Supplemental Digital Content is available in the text
Keywords: clinical epidemiology, dialysis initiation, eGFR, end-stage renal disease, United States Renal Data system
Abstract
The United States Renal Data System (USRDS) registry of end-stage renal disease has often been used to study the timing of dialysis initiation, measured by estimated glomerular filtration rate (eGFR) at dialysis initiation. We conducted an observational study and examined how well variables in the USRDS database explain the trends and variation in eGFR at dialysis initiation.
We identified 971,481 patients who initiated dialysis between 1995 and 2012 in the USRDS registry.
The mean eGFR at dialysis initiation monotonically rose from 7.7 in 1995 to 11.1 in 2009, and then leveled off to 10.9 mL/min/1.73 m2 in 2012. The trend of rising, then leveling off was similar across all subgroups studied. Substantial variation in eGFR at dialysis initiation was observed, with standard deviation of 4.38 (95% CI: 2.0–18.4). A total of 11.4% of the total variation occurred across physicians and 88.6% within physicians. Adjustment for measured factors only modestly decreased the total variation. Of the total variance, 10.7% was explained by measured patient-level variables and 1.2% by measured physician and other factors, while 9.2% of physician-level variation and 78.9% of patient-level variation remained unexplained. The extent of variation explained by measured variables was similar over the entire study period.
The finding that the majority of variation in eGFR at dialysis initiation is unexplained by measured variables casts doubt on how well eGFR serves as a measure for “timing” of dialysis initiation, and it indicates the need to collect more focused data to gain understanding of factors that affect timing of dialysis initiation in the US.
1. Introduction
In the United States (US), about 117,000 patients developed end-stage renal disease (ESRD) requiring dialysis, while more than 663,000 prevalent patients were on dialysis in 2013.[1] In 2012, total Medicare spending for ESRD was about $28 billion, representing 5.6% of the entire Medicare budget.[2] Per standard convention, estimated glomerular filtration rate (eGFR) at dialysis initiation has been used as the principle measure of the “timing” of dialysis initiation, with higher eGFR indicating earlier dialysis initiation. It has served as either the main predictor or endpoint in various research publications on the timing of dialysis initiation.[3–12] In the US, the mean eGFR at dialysis initiation rose for more than two decades, though it may have leveled off in recent years.[13] A number of studies have demonstrated that higher eGFR at dialysis initiation is not associated with longer survival on dialysis.[3,4,8–12] Other studies have linked patient and provider characteristics to eGFR at dialysis initiation,[5] and additional studies have focused on the trend of rising eGFR at dialysis initiation over time.[13–16]
Most of these studies were conducted using the United States Renal Data System (USRDS) ESRD registry database.[5,9–12] However, it is not clear how well the information captured by this database explains the eGFR variation at dialysis initiation, nor how well eGFR serves as a measure for the “timing” of dialysis initiation. Additionally, analyses that focused on eGFR at dialysis initiation were often limited to descriptive statistics, and did not account for temporal changes in patient/provider characteristics. Using USRDS ESRD registry data from 1995 to 2012, our study addresses these questions by examining eGFR at dialysis initiation and its trend over time. It is the largest study of its kind, based on the most recent USRDS data available, and over a long study interval. We investigate the following research questions:
-
(1)
Has the rise in average eGFR at dialysis initiation continued to level off in the US?
-
(2)
Has the overall pattern of change in eGFR over time been consistent across subgroups?
-
(3)
How much variation is there in eGFR at dialysis initiation in the US?
-
(4)
What proportion of this variation can be explained by patient- and nonpatient levels variables recorded in the USRDS ESRD registry database, and has this proportion changed over time?
We hypothesized that eGFR at dialysis initiation has continued to level off overall in the most recent years, but that changes in eGFR over time may not be similar across subgroups. We also hypothesized that there is large variation of eGFR at dialysis initiation, which would indicate that many factors other than eGFR drive the initiation of dialysis. Additionally, we postulated that there is large unexplained variation in eGFR at dialysis initiation, which would imply that the available data are insufficient to understand decisions regarding timing of dialysis initiation at the US population level.
2. Methods
2.1. Study population
The study population included adult patients (18 years or older) who initiated dialysis between January 1, 1995 and December 31, 2012 as identified in the USRDS ESRD database. We excluded patients who received a preemptive kidney transplant instead of dialysis as initial treatment for ESRD. We also excluded patients who had missing values for the variables (see Fig. 1, which demonstrates the patient selection process). Our final study sample included 971,481 patients.
Figure 1.
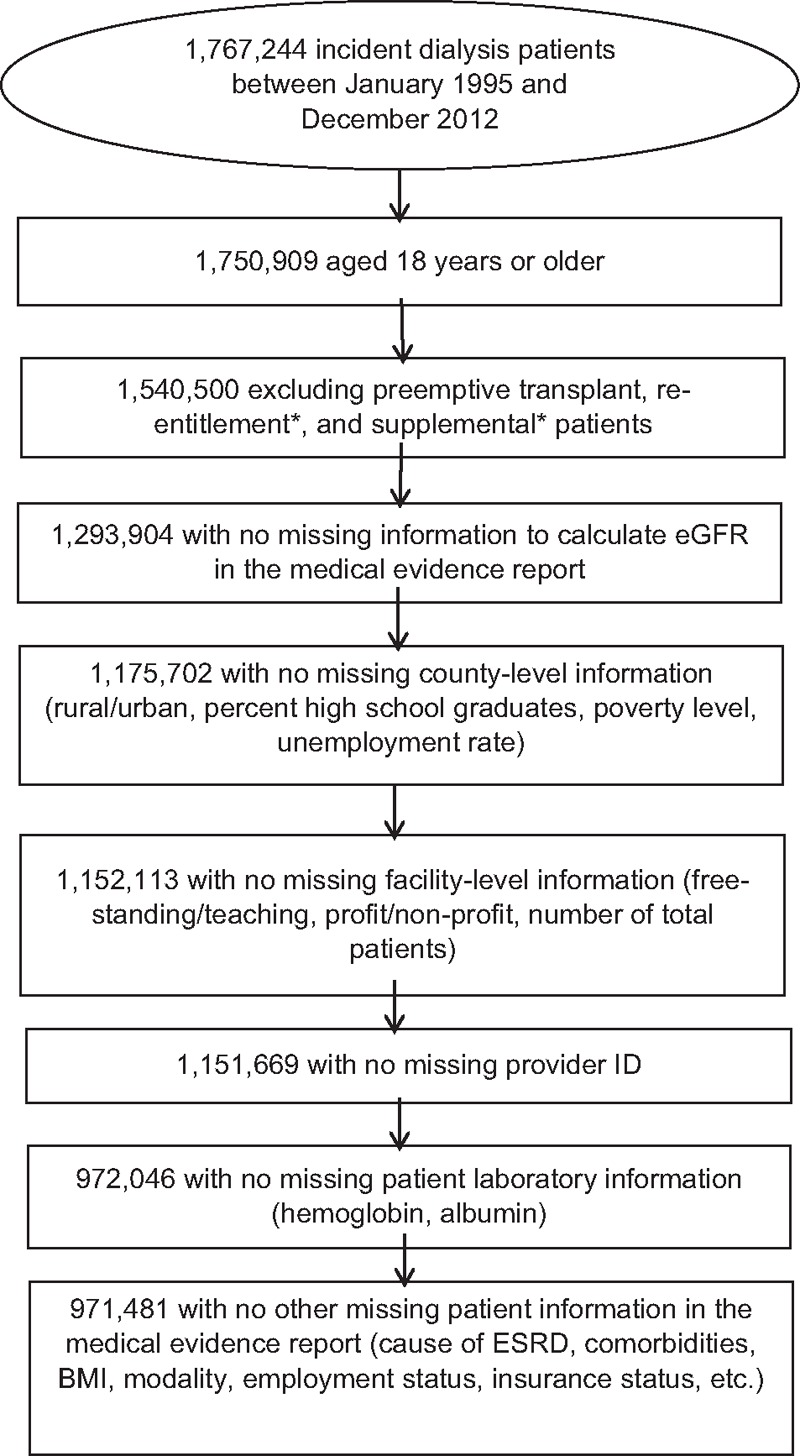
Flow chart of patient selection. ∗Based on CMS Form 2728.
2.2. Patient demographic, clinical, and laboratory measures
We obtained patient-level measures, listed in Table 1, as recorded at the time of patients’ 1st dialysis from the Centers for Medicare & Medicaid Services (CMS) Medical Evidence Report Form 2728. Two versions of Form 2728 were used, due to modifications incorporated in 2005. Data only collected on the new form were used in sensitivity analyses restricted to 2005 to 2012, including the receipt of nephrology care prior to dialysis, vascular access type (catheter, fistula, or graft), and being institutionalized (eg, assisted living, nursing home). eGFR at dialysis initiation was based on serum creatinine reported on Form 2728 (required to be within 45 days before 1st dialysis), and was calculated using the Modification of Diet in Renal Disease equation.
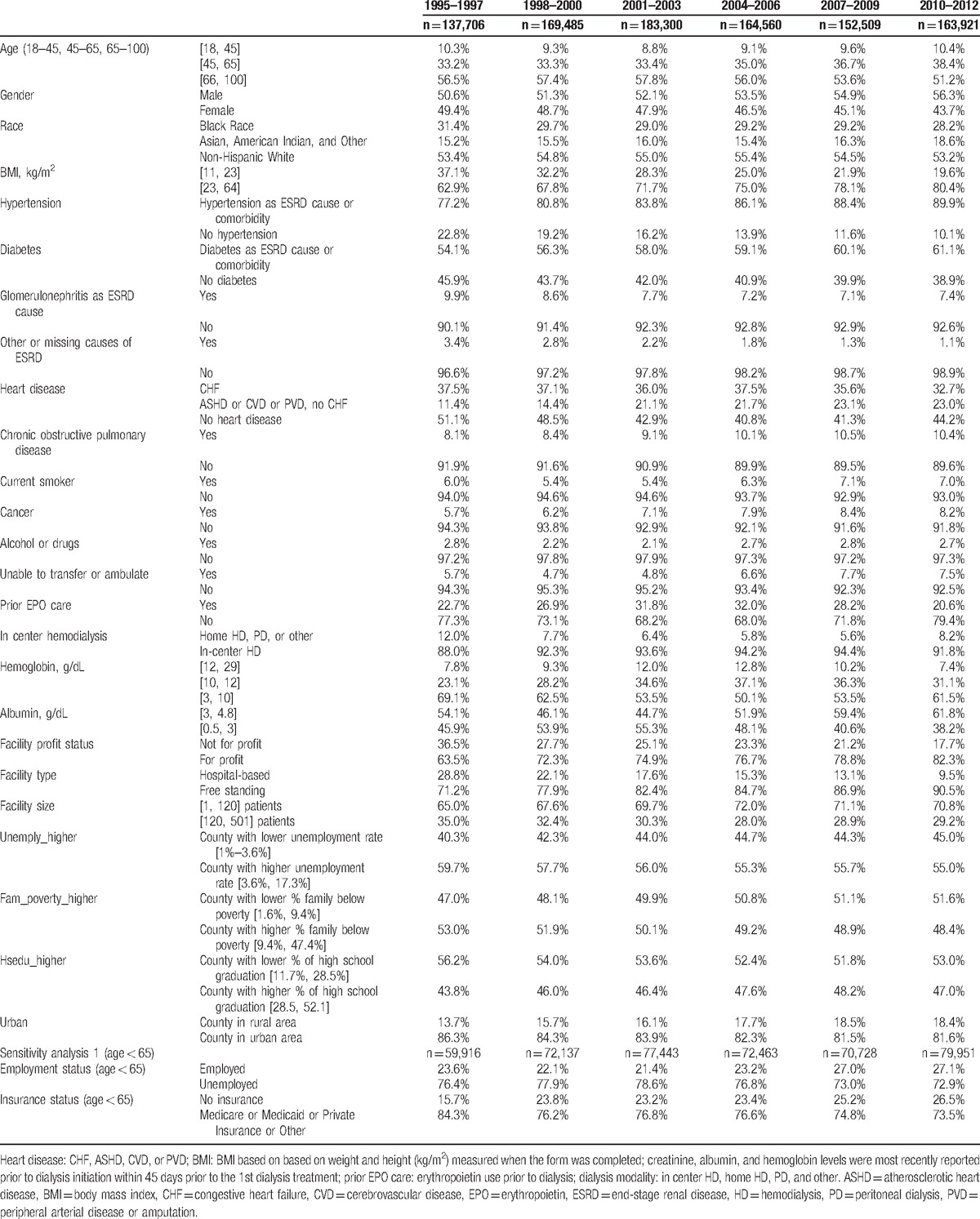
Table 1.
The percentages of patients by subgroups in 3-year intervals from 1995 to 2012.
2.3. Physician-, facility-, and county-level measures
We obtained facility-level variables including the total number of patients, nurse-to-patient ratio, profit versus nonprofit status, free-standing versus hospital-based facilities, and facility ESRD network membership through the CMS Annual Facility Survey 2744. Treating physicians were identified as those signing the CMS Form 2728. Information on counties where facilities were located (including unemployment rate, high school graduation rate, and percentage of households below poverty level) was based on the 2000 US Census, and facility rural status was identified using the 2000 Rural Urban Commuting Area codes.
2.4. Statistical methods
We truncated eGFR values at both tails of the distribution, with the minimum and maximum set at 2 and 20 mL/min/1.73 m2, respectively. About 0.2% of patients had a calculated eGFR < 2 mL/min/1.73 m2 and 5.2% had an eGFR > 20 mL/min/1.73 m2. To obtain adjusted mean eGFR over time, we used linear mixed models (LMMs) to model eGFR as a function of calendar year, the variables provided above, and their respective interactions with year. All LMMs accounted for within-cluster correlations by using treating physicians as the random intercept.
To calculate the proportion of variation in eGFR at dialysis initiation explained by measured variables, we fit a series of LMMs sequentially. First, we used an intercept-only model, which allowed us to estimate the total amount of variation (σ2) in eGFR at initiation and separate its between-provider component, denoted by 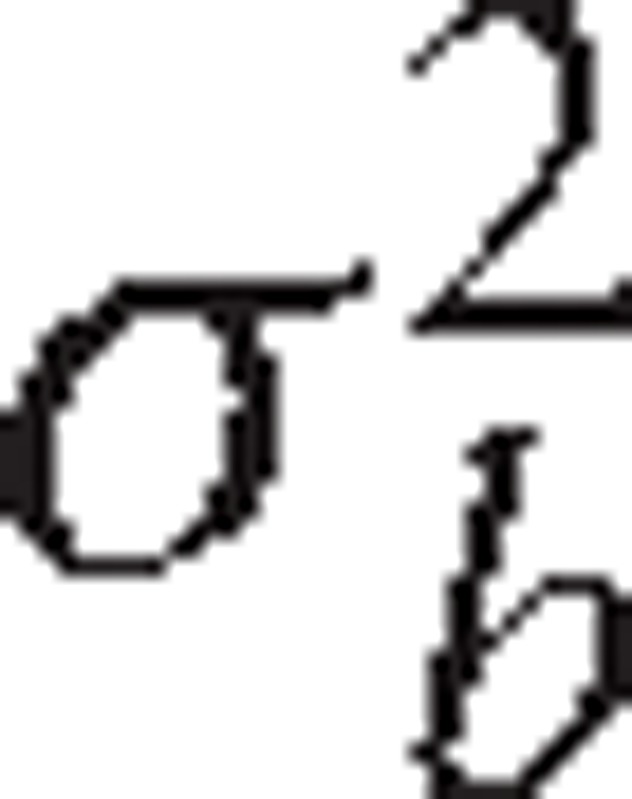 , and within-provider component (ie, patient-level), denoted by
, and within-provider component (ie, patient-level), denoted by 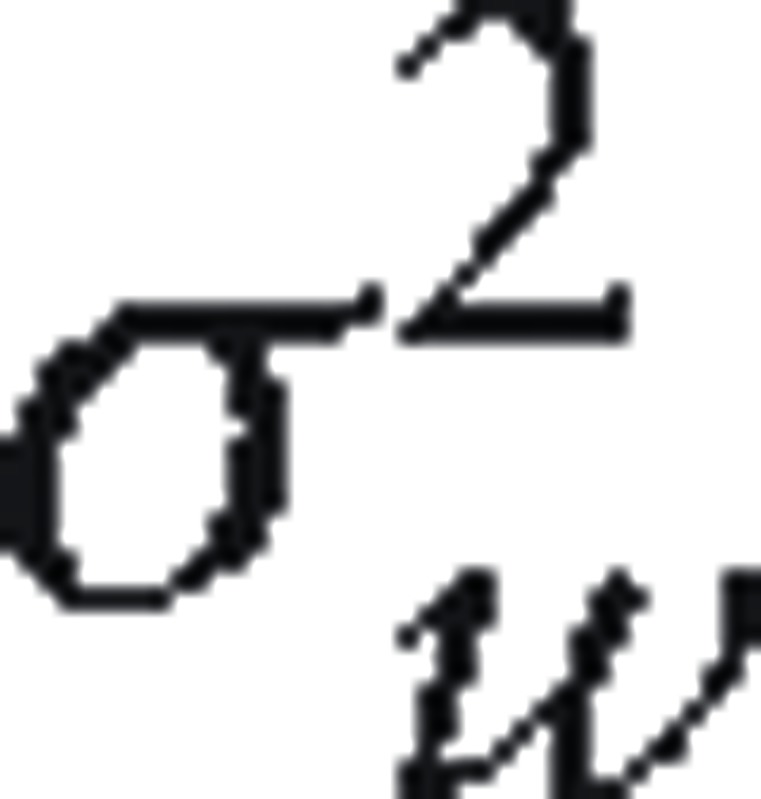 . Here,
. Here, 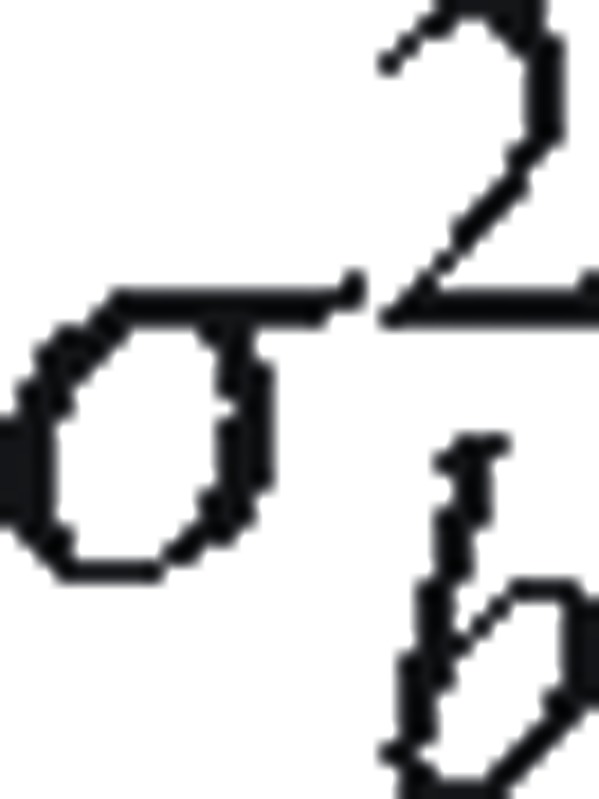 captures variation beyond patient level which includes provider, facility, county, regional, or higher levels. Then we added patient-, treatment-, facility-, and county-level variables to the model sequentially. With each set of variables added, we obtained the total residual (ie, unexplained) variance (π2). The proportion of variance explained by this set of variables was calculated as
captures variation beyond patient level which includes provider, facility, county, regional, or higher levels. Then we added patient-, treatment-, facility-, and county-level variables to the model sequentially. With each set of variables added, we obtained the total residual (ie, unexplained) variance (π2). The proportion of variance explained by this set of variables was calculated as 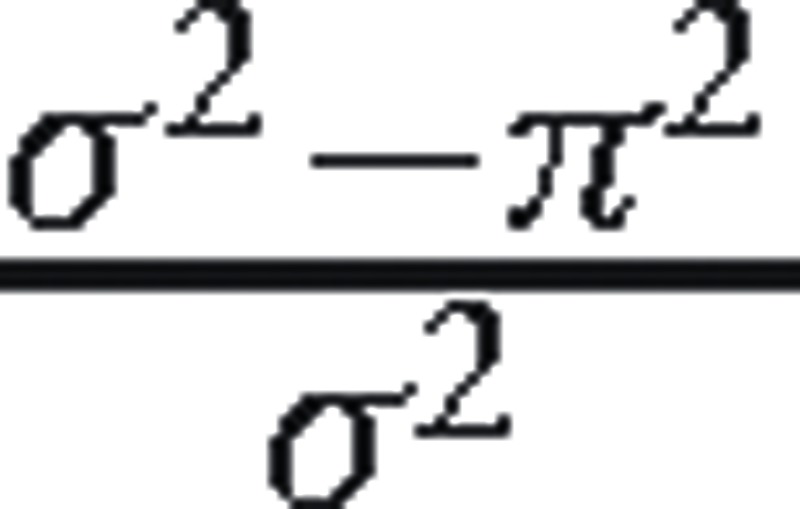 . It measures how well the set of variables predict (explain) the eGFR at dialysis initiation. We varied the order that sets of variables were entered into the models to evaluate how sensitive this approach was to ordering.
. It measures how well the set of variables predict (explain) the eGFR at dialysis initiation. We varied the order that sets of variables were entered into the models to evaluate how sensitive this approach was to ordering.
Sensitivity analyses included: a subset analysis of the 2005 to 2012 USRDS data using the updated CMS Form 2728; LMMs using the dialysis facility (rather than physician) as the random intercept; and a comparison of analyses with and without multiple imputation (based on the 2006–2009 data) to determine if the results were sensitive to missing data. The University of Michigan Institutional Review Board approved this study (HUM0086162); the USRDS database is freely available to the public. The details for data requests can be found at https://www.usrds.org/faq.aspx.
3. Results
3.1. Study population
The reported characteristics of incident dialysis patients and facilities changed over time (Table 1). Compared to 1995 to 1997, patients in 2010 to 2012 were more likely to have body mass index in [23, 64] kg/m2 (80% vs 63%), diabetes (61% vs 54%), or hypertension (90% vs 77%). Patients in 2010 to 2012 were more likely to be treated in facilities that were for-profit (82% vs 64%) and free-standing (91% vs 71%).
3.2. eGFR time trends, unadjusted
Figure 2 shows that mean eGFR (standard deviation) increased from 7.7 mL/min/1.73 m2 (3.5) in 1995 to a peak of 11.0 (4.5) in 2009, before leveling off for the final 3 years, to 10.8 (4.5) in 2012. Over the entire study period, mean eGFR at initiation increased by 3.3 mL/min/1.73 m2 and the standard deviation increased by 1 mL/min/1.73 m2. The 95% confidence interval of eGFR increased from 2.0, 14.6 to 2.2, 19.8. Although this temporal trend is plotted based on our study sample, a similar trend was observed based on all adult USRDS patients with eGFR information (n = 1,293,904; see Figure, Supplemental Content showing temporal trends at dialysis initiation by year).
Figure 2.
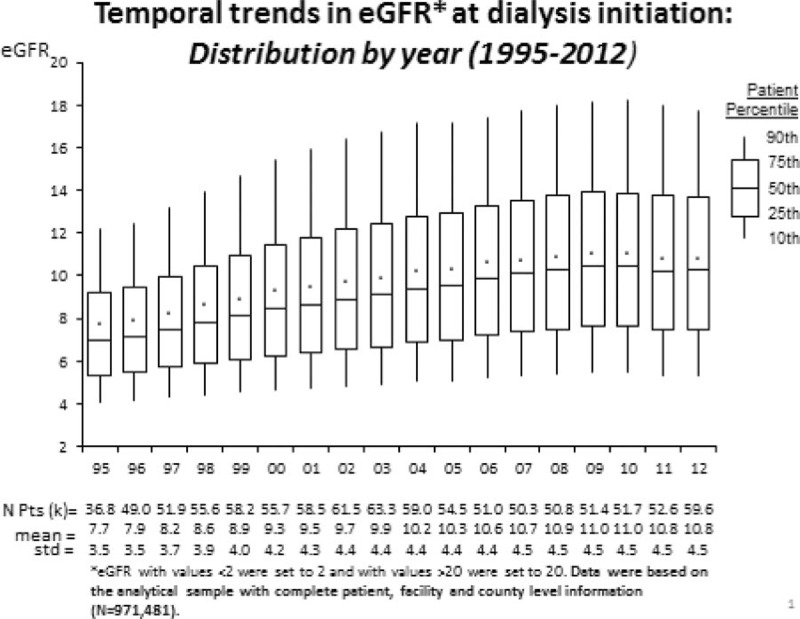
Temporal trends in estimated glomerular filtration rate (eGFR) at dialysis initiation: distribution by year.
3.3. Variation explained in eGFR at dialysis initiation
Figure 3 shows that the partition of variation in eGFR at dialysis initiation in 1995 to 2012 into between- and within-physician components, without and with adjusting for measured covariates. Based on the intercept-only model, the total variance (σ2) of eGFR at initiation was estimated at 20.3 (mL/min/1.73 m2)2, consisting of 11.4% between-physician variance and 88.6% within-physician variance. The intracluster correlation was 0.114. Of the total variation, measured patient-level characteristics explained 10.7% and measured facility/county-level characteristics 1.2%, while 9.2% of between-physician and 78.9% of within-physician variations remained unexplained. Similar proportions were obtained when we varied the order of the variables that entered the models. In sensitivity analyses restricted to 2005 to 2012 (ie, with additional variables on revised Form 2728), measured patient-level characteristics explained 10.6% and measured facility/county-level characteristics 0.8%, while 6.9% of between-physician and 81.7% of within-physician variations remained unexplained.
Figure 3.
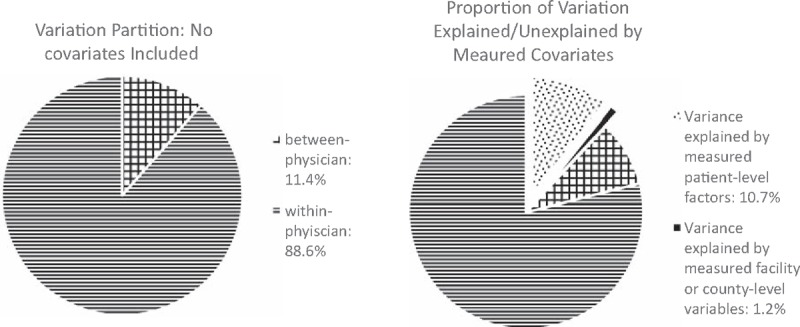
Physcian- and patient-level variation without and with adjusting for measured covariates between 1995 and 2012. Measured covariates include patient-level factors (age, race, sex, insurance, employment status, diabetes, heart disease, chronic obstructive pulmonary disease, cancer, patients’ ability to ambulate or transfer, erythropoietin use prior to dialysis, dialysis modality, creatinine prior to dialysis initiation, albumin/ hemoglobin levels prior to the first dialysis treatment, body mass index) and facility/countylevel factors (total number of patients, nurse-to-patient ratio, profit versus non-profit status, freestanding versus hospital-based facilities, and facility ESRD network membership, unemployment rate, high school graduation rate, and percentage of households below the poverty level, facility rural status).
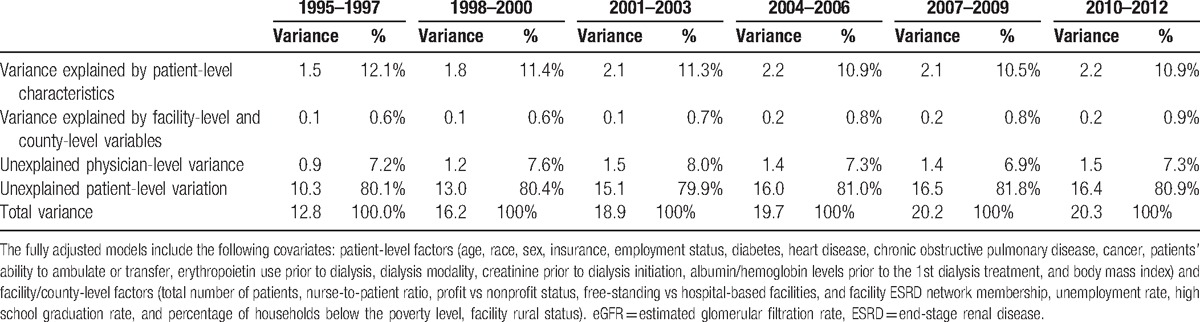
3.4. Temporal trends in variation explained in eGFR at dialysis initiation
Table 2 lists the proportion of variation explained in 3-year increments from 1995 to 2012. The proportions of variation explained were similar across time intervals. Between 2010 and 2012, measured patient-level characteristics explained 10.9% of total variation, and facility/county-level characteristics explained 0.9% of total variation, while 7.3% of between-physician and 80.9% of within-physician variations remained unexplained.
Table 2.
Variation at the patient and physician levels for eGFR at dialysis initiation for every 3 years between 1995 and 2012.
3.5. Trends by patient variables, treatment factors, facility-, and county-level variables
Figure 4 illustrates that the temporal trends in eGFR at dialysis initiation were similar across all subgroups defined by patient demographic and clinical variables (panels A and B), and by dialysis treatment factors, facility-, and county-level variables (panels C and D). Panels A and B show that higher eGFR at dialysis initiation was seen among patients who were older (eg, [65, 100] years old), male, or black. Among younger patients ([18, 65] years old), higher eGFR was seen among patients who were unemployed or insured. In sensitivity analyses of 2005 to 2012 data, institutionalized patients had higher eGFR than noninstitutionalized patients (data not shown). Higher eGFR at dialysis initiation was also seen in patients with lower serum albumin levels ([0.5, 3] g/dL), inability to transfer or ambulate, and with major comorbid conditions such as diabetes, congestive heart failure, atherosclerotic heart disease, cerebrovascular disease, peripheral arterial disease or amputation, and chronic obstructive pulmonary disease (all P-values <.001).
Figure 4.
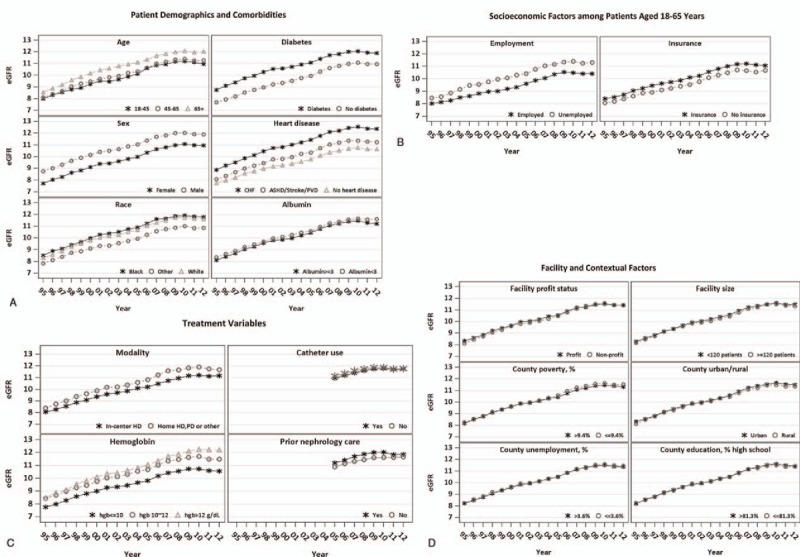
Temporal trend of estimated glomerular filtration rate (eGFR) at dialysis initiation between 1995 and 2012 in subgroups. ∗The adjusted mean eGFR over time was obtained based on linear mixed models (LMMs) with the following variables: patient-level factors (age, race, sex, insurance, employment status, diabetes, heart disease, chronic obstructive pulmonary disease, cancer, patients’ ability to ambulate or transfer, erythropoietin use prior to dialysis, dialysis modality, creatinine prior to dialysis initiation, albumin/hemoglobin levels prior to the first dialysis treatment, body mass index) and facility/county-level factors (total number of patients, nurse-to-patient ratio, profit versus non-profit status, free-standing versus hospital-based facilities, and facility ESRD network membership, unemployment rate, high school graduation rate, and percentage of households below the poverty level, facility rural status), calendar year, respective covariates and calendar year interactions. For catheter use and prior nephrology care in Panel C, the adjusted mean eGFR over time was obtained based on LMMs including the above list of variables and three additional variables: prior nephrology care, vascular access and being institutionalized. ∗∗The LMMs show that the average eGFR at dialysis initiation was significantly different across all subgroups (P < .001). The rate of increase in eGFR at dialysis initiation over time (i.e., the slope) was significantly different across subgroups defined either by age, race, BMI, insurance status, employment status, hypotension, diabetes status, heart disease, alcohol or drug dependence, albumin levels or the status of in-center hemodialysis (P < .01).
Furthermore, Fig. 4 panels C and D show that patients who received home hemodialysis or peritoneal dialysis had higher eGFR at dialysis initiation than in-center hemodialysis patients. In a sensitivity analysis restricted to 2005 to 2012, hemodialysis patients initiating dialysis via a surgical AV access had slightly higher eGFR at dialysis initiation than those using a central venous catheter. Patients receiving prior nephrology care started dialysis at higher eGFR than those without. The temporal trends and the mean eGFR at initiation were very similar across all subgroups defined by facility- and county-level variables.
3.6. Additional sensitivity analyses
When we repeated the analyses with dialysis facilities as the clustering units rather than physicians, the results of all analyses remained similar. Adding the multiple imputation method for missing data for the period 2006 to 2009 yielded very similar results.
4. Discussion
This analysis confirms that mean eGFR at dialysis initiation in the US leveled off during 2009 to 2012, after rising steeply for 2 decades. We demonstrate that this temporal pattern was similar across all subgroups studied, after accounting for temporal changes in measured variables. Examining between-physician (ie, beyond patient-level) and within-physician (patient-level) variation in eGFR at dialysis initiation, we found the total variation and the range of eGFR at dialysis initiation were substantial, with 88.6% of the total variation occurring at the patient level and 11.4% beyond patient levels. After adjusting for the long list of variables recorded in the USRDS ESRD database, it is striking that approximately 88% of total variation in eGFR remains unexplained. This finding has been consistent over the study period. These results suggest that the variables collected in the USRDS ESRD registry database are not sufficient to explain either variation in eGFR at dialysis initiation or its temporal trends, and indicate the need to collect and utilize more focused data to gain better understanding of factors that drive decisions about when to initiate dialysis in the US.
Our analyses provide more comprehensive analyses on the temporal trends in eGFR at dialysis initiation in the US from 1995 to 2012 than previously published. Patients who are older, insured, unemployed (for age <65 years), and with certain major comorbid conditions initiated dialysis at higher eGFR. Patients with surgical arteriovenous vascular access at dialysis initiation and with predialysis nephrology care also initiated dialysis at higher eGFR. The temporal pattern of a monotonic rise before leveling off after 2008 was generally similar across subgroups, indicating that the trend was mostly independent of temporal changes in variables recorded in the USRDS ESRD registry. Although in most related prior studies the trend of eGFR at initiation and/or its association with outcomes before 2009 was examined, Rosansky and Clark[13] focused on descriptive trends of eGFR across age groups between 1996 and 2011. Based on data from the Veterans Affairs health care system, Yu et al[17] found that temporal trends in eGFR at initiation within the Department of Veterans Affairs mirrored those in the US dialysis population between 2000 and 2009; O’Hare et al[18] found that eGFR at initiation increased but the frequencies of clinical signs or symptoms remained similar between 2000 to 2004 and 2005 to 2009.
Our analyses demonstrate that unmeasured factors drove the trends of eGFR at dialysis initiation. Many reasons have been suggested for substantial increases in eGFR at dialysis initiation from the 1990s to 2009.[4,19] Our analyses started in 1995, only 2 years before the 1997 Kidney Disease Outcomes Quality Initiative practice recommendations for dialysis initiation were issued, which recommended beginning dialysis when GFR fell below 10.5 mL/min/1.73 m2, or below 15 mL/min/1.73 m2 in some diabetic patients.[20] In response to these recommendations and published findings from others,[21] it is quite likely that some nephrologists changed practice and based the decision to start dialysis on these relatively high eGFR cut points. Over subsequent years, numerous studies have found no evidence that the trend of rising eGFR at dialysis initiation would, on average, benefit patients.[3,13,22] Indeed, growing concerns that dialysis may have been initiated unnecessarily early for some patients may have contributed to the leveling off in average eGFR at dialysis initiation since 2008.
To our knowledge, this is the first study to examine the variation and its temporal trends in eGFR at dialysis initiation in the US between 1995 and 2012. Our analyses show that the variation of eGFR at dialysis initiation is substantial, and that USRDS data only explain a small fraction of the variation. These findings have been consistent over time between 1995 and 2012. This indicates that even when all measured factors are identical, eGFR at dialysis initiation is still substantially variable and can differ markedly for different patients. Factors not captured by the USRDS database explained most of the variation. Clinical intuition readily generates a list of such factors, such as eGFR trajectory over time, worsening nutrition or frailty, hyperkalemia, metabolic acidosis, volume overload, serum potassium, serum phosphorous, bicarbonate, or blood urea nitrogen, overt or “soft” uremic signs or symptoms, treatment information such as diuretic use, other precipitating medical events, patient/provider preference, or perceived benefits and burden of dialysis.
eGFR has been used as the principle indicator of “timing” of dialysis initiation in prior studies. Many patient factors and provider and care characteristics have been linked to eGFR at initiation.[5,23,24] Although these prior studies focused on identifying statistically significant determinants of eGFR at initiation, our study clearly shows that, despite statistical significance, these factors only explain a very small fraction of the variation in eGFR and do not predict well eGFR at dialysis initiation. The remaining substantial variation in eGFR after adjustment implies many unmeasured factors played substantial roles in decisions about the timing of dialysis initiation. eGFR level is one of many considerations about when to initiate dialysis, and alone it may tell little about the true timing of dialysis initiation.
Another noteworthy finding is that the majority of the variation in eGFR occurred at the patient level, rather than beyond the patient level. This indicates that the mean eGFR at initiation is relatively similar across different physicians, but the eGFR of patients treated by the same physician tend to vary substantially. This suggests that despite possible preferences of physicians to start at relatively lower or higher eGFR, the decision to initiate dialysis is largely determined by patient-related factors not recorded in USRDS data. Based on Canadian data, Sood et al[28] also found the variation in mean eGFR across facilities and regions was small (note that Sood et al used “explained variation” to denote “total variation.”[25] Therefore, special efforts are needed to collect patient-level factors among chronic kidney disease patients approaching the need for dialysis, in order to gain real understanding about determinants of timing of dialysis initiation.
Nonetheless, the observed between-physician variation in eGFR at dialysis initiation highlights the influence of physician preferences. In fact, 9.2% of residual between-provider variation is not trivial, given that the long list of measured patient-level factors accounted for only 10.7% of the total variation in eGFR at dialysis initiation. Residual between-provider variation implies discretionary variation, that is, treatment decisions that were influenced by nonpatient-centered factors (such as physician preference and facility size). In turn, this suggests that individualization of care, that is, initiating dialysis based on patients’ symptoms and wishes, was compromised to some degree.
Our findings can be generalizable to the incident dialysis patient population in the US because we used the USRDS ESRD database, which captures more than 99% of the incident ESRD patients nationally.[26] However, our study is subject to a few limitations.
-
(1)
eGFR may not accurately estimate true GFR (kidney function) especially in patients who are frail, old, undernourished, or with very low kidney function.[4,27–29]
-
(2)
Laboratory measurement and reporting of serum creatinine changed slightly after 2006; however, it is reassuring that we did not see a huge shift in observed eGFR over this time period.[30]
-
(3)
We have 2.5% of patients with acute kidney injury; however, analysis results remain similar after exclusion of these patients.
-
(4)
The physician identified as the attending physician on CMS Form 2728 may not have been the physician overseeing the decision to initiate dialysis. This concern is mostly assuaged by consistent findings when analyzed at the dialysis facility level, rather than physician level.
-
(5)
Reporting of comorbid conditions on CMS Form 2728 may not be uniformly consistent.
-
(6)
The exclusion of patients due to incomplete information is not trivial, and missing data methods on the 18-year dataset were not computationally feasible. However, we found that the distributions of eGFR over time with and without exclusions were similar. Additionally, the results were consistent with and without the multiple imputations in an analysis of 4-year data (2006–2009).
-
(7)
In the USRDS database, there may be under-reporting of high-risk incident patients in the initial weeks of dialysis due to very early death.[31]
In conclusion, we found the temporal trends and the majority of variation in eGFR at dialysis initiation were unexplained by USRDS data. These findings suggest the need to collect data prospectively from advanced chronic kidney disease patients approaching the need for dialysis, rather than rely on data from patients starting dialysis therapy as with the USRDS dataset, to gain better understanding of factors that drive decisions about when to initiate dialysis in the US. Given the potentially dramatic implications for patients’ quality of life, morbidity, mortality, and costs, these efforts should be a priority.
Supplementary Material
Footnotes
Abbreviations: CMS = Centers for Medicare & Medicaid Services, eGFR = estimated glomerular filtration rate, ESRD = end-stage renal disease, LMM = linear mixed model, USRDS = United States Renal Data System.
Funding/support: This project has been funded in whole or in part with Federal funds from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN276201400001C.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].U.S. Renal Data System, USRDS 2015 Annual Data Report: Atlas of chronic kidney disease and end-stage renal disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2015. [Google Scholar]
- [2].U.S. Renal Data System, USRDS 2014 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2013. [Google Scholar]
- [3].Rosansky Glassock RJ, Clark WF. Early start of dialysis: a critical review. Clin J Am Soc Nephrol 2011;6:1222–8. [DOI] [PubMed] [Google Scholar]
- [4].Johansen KL, Delgado C, Bao Y, et al. Frailty and dialysis initiation. Semin Dial 2013;26:690–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Slinin Y, Guo H, Li S, et al. Provider and care characteristics associated with timing of dialysis initiation. Clin J Am Soc Nephrol 2014;9:310–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Streja E, Nicholas SB, Norris KC. Controversies in timing of dialysis initiation and the role of race and demographics. Semin Dial 2013;26:658–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cohen SD, Kimmel PL. Quality of life and mental health related to timing, frequency and dose of hemodialysis. Semin Dial 2013;26:697–701. [DOI] [PubMed] [Google Scholar]
- [8].Traynor JP, Simpson K, Geddes CC, et al. Early initiation of dialysis fails to prolong survival in patients with end-stage renal failure. J Am Soc Nephrol 2002;13:2125–32. [DOI] [PubMed] [Google Scholar]
- [9].Beddhu S, Samore MH, Roberts MS, et al. Impact of timing of initiation of dialysis on mortality. J Am Soc Nephrol 2003;14:2305–12. [DOI] [PubMed] [Google Scholar]
- [10].Wright S, Klausner D, Baird B, et al. Timing of dialysis initiation and survival in ESRD. Clin J Am Soc Nephrol 2010;5:1828–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rosansky SJ, Eggers P, Jackson K, et al. Early start of hemodialysis may be harmful. Arch Intern Med 2011;171:396–403. [DOI] [PubMed] [Google Scholar]
- [12].Scialla JJ, Liu J, Crews DC, et al. An instrumental variable approach finds no associated harm or benefit with early dialysis initiation in the United States. Kidney Int 2014;86:798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rosansky SJ, Clark WF. Has the yearly increase in the renal replacement therapy population ended? J Am Soc Nephrol 2013;24:1367–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].US Renal Data System: USRDS 2009 Annual Data Report, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2009. [Google Scholar]
- [15].US Renal Data System: USRDS 2004 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2004. [Google Scholar]
- [16].US Renal Data System: USRDS 2004 Annual Data Report. The National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2004. [Google Scholar]
- [17].Yu MK, O’Hare AM, Batten A, et al. Trends in timing of dialysis initiation within versus outside the department of veterans affairs. Clin J Am Soc Nephrol 2015;10:1418–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].O’Hare AM, Wong SP, Yu MK, et al. Trends in the timing and clinical context of maintenance dialysis initiation. J Am Soc Nephrol 2015;26:1975–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Rosansky SJ, Clark WF, Eggers P, et al. Initiation of dialysis at higher GFRs: Is the apparent rising tide of early dialysis harmful or helpful? Kidney Int 2009;76:257–61. [DOI] [PubMed] [Google Scholar]
- [20].NKF-DOQI clinical practice guidelines for peritoneal dialysis adequacy. National Kidney Foundation. Am J Kidney Dis 1997;30(3 Suppl 2):S67–136. [DOI] [PubMed] [Google Scholar]
- [21].Mehrotra R, Saran R, Moore HL, et al. Toward targets for initiation of chronic dialysis. Perit Dial Int 1997;17:497–508. [PubMed] [Google Scholar]
- [22].Cooper BA, Branley P, Bulfone L, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med 2010;363:609–19. [DOI] [PubMed] [Google Scholar]
- [23].O’Hare AM, Choi AI, Boscardin WJ, et al. Trends in timing of initiation of chronic dialysis in the United States. Arch Intern Med 2011;171:1663–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Crews DC, Scialla JJ, Liu J, et al. Predialysis health, dialysis timing, and outcomes among older United States adults. J Am Soc Nephrol 2014;25:370–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sood, Manns B, Dart A, et al. Variation in the level of eGFR at dialysis initiation across dialysis facilities and geographic regions. Clin J Am Soc Nephrol 2014;9:1747–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kurella M, Covinsky KE, Collins AJ, et al. Octogenarians and nonagenarians starting dialysis in the United States. Ann Intern Med 2007;146:177–83. [DOI] [PubMed] [Google Scholar]
- [27].Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–56. [DOI] [PubMed] [Google Scholar]
- [28].Grootendorst DC, Michels WM, Richardson JD, et al. The MDRD formula does not reflect GFR in ESRD patients. Nephrol Dial Transplant 2011;26:1932–7. [DOI] [PubMed] [Google Scholar]
- [29].Weiner DE, Stevens LA. Timing hemodialysis initiation: a call for clinical judgment. Am J Kidney Dis 2011;57:562–5. [DOI] [PubMed] [Google Scholar]
- [30].Levey AS, Coresh J, Greene T, et al. Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 2007;53:766–72. [DOI] [PubMed] [Google Scholar]
- [31].Foley RN, Chen S-C, Solid CA, et al. Early mortality in patients starting dialysis appears to go unregistered. Kidney Int 2014;86:392–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


