Abstract
Background:
A number of studies have investigated the roles of excision repair cross complementation group 1 (ERCC1), ERCC2, and ERCC5 genes polymorphisms in the development of glioma; however, the results were inconsistent. Here, we performed a meta-analysis to investigate the association between 6 polymorphisms in the ERCC genes (rs3212986, rs11615, rs13181, rs1799793, rs238406, rs17655) and glioma risk.
Methods:
The PubMed, Embase, and Web of science were searched up to September 6, 2016, for studies on the association between ERCC polymorphisms and glioma risk. A fixed-effects or random-effects model was used to calculate the pooled odds ratios based on the results from the heterogeneity tests. Sensitivity and cumulative meta-analyses were also performed.
Results:
A total of 15 studies were eligible for the pooled analysis, conducted in 2 populations of ethnic descent: 8 Europeans and 7 Asians. The results showed that ERCC1 rs3212986 polymorphism was positively associated with glioma [AA vs CC: odds ratio (OR) = 1.298, 95% confidence interval (95% CI) = 1.043–1.230, P = .025]. Association of the ERCC2 rs13181 and rs1799793 polymorphisms was only observed in Asians (CC vs AA for rs13181: OR = 1.539, 95% CI = 1.122–2.109, P = .007; AA vs GG for rs1799793: OR = 1.474, 95% CI = 1.090–1.994, P = .012). However, no association was observed between glioma risk and ERCC1 rs11615, ERCC2 rs238406, and ERCC5 rs17655 polymorphisms. Moreover, sensitivity and cumulative meta-analyses confirmed the stability of the results.
Conclusions:
Our meta-analysis indicated that the ERCC1 rs3212986 polymorphism and 2 polymorphisms in ERCC2 gene (rs13181 and rs1799793) contributed to the susceptibility of glioma.
Keywords: ercc1, ercc2, ercc5, glioma, meta-analysis, polymorphism
1. Introduction
Gliomas account for more than 70% of all brain tumors, and of which, malignant gliomas, the most common primary brain tumor in adults, are generally associated with poor survival relative to other types of brain tumors.[1] Many environmental and lifestyle factors, including several occupations, ionizing radiation, cellular phones, smoking, and diet, have been considered to be associated with an increased glioma risk. However, the exact etiology remains poorly understood.[2,3] Recently, accumulating evidence suggests that inherited risks may play an important role in glioma.[4–6] Genetic studies, including genome-wide association studies (GWAS), demonstrated that several genetic factors might be associated with glioma, such as CCDC26, EGFR, RTEL, GSTP1, TERT, and PHLDB1 genes.[7–11]
Usually, DNA damage can be induced by exogenous carcinogens, such as ultraviolet rays and ionizing radiation, and contributes to genomic instability. DNA repair, playing an important role in the maintaining genomic integrity, involves several DNA repair pathways, including base excision repair (BER), mismatch repair (MMR), and nucleotide excision repair (NER).[12,13] Previous studies indicated that variants in DNA repair genes might impair the DNA repair capacity and contribute to cancer risk.[14]
Excision repair cross complementation group 1 (ERCC1), ERCC2, and ERCC5 genes are DNA repair genes, whose products are important in NER.[15] Recently, several studies have focused on the association between polymorphisms in ERCC1 gene (rs3212986, rs11615), ERCC2 gene (rs1799793, rs13181, and rs238406), or ERCC5 rs17655 polymorphism and glioma risk. However, the results were inconclusive, which might be due to studies with limited sample sizes or ethnic differences. To date, several meta-analyses reported the association between ERCC1 or ERCC2 polymorphisms and glioma risk, whereas these studies only focused on the 2 polymorphisms (rs3212986 in ERCC1 gene and rs13181 in ERCC2 gene).[16–21] Moreover, some recent studies involving glioma risk and ERCC polymorphisms were not included.[22–25] Thus, we conducted a comprehensive meta-analysis to investigate whether 6 polymorphisms in ERCC1 (rs3212986 and rs11615), ERCC2 (rs13181, rs1799793 and rs238406), and ERCC5 (rs17655) genes are risk factors to the glioma susceptibility.
2. Materials and methods
2.1. Search strategy
We performed this meta-analysis according to the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.[26] A comprehensive literature search was performed through the PubMed, Embase, and Web of science up to September 6, 2016. Search strategies were as follows: “glioma” or “brain tumor,” “polymorphism,” and “ERCC1,” “ERCC2,” “ERCC5,” “rs3212986,” “rs11615,” “rs13181,” “rs1799793,” “rs238406,” or “rs17655.” In addition, the reference lists of all selected articles were checked by hand-search for additional potential studies.
2.2. Inclusion and exclusion criteria
Studies were included in the meta-analysis if they met the following criteria: case–control or cohort studies; association between ERCC1 (rs3212986and rs11615), ERCC2 (rs13181, rs1799793, and rs238406), or ERCC5 (rs17655) polymorphism and glioma risk; available allele and genotype frequencies. Major reasons for exclusion of studies were as follows: articles only with an abstract, review articles, and comments; articles considered overlapped with other studies; and studies that had no control group.
2.3. Data extraction
The following information from each eligible study was extracted independently by 2 investigators: first author's name, publication year, ethnicity (Europeans and Asians), whether cases and controls were matched (for case–control studies), and genotype distribution in cases and controls. If the article did not provide sufficient genotype distribution, the corresponding author was contacted for the detailed data. Disagreements were resolved by discussion between the 2 investigators. Moreover, our analyses were based on previously published studies; thus, no ethical approval and patient consent are required
2.4. Quality score assessment
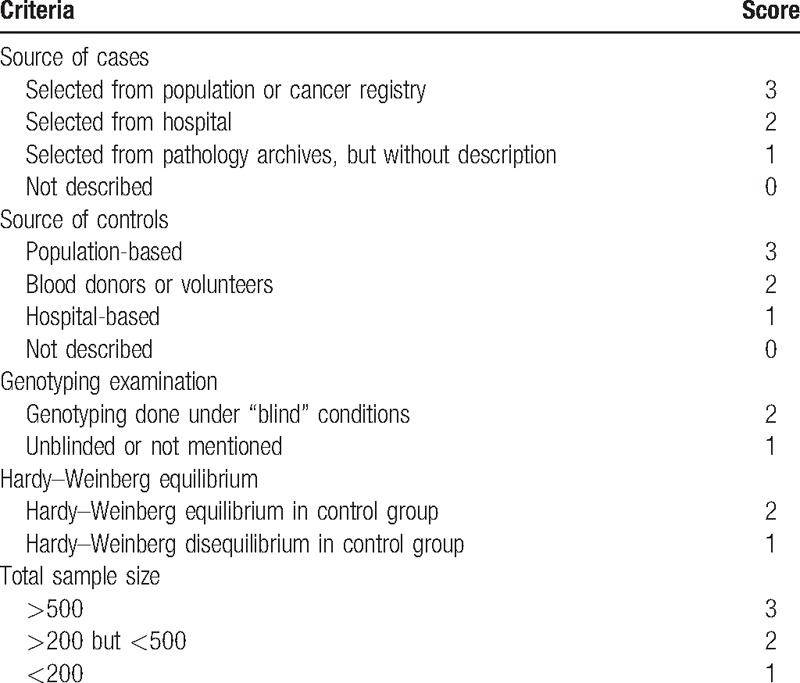
The quality of the studies was independently assessed by 2 authors according to the quality scoring criteria, which is modified from previous meta-analyses (Table 1).[27,28] Quality scores ranged from 0 points (worst) to 13 points (best). Studies scoring less than 9 points were classified as low quality, and those scoring 9 points or higher were classified as high quality.
Table 1.
Scale for quality assessment.
2.5. Statistical analysis
The strength of the association between 6 polymorphisms in ERCC1, ERCC2, and ERCC5 genes and glioma risk was estimated by odds ratios (ORs) with corresponding 95% confidence intervals (CIs). The genetic models evaluated for the pooled OR of rs3212986 polymorphism were allele contrast (A vs C), homozygote comparison (AA vs CC), heterozygote comparison (AC vs CC), dominant model (AA+AC vs CC), as well as recessive model (AA vs AC+CC). Similar models were analyzed for the other polymorphisms. The significance of the pooled OR was determined by the Z-test, and a P value less than .05 was considered as statistically significant. In addition, stratified analysis by ethnicity was also performed. Between-study heterogeneity was assessed by Chi-square based Q test and I2 test. Heterogeneity was considered significant for P < .10, and then the random effect model was selected; otherwise, a fixed-effects model was used. In addition, Galbraith plot was used to visualize the impact of individual studies on the overall heterogeneity, which spotted the outlier as the possible origin of heterogeneity.[29,30] The Hardy–Weinberg equilibrium (HWE) in the control group was also assessed, and a P < .05 was considered as significant disequilibrium.
Sensitivity analysis was performed by sequential excluding a single study each time in an attempt to identify the potential influence of the individual data to the pooled ORs.[31] Cumulative meta-analysis was carried out for each polymorphism in association with glioma to evaluate the trend of the genetic risk effect (OR) of the allele comparisons as evidence accumulates over time.[32] Publication bias was assessed by funnel plots and Egger linear regression test.[33] If significant publication bias was detected, trim and fill methods was used to adjust ORs and 95% CIs.[34] Analyses were performed using STATA software, version 12 (StataCorp LP, College Station, TX).
3. Results
3.1. Characteristics of the included studies
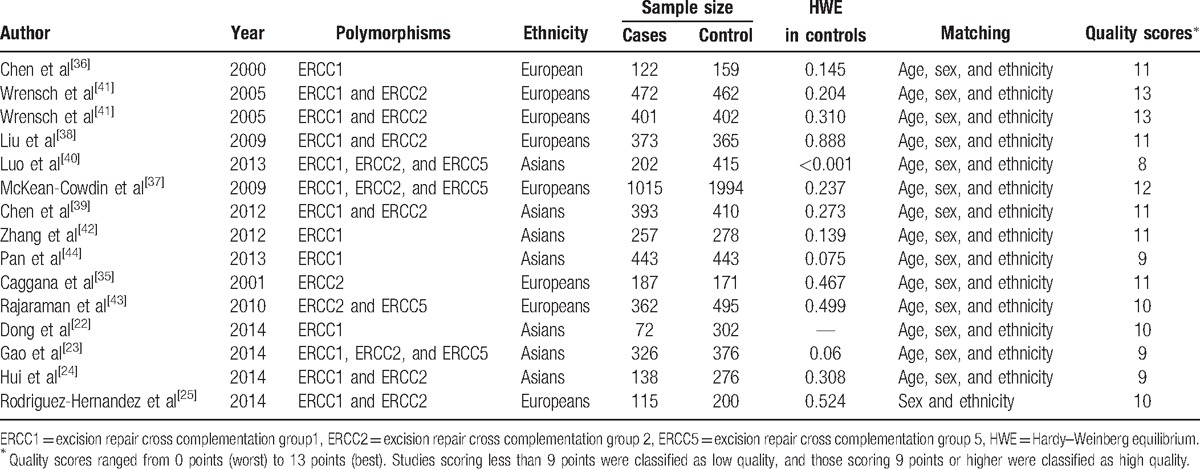
A total of 166 studies were identified during our premature searches. After a review of titles and abstracts, 138 nonrelevant studies were excluded. Of the remaining 28 full-text articles, 1 article only with an abstract, 8 about other tumors, 3 review articles, and 2 articles reported other polymorphisms. Finally, a total of 14 articles met our selection criteria.[22–25,35–44] The flow chart for the study selection process is shown in Fig. 1. Among them, 1 article reported data on 2 different series, and we treated them independently.[41] Finally, 15 studies comprising 4878 cases and 6748 controls were included in the meta-analysis. Studies were conducted in 2 populations of ethnic descent: 8 Europeans and 7 Asians. The distribution of genotypes in the control groups of all studies was in agreement with HWE except one.[40] The characteristics of all eligible studies are summarized in Table 2.
Figure 1.
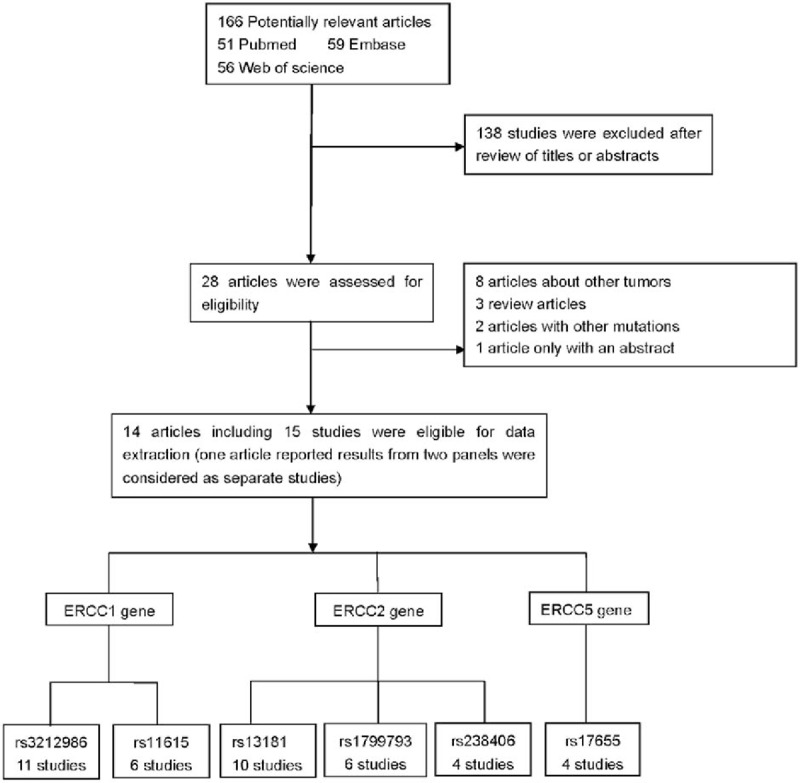
Flow chart for relevant studies.
Table 2.
Summary characteristics for the included studies.
3.2. Association of 2 polymorphisms in ERCC1 gene (rs3212986 and rs11615) with glioma risk
The association between the ERCC1 rs3212986 polymorphism and susceptibility to glioma was assessed in a total of 3539 cases and 5035 controls. As summarized in Table 3 and Fig. 2A, a significant association was observed in allele comparison (A vs C: OR = 1.079, 95% CI = 1.007–1.157, P = .032), homozygote comparison (AA vs CC: OR = 1.280, 95% CI = 1.083–1.514, P = .004), and recessive model (AA vs AC + CC: OR = 1.263, 95% CI = 1.074–1.486, P = .005) in overall population. In the subgroup analysis by ethnicity, a significantly increased glioma risk was found in Asian population (A vs C: OR = 1.132, 95% CI = 1.022–1.254, P = .018; AA vs CC: OR = 1.298, 95% CI = 1.043–1.630, P = .025; and AA vs AA + AC: OR = 1.250, 95% CI = 1.004–1.556, P = .046). However, in Europeans, a significant association between rs3212986 polymorphism and glioma risk was only observed in recessive model (AA vs AA + AC: OR = 1.280, 95% CI = 1.004–1.631, P = .046). Moreover, the results did not show significant association between ERCC1 rs11615 polymorphism and glioma risk. The between-study heterogeneity was not significant in all genetic models.
Table 3.
Meta-analysis for the ERCC1 gene rs3212986 and rs11615 polymorphisms and glioma risk.
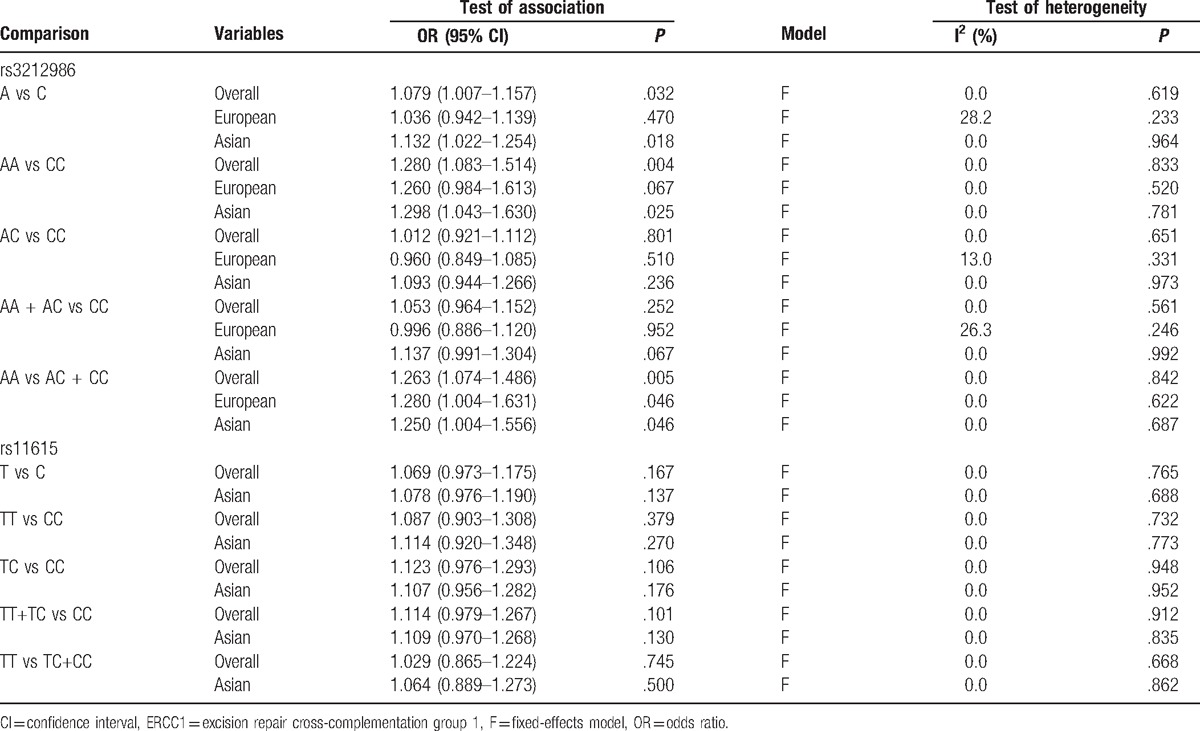
Figure 2.
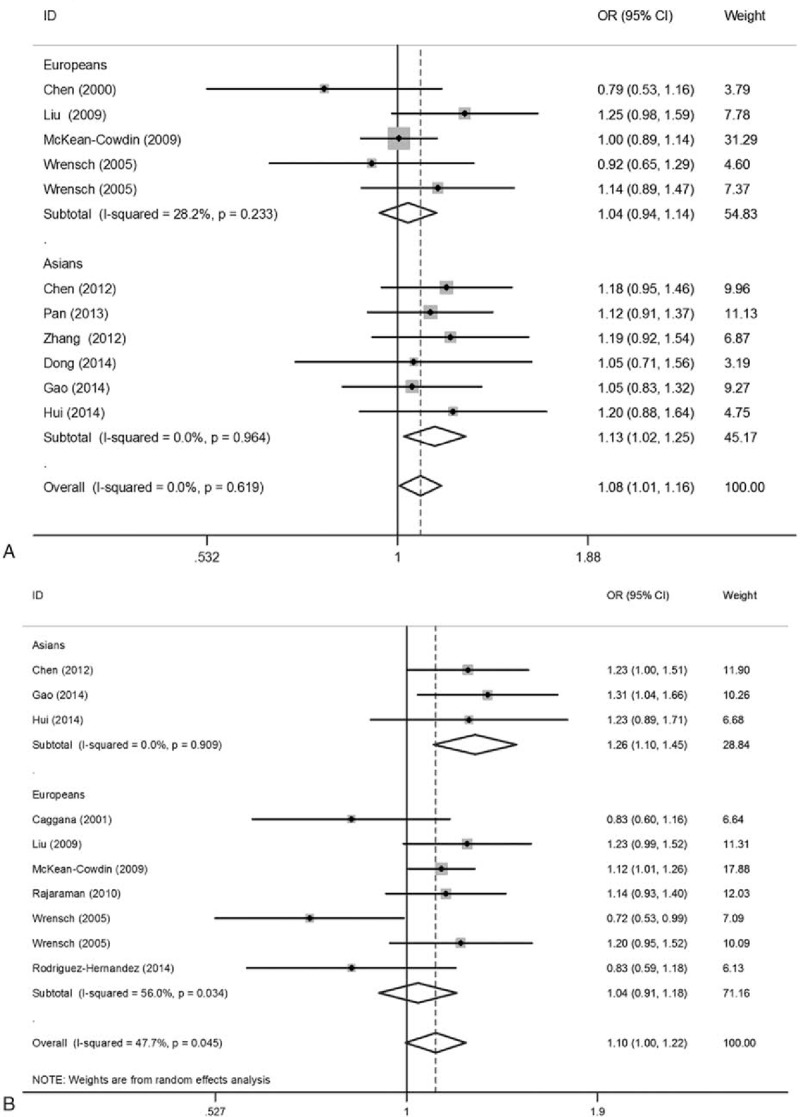
Forest plots for the association between theERCC1 rs3212986 and ERCC2 rs13181 polymorphisms and glioma risk. (A) ERCC1 rs3212986 polymorphism (A vs C); (B) ERCC2 rs13181 polymorphism (C vs A). The sizes of the squares reflect the weighting of included studies; the center of diamonds reflect summary effect, the left and right extremes of diamonds reflect 95% confidence intervals. CI = confidence interval, OR = odds ratio.
3.3. Association of 3 polymorphisms in ERCC2 gene (rs13181, rs1799793, and rs238406) with glioma risk
Meta-analysis findings of association between rs13181 polymorphism and glioma are summarized in Table 4. A total of 10 studies involving 3289 cases and 4718 controls were included. There was no significant association observed in the overall population. When stratified by ethnicity, a significantly increased glioma risk was found in Asians (C vs A: OR = 1.259, 95% CI = 1.095–1.466, P = .001) (Fig. 2B). For the rs1799793 polymorphism, significantly increased glioma risk was also observed in Asians (A vs G: OR = 1.274, 95% CI = 1.118–1.451, P < .001). However, nonsignificant correlation was observed between rs238406 polymorphsim and glioma risk. Chi-square based Q test showed that significant heterogeneity existed in 3 genetic models for rs13181 polymorphism (C vs A: P = .045, CA vs AA: P = .070, CC+CA vs AA: P = .051, CC vs CA+AA: P = .037). Galbraith plots showed that 1 independent study was the possible origin of heterogeneity,[41] and the heterogeneity was removed when this study was excluded (C vs A: Ph = .452, CA vs AA: Ph = .242, CC+CA vs AA: Ph = .254) (Fig. 3).
Table 4.
Meta-analysis results for the ERCC2 gene rs13181 polymorphism and glioma risk.
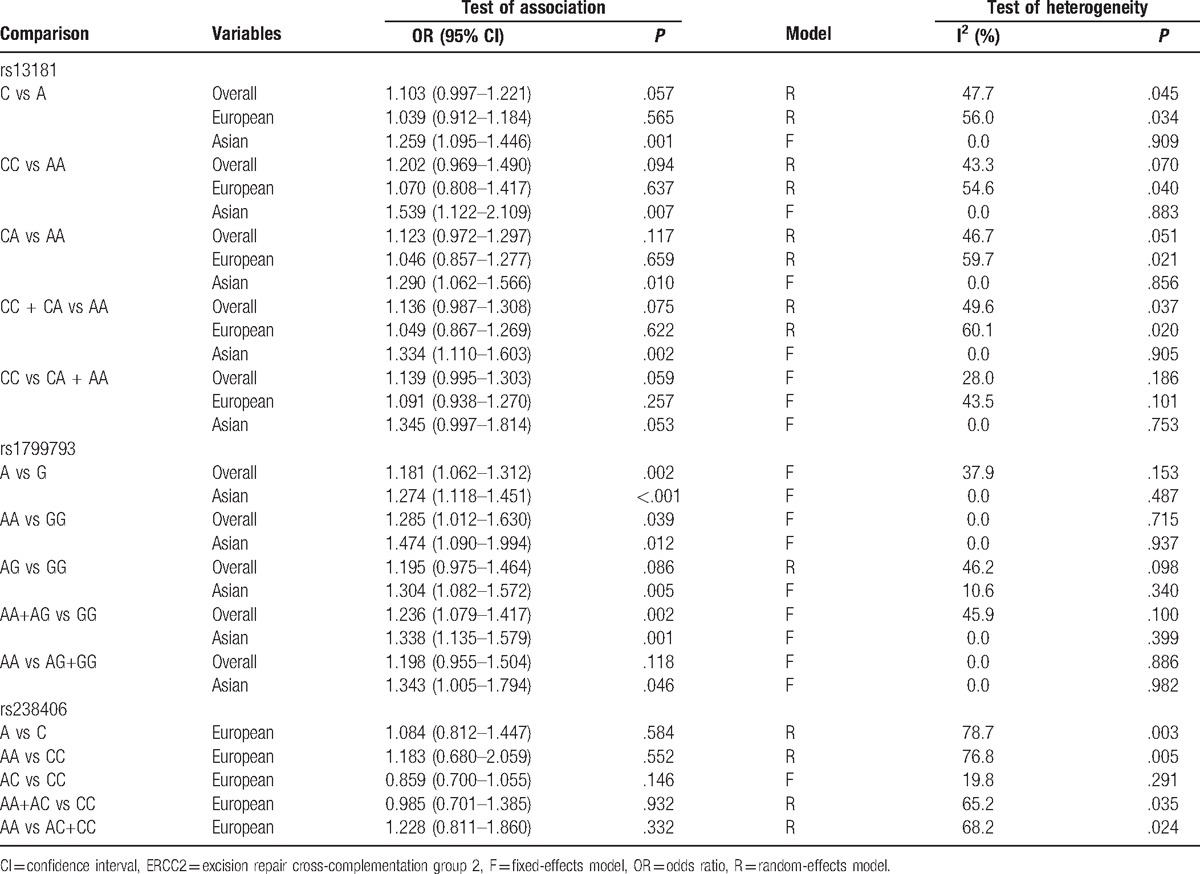
Figure 3.
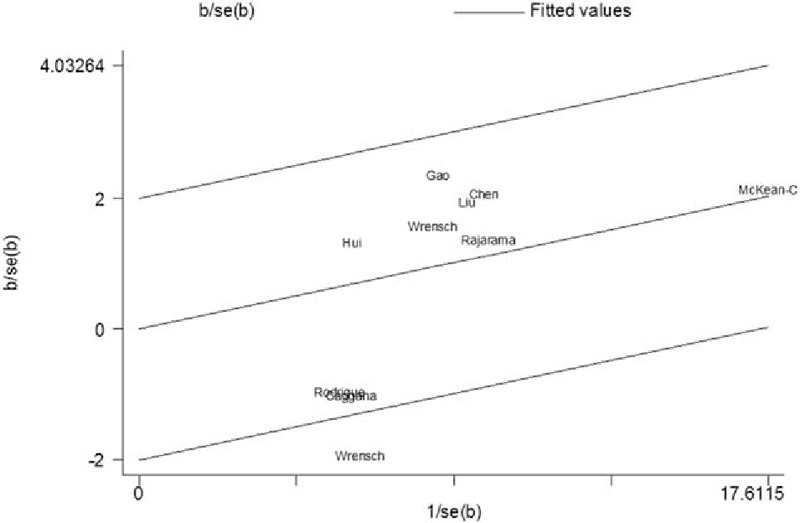
Galbraith plots of ERCC2 rs13181 polymorphism and glioma risk. The regression runs through the origin interval (central solid line). The 95% confidence interval is between the 2 outer parallel lines at 2 units above and below the regression line. One study (Wrensch et al[41]) was the outlier.
3.4. Association of ERCC5 rs17655 polymorphism with glioma risk
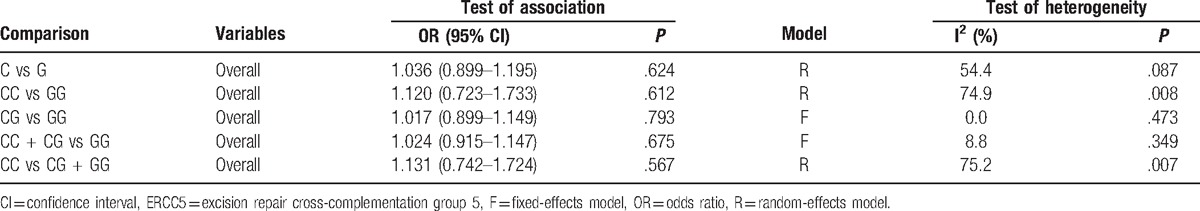
A total of 1989 patients and 3216 controls were analyzed for ERCC5 rs17655 polymorphism and glioma risk. The results showed that the risk for glioma was not significantly increased in persons carrying a C allele compared with those carrying a G allele (C vs G: OR=1.036, 95% CI=0.899–1.195). Similar results were observed in other genetic models (Table 5). Moreover, the Chi-square based Q test and I2 test indicated that between-study heterogeneity was not significant in all genetic models.
Table 5.
Meta-analysis results for the ERCC5 gene rs17655 polymorphism and glioma risk.
3.5. Sensitivity analysis and cumulative meta-analysis
Sensitivity analysis was performed by sequential removal of each study, the results of which showed that the pooled ORs were consistently significant by omitting 1 study at a time (Fig. 4A, B). In the cumulative meta-analysis, pooled ORs tended to be significant and stable with the accumulation of more data over time (Fig. 5A, B). Taken together, these results suggested that the results of this meta-analysis were highly stable.
Figure 4.
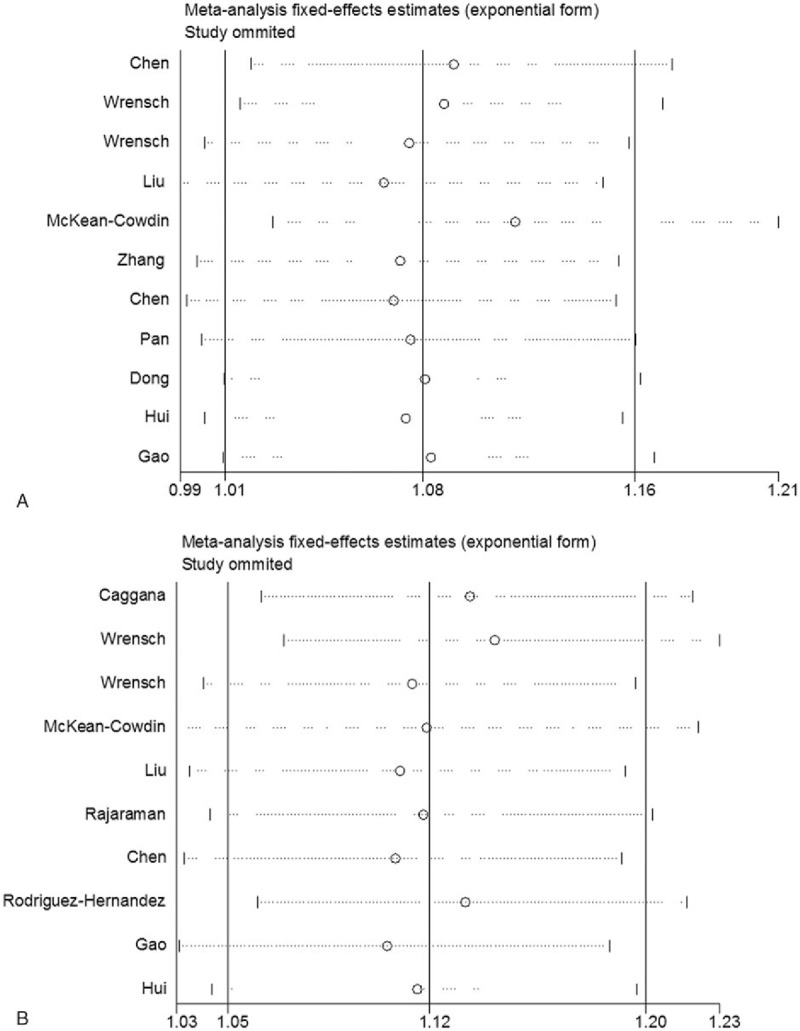
Sensitivity analysis on the association between the ERCC1 rs3212986 and ERCC2 rs13181 polymorphisms and glioma risk. (A) ERCC1 rs3212986 polymorphism (A vs C); (B) ERCC2 rs13181 polymorphism (C vs A). Results were computed by omitting each study (left column) in turn.
Figure 5.
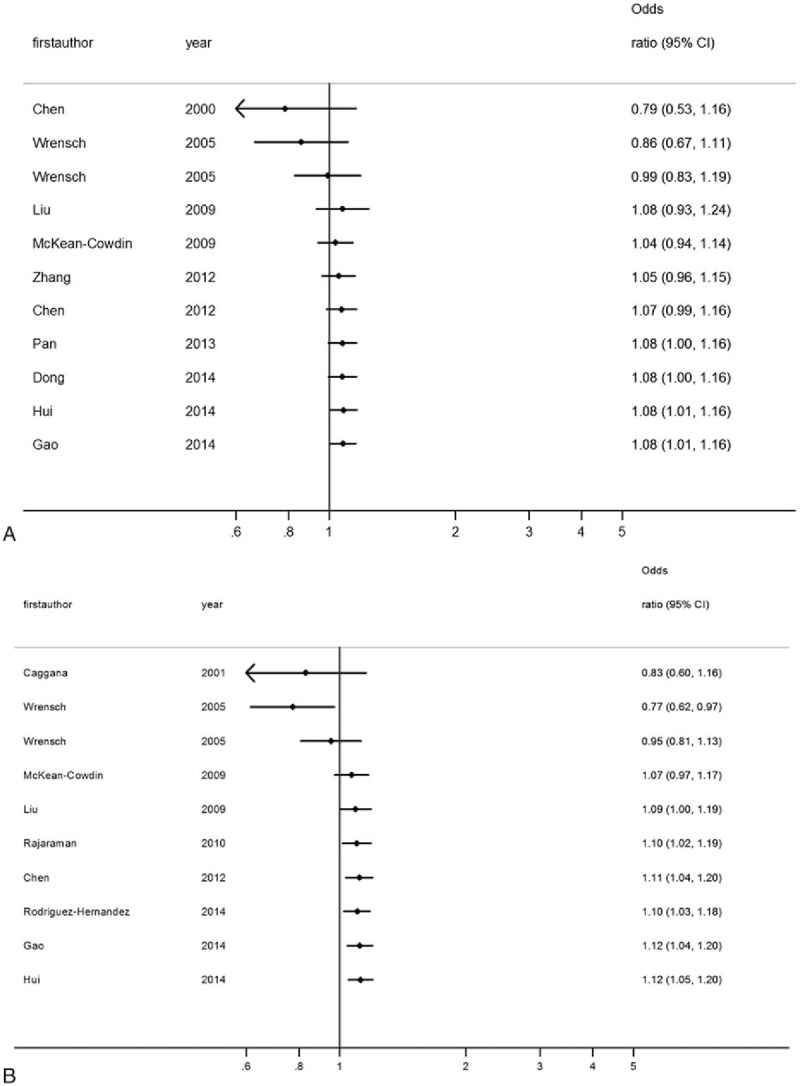
A cumulative meta-analysis on the association between the ERCC1 rs3212986 and ERCC2 rs13181 polymorphisms and glioma risk. (A) ERCC1 rs3212986 polymorphism (A vs C); (B) ERCC2 rs13181 polymorphism (C vs A). Pooled OR estimates with the 95% CI as information accumulates at the end of each year (left column).
3.6. Publication bias
Funnel plots and Egger test were carried out to assess publication bias. The shapes of the funnel plots did not reveal evidence of obvious asymmetry in all comparison models (Fig. 6). Moreover, the results of Egger test confirmed this finding (P = .566 for AA vs CC in rs3212986 polymorphism, P = .163 for TT vs CC in rs11615 polymorphism, P = .311 for CC vs AA in rs13181 polymorphism, P = .973 for AA vs GG in rs1799793 polymorphism, P = .076 for AA vs CC in rs238406 polymorphism, and P = .735 for CC vs GG in rs17655 polymorphism). Figure 6 showed the funnel plots of dominant models in the 2 polymorphisms.
Figure 6.
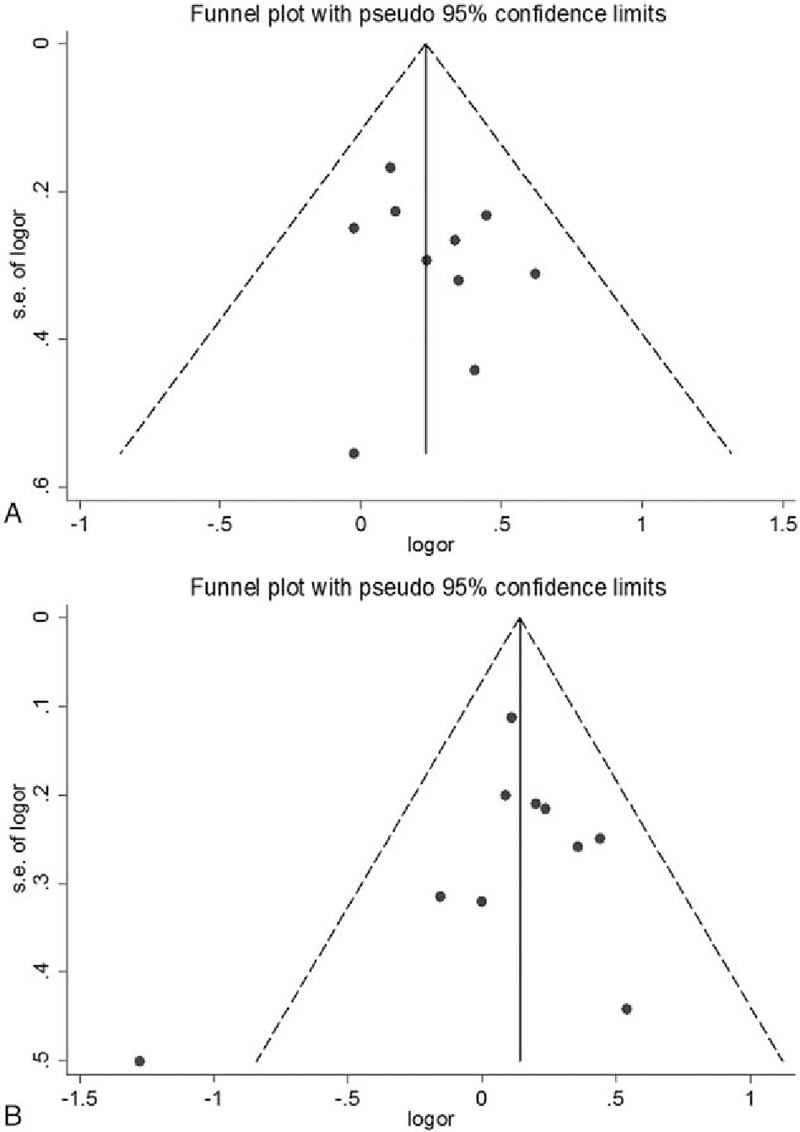
Funnel plots of the association between the ERCC1 rs3212986 and ERCC2 rs13181 polymorphisms and glioma risk. (A) ERCC1 rs3212986 polymorphism (AA vs AC+CC); (B) ERCC2 rs13181 polymorphism (CC vs CA+AA). Nonsignificant funnel asymmetry was observed that could indicate publication bias. The vertical line in the funnel plot indicates the summary estimate, while the sloping lines indicate the expected 95% CI for a given standard error, assuming no heterogeneity between studies. Logor natural logarithm of the OR, s.e. of logor standard error of the logOR.
4. Discussion
DNA repair plays an important role in the maintaining genomic integrity, which consists of several pathways. Recent studies showed that NER was one of the most important pathways during DNA repair.[45] ERCC1, ERCC2, and ERCC5 were core factors that participated in the NER pathway.[46] During NER, the ERCC1 gene codes for a protein that makes the 5’ incision by forming a complex with XPF.[47] Moreover, Melton et al[48] showed that mutant cells from ERCC1-deficient mice showed NER deficiency and had an increased mutation frequency as well as an elevated level of genomic instability. The ERCC2 protein, an evolutionarily conserved helicase, is also essential for NER. Mutations in ERCC2 gene were found to affect the DNA repair proficiency.[49] Moreover, accumulated genetic epidemiological studies have been conducted to explore the association between ERCC1, ERCC2, and ERCC5 polymorphisms and glioma risk; however, the results were inconclusive.[37,38,43] Therefore, we performed a comprehensive meta-analysis with published studies to clarify the role of these polymorphisms in glioma.
This meta-analysis demonstrated that ERCC1 rs3212986 polymorphism was significantly associated with glioma risk under the following genetic models (AA vs CC: OR = 1.280, 95% CI=1.083–1.514, P = .004 and AA vs AC + CC: OR = 1.263, 95% CI = 1.074–1.486, P = .005). When stratified by ethnicity, the significant association was still observed in Asians (AA vs CC: OR = 1.298, 95% CI = 1.043–1.630, P = .025), but not among Europeans in major genetic models, suggesting that the contribution of ERCC1 rs3212986 polymorphism might vary across different populations. Generally, Europeans more frequently suffered from glioma than people of African or Asian descent, which was also observed in children.[50–53] In addition, the pooled OR did not change in the sensitivity analysis by excluding 1 study each time, indicating that the results of this meta-analysis were highly stable. Finally, cumulative meta-analysis indicated that pooled ORs tended to be significant and stable with the accumulation of more data over time.
The ERCC2 rs13181 polymorphism showed significant association with glioma susceptibility (CC vs AA: OR = 1.539, 95% CI = 1.122–2.109, P = .007) in Asians. Similar results were found in rs1799793 polymorphism. (AA vs GG: OR = 1.474, 95% CI = 1.090–1.994, P = .012). In the analysis of rs11381 polymorphism, significant heterogeneity existed in major genetic models when all eligible studies were pooled into analysis. However, the results of Galbraith plots analyses indicated that 1 independent study[41] was the main potential origin of heterogeneity; when excluding, the heterogeneity was removed. Moreover, a sensitivity analysis showed that no single study qualitatively changed the pooled ORs. However, there was no significant association observed between rs11615, rs238406, or rs17655 polymorphism and glioma susceptibility.
Our analyses demonstrated that the ERCC1 rs3212986 and ERCC2 gene (rs13181 and rs1799793) polymorphisms had a moderate increase in glioma susceptibility. However, several limitations need to be considered for interpretation of our results. First, only 3 studies were performed in Asians for rs13181 polymorphism. Therefore, validation of association in other population is required in further studies. Second, it is clear that genetic susceptibility to cancer is complex because of interactions between genes and environmental factors. However, we could not assess gene–environment interactions due to insufficient data in most studies. Recently, Pan et al[54] investigated the association between language biases and selective reporting in human genome epidemiology, which demonstrated that Chinese studies showed more prominent genetic effects than non-Chinese studies, whereas the sample size of Chinese studies was always smaller. Thus, more non-Chinese studies in Asian populations were needed to confirm the significant association in Asians. In addition, GWAS have identified single nucleotide polymorphisms implicating hundreds of replicated loci for common traits and became a powerful tool to detect the susceptibility genes in cancers. Accumulated GWAS have provided strong evidences for the association between glioma risk and numerous genes, including TERT, TERC, EGFR, CCDC26, and RTEL.[10,11,55–58]. However, to date, association of polymorphisms in ERCC genes with susceptibility to glioma has not been investigated in GWAS. Thus, further genetics studies, especially GWAS studies, are required to confirm the possible role of ERCC polymorphisms in glioma.
5. Conclusions
Future studies with larger sample size in different ethnic groups (e.g., Asians and Africans) are needed to clarify the possible roles of ERCC1, ERCC2, and ERCC5 genes in the etiology and progression of glioma. In addition, studies investigating gene–environment may lead to a better understanding of the role of the ERCC gene polymorphisms in glioma.
Footnotes
Abbreviations: CI = confidence interval, ERCC = excision repair cross complementation group, GWAS = genome-wide association studies, NER = nucleotide excision repair, OR = odds ratio, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Funding/support: This study was supported by the Jintan Science and Technology Plan Project (2014059). The funder had no role in the design, execution, or writing of the study.
The authors declare no conflict of interest.
References
- [1].Bondy ML, Scheurer ME, Malmer B, et al. Brain tumor epidemiology: consensus from the Brain Tumor Epidemiology Consortium. Cancer 2008;113(7 Suppl):1953–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Goodenberger ML, Jenkins RB. Genetics of adult glioma. Cancer Genet 2012;205:613–21. [DOI] [PubMed] [Google Scholar]
- [3].Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol 2005;109:93–108. [DOI] [PubMed] [Google Scholar]
- [4].Ramalho-Carvalho J, Pires M, Lisboa S, et al. Altered expression of MGMT in high-grade gliomas results from the combined effect of epigenetic and genetic aberrations. PLoS One 2013;8:e58206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sun G, Wang X, Shi L, et al. Association between polymorphisms in interleukin-4Ralpha and interleukin-13 and glioma risk: a meta-analysis. Cancer Epidemiol 2013;37:306–10. [DOI] [PubMed] [Google Scholar]
- [6].Amankwah EK, Thompson RC, Nabors LB, et al. SWI/SNF gene variants and glioma risk and outcome. Cancer Epidemiol 2013;37:162–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wrensch M, Jenkins RB, Chang JS, et al. Variants in the CDKN2B and RTEL1 regions are associated with high-grade glioma susceptibility. Nat Genet 2009;41:905–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Xu G, Wang M, Xie W, et al. Three polymorphisms of DNA repair gene XRCC1 and the risk of glioma: a case-control study in northwest China. Tumour Biol 2014;35:1389–95. [DOI] [PubMed] [Google Scholar]
- [9].Xie P, Liang Y, Liang G, et al. Association between GSTP1 Ile105Val polymorphism and glioma risk: a systematic review and meta-analysis. Tumour Biol 2014;35:493–9. [DOI] [PubMed] [Google Scholar]
- [10].Rajaraman P, Melin BS, Wang Z, et al. Genome-wide association study of glioma and meta-analysis. Hum Genet 2012;131:1877–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shete S, Lau CC, Houlston RS, et al. Genome-wide high-density SNP linkage search for glioma susceptibility loci: results from the Gliogene Consortium. Cancer Res 2011;71:7568–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med 2004;10:789–99. [DOI] [PubMed] [Google Scholar]
- [13].Popanda O, Schattenberg T, Phong CT, et al. Specific combinations of DNA repair gene variants and increased risk for non-small cell lung cancer. Carcinogenesis 2004;25:2433–41. [DOI] [PubMed] [Google Scholar]
- [14].Lunn RM, Langlois RG, Hsieh LL, et al. XRCC1 polymorphisms: effects on aflatoxin B1-DNA adducts and glycophorin A variant frequency. Cancer Res 1999;59:2557–61. [PubMed] [Google Scholar]
- [15].Smith JS, Tachibana I, Pohl U, et al. A transcript map of the chromosome 19q-arm glioma tumor suppressor region. Genomics 2000;64:44–50. [DOI] [PubMed] [Google Scholar]
- [16].Huang LM, Shi X, Yan DF, et al. Association between ERCC2 polymorphisms and glioma risk: a meta-analysis. Asian Pac J Cancer Prev 2014;15:4417–22. [DOI] [PubMed] [Google Scholar]
- [17].Xin Y, Hao S, Lu J, et al. Association of ERCC1 C8092A and ERCC2 Lys751Gln polymorphisms with the risk of glioma: a meta-analysis. PLoS One 2014;9:e95966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Xu Z, Ma W, Gao L, et al. Association between ERCC1 C8092A and ERCC2 K751Q polymorphisms and risk of adult glioma: a meta-analysis. Tumour Biol 2014;35:3211–21. [DOI] [PubMed] [Google Scholar]
- [19].Yuan G, Gao D, Ding S, et al. DNA repair gene ERCC1 polymorphisms may contribute to the risk of glioma. Tumour Biol 2014;35:4267–75. [DOI] [PubMed] [Google Scholar]
- [20].Adel Fahmideh M, Schwartzbaum J, Frumento P, et al. Association between DNA repair gene polymorphisms and risk of glioma: a systematic review and meta-analysis. Neuro Oncol 2014;16:807–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhou CX, Zhao JH. Systematic review on the association between ERCC1 rs3212986 and ERCC2 rs13181 polymorphisms and glioma risk. Genet Mol Res 2015;14:2868–75. [DOI] [PubMed] [Google Scholar]
- [22].Dong YS, Hou WG, Li XL, et al. Genetic association of CHEK2, GSTP1, and ERCC1 with glioblastoma in the Han Chinese population. Tumour Biol 2014;35:4937–41. [DOI] [PubMed] [Google Scholar]
- [23].Gao K, Mu SQ, Wu ZX. Investigation of the effects of single-nucleotide polymorphisms in DNA repair genes on the risk of glioma. Genet Mol Res 2014;13:1203–11. [DOI] [PubMed] [Google Scholar]
- [24].Hui L, Yue S, Gao G, et al. Association of single-nucleotide polymorphisms in ERCC1 and ERCC2 with glioma risk. Tumour Biol 2014;35:7451–7. [DOI] [PubMed] [Google Scholar]
- [25].Rodriguez-Hernandez I, Perdomo S, Santos-Briz A, et al. Analysis of DNA repair gene polymorphisms in glioblastoma. Gene 2014;536:79–83. [DOI] [PubMed] [Google Scholar]
- [26].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Thakkinstian A, D’Este C, Eisman J, et al. Meta-analysis of molecular association studies: vitamin D receptor gene polymorphisms and BMD as a case study. J Bone Miner Res 2004;19:419–28. [DOI] [PubMed] [Google Scholar]
- [28].Jiang DK, Ren WH, Yao L, et al. Meta-analysis of association between TP53 Arg72Pro polymorphism and bladder cancer risk. Urology 2010;76:765.e761-767. [DOI] [PubMed] [Google Scholar]
- [29].Galbraith RF. A note on graphical presentation of estimated odds ratios from several clinical trials. Stat Med 1988;7:889–94. [DOI] [PubMed] [Google Scholar]
- [30].Huy NT, Thao NT, Diep DT, et al. Cerebrospinal fluid lactate concentration to distinguish bacterial from aseptic meningitis: a systemic review and meta-analysis. Crit Care 2010;14:R240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Copas J, Shi JQ. Meta-analysis, funnel plots and sensitivity analysis. Biostatistics 2000;1:247–62. [DOI] [PubMed] [Google Scholar]
- [32].Xue H, Ni P, Lin B, et al. X-ray repair cross-complementing group 1 (XRCC1) genetic polymorphisms and gastric cancer risk: a HuGE review and meta-analysis. Am J Epidemiol 2011;173:363–75. [DOI] [PubMed] [Google Scholar]
- [33].Liu Y, Li L, Qi H, et al. Survivin -31G>C polymorphism and gastrointestinal tract cancer risk: a meta-analysis. PLoS One 2013;8:e54081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wang W, Wang Y, Gong F, et al. MTHFR C677T polymorphism and risk of congenital heart defects: evidence from 29 case-control and TDT studies. PLoS One 2013;8:e58041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Caggana M, Kilgallen J, Conroy JM, et al. Associations between ERCC2 polymorphisms and gliomas. Cancer Epidemiol Biomarkers Prev 2001;10:355–60. [PubMed] [Google Scholar]
- [36].Chen P, Wiencke J, Aldape K, et al. Association of an ERCC1 polymorphism with adult-onset glioma. Cancer Epidemiol Biomarkers Prev 2000;9:843–7. [PubMed] [Google Scholar]
- [37].McKean-Cowdin R, Barnholtz-Sloan J, Inskip PD, et al. Associations between polymorphisms in DNA repair genes and glioblastoma. Cancer Epidemiol Biomarkers Prev 2009;18:1118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Liu Y, Scheurer ME, El-Zein R, et al. Association and interactions between DNA repair gene polymorphisms and adult glioma. Cancer Epidemiol Biomarkers Prev 2009;18:204–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Chen DQ, Yao DX, Zhao HY, et al. DNA repair gene ERCC1 and XPD polymorphisms predict glioma susceptibility and prognosis. Asian Pac J Cancer Prev 2012;13:2791–4. [DOI] [PubMed] [Google Scholar]
- [40].Luo KQ, Mu SQ, Wu ZX, et al. Polymorphisms in DNA repair genes and risk of glioma and meningioma. Asian Pac J Cancer Prev 2013;14:449–52. [DOI] [PubMed] [Google Scholar]
- [41].Wrensch M, Kelsey KT, Liu M, et al. ERCC1 and ERCC2 polymorphisms and adult glioma. Neuro Oncol 2005;7:495–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhang N, Lin LY, Zhu LL, et al. ERCC1 polymorphisms and risk of adult glioma in a Chinese population: a hospital-based case-control study. Cancer Invest 2012;30:199–202. [DOI] [PubMed] [Google Scholar]
- [43].Rajaraman P, Hutchinson A, Wichner S, et al. DNA repair gene polymorphisms and risk of adult meningioma, glioma, and acoustic neuroma. Neuro Oncol 2010;12:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Pan WR, Li G, Guan JH. Polymorphisms in DNA repair genes and susceptibility to glioma in a Chinese population. Int J Mol Sci 2013;14:3314–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Juenger H, Holst MI, Duffe K, et al. Tetraspanin-5 (Tm4sf9) mRNA expression parallels neuronal maturation in the cerebellum of normal and L7En-2 transgenic mice. J Comp Neurol 2005;483:318–28. [DOI] [PubMed] [Google Scholar]
- [46].Neumann AS, Sturgis EM, Wei Q. Nucleotide excision repair as a marker for susceptibility to tobacco-related cancers: a review of molecular epidemiological studies. Mol Carcinog 2005;42:65–92. [DOI] [PubMed] [Google Scholar]
- [47].Volker M, Mone MJ, Karmakar P, et al. Sequential assembly of the nucleotide excision repair factors in vivo. Mol Cell 2001;8:213–24. [DOI] [PubMed] [Google Scholar]
- [48].Melton DW, Ketchen AM, Nunez F, et al. Cells from ERCC1-deficient mice show increased genome instability and a reduced frequency of S-phase-dependent illegitimate chromosome exchange but a normal frequency of homologous recombination. J Cell Sci 1998;111(Pt 3):395–404. [DOI] [PubMed] [Google Scholar]
- [49].Sung P, Bailly V, Weber C, et al. Human xeroderma pigmentosum group D gene encodes a DNA helicase. Nature 1993;365:852–5. [DOI] [PubMed] [Google Scholar]
- [50].McLendon RE, Robinson JS, Jr, Chambers DB, et al. The glioblastoma multiforme in Georgia, 1977-1981. Cancer 1985;56:894–7. [DOI] [PubMed] [Google Scholar]
- [51].Kuratsu J, Takeshima H, Ushio Y. Trends in the incidence of primary intracranial tumors in Kumamoto, Japan. Int J Clin Oncol 2001;6:183–91. [DOI] [PubMed] [Google Scholar]
- [52].Fan KJ, Pezeshkpour GH. Ethnic distribution of primary central nervous system tumors in Washington, DC, 1971 to 1985. J Natl Med Assoc 1992;84:858–63. [PMC free article] [PubMed] [Google Scholar]
- [53].Stiller CA, Nectoux J. International incidence of childhood brain and spinal tumours. Int J Epidemiol 1994;23:458–64. [DOI] [PubMed] [Google Scholar]
- [54].Pan Z, Trikalinos TA, Kavvoura FK, et al. Local literature bias in genetic epidemiology: an empirical evaluation of the Chinese literature. PLoS Med 2005;2:e334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Walsh KM, Codd V, Smirnov IV, et al. Variants near TERT and TERC influencing telomere length are associated with high-grade glioma risk. Nat Genet 2014;46:731–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Zhang J, Wu G, Miller CP, et al. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet 2013;45:602–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Paugh BS, Broniscer A, Qu C, et al. Genome-wide analyses identify recurrent amplifications of receptor tyrosine kinases and cell-cycle regulatory genes in diffuse intrinsic pontine glioma. J Clin Oncol 2011;29:3999–4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Sanson M, Hosking FJ, Shete S, et al. Chromosome 7p11.2 (EGFR) variation influences glioma risk. Hum Mol Genet 2011;20:2897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]


