Abstract
Despite distinct dissimilarities, diverse cancers express several common protumorigenic traits. We present here evidence that the proapoptotic protein Par-4 utilizes one such common tumorigenic trait to become selectively activated and induce apoptosis in cancer cells. Elevated protein kinase A (PKA) activity noted in cancer cells activated the apoptotic function of ectopic Par-4 or its SAC (selective for apoptosis induction in cancer cells) domain, which induces apoptosis selectively in cancer cells and not in normal or immortalized cells. PKA preferentially phosphorylated Par-4 at the T155 residue within the SAC domain in cancer cells. Moreover, pharmacological-, peptide-, or small interfering RNA-mediated inhibition of PKA activity in cancer cells resulted in abrogation of both T155 phosphorylation and apoptosis by Par-4. The mechanism of activation of endogenous Par-4 was similar to that of ectopic Par-4, and in response to exogenous stimuli, endogenous Par-4 induced apoptosis by a PKA- and phosphorylated T155-dependent mechanism. Enforced elevation of PKA activity in normal cells resulted in apoptosis by the SAC domain of Par-4 in a T155-dependent manner. Together, these observations suggest that selective apoptosis of cancer cells by the SAC domain of Par-4 involves phosphorylation of T155 by PKA. These findings uncover a novel mechanism engaging PKA, a procancerous activity commonly elevated in most tumor cells, to activate the cancer selective apoptotic action of Par-4.
Tumors often show heterogeneity at many levels, including morphology, expression of prosurvival traits, and down-modulation of apoptotic or tumor suppressor traits. Despite tumor heterogeneity, however, several predominant traits exist within similar types of cancers. Such traits are prospective targets for therapeutic intervention strategies. However, because most cancer therapy protocols have an accompanying toxicity for normal cells, there are ongoing efforts directed toward the identification of apoptotic molecules that are highly selective for cancer cells. Our recent studies have identified Par-4 protein as a unique proapoptotic molecule that induces apoptosis in hormone-independent but not hormone-dependent cancer cells or in primary or immortalized normal cells (12). We describe here a novel mechanism underlying the selective activation of the apoptotic function of Par-4 in cancer cells.
The Par-4 gene was identified in a search for genes induced during apoptosis in prostate cancer cells (20). The gene maps to human chromosome 12q21, a region that is unstable and often deleted in pancreatic and gastric cancer (19). Expression of Par-4 is diminished in renal cell carcinoma (7), neuroblastoma (15), acute lymphatic leukemia, and chronic lymphocytic leukemia (2). In addition, the Par-4 gene is down-regulated by oncogenes such as Ras, Raf, or Src (1, 18), and restoration of Par-4 levels results in the inhibition of oncogene-induced cellular transformation (17). Interestingly, ectopic overexpression of Par-4 in cancer cells is sufficient to induce apoptosis in these cells and cause regression of tumors. Direct apoptosis by Par-4 occurs by a unique mechanism that involves coparallel activation of the prodeath FasL-Fas-FADD-caspase-8 pathway and inhibition of the NF-κB prosurvival pathway (3). Par-4 activates the Fas pathway by inducing the translocation of Fas and FasL to the cell membrane and parallel inhibition of the transcription activity of NF-κB to allow the apoptosis pathway to progress unhindered (3). Apoptosis of cancer cells by Par-4 is independent of potential antiapoptotic roadblocks, such as high NF-κB activity, Bcl-xL, or Bcl-2 levels, and the status of tumor suppressors such as p53 or PTEN (3).
Par-4 is a 332-amino-acid protein that is composed of a leucine zipper domain in the carboxy-terminal region and two putative nuclear localization signal (NLS) domains (11). These domains are fully conserved in human, rat, and mouse Par-4 (11). We recently reported that nuclear localization of Par-4 by NLS2 is essential for apoptosis by Par-4 (12). In certain cancer cells and normal or immortalized cells, Par-4 does not enter the nucleus and is unable to induce apoptosis (12). Consistently, a deletion mutant of Par-4 containing amino acids 137 to 195 with an intact NLS2 domain constitutively enters the nucleus and has an expanded range in inducing apoptosis of all cancer cell lines tested (12). In fact, the region containing amino acids 137 to 195 represents a unique core domain of Par-4 that is essential and sufficient for Fas/FasL translocation to the cell membrane, inhibition of NF-κB activity, and induction of apoptosis in cancer cells. Interestingly, although this core domain localizes to the nucleus, it does not induce apoptosis in normal or immortalized cells, and was therefore designated the SAC (selective for apoptosis induction in cancer cells) domain (12). These findings indicate that nuclear entry is essential but not sufficient for apoptosis by Par-4 and that Par-4 may require additional activation events (for example, posttranslational changes in the SAC domain) that occur selectively in cancer cells, leading to induction of apoptosis.
One of the most common posttranslational modifications by which the function of cellular proteins may be rapidly switched on or off is phosphorylation. The phosphorylation events are effected by tyrosine or serine/threonine (S/T) kinases. Cyclic AMP (cAMP)-dependent protein kinase or protein kinase A (PKA) is one such S/T kinase that is involved in the regulation of gene expression, metabolism, cell growth and differentiation. PKA is a broad-spectrum kinase that phosphorylates a wide variety of substrates and is therefore very tightly regulated by differential expression of subunits and subcellular localization. The PKA holoenzyme is a tetramer composed of two catalytic (C) subunits that are held in an inactive state by association with regulatory (R) subunits. Binding of cAMP to the R subunits causes the C subunits to dissociate, allowing phosphorylation of substrates (8, 9, 13, 24). PKA occurs in mammalian cells in two distinct isoforms, PKA-I and PKA-II, which differ only in their regulatory subunits (that are termed RI for PKA-I and RII for PKA-II). PKA-I is generally associated with proliferation and is often overexpressed in human cancer cell lines and in primary tumors. On the other hand, preferential expression of PKA-II is found in normal nonproliferating tissues and in growth-arrested cells (for a review, see reference 23). In addition to the current dogma for PKA regulation, recent discoveries implicate the existence of cAMP-independent activation of PKA (10).
The role of PKA in cancers is not fully understood. On the one hand, germ line mutations in PKA-I lead to the Carney complex, a multiple-neoplasia syndrome associated with a greater PKA response to cAMP, due to compensatory increases in the other PKA subunits (for a review, see reference 22). On the other hand, PKA-I is overexpressed in several cancer cells and is often associated with a poor prognosis in cancer patients (5). In addition, increased expression of PKA-I is seen following cellular transformation by certain growth factors, such as transforming growth factor α or oncogenes, such as ras, myc, and erbB-2 (23). Although PKA is primarily an intracellular enzyme, there is growing evidence that there is a form of PKA that is secreted into the extracellular space. Extracellular PKA functions in a cAMP-independent manner, and the levels of this form of PKA are especially high in the growth media of cultured cancer cells, as well as in plasma samples from cancer patients (4, 10). Furthermore, overexpression of the catalytic subunit of PKA (PKAc) in fibroblast cells leads to transformation of the fibroblasts; this finding directly implicates PKA in the process of oncogenesis (25). In a recent study of breast cancer patients, elevation of PKA activity was found to be the cause of tamoxifen resistance in breast cancer (16). Thus, ample evidence in the literature suggests that PKA plays a critical role in the development of the cancer phenotype. PKA exerts its cellular functions by phosphorylation of various substrate proteins that include transcription factors such as CREB and NF-κB, proapoptotic proteins such as Bad, or tumor suppressor proteins such as LKB1 (6, 14, 26).
In the present study, we investigated the mechanism by which Par-4 or the SAC domain selectively induces apoptosis in cancer cells but not in primary normal or immortalized cells. We report that PKA-mediated phosphorylation of the T155 residue in Par-4 is essential for the apoptotic function of Par-4. Moreover, we found that the enzymatic activity of PKA is elevated in transformed and cancer cell lines, relative to that in normal or immortalized cells, leading to selective activation of the apoptotic function of Par-4 in cancer cells but not in nontransformed cells. Thus, the proapoptotic molecule Par-4 utilizes elevated PKA, a prosurvival alteration commonly occurring in several types of cancer cells, for activation to induce selective apoptosis of the cancer cells.
MATERIALS AND METHODS
Cell lines.
Androgen-dependent prostate cancer cells LNCaP; androgen-independent prostate cancer cells PC-3, DU145, or LNCaP/IGFBP5 (an isogenic derivative of LNCaP); immortalized human prostate epithelial cell line PZ-HPV-7; normal primary prostatic cell line PrE or PrS; mouse embryo fibroblast (MEF) cells, NIH 3T3 fibroblast cells, and NIH 3T3/Ras transformed fibroblast cells; human lung cancer cells A549, H157, H838 and H460; and human breast cancer cells MCF-7, MDA MB 231, and MDA MB 435 have been described previously by us (12). Normal bronchial epithelial (NHBE) cells were purchased from Clonetics Corp. (San Diego, Calif.). Human embryonic lung (HEL) fibroblast cells were from Tim Kowalik (University of Massachusetts, Worcester, Mass.).
Plasmid constructs, chemical reagents, and antibodies.
pCB6+ vector, pCB6+-Par-4, the green fluorescent protein (GFP)-SAC domain, GFP-137-187 constructs, and the NF-κB-luciferase reporter construct were described previously (12). The S154-to-154A or T155-to-155A point mutant of Par-4 was made by site-directed mutagenesis to change the sequence at S154 from TCC to GCC or T155 from AGC to GCC. The GFP-SAC domain/155A and GFP-137-187/155A constructs were prepared by PCR amplification with Par-4/155A as a template, followed by ligation in the pcDNA3.1/CT-GFP-TOPO vector (Invitrogen Corp., Carlsbad, Calif.). The expression construct for PKAC was kindly provided by Sankar Ghosh (Yale Medical School, New Haven, Conn.). PKA-inhibitory peptide PKI and 8-Cl-cAMP were from Calbiochem. Vincristine, doxorubicin, and DAPI (4,6-diamidino-2-phenylindole) were from Sigma Chemical Co. (St. Louis, Mo.). Antibodies for Par-4, PKA, pCREB, and total CREB, as well as the Annexin V kit for apoptosis assays, were from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.). The actin antibody was from Sigma Chemical Co. The phosphorylated (phospho)-T155-Par-4 antibody was raised in rabbits against the peptide KRKLREKRRS(PO4T)GVVNIP (representing amino acids 148 to 161 of Par-4 with a phosphorylated T155 residue, shown in parentheses). Small interfering RNA (siRNA) oligonucleotide duplexes directed against human Par-4 or human PKA α or β catalytic subunits were from Dharmacon, Inc. (Lafayette, Colo.).
Transfection and RelA/NF-κB reporter assays.
Cells were transiently transfected with the indicated plasmid constructs by using Lipofectamine Plus (Invitrogen) according to the manufacturer's instructions. Cells were harvested after 48 h, and whole-cell lysates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidene difluoride (PVDF) membranes, and subjected to Western blot analysis to ascertain expression of the constructs or to luciferase and β-galactosidase assays, to quantify and normalize NF-κB activity, as previously described (3).
PKA activity assay.
The in vitro PKA enzymatic assay was performed with the cAMP-dependent PKA Signatect Assay kit from Promega Corp. (Madison, Wis.). Briefly, cells were suspended in extraction buffer (25 mM Tris [pH 7.4], 0.5 mM EDTA, 0.5 mM EGTA, 10 mM β-mercaptoethanol, leupeptin, aprotinin, and phenylmethylsulfonyl fluoride) and homogenized with a cold homogenizer. Aliquots of cell lysate (each, 5 μl) were incubated with PKA assay buffer (200 mM Tris [pH 7.4], 100 mM MgCl2, 0.5 mg of bovine serum albumin/ml), 25 mM cAMP, PKA-biotinylated substrate peptide, and γ-32P-labeled ATP at 30°C. After 15 min, the reaction was terminated with guanidinium hydrochloride, and the mixture was spotted on streptavidin-coated phosphocellulose paper. Incorporation of γ-32P into PKA-biotinylated substrate peptide was measured with a scintillation counter, and PKA activity was normalized to the protein concentration of the cell lysates and expressed as picomoles of ATP per minute per microgram of protein.
Apoptosis assay and indirect immunofluorescence.
Cells plated in chamber slides were transiently transfected with Par-4, mutant Par-4, or PKAc constructs or subjected to the indicated treatments. The cells were subjected to immunocytochemistry (ICC) with Par-4 or PKA antibody, followed by secondary antibody conjugated to Alexa Fluor 488 (green fluorescence) or Texas red (red fluorescence). Nuclei were stained with DAPI (cyan fluorescence) and visualized by confocal microscopy. In each experiment, a total of at least 300 transfectants expressing the constructs were scored for apoptosis, based on condensed nuclei or by staining with fluorescein isothiocyanate (FITC)-conjugated annexin V, as previously described (3). The data presented are mean values of three independent experiments ± standard deviation.
Metabolic phospholabeling and immunoprecipitation.
Cells were transfected with expression constructs for Par-4 or the indicated point mutants; 36 h posttransfection, the cells were washed with phosphate-free Dulbecco's modified Eagle medium and incubated overnight with [32P]orthophosphate (200 μCi/ml). Cells were lysed with lysis buffer (50 mM Tris-HCl [pH 7.5], 1 mM EGTA, 1 mM EDTA, 1% [wt/wt] Triton X-100, 1 mM sodium orthovanadate, 50 mM sodium fluoride, 5 mM sodium pyrophosphate, 0.27 M sucrose, 1 μM microcystin-LR, 0.1% [vol/vol] 2-mercaptoethanol, and complete proteinase inhibitor mixture) and Par-4 was immunoprecipitated from the cleared lysate with anti-Par-4 antibody (5 μg of antibody conjugated to 40 μl of protein G-Sepharose beads). The immunoprecipitates were washed 10 times with lysis buffer, subjected to SDS-PAGE, and transferred to a nylon membrane, followed by autoradiography. The same blots were subjected to Western blot analysis with anti-Par-4 antibody as a loading control.
In vitro phosphorylation assays on immunoprecipitated Par-4.
NIH 3T3 cells were transiently transfected with Par-4 or the indicated point mutants; at 48 h posttransfection, the cells were lysed and Par-4 was immunoprecipitated as described above. Kinase assays were performed in vitro by adding purified PKA enzyme and [γ-32P]ATP in kinase buffer (10 mM MgCl2 and HEPES [pH 7.2]). Immunoprecipitated proteins were incubated in a total volume of 40 μl at 30°C with 1 U/ml of PKA in kinase (10 mM MgCl2 and HEPES [pH 7.2]) and 100 μM [γ-32P]ATP (1,000 cpm/pmol). After incubation for 15 min, incorporation of phosphate into Par-4 was determined by resolving the samples by SDS-PAGE, followed by transfer to a PVDF nylon membrane and autoradiography. The same blots were subjected to Western blot analysis with anti-Par-4 antibody as a loading control.
Transfections with siRNA.
Cells in six-well plates were transiently transfected with siRNAs with Oligofectamine (Invitrogen) according to the manufacturer's instructions. Briefly, 4 μg of siRNA against Par-4 or PKA α and β catalytic subunits or control siRNA was mixed with 175 μl of Opti-MEM (fresh RPMI medium without antibiotics) and complexed with a mixture of 3 μl of Oligofectamine and 15 μl of Opti-MEM for 20 min at room temperature. The complex was diluted to obtain a final volume of 1 ml and added to the cells. After 4 h, the cells were replenished with 500 μl of Opti-MEM containing 30% fetal calf serum and incubated for 24 h. siRNA transfections were repeated after 24 h; after a total of 48 h, the cells were processed for either apoptosis, PKA activity assays, or Western blot analysis. The siRNA for Par-4 (5′-GAUGCAAUUACACAACAGAdTdT-3′) and the siRNA for PKA catalytic subunit α (catalog number M-004649-00) and PKA catalytic subunit β (catalog number M-004650-00) were purchased from Dharmacon, Inc.
RESULTS
T155 residue is essential for apoptosis by Par-4.
We recently reported that nuclear localization is essential for apoptosis by Par-4 (12). Par-4 does not enter the nucleus in normal cells or in hormone-dependent cancer cells, such as the prostate cancer cell line LNCaP or breast cancer cell line MCF-7, and is unable to induce apoptosis. Moreover, when NLS2 is deleted, Par-4 loses its ability to induce apoptosis. The SAC domain mutant of Par-4 constitutively enters the nucleus and has an expanded range in inducing apoptosis of all cancer cell lines tested. Interestingly, although this mutant localizes to the nucleus, it does not induce apoptosis in nontransformed normal or immortalized cells (12). These findings indicated that besides nuclear entry, Par-4 or the SAC domain requires an additional activation event(s) to induce apoptosis.
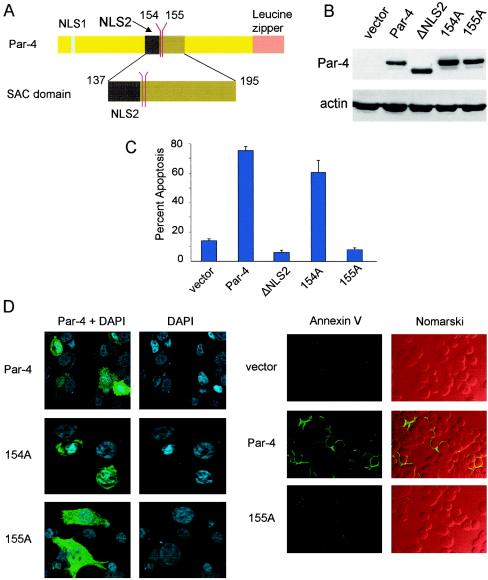
To identify a potential activation switch in Par-4, we focused on the amino acid sequence of the SAC domain and noted the presence of two consensus phosphorylation sites at residues S154 and T155 for PKA (Fig. 1A). To test the functionality of these sites, we mutated the S154 or T155 residues to 154A or 155A, respectively, in full-length Par-4. The ability of these mutants to induce apoptosis was tested in PC-3 cells, which are known to undergo apoptosis with Par-4. The cells were transiently transfected with vector, wild-type Par-4, 154A, or 155A mutant constructs and assayed for apoptosis by scoring either condensed DAPI-stained nuclei (Fig. 1D, left) or annexin V-positive cells (Fig. 1D, right). The deletion mutant ΔNLS2, lacking NLS1 and NLS2 that does not translocate to the nucleus (12), was used as a control. We found that the 154A mutant induced apoptosis similar to wild type Par-4, but the 155A mutant and ΔNLS2 were unable to induce apoptosis (Fig. 1C and D). Data for cytoplasmic retention of ΔNLS2 are similar to those described previously (12) and are therefore not shown. All the constructs were expressed equally well as assessed by Western blot analysis (Fig. 1B) and ICC (Fig. 1D), suggesting that lack of apoptosis by 155A is not due to reduced expression. These findings suggested that T155 is essential for the apoptotic function of Par-4 and that the T155A mutation abolishes its apoptotic activity in PC-3 cells.
FIG. 1.
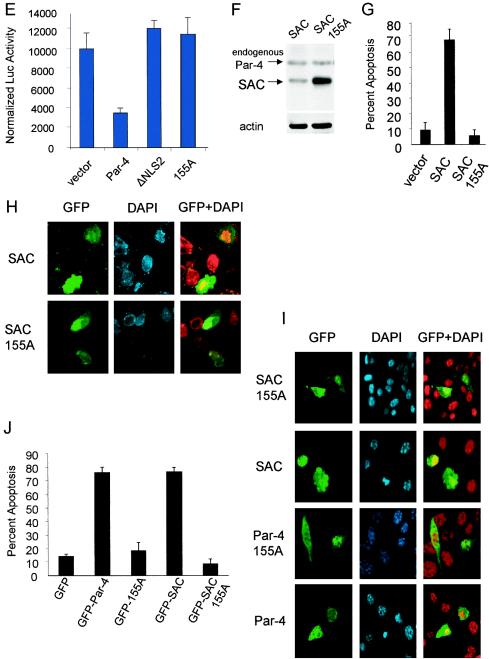
T155 is essential for the apoptotic function of Par-4. (A) Schematic representation of NLS1, NLS2, S154, T155, and the SAC domain of full-length Par-4. PC-3 cells were transiently transfected with vector, Par-4, ΔNLS2, or the point mutant 154A or 155A; expression of Par-4 and mutants was examined by Western blot analysis with the Par-4 antibody or actin antibody (B). The ability of Par-4 or the mutants to induce apoptosis at 48 h posttransfection (C and D) was examined in PC-3 cells by either ICC with the Par-4 antibody and secondary antibody conjugated to Alexa Fluor 488 (green fluorescence) followed by nuclear staining with DAPI (D, left) or by staining with FITC-conjugated annexin V antibody (D, right). Cells expressing Par-4 or mutant proteins were scored for chromatin condensation or annexin V-positive cells indicative of apoptosis by confocal microscopy, as described in Materials and Methods. (C) Quantitative data. Nomarski images show total number of cells in each field (D, right). To determine inhibition of NF-κB transcriptional activity by Par-4 or its mutants (E), PC-3 cells were transfected for 48 h with NF-κB-luc reporter construct and β-galactosidase plasmid together with vector, Par-4, or mutant constructs. Whole-cell lysates were subjected to luciferase assays, and luciferase activity was normalized to the corresponding β-galactosidase activity. PC-3 cells were transiently transfected with expression constructs for GFP-SAC or GFP-SAC/155A mutant. Expression of the mutants was confirmed by Western blot analysis with the Par-4 or actin antibody (F), and the ability of the mutants to induce apoptosis was quantified by confocal microscopy (G and H). In the results shown in panel H, DAPI is pseudocolored red, and the yellow fluorescence resulting from the overlay with the GFP fusion proteins indicates nuclear localization. NIH 3T3/Ras cells were transiently transfected with the indicated expression constructs, and the ability of the constructs to induce apoptosis was quantified by confocal microscopy (I and J).
We then tested whether the functions of Par-4 essential for its apoptotic activity, such as inhibition of NF-κB transcription, were altered by the T155A mutation. PC-3 cells were transiently transfected with Par-4, the 155A mutant, ΔNLS2, vector for control, and the NF-κB-luciferase reporter construct, and whole-cell extracts were tested for the ability of Par-4 or the mutants to inhibit NF-κB activity. As seen in Fig. 1E, relative to vector, Par-4 inhibited NF-κB-dependent luciferase activity, but the 155A mutant or ΔNLS2 was unable to inhibit this activity. A study of the kinetics of inhibition of NF-κB activity and apoptosis over a 48-h period indicated that, similar to our previous findings (12), inhibition of NF-κB activity by Par-4 preceded apoptosis (data not shown). Moreover, the 155A mutant neither inhibited NF-κB activity nor induced apoptosis over this time period (data not shown). These findings suggested that the T155A mutation in Par-4 led to loss of its apoptotic potential.
Next, because the SAC domain of Par-4 was necessary and sufficient for cancer cell apoptosis by Par-4, we tested whether T155 was crucial for apoptosis by the SAC domain. The T155 residue in the SAC domain was mutated to 155A, and PC-3 cells were transiently transfected with the SAC domain construct or the SAC/155A mutant construct and tested for apoptosis induction. Expression of the constructs was ascertained by Western blot analysis (Fig. 1F). As seen in Fig. 1G and H, the 155A mutant of the SAC domain completely lost its ability to induce apoptosis in PC-3 cells. The proximity of T155 to the NLS raised the possibility that phosphorylation of T155 might play a role in nuclear entry of Par-4. However, confocal microscopy indicated that the T155A mutant of Par-4 or of the SAC domain translocated to the nucleus yet failed to induce apoptosis (Fig. 1C and H).
To ascertain that the loss of the apoptotic potential of Par-4 or the SAC domain resulting from the T155A mutation was not limited to the PC-3 cell background, we tested the 155A mutant of Par-4 or of the SAC domain in NIH 3T3/Ras cells (Fig. 1I and J), as well as in various lung cancer, breast cancer, and prostate cancer cell lines (Fig. 2G and H and Fig. 3B to D). Similar to our findings with PC-3 cells, Par-4 or the SAC domain induced apoptosis in all the cells tested, but the Par-4/155A or SAC/155A mutant failed to induce apoptosis in these cells (Fig. 1I and J; Fig. 2 and 3). Together, these findings indicated that the T155 residue is essential for apoptosis by Par-4 or the SAC domain.
FIG. 2.
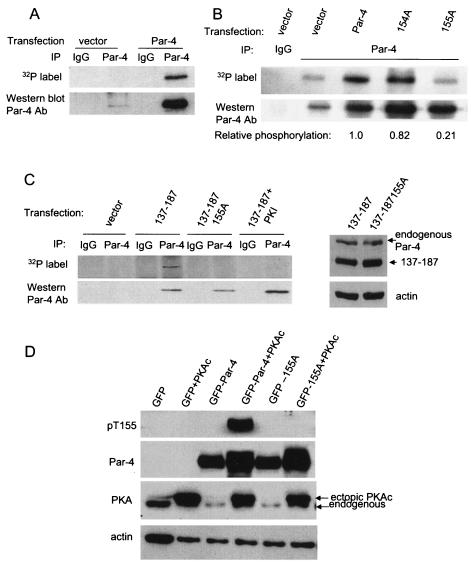
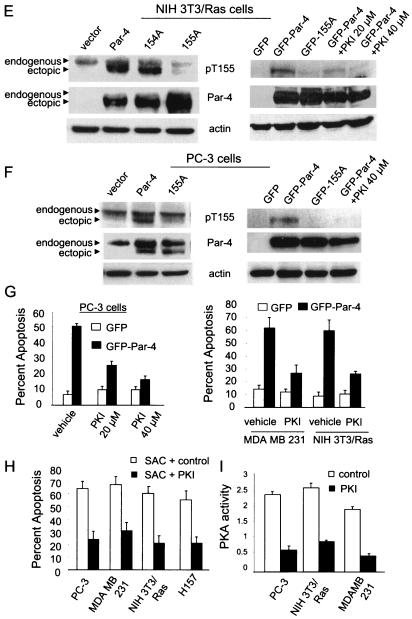
PKA phosphorylates Par-4 at T155. To determine if Par-4 is a phosphorylated protein (A), NIH 3T3/Ras cells were transiently trasfected with vector or Par-4 for 12 h, incubated in phosphate-free medium overnight, and then metabolically labeled with 200 μCi of [32P]orthophosphate for 5 h. Whole-cell extracts were subjected to immunoprecipitation with Par-4 or control IgG antibody, resolved by SDS-PAGE, and transferred to a PVDF membrane. The blot was autoradiographed (top) and finally probed with the Par-4 antibody (bottom). To determine whether PKA phosphorylates Par-4 at T155 (B), NIH 3T3 cells were transiently transfected with vector, Par-4, 154A, or 155A expression construct for 24 h, and whole-cell extracts were immunoprecipitated with the Par-4 antibody or IgG control antibody. The immunoprecipitated proteins were subjected to an in vitro phosphorylation reaction with purifed PKA enzyme and [γ-32P]ATP, and then the radiolabeled proteins were resolved by SDS-PAGE, transferred to a PVDF membrane, and autoradiographed. The blot was finally probed with Par-4 antibody to determine Par-4 or mutant protein levels. Following densitometric scanning of the blots, the amount of 32P label incorporated into ectopic Par-4 or mutant protein by PKA was normalized to the corresponding protein level detected on the Western blots, and the relative amount of 32P label incorporated (i.e., PKA phosphorylation) is shown at the bottom of the panel (B). In experiments aimed at determining whether PKA phosphorylates Par-4 in vivo (C), we used 137-187 and 137-187/155A expression constructs, which were first tested for expression by transient transfection, followed by Western blot analysis with Par-4 or actin antibody (C, right). To examine whether PKA phosphorylates Par-4 in vivo (C, left), NIH 3T3/Ras cells were transiently transfected with a vector or 137-187 or 137-187/155A mutant expression constructs in the presence or absence of PKA-inhibitory peptide PKI for 24 h, then metabolically labeled, and immunoprecipitated with the Par-4 antibody or IgG control antibody. The immunoprecipitates were subjected to SDS-PAGE, transferred to nylon membranes, and autoradiographed (C, top left). Finally, the blots were probed with the Par-4 antibody (C, bottom left), as described above (A and B). To characterize the phospho-T155 (pT155) antibody, MEFs were transfected with GFP, GFP-Par-4, or GFP-155A plasmids with or without the PKAc expression construct for 24 h, and Western blot analysis for phospho-T155, Par-4, the PKA catalytic subunit, and actin was performed (D). NIH 3T3/Ras (E) or PC-3 (F) cells were transiently transfected with vector, Par-4, 154A, or 155A plasmids (E and F, left), or with GFP, GFP-Par-4, or GFP-Par-4/155A plasmid; the cells were then treated for 24 h with vehicle or 20 or 40 μM PKI (E and F, right) and then subjected to Western blot analysis with pT155, Par-4, or actin antibodies. To determine whether inhibition of PKA activity inhibits apoptosis by Par-4 (G), PC-3, NIH 3T3/Ras, or MDA MB 231 cells were transiently transfected with GFP or GFP-Par-4. The PC-3 transfectants were treated with vehicle or with 20 or 40 μM PKI; NIH 3T3/Ras or MDA MB 231 cells were treated with 20 μM PKI. The transfectants and cells were then assayed for apoptosis, as described in the legend to Fig. 1. To ascertain that inhibition of PKA activity by PKI inhibits apoptosis by the SAC domain in diverse cancer cell lines, the cells were transfected with the SAC expression construct, treated with 20 μM PKI peptide or control, and scored for apoptosis (H). To confirm that PKI inhibits PKA activity, PC-3, NIH 3T3/Ras, or MDA MB 231 cells were treated with control or 20 μM PKI peptide for 48 h, and whole-cell lysates were tested for PKA activity with the cAMP-dependent PKA Signatect assay kit (I).
FIG. 3.
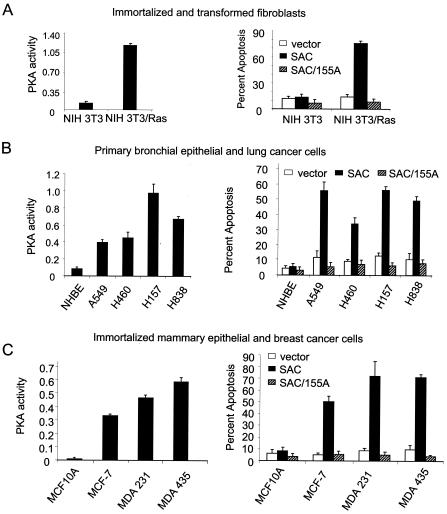
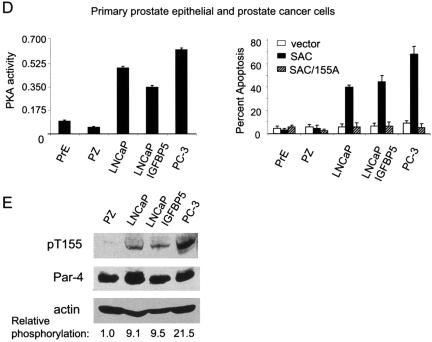
Cancer cells have elevated PKA activity levels. Whole-cell lysates were prepared from various normal or immortalized or cancer cell lines, and an in vitro PKA enzymatic assay was performed with the cAMP-dependent PKA Signatect Assay kit (A to D, left). The cell lines were transiently transfected with vector, GFP-SAC, or GFP-SAC/155A constructs and the transfectants were visualized by confocal microscopy for GFP fluorescence of Par-4 or mutant constructs and DAPI staining for apoptosis (A to D, right). To determine if Par-4 was preferentially phosphorylated in cancer cells compared to normal cells, whole-cell lysates from prostate cancer cell lines LNCaP, LNCaP/IGFBP5, and PC-3 and immortalized prostate epithelial cell line PZ were subjected to SDS-PAGE, transferred to PVDF membranes, and probed with phospho-T155 antibody, Par-4 antibody, and actin antibody as a loading control (E).
PKA phosphorylates Par-4 at T155.
Par-4 protein contains several S and T residues that are potential phosphorylation sites for PKA, PKC, and CKII (11). To determine whether regulation of the apoptotic function of Par-4 is linked to phosphorylation, we examined whether Par-4 is phosphorylated in vivo. NIH 3T3/Ras cells were transfected with the Par-4 expression construct or vector for control and then metabolically labeled with [32P]orthophosphate. The lysates were subjected to immunoprecipitation with the rabbit Par-4 polyclonal antibody or with preimmune serum as a control. The immunoprecipitates were resolved by SDS-PAGE, transferred onto a nylon membrane, and detected by autoradiography. The 32P-labeled protein (approximately 40 kDa) that immunoprecipitated with the Par-4 antibody (Fig. 2A, top) was further confirmed as Par-4 by subjecting the same blot to Western blot analysis with Par-4 antibody (Fig. 2A, bottom).
The amino acid context of T155 (KRRST) suggests that it is a consensus phosphorylation site (RXXS/T) for PKA. To ascertain that Par-4 is indeed phosphorylated by PKA and that Par-4 phosphorylation occurs at T155, we performed an in vitro phosphorylation assay with PKA on Par-4 or mutants immunoprecipitated from NIH 3T3 cells, which are resistant to apoptosis by Par-4. Relative to endogenous Par-4 which showed basal levels of phosphorylation in cells transfected with vector, ectopic wild-type Par-4 and the 154A mutant (but not the 155A mutant) showed elevated levels of PKA phosphorylation (Fig. 2B), suggesting that T155 is a primary site of phosphorylation by PKA.
We next determined whether Par-4 was phosphorylated at the T155 residue by PKA in NIH 3T3/Ras cells. Par-4 has putative phosphorylation sites for several protein kinases throughout its sequence that could potentially overshadow the phosphorylation of T155. To focus on the effect of PKA on T155 in metabolic labeling experiments, we used a deletion mutant of Par-4 that contained amino acids 137 to 187 as an in vivo substrate, in which S154 and T155 are the only two potential phosphorylation sites. The cells were transiently transfected with the 137-187 or 137-187/155A mutant and subjected to Western blot analysis to ascertain expression of the constructs (Fig. 2C, right) or to metabolic labeling, followed by immunoprecipitation with the Par-4 antibody or control immunoglobulin G (IgG) (Fig. 2C, left). As seen in Fig. 2C, top, 137-187 showed phosphorylation that was abolished when the T155 residue was altered to 155A, suggesting that T155 is the primary phosphorylation site in the SAC domain. Moreover, when the cells were treated with PKA inhibitory peptide PKI and then subjected to metabolic labeling, followed by immunoprecipitation of labeled proteins, we noted that inhibition of endogenous PKA activity with PKI abolished phosphorylation of 137-187 (Fig. 2C, top left). Western blot analysis confirmed immunoprecipitation of comparable amounts of Par-4 mutant proteins with the Par-4 antibody (Fig. 2C, bottom left).
To ascertain phospho-labeling of the T155 residue of Par-4, we raised an antibody toward the phospho-T155 residue of Par-4, as described in Materials and Methods. To characterize the specificity of this antibody toward the phospho-T155 residue of Par-4, MEF cells were transfected with GFP, GFP-Par-4, or the GFP-Par-4/155A mutant plasmid in the presence or absence of a plasmid expressing PKAC. As seen in Fig. 2D, the phospho-T155 antibody recognized a Par-4 band only in transfectants that expressed both GFP-Par-4 and PKAC but not in those that expressed GFP-Par-4 in the absence of PKAC or expressed GFP or the GFP-Par-4/155A mutant, indicating that the phospho-T155 antibody is specific for the phospho-T155 residue of Par-4.
We next used the phospho-T155 antibody to test whole-cell lysates of NIH 3T3/Ras or PC-3 cells transfected with either Par-4 or the 155A mutant of Par-4. Consistent with the phospholabeling data, the phospho-T155 antibody recognized a potentially phosphorylated form of Par-4 only when NIH 3T3/Ras or PC-3 cells were transfected with Par-4 but not when transfected with the 155A mutant (Fig. 2E and F, left). Moreover, the phospho-T155 band of Par-4 was abolished when the cells were treated with PKA-specific inhibitor PKI, further confirming that PKA phosphorylates Par-4 at T155 (Fig. 2E and F, right). Together, these findings indicated that in cells such as NIH 3T3/Ras and PC-3 that are susceptible to apoptosis by Par-4, PKA activity phosphorylates Par-4 at T155.
Inhibition of PKA activity inhibits apoptosis by Par-4 or SAC domain.
To ascertain that PKA activity is necessary for the apoptotic function of Par-4, PC-3, NIH 3T3/Ras, or MDA MB 231 cells were transiently transfected with GFP or the GFP-Par-4 construct and treated with either control or PKA inhibitory peptide PKI. The cells were then scored for apoptosis. As seen in Fig. 2G, PKI significantly (P < 0.001) inhibited induction of apoptosis by Par-4 in all three cell lines. We also tested whether apoptosis induced by the SAC domain was regulated by PKI by transfecting PC-3, NIH 3T3/Ras, MDA MB 231, or H157 cells with the SAC domain construct in the presence of control or PKI peptide. As seen in Fig. 2H, PKI inhibited apoptosis by the SAC domain, relative to the control.
To ascertain that PKI indeed inhibited PKA activity, PC-3, NIH 3T3/Ras or MDA MB 231 cells were treated with PKI or control peptide, and intracellular PKA activity was determined. As seen in Fig. 2I, PKI strongly inhibited the endogenous PKA activity relative to the control peptide. These findings suggest that the apoptotic ability of Par-4 is dependent on the presence of active PKA enzyme and inhibition of PKA enzymatic activity abolishes the apoptotic ability of Par-4.
Cancer cells have elevated PKA activity, which correlates with the apoptotic ability of the SAC domain.
The findings at this stage of the study suggested that apoptosis by Par-4 depends on the presence of an intact PKA consensus site T155 and active PKA enzyme. Moreover, the differential apoptotic ability of ectopic Par-4 in cancer versus normal cells raised the possibility that cancer cells may have elevated basal PKA activity levels compared to those in normal counterparts. A literature search indicated that PKA, especially PKA-I, is highly expressed in various transformed and cancer cells compared to corresponding nontransformed normal or immortalized cells (4, 5, 23). However, as PKA is a tightly regulated enzyme, it was not clear whether elevated protein levels of the PKA subunits correlate with elevated catalytic activity. Therefore, we sought to determine whether PKA activity was indeed elevated in cancer cells relative to normal or immortalized cells. We assayed the enzymatic activity of PKA in several panels of transformed or cancer cell lines, as well as their normal or immortalized counterparts. As seen in Fig. 3A, left, NIH 3T3/Ras cells showed over 10-fold-higher levels of PKA activity than the immortalized NIH 3T3 parental cells. A panel of lung cancer cells including A549, H838, H157, and H460 showed 5- to 12-fold-higher levels of PKA activity than the normal primary bronchial epithelial cell line NHBE (Fig. 3B, left). The breast cancer cells MCF-7, MDA MB 231, and MDA MB 435 showed 47- to 82-fold-higher PKA activity levels than normal immortalized mammary epithelial cell line MCF10A (Fig. 3C, left). A panel of prostate cancer cells including LNCaP, LNCaP derivative LNCaP/IGFBP5 and PC3 cells showed 4- to 13-fold-higher PKA activity levels than normal primary prostate epithelial PrE cells or immortalized prostate epithelial PZ cells (Fig. 3D, left). These findings indicated that transformed or tumor cells had elevated PKA activity relative to the corresponding normal or immortalized cells.
The apoptotic ability of Par-4 is mediated by the SAC domain with an intact T155 residue. To test whether elevated PKA activity correlates with susceptibility to apoptosis by the SAC domain, we transfected the normal or immortalized and cancer cells with vector, SAC domain, or the SAC/155A expression construct and assayed for apoptosis 48 h posttransfection. Interestingly, the SAC domain of Par-4 induced apoptosis only in the cells with elevated PKA, i.e., in the transformed or tumor cells and not in the normal or immortalized cells (Fig. 3A to D, right). By contrast, the SAC/155A mutant failed to induce apoptosis in all the cell lines tested (Fig. 3A to D, right). These findings indicated that PKA activity is elevated in transformed and/or cancer cells compared to normal and/or immortalized cells and that elevated PKA activity levels correlate with the ability of the SAC domain to induce apoptosis in a T155-dependent manner. Thus, elevation of PKA activity is a key factor that activates cancer cell-specific apoptosis by Par-4.
To test if elevated PKA activity in cancer cells correlated with increased phosphorylation of Par-4 in cancer compared to normal cells, whole-cell extracts of prostate cancer cells LNCaP, LNCaP/IGFBP5, and PC3 cells along with immortalized prostate epithelial PZ cells were analyzed for phosphorylation of Par-4 at T155 by Western blot analysis with a phospho-T155 Par-4 antibody. As seen in Fig. 3E (left), when the phospho-T155 Par-4 levels were normalized to total Par-4 levels, the cancer cells showed much higher levels of phospho-T155 Par-4 than the PZ cells. The pattern of occurrence of phospho-Par-4 correlated with the PKA activities of the cells and with the ability of the SAC domain to induce apoptosis in the respective cells. The findings from these experiments indicate that Par-4 is preferentially phosphorylated in cancer cells at T155 and that the relative extent of phosphorylation correlates with PKA activity and the apoptotic ability of Par-4 in these cells.
Enforced elevation of PKA activity in normal cells activates the apoptotic potential of the SAC domain of Par-4 in a T155-dependent manner.
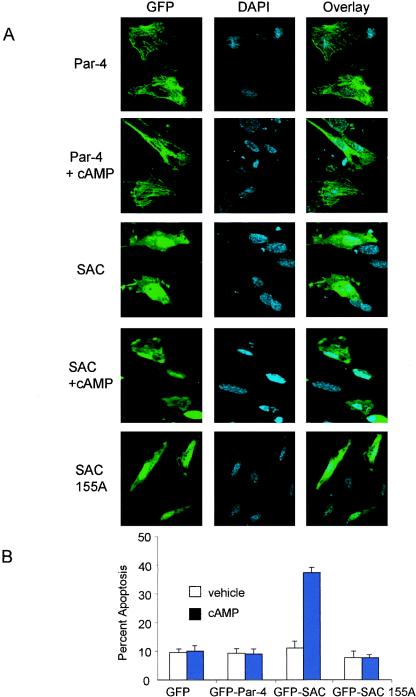
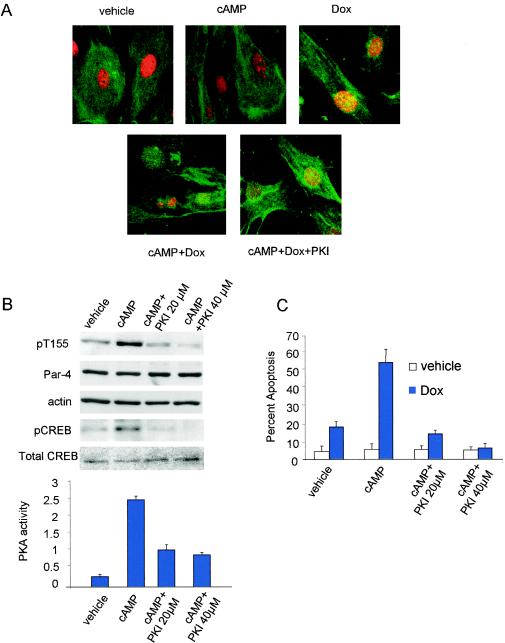
The above experiments indicated that in addition to nuclear entry, Par-4 must be phosphorylated at T155 by PKA to induce apoptosis. Normal or immortalized cells do not allow Par-4 to translocate to the nucleus and may not contain adequate levels of active PKA enzyme for T155 phosphorylation; the cells are therefore resistant to apoptosis by Par-4. This observation of inadequate levels of PKA in normal or immortalized cells is further supported by the fact that the SAC domain construct is unable to induce apoptosis in normal or immortalized cells, despite its predominantly nuclear localization (Fig. 4A). We therefore tested whether enforced elevation of PKA activity in normal cells renders them sensitive to apoptosis by Par-4, the SAC domain, or the SAC domain/T155A mutant. To activate endogenous PKA, normal MEFs were transfected with the Par-4 or SAC domain expression construct and treated with cAMP analog 8-Cl-cAMP or with vehicle as a control, and the cells were examined by confocal microscopy for nuclear translocation of ectopically expressed proteins and apoptosis. Treatment with cAMP significantly increased apoptosis by the SAC domain compared to the vehicle-treated cells (Fig. 4B). By contrast, cAMP treatment did not increase apoptosis by Par-4 or the SAC domain/T155A mutant (Fig. 4B). Consistently, cAMP did not alter the cytoplasmic localization of Par-4 or the nuclear localization of the SAC domain or SAC domain/T155A mutant (Fig. 4A).
FIG. 4.
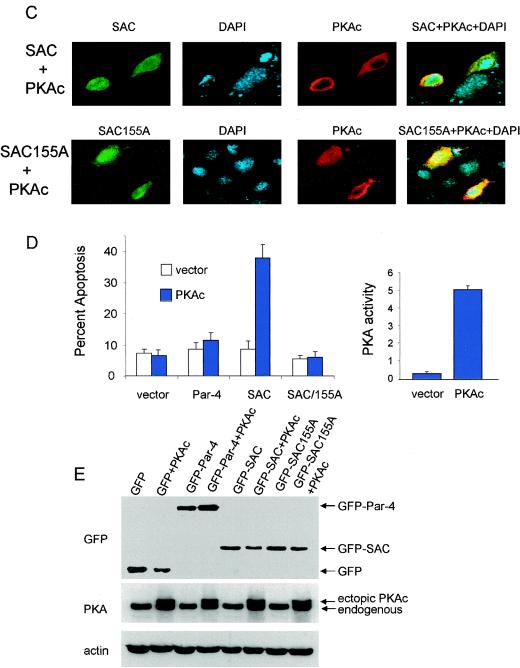
Elevation of PKA activity in normal cells activates the apoptotic potential of the SAC domain of Par-4 in a T155-dependent manner. MEF cells were transiently transfected with vector, GFP-Par-4, GFP-SAC, or GFP-SAC/155A expression constructs, treated with either vehicle or 8-Cl-cAMP (10 μM) for 48 h, and examined for intracellular localization of Par-4 or mutants (A) or apoptosis (B). MEF cells were cotransfected with expression constructs for GFP-Par-4, GFP-SAC, or vector and PKAc for 48 h; the transfected cells were visualized under a confocal microscope by GFP fluorescence for Par-4 or mutants or by immunostaining with PKAc antibody, followed by Texas red-conjugated secondary antibody, and for apoptosis by DAPI staining (C). Apoptotic cells were scored and the data were presented as percent apoptosis (D, left). PKA activity was determined with the cAMP-dependent PKA Signatect assay kit (D, right). Protein expression was examined by Western blot analysis with antibodies for GFP, PKAc, or actin (E).
Similarly, when MEFs were cotransfected with the expression construct for Par-4, the SAC domain, or the SAC domain/155A mutant and PKAc, we noted that the cotransfectants expressing the SAC domain and PKAc underwent apoptosis (Fig. 4C and D). By contrast, PKAc did not induce apoptosis in conjunction with vector, Par-4, or the SAC domain/155A mutant (Fig. 4D, left). Expression of the constructs was ascertained by Western blot analysis (Fig. 4E). Transfection with the PKAc construct resulted in an increase in PKA catalytic activity (Fig. 4D, right). These findings suggest that neither Par-4, which fails to enter the nucleus, nor the SAC domain/T155A mutant, which enters the nucleus but lacks the PKA phosphorylation site, causes apoptosis of the normal cells in the presence of elevated PKA activity. Thus, the localization of Par-4 does not depend upon its phosphorylation status, and nuclear localization of Par-4 is required but not sufficient for ectopic Par-4 to induce apoptosis.
Exogenous apoptotic stimuli activate endogenous Par-4 to induce apoptosis in a PKA- and T155-dependent manner.
Our findings thus far suggested that apoptosis by ectopic Par-4 is critically dependent on the phosphorylation of T155 by PKA. To determine whether endogenous Par-4 also induces apoptosis in a T155- and PKA-dependent manner, we used primary MEFs to study the role of the cAMP/PKA pathway in apoptosis by endogenous Par-4. Consistent with the findings with ectopic Par-4, endogenous Par-4 in MEFs was predominantly cytoplasmic in localization (Fig. 5A). Treatment with cAMP, which induced phosphorylation of the T155 residue of Par-4 (Fig. 5B), did not alter the localization of Par-4 (Fig. 5A) and did not cause apoptosis (Fig. 5C). Treatment with low doses of doxorubicin (which do not elevate PKA levels) (21), caused translocation of Par-4 to the nucleus but did not induce apoptosis (Fig. 5A and C). Interestingly, cotreatment with cAMP (to cause phosphorylation of Par-4 at T155 through the cAMP/PKA pathway) and doxorubicin resulted in translocation of Par-4 to the nucleus with a significant increase in apoptosis (Fig. 5A and C). Importantly, apoptosis inducible by this combination of cAMP and doxorubicin was blocked by inhibiting PKA with PKI treatment (Fig. 5A and C). The efficacy of cAMP or PKI was confirmed by determining phospho-CREB protein levels by Western blot analysis (Fig. 5B, top) and by assaying the PKA enzymatic activity of the cells (Fig. 5B, bottom).
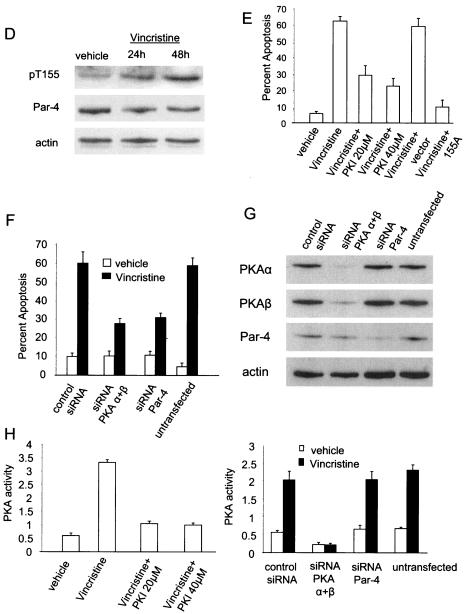
FIG. 5.
Endogenous Par-4 induces T155- and PKA-dependent apoptosis. To study localization of Par-4 and apoptosis in response to cAMP and doxorubicin treatment (A and C), MEFs were treated with vehicle, 10 μM 8-Cl-cAMP, 100 nM doxorubicin, 8-Cl-cAMP plus doxorubicin, or 8-Cl-cAMP plus 20 μM PKI and subjected to ICC analysis with Par-4 antibody followed by FITC-conjugated secondary antibody (green). The nuclei were stained with DAPI (pseudocolored red) and visualized under a confocal microscope (A) and scored for apoptosis (A and C). To study phosphorylation of T155 by cAMP (B), MEFs were treated with 10 μM cAMP, cAMP plus PKI 20 μM, or cAMP plus 40 μM PKI; whole-cell lysates were subjected to Western blot analysis with pT155, Par-4, pCREB, total CREB, or actin antibodies (B, top). PKA activity was determined in the lysates with the cAMP-dependent PKA Signatect assay kit (B, bottom). To determine whether vincristine induces T155 phosphorylation of Par-4 (D), HEL cells were treated with vehicle or 100 nM vincristine for 24 and 48 h, and Western blot analysis was performed on the whole-cell lysates with pT155, Par-4, or actin antibodies. To study vincristine-inducible apoptosis (E), HEL cells were treated with vehicle or vincristine in the presence or absence of 20 or 40 μM PKI peptide or transiently transfected with vector or GFP-Par-4 155A plasmid and assayed for apoptosis as described in the legend to Fig. 1. To determine whether inhibition of Par-4 or PKA expression inhibits apoptosis by vincristine (F), HEL cells were transiently transfected with 10 μM nonspecific control siRNA, siRNA for Par-4, or siRNA for PKA α plus β duplexes, treated with either vehicle or 100 nM vincristine, and assayed for apoptosis as described in the legend to Fig. 1. Whole-cell lysates from the siRNA-transfected cells were subjected to Western blot analysis with antibodies against PKAc α and β, Par-4, and actin (G). To determine the effect of the various treatments on PKA activity, whole-cell lysates prepared from the cells after various treatments, as described above (D and E), were tested for PKA enzymatic assay with the cAMP-dependent PKA Signatect assay kit (H).
Moreover, we studied vincristine for PKA- and phospho-T155/Par-4-dependent apoptosis of the primary human fibroblast cell line HEL. Vincristine, a microtubule inhibitor and widely used chemotherapeutic agent, is a potent inducer of apoptosis and is known to induce PKA to cause apoptosis (21). As the first step in determining whether vincristine activates T155-dependent apoptosis by Par-4, we tested whether vincristine can induce phosphorylation of Par-4 at T155. As seen in Fig. 5D, vincristine treatment led to an increase in phospho-T155 levels in HEL cells, but total Par-4 levels remained unaltered. Vincristine treatment induced apoptosis, and cotreatment with PKI resulted in a significant decrease in vincristine-induced apoptosis (Fig. 5E), indicating that vincristine induces PKA-dependent apoptosis. Moreover, ectopic expression of the 155A mutant of Par-4 but not the control vector completely abrogated vincristine-inducible apoptosis in these cells. The precise mechanism by which the 155A mutant serves as a dominant negative to inhibit endogenous Par-4 function is currently being investigated in our laboratory.
To further ascertain that vincristine-inducible apoptosis was Par-4 and PKA dependent, siRNAs targeted against either Par-4 or the α and β catalytic subunits of PKA were used to knock down endogenous levels of the respective proteins in HEL cells (Fig. 5G), and the apoptotic effect of vincristine on these cells was studied. As seen in Fig. 5F, apoptosis inducible by vincristine was significantly diminished in cells with either siRNA for Par-4 or siRNA for PKA α plus β compared to the nonspecific control siRNA or the untransfected control cells. Transfection of the siRNA for PKA α plus β in the HEL cells led to a decrease in basal PKA activity and abolished vincristine-inducible PKA activity (Fig. 5H, right). PKA activity in cells transfected with control siRNA or siRNA for Par-4 were similar to that with the untransfected control, indicating that the siRNAs used did not exert nonspecific effects on PKA activity. Taken together, these findings imply that in normal cells subjected to an exogenous apoptotic stimulus, endogenous Par-4 induces apoptosis in a T155- and PKA-dependent manner (i.e., similar to ectopic Par-4 in cancer cells) and that inhibition of either PKA or Par-4 can abrogate apoptosis.
We also examined the role of PKA- and phospho-T155 Par-4 in the action of vincristine in lung cancer cells H157. Similar to our observations of HEL cells, vincristine induced apoptosis in H157 cells; this process was inhibited by PKI, siRNA Par-4, siRNA PKA α plus β, or 155A (data not shown). Thus, vincristine induces apoptosis by a mechanism that is dependent on PKA and the T155 residue of Par-4 in both normal and cancer cells.
DISCUSSION
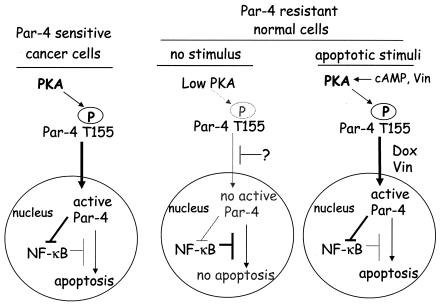
The findings of this study uncover a novel mechanism wherein a procancerous trait such as elevated PKA activity is effectively utilized by Par-4 for selective apoptosis of cancer cells. We noted that cancer cells show a marked elevation in the enzymatic activity of PKA compared to their normal counterparts; PKA phosphorylates Par-4 at the T155 residue within the SAC domain and PKA-mediated phosphorylation of T155 is critical for induction of apoptosis by Par-4 (Fig. 6). Apoptosis by Par-4 requires trafficking of Fas to the cell membrane to activate the Fas death receptor pathway and coparallel inhibition of cell survival NF-κB activity (12). Phosphorylation of Par-4 at T155 is essential for both Fas trafficking and NF-κB inhibition (this study and data not shown). The enzymatic activity of PKA in cancer and normal cells correlates with the phosphorylation status of Par-4 in these cells and with the susceptibility of the cells to apoptosis by Par-4. Normal or immortalized cells contain low levels of basal PKA activity and are unable to phosphorylate Par-4 at T155; moreover, they do not translocate Par-4 to the nucleus and consequently are resistant to apoptosis by Par-4 (Fig. 6). By contrast, cancer cells that show nuclear entry of Par-4 and have elevated PKA activity levels are sensitive to apoptosis by Par-4 (Fig. 6). Consistent with this dual requirement of PKA phosphorylation and nuclear entry for apoptosis, the SAC domain, which translocates to the nucleus in all cell types, induces apoptosis only in cancer cells because they show elevated PKA activity levels. As PKA promotes survival in various cellular contexts and shows elevated expression and activity in a broad range of cancer cells, PKA-dependent phosphorylation represents a common mechanism for activation of the apoptotic function of overexpressed Par-4 in cancer cells.
FIG. 6.
Schematic representation of PKA- and T155-dependent apoptosis by Par-4 in normal and cancer cells. Based on our findings, we propose the following model for differential apoptosis by Par-4 in cancer and normal cells. PKA activity, which is constitutively elevated in cancer cells, causes phosphorylation of Par-4 at the T155 residue. The translocation of phospho-T155 to the nucleus results in inhibition of NF-κB activity and apoptosis. By contrast, PKA activity and T155 phosphorylation levels are relatively low in normal cells. In addition, Par-4 translocation to the nucleus is blocked by an unknown mechanism. Consequently, normal cells fail to undergo apoptosis with Par-4. However, proapoptotic stimuli, such as cAMP or vincristine, cause PKA-dependent phosphorylation of T155; doxorubicin or vincristine can cause translocation of Par-4 to the nucleus. Therefore, vincristine alone or a combination of cAMP and doxorubicin can induce apoptosis of the normal cells in a PKA- and T155 Par-4-dependent mechanism. The mechanism of nuclear translocation of Par-4 is currently under investigation.
The findings of this study implicate the T155 phosphorylation site in the activation of Par-4-mediated cell death by PKA. T155 flanks NLS2 and is conserved in rat, mouse, and human Par-4. An intact T155 site is required for Par-4-mediated cell death, and mutation of T155 to 155A results in loss of apoptosis by ectopic Par-4. By contrast, mutation of S154, a putative PKA phosphorylation site in Par-4, to 154A, had no effect on the ability of ectopic Par-4 to induce apoptosis, indicating that T155 was the primary site of action of PKA in Par-4. Furthermore, inhibition of PKA function with PKI or PKA expression with siRNAs toward the PKA catalytic subunits abrogated apoptosis by ectopic Par-4 or by the SAC domain, suggesting that the presence of an active form of PKA is essential for apoptosis. Both in vivo and in vitro assays indicated that the T-to-A mutation at residue 155 resulted in significantly diminished PKA phosphorylation of Par-4. On the other hand, the S-to-A mutation at residue 154 did not substantially affect the phosphorylation status of the T155 residue. Only exogenous Par-4 induces apoptosis on its own in cancer cells, whereas endogenous Par-4 is necessary for apoptosis when cells are treated with vincristine or cAMP plus doxorubicin (Fig. 6). Similar to exogenous Par-4, endogenous Par-4 is phosphorylated at T155 in response to vincristine or cAMP treatment and induces apoptosis in response to the exogenous stimuli in a phospho-T155/PKA-dependent manner. Together, these observations suggest that PKA triggers Par-4 phosphorylation primarily at the T155 residue.
Several studies have noted that PKA is overexpressed in transformed cells and in various cancers (23). However, because the activity of PKA is tightly controlled by its regulatory subunit and cAMP, it was unclear whether this elevation in expression results in concomitant increase in the enzyme activity. The findings of this study indicated that PKA activity is significantly elevated in various types of cancer cell lines such as lung, breast, and prostate cancer cells, compared to their normal or immortalized counterparts. Moreover, there was a direct correlation between the PKA activity of the cells and apoptotic ability of Par-4 via the SAC domain. This observation is further supported by the recent findings of Dalla-Favera and coworkers that c-myc inducible up-regulation of PKAc is essential for the transformation of normal fibroblasts (25). Moreover, stable transfection of normal fibroblast cells with the PKAc or PKA-I leads to transformation and anchorage-independent colony formation in soft agar (25). Several other reports have shown that free PKAc (also known as extracellular PKA) is elevated and secreted in several cancers, leading to increased cell survival, migration, and metastasis of the cancers (5, 10). By directly activating PKAc, oncogenes can induce the cAMP/PKA signal transduction pathway in the absence of other cAMP-inducing stimuli. Therefore, elevation of PKA expression and activity is an integral part of the process of transformation. This distinct feature of cancer cells is effectively utilized by ectopic Par-4 for its own activation and apoptosis induction. Interestingly, although endogenous Par-4 is phosphorylated by elevated levels of PKA activity in cancer cells, it fails to induce apoptosis, suggesting that the apoptotic action of Par-4 is hindered by an active intracellular mechanism. This constitutive antiapoptotic mechanism directed against endogenous Par-4 is currently under investigation in our laboratory.
The SAC domain construct was able to induce apoptosis in all cancer cells tested but was unable to induce apoptosis in primary normal or immortalized cells. Because PKA elevation is one of the important features contributing to the selective apoptotic action of Par-4 in cancer cells, it was conceivable that enforced elevation of PKA enzymatic activity in normal cells may enable Par-4 to induce apoptosis in normal cells. Indeed, elevation of PKA activity in normal cells induced the ability of the SAC domain, which localized predominantly to the nucleus, to induce apoptosis in MEFs. By contrast, full-length Par-4 is predominantly localized in the cytoplasm and is unable to induce apoptosis despite enforced elevation in PKA activity in normal cells. The mechanism for this selective action of the SAC domain was hitherto unknown. Our findings suggest that Par-4 phosphorylation at T155 by PKA, as well as nuclear translocation, is essential for Par-4 and the SAC domain to induce apoptosis. Cancer cells susceptible to full-length Par-4 have elevated PKA activity levels for phosphorylation of T155 and are also able to translocate Par-4 to the nucleus. On the other hand, hormone-dependent cancer cells, such as LNCaP or MCF-7, are unable to localize full-length Par-4 to the nucleus despite elevated PKA activity; normal cells are unable to phosphorylate T155 owing to low PKA levels or to cause nuclear localization of Par-4. Apoptosis by Par-4, therefore, requires two critical steps: phosphorylation of T155 by PKA and entry into the nucleus (Fig. 6). Both these steps are feasible for the SAC domain in all cancer cells or for Par-4 in hormone-independent cancer cells, but not in normal cells, thus accounting for the tumor-selective activation and apoptotic action of Par-4 via its SAC domain.
In summary, ectopic Par-4 is sufficient to induce apoptosis in cancer cells, whereas endogenous Par-4 is critical for apoptosis induced by vincristine or cAMP plus doxorubicin. PKA activity is constitutively elevated in cancer cells and causes phoshorylation of the T155 residue of Par-4. This phosphorylation event is critical for apoptosis by Par-4. In normal cells, basal PKA activity levels are relatively low and phosphorylation of T155 fails to occur; therefore, normal cells are resistant to apoptosis by ectopic Par-4. Elevation of PKA in normal cells by cAMP-doxorubicin or vincristine induces apoptosis by a mechanism that is dependent on PKA-mediated phosphorylation of the T155 residue of endogenous Par-4. Thus, phosphorylation of the T155 residue of Par-4 by PKA is critical for apoptosis. This work provides a novel insight into the mechanism underlying differential sensitivity of normal and cancer cells.
Acknowledgments
This study was supported by NIH/NCI grants CA60872 and CA84511 (to V.M.R.).
REFERENCES
- 1.Barradas, M., A. Monjas, M. T. Diaz-Meco, M. Serrano, and J. Moscat. 1999. The downregulation of the pro-apoptotic protein Par-4 is critical for Ras-induced survival and tumor progression. EMBO J. 18:6362-6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boehrer, S., K. U. Chow, E. Puccetti, M. Ruthardt, S. Godzisard, A. Krapohl, B. Schneider, D. Hoelzer, P. S. Mitrou, V. M. Rangnekar, and E. Weidmann. 2001. Deregulated expression of prostate apoptosis response gene-4 in less differentiated lymphocytes and inverse expressional patterns of par-4 and bcl-2 in acute lymphocytic leukemia. Hematol. J. 2:103-107. [DOI] [PubMed] [Google Scholar]
- 3.Chakraborty, M., S. G. Qiu, K. M. Vasudevan, and V. M. Rangnekar. 2001. Par-4 drives trafficking and activation of Fas and Fasl to induce prostate cancer cell apoptosis and tumor regression. Cancer Res. 61:7255-7263. [PubMed] [Google Scholar]
- 4.Cho, Y. S., Y. G. Park, Y. N. Lee, M. K. Kim, S. Bates, L. Tan, and Y. S. Cho-Chung. 2000. Extracellular protein kinase A as a cancer biomarker: its expression by tumor cells and reversal by a myristate-lacking Cα and RIIβ subunit overexpression. Proc. Natl. Acad. Sci. USA 97:835-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho-Chung, Y. S., M. Nesterova, K. G. Becker, R. Srivastava, Y. G. Park, Y. N. Lee, Y. S. Cho, M. K. Kim, C. Neary, and C. Cheadle. 2002. Dissecting the circuitry of protein kinase A and cAMP signaling in cancer genesis: antisense, microarray, gene overexpression, and transcription factor decoy. Ann. N. Y. Acad. Sci. 968:22-36. [DOI] [PubMed] [Google Scholar]
- 6.Collins, S. P., J. L. Reoma, D. M. Gamm, and M. D. Uhler. 2000. LKB1, a novel serine/threonine protein kinase and potential tumour suppressor, is phosphorylated by cAMP-dependent protein kinase (PKA) and prenylated in vivo. Biochem. J. 345:673-680. [PMC free article] [PubMed] [Google Scholar]
- 7.Cook, J., S. Krishnan, S. Ananth, S. F. Sells, Y. Shi, M. M. Walther, W. M. Linehan, V. P. Sukhatme, M. H. Weinstein, and V. M. Rangnekar. 1999. Decreased expression of the pro-apoptotic protein Par-4 in renal cell carcinoma. Oncogene 18:1205-1208. [DOI] [PubMed] [Google Scholar]
- 8.Corbin, J. D., and S. L. Keely. 1977. Characterization and regulation of heart adenosine 3′:5′-monophosphate-dependent protein kinase isozymes. J. Biol. Chem. 252:910-918. [PubMed] [Google Scholar]
- 9.Corbin, J. D., T. R. Soderling, and C. R. Park. 1973. Regulation of adenosine 3′,5′-monophosphate-dependent protein kinase. I. Preliminary characterization of the adipose tissue enzyme in crude extracts. J. Biol. Chem. 248:1813-1821. [PubMed] [Google Scholar]
- 10.Cvijic, M. E., T. Kita, W. Shih, R. S. DiPaola, and K. V. Chin. 2000. Extracellular catalytic subunit activity of the cAMP-dependent protein kinase in prostate cancer. Clin. Cancer Res. 6:2309-2317. [PubMed] [Google Scholar]
- 11.El-Guendy, N., and V. M. Rangnekar. 2003. Apoptosis by Par-4 in cancer and neurodegenerative diseases. Exp. Cell Res. 283:51-66. [DOI] [PubMed] [Google Scholar]
- 12.El-Guendy, N., Y. Zhao, S. Gurumurthy, R. Burikhanov, and V. M. Rangnekar. 2003. Identification of a unique core domain of par-4 sufficient for selective apoptosis induction in cancer cells. Mol. Cell. Biol. 23:5516-5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibbs, C. S., D. R. Knighton, J. M. Sowadski, S. S. Taylor, and M. J. Zoller. 1992. Systematic mutational analysis of cAMP-dependent protein kinase identifies unregulated catalytic subunits and defines regions important for the recognition of the regulatory subunit. J. Biol. Chem. 267:4806-4814. [PubMed] [Google Scholar]
- 14.Harada, H., B. Becknell, M. Wilm, M. Mann, L. J. Huang, S. S. Taylor, J. D. Scott, and S. J. Korsmeyer. 1999. Phosphorylation and inactivation of BAD by mitochondria-anchored protein kinase A. Mol. Cell 3:413-422. [DOI] [PubMed] [Google Scholar]
- 15.Kogel, D., C. Reimertz, P. Mech, M. Poppe, M. C. Fruhwald, H. Engemann, K. H. Scheidtmann, and J. H. Prehn. 2001. Dlk/ZIP kinase-induced apoptosis in human medulloblastoma cells: requirement of the mitochondrial apoptosis pathway. Br. J. Cancer 85:1801-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michalides, R., A. Griekspoor, A. Balkenende, D. Verwoerd, L. Janssen, K. Jalink, A. Floore, A. Velds, L. van't Veer, and J. Neefjes. 2004. Tamoxifen resistance by a conformational arrest of the estrogen receptor alpha after PKA activation in breast cancer. Cancer Cell 5:597-605. [DOI] [PubMed] [Google Scholar]
- 17.Nalca, A., S. G. Qiu, N. El-Guendy, S. Krishnan, and V. M. Rangnekar. 1999. Oncogenic Ras sensitizes cells to apoptosis by Par-4. J. Biol. Chem. 274:29976-29983. [DOI] [PubMed] [Google Scholar]
- 18.Qiu, S. G., S. Krishnan, N. el-Guendy, and V. M. Rangnekar. 1999. Negative regulation of Par-4 by oncogenic Ras is essential for cellular transformation. Oncogene 18:7115-7123. [DOI] [PubMed] [Google Scholar]
- 19.Schneider, B. G., S. Y. Rha, H. C. Chung, J. C. Bravo, R. Mera, J. C. Torres, K. T. Plaisance, Jr., R. Schlegel, C. M. McBride, X. T. Reveles, and R. J. Leach. 2003. Regions of allelic imbalance in the distal portion of chromosome 12q in gastric cancer. Mol. Pathol. 56:141-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sells, S. F., D. P. Wood, Jr., S. S. Joshi-Barve, S. Muthukumar, R. J. Jacob, S. A. Crist, S. Humphreys, and V. M. Rangnekar. 1994. Commonality of the gene programs induced by effectors of apoptosis in androgen-dependent and -independent prostate cells. Cell Growth Differ. 5:457-466. [PubMed] [Google Scholar]
- 21.Srivastava, R. K., A. R. Srivastava, S. J. Korsmeyer, M. Nesterova, Y. S. Cho-Chung, and D. L. Longo. 1998. Involvement of microtubules in the regulation of Bcl2 phosphorylation and apoptosis through cyclic AMP-dependent protein kinase. Mol. Cell. Biol. 18:3509-3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stergiopoulos, S. G., and C. A. Stratakis. 2003. Human tumors associated with Carney complex and germline PRKAR1A mutations: a protein kinase A disease! FEBS Lett. 546:59-64. [DOI] [PubMed] [Google Scholar]
- 23.Tortora, G., and F. Ciardiello. 2002. Protein kinase A as target for novel integrated strategies of cancer therapy. Ann. N. Y. Acad. Sci. 968:139-147. [DOI] [PubMed] [Google Scholar]
- 24.Wang, Y. H., J. D. Scott, G. S. McKnight, and E. G. Krebs. 1991. A constitutively active holoenzyme form of the cAMP-dependent protein kinase. Proc. Natl. Acad. Sci. USA 88:2446-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu, K. J., M. Mattioli, H. C. Morse III, and R. Dalla-Favera. 2002. c-MYC activates protein kinase A (PKA) by direct transcriptional activation of the PKA catalytic subunit beta (PKA-Cβ) gene. Oncogene 21:7872-7882. [DOI] [PubMed] [Google Scholar]
- 26.Zhong, H., R. E. Voll, and S. Ghosh. 1998. Phosphorylation of NF-kappa B p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol. Cell 1:661-671. [DOI] [PubMed] [Google Scholar]