Abstract
Aggressive angiomyxoma (AAM) is a rare mesenchymal tumor that usually occurs in the pelvis and perineum of young females. AAM can simulate Bartholin's gland cyst, abscess, lipoma, simple labial cyst, or other pelvic soft tissue tumors. Here we present five cases of AAM with mean age of 42. The patients mainly presented slow-growing mass in the abdomen and perineum (3 cases in the pelvis, 1 in the vulva, and 1 in the buttock). Color Doppler flow imaging revealed blood flow for the 3 pelvic lesions. Enhanced computed tomography and magnetic resonance imaging of the other 2 cases showed the typical “swirled” or “layered” structure characteristic. Through the pathological examination, its positivity to estrogen and progesterone receptors can justify enlargement and recurrence, confirming the tumor is AAM. All 5 patients underwent local tumor resection. Two patients recurred 8 and 15 months after surgery, respectively. The longest follow-up was 42 months. Although few cases are reported, early recognition demands high index of suspicion for both gynaecologists and pathologists. Wide surgical excision with tumor free margins is the basis of curative treatment. Adjuvant therapy may be necessary for residual or recurrent tumors. Long-term follow-up is recommended.
Keywords: aggressive angiomyxoma, pathology, pelvic tumors, surgical treatment
1. Introduction
Aggressive angiomyxoma (AAM) is a rare locally aggressive soft tissue tumor, which primarily occurs in the pelvic–perineal regions. The incidence in females is significantly higher than in males (female-to-male ratio of 6:1).[1,2] The main site of disease is the perineum, followed by the pelvic cavity and vagina. In 1863, Virchow first classified myxomata as a distinct type of soft tissue tumors, whose tissues were similar to the normal human umbilical cord tissues with considerable variation in the organization of the tumor stroma. In 1983, after reviewing the cases reported over a hundred years, based on the clinical and pathological features of 9 patients, Steeper and Rosai[3] named this tumor “aggressive angiomyxoma” for the first time. The 2003 third edition and 2013 fourth edition of the World Health Organization Classification of Bone and Soft Tissue tumors, classified it as a tumor of uncertain differentiation, and named as deep (aggressive) angiomyxoma.[1] At present, the reports of this disease in China are mainly case reports, and there is a lack of summarized analysis of a vast number of cases. Hence, the disease is easily misdiagnosed as vulval abscess, Bartholin gland cyst, or others before surgery. Since the bulk of the tumor is often concealed within the deep soft tissues and it does not lead to rectal, urethral, vaginal, or vascular obstruction, most tumors are quite large as observed during resection. In the present report, 5 cases of AAM of the female pelvic cavity are reported. Furthermore, the pathological morphology and immunophenotype are discussed in order to raise awareness of the disease and avoid misdiagnosis.
2. Case report
The study was approved by the ethics committee of our Hospital. All patients provided a written informed consent. After reviewing the entire pathology database covering January 2012 to December 2015, 5 cases of AAM were diagnosed at the Department of Pathology of the Beijing Chao-Yang Hospital (Jingxi Hospital District), Capital Medical University. This hospital has about 3600 medical and support staff members and room for 1910 patients. All patients were female, aged between 31 and 50 years (mean age of 42). Among them, 3 lesions occurred in the pelvis; these patients were admitted to the Department of Hernia Surgery as “pelvic floor hernia.” One patient case was admitted as “reducible mass in the left buttock.” One lesion occurred in the perineum, and the patient was admitted to the Department of Gynecology and Obstetrics as “perineal mass.” The patients with tumors in the pelvis were discovered while undergoing B-ultrasound because of an abdominal bulge. The patient with a tumor in the perineum was feeling a slight pain and pulsatory feeling. All 5 patients underwent preoperative imaging examination of the related sites, including 3 cases of ultrasound, 1 case of computed tomography (CT), and 1 case of magnetic resonance imaging (MRI). B-ultrasound of the 3 cases suggested a homogeneous hypoechoic area in the perineum with clear border and irregular shape that could contract. Among them, case 1 and case 2 were reducible while case 5 was not. Color Doppler flow imaging signals suggested a small amount of internal blood flow (Fig. 1A and B). The perineal mass on the pelvic floor was considered. CT of case 3 revealed a perineal space-occupying with low-density shadow and visible “layered” structure. MRI examination was performed on case 4, wherein the T2 weighted image (T2WI) image of the pelvic mass showed high-density shadows (Fig. 1C and D).
Figure 1.
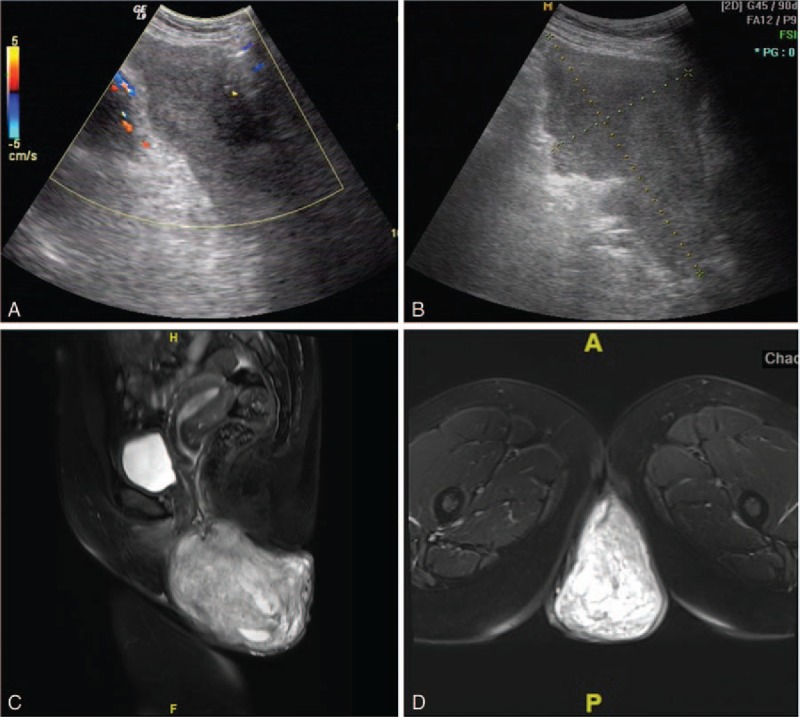
B-ultrasound showed that (A) case 1, pelvic mass with visible internal blood flow signal; and (B) case 2, irregular mass in the left buttock with a clear border. (C) Case 4, sagittal magnetic resonance imaging (MRI) shows a pelvic irregular mass (7 × 4.4 × 3 cm) on T2WI MRI. DWI sequence shows a moderately high signal. (D) Case 4, axial MRI showing heterogeneous enhancement. A “layered” structure can be observed. DWI = diffusion weighted image, T2WI = T2 weighted image.
All 5 patients underwent surgical resection of the tumors. The tumors were gray, lobular, poorly encapsulated masses, measuring from 7 × 4.4 × 3 to 36 × 7.5 × 5 cm, for an average diameter of 18 cm. The tumors were attached by fibrofatty tissues with poorly defined border and soft and firm texture. The cut surface was glistening, myxedematous or gelatinous, and gray reddish-brown. The specimens were fixed with 4% formalin, embedded in paraffin, cut into 4-μm sections, stained with hematoxylin and eosin, and observed under a light microscope. Tumor cells were relatively smooth stellate and spindle-shaped cells with lightly stained or eosinophilic cytoplasm and poorly defined borders. The nuclei were oval-shaped, bland, and lightly stained with a single, small, centrally located nucleolus. Mitotic figures were not observed (Fig. 2A and B). The cells were of low-to-moderate density and were slightly denser in some regions. The cells were localized in the myxedematous matrix, which contained varying numbers of circular or slightly twisted blood vessels of medium caliber, as well as evidently dilated vessels. Some blood vessel walls were either evenly or unevenly thickened (Fig. 2C). The tumor tissue with unclear boundaries pushed its growth in the shape of loose bundles into the surrounding adipose tissue, whereas some infiltrated into the surrounding striated muscle (Fig. 2D).
Figure 2.
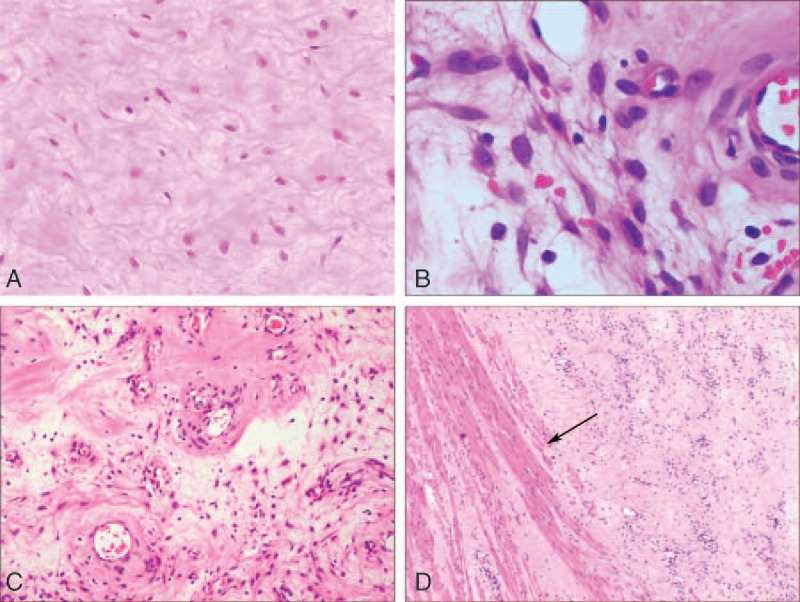
HE staining of tumor and observation under a microscope. (A) Tumor cells were distributed sparsely and scattered in the acidic mucin-rich matrix in the shape of stellate or spindle (magnification × 200). (B) The cytoplasm of the tumor cells was lightly stained with poorly defined borders. The nuclei were oval-shaped with a single, small nucleolus. No mitotic structures were seen (magnification × 400). (C) The myxedematous stroma contained varying numbers of blood vessels with medium caliber and evidently dilated vessels (arrows, magnification × 200). (D) The tumor invaded into the surrounding striated muscle (arrows, magnification × 200). HE = hematoxylin-eosin staining.
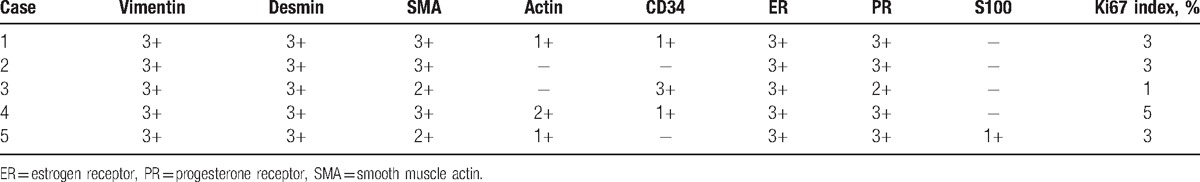
The 2-step EnVision method (HRP-Rabbit/mouse, from Beijing Zhongshan Golden Bridge Biotechnology Co. Ltd., Beijing, China) was used for immunohistochemistry using antibodies against vimentin, desmin, smooth muscle actin (SMA), actin, estrogen receptor (ER), progesterone receptor (PR), S-100, CD34, and Ki67 (all 1:500; all from Beijing Zhongshan Golden Bridge Biotechnology Co. Ltd., Beijing, China). The interpretation criteria were as follows: <10% positive cells were considered as (−), 10% to 30% positive cells were considered as (1+), 31% to 50% positive cells were considered as (2+), and >50% positive cells were considered as (3+). Tumor cells showed strong expression of vimentin (5/5) (Fig. 3A), desmin (5/5) (Fig. 3B), ER (5/5) (Fig. 3C), and PR (5/5). On the other hand, partial or weak expression was observed for SMA (5/5), actin (4/5) (Fig. 3D), CD34 (3/5), and S-100 (2/5), whereas the Ki-67 index was 1% to 3%. The expression of each immunohistochemistry is shown in Table 1.
Figure 3.
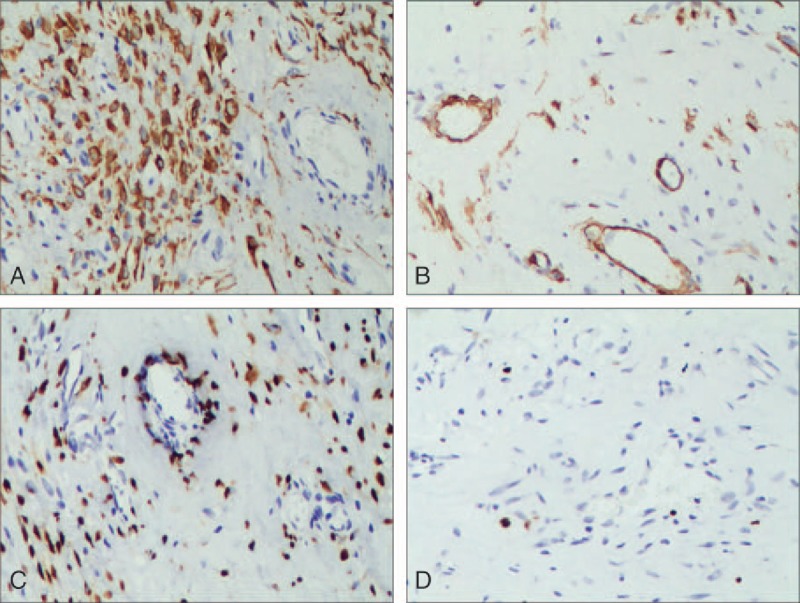
Tumor immunohistochemistry. (A) The tumor cells were positively stained for desmin with the EnVision method (magnification × 200). (B) The tumor cells were partly positively stained for smooth muscle actin with the EnVision method (magnification × 200). (C) The positive rate of estrogen receptor in tumor cells was about 80%, as shown by the EnVision method (magnification × 200). (D) The positive rate of Ki-67 in tumor cells was about <3%, as shown by the EnVision method (magnification × 200).
Table 1.
The immunophenotype of the 5 AAM cases.
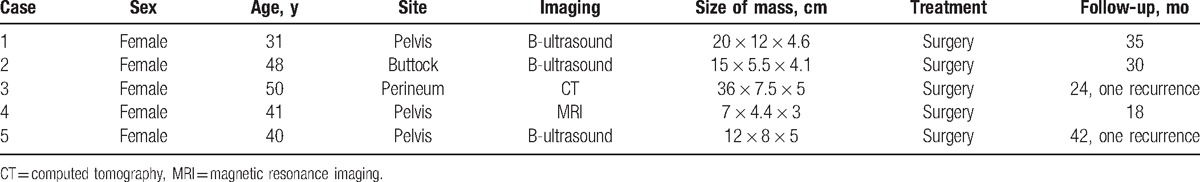
After tumor resection, none of the patients underwent radiotherapy, hormonal therapy, or chemotherapy. The follow-up began after surgery. During the 18 to 42-month follow-up, case 3 and case 5 recurred at 8 and 15 months, respectively. Case 5 was first misdiagnosed as vascular myofibroblastoma after surgery and was diagnosed as AAM when the surgical section from the first surgery was reviewed after recurrence. The remaining patients were in good condition. The clinical data of the 5 patients with AAM are summarized in Table 2.
Table 2.
The clinical data of the 5 AAM patients.
3. Discussion
The 5 female patients in this report, aged between 31 and 50 years, included 3 cases of pelvic AAM, 1 case of vulva AAM, and 1 case of buttock AAM. Clinically, most AAM patients present with a slow-growing mass in the pelvic and perianal region that is either asymptomatic or associated with regional pain, dyspareunia, or a pressure-like sensation. Clinical diagnosis before surgery is difficult since the clinical characteristics are similar to those of a vulval mass, vulval abscess, Bartholin gland cyst, cyst of mesonephric duct, vaginal hernia, or levator ani muscle hernia. Ultrasound suggests a nonspecific heterogeneous hypoechoic area with thin wall and internal intervals, which some cases may show blood flow signal.[4,5] CT shows a mass with low or equal density shadow, which has a tendency to grow around the pelvis, not causing any damage to vaginal or rectal muscle tissue.[2] A specific “swirled” or “layered” structure can be observed on CT.[2] T1 weighted image MRI reveals muscle signals with equivalent strength. T2WI shows high-density shadow, and after enhancement, a “spiral” or “layered” structure will appear.[2] In the present cases, this characteristic structure was observed in the patient who underwent CT, and it facilitated preoperative diagnosis. The low density in CT and high signal in MRI of the tumor may be correlated with the water and loose myxoid matrix inside the tumors. The enhancement is associated with the vessels contained in the tumors.[6]
Since AAM is located deeply, it is difficult to be detected at the early stage. The pathological examination revealed that the maximum diameter of the tumors ranged from 1 to 60 cm, most being 8 to 20 cm. The tumors are neither encapsulated nor partially encapsulated, and are spherical, semicircular, cord-shaped, dumbbell-shaped, or irregularly shaped. Most tumors are lobular and soft with unclear borders and invasive growth. They adhere to the muscle and fat and other surrounding structures and usually coexist in the soft and tough areas. The cut surface is translucent, myxedematous, and gelatinous, with cystic degeneration and hemorrhage areas.[7]
Under low-powered microscopy, the neoplastic cells are distributed sparsely and scattered in the acidic mucin-rich matrix in the shape of stellate or spindle. The blood vessels in the matrix are arranged disorderly and randomly while the vessels have varying caliber, and vessel walls have an uneven thickness. The larger vessels are surrounded by multiple layers of hypertrophic smooth muscle cells to form the thick wall, around which acute or chronic inflammatory cell infiltration and extravasation of erythrocytes can be seen. Under high-powered microscopy, elongated fibroblast-like cells and a significant myxoid background can be seen. Tumor cells have scant and eosinophilic cytoplasm, oval-shaped nuclei, and a clear nucleolus. Nuclear atypia and mitotic structures are absent. In the present study, a significant number of blood vessels with varying caliber could be seen in the matrix of 3 cases along with lymphocytes, plasma cells, and other chronic inflammatory cell infiltration at the vessel wall.
According to the report by Jingping and Chunfu[8] of the immunohistochemistry results of 71 patients, vimentin, SMA, and CD34 were positive, the positive rate of PR was 70%, and that of ER was 65%; S-100 and CD68 were negative. The results of this study showed that the average positive rates were 100% for vimentin, desmin, and SMA, whereas it was 60% for CD34, 100% for ER, 100% for PR, 20% for S-100, and 3% for Ki-67. The immunohistochemistry results supported that the tumor cells may originate from mesenchymal cells with the characteristics of fibroblasts and myofibroblasts. The 3% positive rate of Ki67 was in agreement with the tumor features such as slow growth and inactive cell proliferation. Therefore, the cases reported here were consistent with the available literature.
AAM is locally aggressive because it is locally invasive and recurrent, and it requires extensive removal of the lesion. A small localized mass of the vulva and vagina can be excised by surgery while AAM involving the deep pelvic cavity or even the abdominal cavity is often larger, and its excision involves the removal of part or all of the adjacent pelvic organs (bladder, rectum, and vagina) by an extensive surgery that is required to obtain negative margins. In order to reduce recurrence, surgical resection should expand the scope of resection as large as possible. Local recurrence may be caused by inadequate resection, rather than by malignant lesions.[9] Some studies speculate that since the tumor immunohistochemistry shows positive ER and PR in most patients, and since the tumor is likely to grow during and after pregnancy, the tumor may be considered as a hormone-dependent tumor.[10] Therefore, gonadotropin-releasing hormone agonist (GnRH-α), aromatase inhibitors, and estrogen and PR blocker therapy can be considered. GnRH-α is a commonly used drug for postmenopausal women; however, aromatase inhibitors have also been reported to be effective.[11] Some studies demonstrated that the GnRH-α and other antiestrogen drugs can shrink or abolish the tumor.[12] Nevertheless, it is not yet elucidated whether long-term use of the GnRH-α drug can cure the disease or whether recurrence will appear when the drug is discontinued.[13] It has been reported that with the use of radiation therapy on recurrent patients, the recurrence was not observed for 2 to 3 years.[14]
Local recurrences were frequently seen in AAM, with the recurrence rate ranging from 25% to 47%, and with 85% of the recurrences occurring within 5 years.[10,15] The earliest recurrence was reported to be within 2 months after surgery, and the latest case recurred 20 years after the initial surgery.[10,15] The longest follow-up among the 5 patients in the present study was 42 months, during which there were 2 cases of recurrence, with the first case occurring 7 months after surgery. Distant metastasis from AAM is extremely rare, and currently there are 3 cases reported around the world.[16–18] The first case is a 63-year-old woman with lung and lymph node metastasis. The second case is a 27-year-old woman with multiple postoperative recurrences, who was diagnosed with lung metastasis after a few years and died from her disease. The third case is a recurrent AAM; the tumors invaded into the inferior vena cava and right atrium, as well as in both lungs. These 3 cases had lung metastases. The metastasis was distinctly diagnosed through the pathological examination of the lung lesions followed by comparison with the pathological features of the primary tumor lesions. Because of the unpredictable biological behavior of AAM, some groups recommended classifying AAM as moderate malignant tumors.[17]
It is important to keep in mind that AAM can be misdiagnosed easily. Indeed, it has been reported that both AAM and angiomyofibroblastoma (AMFB) may originate from the perivascular stem cells that are differentiating into myofibroblast.[19] The immunohistochemistry of AMFB can also be positive for vimentin, SMA, desmin, ER, and PR. However, AMFB is relatively small and has abundant tumor cells that are ovoid and arranged around the blood vessels in the form of bundles or nests. The myxoid background of AAM is distinct, and the tumor cells are not like epithelial cells. The folding nucleus or disrupted nucleus can be found. There are obvious muscle-like fiber bundles, localized in the vicinity of the blood vessels, and the vascular wall is thick. From the behavioral perspective, AAM easily infiltrates into the surrounding fat tissue and is invasive, easy to recur but without metastasis. Moreover, AMFB is noninvasive, and hence their treatments are also different. The tumor size and biological behavior of the invasive growth in case 5 of the present study were first ignored, misleading the diagnosis. The patient showed recurrence 15 months after surgery. Superficial angiomyxoma is an uncommon skin or subcutaneous tumor, which often occurs in the body trunk, limbs, head, and neck. It shows single or multiple nodular growths with a clear boundary and is easy to recur. It does not have the thick-walled blood vessels seen in AAM. Intramuscular myxoma frequently occurs among the large muscles of the thigh, shoulder, and upper limbs in adults. The cellular components are less, blood vessels are absent, and acidic mucus is abundant as compared to AAM. Mucinous neurofibroma shows elongated, curved, and corrugated tumor nuclei. The neurological marker S-100 is positive. There are no significant vascular components as seen in AAM.
Of course, the present study is limited by the small number of patients from a single institution. All cases of AAM treated during the study period were identified using the hospital database and validated by reviewing the slides, but it has to be noted that some cases might have been misdiagnosed and could not be included. In addition, after discussion in tumor boards, these patients were not given hormonal treatment, and the added value of these agents to surgery could not be evaluated.
In summary, we reported a group of relatively rare cases and described their clinical manifestation, imaging, and pathological alterations. We aspire to assist the pathologists in learning more about this tumor and avoiding misdiagnosis.
Footnotes
Abbreviations: AAM = aggressive angiomyxoma, AMFB = angiomyofibroblastoma, GnRH-α = gonadotropin-releasing hormone agonist.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Fletcher C, Bridge J, Hogendoorn P, et al. World Health Organization Classification of Tumours of Soft Tissue and Bone. 4th ed.Lyon: IARC Press; 2013. [Google Scholar]
- [2].Outwater EK, Marchetto BE, Wagner BJ, et al. Aggressive angiomyxoma: findings on CT and MR imaging. AJR Am J Roentgenol 1999;172:435–8. [DOI] [PubMed] [Google Scholar]
- [3].Steeper TA, Rosai J. Aggressive angiomyxoma of the female pelvis and perineum. Report of nine cases of a distinctive type of gynecologic soft-tissue neoplasm. Am J Surg Pathol 1983;7:463–75. [DOI] [PubMed] [Google Scholar]
- [4].Tariq R, Hasnain S, Siddiqui MT, et al. Aggressive angiomyxoma: swirled configuration on ultrasound and MR imaging. J Pak Med Assoc 2014;64:345–8. [PubMed] [Google Scholar]
- [5].Li X, Ye Z. Aggressive angiomyxoma of the pelvis and perineum: a case report and review of the literature. Abdom Imaging 2011;36:739–41. [DOI] [PubMed] [Google Scholar]
- [6].Heffernan EJ, Hayes MM, Alkubaidan FO, et al. Aggressive angiomyxoma of the thigh. Skeletal Radiol 2008;37:673–8. [DOI] [PubMed] [Google Scholar]
- [7].Choi H, Park C, Ji YI. Alternative surgical approaches for aggressive angiomyxoma at different sites in the pelvic cavity. Obstet Gynecol Sci 2015;58:525–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jingping Z, Chunfu Z. Clinical experiences on aggressive angiomyxoma in China (report of 93 cases). Int J Gynecol Cancer 2010;20:303–7. [DOI] [PubMed] [Google Scholar]
- [9].Wang Q, Zhao M, Lin X, et al. Aggressive angiomyxoma of the vulva: intra-operative pathological diagnosis is useful in deciding the scope of surgery and reducing recurrence. Acta Chir Belg 2012;112:79–84. [DOI] [PubMed] [Google Scholar]
- [10].Sutton BJ, Laudadio J. Aggressive angiomyxoma. Arch Pathol Lab Med 2012;136:217–21. [DOI] [PubMed] [Google Scholar]
- [11].Giles DL, Liu PT, Lidner TK, et al. Treatment of aggressive angiomyxoma with aromatase inhibitor prior to surgical resection. Int J Gynecol Cancer 2008;18:375–9. [DOI] [PubMed] [Google Scholar]
- [12].Shinohara N, Nonomura K, Ishikawa S, et al. Medical management of recurrent aggressive angiomyxoma with gonadotropin-releasing hormone agonist. Int J Urol 2004;11:432–5. [DOI] [PubMed] [Google Scholar]
- [13].McCluggage WG, Jamieson T, Dobbs SP, et al. Aggressive angiomyxoma of the vulva: dramatic response to gonadotropin-releasing hormone agonist therapy. Gynecol Oncol 2006;100:623–5. [DOI] [PubMed] [Google Scholar]
- [14].Suleiman M, Duc C, Ritz S, et al. Pelvic excision of large aggressive angiomyxoma in a woman: irradiation for recurrent disease. Int J Gynecol Cancer 2006;16(suppl 1):356–60. [DOI] [PubMed] [Google Scholar]
- [15].Kiran G, Yancar S, Sayar H, et al. Late recurrence of aggressive angiomyxoma of the vulva. J Low Genit Tract Dis 2013;17:85–7. [DOI] [PubMed] [Google Scholar]
- [16].Siassi RM, Papadopoulos T, Matzel KE. Metastasizing aggressive angiomyxoma. N Engl J Med 1999;341:1772. [DOI] [PubMed] [Google Scholar]
- [17].Blandamura S, Cruz J, Faure Vergara L, et al. Aggressive angiomyxoma: a second case of metastasis with patient's death. Hum Pathol 2003;34:1072–4. [DOI] [PubMed] [Google Scholar]
- [18].Geng J, Cao B, Wang L. Aggressive angiomyxoma: an unusual presentation. Korean J Radiol 2012;13:90–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wang YF, Qian HL, Jin HM. Local recurrent vaginal aggressive angiomyxoma misdiagnosed as cellular angiomyofibroblastoma: a case report. Exp Ther Med 2016;11:1893–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


