Abstract
Background and objective:
The exploration for the etiology of osteonecrosis of the femoral head (ONFH) has got some promising findings, but the morbidity of ONFH is still not under control. The C3435T polymorphism of ATP-binding cassette subfamily B member 1 (ABCB1) gene has been reported to possess significant influence on ONFH onset, but relevant outcomes remain conflicting rather than conclusive. Therefore, a meta-analysis was useful to pool these results together for a more reliable conclusion.
Methods:
The association of ABCB1 C3435T polymorphism with ONFH susceptibility was estimated through calculated odds ratios (ORs) with their 95% confidence intervals (95% CIs). The Q-test was applied for detecting inter-study heterogeneity, whereas sensitivity analysis for identifying any study owning substantial influence on pooled results. Begg's funnel plot and Egger's test were employed to examine publication bias across included studies.
Results:
ABCB1 C3435T polymorphism significantly decreased the risk of ONFH under TT vs CC (OR = 0.26, 95% CI = 0.13–0.50), TT+CT vs CC (OR = 0.72, 95% CI = 0.52–0.99), TT vs CC+CT (OR = 0.28, 95% CI = 0.15–0.52), and T vs C (OR = 0.64, 95% CI = 0.50–0.81) contrasts.
Conclusion:
The variant C3435T in the ABCB1 gene may offer protection against the attack of ONFH, and more studies with larger sample sizes should be conducted to certify this issue.
Keywords: ATP-binding cassette subfamily B member 1, osteonecrosis of the femoral head, polymorphism
1. Introduction
Osteonecrosis of the femoral head (ONFH) refers to bony changes caused by osteocyte death under the effects of complicated factors, which lead to the fracture and collapse of subchondral bone and the changes in shape and functions of femoral head.[1,2] ONFH is generally classified into trauma-induced ONFH and nontraumatic ONFH, and the latter, also termed as aseptic necrosis of femoral head or avascular necrosis of femoral head, contains cause-specific and idiopathic ONFH.[3,4] At the moment, blood supply insufficiency in femoral head is regarded as one of the direct, major reasons for osteocyte apoptosis.[5] It is estimated that there are more than 20 million ONFH patients all over the world, and 5 to 7.5 million cases are Chinese people.[6] As for the estimates of new ONFH cases each year, there are about 15 to 20 thousand cases in America, 2.5 to 3 thousand in Japan, and 100 to 200 thousand in China.[7–9] Moreover, the morbidity rate of ONFH has shown a rising trend in the few past years. The etiology of ONFH has been extensively researched, and the majority of nontraumatic ONFH cases are associated with hormone use and alcohol consumption.[10] Besides, potential causes of idiopathic ONFH have been proposed, containing slipped capital femoral epiphysis (SCFE), decompression disease, systemic lupus erythematosus (SLE), fat embolism syndrome, tumor chemotherapy, organ transplantation, chronic liver diseases, gout, human immunodeficiency virus (HIV) infection, and other metabolic bone diseases.[11–15]
ATP-binding cassette subfamily B member 1 (ABCB1), or multidrug resistance protein 1 (MDR1) or P-glycoprotein 1 (P-gp) which is encoded by the ABCB1 gene, acts as an important protein pumping foreign substances out of cells, and having critical functions in drug absorption and distribution.[16] ABCB1 has become one of the most studied members in ABC family due to its production of multidrug resistance in tumor cells. ABCB1 expresses widely and possesses momentous physiological functions, so any defect in this protein, caused by the polymorphisms in the ABCB1 gene, may result in severe diseases or affect curative effects, such as Parkinson's disease, childhood acute lymphoblastic leukemia (ALL), ulcerative enteritis, and treatment effects on HIV.[17–19]
Certain correlations between ABCB1 polymorphisms and ONFH risk have been proposed, and various findings have been generated. In this meta-analysis, we collected previous studies estimating the association between ABCB1 C3435T polymorphism and ONFH susceptibility to systematically combine their results.
2. Materials and methods
2.1. Literature sources and searching strategy
This study was approved by the Ethics Committee of the Dalian University Affiliated Zhongshan Hospital.
Potentially relevant reports were identified up to September 1, 2015, from the electronic databases of PubMed, EMBASE, Wanfang, and CNKI, as well as from other sources. Searching strategy adopted the combination of key terms, including “osteonecrosis of the femoral head” or “avascular necrosis of femoral head,” “ATP-binding cassette subfamily B member 1” or “ABCB1” or “multidrug resistance protein 1” or “MDR1” or “P-glycoprotein 1” or “P-gp”, and “polymorphism” or “mutation” or “variant.” The references of relevant reports were manually filtrated for complementary publications.
2.2. Inclusion and exclusion criteria
Indispensable criteria for every included study were formulated in advance and contained the following aspects: (1) with a case-control design; (2) applying validated genotyping methods; (3) appraising the association between ABCB1 C3435T polymorphism and ONFH susceptibility; and (4) genotype distribution consistent with Hardy–Weinberg equilibrium in the control group. Excluded records embraced editorials, conference abstracts, review articles, and case-only studies.
2.3. Assessment of study quality
The Newcastle–Ottawa Scale (NOS) was employed to evaluate the quality of all selected studies. Based on 8 items from 3 dimensions of selection, comparability and exposure, each study could be given a score ranging from 0 to 9. In advance, we defined the quality of studies with a score between 7 and 9 as high, between 4 and 6 as medium, and between 0 and 3 as low. The grading was carried out by 2 reviewers separately, and they would finally reach consensus for each study through discussion if there were differences in study quality scores given by the 2 reviewers.
2.4. Data extraction
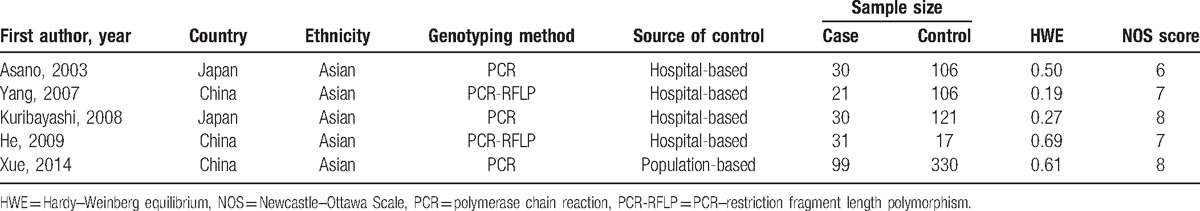
Two reviewers separately took charge of data extraction in accordance with the same tabulation for the main information of selected papers, and resolved all disagreements through discussion between them. The principal information drawn from eligible articles was comprised of first author's name, publication year, original country, ethnicity, numbers of cases and controls, genotype distribution in case and control groups, genotyping method, and P value for the Hardy–Weinberg equilibrium test in the control group.
2.5. Statistical analysis
The intensity of the association between ABCB1 C3435T polymorphism and ONFH susceptibility was assessed by calculated odds ratios (ORs) with their corresponding 95% confidence intervals (95% CIs). In the chi-square-based Q-test for inter-study heterogeneity examination, P < .05 or P ≥ .05 represented the presence or absence of significant heterogeneity, which ensured the choice of random- (DerSimonian–Laird method) or fixed-effects (Mantel–Haenszel method) model for ORs calculation. Sensitivity analysis was conducted through sequential omission of every included study to observe alterations in combined results. Publication bias across selected studies were detected with both Begg's funnel plot and Egger's linear regression test.[20,21]
3. Results
3.1. Study characteristics
A total of 92 potentially relevant publications were retrieved from the above databases, whereas 10 articles were identified from other sources, and 5 eligible articles were picked out through strict standards for inclusion and exclusion.[22–26] The flowchart for this meta-analysis exhibits detailed course of literature screening and particular reasons for exclusion (Fig. 1). Of the included studies, 4 had high quality whereas the other 1 had medium quality. Essential information of eligible reports is listed in Table 1.
Figure 1.
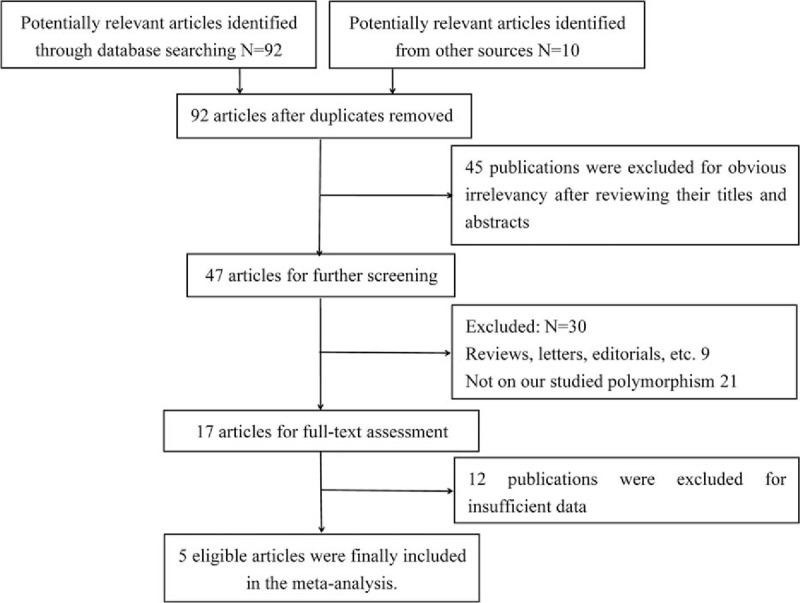
Flowchart for literature selection and exclusion reasons.
Table 1.
Essential information of 5 eligible articles.
3.2. Meta-analysis results
Corresponding results from data analyses are recorded in Table 2. A remarkable correlation of ABCB1 C3435T polymorphism can be found with reduced susceptibility to ONFH in 4 genetic models of TT vs CC (OR = 0.26, 95% CI = 0.13–0.50) (Fig. 2), TT+CT vs CC (OR = 0.72, 95% CI = 0.52–0.99) (Fig. 3), TT vs CC+CT (OR = 0.28, 95% CI = 0.15–0.52) (Fig. 4), and T vs C (OR = 0.64, 95% CI = 0.50–0.81) (Fig. 5).
Table 2.
Effects of ABCB1 C3435T polymorphism on the ONFH risk.
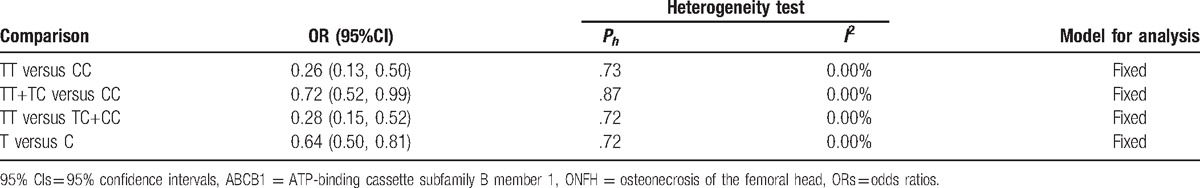
Figure 2.
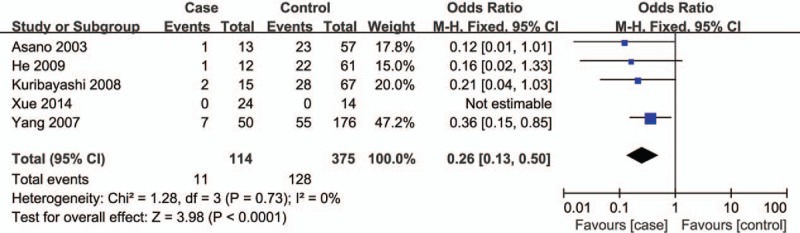
Forest plot for the association between ABCB1 C3435T polymorphism and ONFH susceptibility under TT vs CC contrast. ABCB1 = ATP-binding cassette subfamily B member 1, ONFH = osteonecrosis of the femoral head.
Figure 3.
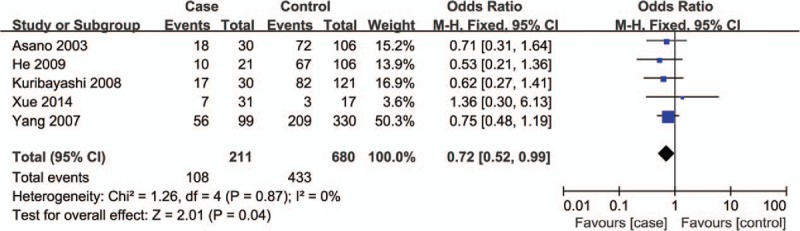
Forest plot for the association between ABCB1 C3435T polymorphism and ONFH susceptibility under TT+CT vs CC contrast. ABCB1 = ATP-binding cassette subfamily B member 1, ONFH = osteonecrosis of the femoral head.
Figure 4.
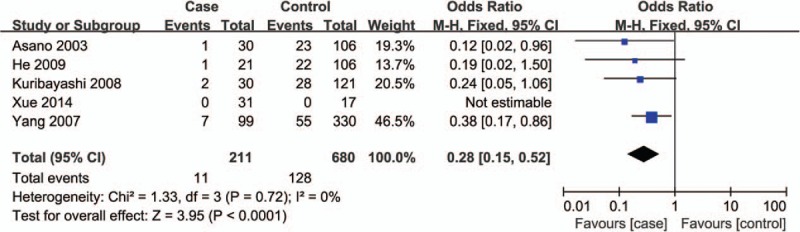
Forest plot for the association between ABCB1 C3435T polymorphism and ONFH susceptibility under TT vs CC+CT contrast. ABCB1 = ATP-binding cassette subfamily B member 1, ONFH = osteonecrosis of the femoral head.
Figure 5.
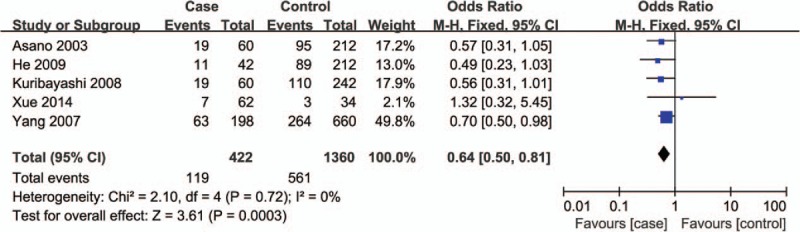
Forest plot for the association between ABCB1 C3435T polymorphism and ONFH susceptibility under T vs C contrast. ABCB1 = ATP-binding cassette subfamily B member 1, ONFH = osteonecrosis of the femoral head.
3.3. Heterogeneity test
As shown in Table 2, there was no significant heterogeneity among included studies (P > 0.05), so the fixed-effects model was determined for ORs calculation.
3.4. Sensitivity analysis
None of the selected studies expressed dramatic impact on pooled results during sensitivity analysis, implying statistical robustness of the results.
3.5. Publication bias examination
Begg's funnel plot provided no evidence for the existence of significant publication bias under any genetic contrast (Fig. 6), nor did Egger's test (data not shown).
Figure 6.
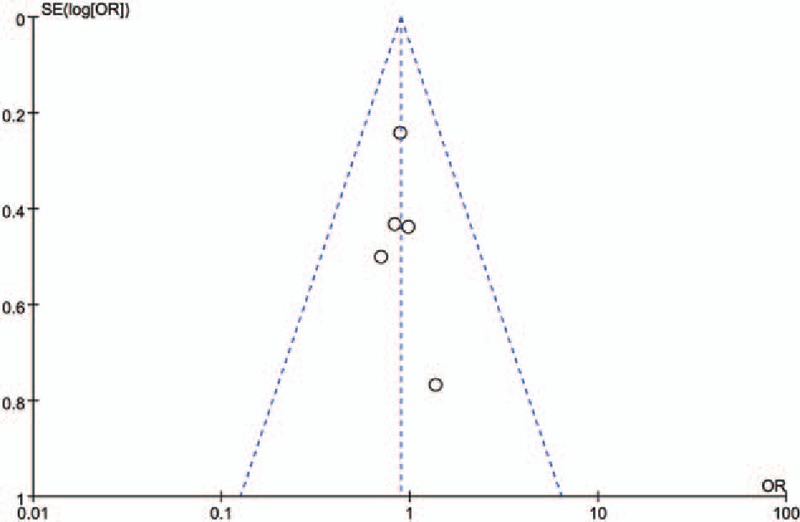
Funnel plot for publication bias among selected studies.
4. Discussion
With the deepened exploration of ONFH etiology, it is widely believed that trauma-induced ONFH occurs due to the direct destruction of blood supply to bone, and microcirculation thrombosis and intravascular coagulation are the common pathways of various causes for nontraumatic ONFH. Amidst the rapid development of molecular biotechniques, heredity is regarded as one of the possible causes for nontraumatic ONFH and leads to ONFH onset independently or together with other high-risk factors (such as like hormones and alcohol). Recent scientific findings show that ONFH attack is related to thrombophilia, decreased ability of fibrinolysis, reduced production of vasoactive substances, and the abnormalities in gene polymorphisms related to drug transporters. All these abnormal factors destroy the blood circulation in femoral head, and result in ONFH occurrence. Multiple synthetic hormones are absorbed and distributed through transportation by ABCB1, a transport protein reflecting drug resistance. There are multiple polymorphisms in the ABCB1 gene, and the variant C3435T in exon 26 is reportedly a functional polymorphism affecting phenotypes. This polymorphism may cause changes in pharmacokinetics and metabolite for hormone,[27] so as to produce various sensitivities of people to hormone.
As for the association between ABCB1 C3435T polymorphism and ONFH susceptibility, Asano et al[22] suggested TT genotype of the polymorphism could reduce the risk of ONFH in Japanese. Moreover, Xue et al[25] found that carriers with T allele or TT genotype had significantly lower risk of glucocorticoid-induced avascular necrosis of the femoral head (GANFH) compared with other carriers. No significant correlation was expressed between C3435T variant and ONFH risk in the research by Yang and Xu,[26] but the variant can be used for predicting the development of ONFH. A totally reverse relevance, however, was revealed in the study by He and Li,[23] that was to say, the ABCB1 C3435T polymorphism increased the susceptibility to ONFH. With regard to discrepancies among these studies, possible reasons might be different ONFH types and genotyping methods, various criteria for the selection of cases and controls, as well as potential effects of other genetic, environmental, and demographic factors.
In our meta-analysis, ABCB1 C3435T polymorphism was significantly related to the reduced risk of ONFH under TT vs CC, TT+CT vs CC, TT vs CC+CT and T vs C genetic models, showing its protective effects against the onset of the disease. As shown in Table 2, the risk of ONFH was reduced to less than one-third in carriers with TT genotype of C3435T polymorphism compared with wild homozygote carriers, displaying a similar tendency to the findings by Asano et al and Xue et al.[22,25] In addition, the genotype distribution of control group was consistent with Hardy–Weinberg equilibrium in every included study.
Although meta-analysis means a more powerful measure than any single study, our findings still need to be interpreted cautiously. Language and source limitations in searching strategy might miss some potent papers, and the limited number of included studies might lessen the comprehensiveness of the results. Additionally, 5 eligible studies provided data merely about Asian populations, which might produce selection bias. Meanwhile, only 1 included study was with population-based control source, and others recruited hospital-based controls, which might be deficient in representativeness for general populations. Moreover, no subgroup analysis was performed according to age, gender, ONFH type, status of alcohol consumption or other relevant respects. Furthermore, the initiation and progression of ONFH involve many aspects, but gene–gene or gene–environment interactions were not incorporated in this study, and this might sway the accuracy of our findings.
In conclusion, this analysis suggests a protective function of ABCB1 C3435T polymorphism against ONFH occurrence. In view of restrictions in the present study, the results should be further verified via studies with more relevant researches and ethnic groups, as well as possible interactions among risk factors for ONFH.
Footnotes
Abbreviations: 95% CIs = 95% confidence intervals, ABCB1 = ATP-binding cassette subfamily B member 1, ALL = acute lymphoblastic leukemia, GANFH = glucocorticoid-induced avascular necrosis of the femoral head, HIV = human immunodeficiency virus, MDR1 = multidrug resistance protein 1, NOS = Newcastle–Ottawa Scale, ONFH = osteonecrosis of the femoral head, ORs = odds ratios, P-gp = P-glycoprotein 1, SCFE = slipped capital femoral epiphysis, SLE = systemic lupus erythematosus.
YZ and HX equally contributed to this study.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Mankin HJ. Nontraumatic necrosis of bone (osteonecrosis). N Engl J Med 1992;326:1473–9. [DOI] [PubMed] [Google Scholar]
- [2].Mont MA, Jones LC, Einhorn TA, et al. Osteonecrosis of the femoral head. Potential treatment with growth and differentiation factors. Clin Orthop Relat Res 1998;355:S314–35. [PubMed] [Google Scholar]
- [3].Mutijima E, De Maertelaer V, Deprez M, et al. The apoptosis of osteoblasts and osteocytes in femoral head osteonecrosis: its specificity and its distribution. Clin Rheumatol 2014;33:1791–5. [DOI] [PubMed] [Google Scholar]
- [4].Karkoulias K, Charokopos N, Kaparianos A, et al. Aseptic femoral head necrosis in a patient receiving long term courses of inhaled and intranasal corticosteroids. Tuberkuloz ve Toraks 2007;55:182–5. [PubMed] [Google Scholar]
- [5].Kerachian MA, Seguin C, Harvey EJ. Glucocorticoids in osteonecrosis of the femoral head: a new understanding of the mechanisms of action. J Steroid Biochem Mol Biol 2009;114:121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Liu TG, Chen WH. Researchprogress of epidemiology aboutnon-traumatic osteonecrosis of femoral head. Contemp Med 2008;14:2638–9. [Google Scholar]
- [7].Mont MA, Zywiel MG, Marker DR, et al. The natural history of untreated asymptomatic osteonecrosis of the femoral head: a systematic literature review. J Bone Joint Surg Am 2010;92:2165–70. [DOI] [PubMed] [Google Scholar]
- [8].Fukushima W, Fujioka M, Kubo T, et al. Nationwide epidemiologic survey of idiopathic osteonecrosis of the femoral head. Clin Orthop Relat Res 2010;468:2715–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Liu LY. Analysis of osteonecrosis of the femoral head. China J Convalescent Med 2007;16:447. [Google Scholar]
- [10].Hamilton TW, Goodman SM, Figgie M. SAS weekly rounds: avascular necrosis. HSS J 2009;5:99–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mont MA, Hungerford DS. Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am 1995;77:459–74. [DOI] [PubMed] [Google Scholar]
- [12].Malizos KN, Karantanas AH, Varitimidis SE, et al. Osteonecrosis of the femoral head: etiology, imaging and treatment. Eur J Radiol 2007;63:16–28. [DOI] [PubMed] [Google Scholar]
- [13].Larson AN, McIntosh AL, Trousdale RT, et al. Avascular necrosis most common indication for hip arthroplasty in patients with slipped capital femoral epiphysis. J Pediatr Orthop 2010;30:767–73. [DOI] [PubMed] [Google Scholar]
- [14].Kim HS, Bae SC, Kim TH, et al. Endothelial nitric oxide synthase gene polymorphisms and the risk of osteonecrosis of the femoral head in systemic lupus erythematosus. Int Orthop 2013;37:2289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Morse CG, Mican JM, Jones EC, et al. The incidence and natural history of osteonecrosis in HIV-infected adults. Clin Infect Dis 2007;44:739–48. [DOI] [PubMed] [Google Scholar]
- [16].Gunes A, Spina E, Dahl ML, et al. ABCB1 polymorphisms influence steady-state plasma levels of 9-hydroxyrisperidone and risperidone active moiety. Ther Drug Monit 2008;30:628–33. [DOI] [PubMed] [Google Scholar]
- [17].Westerlund M, Belin AC, Anvret A, et al. Association of a polymorphism in the ABCB1 gene with Parkinson's disease. Parkinsonism Rel Disord 2009;15:422–4. [DOI] [PubMed] [Google Scholar]
- [18].Gregers J, Green H, Christensen IJ, et al. Polymorphisms in the ABCB1 gene and effect on outcome and toxicity in childhood acute lymphoblastic leukemia. Pharmacogenomics J 2015;15:372–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Reed K, Parissenti AM. The effect of ABCB1 genetic variants on chemotherapy response in HIV and cancer treatment. Pharmacogenomics 2011;12:1465–83. [DOI] [PubMed] [Google Scholar]
- [20].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [21].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Asano T, Takahashi KA, Fujioka M, et al. ABCB1 C3435T and G2677T/A polymorphism decreased the risk for steroid-induced osteonecrosis of the femoral head after kidney transplantation. Pharmacogenetics 2003;13:675–82. [DOI] [PubMed] [Google Scholar]
- [23].He W, Li K. Incidence of genetic polymorphisms involved in lipid metabolism among Chinese patients with osteonecrosis of the femoral head. Acta Orthop 2009;80:325–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kuribayashi M, Fujioka M, Takahashi KA, et al. Combination analysis of three polymorphisms for predicting the risk for steroid-induced osteonecrosis of the femoral head. J Orthop Sci 2008;13:297–303. [DOI] [PubMed] [Google Scholar]
- [25].Xue Y, Zhao ZQ, Hong D, et al. MDR1 gene polymorphisms are associated with glucocorticoid-induced avascular necrosis of the femoral head in a Chinese population. Genet Test Mol Biomarkers 2014;18:196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yang XY, Xu DH. MDR1(ABCB1) gene polymorphisms associated with steroid-induced osteonecrosis of femoral head in systemic lupus erythematosus. Die Pharmazie 2007;62:930–2. [PubMed] [Google Scholar]
- [27].Marino S, Verzegnassi F, Tamaro P, et al. Response to glucocorticoids and toxicity in childhood acute lymphoblastic leukemia: role of polymorphisms of genes involved in glucocorticoid response. Pediatr Blood Cancer 2009;53:984–91. [DOI] [PubMed] [Google Scholar]


