Abstract
Desmosomal adhesion is important for the integrity and protective barrier function of the epidermis and is disregulated during carcinogenesis. Strong adhesion between keratinocytes is conferred by the desmosomal cadherins, desmocollin (Dsc) and desmoglein. These constitute two gene families, members of which are differentially expressed in epidermal strata. It has been suggested that this stratum-specific expression regulates keratinocyte differentiation. We tested this hypothesis by misdirecting the expression of the basally abundant desmosomal cadherins Dsc3a and Dsc3b to suprabasal differentiating keratinocytes in transgenic mice. No phenotype was apparent until adulthood, when mice developed variable ventral alopecia and had altered keratinocyte differentiation within affected areas. The follicular changes were reminiscent of changes in transgenic mice with an altered β-catenin stability. Stabilized β-catenin and increased β-catenin transcriptional activity were demonstrated in transgenic mice prior to the phenotypic change and in transgenic keratinocytes as a consequence of transgene expression. Hence, a link between desmosomal cadherins and β-catenin stability and signaling was demonstrated, and it was shown that desmocollin cadherin expression can affect keratinocyte differentiation. Furthermore, the first function for a “b-type” desmocollin cadherin was demonstrated.
Desmosomes and adherens junctions are multiprotein adhesive complexes located at epithelial membranes. Both impart adhesion through transmembrane cadherins linked via armadillo family members to the cytoskeleton. At adherens junctions, the armadillo members β-catenin and plakoglobin (γ-catenin) are involved in linking classical cadherins to the actin network. Plakoglobin is also a desmosomal component, as it participates with desmoplakin in linking desmosomal cadherins to the keratin intermediate filament cytoskeleton. Additionally, β-catenin and plakoglobin have signaling and transcriptional regulatory roles in the cytoplasm and nucleus, where Wnt signaling induces the transient stabilization of β-catenin, resulting in nuclear translocation and the regulation of downstream genes in association with the Lef1/Tcf (lymphoid enhancer factor/T-cell factor) transcription factors (reviewed in reference 29). However, a role for desmosomes in cell signaling is still being debated (10, 12).
Desmosomal cadherins comprise two families, desmocollins (Dsc) and desmogleins (Dsg), each consisting of multiple isoforms. Isoforms 1 and 3 of Dsc and Dsg are expressed in the stratified layers of the epidermis in a reciprocal graded fashion (32; reviewed in references 9 and 19). Isoform 3 expression is strongest in the basal, proliferative layer, with levels decreasing as keratinocytes differentiate. Isoform 1 levels peak in the upper granular layer, with decreasing levels in the mid-spinous layer, where different cadherins are mixed within individual desmosomes. Dsc2 and Dsg2 are expressed weakly in epidermal basal layers, and recently discovered isoforms homologous to Dsg1 (Dsg4 in humans) are expressed in the upper layers (20, 43).
The functional significance of this differential isoform distribution is unclear, but it may be related to the differential adhesive properties of desmosomes (9, 36). It is possible that strong adhesion is required in the upper layers, which are most subject to abrasive forces and where isoform 1 expression is at its highest. In contrast, more cell motility may be required in the basal layer, where isoform 3 expression peaks. It has also been proposed that the differential expression of desmosomal cadherins mediates signaling appropriate to the differentiation state of the keratinocyte (10, 12).
This hypothesis has been tested by altering Dsg isoform expression in transgenic mice. For example, the redirection of basal Dsg3 to mid-spinous and upper granular layer keratinocytes produced an abnormal stratum corneum and barrier defects, causing neonatal death (7), without detectable defects in underlying keratinocytes. However, when basal Dsg3 was redirected to differentiating suprabasal (spinous) keratinocytes, avoiding the secondary effects of skin barrier disruption, differentiation changes were noted (26), indicating that Dsg isoform distribution does influence differentiation.
Conversely, the misexpression of upper layer Dsc1 in basal layer keratinocytes failed to alter differentiation (17), suggesting that in contrast to Dsg, Dsc cannot partake in intracellular signaling. However, this conclusion leaves the significance of Dsc isoform differential distribution unclear.
In an attempt to clarify the significance of this distribution, we misexpressed the predominantly basal Dsc3 cadherins in the suprabasal epidermis of transgenic mice. The keratin 1 (K1) promoter was used to direct expression to spinous keratinocytes to avoid lethal barrier defects that result from the use of the involucrin promoter (7). Furthermore, K1 does not induce expression until late gestation and thus avoids developmental defects. Untagged endogenous murine Dsc3 isoforms which will incorporate into and interact normally with the multiprotein desmosome complex were used, permitting an unambiguous interpretation of phenotypes.
Desmocollins occur as “a” and “b” splice variants, with the a variant having a slightly longer cytoplasmic domain (Fig. 1). It has been shown that the a variant can support desmosomal assembly (39), but there is no known function for the b form. Therefore, both the a and b forms were used for these experiments.
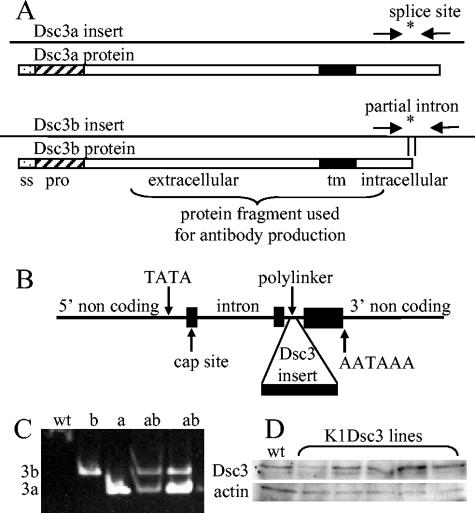
FIG. 1.
(A) Dsc3a and -3b cDNA inserts showing the site of the alternatively spliced C-terminal intron (asterisk) and the partial intron sequence in the Dsc3b construct. The unspliced b isoform terminates within the intron. Arrows show primer sequences used for isoform-specific transgene detection. Below the inserts, boxes show encoded proteins with signal sequences (ss), “pro” sequences (hatched), extracellular domains, transmembrane domains (tm), intracellular domains, and regions used for antibody production. (B) Keratin 1 targeting vector (14, 35) showing Dcs3 insert site. (C) PCR analysis of genomic DNA. wt, wild-type DNA showing absence of amplification; b, Dsc3b transgenic; a, Dsc3a transgenic; ab, Dsc3a/b double transgenic showing isoform-specific transgene detection. (D) Western analysis of Dsc3 showed only minor differences between wild-type and transgenic protein levels in newborn Dsc3a lines.
The results support the hypothesis that desmocollin cadherins can affect signaling in the cell and regulate epidermal differentiation, and they show the first described function for a b-type splice variant.
MATERIALS AND METHODS
Generation and analysis of transgenic mice.
Murine Dsc3a and Dsc3b partial cDNAs containing full coding sequences and part of the Dsc3b differentially spliced intron (Fig. 1) were isolated from a murine skin cDNA library (Stratagene, La Jolla, Calif.) and inserted into the human keratin 1 cassette (HK1 cassette), kindly supplied by Dennis Roop (14, 35), which was modified by subcloning into the EcoRI site of pBluescript SK(+) (Stratagene), which had been previously modified so that the regions between the NotI and SmaI sites in the polylinker were destroyed. This modification supplied flanking BssHII sites for construct excision. Transgenic mice were generated in an ICR or C57BL/6 × CBA F1 hybrid background under British Home Office licenses 40/1556 and 40/2526. The presence of transgenes was confirmed by Southern analysis and PCRs of genomic DNAs by the use of transgene-specific primers which flanked the alternative splice site (Fig. 1) (5′-TGGCACAGTTTCACTCAACC-3′ and 5′-TGCAGGTTTTTGCCAATGTA-3′) and amplified a 209-bp product corresponding to the Dsc3a isoform and a 252-bp product from the Dsc3b isoform (Fig. 1C). For analyses of hair cycles, dorsal and ventral hair (2-cm2 patches) from anesthetized postnatal day 14 mice was depilated by the use of cosmetic wax preparations to induce anagenesis.
Anti-Dsc3 antibody preparation and Western blotting.
Rabbit polyclonal anti-Dsc3 antibodies were generated against a Dsc3 polypeptide-glutathione S-transferase fusion protein comprising 241 amino acids from the extracellular domain, the transmembrane domain, and 53 amino acids from the intracellular domain common to the a and b isoforms (Fig. 1). The antibody specifically labels basal desmosomes (Fig. 2). Epidermal proteins were prepared after the separation of the epidermis from the dermis by incubation in 5 mM EDTA at 50°C for 3 min. The epidermis was boiled in sodium dodecyl sulfate sample buffer for 5 min and then centrifuged.
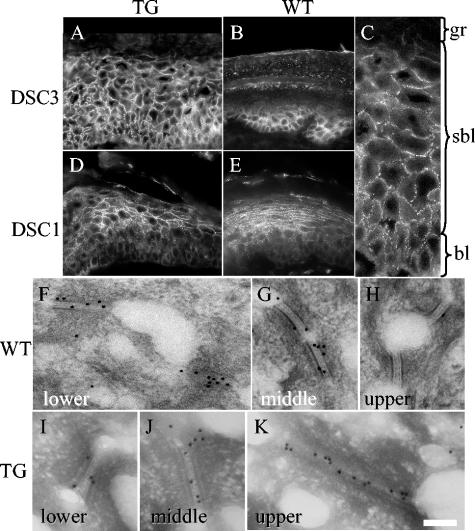
FIG. 2.
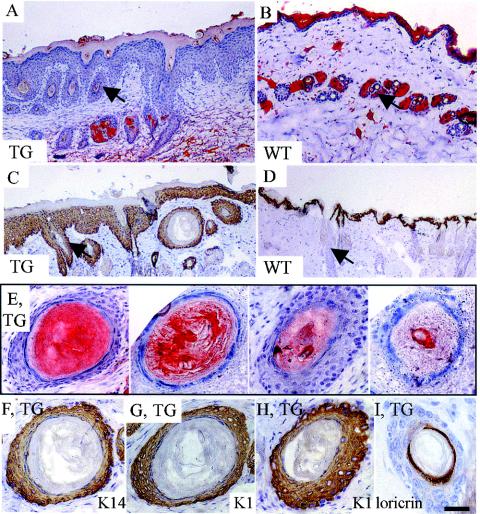
Desmocollin distribution in K1Dsc3 transgenic affected skin. (A) Immunohistochemical detection of Dsc3 protein in transgenic skin showing Dsc3 presence in all strata and a loss of the graded basally abundant expression pattern characteristic of control skin (footpad skin is used to show graded expression in the control [B]). (C) Higher magnification showing Dsc3 punctate membranous expression in basal and suprabasal layers of transgenic skin, indicating desmosomal incorporation. Desmocollin 1 protein distribution appears unaffected in transgenic (D) and littermate control (E) animals, in which it shows reciprocal graded expression compared to Dsc3, with peak expression in the granular layer (compare panels B and E). (F to K) Immunogold detection of Dsc3 in individual desmosomes from distinct epidermal strata in transgenic and control mice. In the wild-type epidermis, Dsc3 is incorporated in desmosomes in lower (F) and middle (G) strata but is almost undetectable in upper strata (H). In contrast, the Dsc3 protein was readily detected in upper strata in the K1Dsc3 epidermis (K), demonstrating that transgene-encoded Dsc3 incorporates into desmosomes. WT, wild type; TG, transgenic; gr, granular layer; sbl, suprabasal layer; bl, basal layer; de, dermis. Bar, 40 μm (A, B, D, and E), 15 μm (C), or 125 nm (F to K).
Histology and immunohistochemistry.
Skin samples were fixed for immunohistochemistry (2% paraformaldehyde in phosphate-buffered saline or Bouin's fixative) and then processed for paraffin embedding (5-μm-thick sections), or unfixed 5-μm-thick frozen sections were used. Sebaceous gland-derived and skin surface lipids were detected with oil red O (Sigma) on unfixed frozen sections after a brief rinse with 50% ethanol. The primary antibodies used were as follows: MK1, MK10, MK14, and MK6, which are antibodies against mouse keratins 1, 10, 14, and 6, respectively; anti-mouse involucrin and anti-mouse loricrin (CoVance); anti-mouse filaggrin (16); 11-5F, an anti-desmoplakin antibody; anti-Dsc1 (32); anti-plakoglobin (Transduction Laboratories); NCL-Ki67p, an anti-Ki67 antigen (Novacastra); anti-β-catenin (Zymed); anti-phospho-β-catenin (37) (Sigma); anti-ABC-β-catenin (42) (Upstate); anti-β-actin (Sigma); and anti-cyclin D (Santa Cruz Biotechnology Inc.).
Secondary antibodies were biotinylated goat anti-rabbit and goat anti-mouse immunoglobulin G or, for immunofluorescence, the equivalent fluorescein isothiocyanate- or Cy3-conjugated antibodies (Vector Laboratories and Jackson Immunological Laboratories, respectively). Binding of the biotinylated antibodies was visualized by the use of ABC-peroxidase and diaminobenzidine substrate, and slides were counterstained with hematoxylin. Images were captured with a Spot RT-slider digital camera attached to a Nikon E600 microscope. Transmission electron microscopy and immuno-transmission electron microscopy were performed as described previously (16).
Keratinocyte culture, transfection, and TOP-flash reporter assay.
Primary keratinocytes were cultured from 3-day-old pups as described previously (15), and N/TERT-1 cells were grown as described previously (5). TOP-flash (40) assays were performed according to the manufacturer's instructions (dual luciferase reporter assay; Promega), with FOP-flash as a negative control, phosphoglycerate kinase from Renilla luciferase as an internal normalization control, and pMβ9 (Xenopus β-catenin) as a positive control. After transfection, murine keratinocytes were incubated in a high-calcium medium (1.5 mM calcium) for 48 h to induce differentiation. N/TERT-1 cells were grown in 0.4 or 1.5 mM calcium for 48 h posttransfection.
RESULTS
Production of transgenic mice expressing suprabasal Dsc3.
Since desmocollin splice variant functions are unclear, both Dsc3a and Dsc3b (Fig. 1A) were incorporated into a K1 expression cassette (14, 35) (Fig. 1B), and transgenic mice that misexpressed the a or b variant in suprabasal epidermal cells were generated. Untagged murine Dsc3 isoforms were used so that phenotypic effects could be unambiguously assigned to transgene expression rather than to a disruptive tagged or foreign protein in the multiprotein desmosome.
Seven lines of transgenic mice were generated (five a lines, two of which were neonatal lethal, and two b lines). Genomic DNAs were analyzed by Southern analysis (not shown) and PCR (Fig. 1C). The a and b lines were also intercrossed, generating double transgenics (Fig. 1C). No phenotypic difference could be detected between mouse lines (see below), so the results presented here are for mice from the a lines. In addition, transgenics generated in both the ICR and C57BL/6 × CBA (F1) backgrounds gave similar phenotypes, implying that the defects observed were unambiguously attributable to transgene action.
To detect transgene expression, we raised a polyclonal antibody against a Dsc3 protein fragment common to the a and b forms (Fig. 1A). Western analysis could not distinguish between endogenous and transgene-encoded Dsc3, and surviving lines showed minor (never more than two- to three-fold) increases in expression levels (Fig. 1D). However, transgene expression monitored by immunohistochemistry showed strong expression in all layers, including the upper layers of the epidermis (Fig. 2A). In contrast, expression was always most abundant in basal layers in littermate controls (Fig. 2B). The expression of spinous layer Dsc3 was punctate at keratinocyte membranes in transgenics (Fig. 2C) and colocalized with desmoplakin, a ubiquitously expressed desmosomal component (not shown), indicating the incorporation of the protein into desmosomes. The expression of other desmosomal components was unaffected in transgenic mice, as shown by immunohistochemical detection (Fig. 2D [Dsc1 transgenic mouse], Fig. 2E [control mouse], and data not shown [Dsg1 and -3]; also, data for desmoplakin and plakoglobin are shown in Fig. 7) and Western analysis (not shown). Furthermore, the incorporation of Dsc3 into upper suprabasal desmosomes was demonstrated at the ultrastructural level by immunogold labeling (Fig. 2F to K). In wild-type animals, Dsc3 was incorporated into desmosomes from the lower (Fig. 2F) and middle (Fig. 2G) epidermal layers but was absent from the upper epidermal layers (Fig. 2H). However, in transgenic skin, desmosomes from all epidermal layers contained Dsc3 (Fig. 2I to K), directly demonstrating transgene-encoded Dsc3 incorporation into desmosomes (Fig. 2K).
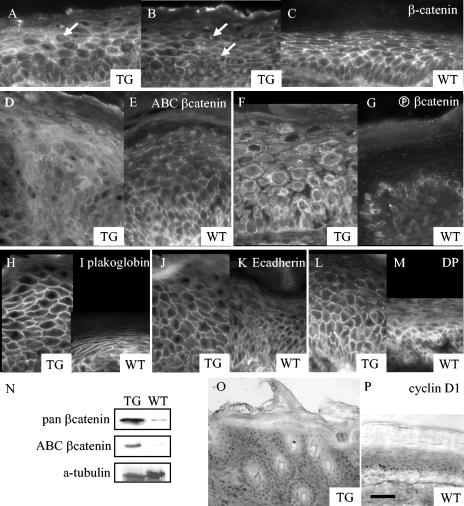
FIG. 7.
Changed β-catenin abundance and distribution in K1Dsc3 transgenic skin. (A and B) A pan-β-catenin antibody demonstrated membranous β-catenin associated with adherens junctions in all living strata of transgenic (A, B) and wild-type (C) skin. However, β-catenin was also detectable cytoplasmically and in nuclei (A and B, arrows) in suprabasal layers of transgenic skin. (D and E) The altered β-catenin pattern in transgenic skin was due to increased cytoplasmic and nuclear active β-catenin (ABC, detected with an antibody to an unphosphorylated N-terminal β-catenin epitope [42]) in the transgenic epidermis (D) compared to the control (E). (F and G) Transgenic skin had substantially increased cytoplasmic and nuclear inactive β-catenin (detected with anti-phospho-β-catenin, an antibody to a phosphorylated N-terminal β-catenin epitope [37]) in all layers of the transgenic epidermis (F). Labeling was confined to the cytoplasm of basal cells in the control epidermis (G). Note the perinuclear staining of keratinocytes in transgenic skin (G). In contrast, other protein components of the adherens junction (H and I, plakoglobin; J and K, E-cadherin; L and M, desmoplakin [DP]) showed similar distributions in transgenic (H, J, and L) and control (I, K, and M) epidermis. (N) Enhanced levels of total (pan) and active β-catenin in K1Dsc3 epidermis were confirmed by Western analysis. (O) Cyclin D immunolocalized to nuclei of suprabasal as well as basal strata in K1Dsc3a transgenic skin but was confined to the basal layer in wild-type epidermis (M [footpad skin was used to provide a similar number of suprabasal layers as the hyperproliferative transgenic control]). Bar, 20 μm (A to G), 30 μm (H to M), or 80 μm (O and P).
Suprabasal Dsc3 expression produces patterned alopecia initiating with the first postnatal hair cycle.
Transgenic mice appeared normal at birth. However, at 10 to 12 weeks the epidermis of approximately 50% of the transgenic mice showed localized progressive ventral alopecia (Fig. 3A to C), with later development in the remainder of the mice. There was considerable phenotypic variability in both the inbred and the outbred background, with severe cases showing more extensive hair loss. The phenotype occurred in animals housed in separate cages, eliminating the possibility that the phenotype arose from interactions, and it was not detected in control mice. Despite housing animals for more than 1 year, we found no detectable increase in overt tumor formation in transgenic mice compared to controls.
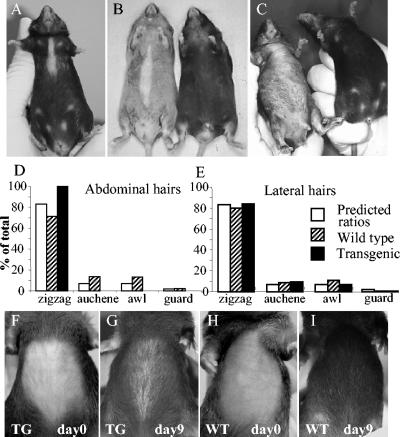
FIG. 3.
Hair defect in K1Dsc3 transgenic mice. (A to C) Transgenic mice developed a progressive ventral alopecia by 10 to 12 weeks which was absent in control littermates (B and C [compare transgenics with control littermates on the right]). The extent of ventral alopecia varied from a small ventral patch (A) to extensive ventral hair loss (C). Hairs in the affected regions were sparse and consisted entirely of zigzag hairs (D), while adjacent unaffected regions contained all four hair types in predicted ratios (E) (6). (F to I) Ventral alopecia accompanies the first postnatal hair cycle. The depilation of ventral skin 2 weeks after birth (F and H) resulted in phenotypic alopecia by 9 days in transgenic skin (G), while wild-type skin recovered (I).
The murine pelage coat consists of four distinct hair types that occur in conserved ratios (6). The development of each hair type appears to be regulated separately (e.g., see reference 8 and references within). A comparison of transgenic hairs from the affected ventral and unaffected adjacent regions showed that ventral transgenic skin hair was sparse and consisted entirely of the most abundant zigzag type (Fig. 3D), while all four hair types were present in unaffected adjacent skin at normal ratios (Fig. 3E). This implies that either (i) the overall number of follicles producing hair shafts was reduced so that the most abundant zigzags were all that remained to be detected or (ii) guard, awl, and auchennes hairs were specifically susceptible to degeneration.
To define the hair defects further, we plucked dorsal and ventral patches of hair from postnatal mice on day 14. This induced a new wave of synchronized anagen (new cycle) growth. There was no detectable difference in regrowth time between K1Dsc3 mice and control littermates on dorsal sites, but ventral hair regrowth was delayed in approximately 40% of transgenic mice, and the density of these regrown hairs was reduced. Ventrally patterned alopecia, typical of the transgenic phenotype (Fig. 3G), appeared immediately after plucking rather than at 10 to 12 weeks, showing that the hair defect was present by the first postnatal hair cycle.
Despite the apparent lack of a defect in dorsal regions, during much later hair cycles plucked transgenic mice could be readily distinguished from their transgenic littermates by a late developing alopecia confined to previously plucked dorsal regions (data not shown), suggesting that hair cycles over the entire animal were subtly affected in transgenic mice.
Suprabasal misexpression of Dsc3 alters epidermal proliferation and early terminal differentiation.
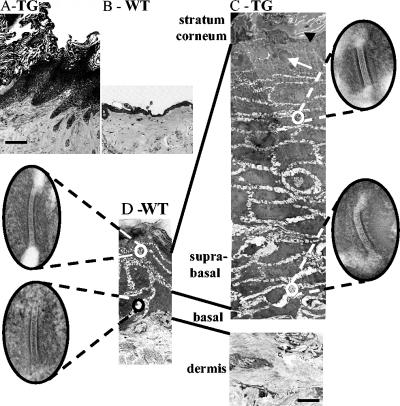
Within the ventral areas where follicles had degenerated (into utriculi and dermal cysts [see below and Fig. 6]), the epidermis was substantially thickened (Fig. 4, compare panels A and B), involving increased suprabasal and granular layers (acanthosis and hypergranulosis, respectively) with a thickened stratum corneum (hyperkeratosis). The flanking regions appeared normal. Electron microscopy showed a substantial increase in the number of suprabasal layers and an expanded granular layer with large keratohyalin granules (Fig. 4C, white arrow). This effect resulted in abnormal keratinization comprising an expanded stratum corneum and incompletely flattened electron-lucent corneocytes (Fig. 4, compare panels C and D). Desmosomes from all layers in the transgenic epidermis were comparable to those from controls (Fig. 4, insets).
FIG. 6.
Altered lipid distribution, follicle degeneration, and associated de- or transdifferentiation of follicles in K1Dsc3 transgenic skin. (A) SSLF was reduced or entirely absent in transgenic skin, as visualized by oil red O staining, compared to the prominent SSLF in site-matched control littermates (B). Also, the follicles were degenerated (A, arrow) and sebaceous glands were abnormally distributed and enlarged in transgenic skin (A) compared to control skin (B, arrow shows a normal cross-sectioned follicle). (C and D) In the transgenic, K1, which is normally expressed in the interfollicular epidermis (IFE) and the uppermost part of the follicle (D, arrow), was expressed in utriculi and dermal cysts deep in the dermis (C, arrow to utriculus; DC, dermal cyst). (E) K1Dsc3 skin showed a range of dermal cyst-like structures, ranging from sebum filled (left) to highly keratinized (right). (E [left], F, and G) Serial sections stained with oil red O, K14, and K1, respectively. This sebum-filled (E) cyst expressed both K14 (F) and, unexpectedly, the exclusively IFE marker K1 (G). (H and I) Cysts deep in the dermis stained with K1 (H) and loricrin (I) markers, which are not expressed in follicles but are characteristic of a differentiated IFE. Bar, 150 μm (A to D) or 40 μm (E to I).
FIG. 4.
K1Dsc3 transgenic mouse epidermis from ventral skin (A) was substantially thickened compared to control epidermis from the same site (B). The K1Dsc3 epidermis also showed significant hyperkeratosis (A). (C) Ultrastructural analysis by electron microscopy showed that the thicker epidermis was due to a substantially increased number of spinous layers (acanthosis) and granular layers (hypergranulosis) in the transgenic epidermis compared to the wild type (D). In addition, the flattened, anucleate keratinocytes of the transgenic stratum corneum (C, black arrow) appeared less compact than control keratinocytes (D, black arrow), and keratohyalin granules were abnormally large (C, white arrow). Desmosomes from all layers of transgenic skin (C, insets) were ultrastructurally comparable to those of control skin (D, insets). Bar, 100 μm (A and B), 5 μm (C and D), or 100 nm (insets).
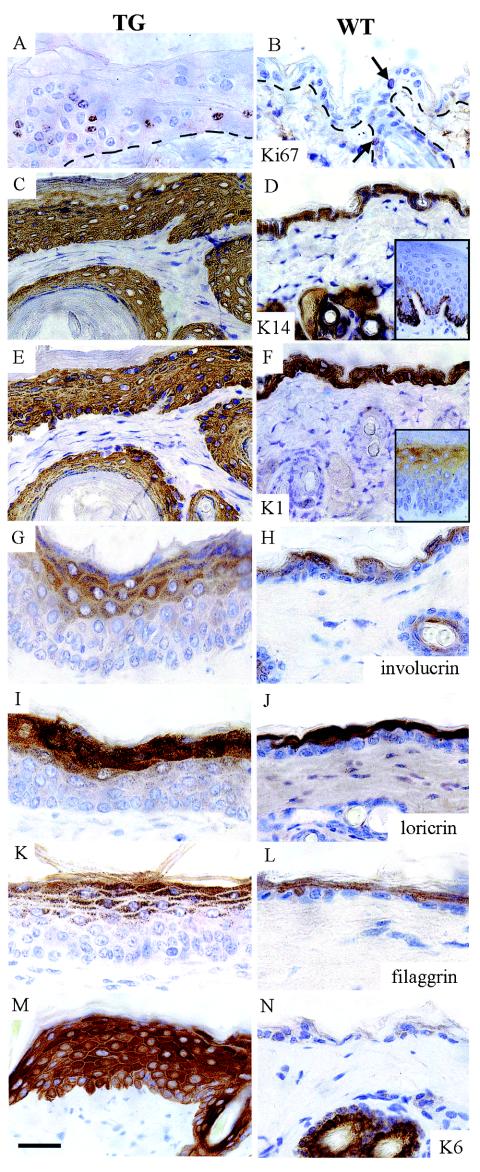
Acanthosis usually indicates increased basal layer proliferation or an altered epidermal turnover rate. Basal keratinocyte proliferation was increased in the transgenic epidermis (Fig. 5A and B). In addition, transgenic skin had proliferating cells in suprabasal layers. This suggests that the suprabasal cells, which then expressed increased Dsc3, had adopted some attributes of basal cells. This interpretation was supported biochemically when keratin 14 (K14), usually confined to basal layers, was expressed in suprabasal keratinocytes (Fig. 5C and D). Keratins 1 and 10 (K1 and K10), usually expressed in the first suprabasal layer and above, showed correct, although broadened, expression in the transgenic epidermis (Fig. 5 E and F [K1]; data not shown for K10) that was associated with an increased number of layers, as did the markers for late keratinocyte terminal differentiation (involucrin, loricrin, and filaggrin) (Fig. 5G to L). Keratin 6 (K6), a marker for a perturbed, diseased, and follicular epidermis, is usually absent from the postnatal interfollicular epidermis (Fig. 5N). However, K6 was expressed abundantly in the transgenic epidermis (Fig. 5M). Hence, the suprabasal expression of Dsc3 causes perturbed early terminal differentiation in transgenic mice, consistent with the proposal that stratum-specific desmocollin expression has functional consequences for differentiation and, possibly, cell positioning. The late terminal differentiation changes were probably consequences of early proliferation changes.
FIG. 5.
Altered terminal differentiation in transgenic epidermis overexpressing Dsc3 suprabasally. (A, C, E, G, I, K, and M) Epidermis from transgenic ventral skin; (B, D, F, H, J, L, and N) Site-matched epidermis from littermate controls. (A and B) Ki67 immunohistochemistry showed that basal layer proliferation was increased in K1Dsc3 epidermis and that proliferative cells were found suprabasally. Arrows show proliferating cells in control epidermis. (C and D) K14immunohistochemistry showed broadened basal and suprabasal expression of keratin 14 in transgenic skin (C). K14 is usually confined to the basal layer in wild-type skin. The inset in panel D shows basal K14 expression in the thicker epidermis from the foot of a control animal, as basal expression is difficult to demonstrate in the thin ventral skin of wild-type mice. (E and F) K1 immunohistochemistry showed broadened expression of suprabasally expressed keratin 1, which was associated with acanthosis in the transgenic epidermis (E). The inset in panel F shows suprabasal K1 expression in the thicker epidermis from the foot of a control animal. Involucrin (G and H), loricrin (I and J), and filaggrin (K and L) also showed broadened expression patterns in the K1Dsc3 epidermis (G, I, and K) which were associated with hypergranulosis compared to control epidermis (H, J, and L), although the differentiation patterns were essentially normal. Keratin 6 was induced in the thickened epidermis of transgenic mice (M) but was undetectable in the control interfollicular epidermis (N). Note the normal follicular expression. Bar, 30 μm (A, B, and G to N), 40 μm (C to F), or 80 μm (insets).
Follicle degeneration and keratinocyte transdifferentiation.
Hair follicles within K1Dsc3-affected areas degenerated into keratinized structures called utriculi (Fig. 6A and C, arrows), which are derived from normal follicles (Fig. 6B and D, arrows). Dermal cysts were also present (Fig. 6C and E to I). A subset of dermal cysts were filled with trapped sebum, presumably derived from degenerating follicles (Fig. 6E). Hair follicle sebaceous gland-derived lipids are normally secreted into the hair shaft and extruded to the epidermal surface, where they mix with epidermal-derived lipids to form the protective skin surface lipid film (SSLF). Affected areas showed a significant reduction in SSLF compared to those in control littermates, as demonstrated by oil red O staining (Fig. 6, compare panels A and B), probably because of reduced production from degenerating follicles. A lack of SSLF may contribute to local skin lesions in response to irritation or infection.
Keratin 1 (K1) is usually expressed only in the interfollicular epithelium (IFE) and the uppermost cells of hair follicles (Fig. 6D). However, transgenic skin cysts and degenerate follicles deep in the dermis, including those with trapped sebum, also showed K1 expression (Fig. 6C, G, and H), which never occurs in control follicles this deep in the normal dermis (Fig. 6D). K1-positive cysts ranged from sebum filled (Fig. 6E, left panel) to highly keratinized (Fig. 6E, right panel). Serially sectioned cysts (Fig. 6E, F, and G) contained trapped sebum, indicating their follicular origin (Fig. 6E); K14, both an IFE and follicular basal cell marker (Fig. 6F); and K1, an exclusively IFE differentiation marker (Fig. 6G). Keratinizing cysts from deep within the dermis expressed both K1 (Fig. 6H) and, adjacent to the keratinized core, loricrin (Fig. 6I), a marker specific for very late terminally differentiated interfollicular keratinocytes (i.e., a nonfollicular marker). This strongly suggested the existence of metaplasia or the transdifferentiation of follicular keratinocyte stem cells to an interfollicular differentiation pathway.
Similar utriculi and dermal cysts have been noted in mice with altered Wnt signaling. Transdifferentiation also occurs in these mice and is attributable to their altered cell lineage (18, 25, 31, 44).
Free β-catenin is elevated in transgenic skin.
K1Dsc3 transgenic mice share similarities with mice that are defective in β-catenin signaling. Hence, β-catenin levels and signaling activity were investigated, with the expectation that β-catenin levels would be similarly reduced. Surprisingly, an antibody to total cellular β-catenin (pan-β-catenin) showed excess suprabasal β-catenin in the cytoplasm and nuclei of transgenic skin cells (Fig. 7A and B, arrows indicate nuclei), although it was undetectable in wild-type skin (Fig. 7C [a thicker wild-type foot epidermis is shown as a control, as it has a similar number of suprabasal layers]) or in skin from nonphenotypic adjacent regions (not shown). To confirm this observation, we used an antibody specific for dephosphorylated active β-catenin (anti-active β-catenin [anti-ABC]) (42) and an antibody specific for phosphorylated β-catenin destined for degradation (anti-phospho-β-catenin) (38). Anti-ABC staining confirmed that both cytoplasmic and nuclear active β-catenin, usually confined to the membrane and a few basal layer cells in the wild-type epidermis (Fig. 7E) (30, 42), was present suprabasally in affected transgenic skin (Fig. 7D). Even more striking was the extensive amount of suprabasal phosphorylated β-catenin, which is confined to basal keratinocytes in wild-type skin (Fig. 7G) but was found extensively in suprabasal cells of transgenic affected skin (Fig. 7F). The thinner transgenic skin from flanking unaffected regions did not show detectable enhanced β-catenin at the immunohistochemical level (data not shown). However, a Western analysis of the total epidermis from unaffected skin (prephenotypic newborn skin) did show an upregulation of both the total and signaling (ABC) forms of β-catenin in transgenics compared to controls (Fig. 7N).
Plakoglobin intracellular localization appeared to be unaffected in transgenic affected skin (Fig. 7H and I), as did that of E-cadherin (Fig. 7J and K) and desmoplakin (Fig. 7L and M), indicating a normal distribution of these desmosomal components.
Since β-catenin was stabilized in suprabasal transgenic keratinocytes, we looked for the upregulation of β-catenin transcriptional targets (reviewed in reference 13). Cyclin D1 showed enhanced expression in transgenic affected skin by Western analysis (not shown) and immunohistochemistry (Fig. 7, compare panels O and P), although c-Myc levels appeared to be unchanged (not shown). Cyclin D1 levels were raised in suprabasal strata, consistent with induction by β-catenin/TCF and suggesting a mechanism for hyperplasia.
Dsc3 regulates β-catenin in cultured keratinocytes.
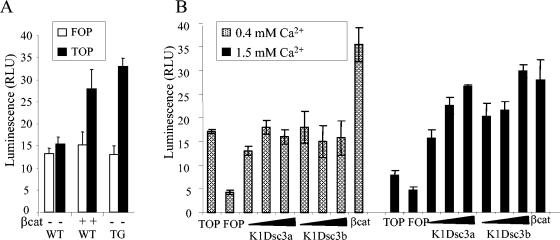
To test directly for enhanced β-catenin/Tcf-mediated transcription, we transfected primary keratinocytes cultured from transgenic and control newborn (3 days postnatal) epidermis with the Tcf optimal promoter (TOP)-flash reporter gene. A FOP-flash vector with mutated Lef sites was used as a control. As a positive control, wild-type (WT) keratinocytes were additionally transfected with Xenopus laevis β-catenin (Fig. 8A). Mouse skin at 3 days is prephenotypic, yet transgenic keratinocytes showed significantly enhanced TOP-flash activity after calcium-induced differentiation compared to nontransgenic control keratinocytes (Fig. 8A). Hence, β-catenin signaling activity preceded the onset of the phenotype.
FIG. 8.
(A) Primary keratinocytes from K1Dsc3a newborn epidermis (TG) show enhanced ability to activate the TOP-flash reporter gene compared to control keratinocytes (WT) or keratinocytes transfected with constitutively active β-catenin (βcat), indicating an enhanced ability to activate Lef/Tcf downstream genes. (B) A human keratinocyte cell line (N/TERT-1) transfected with K1Dsc3a and K1Dsc3b transgenes did not activate ΤΟP-flash in the presence of 0.4 mM calcium, a level in which desmosomes can form but expression of the transgene promoter is minimal. However, upon transfer to a high calcium level (1.5 mM) to activate the transgene promoter, K1Dsc3a and K1Dsc3b both activated TOP-flash activity in a dose-dependent manner compared to cells transfected with TOP-flash alone (TOP), FOP-flash alone (FOP), or a transgene and FOP-flash (not shown). Cells transfected with constitutively active β-catenin/TOP-flash (βcat) provided a positive control. Note the higher endogenous β-catenin activity in keratinocytes transfected with TOP-flash alone and grown in low (0.4 mM) calcium than in the same keratinocytes grown in 1.5 mM calcium. Error bars, standard errors of the means.
To confirm the relationship between K1Dsc3 expression and β-catenin transcriptional activity, we performed transfections by using the N/TERT-1 human keratinocyte line, which is capable of authentic calcium-induced differentiation and the expression of keratin 1 (5). N/TERT-1 cells form adhesion complexes in 0.4 mM calcium, with keratin 1 expression being minimal (5). When the calcium level is raised to 1.5 mM, there is a substantial induction of keratin 1 (keratin 1 is the transgene promoter).
At the lower calcium level (0.4 mM), there was no detectable transgene-induced TOP-flash activation (Fig. 8B). Significantly, at this lower calcium level there was a high level of endogenous β-catenin activity, consistent with β-catenin activity being associated most strongly with basal, undifferentiated keratinocytes in vivo (Fig. 7G and 8B).
At the higher calcium level (1.5 mM, in which keratinocytes had differentiated and the transgenes were most strongly expressed) (Fig. 8B), keratinocytes transfected with either K1Dsc3a or K1Dsc3b showed enhanced, dose-dependent TOP-flash activity, indicating a transgene-dependent activation of β-catenin signaling. In differentiated cells, TOP-flash activation and hence endogenous β-catenin levels were low. This correlated with the lack of immunohistochemical localization of β-catenin in suprabasal keratinocytes noted in wild-type tissue sections (Fig. 7G).
Hence, we have shown by the use of two independent models that transgene-mediated Dsc3a and Dsc3b expression in differentiating keratinocytes enhances β-catenin signaling.
These data show that (i) changed desmosomal cadherin ratios affect signaling through β-catenin in epidermal keratinocytes, (ii) both the Dsc3a and Dsc3b isoforms affect β-catenin signaling, and (iii) this signaling change is dependent on transgene expression and precedes the onset of a phenotype, consistent with the differentiation phenotype being a consequence of enhanced β-catenin signaling.
DISCUSSION
Desmososomal cadherins influence keratinocyte differentiation.
We showed in this study that the misdirected expression of basally abundant Dsc3 to suprabasal keratinocytes induces β-catenin stabilization and transcriptional activity. We used transgenic models with minor changes in Dsc expression levels conferred by untagged murine Dsc genes under the control of a promoter that does not induce expression until late in gestation, thus avoiding developmental effects. The transgene was not expressed in the keratinocyte granular layer cells, avoiding the complication of skin barrier defects. We clearly demonstrated an increased suprabasal desmosomal incorporation of transgene-encoded protein. Hence, the changed β-catenin stability and transcriptional activity can be attributed unambiguously to the dominant molecular change, i.e., the misexpression of Dsc3 in suprabasal keratinocytes.
Since we showed that a change in desmocollin expression induces β-catenin stabilization and signaling, we propose that the consequent differentiation and follicular changes are at least partly attributable to altered β-catenin signaling.
Dsc a and b isoform functions.
Each Dsc isoform exists as a and b forms that are generated by differential splicing and differ at their cytoplasmic C termini. The function of the Dsc b form is unknown. A reasonable hypothesis would be that the b form mediates or regulates Dsc signaling activity through its distinct cytoplasmic domain. However, we found that K1Dsc3a and K1Dsc3b mice have similar phenotypes and that K1Dsc3a and K1Dsc3b constructs have similar β-catenin-inducing activities in culture, implying that a signaling role for the distinct portions of the tails is unlikely and indicating that the signaling function resides elsewhere in the molecule, probably in the conserved portions of the tails. In agreement with this conclusion, mice with deleted a- and b-specific Dsc1 cytoplasmic tails do not show altered differentiation (4).
Both Dsc3a and -b interact with another desmosomal protein, plakophilin 3, (2). Plakophilins are armadillo family desmosomal components that link desmosomal cadherins to the intermediate filament network via a desmoplakin linker. The Dsc3b-plakophilin interaction represents the only known Dsc3b-protein interaction (2), suggesting that a plakophilin may be involved in signaling from Dsc3 isoforms.
The involvement of Dsc3b in epidermal differentiation regulation, as demonstrated in this work, is the first recorded function for a Dsc b isoform.
Link between desmosomal cadherin composition and epidermal β-catenin signaling.
Links between desmosomal components and β-catenin signaling have been noted previously. Plakophilin 2 binds β-catenin in vitro, and experimental upregulation enhances β-catenin signaling (3), although the mechanism is unknown. Intriguingly, plakophilin 2 is basally expressed in the epidermis, where it should interact with Dsc3. Hence, suprabasal Dsc3 expression in transgenic animals may bind and concentrate plakophilin 2 signaling activity suprabasally, suggesting a first step in the pathway linking the desmosome and β-catenin stability. However, available plakophilin 2 antibodies are inadequate for testing this theory because they do not react with the mouse epidermis, and this prediction is a subject of further investigation.
Plakoglobin, an arm-repeat protein that is a constituent of both desmosomes and adherens junctions, is another possible link to β-catenin signaling. It binds preferentially to desmosomal cadherins but also to E-cadherin from the adherens junction. Plakoglobin can partially substitute for the adhesive function of β-catenin in knockout mice, but not for its signaling function (18). However, plakoglobin can apparently substitute for β-catenin signaling activity in some experimental situations by displacing β-catenin from the adherens junctions and releasing it for signaling (27, 46). These findings show that transjunction plakoglobin movement can occur, and they open up the possibility that the perturbation of plakoglobin in transgenic suprabasal desmosomes makes it available for movement to adherens junctions, where it may displace β-catenin for signaling. However, a change in plakoglobin distribution was not detected in K1Dsc3 mice (Fig. 7, compare panels H and I).
Suprabasal β-catenin and follicular defects.
The down-regulation of β-catenin in basal keratinocytes causes similar hair follicle defects as the suprabasal stabilization of β-catenin in K1Dsc3 transgenic mice, i.e., up- and down-regulation produce similar defects (25, 31). These include hair follicle degeneration and the formation of dermal cysts which appear to transdifferentiate into an interfollicular phenotype (Fig. 6). The stabilization of β-catenin in basal keratinocytes results in new follicle formation (11, 24, 41), in contrast to the follicular degeneration associated with suprabasal stabilized β-catenin in K1Dsc3 mice. However, presenilin epidermal null mice with stabilized β-catenin in all epidermal strata resemble K1Dsc3 mice (44) in that they too have degenerating follicles.
Follicular changes in all transgenic models must arise from changes in follicular stem cells, which are located in the bulge region of the follicle (reviewed in reference 33). Follicular degeneration in K1Dsc3 mice, and probably in presenilin mice, could not arise from stabilized β-catenin in the follicular stem cells, as it is well established that this produces de novo follicle formation. It is more likely that follicular degeneration is a secondary effect on these stem cells that is derived from signaling from adjacent transgenic cells. Indeed, the keratin 1 promoter should not be expressed in follicular stem cells, as keratin 1 is expressed in the upper, permanent portion of the hair follicle, or isthmus (35) (Fig. 6D), adjacent to the bulge region.
It is possible that signaling from adjacent transgenic cells destabilizes β-catenin in follicular stem cells, explaining the phenocopy of mice with basally decreased β-catenin signaling, although this would be extremely difficult to demonstrate experimentally. Analyses of follicular phenotypes are complicated by transdifferentiation (Fig. 6G to I), which induces abnormal keratin 1 expression in degenerating follicles and cysts, resulting in de novo follicular transgene expression and the exacerbation and complication of the phenotype.
Confinement of differentiation phenotype to the ventral region.
K1Dsc3 mice showed enhanced β-catenin signaling in prephenotypic skin, and the transgene induced β-catenin signaling in cultured keratinocytes. However, signaling was particularly enhanced and only detectable immunohistologically in the ventral skin of transgenic mice. In addition, the differentiation phenotype was most prominent in ventral skin, although subtle phenotypic defects such as plucking-induced alopecia could be detected dorsally. Thus, K1Dsc3 mice phenocopy a growing range of transgenic and knockout models in which the phenotype is ventrally associated. These include RXRα receptor epidermal null mice (22), vitamin D receptor null mice (23), phosphoinositide-specific phospholipase Cδ1 null mice (28), and K14 parathyroid hormone-related protein transgenic mice (1). In each case, the association of the phenotype to the ventral skin is unexplained.
These ventral phenotypes highlight previously unexplored differences in murine ventral and dorsal skin and imply that the action of desmosomal cadherins on β-catenin must be modulated by additional, regionally associated signaling pathways in skin. This complexity in phenotype manifestation and dependence on regional differences in skin may provide an explanation for conflicting reports on differentiation and signaling roles for desmosomal cadherins in other transgenic or null mice.
Tumor formation.
The epidermal expression of stabilized β-catenin in basal keratinocytes results in tumor formation (11, 30, 44). In addition, natural mutations that interfere with β-catenin degradation, either through the stabilization of β-catenin itself or through a disruption of the degradation machinery, are tumorigenic (reviewed in references 13 and 34). K1Dsc3 mice with enhanced β-catenin stabilization and signaling showed hyperplasia and metaplasia but did not develop tumors at an appreciable rate. This may have been because suprabasal β-catenin stabilization in K1Dsc3 mice mainly involves keratinocytes that are destined to be shed during terminal differentiation rather than the basal or follicle stem cells, whose permanent residence permits the accumulation of additional mutations that are necessary for tumor formation. The need for additional mutations accompanying β-catenin stabilization for tumor formation is highlighted in Notch1 and presenilin epidermal null mice, for which the stabilization of β-catenin was shown to be an initiating event in keratinocyte neoplastic transformation (30, 44). In addition, β-catenin overexpression in cultured keratinocytes is insufficient to induce transformation (45), which is interpreted as a need for an additional mutation(s).
Intriguingly, suprabasal Dsc3 expression has been detected in cases of Bowen's disease (skin carcinoma in situ) and squamous cell carcinoma, but not actinic keratoses (dysplastic precancerous lesions) (e.g., see reference 21), suggesting that the misexpression of desmocollins may contribute to the progression of skin cancer.
In summary, we generated transgenic mice with increased Dsc3a and Dsc3b expression in suprabasal keratinocytes. This expression change produced a primary phenotype of stabilized β-catenin signaling in suprabasal keratinocytes from both cadherins and a downstream phenotype of changed keratinocyte differentiation and follicular defects. Hence, we showed that the Dsc3a and Dsc3b cadherin isoforms can signal in keratinocytes and affect differentiation.
Acknowledgments
This work was supported by a BBSRC grant (G08620) to C.B. and D.G. and by an MRC career establishment grant (G9803920) to C.B. A.A.A. is supported by Cancer Research UK, and A.M. is supported by MRC (99630879).
We thank Damian Marshall and Sarah Kirk for assistance with Dsc3 antibody production and electron microscopy, Dennis Roop for the K1 targeting vector, and Jim Rheinwald for cell lines and protocols.
REFERENCES
- 1.Abdalkhani, A., R. Sellers, J. Gent, H. Wulitich, S. Childress, B. Stein, R. E. Boissy, J. J. Wysolmerski, J. Foley, P. Dann, J. Hong, J. Cosgrove, B. Dreyer, D. Rimm, M. Dunbar, W. Philbrick, and J. Wysolmerski. 2002. Nipple connective tissue and its development: insights from the K14-PTHrP mouse. Mech. Dev. 115:63-77. [DOI] [PubMed] [Google Scholar]
- 2.Bonne, S., B. Gilbert, M. Hatzfeld, X. Chen, K. J. Green, and F. van Roy. 2003. Defining desmosomal plakophilin-3 interactions. J. Cell Biol. 161:403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen, X., S. Bonne, M. Hatzfeld, F. van Roy, and K. J. Green. 2002. Protein binding and functional characterization of plakophilin 2. Evidence for its diverse roles in desmosomes and beta-catenin signaling. J. Biol. Chem. 277:10512-10522. [DOI] [PubMed] [Google Scholar]
- 4.Cheng, X., K. Mihindukulasuriya, Z. Den, A. P. Kowalczyk, C. C. Calkins, A. Ishiko, A. Shimizu, and P. J. Koch. 2004. Assessment of splice variant-specific functions of desmocollin 1 in the skin. Mol. Cell. Biol. 24:154-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dickson, M. A., W. C. Hahn, Y. Ino, V. Ronfard, J. Y. Wu, R. A. Weinberg, D. N. Louis, F. P. Li, and J. G. Rheinwald. 2000. Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol. Cell. Biol. 20:1436-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dry, F. W. 1926. The coat of the mouse (Mus musculus). J. Genet. 16:287-340. [Google Scholar]
- 7.Elias, P. M., N. Matsuyoshi, H. Wu, C. Lin, Z. H. Wang, B. E. Brown, and J. R. Stanley. 2001. Desmoglein isoform distribution affects stratum corneum structure and function. J. Cell Biol. 153:243-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellis, T., I. Smyth, E. Riley, J. Bowles, C. Adolphe, J. A. Rothnagel, C. Wicking, and B. J. Wainwright. 2003. Overexpression of Sonic Hedgehog suppresses embryonic hair follicle morphogenesis. Dev. Biol. 263:203-215. [DOI] [PubMed] [Google Scholar]
- 9.Garrod, D., M. Chidgey, and A. North. 1996. Desmosomes: differentiation, development, dynamics and disease. Curr. Opin. Cell Biol. 8:670-678. [DOI] [PubMed] [Google Scholar]
- 10.Garrod, D. R., A. J. Merritt, and Z. Nie. 2002. Desmosomal cadherins. Curr. Opin. Cell Biol. 14:537-545. [DOI] [PubMed] [Google Scholar]
- 11.Gat, U., R. DasGupta, L. Degenstein, and E. Fuchs. 1998. De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell 95:605-614. [DOI] [PubMed] [Google Scholar]
- 12.Getsios, S., A. C. Huen, and K. J. Green. 2004. Working out the strength and flexibility of desmosomes. Nat. Rev. Mol. Cell Biol. 5:271-281. [DOI] [PubMed] [Google Scholar]
- 13.Giles, R. H., J. H. van Es, and H. Clevers. 2003. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim. Biophys. Acta 5:1-24. [DOI] [PubMed] [Google Scholar]
- 14.Greenhalgh, D. A., X. J. Wang, and D. R. Roop. 1997. Expression of a dominant-negative type II transforming growth factor (TGF) receptor in the epidermis of transgenic mice blocks TGF-mediated growth inhibition. Proc. Natl. Acad. Sci. USA 94:2386-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hager, B., J. R. Bickenbach, and P. Fleckman. 1999. Long-term culture of murine epidermal keratinocytes. J. Investig. Dermatol. 112:971-976. [DOI] [PubMed] [Google Scholar]
- 16.Hardman, M. J., P. Sisi, D. N. Banbury, and C. Byrne. 1998. Patterned acquisition of skin barrier function during development. Development 125:1541-1552. [DOI] [PubMed] [Google Scholar]
- 17.Henkler, F., M. Strom, K. Mathers, H. Cordingley, K. Sullivan, and I. King. 2001. Transgenic misexpression of the differentiation-specific desmocollin isoform 1 in basal keratinocytes. J. Investig. Dermatol. 116:144-149. [DOI] [PubMed] [Google Scholar]
- 18.Huelsken, J., R. Vogel, B. Erdmann, G. Cotsarelis, and W. Birchmeier. 2001. Beta-catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 105:533-545. [DOI] [PubMed] [Google Scholar]
- 19.Ishii, K., and K. J. Green. 2001. Cadherin function: breaking the barrier. Curr. Biol. 11:R569-R572. [DOI] [PubMed] [Google Scholar]
- 20.Kljuic, A., and A. M. Christiano. 2003. A novel mouse desmosomal cadherin family member, desmoglein 1 gamma. Exp. Dermatol. 12:20-29. [DOI] [PubMed] [Google Scholar]
- 21.Kurzen, H., I. Munzing, and W. Hartschuh. 2003. Expression of desmosomal proteins in squamous cell carcinomas of the skin. J. Cutan. Pathol. 30:621-630. [DOI] [PubMed] [Google Scholar]
- 22.Li, M., H. Chiba, X. Warot, N. Messaddeq, C. Gerard, P. Chambon, and D. Metzger. 2001. RXR-alpha ablation in skin keratinocytes results in alopecia and epidermal alterations. Development 128:675-688. [DOI] [PubMed] [Google Scholar]
- 23.Li, Y. C., A. E. Pirro, M. Amling, G. Delling, R. Baron, R. Bronson, and M. B. Demay. 1997. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc. Natl. Acad. Sci. USA 94:9831-9835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lo Celso, C., D. M. Prowse, and F. M. Watt. 2004. Transient activation of beta-catenin signalling in adult mouse epidermis is sufficient to induce new hair follicles but continuous activation is required to maintain hair follicle tumours. Development 131:1787-1799. [DOI] [PubMed] [Google Scholar]
- 25.Merrill, B. J., U. Gat, R. DasGupta, and E. Fuchs. 2001. Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev. 15:1688-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merritt, A. J., M. Y. Berika, W. Zhai, S. E. Kirk, B. Ji, M. J. Hardman, and D. R. Garrod. 2002. Suprabasal desmoglein 3 expression in the epidermis of transgenic mice results in hyperproliferation and abnormal differentiation. Mol. Cell. Biol. 22:5846-5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miravet, S., J. Piedra, J. Castano, I. Raurell, C. Franci, M. Dunach, and A. Garcia De Herreros. 2003. Tyrosine phosphorylation of plakoglobin causes contrary effects on its association with desmosomes and adherens junction components and modulates beta-catenin-mediated transcription. Mol. Cell. Biol. 23:7391-7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura, Y., K. Fukami, H. Yu, K. Takenaka, Y. Kataoka, Y. Shirakata, S. Nishikawa, K. Hashimoto, N. Yoshida, and T. Takenawa. 2003. Phospholipase Cdelta1 is required for skin stem cell lineage commitment. EMBO J. 22:2981-2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelson, W. J., and R. Nusse. 2004. Convergence of Wnt, beta-catenin, and cadherin pathways. Science 303:1483-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicolas, M., A. Wolfer, K. Raj, J. A. Kummer, P. Mill, M. Van Noort, C. C. Hui, H. Clevers, G. P. Dotto, and F. Radtke. 2003. Notch1 functions as a tumor suppressor in mouse skin. Nat. Genet. 33:416-421. [DOI] [PubMed] [Google Scholar]
- 31.Niemann, C., D. M. Owens, J. Hulsken, W. Birchmeier, and F. M. Watt. 2002. Expression of DeltaNLef1 in mouse epidermis results in differentiation of hair follicles into squamous epidermal cysts and formation of skin tumours. Development 129:95-109. [DOI] [PubMed] [Google Scholar]
- 32.North, A. J., M. A. J. Chidgey, J. P. Clarke, W. G. Bardsley, and D. R. Garrod. 1996. Distinct desmocollin isoforms occur in the same desmosomes and show reciprocally graded distributions in bovine nasal epidermis. Proc. Natl. Acad. Sci. USA 93:7701-7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paus, R., and G. Cotsarelis. 1999. The biology of hair follicles. N. Engl. J. Med. 341:491-497. [DOI] [PubMed] [Google Scholar]
- 34.Polakis, P. 2000. Wnt signaling and cancer. Genes Dev. 14:1837-1851. [PubMed] [Google Scholar]
- 35.Rothnagel, J. A., M. A. Longley, D. Bundman, D. A. Greenhalgh, A. M. Dominey, and D. R. Roop. 1990. Targeting gene expression to the epidermis of transgenic mice: potential applications to genetic skin disorders. J. Investig. Dermatol. 95:59S-61S. [DOI] [PubMed] [Google Scholar]
- 36.Runswick, S. K., M. J. O'Hare, L. Jones, C. H. Streuli, and D. R. Garrod. 2001. Desmosomal adhesion regulates epithelial morphogenesis and cell positioning. Nat. Cell Biol. 3:823-830. [DOI] [PubMed] [Google Scholar]
- 37.Sadot, E., M. Conacci-Sorrell, J. Zhurinsky, D. Shnizer, Z. Lando, D. Zharhary, Z. Kam, A. Ben-Ze'ev, and B. Geiger. 2002. Regulation of S33/S37 phosphorylated beta-catenin in normal and transformed cells. J. Cell Sci. 115:2771-2780. [DOI] [PubMed] [Google Scholar]
- 38.Sadot, E., I. Simcha, K. Iwai, A. Ciechanover, B. Geiger, and A. Ben-Ze'ev. 2000. Differential interaction of plakoglobin and beta-catenin with the ubiquitin-proteasome system. Oncogene 19:1992-2001. [DOI] [PubMed] [Google Scholar]
- 39.Troyanovsky, S. M., L. G. Eshkind, R. B. Troyanovsky, R. E. Leube, and W. W. Franke. 1993. Contributions of cytoplasmic domains of desmosomal cadherins to desmosome assembly and intermediate filament anchorage. Cell 72:561-574. [DOI] [PubMed] [Google Scholar]
- 40.van de Wetering, M., R. Cavallo, D. Dooijes, M. van Beest, J. van Es, J. Loureiro, A. Ypma, D. Hursh, T. Jones, A. Bejsovec, M. Peifer, M. Mortin, and H. Clevers. 1997. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell 88:789-799. [DOI] [PubMed] [Google Scholar]
- 41.Van Mater, D., F. T. Kolligs, A. A. Dlugosz, and E. R. Fearon. 2003. Transient activation of beta-catenin signaling in cutaneous keratinocytes is sufficient to trigger the active growth phase of the hair cycle in mice. Genes Dev. 17:1219-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Noort, M., J. Meeldijk, R. van der Zee, O. Destree, and H. Clevers. 2002. Wnt signaling controls the phosphorylation status of beta-catenin. J. Biol. Chem. 277:17901-17905. [DOI] [PubMed] [Google Scholar]
- 43.Whittock, N. V., and C. Bower. 2003. Genetic evidence for a novel human desmosomal cadherin, desmoglein 4. J. Investig. Dermatol. 120:523-530. [DOI] [PubMed] [Google Scholar]
- 44.Xia, X., S. Qian, S. Soriano, Y. Wu, A. M. Fletcher, X. J. Wang, E. H. Koo, X. Wu, and H. Zheng. 2001. Loss of presenilin 1 is associated with enhanced beta-catenin signaling and skin tumorigenesis. Proc. Natl. Acad. Sci. USA 98:10863-10868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu, A. J., and F. M. Watt. 1999. Beta-catenin signalling modulates proliferative potential of human epidermal keratinocytes independently of intercellular adhesion. Development 126:2285-2298. [DOI] [PubMed] [Google Scholar]
- 46.Zhurinsky, J., M. Shtutman, and A. Ben-Ze'ev. 2000. Differential mechanisms of LEF/TCF family-dependent transcriptional activation by beta-catenin and plakoglobin. Mol. Cell. Biol. 20:4238-4252. [DOI] [PMC free article] [PubMed] [Google Scholar]