Abstract
The aim of the study was to evaluate the efficacy of anti-angiogenic therapy with dynamic contrast-enhanced ultrasound (DCE-US) in colorectal cancer (CRC) patients with liver metastasis.
A total of 50 CRC patients with liver metastasis who received bevacizumab (BEV)-based chemotherapy (BEV + FOLFOX6 protocol) were recruited into the present study. Before the study (d0), and 3, 7, 14, and 42 days (d3, d7, d14, and d42) after chemotherapy, DCE-US was performed, and tumor perfusion was evaluated quantitatively by retention time (RT), peak enhancement (PE), and wash-in area under the curve (WiAUC) on the basis of a contrast-uptake curve determined with original linear data.
Routine ultrasonography was used to evaluate metastatic foci in the liver at baseline. A metastatic focus was selected for dynamic monitoring with ultrasound. The metastatic foci were 1.5 to 8 cm (median: 2.5 cm). The results of hemodynamics monitored at different time points, including RT, PE, and WiAUC, showed that RT at baseline was significantly different between groups (P < .001; Responder group: 10.54 seconds; nonresponder group: 15.33 seconds). The2 groups had opposite changes in RT (continuous increase in the responder group and transient reduction in the nonresponder). The RT of metastatic foci was normalized to that of adjacent normal liver as standard RT-quotient, a similar trend was observed, and no marked difference was noted in the standard RT-quotient between the 2 groups. The median progression-free survival was significantly higher in the increased-RT group (10.8 months) than the decreased-RT group (2.5 months) (P = .002). There were no significant differences in peak intensity and WiAUC between the 2 groups.
DCE-US can be used to quantitatively evaluate the hemodynamics of liver metastasis in CRC patients who received bevacizumab-based chemotherapy.
Keywords: anti-angiogenic therapy, bevacizumab, colorectal cancer, dynamic contrast-enhanced ultrasound, liver metastasis
1. Introduction
Colorectal cancer (CRC) is one of the most common malignancies worldwide, and the liver is the most common site of colorectal cancer metastasis. The growth and the metastasis of solid cancers are dependent on angiogenesis. In angiogenesis, vascular endothelial growth factor (VEGF) is one of the most potent pro-angiogenic factors. It plays an important role in the regulation of angiogenesis in cancers. VEGF acts mainly on the VEGF receptor-2 (VEGFR-2) on vascular endothelial cells to promote the proliferation and migration of endothelial cells to induce angiogenesis.[1,2] With the development and application of molecular biological techniques, strategies targeting VEGF/VEGFR have formed the basis of anti-angiogenic therapy, and have become a hot topic in cancer therapeutics.
Bevacizumab (BEV) is a recombinant human monoclonal antibody to VEGF. In first-line chemotherapy and subsequent therapy, combined use of BEV may significantly increase the therapeutic efficacy[3,4]; however, not all patients may benefit from BEV therapy, and anti-angiogenic therapy has specific adverse effects including hypertension, hemorrhage, proteinuria, renal dysfunction, and arterial embolism, which cannot be ignored. Thus, early prediction of the efficacy of BEV therapy in combination with chemotherapy is imperative, to facilitate modulation of the therapeutic protocol as early as possible which may in turn reduce medical cost. To date, no predictive factors have been identified as biomarkers for the evaluation of therapeutic efficacy.[5] Although positron emission tomography (PET), dynamic contrast-enhanced magnetic resonance imaging (MRI), and computed tomography (CT) perfusion imaging have been used to evaluate the therapeutic efficacy of BEV therapy, no consensus has been reached.[6,7] In targeted anti-angiogenic therapy of cancers, it is possible to evaluate the therapeutic efficacy on the basis of the hemodynamics of cancers. Contrast-enhanced ultrasound (CEUS) is a new technique developed in recent years that can be used to acquire information on the blood supply and perfusion, and that can dynamically monitor the hemodynamics of cancers.[8,9] On the basis of these advantages of CEUS, it has been confirmed that CEUS may be used to predict the efficacy of BEV therapy in patients with advanced hepatocellular carcinoma (HCC).[10]
In the present study, dynamic contrast-enhanced ultrasonography (DCE-US) was employed to evaluate the hemodynamics of metastatic CRC in the liver, aiming to identify intrinsic changes in the hemodynamics of the metastatic cancer and to evaluate the efficacy of BEV therapy in combination with chemotherapy.
2. Materials and methods
2.1. Patients
From September 2012 to September 2013, a total of 50 patients with advanced CRC with liver metastasis who had received initial therapy were recruited into the present study (Table 1). Each of the patients was pathologically proven to have advanced CRC. Multidisciplinary consultation concluded that surgical resection of the metastatic foci was not feasible. Of these patients, 18 had been diagnosed with rectal cancer, and 32 with colon cancer. The number of metastatic foci in the liver ranged from 3 to 15. All the patients chosen have written informed consent, and the protocol was authorized by Ethics Committee of Chinese PLA General Hospital.
Table 1.
Characteristics of the patients.

2.2. Protocol for therapy
Chemotherapy consisted of oxaliplatin 85 mg/m2 IV over 2 hours, on day 1; leucovorin 400 mg/m2 over 2 hours, on day 1; 5-FU 400 mg/m2 IV bolus on day 1, and then a 46 hour infusion of 5-FU 2400 mg/m2 (simplified mFOLFOX6). Bevacizumab was administered at a dose of 5 mg/kg. The regimen was repeated every 2 weeks.
2.3. Dynamic contrast-enhanced ultrasound
According to schedule, DCE-US of 1 liver metastasis was carried out before the treatment (day 0), and on days 3, 7, 14, and 42 after administration of chemotherapy and bevacizumab. In patients who had multiple liver metastases, a single metastasis was chosen on the basis of position as assessed by US. US examinations were performed using the Aplio system (Toshiba Medical Systems, Japan), with a 3.5 MHz convex-array abdominal probe, using SonoVue (Bracco SpA, Milan, Italy) as the contrast enhancer. In order to optimize real-time detection of harmonic contrast response, all patients were examined at a rate of 4 frames per second and at a low mechanical index setting. Then, 2.4 mL of SonoVue was injected as a bolus through a peripheral venous catheter over 2 seconds, followed by bolus injection of 10 mL of 0.9% NaCl solution. A 3-minute-long video record was obtained from the beginning of the injection, allowing recording all steps of contrast enhancement characteristics (Fig. 1).
Figure 1.
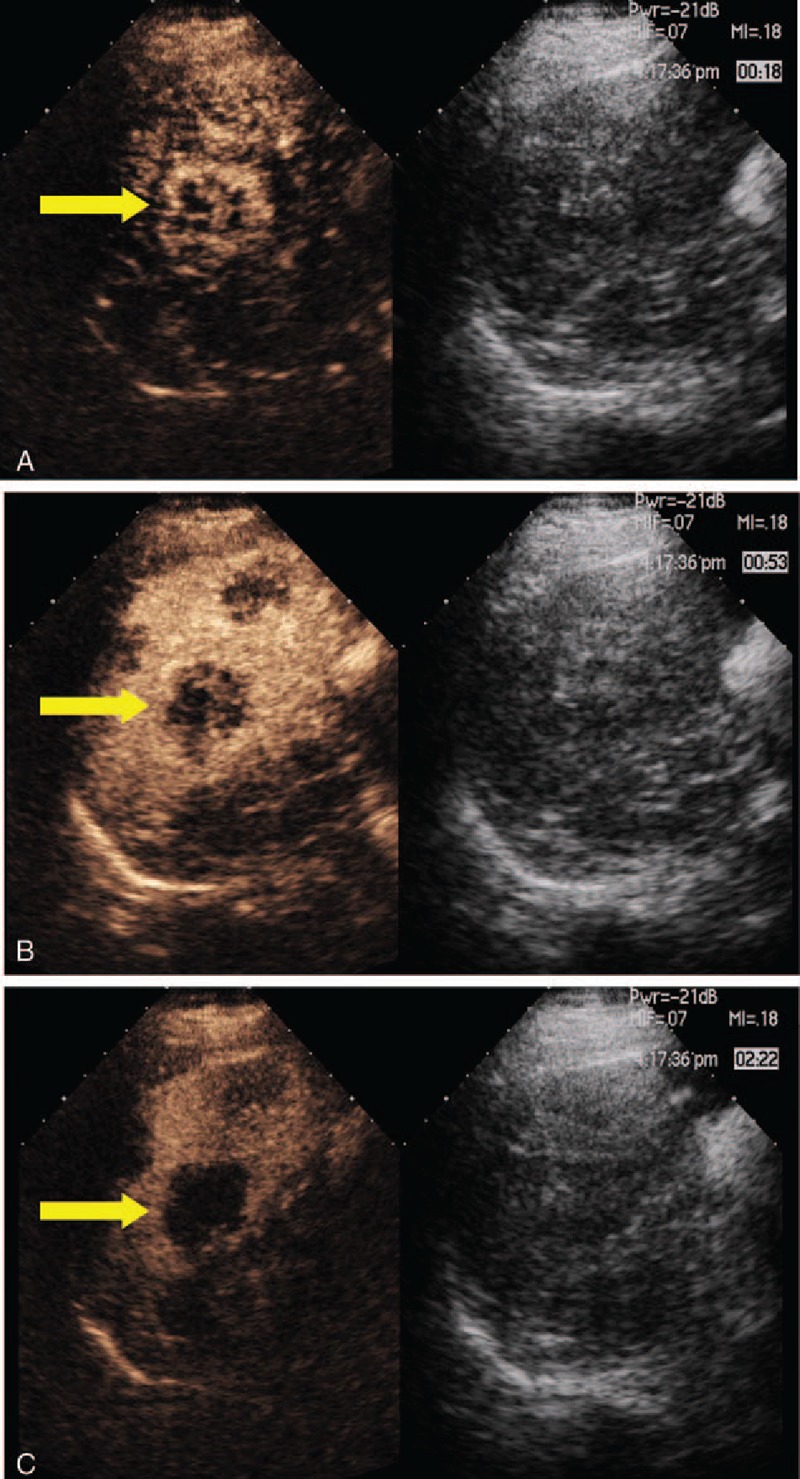
Contrast enhanced ultrasound. (A) A representative liver metastasis in the arterial phase with early contrast enhancement (18 s after contrast injection). (B) A representative liver metastasis in the venous phases of portal perfusion (53 s after contrast injection). (C) A representative liver metastasis in the late phase, becoming markedly hypoechoic (142 s after contrast injection).
In order to quantitatively measure vascularity in the metastasis and normal liver tissue by contrast dye characteristics we used the SonoLiver software. This software is specific sonographic quantification software, based on pixel by pixel signal intensity over time to obtain contrast-enhanced sonographic perfusion maps for each metastasis. In all cases, the region of interest (ROI) covered the whole area of the metastasis. Simultaneously, the ROIs of normal liver tissue were selected in a region at least 2 cm lateral (same deepness) of the metastasis. The measurement began on the first time of contrast enhancement seen in ROI (Fig. 2). Three values were acquired from the time-intensity curve: peak enhancement (PE), the retention time (RT), the area under the time-intensity curve during wash (WiAUC). US examination was analyzed by 2 independent investigators blinded to the cases.
Figure 2.
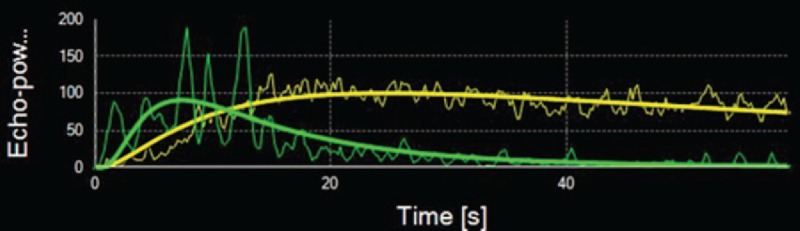
Curves of contrast behavior in a liver metastasis (green line) and normal liver tissue (yellow line) over the time (s = seconds) and contrast enhancement.
2.4. Response evaluation
All patients underwent CT/MRI scan evaluation of tumor load at baseline and after 3 cycles of treatment. In light of the CT/MRI results, we defined clinical response by the change in the longest diameter of each target lesion, according to the Response Evaluation Criteria In Solid Tumors (RECIST 1.1), which consist of 4 response categories: complete response (complete disappearance of tumor lesions), partial response (at least 30% decrease in sum of longest diameter of each tumor), stable disease (between 30% decrease and 20% increase in sum), and progressive disease (more than 20% increase in sum). Those patients with partial remission and complete remission by radiological evaluation were classified as responders, whereas those patients with radiological stable disease and progressive disease as nonresponders.
2.5. Statistical analysis
The findings from DCE-US were compared between the group of patients in whom therapy was effective and those in whom therapy was ineffective. Paired comparisons were performed with the t test. On the basis of dynamic change in RT, patients were divided into 2 groups: those with decreased RT, and those with increased RT. The Kaplan–Meier method was used to evaluate progression-free survival (PFS). A difference of P < .01 was considered to be statistically significant.
3. Results
3.1. Patients and response to therapy
A total of 50 patients (6 women and 44 men) with a mean age of 55.8 (range: 38–78) years received FOLFOX plus bevacizumab for metastatic CRC. According to the RECIST criteria, 1 patient had complete remission, 28 patients had partial remission, 12 patients had stable disease, and 9 patients had progressive disease. Of the 50 patients, 6 underwent resection of liver metastases within 12 weeks after initiation of chemotherapy.
3.2. Findings from DCE-US
Routine ultrasonography was used to evaluate metastatic foci in the liver at baseline. A metastatic focus was selected for dynamic monitoring with ultrasound. The metastatic foci were 1.5 to 8 cm (median: 2.5 cm) in diameter. The hemodynamics was monitored at different time points including RT, PE, and WiAUC. The results showed that RT at baseline was significantly different between groups (P < .001; responder group: 10.54 seconds; nonresponder group: 15.33 seconds). The 2 groups had opposite changes in RT (continuous increase in the responder group and transient reduction in the nonresponder) (Fig. 3). At d42, the RT was comparable between the 2 groups (P = .248; responder group: 18.54 seconds, nonresponder: 16.91 seconds) (Table 2). The RT of metastatic foci was normalized to that of adjacent normal liver as standard RT-quotient, a similar trend was observed, and no marked difference was noted in the standard RT-quotient between the 2 groups (Table 3). According to the trend of dynamic change of RT, patients were divided into the increased-RT group and the decreased-RT group. The median PFS was significantly higher in the increased-RT group (10.8 months) than the decreased-RT group (2.5 months) (P = .002) (Fig. 4). However, there were no significant differences in peak intensity and WiAUC between the 2 groups.
Figure 3.
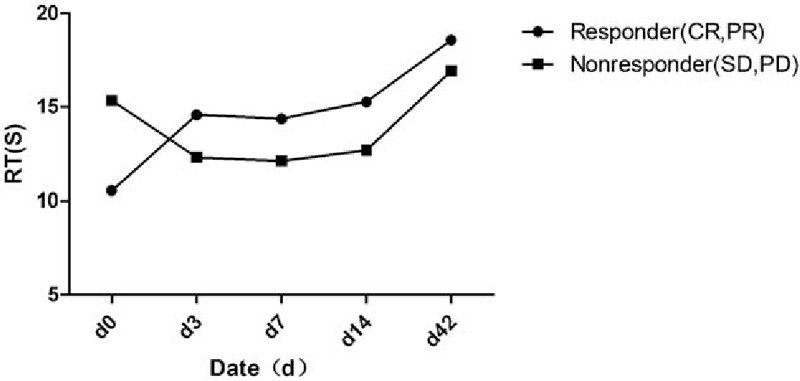
The retention time (RT) values measured in the metastasis between responders and nonresponders on contrast enhanced ultrasound d0, d3, d7, d14, and d42 (responders with complete response [CR] and partial response [PR] [n = 29] vs nonresponder with stable disease [SD] and progressive disease [PD] [n = 21]). CR = complete response, PD = progressive disease, PR = partial response, RT = retention time, SD = stable disease.
Table 2.
Hemodynamic parameters of the metastatic foci in the liver after DCE-US.
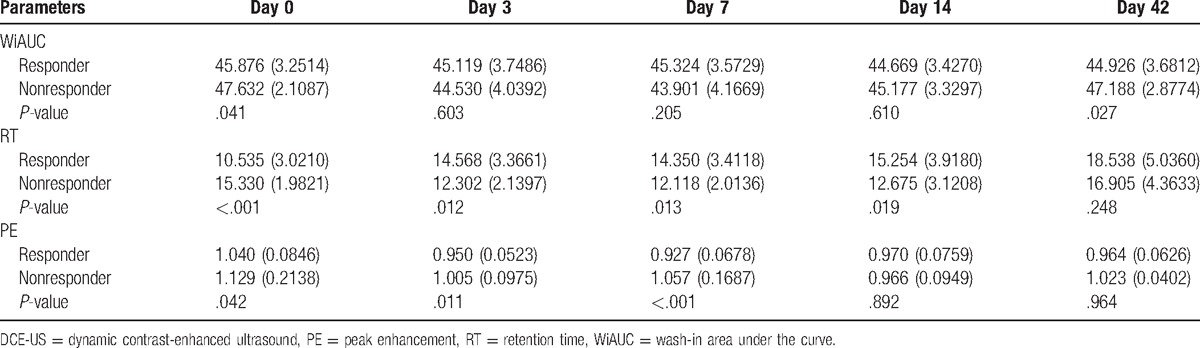
Table 3.
Standard parameter-quotient of hemodynamics of the metastatic foci in the liver after DCE-US.
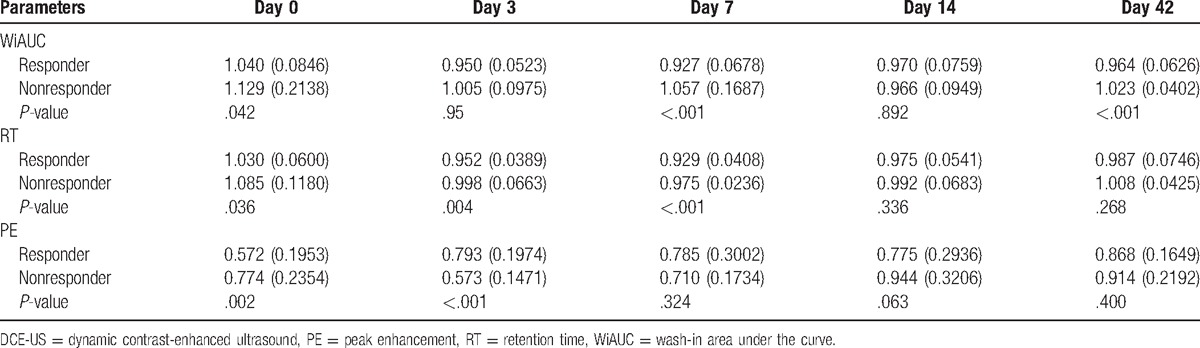
Figure 4.
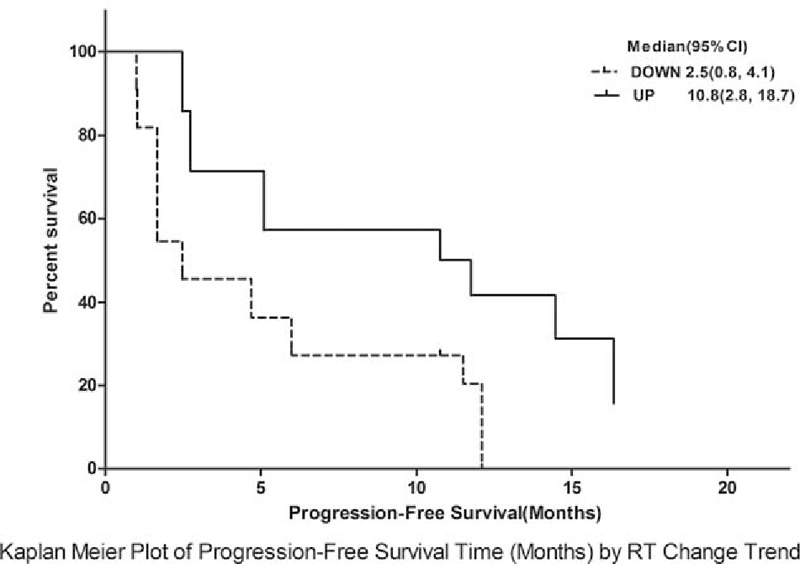
Kaplan–Meier plot for progression-free survival (months) by retention time (RT) change trend; analysis showed a marked difference in progression-free survival between the 2 groups (P = .002). RT = retention time.
4. Discussion
BEV has been included in first- and second-line chemotherapy of advanced CRC. Results from multiple studies have shown that routine chemotherapy in combination with BEV can improve the PFS and overall survival; however, specific toxicities of BEV cannot be ignored. Thus, it is imperative to identify a method to predict the efficacy of anti-angiogenic therapy. Early prediction of therapeutic efficacy may help patients not likely to benefit from anti-angiogenic therapy to avoid unnecessary side effects and the expense of this therapy. In recent years, numerous efforts have been made to predict the efficacy of anti-angiogenic therapy, but no definite conclusions have been reached.[11–14]
Our results suggest that DCE-US could provide additional information to assess the efficacy of BEV-based chemotherapy in patients with advanced CRC even prior to the initiation of therapy. The results also have revealed that the RT at baseline and the standard RT-quotient in the responder group were significantly shorter than that in the nonresponder group. That is, short RT at baseline is associated with therapeutic efficacy. In addition, in the responder group, the RT and standard RT-quotient increased gradually during chemotherapy, and were closely related to the efficacy of anti-angiogenic therapy, which are consistent with findings from the study of Schirin-Sokhan.[15] Dynamic monitoring of hemodynamics showed that RT increased continuously in the responder group and showed transient reduction in the nonresponder group.
Cancer cells obtain nutrients for their growth and for dissemination to distant organs by co-opting host blood vessels, sprouting new blood vessels from pre-existing ones (angiogenesis), and/or recruiting endothelial cells derived from the bone marrow (postnatal vasculogenesis).[16] The structure and function of the resulting vasculature is abnormal. Blood vessels are leaky, dilated, tortuous, and saccular with a haphazard pattern of interconnection. These structural abnormalities result in spatially and temporally heterogeneous tumor blood flow. Due to its special blood supply, metastases in liver from CRC have particular features in DCE-US images. Hypervascular metastases can be achieved perfectly by real-time imaging in the arterial phase. At the beginning of the portal venous phase, the enhancement fades and the entire lesion has become increasingly hypoechoic. During the late phase, the metastases invariably show as dark defects, whereas the parenchymal enhancement of normal liver persists.[17] Based on the time-intensity curve, RT of metastases is significantly shorter than that of the normal liver tissue (Fig. 2).
By reducing mean vessel density in tumors and increasing vessel pericyte coverage, bevacizumab treatment normalized the structure and function of abnormal blood vessels in the cancer. However, sustained antiangiogenic regimens may eventually prune away these vessels.[18,19] This process applies to bevacizumab-sensitive tumors vessels and is consistent with Dynamic Change of the responder group RT (Fig. 3). On the other hand, bevacizumab-resistant tumor vessels were characterized by an increased vessel diameter and normalization of vascular structures.[18] This applies to the nonresponder group, with an extended baseline RT value.
Our findings have shown that measurement of hemodynamics of metastatic foci in the liver of patients with CRC could be used effectively to predict the efficacy of BEV-based chemotherapy. Analysis of change in measurements suggests that the intrinsic mechanisms might be related to the normalization of vasculature in the cancer. Anti-angiogenic therapy is efficacious in those cancers with abnormal vasculature, although the vasculature of such cancers becomes normal for a relatively long time after anti-angiogenic therapy.
Although these findings were encouraging, this study has limitations. We failed to compare the efficacy of anti-angiogenic therapy with that of chemotherapy. There might be patients who were sensitive to chemotherapy, but nonresponsive to BEV. In addition, the sample size was small; randomized, controlled studies with a larger sample size would be required to confirm our findings. Moreover, DCE-US itself has limitations. The detection with DCE-US is influenced by intestine gas, and thus is only applicable in the observation of metastatic foci in the liver. DCE-US is unable to accurately monitor the blood flow of abdominal and pelvic foci. On the basis of findings from monitoring the hemodynamics of tumors, we may purposively perform dynamic contrast-enhanced MRI and CT perfusion imaging, which may be of significant benefit to cancer patients.
Taken together, our findings suggest that DCE-US could be a useful tool to evaluate the efficacy of BEV-based chemotherapy in patients with CRC by assessing the hemodynamics of metastatic foci in the liver. Its clinical value should be confirmed in randomized, controlled trials with a larger sample size. The mechanisms involved might be associated with the normalization of vasculature in the cancer, which needs to be elucidated in more basic studies. Our findings support the use of imaging examinations to evaluate the efficacy of anti-angiogenic therapy.
Footnotes
Abbreviations: BEV = bevacizumab, CEUS = contrast-enhanced ultrasound, CR = complete response, CRC = colorectal cancer, CT = computed tomography, DCE-US = dynamic contrast-enhanced ultrasound, HCC = hepatocellular carcinoma, MRI = magnetic resonance imaging, PD = progressive disease, PE = peak enhancement, PET = positron emission tomography, PFS = progression-free survival, PR = partial response, RECIST = Response Evaluation Criteria In Solid Tumors, ROI = region of interest, RT = retention time, SD = stable disease, VEGF = vascular endothelial growth factor, VEGFR-2 = vascular endothelial growth factor receptor-2, WiAUC = wash-in area under the curve.
Authorship: ZHW and XWY contributed equally to this study.
Ethics approval and consent to participate: All the patients chosen have written informed consent, and the protocol was authorized by Ethics Committee of Chinese PLA General Hospital.
Funding: This work was supported by the National Natural Science Foundation of China (81171358).
The authors have no conflicts of interest to disclose.
References
- [1].Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011;473:298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 2005;23:1011–27. [DOI] [PubMed] [Google Scholar]
- [3].Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–42. [DOI] [PubMed] [Google Scholar]
- [4].Kabbinavar FF, Hambleton J, Mass RD, et al. Combined analysis of efficacy: the addition of bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancer. J Clin Oncol 2005;23:3706–12. [DOI] [PubMed] [Google Scholar]
- [5].Lambrechts D, Lenz HJ, de Haas S, et al. Markers of response for the antiangiogenic agent bevacizumab. J Clin Oncol 2013;31:1219–30. [DOI] [PubMed] [Google Scholar]
- [6].Miles KA. Perfusion CT for the assessment of tumour vascularity: which protocol? Brit J Radiol 2003;76(spec no 1):S36–42. [DOI] [PubMed] [Google Scholar]
- [7].Zhang JZY. Clinic application of CT perfusion technique. J Clin Radiol 2001;20:803–6. [Google Scholar]
- [8].Strobel D, Seitz K, Blank W, et al. Contrast-enhanced ultrasound for the characterization of focal liver lesions—diagnostic accuracy in clinical practice (DEGUM multicenter trial). Ultraschall Der Medizin 2008;29:499–505. [DOI] [PubMed] [Google Scholar]
- [9].Tada T, Kumada T, Toyoda H, et al. Utility of contrast-enhanced ultrasonography with perflubutane for determining histologic grade in hepatocellular carcinoma. Ultrasound Med Biol 2015;41:3070–8. [DOI] [PubMed] [Google Scholar]
- [10].Lassau N, Koscielny S, Chami L, et al. Advanced hepatocellular carcinoma: early evaluation of response to bevacizumab therapy at dynamic contrast-enhanced US with quantification—preliminary results. Radiology 2011;258:291–300. [DOI] [PubMed] [Google Scholar]
- [11].Ferl GZ, Xu L, Friesenhahn M, et al. An automated method for nonparametric kinetic analysis of clinical DCE-MRI data: application to glioblastoma treated with bevacizumab. Magn Res Med 2010;63:1366–75. [DOI] [PubMed] [Google Scholar]
- [12].Jiang T, Kambadakone A, Kulkarni NM, et al. Monitoring response to antiangiogenic treatment and predicting outcomes in advanced hepatocellular carcinoma using image biomarkers, CT perfusion, tumor density, and tumor size (RECIST). Invest Radiol 2012;47:11–7. [DOI] [PubMed] [Google Scholar]
- [13].Koukourakis MI, Mavanis I, Kouklakis G, et al. Early antivascular effects of bevacizumab anti-VEGF monoclonal antibody on colorectal carcinomas assessed with functional CT imaging. Am J Clin Oncol 2007;30:315–8. [DOI] [PubMed] [Google Scholar]
- [14].Morgan BTA, Drevs J. Advanced hepatocellular carcinoma: early evaluation of response to bevacizumab therapy at dynamic contrast-enhanced US with quantification–preliminary results colorectal cancer and liver metastases: results from two phase I studies. J Clin Oncol 2003;21:3955–64.14517187 [Google Scholar]
- [15].Schirin-Sokhan R, Winograd R, Roderburg C, et al. Response evaluation of chemotherapy in metastatic colorectal cancer by contrast enhanced ultrasound. World J Gastroenterol 2012;18:541–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature 2000;407:249–57. [DOI] [PubMed] [Google Scholar]
- [17].Konopke R, Bunk A, Kersting S. The role of contrast-enhanced ultrasound for focal liver lesion detection: an overview. Ultrasound Med Biol 2007;33:1515–26. [DOI] [PubMed] [Google Scholar]
- [18].Arjaans M, Oude Munnink TH, Oosting SF, et al. Bevacizumab-induced normalization of blood vessels in tumors hampers antibody uptake. Cancer Res 2013;73:3347–55. [DOI] [PubMed] [Google Scholar]
- [19].Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 2005;307:58–62. [DOI] [PubMed] [Google Scholar]


