Abstract
BACKGROUND
Periprosthetic hip infection treatment remains a significant challenge for orthopedics. Some studies have suggested that methicillin resistance and gram-negative organism type are associated with increased treatment failure. The aim of this research was to determine if specific organisms were associated with poor outcomes in treatment for hip periprosthetic infection.
METHODS
Records were reviewed of all patients between 2005 and 2015 who underwent treatment for infected partial or total hip arthroplasty. Characteristics of each patient’s treatment course was determined including baseline characteristics, infecting organism(s), infection status at final follow-up, surgeries for infection, and time in hospital. Baseline characteristics and organisms that were associated with clinical outcomes in univariate analysis were incorporated into multivariable outcomes models.
RESULTS
When compared with patients infected with other organism(s), patients infected with the following organisms had significantly decreased infection-free rates: pseudomonas, methicillin-resistant staphylococcus aureus, and proteus. Infection with certain organisms was associated with 1.13 to 2.58 additional surgeries: methicillin-sensitive staphylococcus aureus, coagulase-negative staphylococcus, MRSA, pseudomonas, peptostreptococcus, klebsiella, candida, diphtheroids, propionibacterium acnes, and proteus species. Specific organisms were associated with 8.56 to 24.54 additional days in hospital for infection: MSSA, CoNS, proteus, MRSA, enterococcus, pseudomonas, klebsiella, beta-hemolytic streptococcus, and diphtheroids. Higher comorbidity score was also associated with greater length of hospitalization.
CONCLUSION
MRSA, pseudomonas, and proteus were associated with all three outcomes of lower infection-free rate, more surgery, and more time in hospital in treatment for hip periprosthetic infection. Organism-specific outcome information may help individualize patient-physician discussions about the expected course of treatment for hip periprosthetic infection.
Keywords: hip arthroplasty periprosthetic infection, infection-free, MRSA, pseudomonas, proteus, hip arthroplasty prosthetic joint infection
BACKGROUND
Although hip arthroplasty has been widely viewed as a successful surgery [1], periprosthetic infection remains one of the major challenges for orthopedic surgeons performing joint arthroplasty and represents 15% of all causes for hip revision [2]. In addition to burden on patients and providers, healthcare costs triple with infection [3]. Infection rates have dropped over the last 50 years to approximately 2% [4] largely due to improvements in preventative and peri-operative measures such as laminar flow systems, UV lights, decreased operative time, exhaust suits, routine peri-operative antibiotic administration, and decreased room traffic [5–7]. However, a projected 572,000 THA’s performed per year by 2030 in the United States suggests that there could be as many as 10,000 infections per year in the near future [8].
In addition to surgical techniques used to eradicate infection, orthopedists rely on cultures to guide selection of targeted antibiotics. While a variety of gram-positive and gram-negative bacteria and fungi are associated with hip periprosthetic infection, methicillin-sensitive staphylococcus aureus (MSSA), methicillin-resistant staphylococcus aureus (MRSA), coagulase-negative staphylococci (CoNS), streptococcus species, enterococcus species, aerobic gram-negative bacilli, and anaerobic bacteria are among the most common [9, 10]. Further, a number of organisms such as MRSA, MSSA, acinetobacter, Klebsiella, Enterococcus, and Candida krusei have been reported to produce biofilms making the prospect of eradication more difficult [11].
Although specific infecting organisms are known, the impact of various organism classes as well as patterns of antibiotic resistance on treatment outcomes has only more recently been evaluated.
Several studies have focused on the topic of methicillin resistance. Volin et. al. reviewed a series of 46 hip or knee arthroplasty infections and found no significant difference in infection-free rates between patients with methicillin resistant and methicillin sensitive organisms [12]. On the contrary, Salgado et. al. reported higher risk of treatment failure in a cohort of infected hip and knee arthroplasty patients when comparing 12 patients with methicillin-resistant staphylococcus aureus to 31 patients with methicillin-sensitive staphylococcus aureus [13]. Lim et. al. reviewed a cohort of 2-stage hip arthroplasty revisions infected with resistant vs. non-resistant microorganisms and demonstrated a statistically lower rate of treatment failure with non-resistant microorganisms [14]. Lastly, Leung et. al. reviewed 38 patients with total hip arthroplasty infections due to MRSA or methicillin-resistant staphylococcus epidermidis treated with 2-stage exchange and showed a 21% failure rate. Since their failure rate with the resistant organisms was higher than previously reported averages, they concluded that methicillin resistance may be associated with worse outcomes [15].
Other authors have recognized that certain classes of organisms may predispose to poorer outcomes. Zmitowski and colleagues reported on a cohort of hip or knee arthroplasty infections undergoing incision and drainage. They found that gram-negative infection was associated with greater success than methicillin resistant and methicillin sensitive gram-positive infections. However, they also reported that 2-stage exchange for gram-negative infection was associated with lower infection-free rates than methicillin sensitive gram-positive infections but similar to methicillin resistant gram-positive infections [16]. On the contrary, Hsieh et. al. reported that incision and drainage of gram-negative organisms was associated with higher failure than with gram-positive organisms but that 2-stage exchange is similar in success to that for gram-positive infection in a series of mixed hip or knee arthroplasty infections [17]. Lastly, Buchholz and colleagues determined that certain organisms are more closely associated with failure; specifically pseudomonas species, Group D streptococcus, proteus species, and eschericia coli [18]. While more recent studies have evaluated either antibiotic resistance or broad categories of organism, Buchholz’s study from 1981 is the most recent report to the author’s knowledge to evaluate how specific organisms impact treatment outcomes in patients with hip arthroplasty infection.
Even with meticulous technique and planning, patients remain at risk for infection. While gram negative organisms and methicillin resistance have each been somewhat associated with treatment failure, the relationship between specific organisms and patient outcomes has not been extensively studied. As demand for patient-centered care increases, the need to tailor treatment decisions to individual patient data may increase. Our aim in this study was to determine if particular organisms predispose patients to worse outcomes in treatment for hip periprosthetic infection by evaluating infection free rates, total surgeries for infection, and length of hospitalization for infection treatment.
METHODS
After IRB approval, the clinical content explorer at our institution was used to identify patients who had treatment for periprosthetic hip infection between September 2005 and September 2015. This retrospective, cohort study was analyzed and reported in line with the STROBE statement, a consensus intended to strengthen the reporting of observational studies [19]. The patient’s clinical course was documented including the following factors: organism(s) cultured from the infected hip throughout the duration of diagnosis and treatment; whether or not the patient remained free of infection at final follow-up; total surgeries for infection; and cumulative length of hospitalization for infection. Consistent with the Delphi-based international multidisciplinary consensus statement defining treatment success in periprosthetic infection [20], infection-free was defined as having appropriate arthroplasty components in place without need for further surgery or intravenous antibiotics. Also in line with the consensus statement, patients were only included in the analysis if they had a minimum of 1 year of follow-up with orthopedic providers at our institution after their final surgery or hospitalization for infection. Out of 300 hip arthroplasty infections treated at our institution during the time frame of the study, 149 separate infections met criteria for analysis.
To control for known potential confounding variables, baseline characteristics were measured. These included location of primary implant at outside hospital (OSH) vs this institution, whether or not the patient had previous treatment at OSH of infection, age at first positive hip culture at this institution, race, and gender. Cumulative patient comorbidity was assessed using the Charlson-Deyo comorbidity score, which was calculated using previously-published ICD-9 codes [21]. Briefly, the Charlson-Deyo score is comprised of 22 factors such as history of myocardial infarction, mild liver disease, diabetes with complication, renal disease, etc. that are weighted to yield an overall score ranging from 0 (no comorbidity) to 33 (highest score, maximal comorbidity). Factors in the index are weighted to reflect their contribution to patient morbidity. Many patients fall between 0 and 3 on the comorbidity scale. Scores were calculated based on diagnosis codes entered before the patient’s last hospitalization or surgery for infection in order to capture comorbidities of patients from outside hospital that may not have been fully characterized at the start of treatment. To provide better understanding about the conditions and setting of the infections in this series, infection type and host grade as described by McPherson et. al. were calculated [22]. The authors were able to distinguish between acute post-operative and late post-operative infections. Additionally, systemic host grade could be categorized into “uncompromised,” “compromised,” and “significantly compromised” based on patient diagnoses. Due to the retrospective nature of the investigation and variance of data collected at the time of the infection, lab values, assessment of chronic active infection at another site, and local extremity grade were not included in the staging analysis. In order to report on organisms with broad relevance, analysis was restricted to only those organisms with greater than 3% prevalence in our population.
Using JMP Pro version 12.0.1 from Statistical Analysis Software (SAS), we first performed univariate tests of significance using linear and logistic regression to assess the impact of baseline characteristics and infectious organisms on the chosen outcomes (infection-free status, surgeries for infection, and length of hospitalization for infection). The covariates that first reached a significance threshold of p-value less than 0.05 in univariate analysis were included into multivariable linear and logistic regression main effects models of our outcomes regardless of the direction of their association with the outcomes of interest. Statistical significance in the multivariable models was taken at p<0.05.
RESULTS
Baseline characteristics, McPherson stage, and outcome characteristics are displayed in Table 1. The average patient was 60.2 years old and had an average Charlson-Deyo comorbidity score of approximately 2. Approximately 1/3rd of patients had their initial arthroplasty implanted at outside hospital (OSH), and 1/4th of patients had previous hip infection prior to presenting at our institution. 102 out of the 149 patients (68.5%) were Caucasian. 51.7% of patients were female. 44% of patients had acute post-operative infection while 56% had late post-operative infection. Most patients had a host grade of “B.” 128 out of 149 infections (85.9%) were infection-free. The average patient underwent 2–3 surgeries and spent 2–3 weeks in hospital for treatment. While all patients had at least 12 months of follow-up, the average patient was followed up for approximately 3 years after their final surgery or hospitalization for infection. Further, 147 / 149 (98.7%) patients had Infectious Disease (ID) consultation for management of antibiotic regimen. The other two patients had antibiotics managed by the orthopaedic surgeon and an internal medicine team. 27 / 128 (21.0%) of patients that were infection-free remained on suppressive antibiotics compared to 11 / 21 (52.3%) of patients that were not infection-free. Trimethoprim/sulfamethoxazole, cephalexin, and minocycline were the most commonly-used suppressive antibiotics. Further detail on antibiotic usage is found in the Appendix Tables 4 – 7. Lastly, 91.2% of initial antibiotic treatments were either aligned with results taken directly from culture sensitivities or extrapolated from culture sensitivities by using a same or more potent class of antibiotic (i.e. 3rd generation cephalosporin instead of 2nd generation cephalosporin). The 8.8% of remaining cultures that did not appear to have directed coverage were most commonly cultures of organisms whose pathogenic potential was unclear such as Propionibacterium acnes and diphtherioids. In these cases, joint decisions were made between ID and orthopaedic providers about whether or not to treat these organisms given the tradeoffs inherent in antibiotic treatment and possible under-treatment of infection.
Table 1.
Baseline characteristics, McPherson stage, infection-free rates, surgeries, and days spent in hospital are shown for entire study sample (n=149). Averages with 95% confidence intervals (CI’s) or proportions are shown.
| Baseline characteristic | Average (lower 95% CI, upper 95% CI) or proportion | |
|---|---|---|
| Age at first infection | 60.6 (58.3, 62.9) | |
| Charlson-Deyo score | 2.08 (1.68, 2.51) | |
| Implanted at outside hospital (OSH) | 52 / 149 (34.9%) | |
| Previous infection treatment at OSH | 36 / 149 (24.2%) | |
| Caucasian | 102 / 149 (68.5%) | |
| Female gender | 77 / 149 (51.7%) | |
| McPherson Stage | ||
| Infection type | Host grade | Proportion |
| Stage I (acute post-op, n=66) | A | 19 / 149 (12.8%) |
| B | 35 / 149 (23.5%) | |
| C | 12 / 149 (8.1%) | |
| Stage II/III (late post-op, n=83) | A | 22 / 149 (14.8%) |
| B | 36 / 149 (24.2%) | |
| C | 25 / 149 (16.8%) | |
| Outcome | Average (lower 95% CI, upper 95% CI) or proportion | |
| Infection-free rate | 128 / 149 (85.9%) | |
| Surgeries | 2.65 (2.26, 3.04) | |
| Days in hospital | 17.27 (14.09, 20.45) | |
| Follow-up (months) | 37.90 (33.93, 41.88) | |
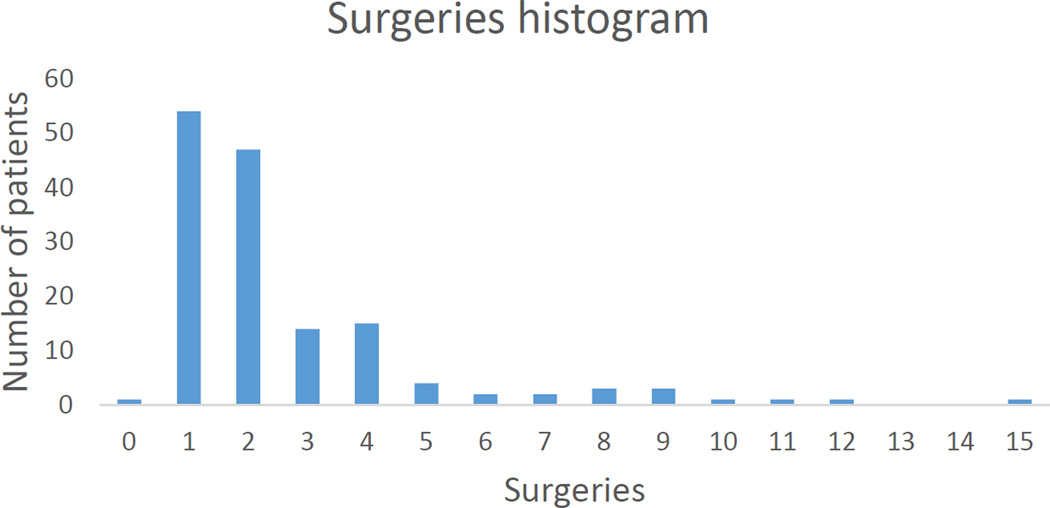
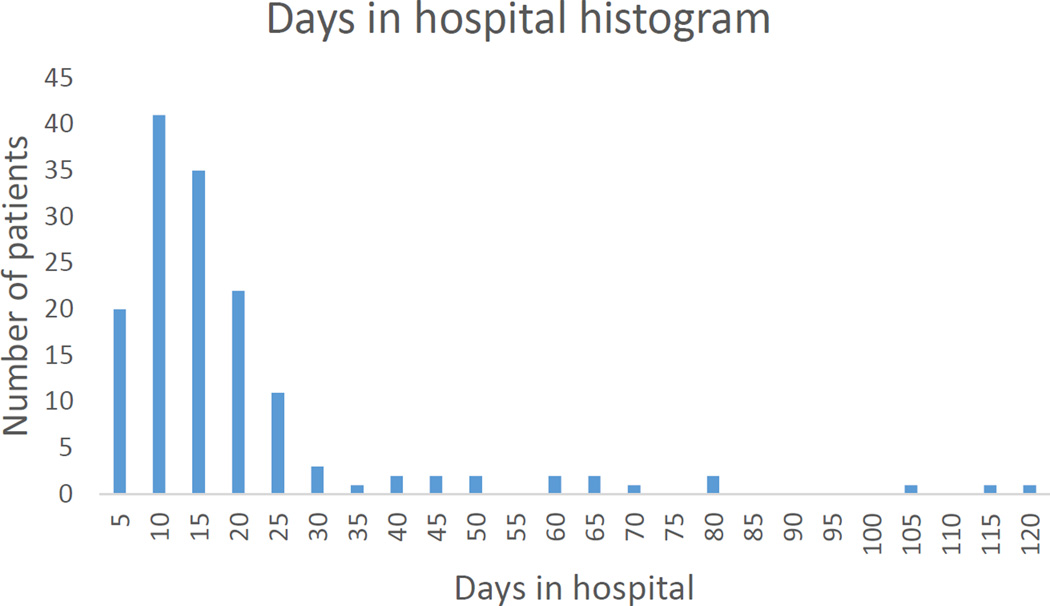
Figures 1 and 2 demonstrate that most patients underwent between 1 and 5 surgeries and spent less than 30 days in hospital for infection treatment.
Figure 1.
Total surgeries for infection. Most patients underwent 1–5 surgeries for infection. Note that 1 patient refused surgery to treat active infection. This patient was not counted as infection free.
Figure 2.
Total days in hospital for related to infection broken down into 5-day increments. Most patients spent less than 30 days in hospital.
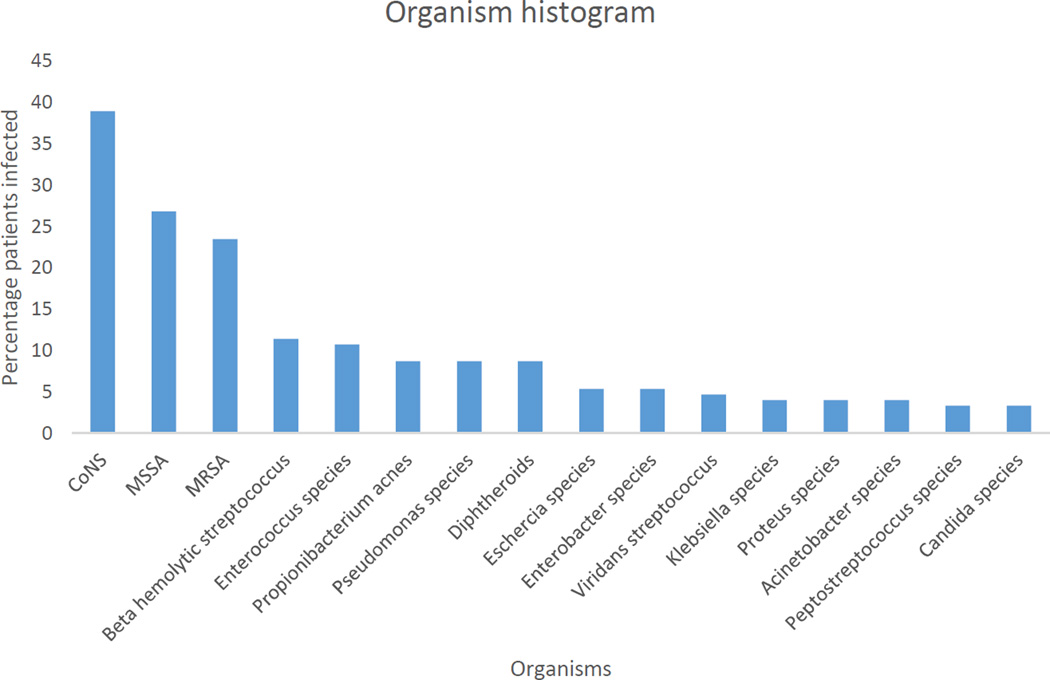
The proportion of patients infected by each organism is shown in Figure 3. Staphlyococcus species such as CoNS, MSSA, and MRSA represented the largest proportion of infecting organism while gram-negative organisms and fungi were relatively less prevalent.
Figure 3.
Proportions of patient sample affected by each organism. Some patients were infected by more than 1 organism over the course of their treatment.
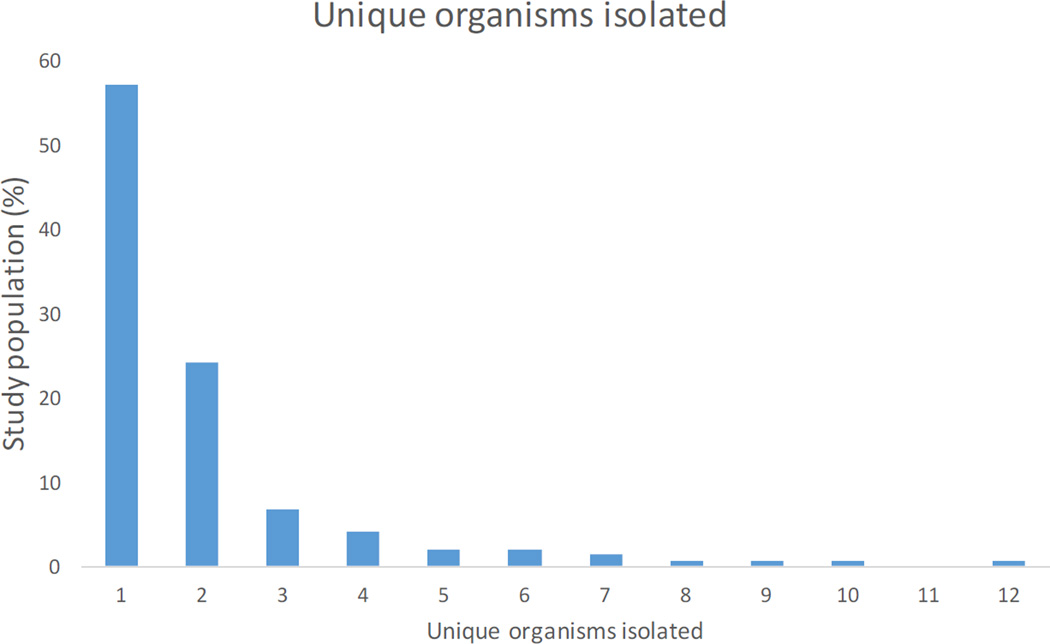
A histogram of the total number of unique infectious organisms cultured from patients’ joint samples during their course of treatment is shown in Figure 4. It indicates that the vast majority of patients (>85%) had between 1 and 3 organisms cultured during their treatment for infection.
Figure 4.
Histogram of the number of unique organisms isolated from patients’ hip cultures over their course of infection.
Table 2 displays outcomes related to treatment strategy. Resections and combinations of resection and I+D had lower infection-free rates, more surgery, and longer hospitalization time than I+D(s) alone.
Table 2.
Operative treatment strategies and outcomes. I+D(s) alone signifies one or multiple incision and drainage procedures with or without head and/or liner exchange with no resection arthroplasty. Resection(s) alone signifies 1-stage or 2-stage resection with or without antibiotic spacer placement without I+D(s). Combination signifies any combination of I+D and resection. Further operative details are characterized in Appendix Tables 1 – 3.
| Operative treatment | Infection-free rate | Surgeries | Hospitalization time |
|---|---|---|---|
| I+D(s) alone (n=68) | 65 / 68 (95.6%) | 1.47 surgeries (1.27, 1.67) |
9.72 (7.96, 11.49) |
| Resection(s) alone (n=42) |
38 / 42 (90.5%) | 2.09 surgeries (1.86, 2.33) |
12.48 days (10.34, 14.61) |
| Combination I+D and resection (n=38) |
25 / 38 (65.8%) | 5.45 procedures (4.44, 6.45) |
36.54 days (27.03, 46.04) |
When compared with patients infected with other organism(s), patients infected with MRSA, pseudomonas species, or proteus species had significantly decreased infection-free rates as shown in Table 3. Although univariate analysis suggested that patients whose initial arthroplasty was performed at an outside institution had higher infection-free rates than patients whose initial arthroplasty was placed at our institution, this correlation did not persist in multivariable analysis.
Table 3.
Multivariable logistic regression comparing infection-free rates between infected patients with the specified organism/baseline characteristic and infected patients without the specified organism/baseline characteristic. All factors in the table below first met a univariate threshold of p<0.05. Odds ratios compare the odds of remaining infection-free without exposure to the organism or baselien characteristic to the odds of remaining infection-free with exposure to the organism or baseline characteristic. An odds ratio greater than 1 indicates higher odds of achieving infection-free status in the group without the organism/baseline characteristic compared to the group with the organism/baseline characteristic. Infection-free rates with and without exposure to the organism/characteristic, adjusted odds ratios with 95% CI’s, and adjusted p-values for achieving Infection-free are displayed.
| Organism or characteristic |
Infection- free with |
Infection- free without |
Adjusted odds ratio for remaining infection-free without (lower 95% CI, upper 95% CI) |
Adjusted p- value |
|---|---|---|---|---|
|
Pseudomona s species |
5 / 13 (38.5%) |
123 / 136 (90.4%) |
13.25 (3.09, 64.87) | 0.0005 |
| MRSA | 23 / 35 (65.7%) |
105 / 114 (92.1%) |
7.89 (2.40, 29.71) | 0.0006 |
|
Proteus species |
2 / 6 (33.3%) |
126 / 143 (88.1%) |
19.68 (2.50, 196.39) | 0.0047 |
| OSH first implant |
49 / 52 (94.2%) |
79 / 97 (81.4%) |
0.79 (0.16, 3.21) | 0.7454 |
As shown in Table 4, infection with MSSA, CoNS. MRSA, pseudomonas species, peptostreptococcus species, klebsiella species, candida species, diphtheroids, propionibacterium acnes or proteus species was significantly associated with the number of surgical procedures for infection when compared with patients infected by other organisms.
Table 4.
Multivariable linear regression comparing total surgeries for infection treatment between patients infected with the specified organism and patients infected with any other organism. All covariates included in the multivariable model first met a univariate threshold of p<0.05. Baseline characteristics were also included if they met the univariate significance threshold. Adjusted estimates, 95% confidence intervals, and adjusted p-values for additional surgery associated with each factor are displayed.
| Organism or characteristic |
Patients with organism or characteristic |
Adjusted additional surgeries (lower 95% CI, upper 95% CI) |
Adjusted p-value |
|---|---|---|---|
| MSSA | 40 / 149 (26.8%) | 1.66 (1.04, 2.28) | <0.0001 |
| CoNS | 58 / 149 (38.9%) | 1.32 (0.75, 1.88) | <0.0001 |
| MRSA | 35 / 149 (23.5%) | 1.20 (0.56, 1.84) | 0.0003 |
|
Pseudomonas species |
13 / 149 (8.7%) | 1.76 (0.73, 2.79) | 0.0009 |
|
Peptostreptococ cus species |
5 / 149 (3.3%) | 2.58 (1.03, 4.13) | 0.0013 |
|
Klebsiella species |
6 / 149 (4.0%) | 2.30 (0.87, 3.73) | 0.0018 |
| Candida species | 5 / 149 (3.3%) | 2.36 (0.75, 3.98) | 0.0045 |
| Diphtheroids | 13 / 149 (8.7%) | 1.36 (0.40, 2.32) | 0.0058 |
|
Propionibacteri um acnes |
13 / 149 (8.7%) | 1.13 (0.15, 2.10) | 0.0239 |
| Proteus species | 6 / 149 (4.0%) | 1.40 (0.01, 2.80) | 0.049 |
| Beta-hemolytic streptococcus |
17 / 149 (11.4%) | 0.70 (-0.18, 1.58) | 0.1194 |
| Age | 60.6 (58.3, 62.9) | -0.01 / year (-0.04, 0.01) | 0.1467 |
| Enterococcus species |
16 / 149 (10.7%) | 0.49 (-0.46, 1.44) | 0.3077 |
As shown in Table 5, MSSA, CoNS, proteus species, MRSA, enterococcus species, pseudomonas species, klebsiella species, beta hemolytic streptococcus, and diphtheroids were each associated with increased time in hospital for infection treatment. Increased Charlson-Deyo score was also associated with increased time in hospital. The remaining organisms and baseline characteristics did not significantly impact the multivariable models.
Table 5.
Multivariable linear regression comparing total hospitalization days for infection treatment between patients infected with the specified organism and patients with any other organism. All covariates included in the multivariable model first met a univariate threshold of p<0.05. Baseline characteristics were included if they met the univariate significance threshold. Adjusted estimates, 95% confidence intervals, and adjusted p-values for additional hospitalization days associated with each factor are displayed.
| Organism or characteristic |
Patients with organism or average |
Adjusted additional days in hospital (lower 95% CI, upper 95% CI) |
Adjusted p-value |
|---|---|---|---|
| MSSA | 40 / 149 (26.8%) | 13.80 (8.73, 18.87) | <0.0001 |
| CoNS | 58 / 149 (38.9%) | 12.16 (7.54, 16.78) | <0.0001 |
| Proteus species | 6 / 149 (4.0%) | 24.54 (13.03, 36.04) | <0.0001 |
| MRSA | 35 / 149 (23.5%) | 9.62 (4.29, 14.95) | 0.0005 |
|
Enterococcus species |
16 / 149 (10.7%) | 13.19 (5.34, 21.01) | 0.0011 |
|
Pseudomonas species |
13 / 149 (8.7%) | 12.79 (4.37, 21.21) | 0.0032 |
| Charlson-deyo | 2.08 (1.68, 2.51) | 1.33 / point (0.44, 2.23) | 0.0036 |
|
Klebsiella species |
6 / 149 (4.0%) | 14.02 (2.38, 25.66) | 0.0186 |
|
Beta-hemolytic streptococcus |
17 / 149 (11.4%) | 8.62 (1.39, 15.85) | 0.0197 |
| Diphtheroids | 13 / 149 (8.7%) | 8.56 (0.66, 16.47) | 0.034 |
| Peptostreptococc us species |
5 / 149 (3.3%) | 13.09 (0.45, 25.74) | 0.0425 |
| Age | 60.6 (58.3, 62.9) | -0.10 / year (-0.27, 0.07) | 0.2353 |
| Candida species | 5 / 149 (3.3%) | -1.44 (-14.51, 11.63) | 0.8275 |
Finally, in order to verify that our exclusion criteria (at least 1 year of follow-up after final surgery or treatment for infection) minimally impacted study findings, we repeated multivariable analyses with the entire possible population of patients undergoing treatment for periprosthetic hip infection at our institution regardless of follow-up length (n=300). We found several relatively minor changes when analyzing this larger population which are likely related to the differences in organism prevalence. Importantly, proteus species no longer remained significantly associated with infection-free rate. However, several other organisms approached statistical significance in impacting infection-free rates including candida, enterococcus, and eschericia species. In addition to factors previously noted, younger patient age, enterococcus species, and beta hemolytic streptococcus were each significantly associated with more surgery for infection. Finally, klebsiella and beta hemolytic streptococcus were no longer significantly associated with increased length of hospitalization. However, candida, acinetobacter, peptostreptococcus, and Propionibacterium acnes achieved statistical significance in this outcome besides the factors previously noted in the smaller analysis. Table 6 displays the most commonly used antibiotics for treatment in each category of organism. Multivariable analyses were repeated excluding patients who remained infection-free at final follow-up but who were on oral antibiotic suppression (n=27). There were no changes in any of the study outcomes other than for beta hemolytic streptococcus, which was no longer associated with increased total length of hospitalization.
Table 6.
Commonly-used antibiotics for each organism category. Complete listing available in Appendix Table 4.
| Organism | Most used antibiotic(s) | Percent usage |
Proportion infection free |
|---|---|---|---|
| Acinetobacter species | ciprofloxacin, vancomycin |
42.8% | 5 / 6 (83.3%) |
| Candida species | fluconazole | 54.5% | 3 / 5 (60%) |
| CoNS | vancomycin | 62.2% | 48 / 58 (82.7%) |
| Diphtheroids | vancomycin | 57.1% | 9 / 13 (69.2%) |
| Enterobacter species | ciprofloxacin | 88.8% | 5 / 8 (62.5%) |
| Enterococcus species | daptomycin | 26.9% | 11 / 16 (68.7%) |
| Eschericia species | ciprofloxacin | 43.7% | 7 / 8 (87.5%) |
| Beta hemolytic streptococcus |
ceftriaxone | 47.8% | 13 / 17 (76.4%) |
| Klebsiella species | ciprofloxacin | 45.4% | 4 / 6 (66.6%) |
| MRSA | vancomycin | 87% | 23 / 35 (65.7%) |
| MSSA | cefazolin | 38.5% | 31 / 40 (77.5%) |
| Peptostreptococcus species | penicillin G | 33.3% | 3 / 5 (60%) |
| Propionibacterium acnes | vancomycin | 30.7% | 12 / 13 (92.3%) |
| Proteus species | ciprofloxacin | 55.5% | 2 / 6 (33.3%) |
| Pseudomonas species | ciprofloxacin | 48% | 5 / 13 (38.4%) |
| Viridans streptococcus | ceftriaxone | 37.5% | 6 / 7 (85.7%) |
CONCLUSION
Despite surgeons’ best efforts, some patients will inevitably have infected prostheses. Many patients achieve infection-free state through surgery and appropriate antibiotics. Treatment for some patients’ infections can include extensive surgery and time in hospital. Although some research has focused on the effects of antibiotic resistance and organism class on periprosthetic hip and/or knee joint infection outcomes, there has not been a recent study to comprehensively evaluate the impact of various infectious organisms in hip arthroplasty while controlling for relevant baseline characteristics. In order to address this question, we retrospectively reviewed cases of hip periprosthetic infection to determine the impact of organisms on outcomes relevant to patients, providers, and healthcare systems. Multivariable analysis of this cohort showed that certain organisms cultured during treatment were associated with lower likelihood of infection-free state, more surgery, or more time in hospital in treatment for hip periprosthetic infection.
The question that is perhaps the most important to provider and patient alike is whether or not the patient’s infection will remain infection-free. MRSA, pseudomonas, and proteus infection were each associated with decreased infection-free rates. Pseudomonas and proteus were associated with strikingly low infection-free rates (38.5% and 33.3%, respectively, compared to approximately 90% of patients infected with any other organism). This current study’s finding of poor outcomes associated with pseudomonas and proteus aligns well with the 1981 findings of Bucholz and colleagues that also suggested that pseudomonas and proteus, among a few other gram-negative organisms (E. coli and klebsiella) were associated with treatment failure [18]. Similarly, Zmitowski et. al. has found that gram-negative infections have a higher overall rate of failure [16]. We did not find, however, that all gram-negative organisms predispose to poor outcomes. This study’s findings suggest that the type of gram-negative organism may also be important. The finding that MRSA infection-free rates are low is similar to several reports of failure with methicillin-resistant organism failure [12–15].
Even though surgical treatment and associated hospitalization for infection likely contributes the vast majority of the direct cost associated with periprosthetic hip infection treatment, there has been limited analysis of these two outcomes. Various organisms across the spectrum of prevalence were associated with increased surgical treatment of their infection (range: 1.13 – 2.58 additional surgeries) when compared with infection by any other organism. While MSSA, CoNS, and MRSA were correlated most strongly with increased surgery, peptostreptococcus infection (2.58 additional surgeries) had the largest impact on total surgeries for infection. Candida species (2.36 additional surgeries) were also associated with a high number of additional surgeries. While this result could be confounded by other unknown, unmeasured covariates relating to immunocompromise, which may sometimes be related to fungal infection, this may be the first report of markedly poor outcomes with fungal periprosthetic hip infection while accounting for baseline characteristics and other organisms.
Only 1 other study has evaluated differences in length of hospitalization between organisms to the author’s knowledge. Salgado et. al. found that patients with MRSA infection stayed in hospital 5 days longer on average than patients with MSSA infection [13]. Similar to the associations with total surgeries, multiple organisms were associated with extended length of hospitalization when compared with infection by any other organism (range: 8.56, 26.54 additional days). As with infection-free rates, pseudomonas and proteus were the gram-negative organisms that most significantly impacted this outcome. Although MRSA infection reduced infection-free rate, the findings from this study do not suggest that MRSA increases length of hospitalization over MSSA as had been previously reported. This study rather found that MRSA infection was associated with 5 fewer days in hospital and about 0.5 fewer operations. These findings may suggest that surgeons are abandoning hope of infection-free status for patients with MRSA rather than putting patients through additional surgery.
We also found that increased Charlson-Deyo comorbidity score correlated with increased time in hospital in treatment for infection although the total number of surgeries and infection-free rate were not impacted. This suggests that those patients with higher comorbidity may spend a longer time in hospital for treatment of infection even if they do not undergo more surgery for infection. This finding supports a role for collaboration with colleagues in medicine who may be able to appropriately manage patients with elevated comorbidity. Notably, this study did not identify a significant difference in outcomes based on infection chronicity.
One of this study’s strengths is its focus on outcomes that matter to patients, providers, and healthcare systems. In addition to evaluating overall infection-free rates, we also compared outcomes such as total surgeries and hospitalization days for infection treatment. Another strength of this study is the rigorous definition of infection-free status, which aligns with the international consensus statement on success after infection treatment. Further enhancing the validity of the study findings, patients had a minimum of 1 year of follow-up with orthopedic providers at our institution after their last surgery or treatment for infection. This is in contrast to including all patients with at least 1 year of follow-up after their first surgical treatment for infection. The conservative inclusion criteria for this study likely selects patients whose treatment is complete rather than still in progress. Additionally, we re-evaluated the entire possible study population regardless of length of follow-up (n=300) and found only minor differences between the study findings with the exclusion criteria. Lastly, we simultaneously evaluated patient characteristics that could impact outcomes including demographics and comorbidity score when these factors demonstrated univariate association with study outcomes.
As with most retrospective studies, this investigation is limited by its reliance on information gathered from chart review. There may be inconsistencies or incomplete data recorded in charts that could impact the quality of data used for research. Retrospective studies also may suffer from unknown and unrecognized confounders. Additionally, since this study was not prospective, surgical and medical treatment and infection chronicity was not standard across all patients and instead was up to the discretion of the treating providers at the time though the vast majority (98.7%) of patients’ medical treatment was decided in consultation with infectious disease specialists. Because treatments were not standardized, the number of surgeries and length of hospitalization were used as a metric of patient and healthcare burden rather than breaking up outcomes by type of intervention. Future studies could expand sample size, collect data prospectively, apply universal infection treatment protocols, and include both academic and community data since the academic setting may not always reflect the characteristics of the broader community.
Overall, MRSA pseudomonas, and proteus infection were associated with all 3 outcomes of interest, which suggests that patients infected by these organisms may be at greatest risk for extensive treatment and potential treatment failure. CoNS, MSSA, diphtheroids, peptostreptococcus, and klebsiella were each associated with increased surgery and length of hospitalization while beta hemolytic streptococcus, candida, propionibacterium acnes, enterococcus, and elevated comorbidity score were associated with 1 of the study outcomes. This retrospective analysis of hip periprosthetic infections treated at a tertiary medical center gives insight into the way in which infectious organisms are associated with granular treatment outcomes for periprosthetic infection. Organism-specific outcome information may help individualize patient-physician discussions about the expected course of treatment for hip periprosthetic infection.
Supplementary Material
Acknowledgments
Funding: This work was supported in part by an NIH training grant through the Clinical and Translational Science Awards, TL1TR001116. The funding source played no role in the investigation.
Appendix
Appendix Table 1.
Patients undergoing only I+D with or without head and/or liner exchange. Averages and 95% confidence intervals displayed when relevant.
| Surgical treatment |
Number (n=68) |
Surgeries | Infection- free rate |
Surgeries | Hospitalization time |
|---|---|---|---|---|---|
| Only I+D without head and/or liner exchange (n=24) |
24 / 68 (35.3%) |
1.79 (1.29, 2.29) |
65 /
68 (95.6%) |
1.47 (1.27, 1.67) |
9.72 (7.96, 11.49) |
| Only I+D with head and/or liner exchange (n=37) |
37 / 68 (54.3%) |
1.12 (1.01, 1.21) |
|||
| Only multiple I+D’s with and without head and/or liner exchange (n=7) |
7 / 68 (10.3%) |
2 (2, 2) |
148 of 149 subjects underwent operative treatment of their infections. One patient refused operative intervention. 68 / 149 (45.6%) underwent only I+D with or without head and/or liner exchange as their surgical treatment. 24 / 68 (35.3%) of these patients underwent only I+D without head and/or liner exchange; 37 / 68 (54.5%) underwent only I+D with head and/or liner exchange; and 7 / 68 (10.3%) underwent a combination of I+D with and without head and/or liner exchange. Overall, 65 / 68 (95.6%) of patients undergoing I+D without any resection procedure remained infection-free. Patients undergoing these strategies underwent a mean of 1.47 procedures and spent a mean of 9.72 days in hospital as part of infection treatment.
Appendix Table 2.
Patients undergoing only 1-stage or 2-stage resection with or without antibiotic spacer placement with or without eventual re-implantation. All spacers included antibiotics. Hemi-radical resection refers to removal of head, liner, and acetabular component but leaving the well-fixed stem. Averages and 95% confidence intervals displayed when relevant.
| Surgical treatment |
Patients (n=42) |
Surgeries | Reimplantation attempted |
Infection -free rate |
Surgeries | Hospitalization time |
|---|---|---|---|---|---|---|
| Only 2- stage resection of components with spacer placement (n=23) |
23 / 42 (54.8%) |
1.04 (0.97, 1.12) |
21 / 23 (91.3%) | 38 / 42 (90.5%) |
2.09 surgeries (1.86, 2.33) |
12.48 days (10.34, 14.61) |
| Only 1- stage resection of components with immediate re- placement of components (n=5) |
5 / 42 (11.9%) |
1 (1, 1) | Not applicable | |||
| Only resection without spacer placement (n=4) |
4 / 42 (9.5%) |
1 (1, 1) | 3 / 4 (75%) | |||
| Only re- placement of spacer (presented to our institution with spacer in place) (n=2) |
2 / 42 (4.7%) | 1.5 (0.98, 2.02) | 1 / 2 (50%) | |||
| Only combination of 1- stage, 2- stage and/or hemi- radical resection |
8 / 42 (19.0%) |
2.38 (1.86, 2.89) |
7 / 8 (87.5%) |
42 / 149 patients (28.2%) underwent only 1-stage or 2-stage resections with or without spacer placement. 23 / 42 (54.8%) of these underwent only 2-stage resections of components with placement of an antibiotic spacer and 21 / 23 (91.3%) of these patients underwent at least 1 attempt at reimplantation; 5 / 42 (11.9%) underwent only 1-stage resections of components and re-placement of components ; 4 / 42 (9.5%) underwent only resections of components without spacer placement and 3 / 4 (75%) of these patients underwent at least 1 reimplantation; and 2 / 42 (4.7%) underwent only re-placements of antibiotic spacer as they had presented to our institution with spacer in place (1 patient had the antibiotic spacer re-placed once at our institution and the other twice at our institution) and 1 of these patients underwent 1 reimplantation attempt. 8 / 42 (19.0%) underwent a combination of 1-stage, 2-stage, and/or hemi-radical and 7 / 8 (87.5%) of these patients underwent 1 reimplantation attempt. Of the patients who had a 2-stage or hemi-radical resection, 32 / 37 (86.5%) underwent a reimplantation attempt. Overall, 38 / 42 (90.5%) of patients undergoing any resection procedure without any I+D remained infection-free. Patients undergoing these procedures underwent a mean of 2.09 procedures and spent a mean of 12.48 days in hospital as part of infection treatment. All spacers incorporated antibiotics in the cement.
Appendix Table 3.
Patients undergoing a combination of I+D and resection. Because patients underwent a combination of procedures, percentages represent the proportion of this population that underwent at least 1 of the listed procedures. Unlike in the previous tables, the percentages will not add to 100%. All spacers included antibiotics. Hemi-radical resection refers to removal of head, liner, and acetabular component but leaving the well-fixed stem. Averages and 95% confidence intervals displayed when relevant.
| Surgical treatment | Patients (n=38) |
Infection- free rate |
Surgeries | Hospitalization time |
|---|---|---|---|---|
| 2-stage resection of components with spacer placement (n=28) |
28 / 38 (73.7%) |
25 /
38 (65.8%) |
5.45 procedures (4.44, 6.45) |
36.54 days
(27.03, 46.04) |
| I+D without head and/or liner exchange (n=24) |
24 / 38 (63.2%) |
|||
| I+D with head and/or liner exchange (n=23) |
23 / 38 (60.5%) |
|||
| One-stage resection of components and re-placement of components (n=9) |
9 / 38 (23.7%) |
|||
| Resection of components without spacer placement (n=6) |
6 / 38 (15.8%) |
|||
| Hemi-radical resection |
2 / 38 (5.3%) |
The remaining 38 / 149 (25.5%) of patients underwent a combination of I+D and resection. 28 / 38 (73.7%) underwent at least one 2-stage resection of components with placement of an antibiotic spacer; 24 / 38 (63.2%) underwent at least one I+D without head and/or liner exchange; 23 / 38 (60.5%) underwent at least one I+D with head and/or liner exchange; 9 / 38 (23.7%) underwent at least one 1-stage resection of components and re-placement of components; 6 / 38 (15.8%) underwent at least one resection of components without placement of any spacer; 2 / 38 (5.3%) underwent at least one hemi-radical resection. Overall, 25 / 38 (65.8%) of patients undergoing any resection procedure as well as any I+D remained infection-free. Patients undergoing this combination of procedures underwent a mean of 5.45 procedures and spent a mean of 36.54 days in hospital as part of infection treatment.
Appendix Table 4.
IV antibiotic usage percentages for each organism.
| Organism | Amikacin | Amoxicillin/clavulanate | Ampicillin | Aztreonam | Cefazolin | Cefepime | Cefoxitin | Ceftaroline | Ceftazidime | Ceftriaxone | Cefuroxime | Daptomycin | Ertapenem | Fluconazole | Gentamycin | Imipenem | Meropenem | Nafcillin | Oxacillin | Penicillin G | Piperacillin/tazobactam | Tobramycin | Amoxicillin | Ampicillin/sulbactam | Vancomycin |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acinetobacter species |
0 | 0 | 0 | 0 | 14.2 | 0 | 0 | 0 | 14.2 | 0 | 0 | 28.5 | 0 | 0 | 0 | 14.2 | 0 | 0 | 0 | 0 | 14.2 | 0 | 0 | 14.2 | 42.8 |
| Candida species | 0 | 0 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 9 | 0 | 54.5 | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 0 | 0 | 0 | 27.2 |
| CoNS | 1.1 | 0 | 0 | 0 | 6.6 | 0 | 0 | 0 | 3.3 | 4.4 | 0 | 5.5 | 1.1 | 1.1 | 1.1 | 0 | 0 | 6.6 | 0 | 0 | 0 | 0 | 0 | 0 | 62.2 |
| Diphtheroids | 0 | 0 | 0 | 7.1 | 7.1 | 0 | 0 | 0 | 7.1 | 7.1 | 0 | 14.2 | 0 | 7.1 | 0 | 0 | 0 | 0 | 0 | 0 | 7.1 | 0 | 0 | 7.1 | 57.1 |
| Enterobacter species |
0 | 0 | 0 | 0 | 11.1 | 0 | 0 | 0 | 0 | 11.1 | 0 | 33.3 | 0 | 0 | 0 | 0 | 11.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11.1 |
| Enterococcus species |
0 | 7.6 | 23 | 0 | 0 | 3.8 | 3.8 | 0 | 3.8 | 0 | 3.8 | 26.9 | 0 | 19.2 | 3.8 | 0 | 0 | 0 | 0 | 0 | 11.5 | 0 | 7.6 | 11.5 | 23 |
| Eschericia species | 0 | 6.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 18.7 | 12.5 | 12.5 | 12.5 | 18.7 | 0 | 0 | 0 | 0 | 0 | 0 | 12.5 | 0 | 6.2 | 6.2 | 31.2 |
| Beta hemolytic streptococcus |
4.3 | 4.3 | 0 | 0 | 13 | 4.3 | 0 | 0 | 0 | 47.8 | 0 | 8.6 | 4.3 | 0 | 0 | 0 | 0 | 8.6 | 0 | 0 | 4.3 | 4.3 | 4.3 | 0 | 4.3 |
| Klebsiella species |
0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 18.1 | 36.3 | 9 | 18.1 | 0 | 0 | 9 | 0 | 0 | 0 | 18.1 | 0 | 0 | 0 | 36.3 |
| MRSA | 0 | 0 | 0 | 0 | 0 | 1.8 | 1.8 | 3.7 | 1.8 | 0 | 0 | 14.8 | 5.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3.7 | 0 | 0 | 1.8 | 87 |
| MSSA | 1.4 | 0 | 0 | 0 | 38.5 | 0 | 0 | 0 | 0 | 0 | 0 | 4.2 | 2.8 | 0 | 0 | 0 | 0 | 28.5 | 1.4 | 0 | 1.4 | 0 | 0 | 0 | 15.7 |
| Peptostreptococ cus species |
0 | 11.1 | 11.1 | 0 | 11.1 | 11.1 | 0 | 0 | 0 | 11.1 | 0 | 0 | 0 | 11.1 | 22.2 | 0 | 0 | 0 | 0 | 33.3 | 0 | 0 | 22.2 | 0 | 22.2 |
| Propionibacteri um acnes |
0 | 0 | 0 | 0 | 7.6 | 7.6 | 0 | 0 | 0 | 0 | 0 | 15.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7.6 | 0 | 0 | 7.6 | 0 | 30.7 |
| Proteus species | 0 | 11.1 | 0 | 0 | 22.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 22.2 | 22.2 |
| Pseudomonas species | 8 | 0 | 0 | 8 | 4 | 24 | 0 | 0 | 12 | 0 | 0 | 20 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 12 | 8 | 4 | 0 | 36 |
| Viridans streptococcus |
0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 37.5 | 0 | 0 | 0 | 12.5 | 0 | 0 | 0 | 0 | 0 | 25 | 0 | 0 | 0 | 0 | 25 |
Appendix Table 5.
Oral antibiotic usage percentages in each organism
| Organism | Azithromycin | Cefixime | Cephalexin | Ciprofloxacin | Clindamycin | Dicloxacillin | Doxycycline | Ethambutol | Levofloxacin | Linezolid | Metronidazole | Minocycline | Penicillin V | Rifampin | Tmp/smx |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acinetobacter species | 0 | 0 | 0 | 42.8 | 14.2 | 0 | 0 | 0 | 28.5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Candida species | 9 | 0 | 0 | 27.2 | 0 | 0 | 0 | 9 | 9 | 0 | 9 | 9 | 0 | 9 | 9 |
| CoNS | 1.1 | 0 | 5.5 | 15.5 | 1.1 | 5.5 | 5.5 | 1.1 | 1.1 | 0 | 1.1 | 2.2 | 0 | 10 | 7.7 |
| Diphtheroids | 0 | 0 | 7.1 | 42.8 | 0 | 0 | 7.1 | 0 | 7.1 | 7.1 | 7.1 | 14.2 | 0 | 0 | 14.2 |
| Enterobacter species | 0 | 0 | 0 | 88.8 | 11.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11.1 |
| Enterococcus species | 0 | 0 | 0 | 19.2 | 0 | 0 | 0 | 0 | 3.8 | 0 | 3.8 | 7.6 | 0 | 0 | 11.5 |
| Eschericia species | 0 | 0 | 12.5 | 43.7 | 0 | 0 | 0 | 0 | 0 | 0 | 6.2 | 0 | 0 | 0 | 0 |
| Beta hemolytic streptococcus | 0 | 0 | 8.6 | 13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8.6 | 4.3 |
| Klebsiella species | 0 | 0 | 9 | 45.4 | 9 | 0 | 0 | 0 | 9 | 0 | 0 | 9 | 0 | 0 | 9 |
| MRSA | 0 | 0 | 7.4 | 9.2 | 0 | 0 | 5.5 | 0 | 1.8 | 1.8 | 0 | 3.7 | 0 | 11.1 | 22.2 |
| MSSA | 0 | 0 | 21.4 | 11.4 | 1.4 | 2.8 | 4.2 | 0 | 2.8 | 1.4 | 0 | 1.4 | 0 | 20 | 21.4 |
| Peptostreptococcus species | 0 | 0 | 0 | 11.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11.1 | 0 | 0 | 11.1 |
| Propionibacterium acnes | 0 | 0 | 0 | 23 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Proteus species | 0 | 0 | 0 | 55.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11.1 | 0 |
| Pseudomonas species | 0 | 4 | 0 | 48 | 4 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 4 |
| Viridans streptococcus | 0 | 0 | 0 | 12.5 | 0 | 0 | 0 | 0 | 0 | 0 | 12.5 | 0 | 12.5 | 0 | 0 |
Appendix Table 6.
Proportions of patients using antibiotic suppression >6 months after final surgery or hospitalization for infection.
| Group | Proportion using antibiotic suppression |
|---|---|
| Overall | 38 / 149 (25.5%) |
| Infection-free | 27 / 128 (21.0%) |
| Not infection-free | 11 / 21 (52.3%) |
Appendix Table 7.
Frequencies of all antibiotics used in chronic suppression.
| Antibiotic | Patient usage |
|---|---|
| Trimethoprim/sulfamethoxazole | 10 |
| Cephalexin | 9 |
| Minocycline | 6 |
| Ciprofloxacin | 4 |
| Doxycycline | 4 |
| Amoxicillin | 1 |
| Amoxicillin/clavulanate | 1 |
| Cefoxitin | 1 |
| Clindamycin | 1 |
| Dicloxacillin | 1 |
| Levofloxacin | 1 |
| Moxifloxacin | 1 |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet. 2007;370(9597):1508–1519. doi: 10.1016/S0140-6736(07)60457-7. [DOI] [PubMed] [Google Scholar]
- 2.Bozic KJ, et al. Patient-related risk factors for periprosthetic joint infection and postoperative mortality following total hip arthropl asty in Medicare patients. J Bone Joint Surg Am. 2012;94(9):794–800. doi: 10.2106/JBJS.K.00072. [DOI] [PubMed] [Google Scholar]
- 3.Edwards C, et al. Early infection after hip fracture surgery: risk factors, costs and outcome. J Bone Joint Surg Br. 2008;90(6):770–777. doi: 10.1302/0301-620X.90B6.20194. [DOI] [PubMed] [Google Scholar]
- 4.Ong KL, et al. Prosthetic joint infection risk after total hip arthroplasty in the Medicare population. J Arthroplasty. 2009;24(6 Suppl):105–109. doi: 10.1016/j.arth.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 5.Gristina AG, Kolkin J. Current concepts review. Total joint replacement and sepsis. J Bone Joint Surg Am. 1983;65(1):128–134. [PubMed] [Google Scholar]
- 6.Bauer TW, et al. Diagnosis of periprosthetic infection. J Bone Joint Surg Am. 2006;88(4):869–882. doi: 10.2106/JBJS.E.01149. [DOI] [PubMed] [Google Scholar]
- 7.Garvin KL, Hanssen AD. Infection after total hip arthroplasty. Past, present, and future. J Bone Joint Surg Am. 1995;77(10):1576–1588. doi: 10.2106/00004623-199510000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Kurtz S, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 9.Tande AJ, Patel R. Prosthetic joint infection. Clin Microbiol Rev. 2014;27(2):302–345. doi: 10.1128/CMR.00111-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsukayama DT, Estrada R, Gustilo RB. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J Bone Joint Surg Am. 1996;78(4):512–523. doi: 10.2106/00004623-199604000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Fernandes A, Dias M. The Microbiological Profiles of Infected Prosthetic Implants with an Emphasis on the Organisms which Form Biofilms. J Clin Diagn Res. 2013;7(2):219–223. doi: 10.7860/JCDR/2013/4533.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Volin SJ, Hinrichs SH, Garvin KL. Two-stage reimplantation of total joint infections: a comparison of resistant and non-resistant organisms. Clin Orthop Relat Res. 2004;(427):94–100. doi: 10.1097/01.blo.0000143559.34143.3d. [DOI] [PubMed] [Google Scholar]
- 13.Salgado CD, et al. Higher risk of failure of methicillin-resistant Staphylococcus aureus prosthetic joint infections. Clin Orthop Relat Res. 2007;461:48–53. doi: 10.1097/BLO.0b013e3181123d4e. [DOI] [PubMed] [Google Scholar]
- 14.Lim SJ, et al. Treatment of periprosthetic hip infection caused by resistant microorganisms using 2-stage reimplantation protocol. J Arthroplasty. 2009;24(8):1264–1269. doi: 10.1016/j.arth.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Leung F, et al. Two-stage total hip arthroplasty: how often does it control methicillin-resistant infection? Clin Orthop Relat Res. 2011;469(4):1009–1015. doi: 10.1007/s11999-010-1725-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zmistowski B, et al. Prosthetic joint infection caused by gram-negative organisms. J Arthroplasty. 2011;26(6 Suppl):104–108. doi: 10.1016/j.arth.2011.03.044. [DOI] [PubMed] [Google Scholar]
- 17.Hsieh PH, et al. Gram-negative prosthetic joint infections: risk factors and outcome of treatment. Clin Infect Dis. 2009;49(7):1036–1043. doi: 10.1086/605593. [DOI] [PubMed] [Google Scholar]
- 18.Buchholz HW, et al. Management of deep infection of total hip replacement. J Bone Joint Surg Br. 1981;63-B(3):342–353. doi: 10.1302/0301-620X.63B3.7021561. [DOI] [PubMed] [Google Scholar]
- 19.von Elm E, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 20.Diaz-Ledezma C, Higuera CA, Parvizi J. Success after treatment of periprosthetic joint infection: a Delphi-based international multidisciplinary consensus. Clin Orthop Relat Res. 2013;471(7):2374–2382. doi: 10.1007/s11999-013-2866-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quan H, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 22.McPherson EJ, et al. Periprosthetic total hip infection: outcomes using a staging system. Clin Orthop Relat Res. 2002;403:8–15. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.