat insulin resistance and diabetes mellitus. PTP-1B directly interacts with and dephosphorylates activated insulin receptors. PTP-1B knockout mice are protected from obesity and insulin resistance, possibly through enhancing effects on leptin signaling in liver (7, 49). Lipid phosphatase SHIP2 has also been shown to modulate insulin action. SHIP2 deletion leads to increased insulin sensitivity associated with severe perinatal hypoglycemia and death, whereas adult mice heterozygous for the SHIP2 mutation have better glucose tolerance and insulin sensitivity (9). However, in these genetic models, it is unclear which insulin target organs contribute primarily to diabetes protection. Since these genetic models were whole-body knockouts, the specific contribution of each insulin-sensitive organ to whole-body glucose control could not be assessed. We show here that amelioration of insulin signaling in skeletal muscle via deletion of Pten in muscle is able to improve the control of whole-body glucose.
Selective Pten deletion in skeletal muscle protects against the development of fat- and age-dependent insulin resistance and diabetes without the development of cancer. Here we show that deletion of Pten in muscle affects metabolism without affecting other facets of Pten physiology that have been shown to be important in other tissues, such as hypertrophy or proliferation. We thus show the exquisite specificity of Pten for tissue type and show mckPten−/− mice to be a good model to study the effect of muscle insulin signaling in insulin resistance. Pten in muscle is a promising therapeutic target to overcome insulin resistance and ameliorate glucose homeostasis in type 2 diabetes.
Acknowledgments
This work was supported by grants to M.W. from the Canadian Institutes of Health Research (OP 57893) and the Banting and Best Diabetes Centre and to A.K. from the Earl DuHane Grant from the Canadian Diabetes Association. M.W. is supported by a Canadian Institutes of Health Research New Investigator Award. D.K. was supported by a fellowship from the Swiss National Science Foundation (grant 81ZH-57433), the Research Institute of The Hospital for Sick Children (Clinician Scientist Award), and the Züricher Diabetes-Gesellschaft.
We thank Paul Dogherty, Armen Manoukian, Stephanie Backman, and Kinh Tung Nguyen for critical editing of the manuscript and helpful discussions. We also thank Xudong Xhu, Kelvin So, and Michelle Sleiman for their technical assistance. We thank Lifescan Johnson and Johnson for providing glucometer strips.
REFERENCES
- 1.Backman, S., V. Stambolic, and T. Mak. 2002. Pten function in mammalian cell size regulation. Curr. Opin. Neurobiol. 12:516-522. [DOI] [PubMed] [Google Scholar]
- 2.Backman, S. A., D. Ghazarian, K. So, O. Sanchez, K. U. Wagner, L. Hennighausen, A. Suzuki, M. S. Tsao, W. B. Chapman, V. Stambolic, and T. W. Mak. 2004. Early onset of neoplasia in the prostate and skin of mice with tissue-specific deletion of Pten. Proc. Natl. Acad. Sci. USA 101:1725-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Backman, S. A., V. Stambolic, A. Suzuki, J. Haight, A. Elia, J. Pretorius, M. S. Tsao, P. Shannon, B. Bolon, G. O. Ivy, and T. W. Mak. 2001. Deletion of Pten in mouse brain causes seizures, ataxia and defects in soma size resembling Lhermitte-Duclos disease. Nat. Genet. 29:396-403. [DOI] [PubMed] [Google Scholar]
- 4.Bruning, J. C., M. D. Michael, J. N. Winnay, T. Hayashi, D. Horsch, D. Accili, L. J. Goodyear, and C. R. Kahn. 1998. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol. Cell 2:559-569. [DOI] [PubMed] [Google Scholar]
- 5.Butler, M., R. A. McKay, I. J. Popoff, W. A. Gaarde, D. Witchell, S. F. Murray, N. M. Dean, S. Bhanot, and B. P. Monia. 2002. Specific inhibition of Pten expression reverses hyperglycemia in diabetic mice. Diabetes 51:1028-1034. [DOI] [PubMed] [Google Scholar]
- 6.Cheatham, B., C. J. Vlahos, L. Cheatham, L. Wang, J. Blenis, and C. R. Kahn. 1994. Phosphatidylinositol 3-kinase activation is required for insulin stimulation of pp70 S6 kinase, DNA synthesis, and glucose transporter translocation. Mol. Cell. Biol. 14:4902-4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng, A., N. Uetani, P. D. Simoncic, V. P. Chaubey, A. Lee-Loy, C. J. McGlade, B. P. Kennedy, and M. L. Tremblay. 2002. Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1B. Dev. Cell 2:497-503. [DOI] [PubMed] [Google Scholar]
- 8.Cho, H., J. Mu, J. K. Kim, J. L. Thorvaldsen, Q. Chu, E. B. Crenshaw III, K. H. Kaestner, M. S. Bartolomei, G. I. Shulman, and M. J. Birnbaum. 2001. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 292:1728-1731. [DOI] [PubMed] [Google Scholar]
- 9.Clement, S., U. Krause, F. Desmedt, J. F. Tanti, J. Behrends, X. Pesesse, T. Sasaki, J. Penninger, M. Doherty, W. Malaisse, J. E. Dumont, Y. Le Marchand-Brustel, C. Erneux, L. Hue, and S. Schurmans. 2001. The lipid phosphatase SHIP2 controls insulin sensitivity. Nature 409:92-97. [DOI] [PubMed] [Google Scholar]
- 10.Crackower, M. A., G. Y. Oudit, I. Kozieradzki, R. Sarao, H. Sun, T. Sasaki, E. Hirsch, A. Suzuki, T. Shioi, J. Irie-Sasaki, R. Sah, H. Y. Cheng, V. O. Rybin, G. Lembo, L. Fratta, A. J. Oliveira-dos-Santos, J. L. Benovic, C. R. Kahn, S. Izumo, S. F. Steinberg, M. P. Wymann, P. H. Backx, and J. M. Penninger. 2002. Regulation of myocardial contractility and cell size by distinct PI3K-Pten signaling pathways. Cell 110:737-749. [DOI] [PubMed] [Google Scholar]
- 11.DeFronzo, R. A., R. Gunnarsson, O. Bjorkman, M. Olsson, and J. Wahren. 1985. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J. Clin. Investig. 76:149-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fink, R. I., P. Wallace, and J. M. Olefsky. 1986. Effects of aging on glucose-mediated glucose disposal and glucose transport. J. Clin. Investig. 77:2034-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gil, E. B., E. Malone Link, L. X. Liu, C. D. Johnson, and J. A. Lees. 1999. Regulation of the insulin-like developmental pathway of Caenorhabditis elegans by a homolog of the Pten tumor suppressor gene. Proc. Natl. Acad. Sci. USA 96:2925-2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hara, K., K. Yonezawa, H. Sakaue, A. Ando, K. Kotani, T. Kitamura, Y. Kitamura, H. Ueda, L. Stephens, T. R. Jackson, et al. 1994. 1-Phosphatidylinositol 3-kinase activity is required for insulin-stimulated glucose transport but not for RAS activation in CHO cells. Proc. Natl. Acad. Sci. USA 91:7415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horie, Y., A. Suzuki, E. Kataoka, T. Sasaki, K. Hamada, J. Sasaki, K. Mizuno, G. Hasegawa, H. Kishimoto, M. Iizuka, M. Naito, K. Enomoto, S. Watanabe, T. W. Mak, and T. Nakano. 2004. Hepatocyte-specific Pten deficiency results in steatohepatitis and hepatocellular carcinomas. J. Clin. Investig. 113:1774-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishihara, H., T. Sasaoka, S. Kagawa, S. Murakami, K. Fukui, Y. Kawagishi, K. Yamazaki, A. Sato, M. Iwata, M. Urakaze, M. Ishiki, T. Wada, S. Yaguchi, H. Tsuneki, I. Kimura, and M. Kobayashi. 2003. Association of the polymorphisms in the 5′-untranslated region of Pten gene with type 2 diabetes in a Japanese population. FEBS Lett. 554:450-454. [DOI] [PubMed] [Google Scholar]
- 17.Jiang, Z. Y., Q. L. Zhou, K. A. Coleman, M. Chouinard, Q. Boese, and M. P. Czech. 2003. Insulin signaling through Akt/protein kinase B analyzed by small interfering RNA-mediated gene silencing. Proc. Natl. Acad. Sci. USA 100:7569-7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kahn, C. R. 2003. Knockout mice challenge our concepts of glucose homeostasis and the pathogenesis of diabetes. Exp. Diabesity Res. 4:169-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katagiri, H., T. Asano, H. Ishihara, K. Inukai, Y. Shibasaki, M. Kikuchi, Y. Yazaki, and Y. Oka. 1996. Overexpression of catalytic subunit p110alpha of phosphatidylinositol 3-kinase increases glucose transport activity with translocation of glucose transporters in 3T3-L1 adipocytes. J. Biol. Chem. 271:16987-16990. [DOI] [PubMed] [Google Scholar]
- 20.Katome, T., T. Obata, R. Matsushima, N. Masuyama, L. C. Cantley, Y. Gotoh, K. Kishi, H. Shiota, and Y. Ebina. 2003. Use of RNA interference-mediated gene silencing and adenoviral overexpression to elucidate the roles of AKT/protein kinase B isoforms in insulin actions. J. Biol. Chem. 278:28312-28323. [DOI] [PubMed] [Google Scholar]
- 21.Kishimoto, H., K. Hamada, M. Saunders, S. Backman, T. Sasaki, T. Nakano, T. W. Mak, and A. Suzuki. 2003. Physiological functions of Pten in mouse tissues. Cell Struct. Funct. 28:11-21. [DOI] [PubMed] [Google Scholar]
- 22.Kitamura, T., C. R. Kahn, and D. Accili. 2003. Insulin receptor knockout mice. Annu. Rev. Physiol. 65:313-332. [DOI] [PubMed] [Google Scholar]
- 23.Kruszynska, Y. T., and J. M. Olefsky. 1996. Cellular and molecular mechanisms of non-insulin dependent diabetes mellitus. J. Investig. Med. 44:413-428. [PubMed] [Google Scholar]
- 24.Li, J., C. Yen, D. Liaw, K. Podsypanina, S. Bose, S. I. Wang, J. Puc, C. Miliaresis, L. Rodgers, R. McCombie, S. H. Bigner, B. C. Giovanella, M. Ittmann, B. Tycko, H. Hibshoosh, M. H. Wigler, and R. Parsons. 1997. Pten, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 275:1943-1947. [DOI] [PubMed] [Google Scholar]
- 25.Lillioja, S., A. A. Young, C. L. Culter, J. L. Ivy, W. G. Abbott, J. K. Zawadzki, H. Yki-Jarvinen, L. Christin, T. W. Secomb, and C. Bogardus. 1987. Skeletal muscle capillary density and fiber type are possible determinants of in vivo insulin resistance in man. J. Clin. Investig. 80:415-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mauvais-Jarvis, F., R. N. Kulkarni, and C. R. Kahn. 2002. Knockout models are useful tools to dissect the pathophysiology and genetics of insulin resistance. Clin. Endocrinol. 57:1-9. [DOI] [PubMed] [Google Scholar]
- 27.Mihaylova, V. T., C. Z. Borland, L. Manjarrez, M. J. Stern, and H. Sun. 1999. The Pten tumor suppressor homolog in Caenorhabditis elegans regulates longevity and dauer formation in an insulin receptor-like signaling pathway. Proc. Natl. Acad. Sci. USA 96:7427-7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miles, P. D., Y. Barak, R. M. Evans, and J. M. Olefsky. 2003. Effect of heterozygous PPARgamma deficiency and TZD treatment on insulin resistance associated with age and high-fat feeding. Am. J. Physiol. Endocrinol. Metab. 284:E618-E626. [DOI] [PubMed] [Google Scholar]
- 29.Nakashima, N., P. M. Sharma, T. Imamura, R. Bookstein, and J. M. Olefsky. 2000. The tumor suppressor Pten negatively regulates insulin signaling in 3T3-L1 adipocytes. J. Biol. Chem. 275:12889-12895. [DOI] [PubMed] [Google Scholar]
- 30.Ogg, S., and G. Ruvkun. 1998. The C. elegans Pten homolog, DAF-18, acts in the insulin receptor-like metabolic signaling pathway. Mol. Cell 2:887-893. [DOI] [PubMed] [Google Scholar]
- 31.Ono, H., H. Katagiri, M. Funaki, M. Anai, K. Inukai, Y. Fukushima, H. Sakoda, T. Ogihara, Y. Onishi, M. Fujishiro, M. Kikuchi, Y. Oka, and T. Asano. 2001. Regulation of phosphoinositide metabolism, Akt phosphorylation, and glucose transport by Pten (phosphatase and tensin homolog deleted on chromosome 10) in 3T3-L1 adipocytes. Mol. Endocrinol. 15:1411-1422. [DOI] [PubMed] [Google Scholar]
- 32.Petersen, K. F., D. Befroy, S. Dufour, J. Dziura, C. Ariyan, D. L. Rothman, L. DiPietro, G. W. Cline, and G. I. Shulman. 2003. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300:1140-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rudich, A., D. Konrad, D. Torok, R. Ben-Romano, C. Huang, W. Niu, R. R. Garg, N. Wijesekara, R. J. Germinario, P. J. Bilan, and A. Klip. 2003. Indinavir uncovers different contributions of GLUT4 and GLUT1 towards glucose uptake in muscle and fat cells and tissues. Diabetologia 46:649-658. [DOI] [PubMed] [Google Scholar]
- 34.Scheid, M. P., and J. R. Woodgett. 2001. PKB/AKT: functional insights from genetic models. Nat. Rev. Mol. Cell. Biol. 2:760-768. [DOI] [PubMed] [Google Scholar]
- 35.Somwar, R., M. Perreault, S. Kapur, C. Taha, G. Sweeney, T. Ramlal, D. Y. Kim, J. Keen, C. H. Cote, A. Klip, and A. Marette. 2000. Activation of p38 mitogen-activated protein kinase alpha and beta by insulin and contraction in rat skeletal muscle: potential role in the stimulation of glucose transport. Diabetes 49:1794-1800. [DOI] [PubMed] [Google Scholar]
- 36.Song, X. M., J. W. Ryder, Y. Kawano, A. V. Chibalin, A. Krook, and J. R. Zierath. 1999. Muscle fiber type specificity in insulin signal transduction. Am. J. Physiol. 277:R1690-R1696. [DOI] [PubMed] [Google Scholar]
- 37.Stambolic, V., A. Suzuki, J. L. de la Pompa, G. M. Brothers, C. Mirtsos, T. Sasaki, J. Ruland, J. M. Penninger, D. P. Siderovski, and T. W. Mak. 1998. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor Pten. Cell 95:29-39. [DOI] [PubMed] [Google Scholar]
- 38.Stambolic, V., M. S. Tsao, D. Macpherson, A. Suzuki, W. B. Chapman, and T. W. Mak. 2000. High incidence of breast and endometrial neoplasia resembling human Cowden syndrome in pten+/− mice. Cancer Res. 60:3605-3611. [PubMed] [Google Scholar]
- 39.Steck, P. A., M. A. Pershouse, S. A. Jasser, W. K. Yung, H. Lin, A. H. Ligon, L. A. Langford, M. L. Baumgard, T. Hattier, T. Davis, C. Frye, R. Hu, B. Swedlund, D. H. Teng, and S. V. Tavtigian. 1997. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat. Genet. 15:356-362. [DOI] [PubMed] [Google Scholar]
- 40.Stiles, B., Y. Wang, A. Stahl, S. Bassilian, W. P. Lee, Y. J. Kim, R. Sherwin, S. Devaskar, R. Lesche, M. A. Magnuson, and H. Wu. 2004. Liver-specific deletion of negative regulator Pten results in fatty liver and insulin hypersensitivity. Proc. Natl. Acad. Sci. USA 101:2082-2087. (Erratum, 101:5180.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki, A., J. L. de la Pompa, V. Stambolic, A. J. Elia, T. Sasaki, I. del Barco Barrantes, A. Ho, A. Wakeham, A. Itie, W. Khoo, M. Fukumoto, and T. W. Mak. 1998. High cancer susceptibility and embryonic lethality associated with mutation of the Pten tumor suppressor gene in mice. Curr. Biol. 8:1169-1178. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki, A., S. Itami, M. Ohishi, K. Hamada, T. Inoue, N. Komazawa, H. Senoo, T. Sasaki, J. Takeda, M. Manabe, T. W. Mak, and T. Nakano. 2003. Keratinocyte-specific Pten deficiency results in epidermal hyperplasia, accelerated hair follicle morphogenesis and tumor formation. Cancer Res. 63:674-681. [PubMed] [Google Scholar]
- 43.Suzuki, A., T. Kaisho, M. Ohishi, M. Tsukio-Yamaguchi, T. Tsubata, P. A. Koni, T. Sasaki, T. W. Mak, and T. Nakano. 2003. Critical roles of Pten in B cell homeostasis and immunoglobulin class switch recombination. J. Exp. Med. 197:657-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanti, J. F., T. Gremeaux, S. Grillo, V. Calleja, A. Klippel, L. T. Williams, E. Van Obberghen, and Y. Le Marchand-Brustel. 1996. Overexpression of a constitutively active form of phosphatidylinositol 3-kinase is sufficient to promote Glut 4 translocation in adipocytes. J. Biol. Chem. 271:25227-25232. [DOI] [PubMed] [Google Scholar]
- 45.Totsuka, Y., Y. Nagao, T. Horii, H. Yonekawa, H. Imai, H. Hatta, Y. Izaike, T. Tokunaga, and Y. Atomi. 2003. Physical performance and soleus muscle fiber composition in wild-derived and laboratory inbred mouse strains. J. Appl. Physiol. 95:720-727. [DOI] [PubMed] [Google Scholar]
- 46.Tsakiridis, T., M. Vranic, and A. Klip. 1994. Disassembly of the actin network inhibits insulin-dependent stimulation of glucose transport and prevents recruitment of glucose transporters to the plasma membrane. J. Biol. Chem. 269:29934-29942. [PubMed] [Google Scholar]
- 47.Vaag, A., J. E. Henriksen, and H. Beck-Nielsen. 1992. Decreased insulin activation of glycogen synthase in skeletal muscles in young nonobese Caucasian first-degree relatives of patients with non-insulin-dependent diabetes mellitus. J. Clin. Investig. 89:782-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang, Q., R. Somwar, P. J. Bilan, Z. Liu, J. Jin, J. R. Woodgett, and A. Klip. 1999. Protein kinase B/Akt participates in GLUT4 translocation by insulin in L6 myoblasts. Mol. Cell. Biol. 19:4008-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zabolotny, J. M., K. K. Bence-Hanulec, A. Stricker-Krongrad, F. Haj, Y. Wang, Y. Minokoshi, Y. B. Kim, J. K. Elmquist, L. A. Tartaglia, B. B. Kahn, and B. G. Neel. 2002. PTP1B regulates leptin signal transduction in vivo. Dev. Cell 2:489-495. [DOI] [PubMed] [Google Scholar]
- 50.Zierath, J. R., K. L. Houseknecht, L. Gnudi, and B. B. Kahn. 1997. High-fat feeding impairs insulin-stimulated GLUT4 recruitment via an early insulin-signaling defect. Diabetes 46:215-223. [DOI] [PubMed] [Google Scholar]
- 51.Zisman, A., O. D. Peroni, E. D. Abel, M. D. Michael, F. Mauvais-Jarvis, B. B. Lowell, J. F. Wojtaszewski, M. F. Hirshman, A. Virkamaki, L. J. Goodyear, C. R. Kahn, and B. B. Kahn. 2000. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat. Med. 6:924-928. [DOI] [PubMed] [Google Scholar]