Abstract
We investigate the surgical outcomes of patients undergoing hepatectomy according to different age intervals, identify the clinical factors related to surgical outcomes, and propose clinical risk scores for severe morbidity and mortality based on the clinical factors.
Eight hundred three patients undergoing liver resection were divided into 3 groups: young patients (YP), <65 years (n = 387), elderly patients (EP), from 65 to 74 years (n = 279); very-elderly patients (VEP), ≥75 years (n = 137).
Severe morbidity was 10.6%, 12.2%, and 17.5% (P = .103), and mortality was 0.3%, 1.4%, and 4.4% (P = .002) in group YP, EP, and VEP, respectively. Ischemic heart disease, cirrhosis, major hepatectomy, biliary tract-associated procedure, and red blood cells (RBC) transfusion ≥3 U were related with severe morbidity. Ischemic heart disease, cirrhosis, major hepatectomy, and RBC transfusion were independent risk factors for postoperative mortality. Age did not result an independent factor related to mortality and severe morbidity. Two different scores were developed and have proved to be statistically related with severe morbidity and mortality. Moreover, in patients with score ≥2, severe morbidity increased from 24.2% in YP, to 29.3% in EP, and to 40.0% in VEP, P = .047. Likewise, mortality increased from 2.3% in YP, to 7.0% in EP, and to 22.7% in VEP, in patients with score ≥2, P = .017.
Age alone should not be considered a contraindication for hepatectomy. We identified factors and proposed 2 scores that can be useful to stratify the risk of morbidity and mortality after hepatectomy. Moreover, severe morbidity and mortality increases according to the different age intervals in patients with scores ≥2.
Keywords: elderly, hepatectomy, increased age, liver resection, liver surgery, postoperative mortality, surgical outcomes
1. Introduction
The increasing mean age of the population and prolonged life expectancy have resulted in a widening of indications for liver surgery. Elderly patients (EP), in particular, are expected to have a higher risk of postoperative complications.[1,2] However, recent improvements in the perioperative management of liver surgery have increased its safety.[3] Several studies investigated the outcomes of liver resection in EP. Apparently, minor liver resections can be safely performed. In EP undergoing major hepatectomy, future remnant liver volume and liver function remain the most important factors regarding short-term outcomes.[2,4]
Across the literature, there are different age cut-off values for the definition of elderly, ranging from 65 to 80 years. However, in most studies, the cut-off age is 70 years.[5–12] A significant reduction in liver mass and portal blood flow were demonstrated in patients aged 70 years or older,[13] but liver regeneration does not seem to be significantly affected by age.[14]
In recent years, eastern clinical studies of liver surgery in patients older than 75 years have increased significantly, and the short-term results reported between elderly and younger patients were similar.[11,15,16] In contrast, a western study reported an increase in the complication rate in elderly population.[17]
Several clinical risk scores, including age and other perioperative factors, have been proposed as predictors of the outcome of patients undergoing surgery. Despite clinical studies demonstrated good predictive value of these scores, their use in clinical practice is limited. The high number of variables taken into account and the complexity are the major limitation for their application.[18–20] Therefore, the aims of this study were: to compare the clinical characteristics and surgical outcomes of patients who underwent liver resection according to different age intervals; to identify the clinical factors related with increased risk of postoperative morbidity and mortality; to propose a clinical score useful in the stratification of postoperative risk of morbidity and mortality based on the clinical factors identified and to apply it in different age intervals.
2. Patients and methods
2.1. Study population and data collection
All consecutive patients who underwent liver resection with curative intent in a European high-volume tertiary referral hepatobiliary (HB) centre between January 2006 and January 2015 were included in the study. Data were retrieved from a prospectively collected database, and this analysis was performed retrospectively. The ethical standards of the World Medical Association (Declaration of Helsinki) were followed during the conduction of this study.
The study population was divided into 3 groups: young patients (YP), patients aged <65 years; EP, patients aged between 65 and 74 years; and very-elderly patients (VEP), patients aged 75 years or older.
Our study population included both patients with malignancies, such as colorectal liver metastases (CRLM), hepatocellular carcinoma (HCC), cholangiocarcinoma (CCA), noncolorectal liver metastases (NCRLM), and gallbladder cancer (GBC), and those with benign liver disease or other rare liver tumors.
The indication for surgery was made after a multidisciplinary team discussion, including experienced HB surgeons, anesthesiologists, gastroenterologists, hepatologists, oncologists, and radiologists. Age was not considered per se as a selection criterion for the type of treatment. Preoperative staging imaging techniques included computed tomography (CT), magnetic resonance imaging (MRI), ultrasonography (US), and contrast-enhanced ultrasonography (CE-US) as appropriate. Preoperative patient investigations included electrocardiogram, chest radiography, and blood samples for all patients (hemoglobin, platelets, bilirubin, aspartate aminotransferase, alanine aminotransferase, gamma glutamyl transferase, sodium, creatinine, albumin, and prothrombin time-international normalized ratio). In selected cases, experienced cardiologists performed a complete clinical and instrumental cardiac examination. Indocyanine green (ICG) test was performed in selected patients with liver disease and those who were candidates for major procedures.
The collected clinical data included age, sex, body mass index (BMI), HCV or HBV infection, alcohol abuse (consumption ≥40 g/day), liver cirrhosis, and presence of comorbidities (hypertension, ischemic heart disease, type II diabetes, chronic obstructive pulmonary disease, asthma, and chronic renal failure). The American Society of Anaesthesiologists (ASA) score was applied to evaluate the surgical risk for each patient. Charlson comorbidity index (CCI) was calculated based on comorbidity and diagnosis; all patients with liver resectable malignancies, primary or secondary, were awarded 2 points. Patients with diffuse metastatic disease were awarded 6 points.[21]
During surgery, intraoperative ultrasound was routinely performed to confirm preoperative diagnosis and to evaluate the relationship between lesions, blood vessels, and biliary ducts. Intraoperative vascular control of in-flow and out-flow was achieved either by a selective clamping or by Pringle's maneuver. The transection of liver parenchyma was performed with ultrasonic aspirator and selective ligation of all identifiable vessels.
The extent of liver resection was classified according to the Brisbane 2000 terminology.[22] In case of malignancies, curative liver resection was defined as the removal of all the recognizable tumors. Complete tumor removal was confirmed by histopathology. Associated procedures were defined as the resection of at least one further organ and were classified according to the organ resected as colon, rectum, common bile duct, major vessels (portal vein, cava vein, and hepatic artery), pancreas, or other organs.
Postoperative complications were assessed according to the Clavien-Dindo classification,[23] and divided into liver-related and nonliver-related (pulmonary and cardiovascular) complications.
Additionally, the following specific liver complications were recorded: posthepatectomy liver failure (PHLF), biliary fistula, ascites, abdominal collections, sepsis, and acute renal failure. A PHLF was defined according to the “fifty-fifty” criteria or postoperative peak of serum bilirubin concentration >7 mg/dL.[24,25] Thirty-day mortality was defined as death within 30 days after surgery; 90-day/in-hospital mortality was defined as death within 90 days after surgery or during the hospital stay and was considered for the univariate and multivariate analysis.
2.2. Statistical analysis
The study data were prospectively collected and analyzed using SPSS statistical software (version 20.0; SPSS, Inc., Chicago, IL). The Fisher exact or Pearson square tests were used for categorical variables and the Student t test or 1-way ANOVA was used for continuous variables where appropriate. The results were expressed as mean and standard deviation (SD) or median and range. Independent risk factors for severe morbidity and 90-day/in-hospital mortality resulted from a multivariate logistic regression model; the selection criteria for entering variables into multivariate analysis was a P-value <.05 at univariate analysis. To identify the clinical role of increasing in age, the age variable categorized into the 3 groups (YP, EP, VEP) entered into the multivariate analysis also with a P-value >.05. The P-value < .05 was regarded as statistically significant. Variables identified at the multivariate analysis for both severe morbidity and 90-day/in-hospital mortality were chosen to built a score for severe morbidity (Severe Morbidity Score, SMS) and for mortality (Mortality Score, MS), respectively. The scores were calculated assigning one point for the presence each predictive variable. Receiver operating characteristic (ROC) curves were generated and the area under the curves (AUC) calculated to test the predictive ability of SMS and MS, respectively.
3. Results
3.1. Demographics and preoperative characteristics
A total of 803 patients were included in the present study. Of these, 387 were younger than 65 years (YP, 48.2%), 279 were between 65 and 74 years (EP, 34.7%), and 137 were 75 years or older (VEP, 17.1%). Demographics and preoperative characteristics of the study population are summarized in Table 1.
Table 1.
Preoperative characteristics.
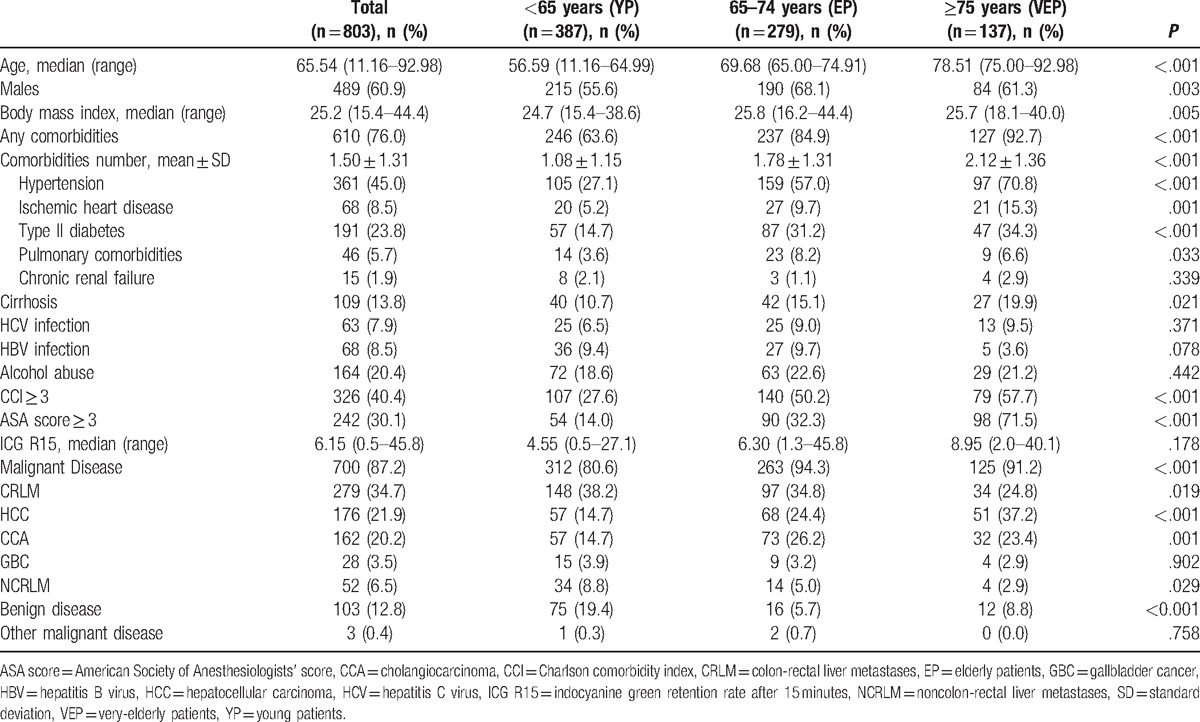
VEP had a higher rate of comorbidities compared with YP and EP (92.7%, 63.6%, and 84.9%, respectively), P < .001. In particular, ischemic heart disease was present in 15.3% of VEP, 9.7% in EP, and 5.2% in YP (P = .001). Type II diabetes was present in 34.3% of VEP, 31.2% in EP, and 14.7% in YP, P < .001. One hundred nine patients (13.8% of the total) had liver cirrhosis, and the frequency increased with age (P = .021). There were no differences between the 3 groups in terms of HBV or HCV infection and alcohol consumption.
The ASA score was significantly higher in VEP: 71.5% of patients had an ASA score ≥3 compared with 32.3% of EP and 14.0% in YP (P < .001). The CCI was higher in VEP: 57.7% of patients had CCI ≥3 compared with 50.2% in EP and 27.6% in YP (P < .001). Surgical outcomes are summarized in Table 2. There were no differences between the 3 groups in terms of extension of hepatectomy and type of associated procedures.
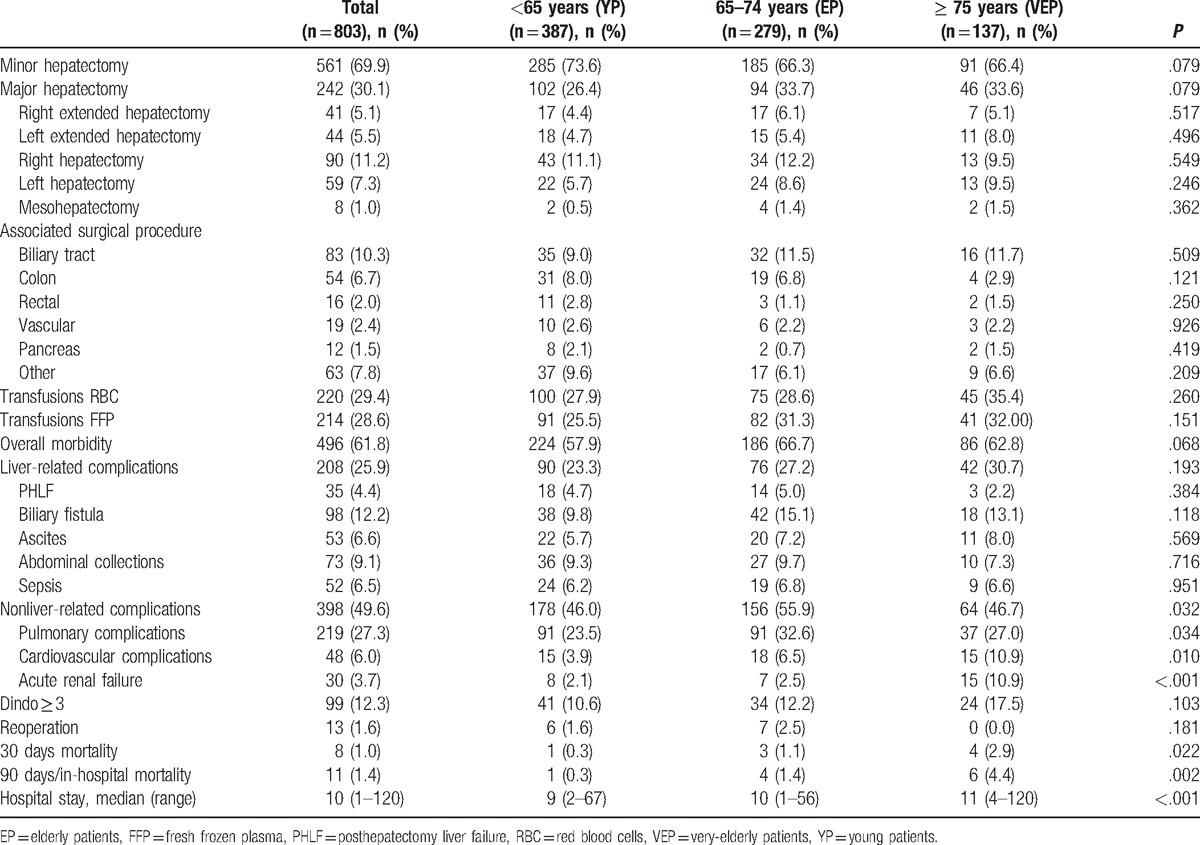
Table 2.
Surgical procedures and outcomes.
3.2. Morbidity and mortality
Overall morbidity, severe morbidity (Dindo score ≥ 3), 30-day mortality and 90-day/in-hospital mortality were 61.8%, 12.3%, 1.0%, and 1.4%, respectively (Table 2). Overall morbidity was 57.9% in YP, 66.7% in EP and 62.8% in VEP, and the difference among the groups was not statistically significant (P = .068).
Liver-related complications occurred in 25.9% of the patients without significant differences in the 3 groups. In contrast, the rate of nonliver-related complications was higher in EP (55.9%) compare with YP and VEP (46.0% and 46.7%, respectively, P = .032). Severe complications (Dindo ≥ 3) occurred in 10.6% of patients <65 years (YP), 12.2% of patients 65 to 74 years (EP), and 17.5% of patients ≥75 years (VEP) (P = .103). The 30-day mortality rate and 90-day/in-hospital mortality rate were 0.3% and 0.3% in YP, 1.1% and 1.4% in EP, and 2.9% and 4.4% in VEP (P = .022 and P = .002), respectively.
The cause of death of the patient who died in YP was acute coronary syndrome (ACS). A total of 4 of EP died, 2 deaths were related to ACS and 2 to PHLF. Six patients died in VEP, 2 for ACS, 3 for sepsis, and 1 for aspiration pneumonia.
3.3. Risk factors of severe morbidity and mortality
Results of the univariate and multivariate analyses of risk factors associated with severe morbidity are summarized in Table 3.
Table 3.
Univariate and multivariate analysis of risk factors associated with severe morbidity (Dindo ≥ 3).
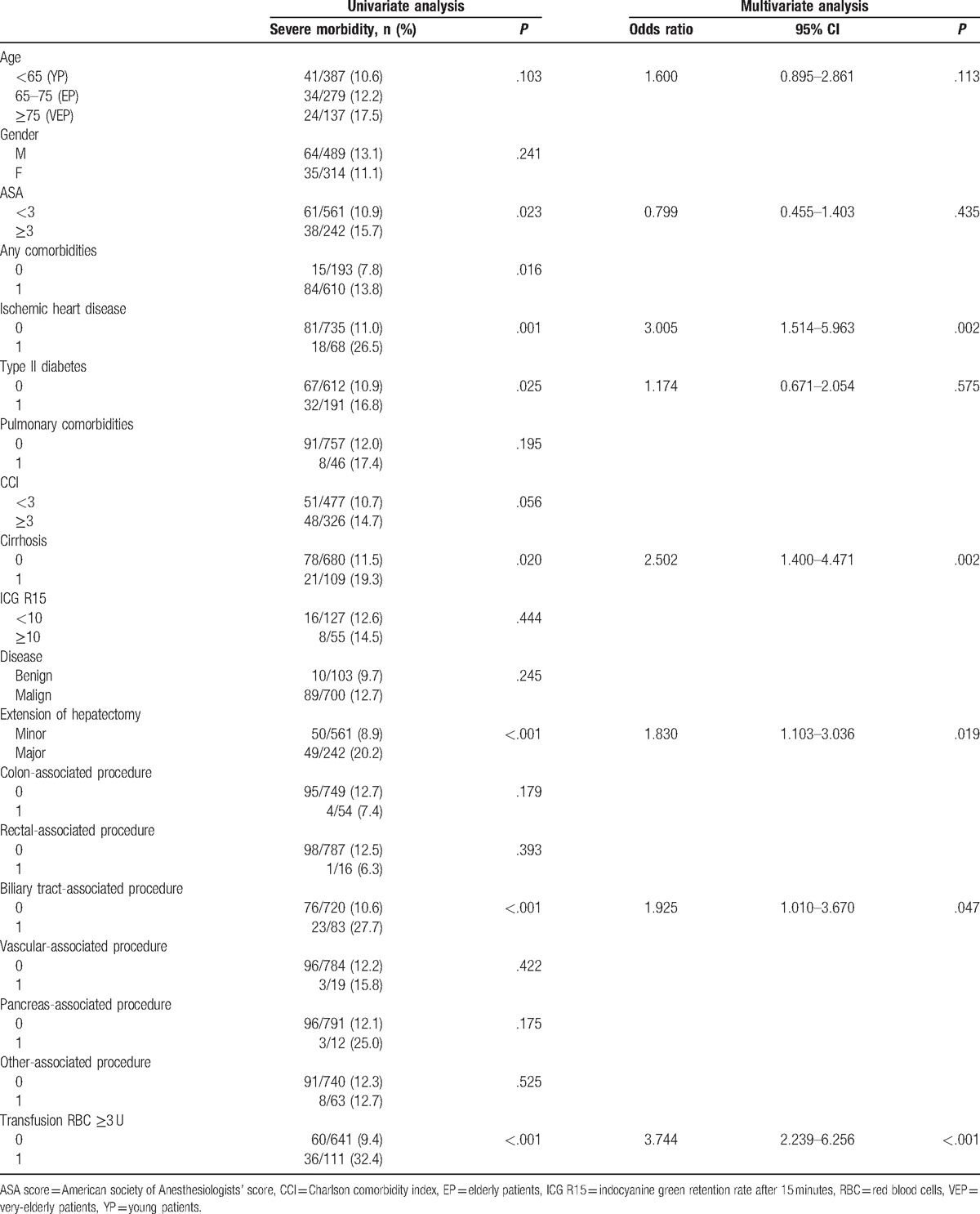
Presence of any comorbidities, ischemic heart disease, type II diabetes, cirrhosis, major hepatectomy, bile duct resection, and red blood cells (RBC) transfusion ≥3 U were factors related with a higher rate of severe morbidity in univariate analysis. In multivariate analysis, ischemic heart disease (OR 3.005, 95% CI: 1.514–5.963, P = .002), cirrhosis (OR 2.502, 95% CI: 1.400–4.471, P = .002), major hepatectomy (OR 1.830, 95% CI: 1.103–3.036, P = .019), biliary tract-associated procedure (OR 1.925, 95% CI: 1.010–3.670, P = .047), and RBC transfusion ≥3 U (OR 3.744, 95% CI: 2.239–6.256, P < .001) were confirmed to be independent factors related with severe morbidity. Age was not significantly related to severe morbidity in univariate or multivariate analysis (OR 1.600, 95% CI: 0.895–2.861, P = .113).
Univariate analysis and multivariate analysis of risk factors associated with 90-day/in-hospital mortality are summarized in Table 4.
Table 4.
Univariate and multivariate analysis of risk factors associated with 90-day/in-hospital mortality.
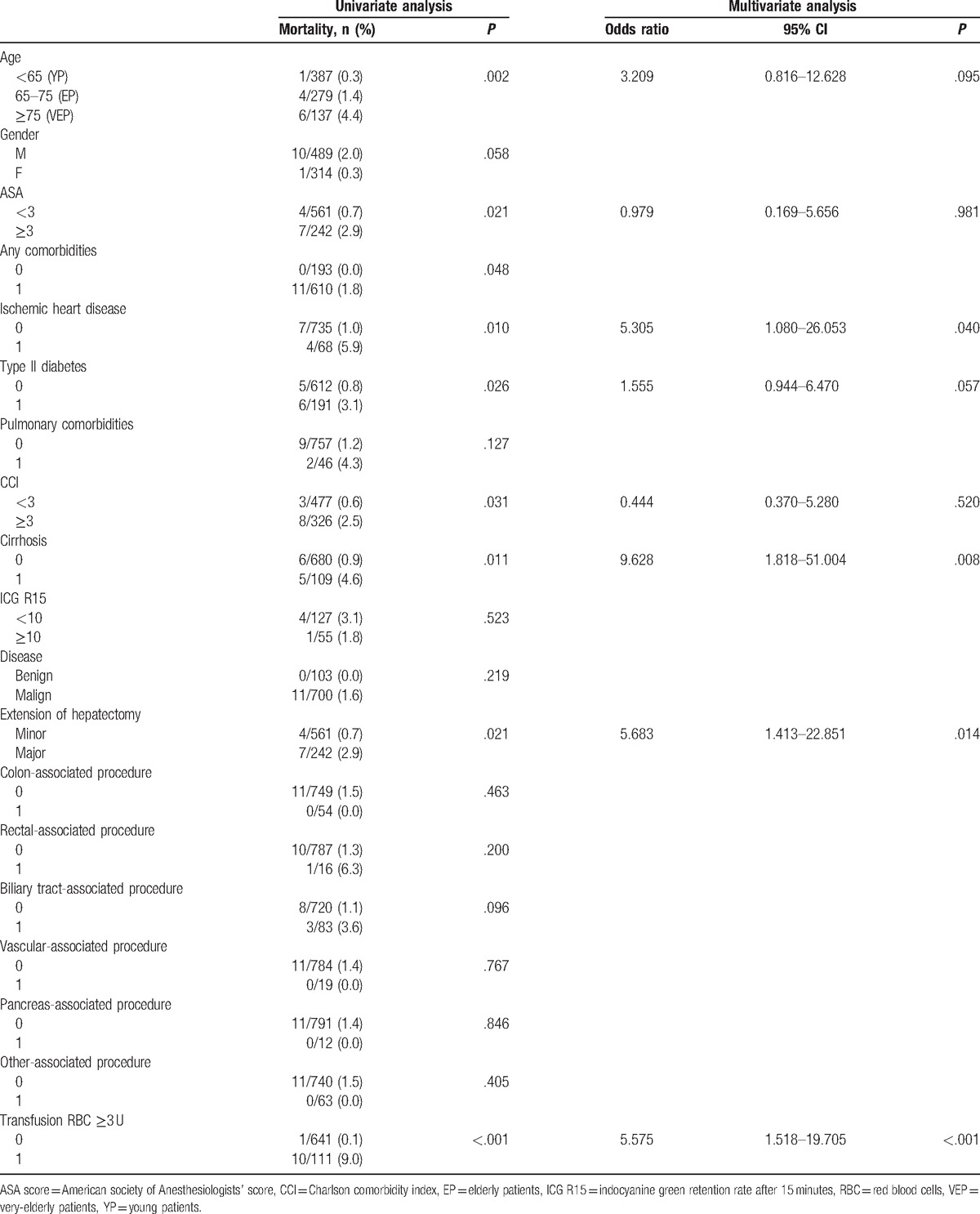
Age, presence of any comorbidities, ischemic heart disease, type II diabetes, ASA ≥ 3, CCI ≥ 3, cirrhosis, major hepatectomy, and RBC transfusion ≥ 3 U were factors associated with a higher rate of 90-day/in-hospital mortality in univariate analysis. In multivariate analysis, ischemic heart disease (OR 5.305, 95% CI: 1.080–26.053, P = .040), cirrhosis (OR 9.628, 95% CI: 1.818–51.004, P = .008), major hepatectomy (OR 5.683, 95% CI: 1.413–22.851, P = .014), and RBC transfusion ≥3 U (OR 5.575, 95% CI: 1.518–19.705, P < .001) were confirmed to be independent factors associated with increased risk of mortality. Age and type II diabetes were not significantly related to mortality in multivariate analysis (OR 3.209, CI: 0.816–12.628, P = .095, and OR 1.555, CI: 0.944–6.470, P = .057, respectively).
3.4. Clinical risk scores for severe morbidity and mortality
The variables identified at multivariate analysis for severe morbidity (ischemic heart disease, cirrhosis, major hepatectomy, biliary tract-associated procedure, and RBC transfusion ≥3 U) were chosen to build a score for severe morbidity (SMS). The SMS was calculated assigning 1 point for the presence of each predictive variable. Similarly, variable identified at multivariate analysis for 90-day/in-hospital mortality (ischemic heart disease, cirrhosis, major hepatectomy, and RBC transfusion ≥3 U) were chosen to build a score for mortality (MS). Also for MS 1 point was assigned for the presence of each predictive variable.
The severe morbidity rate increased according to the SMS, from 4.5% in patients with 0 points, to 14.0% in patients with 1 point, and to 29.3% in patients with ≥2 points, P < .001, ROC curve analysis showed an AUC of 0.724 (Figs. 1A and 2A). Also, the 90-day/in-hospital mortality rate increased according to MS, from 0.0% in patients with 0 points, to 0.7% in patients with 1 point, and to 8.3% in patients with ≥2 points, P < .001. ROC curve analysis showed an AUC of 0.915 (Figs. 2B and 3A).
Figure 1.
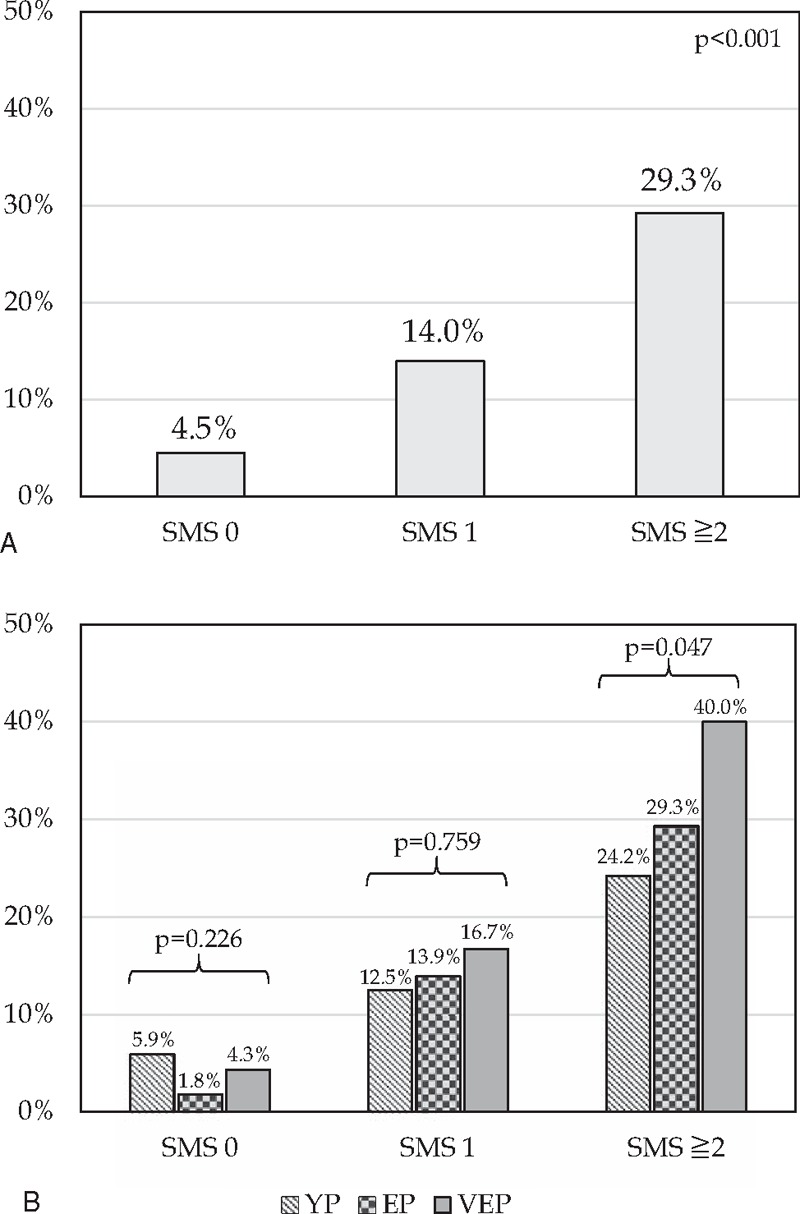
Frequency of severe morbidity (Dindo ≥ 3) rate according to the Severe Morbidity Score (SMS). For each of the following variables was assigned 1 point: ischemic heart disease, cirrhosis, major hepatectomy, biliary tract-associated procedure, and transfusion of RBC ≥ 3 U. (A) Univariate analysis of severe morbidity rate comparing patients with 0, 1, or ≥2 points; (B) univariate analysis of severe morbidity rates in patients with 0, 1, or ≥2 points according to the different age interval. YP, young patients, <65 years; EP, elderly patients, 65–74 years; VEP, very-elderly patients, ≥75 years.
Figure 2.
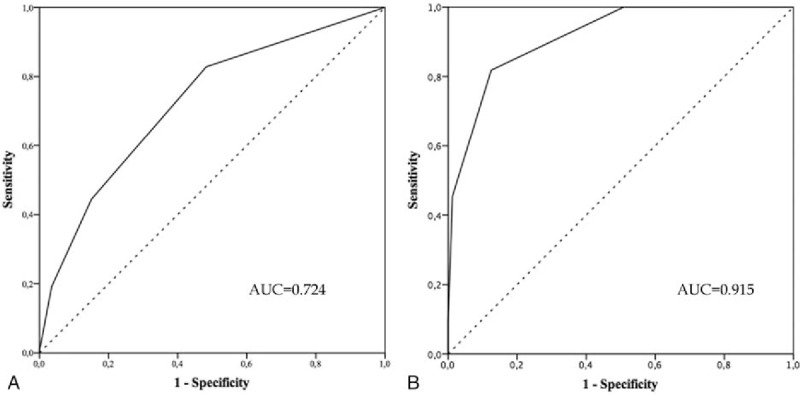
Receiver operating characteristic (ROC) curves of Severe Morbidity Score (SMS) (A) and Mortality Score (MS) (B). Curves represent the performances ability of SMS and MS to predict severe morbidity and 90-day/in-hospital mortality, respectively.
Moreover, we applied the 2 scores (SMS and MS) in the different age intervals (YP, EP, and VEP). We observed no statistically significant differences in severe morbidity or mortality rates in patients with a score of 0 or 1 point according to the 3 age intervals. Conversely, for patients with scores ≥2 the short-term outcome after liver resection were significantly worse in older patients. In particular, severe morbidity rate raised from 24.2% in YP, to 29.3% in EP, and to 40.0% in VEP, respectively, P = .047 (Fig. 1B). Furthermore, 90-day/in-hospital mortality rate increased from 2.3% in YP, to 7.0% in EP, and to 22.7% in VEP, respectively, P = .017 (Fig. 3B).
Figure 3.
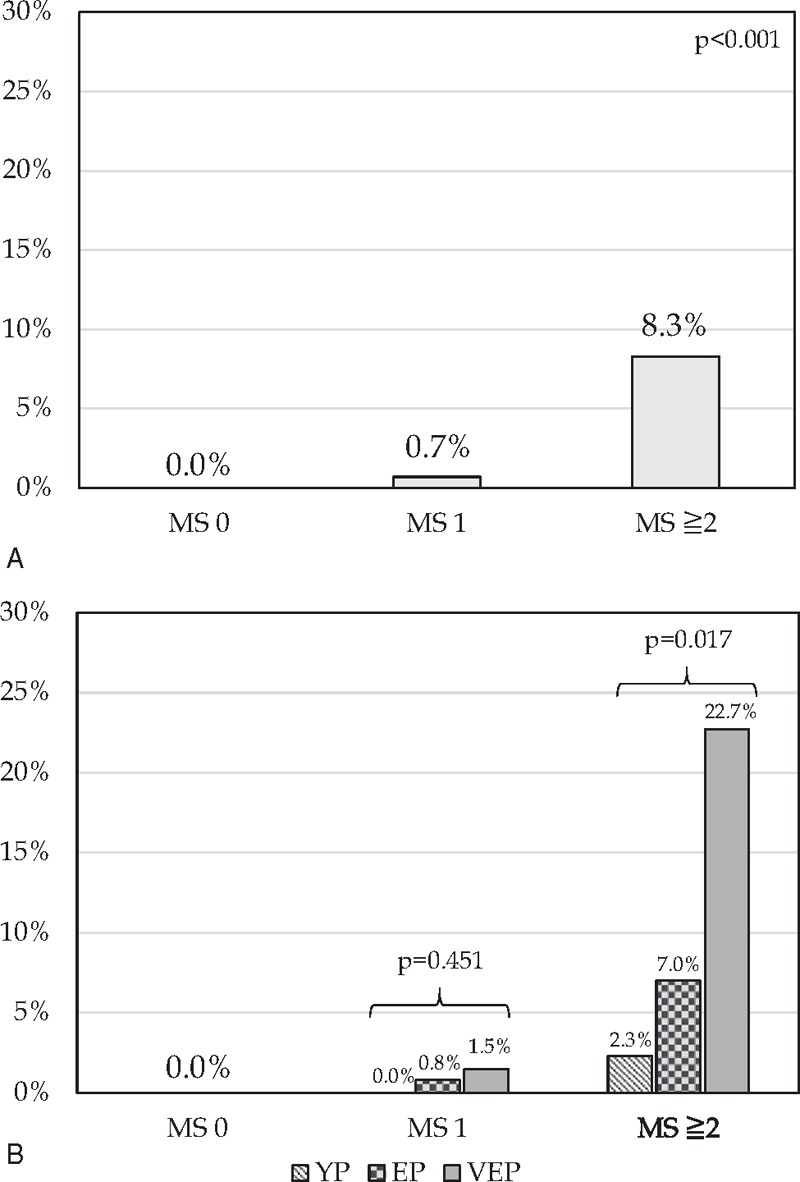
Frequency of 90-day/in-hospital mortality rate according to the Mortality Score (MS). For each of the following variables was assigned 1 point: ischemic heart disease, cirrhosis, major hepatectomy and transfusion of RBC ≥ 3 U. (A) Univariate analysis of 90-day/in-hospital mortality rate comparing patients with 0, 1, or ≥2 points; (B) univariate analysis of 90-day/in-hospital mortality rates in patients with 0, 1, or ≥2 points according to the different age interval. YP, young patients, <65 years; EP, elderly patients, 65–74 years; VEP, very-elderly patients, ≥75 years.
4. Discussion
In our study, major hepatectomy, bile duct resection, perioperative transfusion of 3 U or more of RBC, ischemic heart disease, and cirrhosis were the main factors related with severe morbidity and 90-day/in-hospital mortality. These results suggest that age is not a contraindication for liver resection. However, postoperative outcomes were worse among VEP (≥75 years) who underwent aggressive surgery, such as major hepatectomy, bile duct resection, those who required perioperative transfusion of 3 U or more of RBC, or with severe preexisting comorbidities, such as ischemic heart disease and cirrhosis. These findings have clinical relevance considering the growing number of EP and VEP with liver malignancies who require complex surgical procedures. The rate of EP included in surgical series is increasing steadily because of the prolonged mean age of the population and the improvements in the perioperative management.[1–3] However, in several surgical series, advanced age has been related to a higher risk of postoperative morbidity and mortality.[4,10]
In the existing literature, there is a lack of agreement regarding the definition of elderly making it difficult to compare the results of the different studies. Therefore, the identification of patients at higher risk can be useful to select surgical candidates more accurately. Additionally, preoperative treatments can be planned to decrease the risks associated with surgery.
Commonly, the number of comorbidities increases with age.
In our study, the incidence of ischemic heart disease, hypertension, type II diabetes, and pulmonary comorbidities was significantly higher in EP and VEP compared with YP. The number and severity of comorbidities increased with age; accordingly, ASA score and CCI were proportionally higher with age. Further, these findings are consistent with those of previous studies.[4,10] Moreover, cirrhosis was significantly more frequent in patients older than 75 years. Nevertheless, liver function assessed with common blood test and ICG R15 test was similar across the 3 groups.
Indications for surgery were significantly different between groups. Malignant diseases were a more common surgical indication in EP and VEP compared with YP (94.3%, 91.2%, and 80.6%, P < .001), respectively. There were no significant differences in the type of hepatectomy and associated surgical procedures among the 3 groups.
Experimental studies have demonstrated that aging is related to a reduction of liver volume, portal blood flow,[13,26] and an overall reduction of HB functions.[27] Moreover, liver regeneration following injury or partial resection seems to be decreased with age.[28,29]
These animal studies are the bases to explain the increased rates of postoperative complications among EP after major hepatectomy as reported in past clinical studies. In fact, in the 1990s the reported mortality rate after major hepatectomy was as high as 11.1%, with a cut-off value of 65 years.[30] A case–control study that compared liver regeneration after portal vein occlusion (PVO) in patients older or younger than 70 years demonstrated that liver regeneration is not significantly affected by age.[14] Recent clinical surgical series confirmed these data, showing similar short-term outcomes between elderly and younger patients after liver resection.[4,7,9] Reddy et al analyzed 856 patients who underwent major hepatectomy in 2 high-volume centers and concluded that each 1- and 10-year increase in age resulted in odds ratios of postoperative mortality of 1.036 (95% CI: 1.003–1.071, P = .034) and 1.426 (95% CI: 1.026–1.982, P = .034), respectively. In this study, however, the mortality rate for patients over 75 years and between 65 and 74 years was the same (8.4%). It was 7.0% in patients from 50 to 64 years, and was significantly decreased only in patients younger than 50 years (1.5%). Moreover, increasing age was associated with postoperative sepsis, but not with overall postoperative morbidity.[2]
Another large series reported by Adam et al analyzed the data from a multicenter registry of patients with CRLM who underwent liver resection. The study included 7764 patients and showed that the postoperative mortality was significantly more common in patients older than 70 years (3.8% vs 1.6%, P < .001).[31]
In our study, the mortality rate showed an increase with age: 0.3% in YP, 1.4% in EP, and 4.4% in VEP. However, in multivariate analysis, age was not significantly related to increased mortality, suggesting that other factors can also influence postoperative outcomes.
In the literature, the relationship between postoperative morbidity and age has not been investigated extensively. A recent study by Schiergens et al analyzed outcomes of elective liver resection in 879 patients between 2003 and 2012. Patients were stratified into 3 age cohorts: >70 years, 60 to 69 years, and <60 years. The 30-day mortality rate of all patients was 8%, with a statistically significant difference between the groups: 14% in patients >70 years, 7% in patients 60 to 69 years, and 5% in the younger group, P = .001. The incidence of severe morbidity (P < .01) and nonsurgical complications (P < .001) was higher in older patients compared with younger ones. Moreover, this study showed that blood loss and presence of comorbidities, based on a CCI > 2, were factors related to an increased risk of postoperative morbidity. However, that study did not report an analysis of factors related to postoperative mortality.[10]
In the present study, we performed an extensive analysis of factors related to severe morbidity and with 90-day/in-hospital mortality to improve the safety and feasibility of HB surgery for EP. According to our results, the short-term outcomes of liver resection were related to the type of surgical procedure (major hepatectomy, biliary tract-associated procedure), complexity of surgical procedure (RBC transfusions), presence of underlying liver diseases (cirrhosis), and presence of comorbidities (ischemic heart disease).
Moreover, in our study, we proposed simple and reliable scores for severe morbidity (SMS) and for 90-day/in-hospital mortality (MS) based on the variables identified by multivariate analysis. The severe morbidity rate increased according to the SMS, from 4.5% in patients awarded with 0 points, to 14.0% in patients with 1 point, and to 29.3% in patients with ≥2 points, P < .001 (Fig. 1A). Also, the 90-day/in-hospital mortality rate increased according to MS, from 0.0% in patients awarded with 0 points, to 0.7% in patients with 1 point, and to 8.3% in patients with ≥2 points, P < .001 (Fig. 3A).
Furthermore, age seem to be related with a significant increasing of the risk of severe morbidity and mortality only in patients with a SMS and MS ≥2, respectively.
In particular, severe morbidity rate raised from 24.2% in YP, to 29.3% in EP, and to 40.0% in VEP, respectively, P = .047 (Fig. 1B), and also 90-day/in-hospital mortality rate increased from 2.3% in YP, to 7.0% in EP, and to 22.7% in VEP, respectively, P = .017 (Fig. 3B).
A previous study by Breitenstein et al proposed a simple method to assess the risk of severe complications (Dindo ≥ 3) after liver resection. Four clinical factors were identified as independent predictors of severe complications, including ASA score, preoperative aspartate aminotransferase serum level, major hepatectomy, and the need for associated extrahepatic procedure. A prediction score was calculated for the study population of 369 patients using the 4 independent predictive variables ranging from 0 to 10 points. The risk of developing severe complications was 16% in “low-risk” patients (0–2 points), 37% in “intermediate-risk” patients (3–5 points), and 60% in “high-risk” patients (6–10 points).[32] Although these results were interesting, the results were not stratified according to age groups.
Several limitations should be considered when interpreting present study. We analyzed a large series of 803 consecutive patients underwent liver surgery but the statistical analysis conducted may result subobtimal, in particular, due to the relatively low rate of postoperative mortality rate (1.4%, n = 11 patients). Another limitation of the study could be its retrospective nature.
In our study, we found that age per se is not a contraindication for liver surgery. However, VEP have increased postoperative severe morbidity and mortality, particularly when multiple risk factors are present. The proposed scoring system and multidisciplinary approach should be applied to obtain an accurate preoperative selection of patients in order to improve the postoperative outcomes. Moreover, preoperative and intraoperative management should aim to reduce blood loss as much as possible because this was one of the major negative factors. Nevertheless, our results should be confirmed and validated with larger series by other institutions.
5. Conclusion
In conclusion, we conducted a study that investigated the clinical variables and postoperative outcomes of patients who underwent liver resection according to 3 age intervals. Age alone should not be considered a contraindication for hepatectomy. Liver resection in EP can be safely performed by both an accurate patient selection and a careful surgical strategy. We identified clinical factors that can be useful to stratify the risk of postoperative severe morbidity and mortality. The scores proposed (SMS and MS) are simple tools that can be useful to stratify the risk of morbidity and mortality after hepatectomy. Moreover, severe morbidity and mortality increases according to the different age intervals in patients with scores ≥2.
Footnotes
Abbreviations: ACS = acute coronary syndrome, ASA = American Society of Anaesthesiologists, AUC = area under the curves, BMI = body mass index, CCA = cholangiocarcinoma, CCI = Charlson comorbidity index, CE-US = contrast-enhanced ultrasonography, CRLM = colorectal liver metastases, CT = computed tomography, EP = elderly patients, GBC = gallbladder cancer, HB = hepatobiliary, HCC = hepatocellular carcinoma, ICG = indocyanine green, MRI = magnetic resonance imaging, MS = Mortality Score, NCRLM = noncolorectal liver metastases, PHLF = posthepatectomy liver failure, RBC = red blood cells, ROC = receiver operating characteristic, SMS = Severe Morbidity Score, US = ultrasonography, VEP = very-elderly patients, YP = young patients.
The authors have no conflicts of interest to disclose.
References
- [1].Fong Y, Brennan MF, Cohen AM, et al. Liver resection in the elderly. Br J Surg 1997;84:1386–90. [PubMed] [Google Scholar]
- [2].Reddy SK, Barbas AS, Turley RS, et al. Major liver resection in elderly patients: a multi-institutional analysis. J Am Coll Surg 2011;212:787–95. [DOI] [PubMed] [Google Scholar]
- [3].Poon RT, Fan ST, Lo CM, et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg 2004;240:698–708. discussion 708–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Melloul E, Halkic N, Raptis DA, et al. Right hepatectomy in patients over 70 years of age: an analysis of liver function and outcome. World J Surg 2012;36:2161–70. [DOI] [PubMed] [Google Scholar]
- [5].Oishi K, Itamoto T, Kobayashi T, et al. Hepatectomy for hepatocellular carcinoma in elderly patients aged 75 years or more. J Gastrointest Surg 2009;13:695–701. [DOI] [PubMed] [Google Scholar]
- [6].Shirabe K, Kajiyama K, Harimoto N, et al. Early outcome following hepatic resection in patients older than 80 years of age. World J Surg 2009;33:1927–32. [DOI] [PubMed] [Google Scholar]
- [7].Portolani N, Baiocchi GL, Coniglio A, et al. Limited liver resection: a good indication for the treatment of hepatocellular carcinoma in elderly patients. Jpn J Clin Oncol 2011;41:1358–65. [DOI] [PubMed] [Google Scholar]
- [8].Lee CR, Lim JH, Kim SH, et al. A comparative analysis of hepatocellular carcinoma after hepatic resection in young versus elderly patients. J Gastrointest Surg 2012;16:1736–43. [DOI] [PubMed] [Google Scholar]
- [9].Tsujita E, Utsunomiya T, Yamashita Y, et al. Outcome of hepatectomy in hepatocellular carcinoma patients aged 80 years and older. Hepatogastroenterology 2012;59:1553–5. [DOI] [PubMed] [Google Scholar]
- [10].Schiergens TS, Stielow C, Schreiber S, et al. Liver resection in the elderly: significance of comorbidities and blood loss. J Gastrointest Surg 2014;18:1161–70. [DOI] [PubMed] [Google Scholar]
- [11].Ueno M, Hayami S, Tani M, et al. Recent trends in hepatectomy for elderly patients with hepatocellular carcinoma. Surg Today 2014;44:1651–9. [DOI] [PubMed] [Google Scholar]
- [12].Wang HQ, Yang J, Yan LN, et al. Liver resection in hepatitis B related-hepatocellular carcinoma: clinical outcomes and safety in elderly patients. World J Gastroenterol 2014;20:6620–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zoli M, Iervese T, Abbati S, et al. Portal blood velocity and flow in aging man. Gerontology 1989;35:61–5. [DOI] [PubMed] [Google Scholar]
- [14].Russolillo N, Ratti F, Vigano L, et al. The influence of aging on hepatic regeneration and early outcome after portal vein occlusion: a case-control study. Ann Surg Oncol 2015;22:4046–51. [DOI] [PubMed] [Google Scholar]
- [15].Ide T, Miyoshi A, Kitahara K, et al. Prediction of postoperative complications in elderly patients with hepatocellular carcinoma. J Surg Res 2013;185:614–9. [DOI] [PubMed] [Google Scholar]
- [16].Taniai N, Yoshida H, Yoshioka M, et al. Surgical outcomes and prognostic factors in elderly patients (75 years or older) with hepatocellular carcinoma who underwent hepatectomy. J Nippon Med Sch 2013;80:426–32. [DOI] [PubMed] [Google Scholar]
- [17].Caratozzolo E, Massani M, Recordare A, et al. Liver resection in elderly: comparative study between younger and older than 70 years patients. Outcomes and implications for therapy. G Chir 2007;28:419–24. [PubMed] [Google Scholar]
- [18].Copeland GP, Jones D, Walters M. POSSUM: a scoring system for surgical audit. Br J Surg 1991;78:355–60. [DOI] [PubMed] [Google Scholar]
- [19].Prytherch DR, Whiteley MS, Higgins B, et al. POSSUM and Portsmouth POSSUM for predicting mortality. Physiological and Operative Severity Score for the enumeration of Mortality and morbidity. Br J Surg 1998;85:1217–20. [DOI] [PubMed] [Google Scholar]
- [20].Haga Y, Ikei S, Ogawa M. Estimation of Physiologic Ability and Surgical Stress (E-PASS) as a new prediction scoring system for postoperative morbidity and mortality following elective gastrointestinal surgery. Surg Today 1999;29:219–25. [DOI] [PubMed] [Google Scholar]
- [21].Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- [22].Pang YY. The Brisbane 2000 terminology of liver anatomy and resections. HPB 2000;2:333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187–96. [DOI] [PubMed] [Google Scholar]
- [24].Balzan S, Belghiti J, Farges O, et al. The “50-50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg 2005;242:824–8. discussion 828–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mullen JT, Ribero D, Reddy SK, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg 2007;204:854–62. discussion 862–864. [DOI] [PubMed] [Google Scholar]
- [26].Wynne HA, Cope LH, Mutch E, et al. The effect of age upon liver volume and apparent liver blood flow in healthy man. Hepatology 1989;9:297–301. [DOI] [PubMed] [Google Scholar]
- [27].Regev A, Schiff ER. Liver disease in the elderly. Gastroenterol Clin North Am 2001;30:547–63. x–xi. [DOI] [PubMed] [Google Scholar]
- [28].Sanz N, Diez-Fernandez C, Alvarez AM, et al. Age-related changes on parameters of experimentally-induced liver injury and regeneration. Toxicol Appl Pharmacol 1999;154:40–9. [DOI] [PubMed] [Google Scholar]
- [29].Sanchez-Hidalgo JM, Naranjo A, Ciria R, et al. Impact of age on liver regeneration response to injury after partial hepatectomy in a rat model. J Surg Res 2012;175:e1–9. [DOI] [PubMed] [Google Scholar]
- [30].Fortner JG, Lincer RM. Hepatic resection in the elderly. Ann Surg 1990;211:141–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Adam R, Frilling A, Elias D, et al. Liver resection of colorectal metastases in elderly patients. Br J Surg 2010;97:366–76. [DOI] [PubMed] [Google Scholar]
- [32].Breitenstein S, DeOliveira ML, Raptis DA, et al. Novel and simple preoperative score predicting complications after liver resection in noncirrhotic patients. Ann Surg 2010;252:726–34. [DOI] [PubMed] [Google Scholar]


