Supplemental Digital Content is available in the text
Keywords: continuous venovenous hemofiltration, CVVH, meta-analysis, PQ poisoning
Abstract
Background:
Paraquat (PQ) poisoning is a widespread occurrence, especially in underdeveloped areas. The treatment of PQ poisoning has always been difficult, and there is currently no definite effective treatment. Continuous venovenous hemofiltration (CVVH) treatment for PQ poisoning has been widely used in clinical practice; however, its effect remains uncertain. Accordingly, the purpose of this meta-analysis was to evaluate the efficacy of CVVH in the treatment of PQ poisoning.
Methods:
We searched for relevant trials using PubMed, Embase, the Cochrane Library, and 3 Chinese databases, the Chinese BioMedical Literature Database, National Knowledge Infrastructure Database, and Wanfang Database. We included all qualified randomized controlled trials (RCTs) of CVVH treatment for patients with PQ poisoning. The primary outcome was mortality, while the secondary outcomes included the survival time and constituent ratios of death due to respiratory failure and circulatory failure.
Results:
Three RCTs involving 290 patients were included. The mortality rates of the intervention and control groups were 57.9% and 61.0%, respectively. Pooled analysis demonstrated no significant difference in mortality between the CVVH treatment and control groups (risk ratio [RR] 0.94, 95% confidence interval [CI]: 0.78–1.15, P = .56), with a low level of heterogeneity (X2 = 1.75, I2 = 0%). However, the CVVH group was associated with a longer survival time compared to the control group (weighted mean difference 1.73, 95% CI: 0.56–2.90, P = .004). Respiratory failure as the cause of death was more common in the CVVH group, as compared with the control group (RR 1.66, 95% CI: 1.24–2.23, P = .0008), whereas patients in the control group were more likely to die from circulatory failure than in the CVVH group (RR 0.56, 95% CI: 0.40–0.81, P = .002).
Conclusion:
Although CVVH treatment might not noticeably reduce mortality for patients with PQ poisoning, it can prolong the survival time of the patients and improve the stability of the circulatory system, thereby enabling further treatment.
1. Introduction
Paraquat (PQ; 1,1-dimethyl-4,4-bipyridine cationic salt), a high-performance, nonselective contact herbicide, is highly toxic to humans and animals. Numerous accidental and suicidal PQ poisonings have been reported worldwide, especially in developing countries.[1,2] Due to the high toxicity of PQ and the lack of a special antidote, a number of European countries have now banned the use of PQ, and some developing countries have joined the refusal to use PQ. However, in China, PQ is still used in many areas, especially in rural areas, and it is hard to ban the use of PQ completely.[3–6]
Especially, although the traditionally used PQ solution has been prohibited, a granule and emulsion preparation instead prevails on the Chinese market,[7] indicating that the risk of PQ exposure still exists. Recently, we have experienced several cases of poisoning due to these PQ granules in our department. Therefore, it is of great clinical importance to determine the most effective and appropriate therapy for PQ poisoning.
The exact toxicological mechanisms of PQ poisoning are not entirely clear, although lipid peroxidation damage is considered the main injury mechanism of PQ poisoning. Further, lung injury due to inflammatory and redox reactions has been suggested as another major mechanism.[1,8–11] Various potential therapies for PQ poisoning are also being explored. The current treatment strategy mainly includes preventing the absorption of the poison, promoting the discharge of the toxin, glucocorticoid therapy for immunosuppression, and symptomatic supportive treatment.[1,10,12]
As one commonly used method of poison clearance, blood purification has been demonstrated to be useful for the removal of PQ from the patient's body and for improving the prognosis of the patients. There are various types of blood purification, among which the efficacy of hemoperfusion (HP) for PQ poisoning has been widely recognized.[13–17] Compared to HP, continuous venovenous hemofiltration (CVVH), the most commonly used type of continuous renal replacement therapy, possesses diffusive, convective, and adsorptive functions.[18,19] Theoretically, these features of CVVH cannot only help remove the toxin from the blood, but can also help clear the large number of inflammatory mediators and inflammatory factors produced upon PQ poisoning. However, although CVVH has already been applied for the treatment of PQ poisoning in clinical practice, its efficacy remains controversial.[9,18–21] Therefore, in the present study, we conducted a meta-analysis of reported randomized controlled trials (RCTs) to further clarify the efficacy of CVVH in the treatment of PQ poisoning.
2. Materials and methods
2.1. Study selection
We used the preferred reporting items for systematic reviews and meta-analyses statement methodology to report this meta-analysis.[22] Two investigators (GL and JL) independently conducted a literature search using PubMed, Embase, the Cochrane library, and 3 Chinese databases (the Chinese BioMedical Literature Database, National Knowledge Infrastructure Database, and Wanfang Database). All RCTs were considered, without language or date restrictions. We performed our latest search on December 28, 2016. Relevant text words were used as the search terms, including “PQ,” “CVVH,” “Hemofiltration,” etc. in the English databases, whereas “paraquat [in Chinese],” “continuous venovenous hemofiltration [in Chinese],” etc. were used for the searches in the Chinese databases. (see Word file, Supplemental Content 1, in which the detailed search strategy is provided). In addition, to avoid omissions, the reference lists of the identified trials and all related articles were manually searched.
2.2. Data extraction
The inclusion criteria were as follows: RCTs meeting the standard diagnostic criteria of PQ poisoning; investigating the efficacy of CVVH treatment based on HP as the control; and with sufficient outcomes available to calculate the risk ratios (RRs) or weighted mean differences (WMDs) with 95% confidence intervals (CIs), including mortality, survival time, and cause of death, among others.
The exclusion criteria included: studies in which all patients were treated with CVVH, without a control group; studies including pediatric patients; case reports or case series; nonhuman studies; and reviews. For trials using repeated research data, we included the study with the largest sample size or the latest study, as appropriate.
The 2 investigators (GL and JL) independently assessed each of the studies and extracted the outcomes. To resolve any disagreements, a 3rd investigator (LY) was brought in for discussion. The following features were extracted from the studies: first author, publication year, country, number of participants, oral dose of PQ or concentration of toxin in blood or urine, protocol of CVVH, other treatments including methylprednisolone and HP, mortality, survival time, and constituent ratios of death.
2.3. Data synthesis and statistical analysis
To assess the risk of bias in the included RCTs, the Cochrane collaboration tool was used by 2 reviewers. For each included study, a rating of “high,” “unclear,” or “low” risk of bias was provided for each of the following domains: adequate random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. Studies with a high risk of bias for one or more key domains were considered as being at high risk of bias. Studies with a low risk of bias for all key domains were considered as being at low risk of bias. Otherwise, they were considered as having an unclear risk of bias.[23]
For dichotomous variables, such as mortality and the constituent ratios of death due to respiratory failure and circulatory failure, the RRs and 95% CIs were calculated. For continuous variables, such as the survival time, the WMDs, and 95% CIs were calculated for each study. Heterogeneity was evaluated using the chi-square test, with P < .1 indicating significance, and using I2 statistics, with an I2 value >50% indicating significant heterogeneity. We also assessed clinical heterogeneity by considering the design of each study.[23,24] Due to the small number of included trials, funnel plots could not be used to analyze publication bias. Sensitivity analyses were performed by sequentially removing a single trial and analyzing the potential effect on the overall results. This meta-analysis was performed using Review Manager 5.3 (Cochrane Collaboration, Oxford, UK). Unless otherwise specified, P < .05 was considered to indicate statistical significance. Since our study was a review of previous published studies, ethical approval or patient consent was not required.
3. Results
3.1. Eligible studies
The study selection flow chart is presented in Fig. 1. The literature search generated 451 potentially relevant records. After screening the titles and authors, 59 duplicate studies were removed (see Word file, Supplemental Content 2, which illustrates the results after removing the duplicate studies). By evaluating the titles and abstracts of the identified papers, we further excluded 372 studies not meeting the inclusion criteria. Subsequently, we carefully reviewed the full texts of the remaining 20 studies (see Word file, Supplemental Content 3, which illustrates the full-text articles assessed for eligibility). As a result, we excluded 17 trials, including 11 undefined studies, 5 retrospective studies, and 1 study without a full text. Thus, we finally included 3 RCTs comparing the efficacy of CVVH treatment with a control group for patients with acute PQ poisoning.[25–27] The detailed interventions and primary outcomes of the selected trials are shown in Table 1.
Figure 1.
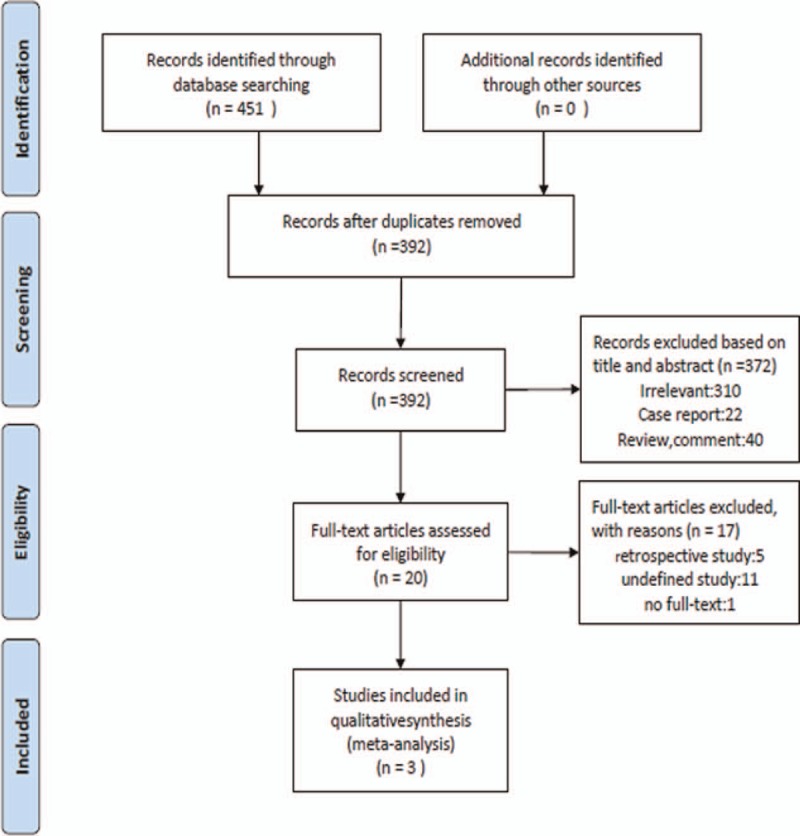
Flow chart of selection of studies.
Table 1.
Interventions and primary outcome of selected trials.
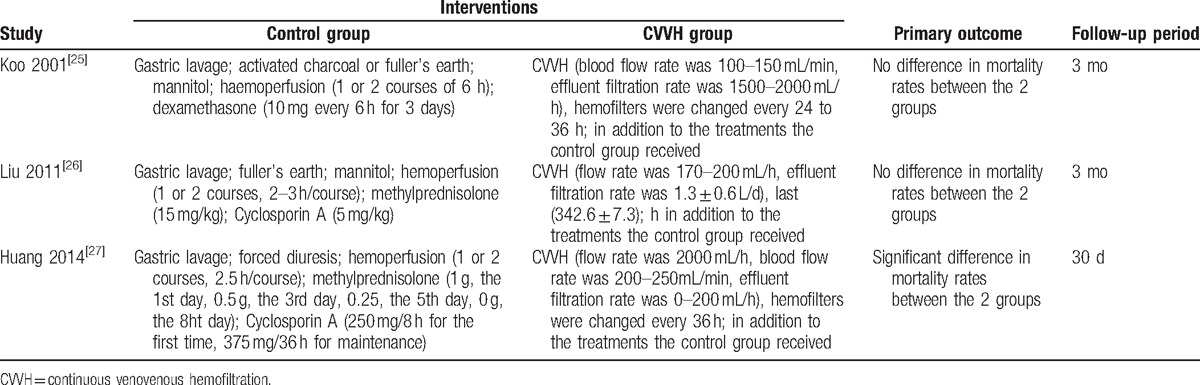
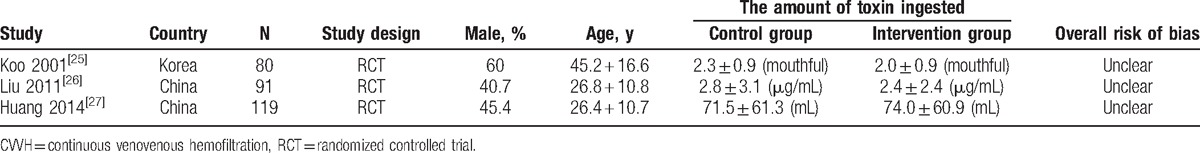
A total of 290 patients were included in this study, with 126 and 164 patients in the intervention (CVVH) and control (HP) groups, respectively. Among the included RCTs, there were 2 studies from China and 1 from Korea (written in English). The primary and secondary outcomes recorded in these studies included mortality (3 trials), survival time (2 trials), the constituent ratio of death due to respiratory failure (3 trials), and the constituent ratio of death due to circulatory failure (3 trials). The characteristics of the RCTs meeting the inclusion criteria are presented in Table 2.
Table 2.
Baseline characteristics of selected trials of CVVH treatment in paraquat poisoning patients.
3.2. Assessment of methodological quality
The results of the assessments of the risk of bias are summarized in Figs. 2 and 3. All studies were judged to be at unclear risk of bias. In these studies, blinding of patients and personnel was not practicable because of the specificity of CVVH treatment, although the outcomes are not likely to be influenced by this lack of blinding. Some information, such as allocation concealment and incomplete outcome data, was not available. We attempted to contact the corresponding authors to obtain further information, but received no responses.
Figure 2.
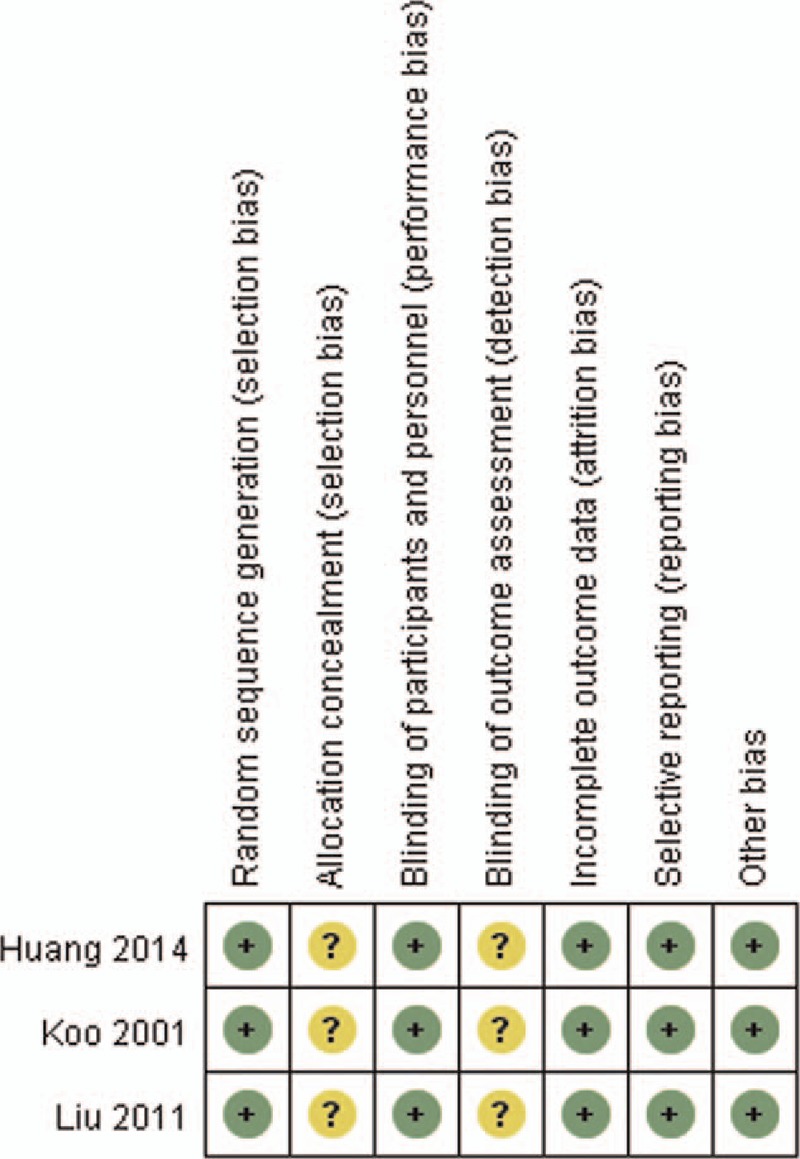
Risk of bias summary: review authors’ judgements about each risk of bias item for each included study.
Figure 3.
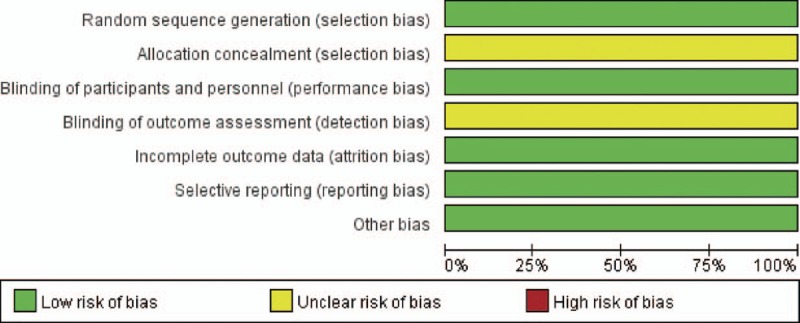
Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies.
3.3. Mortality
Data on mortality were reported in all 290 patients included in the 3 RCTs. The overall mortality rate was 60.3%. The mortality rates of the intervention and control groups were 57.9% and 61.0%, respectively. The pooled analysis of all studies showed that there was no significant difference in mortality between the intervention and control groups (RR 0.94, 95% CI: 0.78–1.15, P = .56), with a low level of heterogeneity (X2 = 1.75, I2 = 0%) (Fig. 4).
Figure 4.
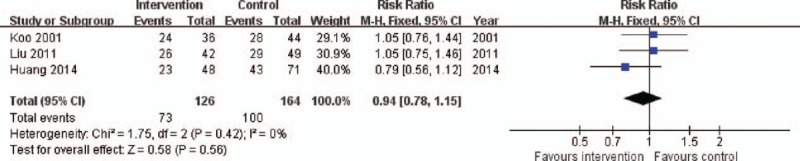
Forest plot for overall mortality. CI = confidence interval, M-H = Mantel–Haenszel.
3.4. Survival time
There were 2 RCTs including 107 patients that presented data on survival time. As shown in Fig. 5, the intervention group was associated with a longer survival time than the control group (WMD 1.73, 95% CI: 0.56–2.90, P = .004), and there was a low level of heterogeneity (X2 = 0.71, I2 = 0%).
Figure 5.
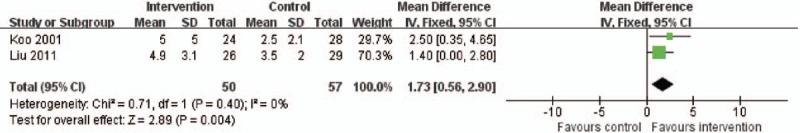
Forest plot for survival time. CI = confidence interval, M-H = Mantel–Haenszel.
3.5. Constituent ratio of death due to respiratory failure
As shown in Fig. 6, the 3 RCTs described the proportion of death due to respiratory failure in 173 patients, with a low level of heterogeneity (X2 = 1.42, I2 = 0%). Respiratory failure as the cause of death was more common in the intervention group than in the control group (RR 1.66, 95% CI: 1.24–2.23, P = .0008).
Figure 6.
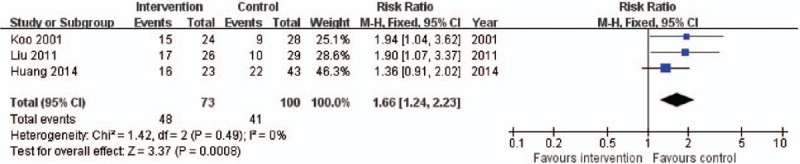
Forest plot for constituent ratio of death due to respiratory failure. CI = confidence interval, M-H = Mantel–Haenszel.
3.6. Constituent ratio of death due to circulatory failure
As shown in Fig. 7, the 3 RCTs provided information on the proportion of death due to circulatory failure in 173 patients. Circulatory failure as the cause of death was more common in the control group than in the intervention group (RR 0.56, 95% CI: 0.40–0.81, P = .002). There was a low level of heterogeneity (X2 = 0.13, I2 = 0%).
Figure 7.
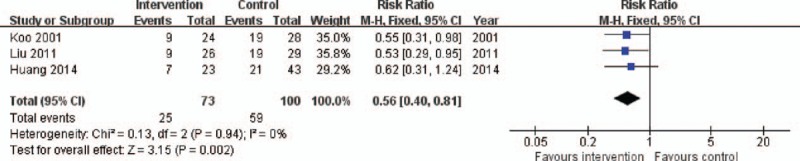
Forest plot for constituent ratio of death due to circulatory failure. CI = confidence interval, M-H = Mantel–Haenszel.
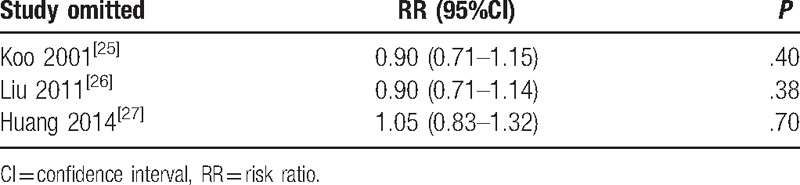
3.7. Sensitivity analysis
To assess the stability of the results of our meta-analysis, we conducted a sensitivity analysis for each outcome by removing 1 study at the time (Table 3). After omitting each of the studies, we found similar results statistically, indicating that the results of this meta-analysis have considerable stability.
Table 3.
Sensitivity analysis for mortality by omitting each study in random-effects model.
4. Discussion
In the present meta-analysis of 3 RCTs including a total of 290 patients, we found that there was no significant difference in mortality between the intervention and control groups, indicating that CVVH cannot improve the survival rate of patients with PQ poisoning compared to HP. However, on the other hand, we found that the survival time of the patients in the intervention group was significantly longer than that of the patients in the control group. In addition, it was also found that the constituent ratio of death in the intervention group was more likely to be respiratory failure compared with in the control group, whereas the constituent ratio of death in the control group tended to be circulatory failure more frequently than in the intervention group, indicating that CVVH may be helpful for the treatment of PQ-induced multiple organ dysfunction, except for lung injury.
The PQ-induced early inflammatory response is an essential mechanism of injury for patients with PQ poisoning. Although HP can remove PQ from the blood of the patients, it cannot protect our body from damage caused by toxic substances entering the tissues and organs, especially the inflammatory reaction in the acute phase. Hence, the use of HP still has many shortcomings. Conversely, CVVH, widely used in critically ill patients with multiple organ failure, can remove poison, regulate the body fluid balance, and clear the body of inflammatory mediators and factors, which is theoretically helpful to improve the condition of patients with PQ poisoning.[20,25,26]
Of note, in cases of severe to acute fulminant toxicity, the patients generally suffer multiorgan failure (hepatic, renal, adrenal, pancreatic, central nervous system, cardiac, and respiratory failures) at an early stage, and they do not survive long enough to demonstrate pulmonary fibrosis.[28] Our results suggest that CVVH treatment might help in the treatment of these early injuries by prolonging the survival time of the patients. In addition, CVVH might help remove PQ that is redistributed from the lungs into the blood, even if the removal effect is not as good as that of HP. However, the clinical efficiency of CVVH for PQ poisoning remains controversial, owing to a lack of adequate clinical evidence. As mentioned above, the present analysis suggests that, while CVVH treatment for acute PQ poisoning cannot improve the survival rate, it can extend the survival time, which might provide a chance for further treatment such as lung transplantation. Accordingly, it has been previously reported that, by maintaining hemodynamic stability, CVVH treatment contributed to longer survival time and circulatory stability, laying a foundation for lung transplantation or other treatments,[29,30] and our department has successful cured a patient with PQ poisoning by lung transplantation.[29] As many cases of deliberate ingestion are not suitable for lung transplantation due to the unknown psychiatric history of the patients, and since the compliance with the essential immunosuppressive therapy and follow-up is likely to be low in such patients, bridging the time to complete depletion of PQ from the body could render this exceptional therapy strategy possible in a subset of patients in whom ingestion was accidental.[31] However, unless the appropriate follow-up treatments can be administered, a prolonged survival time may simply result in the patients dying slowly of respiratory failure, and this is clearly not a positive outcome.
To our knowledge, this study is the first meta-analysis to systematically evaluate the effect of CVVH in patients with PQ poisoning based on RCTs. Our search strategy was precise and repeated, and the final meta-analysis included 3 RCTs from 2 countries, written in both English and Chinese, with a total of 290 patients. Further, 2 investigators carefully assessed the methodological quality of each included RCT independently.
This study provides evidence and may serve as a basis for clinical practical work in the future. However, there are also several potential limitations of our study. First, only 3 RCTs were included in the present analysis, owing to the poor methodological quality in the majority of studies identified, and none of the studies described allocation concealment, which may lead to selection, performance, and detection biases. Second, subgroup analyses, which may have made the results more convincing, were not performed, owing to insufficient information. Especially, only 1 included study analyzed the outcomes according to the severity of acute PQ poisoning. Third, we were unable to investigate publication bias using funnel plots due to only 3 studies being included in the review; however, publication bias is always a consideration in any systematic review. Finally, none of the included studies investigated the Acute Physiology and Chronic Health Evaluation scores of the patients, and this may have influenced the outcomes.
5. Conclusion
In conclusion, our study suggests that, while the use of CVVH may not reduce mortality for patients with PQ poisoning, it can prolong the survival time of the patients and improve the function of multiple organs, except the lungs, thereby enabling additional treatments. Further well-designed, high-quality RCTs, especially studies evaluating the outcomes according to the severity of acute PQ poisoning, are needed to confirm our findings and investigate the treatment efficacy of CVVH.
Supplementary Material
Acknowledgments
The authors thank Special Fund for MOH Research in the Public Interest (grant No. 2015SQ00192-4), Military Medical Scientific (grant No. AWS11C004), and Technological Project for the “Twelfth Five-year Plan” (grant No. BWS12J042) for the support.
Footnotes
Abbreviations: CI = confidence interval, CVVH = continuous venovenous hemofiltration, HP = hemoperfusion, PQ = paraquat, RCT = randomized controlled trial, RR= risk ratio, WMD = weighted mean difference.
Funding/support: This work was supported by Special Fund for MOH Research in the Public Interest (grant No. 2015SQ00192-4), Military Medical Scientific (grant No. AWS11C004), and Technological Project for the “Twelfth Five-year Plan” (grant No. BWS12J042).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Zewu Q. The diagnosis and treatment of paraquat poisoning. Chin Clin Doctors 2012;40:3–5. [Google Scholar]
- [2].Delirrad M, Majidi M, Boushehri B. Clinical features and prognosis of paraquat poisoning: a review of 41 cases. Int J Clin Exp Med 2015;8:8122–8. [PMC free article] [PubMed] [Google Scholar]
- [3].Hou YH, Zhao Q, Wu YX, et al. An analysis of the clinical and epidemiological characteristics of acute poisoning patients in a general hospital. Chin J Indust Hyg Occupat Dis 2016;34:506–9. [DOI] [PubMed] [Google Scholar]
- [4].Zhao Y, Jian XD. Clinical effect of compound monoammonium glycyrrhizinate combined with dandelion in treatment of acute paraquat poisoning. Chin J Indust Hyg Occupat Dis 2016;34:535–7. [DOI] [PubMed] [Google Scholar]
- [5].Xie Y, Xu Z, Yang Y. Analysis of 84 cases treatment of paraquat poisoning. Zhonghua wei zhong bing ji jiu yi xue 2015;27:312–3. [PubMed] [Google Scholar]
- [6].Zhao B, Dai J, Li J, et al. Clinical study on the treatment of acute paraquat poisoning with sequential whole gastric and bowel irrigation. Chin J Indust Hyg Occupat Dis 2015;33:213–5. [PubMed] [Google Scholar]
- [7].International WB. Transformation of Chinese paraquat production enterprice to the development of granules paraquat. Pesticide Market Information 2013;6:9. [Google Scholar]
- [8].Wang Y, Jin B, Liu M, et al. Advance of study on pathological mechanism of paraquat poisoning. J Hyg Res 2006;35:366–9. [PubMed] [Google Scholar]
- [9].Spangenberg T, Grahn H, van der Schalk H, et al. Paraquat poisoning. Case report and overview. Medizinische Klinik, Intensivmedizin und Notfallmedizin 2012;107:270–4. [DOI] [PubMed] [Google Scholar]
- [10].Dinis-Oliveira RJ, Duarte JA, Sanchez-Navarro A, et al. Paraquat poisonings: mechanisms of lung toxicity, clinical features, and treatment. Crit Rev Toxicol 2008;38:13–71. [DOI] [PubMed] [Google Scholar]
- [11].Yao D, Yu X, Tian Y, et al. Progress in the mechanism of cytokine on lung injury caused by acute paraquat poisoning. Chin J Indust Hyg Occupat Dis 2014;32:865–8. [PubMed] [Google Scholar]
- [12].Luo ZY. Treatment 16 cases of acute paraquat poisoning. Chin J Indust Hyg Occupat Dis 2007;25:330. [PubMed] [Google Scholar]
- [13].Hsu CW, Lin JL, Lin-Tan DT, et al. Early hemoperfusion may improve survival of severely paraquat-poisoned patients. PloS One 2012;7:e48397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yang TS, Chang YL, Yen CK. Haemoperfusion treatment in pigs experimentally intoxicated by paraquat. Hum Exp Toxicol 1997;16:709–15. [DOI] [PubMed] [Google Scholar]
- [15].Zhang Q, Wu WZ, Lu YQ, et al. Successful treatment of patients with paraquat intoxication: three case reports and review of the literature. J Zhejiang Univ Sci B 2012;13:413–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hong SY, Yang JO, Lee EY, et al. Effect of haemoperfusion on plasma paraquat concentration in vitro and in vivo. Toxicol Ind Health 2003;19:17–23. [DOI] [PubMed] [Google Scholar]
- [17].Peng ZY, Chang P, Wang H, et al. Intensive hemoperfusion and long-term hemofiltration for treatment of paraquat poisoning: a case report. J Southern Med Univ 2015;35:1515–8. [PubMed] [Google Scholar]
- [18].Gao Y, Zhang X, Yang Y, et al. Early haemoperfusion with continuous venovenous haemofiltration improves survival of acute paraquat-poisoned patients. J Int Med Res 2015;43:26–32. [DOI] [PubMed] [Google Scholar]
- [19].Li A, Li W, Hao F, et al. Early stage blood purification for paraquat poisoning: a multicenter retrospective study. Blood Purif 2016;42:93–9. [DOI] [PubMed] [Google Scholar]
- [20].Fertel BS, Nelson LS, Goldfarb DS. Extracorporeal removal techniques for the poisoned patient: a review for the intensivist. J Intens Care Med 2010;25:139–48. [DOI] [PubMed] [Google Scholar]
- [21].Peng L, Hong-Tao Z, Yu-Guang L, et al. Studying the therapeutic effects of hemoperfusion with continuous venovenous hemofiltration in paraquat-poisoned patients by the ratio of residual normal lung in 3D-CT image. BMC Emerge Med 2012;12. [Google Scholar]
- [22].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hartling L, Ospina M, Liang Y, et al. Risk of bias versus quality assessment of randomised controlled trials: cross sectional study. BMJ 2009;339:b4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Higgins J, S. G. Cochrane handbook for systematic reviews of interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. 2011; http://www.cochrane-handbook.org. [Google Scholar]
- [25].Koo JR, Kim JC, Yoon JW, et al. Failure of continuous venovenous hemofiltration to prevent death in paraquat poisoning. Am J Kidney Dis 2002;39:55–9. [DOI] [PubMed] [Google Scholar]
- [26].Liu P, He YZ, Zhang XG, et al. Studying the therapeutic effects of hemoperfusion with continuous venovenous hemofiltration on the patients with acute paraquat poisoning. Chin J Indust Hyg Occupat Dis 2011;29:266–9. [PubMed] [Google Scholar]
- [27].Changbao H, Yun J, Ruochen T, et al. The efficiency of continuous venovenous hemofiltration for the treatment of oral acute paraquat poisoning. Chin J Emerg Recovery Disaster Med 2014;7:605–8. [Google Scholar]
- [28].Dinis-Oliveira RJ, Duarte JA, Sanchez-Navarro A, et al. Paraquat poisonings: mechanisms of lung toxicity, clinical features, and treatment. Crit Rev Toxicol 2008;38:13–71. [DOI] [PubMed] [Google Scholar]
- [29].Tang X, Sun B, He H, et al. Successful extracorporeal membrane oxygenation therapy as a bridge to sequential bilateral lung transplantation for a patient after severe paraquat poisoning. Clin Toxicol 2015;53:908–13. [DOI] [PubMed] [Google Scholar]
- [30].Walder B, Brundler MA, Spiliopoulos A, et al. Successful single-lung transplantation after paraquat intoxication. Transplantation 1997;64:789–91. [DOI] [PubMed] [Google Scholar]
- [31].Bertram A, Haenel SS, Hadem J, et al. Tissue concentration of paraquat on day 32 after intoxication and failed bridge to transplantation by extracorporeal membrane oxygenation therapy. BMC Pharmacol Toxicol 2013;14:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


