Abstract
The presence of a network of areas in the parietal and premotor cortices, which are active both during action execution and observation, suggests that we might understand the actions of other people by activating those motor programs for making similar actions. Although neurophysiological and imaging studies show an involvement of the somatosensory cortex (SI) during action observation and execution, it is unclear whether SI is essential for understanding the somatosensory aspects of observed actions. To address this issue, we used off-line transcranial magnetic continuous theta-burst stimulation (cTBS) just before a weight judgment task. Participants observed the right hand of an actor lifting a box and estimated its relative weight. In counterbalanced sessions, we delivered sham and active cTBS over the hand region of the left SI and, to test anatomical specificity, over the left motor cortex (M1) and the left superior parietal lobule (SPL). Active cTBS over SI, but not over M1 or SPL, impaired task performance relative to sham cTBS. Moreover, active cTBS delivered over SI just before participants were asked to evaluate the weight of a bouncing ball did not alter performance compared to sham cTBS. These findings indicate that SI is critical for extracting somatosensory features (heavy/light) from observed action kinematics and suggest a prominent role of SI in action understanding.
Keywords: Somatosensory cortex (SI), Transcranial magnetic stimulation (TMS), Causality, Action observation, Weight lifting
Highlights
-
•
TMS over the somatosensory cortex disrupts performance on a weight judgment task.
-
•
Disruption is specific for judgements based on observed human actions.
-
•
No TMS effect is found for judgements based on observed non-human motion.
-
•
No effect is found when TMS is administered over nearby frontal and parietal region.
Introduction
When we observe somebody lifting a box, we can typically judge if the load is heavy or light. What are the brain mechanisms supporting this computation? Years of research on the mirror system, areas of the brain active both when we perform an action and when we observed a similar action performed by another individual, suggest the mirror mechanism as a possible basis for action understanding (Rizzolatti and Sinigaglia, 2010, Rizzolatti and Sinigaglia, 2016). Motor mirroring engages a system of reciprocally connected cortical motor areas, including the inferior frontal cortex (IFC) and inferior parietal lobule (IPL) (di Pellegrino et al., 1992, Fogassi et al., 2005, Gallese et al., 1996, Keysers and Perrett, 2004; Gazzola and Keysers, 2009; Casile, 2013; Bonini, 2016). Lesion and transcranial magnetic stimulation (TMS) studies provide evidence for the functional relevance of IFC and IPL to action perception. For instance, as a consequence of a brain damage affecting the IFC, people become less accurate at detecting errors in action videos (Pazzaglia et al., 2008), ordering pictures of actions in temporal sequences (Fazio et al., 2009), and identifying what action was performed in point-light displays (Saygin, 2007). Also, lesions of the IPL impair detection of spatio–temporal errors in action sequences (Buxbaum et al., 2005, Weiss et al., 2008, Kalénine et al., 2010; see Urgesi et al. (2014) for a meta-analysis). Non-invasive transcranial stimulation of IFC and IPL transiently affects the discrimination of observed actions (Urgesi et al., 2007, Candidi et al., 2008, Cattaneo, 2010, Cattaneo et al., 2010, Cattaneo et al., 2011, Tidoni et al., 2013; Michael et al., 2014; Jacquet and Avenanti, 2015; Avenanti et al., 2017), and particularly relevant to our study, Pobric and Hamilton (2006) found that TMS interference with IFC reduced participants' ability to judge the weight of a box when seen lifted (see Avenanti et al., 2013b for a review).
Mounting functional magnetic resonance imaging (fMRI) and neurophysiological evidence suggests that the somatosensory cortices are also consistently activated when people observe the actions of others (Rossi et al., 2002, Avikainen et al., 2002, Raos et al., 2007, Gazzola et al., 2007a, Gazzola et al., 2007b, Hihara et al., 2015, McGregor et al., 2016, Valchev et al., 2016; see Keysers et al., 2010 for a review). Based on the observation that activity of the somatosensory cortices is strongly increased when seeing hands grasping objects (Pierno et al., 2009, Gazzola and Keysers, 2009, Caspers et al., 2010) or extreme joint stretching (Costantini et al., 2005), we proposed that while the parietal and premotor nodes of the mirror system could encode motor aspects of the observed actions, somatosensory regions might encode the intensity of the proprioceptive and tactile feedback experienced by that person (Avenanti et al., 2007, Gazzola and Keysers, 2009, Keysers et al., 2010). The work of Kim et al. (2015) supports this idea by showing a direct evidence for a multimodal integration of proprioceptive and tactile information in all compartments of SI. However, empirical evidence for whether and how the robust activation of SI during action observation contributes to the perception of an observed action remains scarce.
Indirect evidence for the relevance of SI to ‘social’ perception stems from studies reporting somatosensory activation when participants view others’ tactile or painful bodily states (Keysers et al., 2004, Bufalari et al., 2007; lamm et al., 2011, Lamm et al., 2007; Schaefer et al., 2008, 2012; Valeriani et al., 2008; Holle et al., 2013; Kuehn et al., 2013). More direct evidence stems from Bolognini et al. (2011) who showed that TMS perturbation over SI makes people less accurate at judging whether an observed hand was touched or not (see also Bolognini et al., 2013, Bolognini et al., 2014). Additionally, Jacquet and Avenanti (2015) repeatedly showed participants movies displaying an actor performing one of two different goal-directed hand actions. A TMS pulse over the hand representation in SI (or IFC) facilitated the recognition of repeated goals (via matching to a test picture) suggesting a role of SI in the perception of action goals. Finally, Adolphs et al. (2000), showed that lesions of the somatosensory system impair the ability to perceive facial expressions, and Paracampo et al. (2016) showed that repetitive TMS over SI (and IFC) disrupts the ability to infer amusement authenticity from observed facial movements. Although these studies suggested a causal role for SI in processing touch and high-level aspects of observed actions (i.e., the goal of an action or the emotion underlying a facial expression), it remains untested whether SI contributes to the perception of proprioceptive aspects of observed actions such as weight.
The goal of the present study was to test the functional relevance of SI to perceiving this proprioceptive information from observed actions using off-line TMS. To test the accuracy of weight perception from observed actions we used the paradigm developed by Pobric and Hamilton (2006) in four new TMS experiments. Participants had to estimate the weight of a box by observing it being lifted. The task was performed in two counterbalanced sessions carried out immediately after active or sham continuous theta-burst stimulation (cTBS; Huang et al., 2005) over the target area. The cTBS protocol was used to alter neural activity of the stimulated areas for several minutes after stimulation (Huang et al., 2005, Huang et al., 2011; Franca et al., 2006; Bertini et al., 2010), and test its critical role on the ability to judge the weight of a box from observed actions. In the first three experiments, we targeted SI to test its critical role in action understanding, and two neighboring sensorimotor regions, M1 and SPL, to test for anatomo-functional specificity (Chouinard et al., 2009, Eidenmüller et al., 2014). Both M1 and SPL, possess functional and reciprocal connections with SI, and are found to respond to action execution (Vigneswaran et al., 2013, Bonini, 2016, Lloyd et al., 2002, Gazzola and Keysers, 2009, Keysers and Gazzola, 2009). In the fourth experiment, we applied cTBS over SI before participants judged the weight of a bouncing ball, to test for SI specificity to weight estimation when the action of a human agent is involved. Our results extend those of Bolognini et al., 2011, Bolognini et al., 2013, Bolognini et al., 2014 by showing that in addition to processing purely tactile information, SI also contributes to the processing of more proprioceptive information derived from action kinematics to infer weight; and those of Pobric and Hamilton (2006) by showing that weight judgment by observation requires SI in addition to IFC, supporting a behavioural relevance for the functional interplay between motor and somatosensory regions/representations in action perception suggested by our combined fMRI/TMS study (Valchev et al., 2016). Lastly, our results expand those of Jacquet and Avenanti (2015), Adolphs et al. (2000) and Paracampo et al. (2016) by showing that SI is critical not only for goal inference, but also for inferring proprioceptive qualities from observed kinematics.
Materials and methods
Participants
A total of 91 students from the University of Bologna took part in one of four TMS experiments (67 participants, 35 females, mean age±S.D: 23.3 y±1.9; see Table 1 for sample details) or in two psychophysical pilot studies (Pilot study 1: 12 participants, 8 females, mean age 22.8 y±2.0; Pilot study 2: 12 participants, 6 females, mean age 26.6 y±2.2). All participants provided written informed consent. All of them were right-handed (as assessed by verbal report of their manual preference) with normal or corrected to normal vision. None of them had neurological, psychiatric, or other medical problems, or had any contraindication to TMS (Rossi et al., 2009). The protocol was approved by the local ethics committee at University of Bologna and was carried out in accordance with the ethical standards of the 1964 Declaration of Helsinki. No discomfort or adverse effects during TMS were reported or noticed.
Table 1.
From left to right the table indicates, for each of the four experiments (Exp. 1 to 4), the anatomical description of the stimulation site, followed by the Talairach (upper raw) and estimated MNI (lower raw) coordinates of the target point, the type of task participants had to perform (weight estimation of a Box lifted by another individual or of a bouncing Ball), the number of participants, the average age, and the intensity of the TMS stimulation applied on the target point. MNI coordinates were estimated using the tal2mni matlab function developed by Matthew Brett (e.g. http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach). *including one outlier (see method section for details).
| anatomical description |
Mean Talairach and |
Task | No of participants | Mean participant's age in y (±SD) | Stimulation intensity as % of max stim. output (±SD) | |||
|---|---|---|---|---|---|---|---|---|
|
MNI coordinates in mm (±SD) | ||||||||
| x | y | z | (no of female) | |||||
| Exp. 1 | SI | −41.3 (±5.8) | −34.7 (±3.8) | 57.8 (±3.5) | Box | 17* (9) | 23.1 (±1.6) | 43.5 (±5.1) |
| −41.9 (±5.9) | −38.8 (±4.1) | 60.9 (±3.7) | ||||||
| Exp. 2 | M1 | −41.5 (±4.5) | −16.7 (±3.8) | 56.8 (±3.3) | Box | 16 (9) | 23.5 (±1.8) | 43.5 (±6.7) |
| −41.9 (±4.5) | −20.2 (±3.9) | 61.0 (±3.7) | ||||||
| Exp. 3 | SPL | −40.5 (±2.9) | −56.2 (±4.7) | 51.1 (±4.4) | Box | 17 (8) | 24.1 (±2.1) | 43.4 (±7.3) |
| −41.0 (±2.9) | −60.5 (±4.6) | 52.5 (±5.0) | ||||||
| Exp. 4 | SI | −40.7 (±5.1) | −34.2 (±3.3) | 57.7 (±4.3) | Ball | 17 (9) | 22.4 (±2.0) | 43.7 (±5.8) |
| −41.3 (±5.2) | −38.2 (±3.6) | 60.8 (±4.8) | ||||||
Weight estimation task
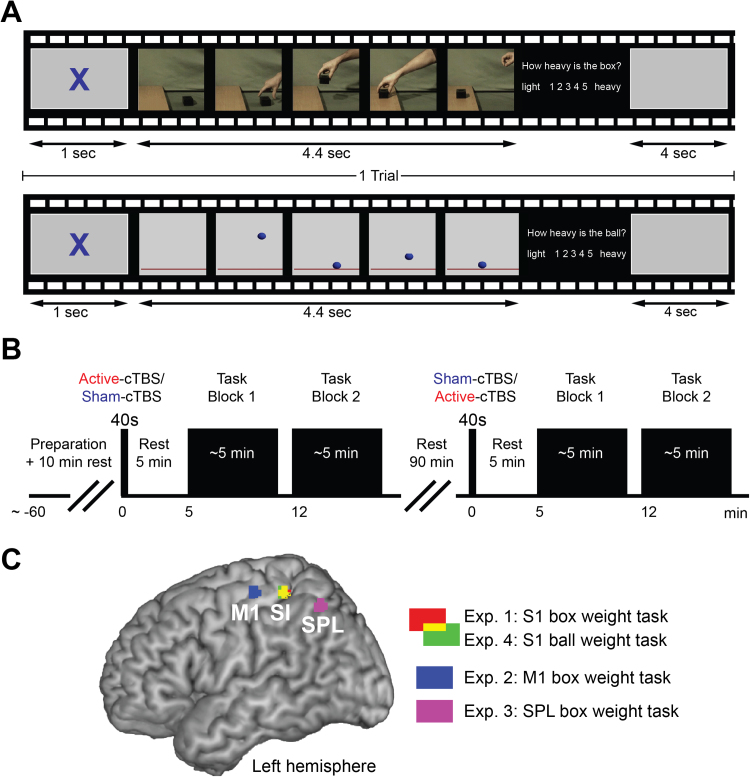
Participants watched 4.4 s movies showing either (i) a human hand lifting a box to place it on a shelf, or (ii) a ball falling from the top of the screen to then bounce at the bottom until it stops (no hand throwing the ball was visible) (Fig. 1A). The task consisted in judging, after each video, the weight of either the box or the ball by answering the question "How heavy is the box (or ball)?" on a 5 points scale, with 1 corresponding to the lightest and 5 to the heaviest weight estimation. Five different movies, representing 5 different box (or ball) weights were shown to the participants in a randomized order. Each movie was presented 12 times, 6 for each block, for a total of 30 movies per block. For both the box and ball task, each video was preceded by a 1 s fixation cross, and participants answered by pressing one of 5 keys with the left hand (ipsilateral to the stimulation site) to indicate a number from one to five. They were instructed to answer as quickly and accurately as possible. Participants wore headphones providing white noise to eliminate auditory information during task performance.
Fig. 1.
(A) Experimental weight estimation tasks, with frames extracted from one of the box and ball presented videos. (B) Experimental design for the four TMS experiments. (C) TMS targeted regions of interests for Experiments 1 to 4.
Visual stimuli
All the video stimuli came from previous experiments (Pobric and Hamilton, 2006, Hamilton et al., 2007). Briefly, the five different videos of the hand lifting a box (Experiment 1–3) were generated by down sampling a single high-speed movie of a lifting hand to create the perception of 5 different box weights, ranging from approximately 50 g to 850 g. Since they all derived from the same video, they are very well controlled for visual differences not relevant for the task. The videos of the bouncing balls were generated using Matlab (www.mathworks.com/) as in previous research (Pobric and Hamilton 2006). The perception of 5 different weights was created by modifying two parameters which affect the elasticity of the ball, thus creating the perception of observing balls of different weights. All movies were presented using custom-made software written in Matlab (www.mathworks.com/) at a resolution of 512×480 pixels and 30 frames per s on a 17 in. monitor. For both the box and the ball videos, we thus attributed value of 1, 2, 3, 4 or 5 to the ‘weight’ of the box or ball, based on these prior studies. These values were then directly compared against the reported weight (from 1 to 5) from the current study to yield accuracies (see below).
Experimental design and procedure
The study included two pilot and four TMS experiments, in which participants had to estimate the weight of the box or the ball (Fig. 1A).
The first pilot experiment was conducted on 12 volunteers (8 females, mean age 22.8 y±2.0) not participating in the TMS experiments to check if the accuracy in judging the weight of the ball was comparable to that of the box. Participants were asked to complete the box and ball weight estimation tasks in two separate sessions whose order was counterbalanced across subjects. Each session started with 60 practice trials, and continued with two blocks of 30 experimental trials each.
The box and ball task might not only differ in difficulty, but also have a different susceptibility to TMS interference. The second pilot experiment, on 12 additional volunteers (6 females, mean age 26.6 y±2.2), was therefore performed to test whether the box and ball weight estimation tasks presented comparable sensitivity to external interference. To this aim, we experimentally manipulated stimulus visibility in both tasks by visually masking the phases critical to the estimation of the box's and ball's weight. The informative time window (IFT) for the box weight estimation's videos started from the frame in which the hand touched the cube before lifting it, and lasted until the frame in which the hand released the cube. The IFT for the ball weight estimation's videos started from the frame in which the ball touched ground for the first time and ended with the frame in which the ball stopped. For each video, we applied two static masks of different duration (15% and 30% of the IFT), starting at 6 different onsets (at 0-10-20-30-40-50% of IFT duration), which were obtained by freezing the very last frame before mask onset. At the end of the mask, the video resumed showing the remaining frames. In this way, we obtained 60 new movies (2 masks×6 onsets×5 weights) for each task. Participants in the pilot study performed the box and ball weight estimation tasks in two separate sessions whose order was counterbalanced across subjects. For each task, the original version of the videos (0% of the IFT) and the two masking conditions (15–30% of the IFT) were presented in separate blocks. Each session started with 60 practice trials, and continued with the three blocks of 60 experimental trials (180 experimental trials in total). By using the masking manipulation, we provide an independent measure of task sensitivity that can be used to better interpret the results of the TMS experiments. A similar sensitivity in the two tasks will make it less likely that the changes in performance induced by TMS in one of the tasks could be simply explained by differences in sensitivity to external manipulation.
All four TMS experiments were composed of three parts: a preparatory, an active and a sham cTBS session (Fig. 1B). The order of the active and sham cTBS sessions was counterbalanced across participants. Additionally, active and sham cTBS sessions were separated by 90 min to ensure that no cTBS effects were carried over from one session to the other. During these 90 min participants were asked to remain relaxed and seated on a comfortable chair. Participants were randomly assigned to the different experiments.
During the preparatory session the optimal scalp position and the resting motor threshold were evaluated by means of motor-evoked potentials (MEPs) recording (see Transcranial magnetic stimulation paragraph for more details). Once the target site was individuated (Fig. 1C), it was marked on the scalp, and Talairach coordinates were estimated using the neuro-navigation system (see Target sites and neuro-navigation paragraph for more details; Table 1). Participants were then familiarized with the experimental task by performing a practice block of 60 trials. At the end of the practice, there was a 10 min rest period in which participants were required to remain in front of the computer.
During the active cTBS session the experimenter administered 40 s of off-line continuous theta-burst stimulation over the target site, by placing the intersection of the coil tangentially to the scalp with the handle pointing backward and laterally at a 45° angle away from the midline (Balslev et al., 2004, Azañón and Haggard, 2009, Jacquet and Avenanti, 2015). Two blocks of 30 trials (~5 min duration each) were performed at five and twelve minutes after the stimulation (Fig. 1B). Between blocks and trials, participants were asked to rest. Active cTBS is known to disrupt functions related to the target area for about 30–60 min (Huang et al., 2005, Huang et al., 2011; Franca et al., 2006; Bertini et al., 2010; Wischnewski and Schutter, 2015). Since the task was completed within 20 min after active cTBS administration, performance should reflect the influence of active cTBS over the stimulated site. The sham cTBS session was exactly the same as the active cTBS session except that the coil was positioned, over the target site, perpendicular to the scalp, so that no current was induced in the brain.
In Experiments 1–3, participants performed the weight lifting task on the box videos after receiving stimulation over the left SI, left M1 and left SPL respectively (Fig. 1C). In Experiment 4, stimulation was delivered over the left SI and participants performed the weight estimation task on the falling ball videos. The number of trials was the kept the same throughout Experiments 1 to 4.
Transcranial magnetic stimulation protocol
The cTBS protocol lasted 40 s and consisted of bursts of 3 TMS pulses delivered at 50 Hz, with each train burst repeated every 200 ms (5 Hz) for a total of 600 pulses (Huang et al., 2005). Stimulation was administered with a 70 mm figure-eight stimulation coil connected to a Magstim Rapid2 (The Magstim Company, Carmarthenshire, Wales, UK).
Previous studies have suggested that motor experience before or after the administration of cTBS may alter its effect on cortical excitability (Iezzi et al., 2008, Iezzi et al., 2011, Todd et al., 2009), possibly leading to large inter-individual differences in the cTBS effect. To minimize the influence of motor activity before task execution, participants rested for at least 10 min before active cTBS in all the TMS experiments. After active cTBS, they rested for 5 min before performing the task to allow the active cTBS’ effect to reach its maximum level (Huang et al., 2005). To minimize the influence of motor activity after cTBS, participants performed the weight estimation task using their left hand, ipsilateral to the stimulated sites. To be consistent, the same rest periods were included in the sham cTBS sessions.
Pulse intensity was set at 80% of the resting motor threshold (rMT) and was comparable in the four experiments (F3,63=0.003, P>0.99; Table 1). In those participants with rMT>64% of maximum stimulator output (2, 3, 3 and 3 participants in Experiment 1, 2, 3 and 4 respectively) the intensity was set at the maximum allowed by the stimulator (51%; on average this intensity corresponded to 76%±3 of rMT; Bertini et al., 2010). The rMT evaluation was performed by recording motor-evoked potentials (MEPs) induced by single-pulse TMS of the left motor cortex. MEPs were recorded from the right first dorsal interosseous (FDI) by means of a Biopac MP-150 electromyograph (Biopac Corp, Goletta, CA.). EMG signals were band-pass filtered (20 Hz to 1.0 kHz, sampled at 5 kHz), digitized and displayed on a computer screen. Pairs of silver/silver chloride surface electrodes were placed over the muscle belly (active electrode) and over the associated joint of the FDI muscle (reference electrode). A ground electrode was placed on the ventral surface of the right wrist. The optimum scalp position (OSP) was chosen so as to produce maximum amplitude MEPs in the FDI muscle. The rMT was defined as the lowest level of stimulation able to induce MEPs of at least 50 µV with 50% probability (Rossini et al., 1994, Rossini et al., 2015).
Target sites and neuronavigation
In Experiment 1 and 4 scalp locations corresponding to the left SI were targeted by moving the coil 2.5 cm back with respect to the OSP (corresponding to the M1 hand area). TMS studies that successfully targeted the somatosensory hand area positioned the coil 1–4 cm posterior to the motor hotspot (Avenanti et al., 2007, Harris et al., 2002, Balslev et al., 2004, Merabet et al., 2004, Fiorio and Haggard, 2005, Azañón and Haggard, 2009, Jacquet and Avenanti, 2015). We therefore assumed that positioning the coil 2.5 cm from the previously marked optimal scalp position (OSP) for activation of the right FDI muscle would interfere with the activity of the hand representation area of SI with minimum effects on M1. To test this assumption directly, we checked that TMS pulses at 105% rMT with the coil in the above position did not elicit any detectable MEPs.
To directly test the anatomical and functional specificity of the effect of cTBS on SI activity, in Experiments 2 and 3, we applied cTBS over M1 and SPL, two brain areas recruited during action observation (Keysers and Gazzola, 2009, Casile, 2013, Bonini, 2016), respectively. In Experiment 2, left M1 was stimulated by placing the coil over the OSP, corresponding to the scalp projection of motor cortex hand area (Rossini et al., 1994, Rossini et al., 2015). In Experiment 3, left SPL was stimulated by moving the coil 5 cm back with respect to the OSP (Balslev et al., 2004). Thus, stimulation of M1 and SPL occurred 2.5 cm forward and backward to SI, respectively.
In all the TMS experiments, we only stimulated brain regions (SI, M1 or SPL) in the left hemisphere. This choice was dictated by several factors including: (i) being able to directly compare our results with those of previous studies, which also stimulated the left hemisphere (Pobric and Hamilton, 2006, Cattaneo, 2010, Cattaneo et al., 2010, Cattaneo et al., 2011, Tidoni et al., 2013, Jacquet and Avenanti, 2015, Avenanti et al., 2017, Valchev et al., 2016); (ii) the use of right-hand actions in the box videos; (iii) the inclusion of right-handed participants in our sample. Indeed, although responses to action observation is bilaterally distributed (van Overwalle and Baetens, 2009; Grosbras et al., 2012; Borgomaneri et al., 2015), studies have shown a gradient of lateralization which depends on the laterality of the body part involved in the observed action, as well as the observers’ hand preference. In particular, during observation of right hand actions, activations of right-handers tend to be stronger (Aziz-Zadeh et al., 2002, Shmuelof and Zohary, 2005, van Schie et al., 2004; Gazzola and Keysers, 2009; Cabinio et al., 2010; Caspers et al., 2010) in the left, relative to the right, hemisphere, and some studies showed that stimulation of the left hemisphere is more effective than the right hemisphere to modulate perception of observed right hand actions (Avenanti et al., 2017; but see Urgesi et al., 2007). Thus, by targeting the left hemisphere, we could test whether performance in the box weight estimation task was affected by interference with observer's sensorimotor regions representing the very same hand shown in the action movies.
Brain surface Talairach coordinates corresponding to projection of the stimulated sites in SI (Experiments 1 and 4), M1 (Experiment 2) or SPL (Experiment 3) were identified on each participant's scalp with the SofTaxic Navigator system (Electro Medical Systems, Bologna, Italy) as in previous research (Avenanti et al., 2007, Avenanti et al., 2013a, Avenanti et al., 2017, Bertini et al., 2010, Serino et al., 2011, Tidoni et al., 2013, Jacquet and Avenanti, 2015, Paracampo et al., 2016). Skull landmarks (nasion, inion, and two preauricular points) and about 100 points providing a uniform representation of the scalp were digitized by means of a Polaris Vicra digitizer (Northern Digital Inc., Ontario, Canada). An individual estimated magnetic resonance image (MRI) was obtained for each subject through a 3D warping procedure fitting a high-resolution MRI template with the participant's scalp model and craniometric points. This procedure has been proven to ensure a global localization accuracy of roughly 5 mm, a level of precision closer to that obtained using individual MRIs than can be achieved using other localization methods (Carducci and Brusco, 2012). Coordinates of target regions in Talairach space (corresponding scalp projections on brain surface) were automatically estimated by the SofTaxic Navigator from the MRI-constructed stereotaxic template and later transformed to the MNI space for better visualisation (Table 1). For illustrative purpose, spherical rois of diameter 4 mm around the mean target point from each TMS experiment were created using Marsbar (Brett et al., 2002) running in MATLAB 7.5 (Mathworks Inc., Sherborn, MA, USA) and then overlaid on the MNI brain template from MRIcron (http://www.cabiatl.com/mricro/mricron/index.html; Fig. 1C).
Data analysis
In all experiments, separately for each session, we calculated participants’ performance as the sum of squared errors (SSE), which was calculated on the difference between the subjects’ reported weight, and the weight associated with the stimulus based on Pobric and Hamilton (2006) and Hamilton et al. (2007). SSE was preferred to the R2 measure used in previous studies (e.g. Pobric and Hamilton, 2006) for two reasons. First, by subtracting averages, R2 ignores possible differences in the mean values between reported and stimulus weights, and thus fails to capture systematic shifts in reports. Second, while R2 is good at estimating linear relationships between reported and stimulus weights, it does not necessarily reflect performance. For instance, a participant reporting values of 1 1 1 1 5 to stimuli with assigned values of 1 2 3 4 5, would receive R2=0.5; one reporting 1 2 1 1 5, R2=0.4. Hence, the latter reporting 3 weights correctly would have a performance lower than the former, reporting only 2 weights correctly. The SSE does not suffer from these limitations, as it directly sums the deviations from the stimulus weights (for the two examples above SSE(11115)=14, and SSE(12115)=13). Importantly, all our participants performed the exact same number of trials, which is why sums can be directly compared.
A paired t-test was used in the first pilot study to compare the performance (SSE) in estimating the box weight to that of the bouncing ball. In the second pilot study a Task (hand, ball)×Masks (0%, 15%, 30%) repeated measures ANOVA was used on the SSE (Table 2) to compare the effect the different masks had on the performance in the different conditions.
Table 2.
Average perceived weight and SSE for each % of masking for the box and ball weight estimation task, from the second pilot. Standard error of the mean is indicated within brackets.
|
Box |
Ball |
|||||
|---|---|---|---|---|---|---|
| 0% | 15% | 30% | 0% | 15% | 30% | |
| Mean weight (± s.e.m.) | 3.02 (±0.08) | 3.14 (±0.04) | 3.03 (±0.09) | 2.92 (±0.09) | 2.97 (±0.07) | 3.04 (±0.08) |
| Mean SSE (± s.e.m.) | 45.17 (±3.86) | 53.33 (±3.11) | 59.17 (±2.05) | 46.08 (±3.14) | 52.25 (±3.37) | 59.92 (±3.23) |
The effect of cTBS on our regions of interest, SI, was directly tested by using a paired t-test on the SSE. Site- and task-specificity of the observed effects were then tested by examining the interaction term of a general linear model implementation of mixed models ANOVAs with factor Conditions (SI box, M1 box, SPL box, SI ball) and cTBS (sham, active), i.e. by examining if the cTBS effect on performance was different depending on condition or site. To further understand the interaction, we used two series of Duncan post-hoc tests. The first series, fully exploratory, compared performance after sham vs active cTBS across the four conditions. The second series compared the sham-active contrast across the 4 conditions to test whether the cTBS effect, as measured using the sham-active difference, varied across conditions. This directly follows up on the significant interaction.
To confirm that a parametric test was suited for analyzing our SSE values, we checked for normality. A Lilliefors tests on our SSE differences did not reveal a deviation from normality for any of our condition (all p>0.2). To maximize the power to detect deviation from normality we also pooled all but the SI box conditions (as their means did not differ), but even in this case the Lilliefors test found no evidence to reject normality (p>0.5). We also repeated the normality analysis using the ChiSquare goodness of fit, which confirmed the Lilliefors test results.
The same analyses (paired t-tests and ANOVAs) used for our main outcome measure were performed on other indices of task performance, namely the Pearson R2 (to keep the comparability of current results with that of Pobric and Hamilton (2006)), mean weight estimation values and response times (RTs). Only one participants in Exp. 1 had values above 2 SD for both SSE (SSEP17=125; SSEgroup=67.2±25.38 SD) and RTs (RTP17=2.18; RTgroup=0.63±0.06 SD) during the sham cTBS condition, indicating the possibility of being an outlier. Because the relatively contained sample size for each group, which limits the confidence of identifying outliers, the significance of the ANOVAs are reported first excluding that participant, then including the participant.
Results
Behavioral pilot experiments
In the first pilot experiment a t-test confirmed that performance was indeed comparable in the box (mean SSE±s.e.m.=86.92±8.5) and ball (93.58±14.77) weight estimation tasks (t11<1, p=0.48).
The two conditions (hand, ball)×three masks (0%, 15%, 30%) one-way ANOVA applied to the data from the second pilot experiment (Table 2) showed a main effect of masks, indicating that the task became more difficult as the percentage of mask increased (F2,22=14.55; p<0.0001), independently of whether participants estimated the weight of a box being lifted or a bouncing ball. No other significant effects were observed. In particular, there was no interaction between conditions and masks (F2,22=0.09, p>0.9), suggesting that the box and the ball weight estimation tasks were comparably sensitive to this manipulation.
One may note that performance in the no mask condition of the second pilot study appears greater than performance in the first pilot study. Importantly, this between-experiment difference occurred for both tasks (which in turn did not differ from each other, all p>0.48), and is likely due to the difference in total number of experimental trials (180 experimental trials in Pilot 2, and 60 in Pilot 1) enabling different levels of learning. Such an effect of trial number is compatible with SSE during the four TMS experiments, in which participants performed 120 experimental trials, falling between the first and second pilot. Despite different practice, the two pilot studies confirmed that the box and ball weight estimation tasks were matched for difficulty and were similarly sensitive to visual interference.
TMS experiments: is SI functionally relevant to weight estimation?
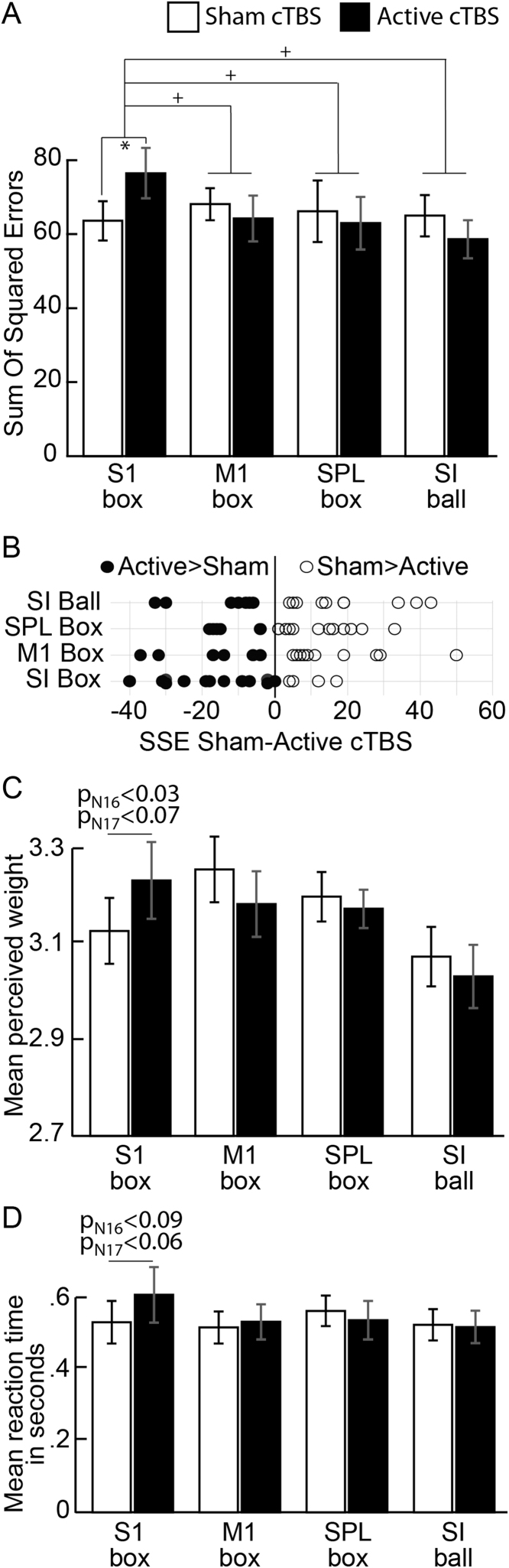
With our experiment, we wanted to test two main questions. First, we tested if SI carries information relevant to deducing the weight of a box from the observation of human actions, which we tested by investigating whether cTBS had an effect in the SI-box condition - i.e. by directly comparing the SSE in the box weight estimation task after sham and active cTBS over SI (Experiment 1). A paired t-test revealed greater SSE values after active relative to sham cTBS and this difference could be detected regardless of whether the outlier participant was included in the analysis or not (N16: t15=−3.35, p=0.004; N17: t16=−2.77, p=0.013; Fig. 2A).
Fig. 2.
For each experiment (SI box, M1 box, SPL box and SI ball weight estimation task) we show: (A) the SSE for the active cTBS (in black) and sham cTBS (white with black contours) sessions (* indicates a significant difference between Sham and Active, + indicates significant difference between the sham-active contrast across experiments; see text for p-values); (B) the distribution of SSE differences (sham – active cTBS) for the four experiments; (C) the mean perceived weights and (D) the mean RTs.
Second, we wanted to know if the observed effect was site- and task-specific. To this aim we performed a 4 condition (SI box, M1 box, SPL box, SI ball)×2 cTBS (Sham vs Active) ANOVA. The ANOVA revealed a significant condition×cTBS interaction (N16: F3,62=3.46, p=0.02; N17: F3,63=2.88, p=0.04; Fig. 2A). Duncan post-hoc tests indicated that the SI box condition (Experiment 1) showed the only significant SSE difference between sham and active cTBS (N16: p=0.02; N17: p=0.04), whereas no difference between sham and active cTBS were found in the other three conditions (Experiment 2–4, all paired wise comparisons p>0.34). Comparable SSE was found across the sham condition of the four experiments (all p>0.88).
Directly comparing sham and active cTBS differences across all conditions confirmed that this difference was significantly larger in the SI box condition (Experiment 1) than in the other three conditions (Experiment 2–4; N16: all p<0.014; N17: all p<0.028) which in turn did not differ from one another (all p>0.84 for N16 and N17). These data indicate a selective reduction of performance in the SI box condition after active cTBS. Fig. 2B shows the distribution of the SSE differences (sham – active cTBS) for the four experiments. While a clear disruption could be observed at the group level, the cTBS effect was variable across participants in the SI box condition (Experiment 1): 11 out of the 17 participants showed larger SSE during active relative to sham cTBS whereas the remaining 6 participants showed an opposite trend although smaller in size (average SSE sham-active cTBS SI box±s.e.m.=−11±4.0). The SI ball (Experiment 4) and M1 box (Experiment 2) conditions showed a distribution of SSE values more centred at zero, with 9 out of 17, and 9 out of 16 participants showing a decrease in SSE during active cTBS respectively (average SSE sham -active cTBS SI ball±s.e.m.=5.29±5.28; average SSE sham-active cTBS M1 box±s.e.m.=3.88±5.6). The other condition, PPC box (Experiment 3), showed greater SSE values during sham compared to active cTBS in 5 out of 17 participants, little or no changes in 3 participants (i.e., abs SSE changes<±3) and greater SSE during sham relative to active cTBS in the remaining participants (average SSE sham-active cTBS PPC box±s.e.m.=4.94±3.76). In sum, there was behavioural variability in the sham and active cTBS session across the four experiments. This was also true for Experiment 1 where not all the participants showed the disruptive effect of SI perturbation that, nonetheless, could be clearly observed at the group level.
In summary, the SSE performance decreased after active cTBS only and most for the SI box condition (Experiment 1), indicating a specific causal role of SI when the weight of an object is deduced from biological motions.
TMS experiments: control analyses
These findings on SSE were confirmed by the condition x cTBS ANOVA performed on another index of task performance, namely the Pearson R2 (as used in Pobric and Hamilton (2006)). This ANOVA showed a condition×cTBS interaction (N16, F3,62=3.03, p=0.036; N17, F3,63=2.6, p=0.054). Duncan post-hoc tests indicated SI box condition as the only condition with a significant R2 difference between sham and active cTBS (N16, p=0.03). No difference was found between sham and active cTBS in the other conditions (all p>0.22). Directly comparing sham and active cTBS differences across all conditions using the same Duncan post-hoc procedure confirmed that this difference was significantly larger in the SI box condition than in the other three conditions (N16: p<0.031) which in turn did not differ from one another (all p>0.6). As expected, the SSE and R2 measures were highly and inversely correlated across the four conditions and 2 types of cTBS (all r>.86, all p<.001). Moreover, in Experiment 1, changes in SSE (sham – active) significantly correlated with changes in R2 (r>−.74, p<.001), suggesting that disruption of performance could be similarly detected with the two indices of performance.
To further explore whether the increase of errors in the SI box condition (Experiment 1) was due to a systematic change in perceived weight, or a reduction in the reliability of estimation, we also compared the mean estimated box weight after sham cTBS (mean weight±s.e.m: 3.13±0.07 for both N16 and N17) and active cTBS (3.24±0.08 for N16; and 3.22±0.08 for N17) in the SI box condition (paired t-test, for N16: p=0.027, and for N17: p=0.074), suggesting a tendency for perceiving greater weight after active cTBS over SI (Fig. 2C). No sham-active cTBS differences in box weight estimation was found in the other conditions (all p<.40). However, the condition×cTBS ANOVA did not show significant main effects or interaction (N16: all F<2.05, all p>0.11; N17: all F<1.89, all p>0.14), suggesting that changes in the mean estimated weight were less consistent than changes in SSE. Further t-tests suggested that in all conditions participants tended to overestimate the box weight (Supplementary Table 1). Moreover, in Experiment 1, changes in SSE (sham-active) correlated with changes in the estimated mean box weight (r=.53, p=0.028). Thus, in Experiment 1, the disruption of performance induced by active cTBS over SI was at least in part due to an increased bias toward weight overestimation (a bias that was present in all groups but increased only after SI perturbation).
The ANOVA on RTs during sham and active cTBS (Fig. 2D) did not show a significant condition×cTBS interaction (N16: F3,62=1.11, p=0.35; N17: F3,63=1.3, p=0.28) suggesting that active cTBS over SI impaired the accuracy in the weight estimation of observed lifted box and not the speed of the response (as measured by RT). The direct comparison between the mean RTs for the sham (mean RTs±s.e.m.: N16: 514 ms±60; N17: 632 ms±112) and active cTBS (N16: 601 ms±81; N17: 714 ms±125) conditions showed a non-significant trend towards an increase of RTs after active cTBS in the SI box condition (paired t-test, N16: p=0.09; N17: p=0.06). No similar trend was observed in the other conditions (all p>0.64). Importantly, that cTBS in the SI box condition reduced accuracy (significantly) and increased RTs (marginally), speaks against the possibility of a speed-accuracy trade-off. Indeed, there was also a significant correlation between changes (sham-active) in SSE and RTs (r=0.59, p=0.012), suggesting that in Experiment 1 greater impairments in accuracy induced by SI cTBS were associated with slower response.
Discussion
Our results show that, compared to sham stimulation, active cTBS perturbation of SI selectively worsened participant's ability to estimate the weight of a box when seen lifted (Experiment 1). In contrast, participants’ performance after active cTBS remained comparable to sham stimulation when (i) participants judged the weight of a bouncing ball (Experiment 4), and (ii) stimulation was applied over the adjacent M1 (Experiment 2) or (iii) SPL (Experiment 3). Notably, disruption of performance after active cTBS over SI consisted of a clear reduction in the accuracy of estimations in the box weight, with an increased tendency for weight overestimation and slower responses. This suggests SI is a critical part of a system of brain regions sub-serving weight estimation when a human agent is involved, and supports the idea that SI may enrich action understanding by providing vicarious representations of the proprioceptive consequences of the observed actions (Gazzola et al., 2007a, Gazzola et al., 2007b, Avenanti et al., 2007, Raos et al., 2007, Caspers et al., 2010, Keysers et al., 2010, McGregor et al., 2016, Valchev et al., 2016).
This extends the network of brain regions necessary for optimal action perception, as evidence for necessity was so far mostly restricted to the IFC and IPL, as shown by TMS (Pobric and Hamilton, 2006, Urgesi et al., 2007, Cattaneo, 2010, Cattaneo et al., 2010, Cattaneo et al., 2011, Tidoni et al., 2013, Avenanti et al., 2013b, Jacquet and Avenanti, 2015), transcranial direct current stimulation (Avenanti et al., 2017), and neurological lesion studies (Tranel et al., 2003, Battelli et al., 2003, Saygin et al., 2004, Buxbaum et al., 2005, Saygin, 2007, Pazzaglia et al., 2008, Weiss et al., 2008, Fazio et al., 2009, Kalénine et al., 2010, Urgesi et al., 2014).
If the effect of cTBS over SI were not the result of a perturbation of SI but of a spread of the magnetic impulse onto nearby motor or parietal regions, moving the coil forward or backwards should increase rather than decrease its detrimental effects on perception. This was not the case, supporting the conclusion that the effect was indeed mediated by SI. However, imaging and neurophysiological studies show SI does not work in isolation during action observation, but is rather part of an entire network composed of ventral and dorsal premotor, anterior and posterior parietal cortices activated in both action observation and execution (Avikainen et al., 2002, Rossi et al., 2002, Hasson et al., 2004, Caetano et al., 2007, Gazzola et al., 2007a, Gazzola et al., 2007b, Raos et al., 2007, Kilner et al., 2009, Pierno et al., 2009, Caspers et al., 2010, Arnstein et al., 2011, Turella et al., 2012). Indeed, we found that cTBS over SI alters brain activity in the premotor cortices (Valchev et al., 2015a, Valchev et al., 2016), known also to contribute to weight estimation (Hamilton et al., 2006, Pobric and Hamilton, 2006). Accordingly, the current results should not be interpreted to suggest that the impairment of performance reflects cTBS-induced changes of activity in SI alone, but rather that disrupting SI activity using cTBS is likely to have disrupted the functioning of a somatosensory-motor, parieto-frontal network of which SI is an active element.
Given the importance of both IFC (Pobric and Hamilton, 2006) and SI (this paper) to weight estimation by observation, as well as the exchange of information between these regions during action observation (Kokal and Keysers, 2010, Schippers and Keysers, 2011, Valchev et al., 2016, McGregor et al., 2016), it is relevant to consider what the roles of each region may be. TMS studies show that seeing biomechanically possible and extremely overstretching movements facilitates the corticospinal representation of the muscles involved in the observed movements (Romani et al., 2005). Notably, rTMS over IFC disrupted motor facilitation during the observation of possible actions, while rTMS over SI disrupted the facilitation during observation of overstretching movements (Avenanti et al., 2007, Avenanti et al., 2013a). The IFC could therefore provide primarily vicarious motor representations derived from the kinematics that would enable the observer to produce a similar action, if the movement is biomechanically possible. SI, on the other side, could primarily contribute vicarious somatosensory (tactile and/or proprioceptive) action components, that emerge for instance during observation of overstretching finger movements. The contribution of SI in mapping somatosensory consequences of observed actions is supported by the findings that SI activity is increased when seeing other people grasping or manipulating objects (Keysers et al., 2010) or when seeing extreme joint stretching movements (Costantini et al., 2005). Evidence that somatosensory cortices are recruited both when sensing the body and during perception of others being touched or painfully stimulated (Bufalari et al., 2007, Valeriani et al., 2008, Keysers et al., 2010; Lamm et al., 2011), and that rTMS over SI impairs the ability to detect touch in others (Bolognini et al., 2011, Bolognini et al., 2014) further supports this interpretation.
While manipulation of biomechanical plausibility may dissociate somatosensory and motor components during action observation, typically these two components are tightly interlinked. This is particularly evident when observing somebody else lifting objects. Recently, Alaerts et al. (2010) found that when participants observe an actor lift objects of different weights, motor-evoked potentials are facilitated mainly by two factors: the kinematics of the movement and the degree of contraction of the hand (see also Tidoni et al. (2013) and Valchev et al. (2015b)). This facilitation could be the result of the integration in M1 of motor plans inferred via IFC and proprioceptive/tactile information inferred via SI. In our experiments, as the stimuli were generated by modifying kinematics alone, the source of somatosensory information has to be kinematic. Importantly, we propose that SI main contribution to action perception relates to the extraction of proprioceptive/tactile information derived from observed action kinematics, rather than to encoding action kinematics per se. On the other hand, observed kinematics is likely processed in other (visual and/or motor) brain regions including the IFC. This proposal fits with previous studies showing impaired recognition of action kinematics following lesion or interference with the IFC (Avenanti et al., 2013b, Urgesi et al., 2014), and with the recent study of Jacquet and Avenanti (2015) showing that visual discrimination of observed hand kinematics (i.e., grip aperture) was disrupted by IFC but not SI online TMS interference.
Our study supports the notion that SI provides a vicarious somatosensory representation of seen actions and this representation is necessary for accurate performance in the box weight estimation task. However, we argue that the contribution of SI to action understanding may be more general. Indeed, studies using causal methods have shown that SI is critical for recognizing the facial or vocal emotional expressions of others (Adolphs et al., 2000, Pitcher et al., 2008, Banissy et al., 2010, Paracampo et al., 2016). While the TMS adaptation study of Jacquet and Avenanti (2015) suggested no critical role of SI for accurate perception of action kinematics, the same study also highlighted a state-dependent effect of SI (and IFC) stimulation in a task requiring to discriminate between action goals. TMS pulses over SI and IFC hand representations (but not over control regions) facilitated the recognition of test pictures showing a repeated (adapted) action goal, regardless of the kinematics used to perform the action (Jacquet and Avenanti, 2015). These state-dependent effects suggested a causal role of SI and IFC in the encoding of action goal. Remarkably, this role is not in contrast with the proposal that SI and IFC may mirror somatosensory and motor components of observed actions, respectively (Gazzola et al., 2007; Avenanti et al., 2007; Keysers et al., 2010; McGregor et al., 2016). Indeed, goal processing may involve the prediction of both motor and somatic afferent action components which could be processed in partially separated networks (Christensen et al., 2007, Etzel et al., 2008, Gazzola and Keysers, 2009).
Our study significantly expands this previous evidence by showing that disruption of SI with active cTBS impairs performance in the box weight estimation task (Experiment 1), and this provides strong evidence that SI is causally essential for accurate action perception. On the other hand, active cTBS over SI did not affect the ability to judge the weight of a bouncing ball (Experiment 4). The selectivity of the cTBS disruption cannot be accounted by a difference in the difficulty between the box and ball weight estimation tasks. Indeed, the two tasks were matched for difficulty (as shown in pilot study 1 and 2) and presented similar sensitivity to external (visual masking) interference (as shown in pilot study 2). Thus, the selective interference with box weight estimation by SI perturbation cannot be accounted by confounding effects like a different difficulty of the two tasks and rather suggests that SI is critically involved in inferring somatosensory qualities (i.e., light/heavy) from human actions and not from non-human motion.
In all the conditions, participants tended to slightly overestimate the ‘correct’ weight in the box weight estimation task. Interestingly, active cTBS over SI tended to further increase such overestimation relative to sham cTBS, suggesting that the disruption in accuracy was in part due to a tendency to perceive the weight of the box as heavier. Thus, altered signals in SI may make people less accurate in judging the weight of lifted objects, by biasing their weight judgment. Again, these effects were specific for human action as no weight bias was induced in the ball estimation task. Notably, a contribution of SI, IFC and other sensorimotor regions to perceiving the weight of objects seen to be lifted was suggested by previous studies showing that: i) lifting a box influences participant's perceptual judgments of the weight of a box lifted by others (Hamilton et al., 2004); and, ii) the strength of this perceptual bias correlated with neural activity in a network of cortical regions including SI, IFC, M1 and SPL (Hamilton et al., 2006). However, these methods could not establish whether activity in SI was necessary for accurate action perception. While previous evidence showed that IFC is necessary for correct performance in the box weight estimation task (Pobric and Hamilton, 2006), the present study provides the first causative evidence that also SI, but not M1 or SPL, is critical for the social perception of weight.
The lack of a significant effect with M1 stimulation (Experiment 2) is not surprising. First, although SI and M1 are anatomically close and highly interconnected, there is physiological and behavioral evidence that the aftereffect of SI and M1 cTBS can dissociate (Ishikawa et al., 2007, Mochizuki et al., 2007, Ragert et al., 2008, Schabrun et al., 2008). Second, although neural activity in M1 may be modulated by action observation (Nishitani and Hari, 2000, Fadiga et al., 2005, Caetano et al., 2007; Schütz–Bosbach et al., 2009; Gazzola and Keysers, 2009; Vigneswaran et al., 2013), it is debated whether such activity might play a major role in action perception (Lepage et al., 2008, Pineda, 2008, Bonini, 2016). On the one hand, the activity may be a simple downstream consequence of the strong reciprocal cortico-cortical connections, for example with IFC and/or SI (Geyer et al., 2000, Rizzolatti and Luppino, 2001). Similarly, previous TMS studies reported that offline inhibition of M1 excitability did not influence mirror-like motor facilitation (Avenanti et al., 2007), and online M1 interference did not affect perceptual judgments of seen actions (Cattaneo et al., 2011), whereas these processes were affected by stimulation of IFC or SI (Pobric and Hamilton, 2006; Avenanti et al., 2007; Cattaneo et al., 2011; Jacquet and Avenanti, 2015; and present study). On the other hand, a few recent studies have documented a disruption of effector recognition (Naish et al., 2016) or body posture recognition (Borgomaneri et al., 2015) after online TMS of the hand representation in M1. However, online M1 stimulation may cause peripheral motor responses and the lack of control area eliciting similar responses in a different body part or the lack of control task for assessing nonspecific, distracting effects of online TMS makes the interpretation of such studies not conclusive. Recently, Palmer et al. (2016) found no net change in action perception following offline cTBS over M1, a null result that is in keeping with the present data. However, Palmer et al. (2016) also showed that the physiological effect of cTBS was variable across participants, leading to inhibitions of M1 excitability in some and increases in other participants. Interestingly, participants showing M1 inhibition after cTBS also showed a clear disruptive effect on action perception. It should be noted that also in our data, the cTBS effect was variable across participants. Variability was observed in all the experiments, including Experiment 2 (M1 box condition) where approximately half of the participants showed improved performance and the remaining participants showed decreased performance, thus resembling the proportion observed by Palmer et al. (2016). Thus, although current and previous data suggest no major action perception impairment when M1 is stimulated, further studies combining cTBS with physiological and behavioral assessment might test whether effective inhibition of M1 affects performance in the box weight estimation task. Nerveless, the inconsistency in the effect of M1 stimulation is at variance with the disruption we found after SI cTBS (Experiment 1). Although also in this case cTBS effects were variable, a clear reduction of performance was observed at the group level, suggesting that SI may be more important than M1 for estimating weight from seen actions.
The absence of effects after rTMS over SPL (Experiment 3) may be less expected. The SPL is a high-order multisensory region integrating visual and somatosensory information about limb position (Lloyd et al., 2002). Direct stimulation of SPL (area 7) in awake neurosurgery patients produces sensations on the body but not motor output (Desmurget et al., 2009). Moreover, rTMS over this region impairs performance in proprioceptive tasks, although less than rTMS over SI (Balslev et al., 2004). Also, studies show activation in SPL both during action execution and observation (Raos et al., 2007, Keysers and Gazzola, 2009) and that the effect of cTBS over SI spreads to SPL (Valchev et al., 2016), suggesting a possible role of SPL in action perception. However, during action observation this region is less consistently activated relative to other sectors of the parietal cortex (Van Overwalle and Baetens, 2009). It may thus be that SPL (and in particular area 7, the target of Experiment 3), plays a role in action perception that is relatively minor relative to nearby parietal regions, including SI and IPL that appears more critical for action perception (Cattaneo et al., 2010, Urgesi et al., 2014, Jacquet and Avenanti, 2015).
Ours is one of the first studies showing that offline transcranial stimulation can affect action perception. Indeed, most of previous studies investigating action perception implemented online rTMS protocols that induce distracting scalp sensations and auditory stimulations (i.e., the coil click) during task performance. Offline protocols overcome these confounding effects and provide insights into the plasticity of the targeted areas. In a first study, Michael et al. (2014) showed that cTBS over the hand and mouth representations of the IFC slowed recognition of hand and mouth actions, respectively. More recently, Avenanti et al. (2017) showed that cathodal (inhibitory) and anodal (excitatory) transcranial direct currents over IFC hindered and enhanced accuracy in an action prediction task, while leaving unaffected performance in a non-human motion prediction task. Our study expands on this previous evidence by showing that offline transcranial stimulation affects action perception not only when it is administered over frontal motor areas, but also when SI is targeted.
Yet, it should be noted that the use of offline protocols limits the number of conditions that can be tested within a single session. Indeed, cTBS can affect cortical excitability for up to 50 min, but most of the after-effects likely occur between 5 and 30 min after stimulation (Huang et al., 2005, Huang et al., 2011; Franca et al., 2006; Bertini et al., 2010; Wischnewski and Schutter, 2015). Because of these temporal constraints, we opted for having the critical sham vs. active cTBS factor as the only within-subjects factor. We did not systematically test performance at both experimental and control tasks across the three tested areas (SI, M1 and PPC). Rather, we implemented the control task (ball weight estimation) in a separate experiment (Experiment 4) and limited this experimental control to the only area (SI) that showed an effect on action perception across Experiment 1–3.
In our study, we tilted the TMS coil by 90° during sham cTBS. Although participants could have noted different scalp sensations between sham and active cTBS, the selectivity of our findings cannot be simply accounted for by such perceived differences, as participants, who were naïve to TMS, cannot predict whether the cTBS effect is supposed to increase, decrease or leave brain activity unaltered. That we observed a change in performance in Experiment 1 but not in Experiments 2–4, despite the fact that differences in scalp sensations between sham and active cTBS would have been shared across all four experiments, makes such unspecific sensations explanation of our data unlikely.
Our study does not clarify whether the observed decrease in performance caused by cTBS on SI was driven by excitatory or inhibitory modulations. The effect of cTBS is a complex combination of suppression and excitation (Gentner et al., 2008; Huang et al., 2011; Iezzi et al., 2008, Iezzi et al., 2011) and may be highly variable across individuals (Hamada et al., 2012; Ridding and Ziemann, 2010; Jones et al., 2016). Indeed, we showed, using fMRI, that cTBS over SI leads to reductions in BOLD responses to the observation of actions in some and increases in response in other participants (Valchev et al., 2016). To minimize interindividual variability, in the present research we limited motor activity of participants’ right hand (i.e., the hand contralateral to the target sites and corresponding to the actor's hand depicted in the movies) before and after cTBS, possibly leading to a consistent suppression of neural activity in the stimulated sites (Huang et al., 2005, Ishikawa et al., 2007, Poreisz et al., 2008). However, even if we observed a disruptive effect at the group level, the effect of SI cTBS was variable across participants of Experiment 1 and not all showed a disruption (see Jones et al., 2016). Additionally, we do not rule out that either increases or decreases of neural activity could move brain activity away from its optimal state, and thereby could reduce behavioural accuracy. Future studies combining brain stimulation and brain imaging techniques will directly address these issues and clarify the relationship between physiological and behavioural effects induced by SI cTBS.
In conclusion, earlier evidence supported the claim that somatosensory cortices are activated not only during action execution, but also during perception of others’ actions, but whether such activation of SI is necessary to efficiently judge the somatosensory aspects of the actions of others remained unclear (Gazzola et al., 2007b, Pernigo et al., 2012, Vannuscorps and Caramazza, 2015). Indirect evidence came from sensory neuropathy patients that lack a sense of touch on their own body. These patients showed impaired performance in a task requiring weight estimation from lifting actions (Miall et al., 2000) and inference of another's expectation of a weight when seeing him lifting a box (Bosbach et al., 2005). Our findings, that cTBS over SI negatively influences the capacity to judge the weight of a box by observing the action (lifting) of other people, now provides direct evidence that SI is a crucial part of a system of brain areas necessary for the optimal perception of at least certain aspects of other people's hand actions. Together with evidence that SI is also critical for recognizing the facial expressions of others (Adolphs et al., 2000, Pitcher et al., 2008, Banissy et al., 2010, Paracampo et al., 2016) and encoding action goals (Jacquet and Avenanti, 2015), this suggests that SI seems to play a more important role in action understanding than previously thought.
Fundings
The work was supported by a grant from the Portuguese Foundation for Science and Technology SFRH/BD/47576/2008 to N.V.; a grant from the European Research Council of the European Commission (ERC consolidator grant INTERACT 313398) to A.H.; grants from the Netherlands Organisation for Scientific Research (VENI 451-09-006, VIDI 452-14-015) to V.G.; and grants from the Ministero della Salute (GR-2010-2319335), Cogito Foundation (R-117/13; and 14-139-R), Ministero Istruzione, Università e Ricerca (RBFR12F0BD) and BIAL Foundation (298/16) awarded to A.A.
Conflict of interest
The authors declare that they have no conflicts of interest for this study.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.neuroimage.2017.02.075.
Contributor Information
Valeria Gazzola, Email: v.gazzola@nin.knaw.nl.
Alessio Avenanti, Email: alessio.avenanti@unibo.it.
Appendix A. Supplementary material
Supplementary material
.
References
- Adolphs R., Damasio H., Tranel D., Cooper G., Damasio A.R. A role for somatosensory cortices in the visual recognition of emotion as revealed by three–dimensional lesion mapping. J. Neurosci. 2000;20:2683–2690. doi: 10.1523/JNEUROSCI.20-07-02683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaerts K., Swinnen S.P., Wenderoth N. Observing how others lift light or heavy objects: which visual cues mediate the encoding of muscular force in the primary motor cortex? Neuropsychologia. 2010;48:2082–2090. doi: 10.1016/j.neuropsychologia.2010.03.029. [DOI] [PubMed] [Google Scholar]
- Arnstein D., Cui F., Keysers C., Maurits N.M., Gazzola V. μ–suppression during action observation and execution correlates with BOLD in dorsal premotor, inferior parietal, and SI cortices. J Neurosci. 2011;31:14243–14249. doi: 10.1523/JNEUROSCI.0963-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avenanti A., Bolognini N., Maravita A., Aglioti S.M. Somatic and motor components of action simulation. Curr. Biol. 2007;17:2129–2135. doi: 10.1016/j.cub.2007.11.045. [DOI] [PubMed] [Google Scholar]
- Avenanti A., Annella L., Candidi M., Urgesi C., Aglioti S.M. Compensatory plasticity in the action observation network: virtual lesions of STS enhance anticipatory simulation of seen actions. Cereb. Cortex. 2013;23:570–580. doi: 10.1093/cercor/bhs040. [DOI] [PubMed] [Google Scholar]
- Avenanti A., Candidi M., Urgesi C. Vicarious motor activation during action perception: beyond correlational evidence. Front. Hum. Neurosci. 2013;7:185. doi: 10.3389/fnhum.2013.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avenanti A., Paracampo R., Annella L., Aglioti S.M. Boosting and decreasing action prediction abilities through excitatory and inhibitory tDCS of inferior frontal cortex. Cereb. Cortex. 2017:1–15. doi: 10.1093/cercor/bhx041. (Advance Article) [DOI] [PubMed] [Google Scholar]
- Avikainen S., Forss N., Hari R. Modulated activation of the human SI and SII cortices during observation of hand actions. Neuroimage. 2002;15:640–646. doi: 10.1006/nimg.2001.1029. [DOI] [PubMed] [Google Scholar]
- Azañón E., Haggard P. Somatosensory processing and body representation. Cortex. 2009;45:1078–1084. doi: 10.1016/j.cortex.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Aziz-Zadeh L., Maeda F., Zaidel E., Mazziotta J., Iacoboni M. Lateralization in motor facilitation during action observation: a TMS study. Exp. Brain Res. 2002;144:127–131. doi: 10.1007/s00221-002-1037-5. [DOI] [PubMed] [Google Scholar]
- Balslev D., LOD Christensen, Lee J.H., Law I., Paulson O.B., Miall R.C. Enhanced accuracy in novel mirror drawing after repetitive transcranial magnetic stimulation–induced proprioceptive deafferentation. J. Neurosci. 2004;24:9698–9702. doi: 10.1523/JNEUROSCI.1738-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banissy M.J., Sauter D.A., Ward J., Warren J.E., Walsh V., Scott S.K. Suppressing sensorimotor activity modulates the discrimination of auditory emotions but not speaker identity. J. Neurosci. 2010;30:13552–13557. doi: 10.1523/JNEUROSCI.0786-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battelli L., Cavanagh P., Thornton I.M. Perception of biological motion in parietal patients. Neuropsychologia. 2003;41:1808–1816. doi: 10.1016/s0028-3932(03)00182-9. [DOI] [PubMed] [Google Scholar]
- Bertini C., Leo F., Avenanti A., Ladavas E. Independent mechanisms for ventriloquism and multisensory integration as revealed by theta–burst stimulation. Eur. J. Neurosci. 2010;10:1791–1799. doi: 10.1111/j.1460-9568.2010.07200.x. [DOI] [PubMed] [Google Scholar]
- Bolognini N., Rossetti A., Maravita A., Miniussi C. Seeing touch in the somatosensory cortex: a TMS study of the visual perception of touch. Hum. Brain Mapp. 2011;32:2104–2114. doi: 10.1002/hbm.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognini N., Rossetti A., Convento S., Vallar G. Understanding others' feelings: the role of the right primary somatosensory cortex in encoding the affective valence of others' touch. J. Neurosci. 2013;33:4201–4205. doi: 10.1523/JNEUROSCI.4498-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognini N., Rossetti A., Fusaro M., Vallar G., Miniussi C. Sharing social touch in the primary somatosensory cortex. Curr. Biol. 2014;24:1513–1517. doi: 10.1016/j.cub.2014.05.025. [DOI] [PubMed] [Google Scholar]
- Bonini L. The extended mirror neuron network: anatomy, origin, and functions. Neuroscientist. 2016:8. doi: 10.1177/1073858415626400. (pii: 1073858415626400) [DOI] [PubMed] [Google Scholar]
- Borgomaneri S., Gazzola V., Avenanti A. Transcranial magnetic stimulation reveals two functionally distinct stages of motor cortex involvement during perception of emotional body language. Brain Struct. Funct. 2015;220:2765–2781. doi: 10.1007/s00429-014-0825-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosbach S., Cole J., Prinz W., Knoblich G. Inferring another's expectation from action: the role of peripheral sensation. Nat. Neurosci. 2005;8:1295–1297. doi: 10.1038/nn1535. [DOI] [PubMed] [Google Scholar]
- Brett M., Anton J.L., Valabregue R., Poline J.B. Region of interest analysis using an SPM toolbox. Neuroimage. 2002;16:1140–1141. [Google Scholar]
- Bufalari I., Aprile T., Avenanti A., Di Russo F., Aglioti S.M. Empathy for pain and touch in the human somatosensory cortex. Cereb. Cortex. 2007;17:2553–2561. doi: 10.1093/cercor/bhl161. [DOI] [PubMed] [Google Scholar]
- Buxbaum L.J., Kyle K.M., Menon R. On beyond mirror neurons: internal representations subserving imitation and recognition of skilled object–related actions in humans. Cogn. Brain Res. 2005;25:226–239. doi: 10.1016/j.cogbrainres.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Cabinio M., Blasi V., Borroni P., Montagna M., Iadanza A., Falini A., Cerri G. The shape of motor resonance: right- or left-handed? Neuroimage. 2010;51:313–323. doi: 10.1016/j.neuroimage.2010.01.103. [DOI] [PubMed] [Google Scholar]
- Caetano G., Jousmäki V., Hari R. Actor's and observer's primary motor cortices stabilize similarly after seen or heard motor actions. Proc. Natl. Acad. Sci. 2007;104:9058–9062. doi: 10.1073/pnas.0702453104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candidi M., Urgesi C., Ionta S., Aglioti S.M. Virtual lesion of ventral premotor cortex impairs visual perception of biomechanically possible but not impossible actions. Soc. Neurosci. 2008;3(3-4):388-–3400. doi: 10.1080/17470910701676269. [DOI] [PubMed] [Google Scholar]
- Carducci F., Brusco R. Accuracy of an individualized MR–based head model for navigated brain stimulation. Psychiatry Res. 2012;203:105–108. doi: 10.1016/j.pscychresns.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Casile A. Mirror neurons (and beyond) in the macaque brain: an overview of 20 years of research. Neurosci. Lett. 2013;540:3–14. doi: 10.1016/j.neulet.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Caspers S., Zilles K., Laird A.R., Eickhoff S.B. ALE meta–analysis of action observation and imitation in the human brain. Neuroimage. 2010;50:1148–1167. doi: 10.1016/j.neuroimage.2009.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo L. Tuning of ventral premotor cortex neurons to distinct observed grasp types: atms–priming study. Exp. Brain Res. 2010;207:165–172. doi: 10.1007/s00221-010-2454-5. [DOI] [PubMed] [Google Scholar]
- Cattaneo L., Sandrini M., Schwarzbach J. State–dependent TMS reveals a hierarchical representation of observed acts in the temporal, parietal and premotor cortices. Cereb. Cortex. 2010;20:2252–2258. doi: 10.1093/cercor/bhp291. [DOI] [PubMed] [Google Scholar]
- Cattaneo L., Barchiesi G., Tabarelli D., Arfeller C., Sato M., Glenberg A.M. One's motor performance predictably modulates the understanding of others' actions through adaptation of premotor visuo–motor neurons. Soc. Cogn. Affect. Neurosci. 2011;6:301–310. doi: 10.1093/scan/nsq099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouinard P.A., Large M.E., Chang E.C., Goodale M.A. Dissociable neural mechanisms for determining the perceived heaviness of objects and the predicted weight of objects during lifting: an fMRI investigation of the size–weight illusion. Neuroimage. 2009;44:200–212. doi: 10.1016/j.neuroimage.2008.08.023. [DOI] [PubMed] [Google Scholar]
- Christensen M.S., Lundbye–Jensen J., Geertsen S.S., Petersen T.H., Paulson O.B., Nielsen J.B. Premotor cortex modulates somatosensory cortex during voluntary movements without proprioceptive feedback. Nat. Neurosci. 2007;10:417–419. doi: 10.1038/nn1873. [DOI] [PubMed] [Google Scholar]
- Costantini M., Galati G., Ferretti A., Caulo M., Tartaro A., Romani G.L., Aglioti S.M. Neural systems underlying observation of humanly impossible movements: an fMRI study. Cereb. Cortex. 2005;15:1761–1767. doi: 10.1093/cercor/bhi053. [DOI] [PubMed] [Google Scholar]
- Desmurget M., Reilly K.T., Richard N., Szathmari A., Mottolese C., Sirigu A. Movement intention after parietal cortex stimulation in humans. Science. 2009;324:811–813. doi: 10.1126/science.1169896. [DOI] [PubMed] [Google Scholar]
- di Pellegrino G., Fadiga L., Fogassi L., Gallese V., Rizzolatti G. Understanding motor events: a neurophysiological study. Exp. Brain Res. 1992;91:176–180. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- Eidenmüller S., Randerath J., Goldenberg G., Li Y., Hermsdörfer J. The impact of unilateral brain damage on anticipatory grip force scaling when lifting everyday objects. Neuropsychologia. 2014;61:222–234. doi: 10.1016/j.neuropsychologia.2014.06.026. [DOI] [PubMed] [Google Scholar]
- Etzel J.A., Gazzola V., Keysers C. Testing simulation theory with cross–modal multivariate classification of fMRI data. PLoS One. 2008;3:e3690. doi: 10.1371/journal.pone.0003690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadiga L., Craighero L., Olivier E. Human motor cortex excitability during the perception of others' action. Curr. Opin. Neurobiol. 2005;15:213–218. doi: 10.1016/j.conb.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Fazio P., Cantagallo A., Craighero L., D’Ausilio A., Roy A.C., Pozzo T., Calzolari F., Granieri E., Fadiga L. Encoding of human action in broca's area. Brain. 2009;132:1980–1988. doi: 10.1093/brain/awp118. [DOI] [PubMed] [Google Scholar]
- Fiorio M., Haggard P. Viewing the body prepares the brain for touch: effects of TMS over somatosensory cortex. Eur. J. Neurosci. 2005;22:773–777. doi: 10.1111/j.1460-9568.2005.04267.x. [DOI] [PubMed] [Google Scholar]
- Fogassi L., Ferrari P.F., Gesierich B., Rozzi S., Chersi F., Rizzolatti G. Parietal lobe: from action organization to intention understanding. Science. 2005;308:662–667. doi: 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- Franca M., Koch G., Mochizuki H., Huang Y.Z., Rothwell J.C. Effects of theta burst stimulation protocols on phosphene threshold. Clin. Neurophysiol. 2006;117:1808–1813. doi: 10.1016/j.clinph.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Gallese V., Fadiga L., Fogassi L., Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119:593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- Gazzola V., Keysers C. The observation and execution of actions share motor and somatosensory voxels in all tested subjects: single subject analyses of unsmoothed fMRI data. Cereb. Cortex. 2009;19:1239–1255. doi: 10.1093/cercor/bhn181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzola V., Rizzolatti G., Wicker B., Keysers C. The anthropomorphic brain: the mirror neuron system responds to human and robotic actions. Neuroimage. 2007;35:1674–1684. doi: 10.1016/j.neuroimage.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Gazzola V., van der Worp H., Mulder T., Wicker B., Rizzolatti G., Keysers C. Aplasics born without hands mirror the goal of hand actions with their feet. Curr. Biol. 2007;17:1235–1240. doi: 10.1016/j.cub.2007.06.045. [DOI] [PubMed] [Google Scholar]
- Gentner R., Wankerl K., Reinsberger C., Zeller D., Classen J. Depression of human corticospinal excitability induced by magnetic theta-burst stimulation: evidence of rapid polarity-reversing metaplasticity. Cereb. Cortex. 2008;18:2046–2053. doi: 10.1093/cercor/bhm239. [DOI] [PubMed] [Google Scholar]
- Geyer S., Schormann T., Mohlberg H., Zilles K. Areas 3a, 3b, and 1 of human primary somatosensory cortex: 2. spatial normalization to standard anatomical space. Neuroimage. 2000;11:684–696. doi: 10.1006/nimg.2000.0548. [DOI] [PubMed] [Google Scholar]
- Grosbras M.-H., Beaton S., Eickhoff S.B. Brain regions involved in human movement perception: a quantitative voxel-based meta-analysis. Hum. Brain Mapp. 2012;33:431–454. doi: 10.1002/hbm.21222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada M., Murase N., Hasan A., Balaratnam M., Rothwell J.C. The role of interneuron networks in driving human motor cortical plasticity. Cereb. Cortex. 2013;23:1593–1605. doi: 10.1093/cercor/bhs147. [DOI] [PubMed] [Google Scholar]
- Hamilton A., Wolpert D., Frith U. Your own action influences how you perceive another person's action. Curr. Biol. 2004;14:493–498. doi: 10.1016/j.cub.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Hamilton A., Wolpert D.M., Frith U., Grafton S.T. Where does your own action influence your perception of another person's action in the brain? Neuroimage. 2006;29:524–535. doi: 10.1016/j.neuroimage.2005.07.037. [DOI] [PubMed] [Google Scholar]
- Hamilton A., Joyce D., Flanagan J., Frith C., Wolpert D. Kinematic cues in perceptual weight judgement and their origins in box lifting. Psychol. Res. 2007;71:13–21. doi: 10.1007/s00426-005-0032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J.A., Miniussi C., Harris I.M., Diamond M.E. Transient storage of a tactile memory trace in primary somatosensory cortex. J. Neurosci. 2002;22:8720–8725. doi: 10.1523/JNEUROSCI.22-19-08720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U., Nir Y., Levy I., Fuhrmann G., Malach R. Intersubject synchronization of cortical activity during natural vision. Science. 2004;303:1634–1640. doi: 10.1126/science.1089506. [DOI] [PubMed] [Google Scholar]
- Hihara S., Taoka M., Tanaka M., Iriki A. Visual responsiveness of neurons in the secondary somatosensory area and its surrounding parietal operculum regions in awake macaque monkeys. Cereb. Cortex. 2015;25(11):4535–4550. doi: 10.1093/cercor/bhv095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holle H., Banissy M.J., Ward J. Functional and structural brain differences associated with mirror-touch synaesthesia. Neuroimage. 2013;83:1041–1050. doi: 10.1016/j.neuroimage.2013.07.073. [DOI] [PubMed] [Google Scholar]
- Huang Y.Z., Edwards M.J., Rounis E., Bhatia K.P., Rothwell J.C. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Huang Y.-Z., Rothwell J.C., Chen R.-S., Lu C.-S., Chuang W.-L. The theoretical model of theta burst form of repetitive transcranial magnetic stimulation. Clin. Neurophysiol. 2011;122:1011–1018. doi: 10.1016/j.clinph.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iezzi E., Conte A., Suppa A., Agostino R., Dinapoli L., Scontrini A., Berardelli A. Phasic voluntary movements reverse the aftereffects of subsequent theta–burst stimulation in humans. J. Neurophysiol. 2008;100:2070–2076. doi: 10.1152/jn.90521.2008. [DOI] [PubMed] [Google Scholar]
- Iezzi E., Suppa A., Conte A., Voti P.L., Bologna M., Berardelli A. Short‐term and long‐term plasticity interaction in human primary motor cortex. Eur. J. Neurosci. 2011;33:1908–1915. doi: 10.1111/j.1460-9568.2011.07674.x. [DOI] [PubMed] [Google Scholar]
- Ishikawa S., Matsunaga K., Nakanishi R., Kawahira K., Murayama N., Tsuji S., Huang Y.Z., Rothwell J.C. Effect of theta burst stimulation over the human sensorimotor cortex on motor and somatosensory evoked potentials. Clin. Neurophysiol. 2007;118:1033–1043. doi: 10.1016/j.clinph.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Jacquet P.O., Avenanti A. Perturbing the action observation network during perception and categorization of actions' goals and grips: state-dependency and virtual lesion TMS effects. Cereb. Cortex. 2015;25:598-–5608. doi: 10.1093/cercor/bht242. [DOI] [PubMed] [Google Scholar]
- Jones C.B., Lulic T., Bailey A.Z., Mackenzie T.N., Mi Y.Q., Tommerdahl M., Nelson A.J. Metaplasticity in human primary somatosensory cortex: effects on physiology and tactile perception. J. Neurophysiol. 2016;115:2681–2691. doi: 10.1152/jn.00630.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalénine S., Buxbaum L.J., Coslett H.B. Critical brain regions for action recognition: lesion symptom mapping in left hemisphere stroke. Brain. 2010;133:3269–3280. doi: 10.1093/brain/awq210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keysers C., Gazzola V. Expanding the mirror: vicarious activity for actions, emotions, and sensations. Curr. Opin. Neurobiol. 2009;19:666–671. doi: 10.1016/j.conb.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Keysers C., Perrett D.I. Demystifying social cognition: a Hebbian perspective. Trends Cogn. Sci. 2004;8:501–507. doi: 10.1016/j.tics.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Keysers C., Wicker B., Gazzola V., Anton J.L., Fogassi L., Gallese V. A touching sight: sii/pv activation during the observation and experience of touch. Neuron. 2004;42:335–346. doi: 10.1016/s0896-6273(04)00156-4. [DOI] [PubMed] [Google Scholar]
- Keysers C., Kaas J.H., Gazzola V. Somatosensation in social perception. Nat. Rev. Neurosci. 2010;11:417–428. doi: 10.1038/nrn2833. [DOI] [PubMed] [Google Scholar]
- Kilner J.M., Marchant J.L., Frith C.D. Relationship between activity in human primary motor cortex during action observation and the mirror neuron system. PLoS One. 2009;4:e4925. doi: 10.1371/journal.pone.0004925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.S., Gomez–Ramirez M., Thakur P.H., Hsiao S.S. Multimodal Interactions between proprioceptive and cutaneous signals in primary somatosensory cortex. Neuron. 2015;86:555–566. doi: 10.1016/j.neuron.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokal I., Keysers C. Granger causality mapping during joint actions reveals evidence for forward models that could overcome sensory–motor delays. PLoS One. 2010;5:e13507. doi: 10.1371/journal.pone.0013507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn E., Trampel R., Mueller K., Turner R., Schütz-Bosbach S. Judging roughness by sight--a 7-Tesla fMRI study on responsivity of the primary somatosensory cortex during observed touch of self and others. Hum. Brain Mapp. 2013;34(8):1882–1895. doi: 10.1002/hbm.22031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C., Nusbaum H.C., Meltzoff A.N., Decety J. What are you feeling? Using functional magnetic resonance imaging to assess the modulation of sensory and affective responses during empathy for pain. PLoS One. 2007;2:e1292. doi: 10.1371/journal.pone.0001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C., Decety J., Singer T. Meta–analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54:2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Lepage J.–F., Lortie M., Champoux F. Action–coding neurons in primary motor cortex: making sense of M1 activity during action perception. J. Neurosci. 2008;28:1995–1996. doi: 10.1523/JNEUROSCI.5422-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd D.M., Shore D.I., Spence C., Calvert G.A. Multisensory representation of limb position in human premotor cortex. Nat. Neurosci. 2002;6:17–18. doi: 10.1038/nn991. [DOI] [PubMed] [Google Scholar]
- McGregor H.R., Cashaback J.G.A., Gribble P.L. Functional plasticity in somatosensory cortex supports motor learning by observing. Curr. Biol. 2016;26:921–927. doi: 10.1016/j.cub.2016.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merabet L., Thut G., Murray B., Andrews J., Hsiao S., Pascual–Leone A. Feeling by sight or seeing by touch? Neuron. 2004;42:173–179. doi: 10.1016/s0896-6273(04)00147-3. [DOI] [PubMed] [Google Scholar]
- Miall R.C., Ingram H. a, Cole J.D., Gauthier G.M. Weight estimation in a “deafferented” man and in control subjects: are judgements influenced by peripheral or central signals? Exp. Brain Res. 2000;133:491–500. doi: 10.1007/s002210000433. [DOI] [PubMed] [Google Scholar]
- Michael J., Sandberg K., Skewes J., Wolf T., Blicher J., Overgaard M., Frith C.D. Continuous theta-burst stimulation demonstrates a causal role of premotor homunculus in action understanding. Psychol. Sci. 2014;25:963–972. doi: 10.1177/0956797613520608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki H., Furubayashi T., Hanajima R., Terao Y., Mizuno Y., Okabe S., Ugawa Y. Hemoglobin concentration changes in the contralateral hemisphere during and after theta burst stimulation of the human sensorimotor cortices. Exp. Brain Res. 2007;180:667–675. doi: 10.1007/s00221-007-0884-5. [DOI] [PubMed] [Google Scholar]
- Naish K.R., Barnes B., Obhi S.S. Stimulation over primary motor cortex during action observation impairs effector recognition. Cognition. 2016;149:84–94. doi: 10.1016/j.cognition.2016.01.008. [DOI] [PubMed] [Google Scholar]
- Nishitani N., Hari R. Temporal dynamics of cortical representation for action. Proc. Natl. Acad. Sci. USA. 2000;97:913–918. doi: 10.1073/pnas.97.2.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer C.E., Bunday K.L., Davare M., Kilner J.M. A causal role for primary motor cortex in perception of observed actions. J. Cogn. Neurosci. 2016;28:2021–2029. doi: 10.1162/jocn_a_01015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paracampo R., Tidoni E., Borgomaneri S., di Pellegrino G., Avenanti A. Sensorimotor network crucial for inferring amusement from smiles. Cereb. Cortex. 2016:1–14. doi: 10.1093/cercor/bhw294. (Advance Article) [DOI] [PubMed] [Google Scholar]
- Pazzaglia M., Smania N., Corato E., Aglioti S.M. Neural underpinnings of gesture discrimination in patients with limb apraxia. J. Neurosci. 2008;28:3030–3041. doi: 10.1523/JNEUROSCI.5748-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernigo S., Moro V., Avesani R., Miatello C., Urgesi C., Aglioti S.M. Massive somatic deafferentation and motor deefferentation of the lower part of the body impair its visual recognition: a psychophysical study of patients with spinal cord injury. Eur. J. Neurosci. 2012;36:3509–3518. doi: 10.1111/j.1460-9568.2012.08266.x. [DOI] [PubMed] [Google Scholar]
- Pierno A.C., Tubaldi F., Turella L., Grossi P., Barachino L., Gallo P., Castiello U. Neurofunctional modulation of brain regions by the observation of pointing and grasping actions. Cereb. Cortex. 2009;19:367–374. doi: 10.1093/cercor/bhn089. [DOI] [PubMed] [Google Scholar]
- Pineda J.A. Sensorimotor cortex as a critical component of an “extended” mirror neuron system: does it solve the development, correspondence, and control problems in mirroring? Behav. Brain Funct. 2008;4:47. doi: 10.1186/1744-9081-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher D., Garrido L., Walsh V., Duchaine B.C. Transcranial magnetic stimulation disrupts the perception and embodiment of facial expressions. J. Neurosci. 2008;28:8929–8933. doi: 10.1523/JNEUROSCI.1450-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobric G., Hamilton A. Action understanding requires the left inferior frontal cortex. Curr. Biol. 2006;16:524–529. doi: 10.1016/j.cub.2006.01.033. [DOI] [PubMed] [Google Scholar]
- Poreisz C., Antal A., Boros K., Brepohl N., Csifcsak G., Paulus W. Attenuation of N2 amplitude of laser-evoked potentials by theta burst stimulation of primary somatosensory cortex. Exp. Brain Res. 2008;185:611–621. doi: 10.1007/s00221-007-1188-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragert P., Franzkowiak S., Schwenkreis P., Tegenthoff M., Dinse H.R. Improvement of tactile perception and enhancement of cortical excitability through intermittent theta burst rTMS over human primary somatosensory cortex. Exp. Brain Res. 2008;184:1–11. doi: 10.1007/s00221-007-1073-2. [DOI] [PubMed] [Google Scholar]
- Raos V., Evangeliou M.N., Savaki H.E. Mental simulation of action in the service of action perception. J. Neurosci. 2007;27:12675–12683. doi: 10.1523/JNEUROSCI.2988-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridding M.C., Ziemann U. Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. J. Physiol. 2010;588:2291–2304. doi: 10.1113/jphysiol.2010.190314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G., Luppino G. The cortical motor system. Neuron. 2001;31:889–901. doi: 10.1016/s0896-6273(01)00423-8. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G., Sinigaglia C. The functional role of the parieto–frontal mirror circuit: interpretations and misinterpretations. Nat. Rev. Neurosci. 2010;11:264–274. doi: 10.1038/nrn2805. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G., Sinigaglia C. The mirror mechanism: a basic principle of brain function. Nat. Rev. Neurosci. 2016;17:757–765. doi: 10.1038/nrn.2016.135. [DOI] [PubMed] [Google Scholar]
- Romani M., Cesari P., Urgesi C., Facchini S., Aglioti S.M. Motor facilitation of the human cortico–spinal system during observation of bio–mechanically impossible movements. Neuroimage. 2005;26:755–763. doi: 10.1016/j.neuroimage.2005.02.027. [DOI] [PubMed] [Google Scholar]
- Rossi S., Tecchio F., Pasqualetti P., Ulivelli M., Pizzella V., Romani G., Passero S., Battistini N., Rossini P. Somatosensory processing during movement observation in humans. Clin. Neurophysiol. 2002;113:16–24. doi: 10.1016/s1388-2457(01)00725-8. [DOI] [PubMed] [Google Scholar]
- Rossi S., Hallett M., Rossini P.M., Pascual–Leone A., Safety of TMS Consensus Group Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini P., Barker A., Berardelli A., Caramia M., Caruso G., Cracco R., Dimitrijević M.R., Hallett M., Katayama Y., Lücking C.H., Maertens de Noordhout A.L., Marsden C.D., Murray N.M.F., Rothwell J.C., Swash M., Tomberg C. Non–invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. report of an IFCN committee. Electroencephalogr. Clin. Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Rossini P.M., Burke D., Chen R., Cohen L.G., Daskalakis Z., Di Iorio R., Di Lazzaro V., Ferreri F., Fitzgerald P.B., George M.S., Hallett M., Lefaucheur J.P., Langguth B., Matsumoto H., Miniussi C., Nitsche M.A., Pascual–Leone A., Paulus W., Rossi S., Rothwell J.C., Siebner H.R., Ugawa Y., Walsh V., Ziemann U. Non–invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin. Neurophysiol. 2015;126:1071–1107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saygin A.P. Superior temporal and premotor brain areas necessary for biological motion perception. Brain. 2007;130:2452–2461. doi: 10.1093/brain/awm162. [DOI] [PubMed] [Google Scholar]
- Saygin A.P., Wilson S.M., Dronkers N.F., Bates E. Action comprehension in aphasia: linguistic and non–linguistic deficits and their lesion correlates. Neuropsychologia. 2004;42:1788–1804. doi: 10.1016/j.neuropsychologia.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Schabrun S.M., Ridding M.C., Miles T.S. Role of the primary motor and sensory cortex in precision grasping: a transcranial magnetic stimulation study. Eur. J. Neurosci. 2008;27:750–756. doi: 10.1111/j.1460-9568.2008.06039.x. [DOI] [PubMed] [Google Scholar]
- Schaefer M., Heinze H.J., Rotte M. Observing the touched body magnified alters somatosensory homunculus. Neuroreport. 2008;19(9):901–905. doi: 10.1097/WNR.0b013e328301a629. [DOI] [PubMed] [Google Scholar]
- Schaefer M.1, Heinze H.J., Rotte M. Embodied empathy for tactile events: interindividual differences and vicarious somatosensory responses during touch observation. Neuroimage. 2012;60:952–957. doi: 10.1016/j.neuroimage.2012.01.112. [DOI] [PubMed] [Google Scholar]
- Schippers M.B., Keysers C. Mapping the flow of information within the putative mirror neuron system during gesture observation. Neuroimage. 2011;57:37–44. doi: 10.1016/j.neuroimage.2011.02.018. [DOI] [PubMed] [Google Scholar]
- Schütz–Bosbach S., Avenanti A., Aglioti S.M., Haggard P. Don't do it! Cortical inhibition and self–attribution during action observation. J. Cogn. Neurosci. 2009;21:1215–1227. doi: 10.1162/jocn.2009.21068. [DOI] [PubMed] [Google Scholar]
- Serino A., Canzoneri E., Avenanti A. Fronto–parietal areas necessary for a multisensory representation of peripersonal space in humans: an rTMS study. J. Cogn. Neurosci. 2011;23:2956–2967. doi: 10.1162/jocn_a_00006. [DOI] [PubMed] [Google Scholar]
- Shmuelof L., Zohary E. Dissociation between ventral and dorsal fMRI activation during object and action recognition. Neuron. 2005;47:457–470. doi: 10.1016/j.neuron.2005.06.034. [DOI] [PubMed] [Google Scholar]
- Tidoni E., Borgomaneri S., di Pellegrino G., Avenanti A. Action simulation plays a critical role in deceptive action recognition. J. Neurosci. 2013;33:611–623. doi: 10.1523/JNEUROSCI.2228-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd G., Flavel S.C., Ridding M.C. Priming theta–burst repetitive transcranial magnetic stimulation with low–and high–frequency stimulation. Exp. Brain Res. 2009;195:307–315. doi: 10.1007/s00221-009-1791-8. [DOI] [PubMed] [Google Scholar]
- Tranel D., Kemmerer D., Adolphs R., Damasio H., Damasio A.R. Neural correlates of conceptual knowledge for actions. Cogn. Neuropsychol. 2003;20:409–432. doi: 10.1080/02643290244000248. [DOI] [PubMed] [Google Scholar]
- Turella L., Tubaldi F., Erb M., Grodd W., Castiello U. Object presence modulates activity within the somatosensory component of the action observation network. Cereb. Cortex. 2012;22:668–679. doi: 10.1093/cercor/bhr140. [DOI] [PubMed] [Google Scholar]
- Urgesi C., Candidi M., Ionta S., Aglioti S.M. Representation of body identity and body actions in extrastriate body area and ventral premotor cortex. Nat. Neurosci. 2007;10:30–31. doi: 10.1038/nn1815. [DOI] [PubMed] [Google Scholar]
- Urgesi C., Candidi M., Avenanti A. Neuroanatomical substrates of action perception and understanding: an anatomic likelihood estimation meta–analysis of lesion–symptom mapping studies in brain injured patients. Front. Hum. Neurosci. 2014;8:344. doi: 10.3389/fnhum.2014.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valchev N., Ćurčić–Blake B., Renken R.J., Avenanti A., Keysers C., Gazzola V., Maurits N.M. cTBS delivered to the left somatosensory cortex changes its functional connectivity during rest. Neuroimage. 2015;114:386–397. doi: 10.1016/j.neuroimage.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valchev N., Zijdewind I., Keysers C., Gazzola V., Avenanti A., Maurits N.M. Weight dependent modulation of motor resonance induced by weight estimation during observation of partially occluded lifting actions. Neuropsychologia. 2015;66:237–245. doi: 10.1016/j.neuropsychologia.2014.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valchev N., Gazzola V., Avenanti A., Keysers C. Primary Somatosensory contribution to action observation brain activity–combining fMRI and cTBS. Soc. Cogn. Aff. Neurosci. 2016 doi: 10.1093/scan/nsw029. (nsw029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeriani M., Betti V., Le Pera D., De Armas L., Miliucci R., Restuccia D., Avenanti A., Aglioti S.M. Seeing the pain of others while being in pain: a laser–evoked potentials study. Neuroimage. 2008;40:1419–1428. doi: 10.1016/j.neuroimage.2007.12.056. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F., Baetens K. Understanding others' actions and goals by mirror and mentalizing systems: a meta–analysis. Neuroimage. 2009;48:564–584. doi: 10.1016/j.neuroimage.2009.06.009. [DOI] [PubMed] [Google Scholar]
- van Schie H.T., Mars R.B., Coles M.G.H., Bekkering H. Modulation of activity in medial frontal and motor cortices during error observation. Nat. Neurosci. 2004;7:549–554. doi: 10.1038/nn1239. [DOI] [PubMed] [Google Scholar]
- Vannuscorps G., Caramazza A. Typical action perception and interpretation without motor simulation. Proc. Natl. Acad. Sci. USA. 2015;113:86–91. doi: 10.1073/pnas.1516978112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneswaran G., Philipp R., Lemon R.N., Kraskov A. M1 corticospinal mirror neurons and their role in movement suppression during action observation. Curr. Biol. 2013;23:236–243. doi: 10.1016/j.cub.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss P.H., Rahbari N.N., Hesse M.D., Fink G.R. Deficient sequencing of pantomimes in apraxia. Neurology. 2008;70:834–840. doi: 10.1212/01.wnl.0000297513.78593.dc. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material