Abstract
We have previously shown that long tone burst high mechanical index ultrasound (US) and microbubble (MB) therapy can restore perfusion in both an in vitro and in vivo model of microvascular obstruction (MVO). Addition of MB to US has been shown to potentiate the efficacy of thrombolytics on large venous thrombi, however the optimal US parameters for achieving microvascular reperfusion of MVO caused by microthrombi, when combined with tissue plasminogen activator (tPA) are unknown. We sought to elucidate the specific effects of US, with and without tPA, for effective reperfusion of MVO in an in vitro model using both venous and arterial microthrombi. Venous and arterial type microthrombi were infused onto a mesh with 40-μm pores to simulate MVO. Pulsed US (1 MHz) was delivered with inertial cavitation (IC) (1.0 MPa, 1000 cycles, 0.33 Hz) and stable cavitation (SC) US (0.23 MPa, 20% duty, 0.33 Hz) regimes while MB suspension (2×106 MBs/mL) was infused. The efficacy of sonoreperfusion with these parameters was tested with and without tPA. Sonoreperfusion efficacy was significantly greater for IC+tPA when compared to tPA alone, IC, SC, and SC+tPA, suggesting lytic synergism between tPA and US for both venous and arterial type microthrombi. In contrast to our previous in vitro studies using 1.5 MPa at 5000 US cycles without tPA, the IC regime used herein used 90% less US energy. These findings suggest an IC regime can be used with tPA synergistically to achieve a high degree of fibrinolysis for both thrombus types.
Keywords: Ultrasound, Sonothrombolysis, Microbubbles, Ultrasound contrast agents, Microvascular obstruction, Myocardial infarction, Microcirculation
Introduction
Percutaneous coronary intervention (PCI) to restore epicardial coronary artery patency during acute myocardial infraction is successful, but its overall efficacy is limited by hypoperfusion of the distal microvasculature due to embolization of atherothrombotic debris. This phenomenon, known as microvascular obstruction (MVO), is a major contributor to the ‘no-reflow’ phenomenon, occurs in up to 80% of cases, and remains an important obstacle for fully successful myocardial reperfusion (Wu et al. 1998; Morishima et al. 2000; Costantini et al. 2004; Jaffe et al. 2008).
Myocardial contrast echocardiography and magnetic resonance imaging have demonstrated MVO to be an independent predictor of long-term left ventricular dysfunction and subsequent mortality (Khumri et al. 2006; Klug et al. 2012) further emphasizing the importance of adequate microvascular perfusion. Studies evaluating positive effects of thrombus aspiration post-PCI including the TAPAS trial suggest that reduction of MVO may improve one-year clinical outcomes (Vlaar et al. 2008).
Preventive therapy before PCI with various pharmacological agents including anti-platelets (e.g. abciximab), vasodilators (e.g. adenosine, verapamil, and nitroprusside), and fibrinolytics (e.g. tissue-plasminogen activator and streptokinase) has been shown to reduce MVO, but there is currently no consistently efficacious pharmacologic therapy for MVO (Jaffe et al. 2010). Streptokinase added post-PCI did demonstrate improved immediate myocardial reperfusion but no difference in 6-month left ventricular function was noted (Sezer et al. 2007). In addition to an increased risk of bleeding with fibrinolytics, such agents may be limited by the lack of adequate penetration into microthrombi at administered doses, an issue that may be potentially addressed by the addition of ultrasound (US) and microbubbles (MBs).
The benefits of applying localized US energy to enhance reperfusion, with and without the addition of MBs, has been established in vitro and in vivo using different ultrasound conditions for treating acute ischemic stroke, peripheral artery disease, deep venous thrombosis, acute coronary syndrome and MVO. Numerous strategies have been employed for the recanalization of occluded large vessels using mechanical dissolution with focused US with or without MBs (Siegel et al. 1989), chemical dissolution with fibrinolytics (Bookstein et al. 1989; GUSTO 1993; Wan et al. 2004; Hacke et al. 2008) or a combination of both approaches (Kudo 1989; Lauer et al. 1992; Alexandrov et al. 2004; Parikh et al. 2008). For example, reperfusion was enhanced in an in vitro model when tissue plasminogen activator (tPA) was administered concurrently with US (0.5–1 MHz) (Lauer et al. 1992; Blinc et al. 1993; Harpaz et al. 1993; Harpaz et al. 1994). This interactive effect was mechanistically attributed to the ability of US to increase tPA access to fibrin binding sites upon which plasmin can act (Francis et al. 1995; Siddiqi et al. 1998).
The effectiveness of tPA-mediated reperfusion was enhanced by insonation of either MBs embedded within clot architecture (Everbach and Francis 2000) or MBs exogenously administered (Datta et al. 2006). The ability of such US-stimulated MBs to potentiate thrombolysis varies on the specific US parameters used, particularly that of acoustic frequency and pressure, which have profound effects on MB activity (Datta et al. 2008). Inertial cavitation (IC) and stable cavitation (SC) US regimes have both independently been proposed to be optimal for tPA synergism with MBs for effective thrombolysis (Bader et al. 2015; Petit et al. 2015), yet there remains to be a thorough, direct comparison between both regimes.
We have previously demonstrated the efficacy of long tone burst, high acoustic pressure US and MB therapy without fibrinolytics in relieving such MVO in an in vitro model (Leeman et al. 2012) and more recently in an in vivo rat hindlimb model of MVO (Pacella et al. 2015). MB-thrombus interaction was directly visualized using an ultrafast microscopy system (Chen et al. 2013) where high pressure US pulses caused disruption of a microthrombus positioned in close proximity to a MB undergoing inertial cavitation (Chen et al. 2014). Such thrombus penetration suggests a possible mechanism by which MBs and US may potentiate the transport of fibrinolytic agents to facilitate both mechanical and pharmacological thrombolysis. In these studies, our group investigated the effects of sonoreperfusion with US and MBs on venous type microthrombi. However, known structural differences between venous and arterial type microthrombi, in particular differences in fibrin content, may influence the efficacy of sonoreperfusion. Specifically, thin and highly branched fibrin networks as seen may be observed in arterial type thrombi are typically less permeable and susceptible to lysis (Undas and Ariens 2011; Black et al. 2016). Indeed, differences in sonoreperfusion between thrombus origins have been recently reported possibly due to the tighter fibrin network and greater fibrin density of arterial microthrombi when compared to that of venous microthrombi (Undas and Ariens 2011; Black et al. 2016). However, the efficacy of optimal sonoreperfusion parameters, whether SC or IC regime, in the presence of fibrinolytic agents may further depend on thrombus type, which has not be well studied.
Our current study aims to compare the efficacy of sonoreperfusion between SC and IC, each with and without tPA, for reperfusion of MVO created with venous and arterially derived microthrombi. The purpose of the study was (1) to determine whether SC or IC demonstrated improved synergy with tPA in microthrombi dissolution when compared to the corresponding US parameter alone and (2) to assess whether such an effect would be preserved in fibrin-rich microthrombi (e.g. arterial type) when compared to venous microthrombi.
Methods
Thrombus preparation
Microthrombi were prepared to simulate venous and arterial type microthrombi. Porcine blood anticoagulated with acid citrate dextrose was purchased (Lampire Biological Labs, Ottsville, PA, USA) and used within 3 weeks of withdrawal. Venous microthrombi were prepared by adding 1.5 mL of porcine blood to 150 μL 0.25 M CaCl2 into a 2.5 mL glass vial (Borosilicate type I, Supelco Analytical, Bellefonte, PA, USA) (Poole 1959). The contents were mixed initially by inversion and allowed to sit under static conditions at room temperature for 2 hours (Nishioka et al. 1997). The vial was then shaken in a vial mixer (Bristol-Myers Squibb Medical Imaging, North Billerica, MA, USA) for 20 seconds at 4530 ± 100 oscillations/min to break the clot into small fragments. Phosphate buffered saline (PBS) was added to the vial, and the thrombus suspension was passed through a mesh with 200-μm pores in a syringe driven filter holder to yield a suspension of <200 μm venous microthrombi. For arterial type microthrombi, 3 mL of porcine blood was added to 300 μL of 0.25 M CaCl2 solution, and 1 mL was transferred to each of 3 PVC tubes (1/8 inch lumen). The tubing was used to create a Chandler’s loop and mimic shear rate as seen in a physiologic, high pressure arterial system (Robbie et al. 1997). The loop was rotated at 70 RPM, causing the column of blood to flow at a linear velocity of 41 cm/sec for a shear rate of 1032 sec−1. After 1 hour, the white thrombi were removed and placed in a 2.5 mL vial with 1.5 mL PBS. As with venous type microthrombi, the vial was shaken for 20 seconds and filtered through a mesh with 200-μm pores.
Microbubble preparation
Perfluorobutane-filled, lipid-encapsulated MBs were prepared for the sonoreperfusion treatment as previously described (Weller et al. 2002; Pacella et al. 2015). Briefly, a mixture of 1,2-distearoyl-sn-glycero-3-phosphocholine (4 mg/mL), 1,2-distearoyl-sn-glycero-3-phosphoethanolamine (2 mg/mL) and polyethylene glycol (2 mg/mL) was sonicated using a sonicator apparatus at a power setting of 5 (XL2020, Misonix, Farmingdale, NY, USA) for a total duration of 1.5 min in the presence of perfluorobutane gas to produce MBs with a mean diameter of 3 μm and a concentration of 1–2×109 MBs/mL. MBs were diluted to a final concentration of 2×106 MBs/mL in PBS with or without tPA before each experiment.
In vitro experimental setup
An in vitro model of microvascular obstruction previously described is shown in Figure 1 (Leeman et al. 2012). In this setup, a 15-μm thick filter with 40-μm pores (Cell strainer; Falcon, Franklin Lakes, NJ, USA) was mounted across the lumen of a custom built 4-mm inner diameter artificial blood vessel casted from rubber (M-F Manufacturing Co, Fort Worth, TX), placed in a water bath maintained at 37±1°C (VWR 1230; VWR Scientific, West Chester, PA, USA). This filter was subsequently partly occluded with either venous or arterial microthrombi under constant flow rate (1.5 mL/min). To assess thrombus burden, pressure upstream of the partially occluded mesh was monitored with a fluid filled pressure transducer (BD, Franklin Lakes, NJ, USA) connected to a physiologic monitoring system with a 16-bit digitizer (Ponemah, Science Inc., Ontario, Canada). A 1 MHz transducer for US delivery (A302S, 1 inch diameter, 1.63 inch focus, -6 dB beam diameter 3.5 mm, Olympus NDT, Waltham, MA, USA) and a 3.5 MHz US transducer (V383, 0.375 inch diameter, 1 inch focus, -6 dB beam diameter 1.2 mm, Olympus NDT) for detection of MB cavitation events were mounted in the water tank under confocal alignment over the mesh treatment site. The 1 MHz delivery transducer was driven by a function generator (33250A; Agilent, Santa Clara, CA, USA) and power amplifier (100A250A; Amplifier Research, Souderton, PA, USA). The passively detected signal was amplified by 10 dB, band pass filtered with a 2–20 MHz cutoff (5073PR; Olympus) and digitized at 50 MHz sampling frequency on an oscilloscope (WaveRunner 6051A; Lecroy, Chestnut Ridge, NY, USA). Joint time-frequency analysis was performed on 250 ms windows using a time step of 100 ms (60% overlapping) in MATLAB (The MathWorks Inc., Natick, MA, USA) software.
Figure 1.
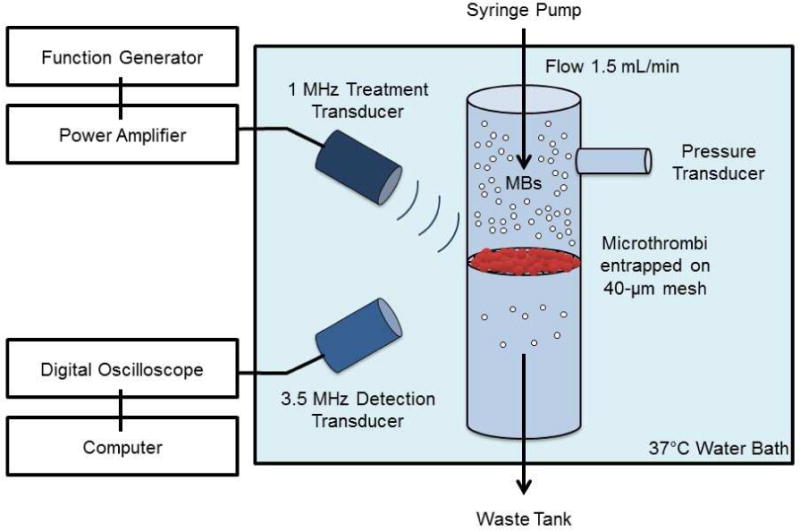
Schematic diagram of the in-vitro model of microvascular obstruction.
Ultrasound parameters
Two US treatment regimes were selected that produced either inertial cavitation (IC) or stable cavitation (SC) MB behavior in PBS. The IC regime (1.0 MPa, 1000 cycles, 0.33 Hz pulse repetition frequency (PRF)) had previously been shown to yield moderately effective sonoreperfusion (Leeman et al. 2012), but incomplete reperfusion, thus allowing for potential improvement. The SC regime (0.23 MPa, 20% duty, 0.33 Hz PRF) was chosen for its ability to generate sustained stable cavitation during a large number of cycles (up to 600k cycles) favoring generation of acoustic streaming and radiation force to enhance tPA transport.
Experimental protocol
PBS with lipid-encapsulated MBs at a concentration of 2×106 MBs/mL was loaded into a syringe pump and perfused through the vessel at a flow rate of 1.5 mL/min (Harvard Apparatus, Holliston, MA, USA). To test the differential effect on various thrombi type, either venous or arterial microthrombi were hand injected into the vessel through a port upstream of the mesh with 40-μm pores, thus entrapping the microthrombi to simulate microvascular obstruction. This was performed until upstream pressure reached 40 ± 5 mmHg, a representative mean arteriolar pressure of small cardiac arterioles. To test the effect of tPA on reducing thrombus burden, 3 μg/mL of alteplase (Genentech Inc, San Francisco, CA, USA) was added to the perfusate in some treatment conditions upon the start of treatment, corresponding to previously described steady state concentrations in humans during the first 30 min of tPA infusion (Tanswell et al. 1992). Using these parameters, five total treatment groups were designed: IC regime, SC regime, tPA alone, IC+tPA, and SC+tPA. Each treatment was 20 min during which continuous upstream pressure was monitored.
Data Analysis
Pressure curves were normalized such that the starting pressure for each trial was 40 mmHg. Lytic efficacy of each US treatment parameter was quantified using the lytic index, defined as the inverse of the area beneath the pressure versus time curve. Lytic index reflects an overall measurement of thrombus burden during the treatment and is related to the amount of thrombolysis (i.e. the higher the lytic index, the greater the amount of thrombolysis). To assess the initial rate of such thrombolysis, early lytic rate (defined as the slope of the pressure versus time curve for the first 3 min of treatment) was measured. An additional measure of lytic rate, termed late lytic rate that analyzed the slope of pressure versus time curve for the first 3 min immediately following the first ten percent pressure drop from 40 mmHg, was performed. This measure was intended to capture the lytic rate in conditions that demonstrated delayed reperfusion.
Statistical analysis
Data are presented as mean ± standard deviation. Two-way analysis of variance (ANOVA) using the Games-Howell approach was used to test differences across each combination of treatment and microthrombus type; the Games-Howell test was used to account for the heterogeneity of variances and properly adjust for multiple comparisons. Statistical significance was defined as p<0.05.
Results
Type of thrombus
Figure 2 shows representative arterial and venous microthrombi in SEM imaging. Microthrombi formed under static conditions (red venous clots) were composed of a loose fibrin mesh entrapping echinocytic red blood cells. Conversely, microthrombi formed under high shear flow in the Chandler loop (white arterial clots) had a denser fibrin mesh entrapping very few red blood cells.
Figure 2.
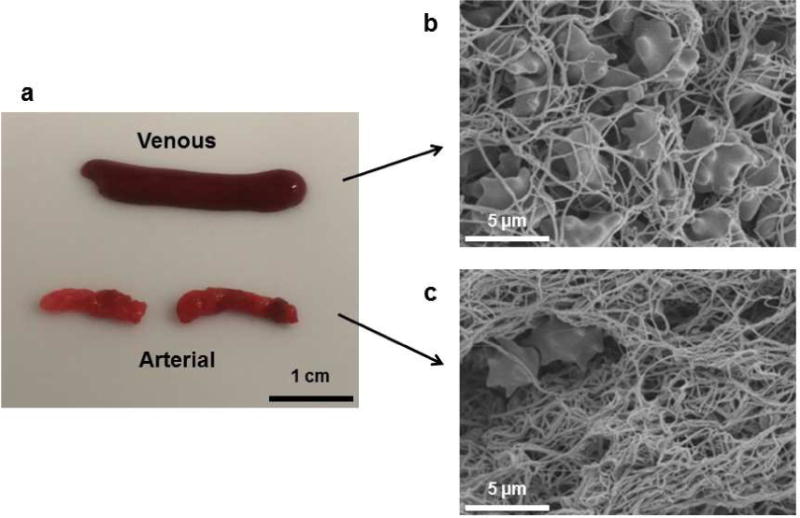
Characteristics of thrombi. (a) Photographs of venous and arterial thrombi. (b) Scanning electron microscope (SEM) image (×2000) of venous thrombus. (c) SEM image of arterial thrombus.
Cavitation detection
Figure 3 shows typical spectrograms with passive cavitation detection of MBs at the two treatment US conditions. At IC parameters (1.0 MPa, 1000 cycles), broadband acoustic cavitation was detected throughout the duration of the 1000 cycles, whereas SC parameters (0.23 MPa, 20% duty) produced sustained ultraharmonics for > 80 ms in duration. Broadband energy was present at the onset of the SC pulse, but at a level that was ~15 dB below the broadband activity seen with the IC pulse. However, this activity disappeared after the first 10 ms of the SC pulse.
Figure 3.
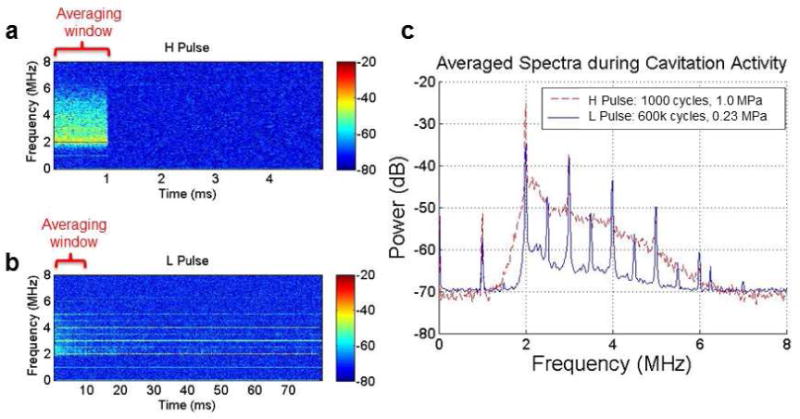
PCD data. (a) Representative power spectra of inertial cavitation (IC) regime. (b) Representative power spectra of stable cavitation regime (SC). (c) Comparison of the averaged power spectra during the first 1 ms for IC regime and during the first 10 ms for SC regime.
Effect of US parameters on sonoreperfusion efficacy for different clot types with and without tPA
Pressure change as a function of time for both venous and arterial type microthrombi can be visualized in Figure 4. IC regime (1.0 MPa, 1000 cycles) reached a final pressure of 21.4 ± 3.9 mmHg for venous clot and 31.0 ± 2.5 mmHg for arterial clot at 20 min, whereas a SC regime (0.23 MPa, 20% duty) produced a non-significant change in upstream pressure (37.0 ± 5.9 mmHg for venous clot and 42.4 ± 3.3 mmHg for arterial clot). When testing the effect of tPA alone without US treatment, minimal change in pressure was seen for both thrombus types after 20 min (30.3 ± 6.0 mmHg for venous clot and 39.9 ± 3.5 mmHg for arterial clot).
Figure 4.
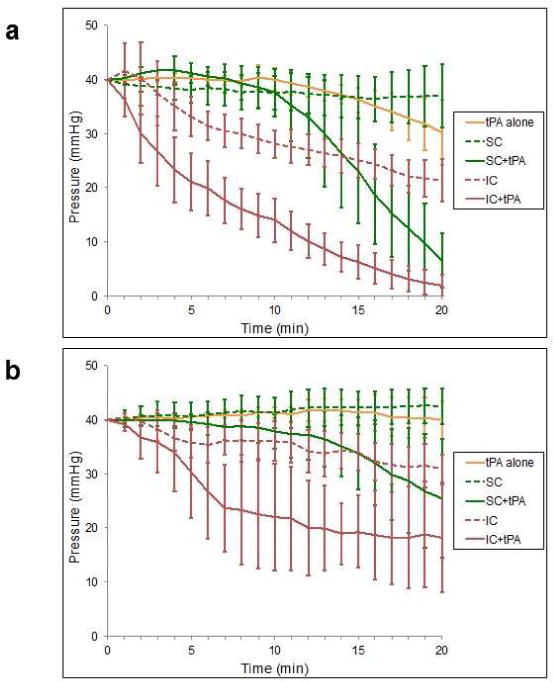
Time course of upstream pressure during ultrasound and microbubble treatment for venous thrombi (A) and arterial thrombi (B). Values presented as mean ± standard deviation (n=4–5 for each experimental condition).
Lytic index, a surrogate for total degree of sonoreperfusion, is shown in Figure 5 at various treatment conditions. SC regime [(1.3 ± 0.1)×10−3 (mmHg.min)−1 for venous clot and (1.2 ± 0.1)×10−3 (mmHg.min) −1 for arterial clot] did not differ significantly from tPA alone [(1.3 ± 0.1)×10−3 (mmHg.min) −1 for venous clot and (1.2 ± 0.0)×10−3 (mmHg.min) −1 for arterial clot], p>0.05 for both clot types. Further, addition of tPA as seen in SC+tPA did not differ when compared to tPA alone for venous clot [(1.6 ± 0.2)×10−3 (mmHg.min) −1, p>0.05] or for arterial clot [(1.4 ± 0.2)×10−3 (mmHg.min) −1, p>0.05]. IC+tPA demonstrated a marked improvement in lytic index for venous clot [(3.5 ± 0.8)×10−3 (mmHg.min) −1, p<0.05] when compared to all other treatment regimes and for arterial clot [(2.1 ± 0.5)×10−3 (mmHg.min) −1, p<0.05] when compared to tPA alone and the SC regime.
Figure 5.
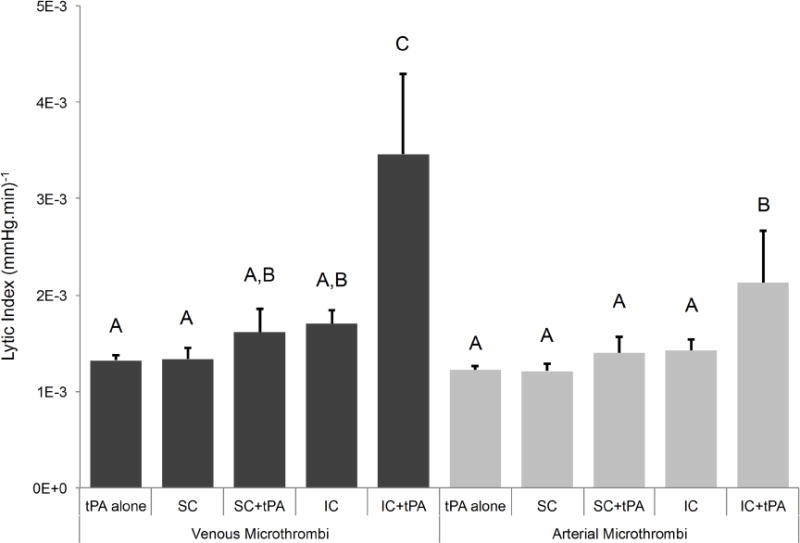
Lytic index for venous and arterial microthrombi under various conditions (n=4–5 for each experimental condition). Means with the same letter are not statistically different from each other. Lytic index was greater for IC+tPA than all other treatment conditions for venous microthrombi (p<0.05) and than tPA alone and SC for arterial microthrombi (p<0.05). Comparing across microthrombus type, lytic index for IC+tPA was greater for venous type than arterial type (p<0.05).
Early lytic rate is shown in Figure 6 at various treatment conditions. Again IC+tPA had a higher early lytic rate (4.6 ± 2.1 mmHg/min) when compared to all other treatment conditions (p<0.05), however this finding was limited to venous type microthrombi. For arterial microthrombi, the early lytic rate for was numerically greatest for the IC+tPA (1.5 ± 2.0 mmHg/min) but was not statistically significant when compared to all other treatment conditions (p>0.05).
Figure 6.
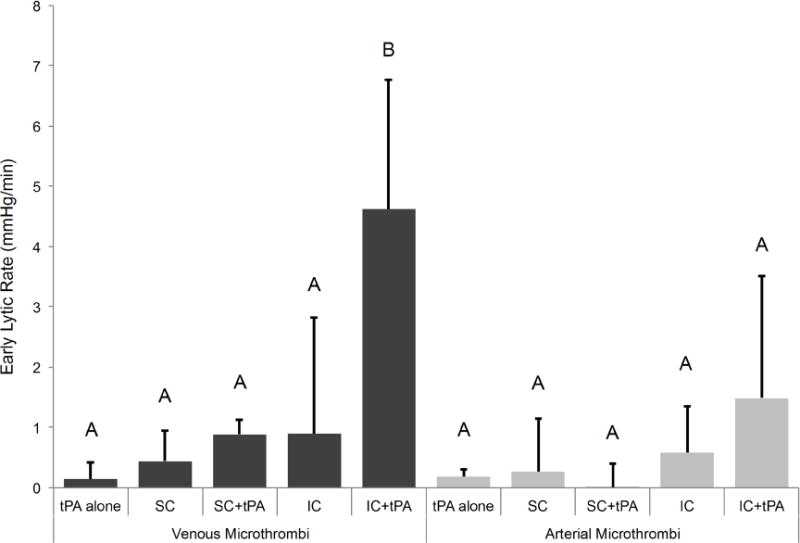
Early lytic rate for venous and arterial microthrombi under various conditions (n=4–5 for each experimental condition). Means with the same letter are not statistically different from each other. Early lytic rate was greater for IC+tPA for venous microthrombi than all other treatment conditions (p<0.05). No statistical differences were noted across microthrombus type (p>0.05).
When considering late lytic rate shown in Figure 7, IC+tPA showed increased late lytic rate (4.8 ± 1.8 mmHg/min for venous clot and 3.1 ± 2.0 mmHg/min for arterial clot) compared to tPA alone (1.0 ± 0.5 mmHg/min for venous clot and 0.2 ± 0.6 mmHg/min for arterial clot), p<0.05 for both clot types. There was a difference between late lytic rate for SC+tPA (2.6 ± 1.2 mmHg/min) compared to the SC regime (0.0 ± 0.7 mmHg/min) for venous clot (p<0.05), but not for arterial clot (SC+tPA, 1.1 ± 0.8 mmHg/min and SC regime, 0.0 ± 0.2 mmHg/min, p>0.05). Although the late lytic rate for IC+tPA was numerically greater than that of the SC+tPA for both clot types, there was no statistical significance in the differences (p>0.05).
Figure 7.
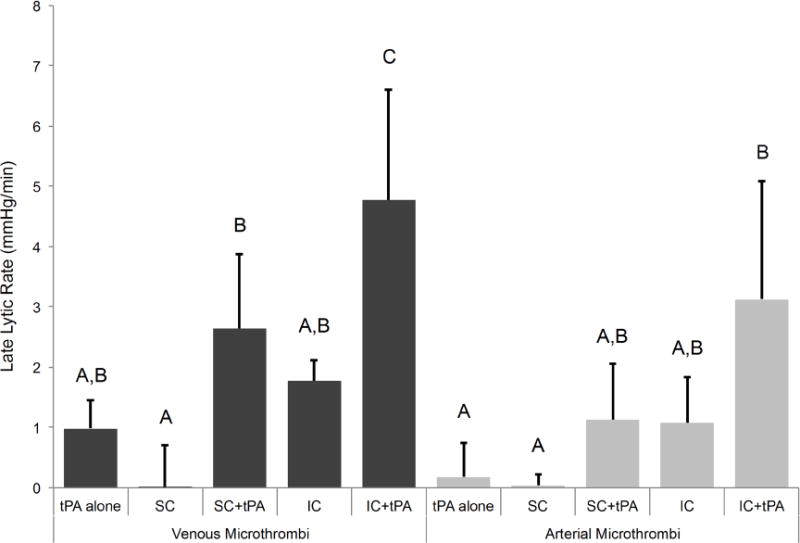
Late lytic rate for venous and arterial microthrombi (n=4–5 for each experimental condition). Means with the same letter are not statistically different from each other. Late lytic rate was greater for IC+tPA for venous microthrombi than tPA alone, SC, and IC (p<0.05), while late lytic rate for IC+tPA for arterial microthrombi was greater than tPA alone and SC (p<0.05). Late lytic rate for SC+tPA was greater than SC for venous microthrombi (p<0.05) while not statistically different for arterial microthrombi (p<0.05). As a group, late lytic rate was greater for venous microthrombi than arterial microthrombi (p<0.05), but no specific treatment group was significantly different.
Finally, for IC+tPA, a decreased lytic index, early and late lytic rate was observed with arterial type microthrombi over venous type microthrombi (p<0.05).
Discussions
In this study, we followed the pressure kinetics in an in vitro model of MVO during sonoreperfusion therapy while varying the following variables: (1) the clot type, (2) the cavitation regime in the presence of MBs, and (3) the addition of tPA. These upstream pressure-time curves were quantified in terms of 3 parameters capturing: (1) the overall efficacy of the treatment (lytic index); (2) the initial rate of microvascular reperfusion from the onset of US application (early lytic rate); and (3) the later rate of reperfusion following a ten percent pressure drop (late lytic rate). Collectively, our data supports that for MVO created by venous type microthrombi, microvascular reperfusion was enhanced when tPA was added to an IC regime when compared to either tPA or IC regime alone as evidenced by increased lytic index, early lytic rate, and late lytic rate. These findings for IC+tPA further extended to arterial type microthrombi when compared against tPA alone, specifically for lytic index and late lytic rate. However, when examining the efficacy of SC+tPA, our results were not able to demonstrate the same degree of synergism seen in our IC regimes for either type of microthrombus. Notably, the only difference detected for SC+tPA was in its improved late lytic index over SC without tPA in venous microthrombi. Further, IC+tPA directly demonstrated enhanced lytic index when compared to that of the SC+tPA for venous microthrombi. For the reasons of generalizability across different thrombus types and uniformly increased lytic index, our results suggested that an IC+tPA was superior to SC+tPA in our in vitro system of MVO. Importantly, similar reperfusion efficacy was seen between IC+tPA when compared to previously studied high mechanical index IC regime (1.5 MPa, 5000 cycles) without tPA in venous type microthrombi (Leeman et al. 2012), but corresponded to 90% less ultrasound energy required to achieve reperfusion.
There has been significant debate in the literature regarding the relative efficacy of IC versus SC US when combined with tPA for optimal sonoreperfusion. However, to our knowledge, no study to date has addressed this question in the presence of continuous flow and specifically within a microvascular model. Our current study directly addresses these conditions while providing kinetic pressure measurements during insonation in an effort to optimize sonoreperfusion in the context of resolving MVO.
The reperfusion improvement of US with MBs (an IC regime and to a lesser degree a SC regime) and tPA over either US alone or tPA alone suggests a synergistic effect between the mechanical and pharmacological dissolution of microthrombi. Such synergism has been previously implicated to be due to possibly increased mechanical interaction of tPA with clot fibrin strands upon which tPA can chemically act (Francis et al. 1995; Siddiqi et al. 1998). When looking specifically at the arterial type microthrombi, we find non-statistically significant differences in early lytic rate. However, a significant improvement was seen in late lytic rate for IC+tPA, particularly when compared to tPA alone. We hypothesize that this could be due to arterial microthrombi having more robust and denser fibrin networks as seen in the SEM images (Figure 2) that require more time to be lysed than venous microthrombi.
To further understand the mechanical (US with MB) and chemical (tPA) interaction in sonoreperfusion, research efforts have been made to stratify the effect by specific type of mechanical interaction, namely IC versus SC. For our experimental design, the IC regime resulted in greater sonoreperfusion efficacy when compared to the SC regime as stated previously. We speculate that this is due to the enhanced sonoporation of fibrin provided by microbubble collapse causing exposure of more fibrin binding sites for tPA. MB penetration into fibrin architecture and subsequent destruction by inertial cavitation not only allows for new microbubbles to enter the clot, but also introduces access for chemical modulators like tPA. Effective sonoreperfusion with an IC+tPA has been demonstrated both in vitro with macrothrombi (Petit et al. 2015) and in vivo with a porcine model post myocardial infarction (Wu et al. 2014) and a rodent hindlimb model of MVO without addition of tPA (Pacella et al. 2015).
Other groups have suggested that the sustained stable oscillations of an SC regime effectively mix the tPA helping it to gain physical interaction with the surface of thrombus (Datta et al. 2006; Datta et al. 2008; Bader et al. 2015). They suggest that this interaction could be superior to the intermittent, clot penetrating nature of microbubble collapse creating broadband emissions seen in an IC regime. Specifically, (Datta et al. 2008) observed enhanced thrombus destruction in a dose-dependent fashion of US when added to tPA. Their most effective thrombolytic regime was observed with 0.32 MPa peak-to-peak US; notably this regime exhibited broadband acoustic emission suggestive of some IC activity. Further, the absence of flow in this system did not allow for microbubble replenishment after being destroyed by IC-generating US pulses. (Bader et al. 2015) added a flow component, addressing this issue, but the treatment condition of IC+tPA was not assessed as comparison. (Petit et al. 2015) also demonstrated improved thrombolysis in large thrombi with an IC regime showing maximum synergism between tPA and US at 1.3 MPa peak negative pressure, similar to our findings. However, these findings were reported in larger thrombi and they were not compared against an IC only regime.
Although the greatest sonoreperfusion efficacy in our study was observed with an IC+tPA, we did not observe a significantly enhanced efficacy using an SC+tPA over tPA alone but did observe a significantly improved late lytic rate over a SC regime for venous microthrombi,, which partially aligns with the findings of (Bader et al. 2015). Additionally, the late lytic rate of the SC+tPA was similar to that of the IC+tPA for venous microthrombi, suggesting a “catch up” effect of the SC+tPA, which ultimately may render it effective. The relative benefit of an IC+tPA likely derives from the fact that sonoreperfusion has an earlier onset than SC+tPA, as seen in the pressure-time curves.
Clinically, the argument for an IC+tPA over a SC+tPA is three-fold. Primarily, the current study demonstrated that IC has improved sonoreperfusion over SC when used with tPA, presumably due to the more rapid opening of fibrin binding sites for tPA, specifically in venous type microthrombi. Second, the spatial peak temporal averaged intensity (ISPTA) for our IC and SC regimes were 0.011 W/cm2 and 0.353 W/cm2 respectively, which corresponds to roughly 97% less energy used in the IC regime. Considering the upper limit for acoustic output ISPTA is cited at 0.72 W/cm2 for cardiac tissue (Lee and Garra 2004), the IC regime has significantly less potential than SC for heat induced off-target effects despite operating at higher acoustic pressures. Third, based on SEM images (Figure 2), arterial microthrombi demonstrated more fibrin content than venous microthrombi. Microthrombi found in MVO are likely a combination of arterial microthrombi that form under high shear rate and venous microthrombi that form under low shear rate. (Acconcia et al. 2014) demonstrated that in fibrin only clots, there was minimal bubble penetration through fibrin at 0.2 MPa, but significant penetration at 0.4 MPa and above. Work from our group further examined sonoreperfusion efficacy without the use of fibrinolytics as a function of clot architecture from either arterial or venous sources (Black et al. 2016) and determined that higher acoustic pressure regimes (e.g. IC regime) may be necessary for effective reperfusion with arterial microthrombi. This further argues for the use of high pressure US parameters in sonoreperfusion, especially in settings where the targeted microthrombi may be rich in fibrin content and use of fibrinolytics is being considered.
Limitations
This study employs changes in upstream pressure as a surrogate for reduction in thrombus burden on the mesh to characterize sonoreperfusion. Direct quantification of thrombus dissolution was not investigated in this study. However, previous optical study using our in vitro model has confirmed that the observed phenomenon was due to thrombus dissolution as opposed to thrombus reorganizations (Leeman et al. 2012). This is further corroborated in the current study by observed differences in sonoreperfusion effect seen between arterial and venous microthrombi. With thrombus displacement instead of dissolution, we might expect to find no difference between the two thrombus types as thrombus reorganization should not vary based on thrombus characteristics.
Furthermore, PBS was used as the perfusate in this study, which allowed for the direct analysis of US, MB, and tPA interactions using various ultrasound conditions and clot types, but did not recapitulate the complex biochemical environment of whole blood. The efficacy of MB+US+tPA on sonoreperfusion using whole blood as the perfusate was previously reported by our group (Roos et al. 2016). It is worth noting that in vivo homeostasis is hard to reproduce in vitro and our study did not recapitulate the complex dynamic process of thrombus formation-dissolution that occurs in hemostasis in vivo.
Conclusions
Our current data suggest that an IC regime can be used with tPA to achieve synergistically a high degree of fibrinolysis for both thrombus types. Furthermore, for all conditions with tPA, greater sonoreperfusion efficacy was observed in venous rather than arterial type microthrombi, which is likely due to differences in fibrin content among other components of thrombus content and architecture. Future studies may consider addition of tPA to sonoreperfusion regimes as it allows for less total US energy delivery, which has important implications for optimizing this therapeutic technique.
Acknowledgments
We thank Linda Lavery and Andrew Carson for providing valuable technical assistance. SEM imaging was performed with equipment graciously provided by Dr. Simon Watkins at the Center for Biological Imaging (CBI) at the University of Pittsburgh. This study was supported by the Center for Ultrasound Molecular Imaging and Therapeutics, University of Pittsburgh Medical Center, Pittsburgh, PA, and by the National Institutes of Health (NIH) (R01 EB016516-01A1, R01 HL125777).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acconcia C, Leung BY, Manjunath A, Goertz DE. Interactions between individual ultrasound-stimulated microbubbles and fibrin clots. Ultrasound Med Biol. 2014;40:2134–50. doi: 10.1016/j.ultrasmedbio.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Alexandrov AV, Molina CA, Grotta JC, Garami Z, Ford SR, Alvarez-Sabin J, Montaner J, Saqqur M, Demchuk AM, Moye LA, Hill MD, Wojner AW, Investigators C Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004;351:2170–8. doi: 10.1056/NEJMoa041175. [DOI] [PubMed] [Google Scholar]
- Bader KB, Gruber MJ, Holland CK. Shaken and stirred: mechanisms of ultrasound-enhanced thrombolysis. Ultrasound Med Biol. 2015;41:187–96. doi: 10.1016/j.ultrasmedbio.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JJ, Yu FT, Schnatz RG, Chen X, Villanueva FS, Pacella JJ. Effect of Thrombus Composition and Viscosity on Sonoreperfusion Efficacy in a Model of Micro-Vascular Obstruction. Ultrasound Med Biol. 2016;42:2220–31. doi: 10.1016/j.ultrasmedbio.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinc A, Francis CW, Trudnowski JL, Carstensen EL. Characterization of ultrasound-potentiated fibrinolysis in vitro. Blood. 1993;81:2636–43. [PubMed] [Google Scholar]
- Bookstein JJ, Fellmeth B, Roberts A, Valji K, Davis G, Machado T. Pulsed-spray pharmacomechanical thrombolysis: preliminary clinical results. AJR Am J Roentgenol. 1989;152:1097–100. doi: 10.2214/ajr.152.5.1097. [DOI] [PubMed] [Google Scholar]
- Chen X, Leeman JE, Wang J, Pacella JJ, Villanueva FS. New insights into mechanisms of sonothrombolysis using ultra-high-speed imaging. Ultrasound Med Biol. 2014;40:258–62. doi: 10.1016/j.ultrasmedbio.2013.08.021. [DOI] [PubMed] [Google Scholar]
- Chen X, Wang J, Versluis M, de Jong N, Villanueva FS. Ultra-fast bright field and fluorescence imaging of the dynamics of micrometer-sized objects. Review of Scientific Instruments. 2013;84:063701. doi: 10.1063/1.4809168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini CO, Stone GW, Mehran R, Aymong E, Grines CL, Cox DA, Stuckey T, Turco M, Gersh BJ, Tcheng JE, Garcia E, Griffin JJ, Guagliumi G, Leon MB, Lansky AJ. Frequency, correlates, and clinical implications of myocardial perfusion after primary angioplasty and stenting, with and without glycoprotein IIb/IIIa inhibition, in acute myocardial infarction. J Am Coll Cardiol. 2004;44:305–12. doi: 10.1016/j.jacc.2004.03.058. [DOI] [PubMed] [Google Scholar]
- Datta S, Coussios CC, Ammi AY, Mast TD, de Courten-Myers GM, Holland CK. Ultrasound-enhanced thrombolysis using Definity as a cavitation nucleation agent. Ultrasound Med Biol. 2008;34:1421–33. doi: 10.1016/j.ultrasmedbio.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Coussios CC, McAdory LE, Tan J, Porter T, De Courten-Myers G, Holland CK. Correlation of cavitation with ultrasound enhancement of thrombolysis. Ultrasound Med Biol. 2006;32:1257–67. doi: 10.1016/j.ultrasmedbio.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everbach EC, Francis CW. Cavitational mechanisms in ultrasound-accelerated thrombolysis at 1 MHz. Ultrasound Med Biol. 2000;26:1153–60. doi: 10.1016/s0301-5629(00)00250-7. [DOI] [PubMed] [Google Scholar]
- Francis CW, Blinc A, Lee S, Cox C. Ultrasound accelerates transport of recombinant tissue plasminogen activator into clots. Ultrasound Med Biol. 1995;21:419–24. doi: 10.1016/0301-5629(94)00119-x. [DOI] [PubMed] [Google Scholar]
- GUSTO I. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. The GUSTO investigators. N Engl J Med. 1993;329:673–82. doi: 10.1056/NEJM199309023291001. [DOI] [PubMed] [Google Scholar]
- Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D, Investigators E Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–29. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- Harpaz D, Chen X, Francis CW, Marder VJ, Meltzer RS. Ultrasound enhancement of thrombolysis and reperfusion in vitro. J Am Coll Cardiol. 1993;21:1507–11. doi: 10.1016/0735-1097(93)90331-t. [DOI] [PubMed] [Google Scholar]
- Harpaz D, Chen X, Francis CW, Meltzer RS. Ultrasound accelerates urokinase-induced thrombolysis and reperfusion. Am Heart J. 1994;127:1211–9. doi: 10.1016/0002-8703(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Jaffe R, Charron T, Puley G, Dick A, Strauss BH. Microvascular obstruction and the no-reflow phenomenon after percutaneous coronary intervention. Circulation. 2008;117:3152–6. doi: 10.1161/CIRCULATIONAHA.107.742312. [DOI] [PubMed] [Google Scholar]
- Jaffe R, Dick A, Strauss BH. Prevention and treatment of microvascular obstruction-related myocardial injury and coronary no-reflow following percutaneous coronary intervention: a systematic approach. JACC Cardiovasc Interv. 2010;3:695–704. doi: 10.1016/j.jcin.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Khumri TM, Nayyar S, Idupulapati M, Magalski A, Stoner CN, Kusnetzky LL, Kosiborod M, Spertus JA, Main ML. Usefulness of myocardial contrast echocardiography in predicting late mortality in patients with anterior wall acute myocardial infarction. Am J Cardiol. 2006;98:1150–5. doi: 10.1016/j.amjcard.2006.05.045. [DOI] [PubMed] [Google Scholar]
- Klug G, Mayr A, Schenk S, Esterhammer R, Schocke M, Nocker M, Jaschke W, Pachinger O, Metzler B. Prognostic value at 5 years of microvascular obstruction after acute myocardial infarction assessed by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2012;14:46. doi: 10.1186/1532-429X-14-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo S. Thrombolysis with Ultrasound Effect. Tokyo Jikeikai Medical Journal. 1989;104:1005–12. [Google Scholar]
- Lauer CG, Burge R, Tang DB, Bass BG, Gomez ER, Alving BM. Effect of ultrasound on tissue-type plasminogen activator-induced thrombolysis. Circulation. 1992;86:1257–64. doi: 10.1161/01.cir.86.4.1257. [DOI] [PubMed] [Google Scholar]
- Lee W, Garra B. AIUM Technical Bulletin. How to interpret the ultrasound output display standard for higher acoustic output diagnostic ultrasound devices: version 2. J Ultrasound Med. 2004;23:723–6. [PubMed] [Google Scholar]
- Leeman JE, Kim JS, Yu FT, Chen X, Kim K, Wang J, Chen X, Villanueva FS, Pacella JJ. Effect of acoustic conditions on microbubble-mediated microvascular sonothrombolysis. Ultrasound Med Biol. 2012;38:1589–98. doi: 10.1016/j.ultrasmedbio.2012.05.020. [DOI] [PubMed] [Google Scholar]
- Morishima I, Sone T, Okumura K, Tsuboi H, Kondo J, Mukawa H, Matsui H, Toki Y, Ito T, Hayakawa T. Angiographic no-reflow phenomenon as a predictor of adverse long-term outcome in patients treated with percutaneous transluminal coronary angioplasty for first acute myocardial infarction. J Am Coll Cardiol. 2000;36:1202–9. doi: 10.1016/s0735-1097(00)00865-2. [DOI] [PubMed] [Google Scholar]
- Nishioka T, Luo H, Fishbein MC, Cercek B, Forrester JS, Kim CJ, Berglund H, Siegel RJ. Dissolution of thrombotic arterial occlusion by high intensity, low frequency ultrasound and dodecafluoropentane emulsion: an in vitro and in vivo study. J Am Coll Cardiol. 1997;30:561–8. doi: 10.1016/s0735-1097(97)00182-4. [DOI] [PubMed] [Google Scholar]
- Pacella JJ, Brands J, Schnatz FG, Black JJ, Chen X, Villanueva FS. Treatment of microvascular micro-embolization using microbubbles and long-tone-burst ultrasound: an in vivo study. Ultrasound Med Biol. 2015;41:456–64. doi: 10.1016/j.ultrasmedbio.2014.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh S, Motarjeme A, McNamara T, Raabe R, Hagspiel K, Benenati JF, Sterling K, Comerota A. Ultrasound-accelerated thrombolysis for the treatment of deep vein thrombosis: initial clinical experience. J Vasc Interv Radiol. 2008;19:521–8. doi: 10.1016/j.jvir.2007.11.023. [DOI] [PubMed] [Google Scholar]
- Petit B, Bohren Y, Gaud E, Bussat P, Arditi M, Yan F, Tranquart F, Allemann E. Sonothrombolysis: the contribution of stable and inertial cavitation to clot lysis. Ultrasound Med Biol. 2015;41:1402–10. doi: 10.1016/j.ultrasmedbio.2014.12.007. [DOI] [PubMed] [Google Scholar]
- Poole JC. A study of artificial thrombi produced by a modification of Chandler’s method. Q J Exp Physiol Cogn Med Sci. 1959;44:377–84. doi: 10.1113/expphysiol.1959.sp001419. [DOI] [PubMed] [Google Scholar]
- Robbie LA, Young SP, Bennett B, Booth NA. Thrombi formed in a Chandler loop mimic human arterial thrombi in structure and RAI-1 content and distribution. Thromb Haemost. 1997;77:510–5. [PubMed] [Google Scholar]
- Roos ST, Yu FT, Kamp O, Chen X, Villanueva FS, Pacella JJ. Sonoreperfusion Therapy Kinetics in Whole Blood Using Ultrasound, Microbubbles and Tissue Plasminogen Activator. Ultrasound Med Biol. 2016;42:3001–9. doi: 10.1016/j.ultrasmedbio.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sezer M, Oflaz H, Goren T, Okcular I, Umman B, Nisanci Y, Bilge AK, Sanli Y, Meric M, Umman S. Intracoronary streptokinase after primary percutaneous coronary intervention. N Engl J Med. 2007;356:1823–34. doi: 10.1056/NEJMoa054374. [DOI] [PubMed] [Google Scholar]
- Siddiqi F, Odrljin TM, Fay PJ, Cox C, Francis CW. Binding of tissue-plasminogen activator to fibrin: effect of ultrasound. Blood. 1998;91:2019–25. [PubMed] [Google Scholar]
- Siegel RJ, Cumberland DC, Myler RK, DonMichael TA. Percutaneous ultrasonic angioplasty:initial clinical experience. Lancet. 1989;2:772–4. doi: 10.1016/s0140-6736(89)90832-5. [DOI] [PubMed] [Google Scholar]
- Tanswell P, Tebbe U, Neuhaus KL, Glasle-Schwarz L, Wojcik J, Seifried E. Pharmacokinetics and fibrin specificity of alteplase during accelerated infusions in acute myocardial infarction. J Am Coll Cardiol. 1992;19:1071–5. doi: 10.1016/0735-1097(92)90297-z. [DOI] [PubMed] [Google Scholar]
- Undas A, Ariens RA. Fibrin clot structure and function: a role in the pathophysiology of arterial and venous thromboembolic diseases. Arterioscler Thromb Vasc Biol. 2011;31:e88–99. doi: 10.1161/ATVBAHA.111.230631. [DOI] [PubMed] [Google Scholar]
- Vlaar PJ, Svilaas T, van der Horst IC, Diercks GF, Fokkema ML, de Smet BJ, van den Heuvel AF, Anthonio RL, Jessurun GA, Tan ES, Suurmeijer AJ, Zijlstra F. Cardiac death and reinfarction after 1 year in the Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study (TAPAS): a 1-year follow-up study. Lancet. 2008;371:1915–20. doi: 10.1016/S0140-6736(08)60833-8. [DOI] [PubMed] [Google Scholar]
- Wan S, Quinlan DJ, Agnelli G, Eikelboom JW. Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: a meta-analysis of the randomized controlled trials. Circulation. 2004;110:744–9. doi: 10.1161/01.CIR.0000137826.09715.9C. [DOI] [PubMed] [Google Scholar]
- Weller GE, Villanueva FS, Klibanov AL, Wagner WR. Modulating targeted adhesion of an ultrasound contrast agent to dysfunctional endothelium. Ann Biomed Eng. 2002;30:1012–9. doi: 10.1114/1.1513565. [DOI] [PubMed] [Google Scholar]
- Wu J, Xie F, Kumar T, Liu J, Lof J, Shi W, Everbach EC, Porter TR. Improved sonothrombolysis from a modified diagnostic transducer delivering impulses containing a longer pulse duration. Ultrasound Med Biol. 2014;40:1545–53. doi: 10.1016/j.ultrasmedbio.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KC, Zerhouni EA, Judd RM, Lugo-Olivieri CH, Barouch LA, Schulman SP, Blumenthal RS, Lima JA. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation. 1998;97:765–72. doi: 10.1161/01.cir.97.8.765. [DOI] [PubMed] [Google Scholar]


