Abstract
Histotripsy is a noninvasive therapeutic technique that uses ultrasound generated from outside the body to create controlled cavitation in targeted tissue, and fractionates it into acellular debris. We have developed a new histotripsy approach, termed microtripsy, to improve targeting accuracy and to avoid collateral tissue damage. This in vivo study evaluates the safety and efficacy of microtripsy for noninvasive thrombolysis in a porcine deep vein thrombosis (DVT) model. Acute thrombi were formed in left femoral veins of pigs (~35 kg) by occluding the vessel using two balloon catheters and infusing with thrombin. Guided by real-time ultrasound imaging, microtripsy thrombolysis treatment was conducted in 14 pigs. 10 pigs were euthanized on the same day (acute) and 4 at 2 weeks (subacute). To evaluate vessel damage, 30-min free-flow treatment in right femoral vein (no thrombus) was also conducted in 8 acute pigs. Blood flow was successfully restored or significantly increased after treatment in 13 out of the 14 pigs. The flow channels reopened by microtripsy had a diameter up to 64% of the vessel diameter (~6 mm). The average treatment time was 16 minute per cm-long thrombus. Only mild intravascular hemolysis was induced during microtripsy thrombolysis. No damage was observed on vessel walls after two weeks of recovery, venous valves were preserved, and there was no sign of pulmonary embolism. The results of this study indicate that microtripsy has the potential to be a safe and effective treatment for DVT in a porcine model.
Keywords: Histotripsy, Microtripsy, Thrombolysis, Sonothrombolysis, In Vivo, Deep Vein Thrombosis
Introduction
Deep vein thrombosis (DVT) is the most common form of venous thrombosis and can lead to pulmonary embolism (PE). DVT/PE affects over 300,000 people and results in the deaths of 60,000 to 100,000 people each year in the United States (Beckman et al. 2010). In addition to anticoagulation, some DVT patients, especially those with severe symptoms, may require thrombolytic treatments, including systemic administration of thrombolytic drugs (Adams et al. 1996; Bates and Ginsberg 2004), catheter-directed infusion of thrombolytic drugs, or mechanical thrombectomy. Systemic administration of thrombolytic drugs has limited effectiveness and requires prolonged time for affect (several hours to days) (Friedman et al. 1996). Catheter-directed thrombolysis can deliver drugs locally at the thrombosis site, but it is invasive and carries risks of bleeding, vascular damage, clot detachment, and infection (Sharafuddin et al. 2003). In more severe cases, such as phlegmasia cerulea dolens, surgical or percutaneous mechanical thrombectomy may be performed (Karthikesalingam et al. 2011).
Histotripsy is a noninvasive tissue ablation method that mechanically fractionates soft tissue using ultrasound (Xu et al. 2007; Xu et al. 2008; Xu et al. 2010). High-intensity, microsecond-long ultrasound pulses are focused to generate well-controlled acoustic cavitation to fractionate target tissue without thermal necrosis. The feasibility of using histotripsy as a noninvasive and image-guided thrombolysis method was first shown by Maxwell et al. (Maxwell et al. 2009; Maxwell et al. 2011a). Histotripsy was used to fractionate blood clots into acellular debris using ultrasound alone both in vitro and in an in vivo porcine DVT model. The safety concerns (primarily hemolysis) of histotripsy in circulating blood were also addressed by Devanagondi et al. (Devanagondi et al. 2015). In the previous studies, multi-cycle (usually ≥ 5 cycle) ultrasound pulses were used to generate acoustic cavitation via a shock scattering mechanism (Maxwell et al. 2011b). Using the shock scattering mechanism, a shock front scatters from individual sparse bubbles formed from weak nuclei resulting in an inverted shockwave. This inverted shockwave combines with the incoming negative pressure phase to create very high negative pressures exceeding the cavitation threshold and generates a cavitation cloud. Because the shock scattering approach relies on the weak nuclei which often reside in the vessel wall, cavitation generated by this approach is usually less-confined and forms near the vessel wall. This poor localization within the clot can result in vessel damage and hemolysis.
Microtripsy is a new histotripsy approach that has recently been evaluated for its use in thrombolysis (Zhang et al. 2015a; Zhang et al. 2015c; Zhang et al. 2016). Microtripsy uses the intrinsic threshold mechanism where acoustic cavitation is generated via single-cycle ultrasound pulses with the negative pressure phase directly exceeding the cavitation threshold intrinsic to the media. The intrinsic threshold mechanism does not rely on pre-existing weak nuclei and is more reproducible and predictable than the shock scattering mechanism (Maxwell et al. 2013; Lin et al. 2014). Our in vitro microtripsy thrombolysis study showed that cavitation can be precisely generated and confined to the vessel lumen without contacting the vessel wall, which allows for creation of a precise flow channel within the clot while minimizing the risk of vessel damage (Zhang et al. 2015c).
This in vivo study investigated the safety and efficacy of microtripsy-mediated thrombolysis in a porcine DVT model. Thrombi were created in the femoral veins of juvenile pigs and then treated using an integrated, portable microtripsy thrombolysis system. Ultrasound images were used to guide and monitor the treatment. Ultrasound cross-sectional scans of the femoral vein were acquired before and after each thrombolysis treatment for qualitative and quantitative assessments of treatment efficacy. To evaluate the treatment safety, vessel damage was examined by histology. Blood samples were collected to evaluate the degree of hemolysis induced during microtripsy thrombolysis. In addition to acute pigs, which were euthanized right after therapy, four pigs were survived for two weeks to assess the subacute extent of any vessel damage and/or hemolysis.
Materials and Methods
Animal Preparation and Thrombus Formation
The protocols involved in this study have been approved by the University Committee on Use and Care of Animals at our university. A porcine DVT model, previously described by Maxwell et al. (Maxwell et al. 2011a), was used in this study. Juvenile pigs (mixed breed) weighing approximately 35 kg were selected as the subjects of the study. The animal was first sedated with 6 mg/kg tiletamine + zolazepam (Telazol, Fort Dodge Animal Health, Fort Dodge, Iowa, USA) and 2.2 mg/kg xylazine (Lloyd Laboratories, Shenandoah, Iowa, USA). The animal was then endotracheally intubated and rotated to a supine position onto a medical grade, water-filled heating pad to maintain body temperature. 0.5%–3.5% isoflurane (Vet-One, Meridian, Idaho, USA) was administered through the endotracheal tube for anesthesia. The animal was attached to monitoring equipment for continuous monitoring of core body temperature, pulse, SpO2 levels, and respiratory rate throughout the procedure. A chemical depilatory (Nair, Church & Dwight Co, Princeton, New Jersey, USA) was applied on the legs and the lower quadrant for 10 minutes, followed by a surgical preparation consisting of three applications of betadine scrub followed by a sterile saline rinse and an application of iodine. The area was then draped with a sterile drape.
Two 5-Fr wedge occlusion balloon catheters (AI-07124, Arrow International, Reading, Pennsylvania, USA) were introduced percutaneously into the left femoral vein from the distal side of the desired location for thrombus formation (Maxwell et al. 2011a). The two balloons were positioned and inflated to occlude a 1.5-cm segment at the desired location in the vein. 0.5 mL thrombin (1000 IU/mL) was then infused through the distal catheter into the occluded region in the vein to stimulate thrombus formation. Heparin (200 U/kg, IV) was administered systemically through an ear-vein catheter immediately after thrombin infusion to prevent blood coagulation outside the occluded region. The balloons remained inflated for two hours to allow the thrombus to become fully formed (Ryan et al. 1999). The balloons were then deflated and the catheters were removed from the vein. Blood pressure was continuously monitored from the carotid artery. Additional heparin (200 U/kg, IV) was administered every hour from the start of thrombus formation to the end of treatment to maintain an activated clotting time (ACT) of blood greater than 200 seconds. For the animals to be euthanized right after treatment, high dose pentobarbital (140–160 mg/kg, IV) was given. For the animals that were euthanized at two weeks after treatment, carprofen (2–4 mg/kg, SC) was given before treatment for pain relief, and protamine (0.5 mg/kg, IV) was given at the end of treatment to lower the ACT. The animals were continuously monitored until they were recovered from anesthesia and returned to the housing facility when they were fully mobile. Animals were observed twice daily for 48 hours for pain levels, activity levels, feeding activity, and any evidence of bleeding. Carprofen (2–4 mg/kg, PO) was given every 12–24 hours as needed until the animal returned to baseline activity levels.
Microtripsy Thrombolysis System and Setup
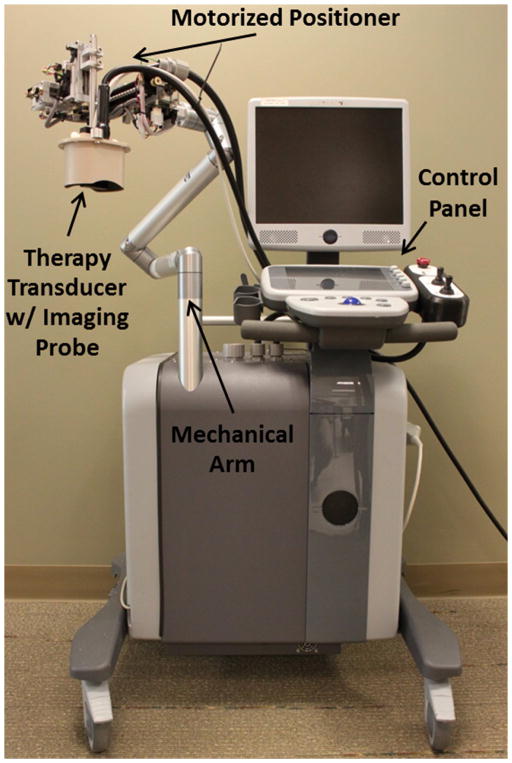
Thrombolysis treatments were performed with an integrated and portable microtripsy system, which was developed in-house for this specific application (Zhang et al. 2015c). It consisted of three subsystems: a microtripsy therapy system, an ultrasound imaging system, and a positioning system (Figure 1). Microtripsy pulses were generated by a 1-MHz, 18-element therapy transducer (Imasonic, Besancon, France) with an effective 10×8-cm aperture and a 7-cm focal length. A 7.5-MHz ultrasound imaging probe (Vermon, Tours, France) was embedded in the rectangular central hole of the therapy transducer to guide and monitor microtripsy thrombolysis treatment. The positioning system, including a compact motorized positioner and a multi-degree-of-freedom mechanical arm, provided high-resolution and large-range mobility for the therapy transducer. Control software with a clinician-friendly interface was developed to manage treatments.
Figure 1.
The integrated microtripsy thrombolysis system. It consists of an ultrasound imaging system, a microtripsy therapy system, and a motorized positioning system. A linear imaging probe was embedded at the center of the therapy transducer. The therapy transducer is mounted on the top of the positioning system. Treatment is controlled using touch screen interface and physical buttons on the control panel.
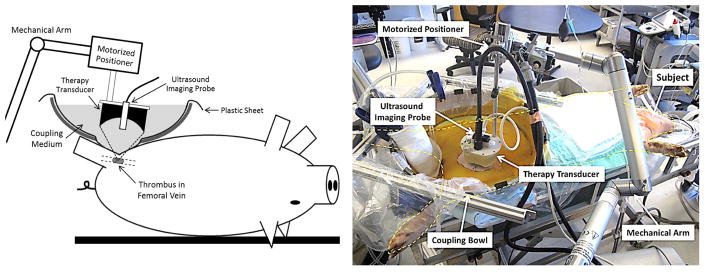
To enable direct ultrasound transmission between the therapy transducer and the subject, an IobanR sheet (3M, St Paul, Minnesota, USA) was first pasted onto a larger polyethylene sheet from which a 25-cm-diameter circular piece was previously removed. The IobanR, exposed by the central hole, was then pasted on to the subject’s skin. The IobanR sheet was then cut away around the area of treatment. A bottomless bowl was placed around the combined sheet and was then filled with a water-based coupling medium developed in-house that was set to body-temperature and degassed overnight. This provided a clear acoustic window while still maintaining a water-tight seal that prevented the coupling medium from leaking. The experimental setup is shown in Figure 2.
Figure 2.
Schematic experimental setup (Left) and a picture of the actual setup during one thrombolysis treatment (Right).
Pre-treatment Planning
The focus of the therapy transducer was first calibrated in the coupling bath. Cavitation was generated by firing microtripsy pulses in the coupling media, and the cavitational bubble cloud was shown as a bright (hyperechoic), dynamic region on the ultrasound image. The center of the cavitation region was then marked as the therapy focus on the ultrasound imaging window. The therapy transducer was re-positioned to align the therapy focus within the left femoral vein where the thrombus was formed. The therapy focus, aiming at the center of the vessel lumen, was moved from the distal end to the proximal end of the thrombus. The moving path was linearly interpolated into a treatment path with treatment locations spaced by 0.3 mm.
Treatments
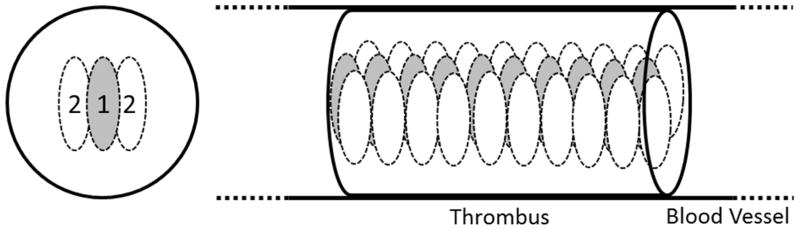
A dual-pass strategy, previously developed in our lab (Zhang et al. 2016), was used for the thrombolysis treatments in this study. The thrombus was treated with microtripsy in two passes (Figure 3). In the first pass, the treatment zone was covered by one focus (the original therapy focus) at each treatment location and scanned through the thrombus following the preset treatment path. In the second pass, the treatment zone followed the preset treatment path and scanned through the thrombus again but was covered by two foci electronically steered to the lateral sides of the first-pass focus. A pulse repetition frequency (PRF) of 100 Hz and a dose of 1000 pulses (10 seconds) at each focus was used for microtripsy treatments. The treatment time for each two-pass treatment was 16 min per cm length clot. With 1.5 to 2 cm overlying tissue and 0.5 dB/cm-MHz acoustic attenuation of the overlying tissue, the in situ peak negative pressure (P-) of the microtripsy pulses was estimated to be 34 to 35 MPa. In total, fourteen pigs were treated with microtripsy. Ten pigs were euthanized (Pentobarbital, 140–160 mg/kg, IV) within one hour after treatment (acute) and four pigs were recovered and euthanized two weeks later (subacute).
Figure 3.
Illustration of the dual-pass treatment. Each ellipse represents a focal zone with the number of the treatment pass. The gray foci were treated in the first treatment pass and the white foci were treated in the second treatment pass.
In 8 acute pigs, microtripsy treatment was also applied to the right femoral vein with no thrombi. Exposure of microtripsy in free blood flow in the femoral vein was considered to be the worst-case scenario test to evaluate the safety of microtripsy thrombolysis treatment, as it maximized the potential contact between cavitation and vessel wall (without the clot barrier between the two) and thus would lead to maximal vessel damage. In addition, the maximal volume of blood would be exposed to cavitation that could induce increased hemolysis without the thrombus barrier (Maxwell et al. 2011a; Devanagondi et al. 2015). The same dual-pass strategy and microtripsy parameters used in the thrombolysis treatment in the left femoral vein were used in the free-flow treatments in the right femoral vein. An 18-mm segment of the right femoral vein was treated for each pig (30 minutes).
Measurements and Evaluations
To qualitatively evaluate the efficacy of the microtripsy thrombolysis treatments, ultrasound B-mode and color Doppler images of the left femoral vein were collected before, right after and two weeks after the treatments to compare the volumes of the thrombi and the blood flows in the veins. A 10-MHz linear imaging probe (L14-5, Ultrasonix, Vancouver, Canada) was placed directly on the skin to image the thrombi and the veins. For quantitative analysis, the thrombi were 3D-scanned before and right after the treatments. Before each treatment, the 10-MHz imaging probe was mounted to a positioning system and positioned in the coupling bath to image the cross sections of the thrombus. A path was set to scan the imaging probe through the thrombus, acquiring a scan image every 0.2 mm. There was no direct contact between the imaging probe and the skin to avoid tissue movements during the scanning. After treatment, the imaging probe was scanned through the thrombus using the exact same path. The contours of the thrombus and the vessel lumen on each scan image were depicted manually in Matlab (The Mathworks, Natick, MA, USA). The diameter of the generated flow channel was quantified as the diameter of a circle with equivalent cross-sectional area on scan images.
Blood samples were collected before and after the thrombolysis treatments, after the free-flow treatments, and two weeks later for the subacute pigs. Serum laboratory studies and hemodynamic values were reported from each sample. Since the free-flow treatments always followed the thrombolysis treatments, the post-thrombolysis data were also used as the baseline data for the free-flow treatments. Carotid artery pressure was obtained at the start of each treatment and every 10 minutes during the treatment for all subjects.
The left femoral bundles, where the thrombolysis treatments were conducted, were harvested together with surrounding tissue from the 4 subacute pigs. The right femoral bundles, where the free-flow treatments were conducted, were harvested with surrounding tissue from the 8 acute pigs. Since the catheter and balloon procedures for thrombus formation caused significant vessel damage and would confound the data (Maxwell et al. 2011a), the left femoral veins of all the acute pigs were not collected for evaluation of vessel damage. The harvested tissues were immersed in 10% buffered formalin for fixation. The cross sections of the femoral bundles were then embedded in paraffin, sectioned, stained with hematoxylin and eosin and examined with a light microscope for signs of damage. The lungs from one acute pig and one subacute pig were fixed with 10% formalin and grossly examined for emboli.
Statistical Analysis
All statistical comparisons in this study were performed using Student’s t-test. P-values < 0.01 were considered significant. Numerical data are expressed as mean ± standard deviation.
Results
Thrombus
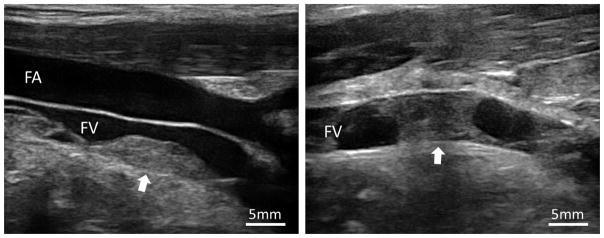
Thrombi were formed in the left femoral veins of the 14 subjects at a depth of 16 to 22 mm from skin (Figure 4). The lengths of the thrombi ranged from 7.5 to 28.6 mm, with an average length of 16.0 mm. The diameters of the veins where the thrombi were formed had a mean of 6.7 mm and a standard deviation (SD) of 1.1 mm. Nine thrombi were completely occlusive and five were partially occlusive with an occlusion percentage of 81.9 ± 12.2 % (mean ± SD). The morphological characteristics of the thrombi are summarized in Table 1.
Figure 4.
Partially and fully occlusive thrombi (Arrow) formed in femoral veins. FV: Femoral Vein; FA: Femoral Artery.
Table 1.
Summary of Thrombus Formations and Treatment Outcomes
| Partial | Occlusive | Total | |
|---|---|---|---|
| No. of Treatments | 5 | 9 | 14 |
| Thrombus Length (mm) | 15.3 ± 1.9 | 16.3 ± 6.9 | 16.0 ± 5.6 |
| Vessel Inner Diameter (mm) | 7.5 ± 1.2 | 6.2 ± 0.8 | 6.7 ± 1.1 |
| Vessel Occlusion (Area %)* | 81.9 ± 12.2 | 100 | 93.5 ± 11.3 |
| No. of Treatments with Flow Improved | 5 | 8 | 13 |
| Increase in Channel Diameter (mm) | 2.8 ± 1.2 | 3.6 ± 0.8 | 3.3 ± 1.0 |
| Occlusion Reduction (Area %)** | 13.3 ± 4.7 | 32.6 ± 6.1 | 26.7 ± 10.8 |
| Channel Opening (Diameter %)*** | 34.4 ± 12.8 | 56.2 ± 9.4 | 50.0 ± 14.4 |
Vessel Occlusion = occluded cross-sectional area / total vessel lumen area × 100%
Occlusion Reduction (Area %) = (pre-treatment vessel occlusion – post-treatment vessel occlusion) / pre-treatment vessel occlusion × 100%
Channel Opening (Diameter %) = generated channel diameter / vessel diameter × 100%
Cavitation
In all 14 subjects, during the thrombolysis treatments, cavitation bubble clouds were generated at the marked therapy focus without any spatial shift and were well confined within the target thrombi without contacting the femoral veins (Video). There was no pre-focal or post-focal cavitation observed on the vessel walls or surrounding tissues. The cavitation bubble cloud remained at the therapy focus when it scanned through thrombus. During the free-flow treatments, cavitation behaved differently. Although the therapy focus was targeted at the center of the vessel lumen, the cavitation bubble clouds were only sporadically visible on the B-mode image but occurred in both the free blood flow and on the post-focal vessel walls and tissues.
Recanalization
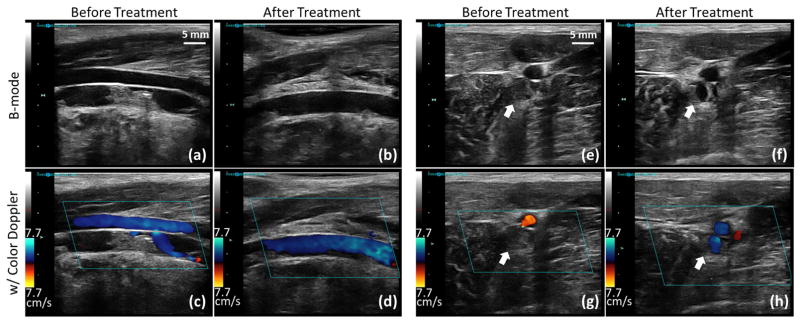
As shown by color Doppler imaging, blood flow was restored or significantly improved by the microtripsy treatments in 13 of the 14 subjects (Figure 5 and 6). In the one treatment where no flow was restored, the thrombus could not be completely scanned through by microtripsy due to the extension of the thrombus to regions with limited acoustic access. Thrombus volume reductions, indicated by brightness decrease on B-mode imaging, were observed in all treatments. Figure 5 shows ultrasound images of the femoral vein taken before and right after one thrombolysis treatment in an acute pig.
Figure 5.
Representative US B-mode images (first row) and color Doppler images (second row) taken before and right after microtripsy thrombolysis treatment in an acute pig. (a)–(d): Longitudinal sections of the femoral vein. (e)–(h): Cross sections of the femoral veins (indicated by the block arrows).
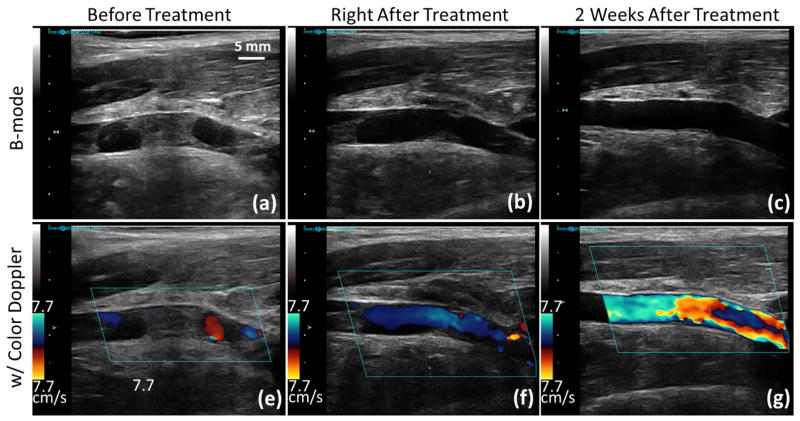
Figure 6.
Representative US B-mode images (first row) and color Doppler images (second row) taken before, right after and two weeks after microtripsy thrombolysis treatment in a subacute pig. Blood flow was increased after two weeks of recovery compared to that right after the treatment. Since a relatively smaller velocity scale was chosen to be consistent throughout all the color Doppler images, velocity aliasing occurred on the two-week color Doppler image (g) with an increased blood flow.
Figure 6 shows ultrasound images of the femoral vein taken before, right after and two weeks after one thrombolysis treatment in a subacute pig. For the subacute pigs, no remaining thrombus was observed in the femoral veins on ultrasound B-mode or color Doppler images after two weeks of recovery. The femoral veins appeared normal on the B-mode images. As shown on the color Doppler images of Figure 6(g) and (f), the blood flow after two weeks of recovery was further increased compared to the blood flow right after treatment, which was due to dissolution of both the remaining thrombus at the treatment site and the untreated thrombus outside the treatment site over two weeks.
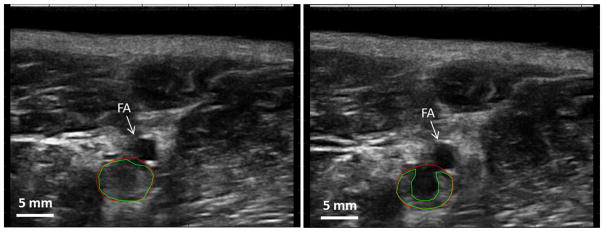
Quantitative evaluation of the thrombolysis efficacy was conducted using the pre- and post-treatment scan images of the thrombi (Figure 7). Cross sections of the flow channels generated in thrombi were distinguished as regions with significant echogenicity reduction between the pre- and post-treatment scan images. The flow channels generated in the fully occlusive thrombi had a diameter of 3.6 ± 0.8 mm, in comparison to a mean vessel diameter of 6.2 mm. The increase in channel diameter in the partially occlusive thrombi was 2.8 ± 1.2 mm. Occlusion (percent of cross-sectional area) was reduced by 32.6 ± 6.1% and 13.3 ± 4.7 % in the fully and partially occlusive thrombi, respectively. The vessels were opened by 56.2 V 9.4 % and 34.4 ± 12.8 % (generated channel diameter vs. vessel diameter) in the fully and partially occlusive thrombi, respectively. The average treatment time was 16 minutes per cm-long thrombus. The quantitative results are also presented in Table 1 and Table 2.
Figure 7.
Quantification of flow channel generated by microtripsy. On the left is the ultrasound image taken before treatment. On the right is the ultrasound image taken at the exact same location right after treatment. Green lines indicate the boundaries of the thrombi and red lines indicate the vessel lumen.
Table 2.
Blood Flow Rates (cm/s) Before and After Treatments, and After Two Weeks of Recovery
| Partial (N = 5) | Occlusive (N = 9) | Total (N = 14) | |
|---|---|---|---|
| Before Treatment | 0.87 ± 0.58 | 0.00 ± 0.00 | 0.31 ± 0.54 |
| After Treatment | 4.78 ± 1.93 | 5.20 ± 2.27 | 5.05 ± 2.09 |
| Increase in Flow Rate | 3.90 ± 1.53 | 5.20 ± 2.27 | 4.74 ± 2.08 |
| Two Weeks Later | -- | 7.45 ± 0.32* | 7.45 ± 0.32* |
N = 4
Blood
Hemodynamic and hematological effects of the microtripsy treatments are shown in Table 3. Given the concern for thrombolysis-induced hemolytic anemia, markers of hemolytic anemia were monitored during microtripsy therapy. The hematocrit was monitored to assess for the development of anemia. Plasma free hemoglobin, lactate dehydrogenase, and serum potassium were also monitored as they are indicators of red cell damage (McPherson and Pincus 2016). Serum chemistries analysis was performed in core clinical or veterinary laboratories at the University of Michigan with equipment continually calibrated and validated to ensure the most accurate results. Over the 14 thrombolysis treatments, there were significant increases in free hemoglobin (3.8 ± 3.4 mg/dL baseline vs 63.2 ± 21.6 mg/dL after treatment, P < 0.0001) and lactate dehydrogenase (LDH) (404.4 ± 104.3 U/L baseline vs 524.6 ± 130.1 U/L after treatment, P < 0.01). There was a small but statistically significant increase (P <0.0001) in serum potassium (4.1 ± 0.2 mmol/L baseline vs 4.9 ± 0.5 mmol/L after treatment). No significant difference was shown in serum hematocrit (P = 0.32) between the baseline (26.6 ± 2.5%) and post-treatment (27.1 ± 3.2%). With regard to hemodynamic responses, there were no significant changes in system systolic blood pressure (81.4 ± 11.2 mmHg vs 86.6 ± 10.8 mmHg, P = 0.11) or heart rate (79.8 ± 11.2 BPM vs 79.9 ± 12.5 BPM, P = 0.49).
Table 3.
Blood Contents Change. “*” indicates significant difference.
| Thrombolysis Treatments (N = 14) | Free-flow Treatments (N = 8) | Two Weeks of Recovery (N = 4) | ||||
|---|---|---|---|---|---|---|
| Baseline1 | Post | Baseline2 | Post | Baseline3 | Two weeks later | |
| Free Hgb (mg/dL) | 3.8 ± 3.4 | 63.2± 21.6* | 56.7 ± 25.1 | 78.1 ± 13.9 | 2.3 ± 0.8 | 2.1 ± 1.0 |
| LDH (U/L) | 404.4 ± 104.3 | 524.6 ± 130.1* | 554.1 ± 132.0 | 608.4 ± 164.5 | 355.0 ± 78.0 | 334.5 ± 67.0 |
| K (mmol/L) | 4.1 ± 0.2 | 4.9 ± 0.5* | 4.7 ± 0.4 | 4.9 ± 0.5 | 4.0 ± 0.3 | 3.6 ± 0.2 |
| Serum Hematocrit (%PCV) | 26.6 ± 2.5 | 27.1 ± 3.2 | 27.3 ± 3.9 | 27.3 ± 3.1 | 28.3 ± 1.0 | 25.3 ± 3.6 |
| pH | 7.3 ± 0.1 | 7.3 ± 0.1 | 7.4 ± 0.1 | 7.4 ± 0.1 | 7.3 ± 0.1 | 7.4 ± 0.03 |
| SBP (mmHg) | 81.4 ± 11.2 | 86.6 ± 10.8 | 84.5 ± 9.2 | 86.0 ± 11.3 | NA | NA |
| HR (BPM) | 79.8 ± 11.2 | 79.9 ± 12.5 | 76.6 ± 13.6 | 78.4 ± 14.5 | NA | NA |
The baselines are the measurements before any treatments and calculated using all 14 samples.
The baselines are the measurements after the thrombolysis treatments and before the free-flow treatments and calculated only using the corresponding 8 samples.
The baselines are the measurements before any treatments and calculated only using the corresponding 4 samples.
After two weeks of recovery for the 4 subacute pigs, all the parameters returned back to the pre-treatment baseline. Free hemoglobin decreased back to 2.1 ± 1.0 mg/dL, which showed no significant difference from its baseline (P = 0.41). LDH decreased to 334.5 ± 67.0 U/L and showed no difference from its baseline (P = 0.35). There was also no significant difference in serum potassium (P = 0.035) and serum hematocrit (P = 0.08) between the recoveries and their baselines.
For the 8 free-flow treatments, there was no significant change in all variables compared to the pre-free-flow treatment baselines: free hemoglobin (P = 0.027), LDH (P = 0.24), serum potassium (P = 0.18), system systolic blood pressure (P = 0.39), hear rate (P = 0.40) and serum hematocrit (P = 0.50).
Gross and Histology Evaluation
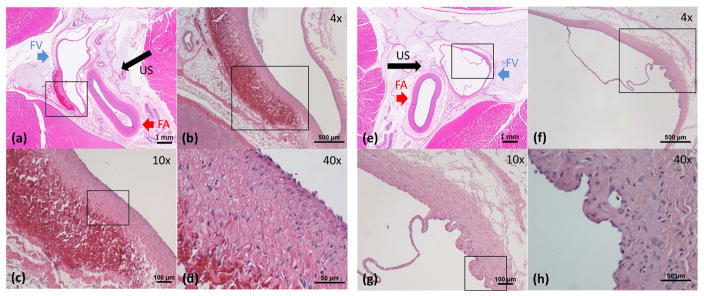
The hematoxylin and eosin stained sections of the femoral bundles were examined to assess vessel damage caused by microtripsy. Acute vessel damage was evaluated on the right femoral veins harvested right after the free-flow treatments (Figure 8(a)–(d)), and subacute vessel damage was evaluated on the left femoral veins recovered after two weeks from the thrombolysis treatments (Figure 8(e)–(h)). In Figure 8(a), the lumen with thin vessel wall on the left is the femoral vein and the lumen with thick wall on the right is the femoral artery. The tissue that immediately surrounds the vessels is connective tissue and the dense, outer surrounding is skeletal muscle. Figure 8(b)–(d) are magnified images of the area in the box in the previous image. In the acute vessel samples, there was focal venular wall thickening with corresponding hemorrhage in the tunica adventitia and thickening of the tunica media distal to the ultrasound source, as shown in Figure 8(a)–(d). Most of the tunica intima of the femoral veins was intact, with no evidence of perforation through vessel walls. In rare cases, there was tunica intima and media injury with hemorrhage into the tunica adventitia. No thrombus was noted in the lumen. No injury was found in or around the femoral arteries. In the subacute vessel samples (Figure 8(e)–(h)), there was a mild thickening of the tunica media with granulation tissue composed of small vascular channels and inflammatory cells, presumably secondary to the microtripsy injury. Otherwise, there were no other significant abnormalities. There was no hemorrhage or evidence of tunica intima injury. No injury to the femoral arteries and surrounding tissues was observed as well. Valves in the femoral veins were preserved from the thrombolysis treatments as shown in Figure 8(f)–(g). Grossly, there was no pulmonary embolus or infarct in the lung parenchyma.
Figure 8.
Histology of Femoral Veins. The direction of ultrasound propagation is indicated by black arrow. (a)–(d): Femoral vein wall right after a free-flow treatment. In (a), the lumen with thin vessel wall on the left is the femoral vein (FV, blue arrow) and the lumen with thick wall on the right is the femoral artery (FA, red arrow). The tissue that immediately surrounds the vessels is connective tissue and the dense, outer surrounding is skeletal muscle. (b)–(d) are magnified images of the area in the box in the previous image. There was focal venular wall thickening with corresponding hemorrhage in the tunica adventitia and thickening of the tunica media. Most of the tunica intima of the femoral vein was intact. No thrombus was noted in the lumen. No injury was found in or around the femoral arteries. (e)–(h): Femoral vein wall two weeks after a thrombolysis treatment. (f)–(h) are magnified images of the area in the box in the previous image. There was a mild thickening of the tunica media with granulation tissue composed of small vascular channels and inflammatory cells. There was no hemorrhage or evidence of tunica intima injury. No injury to the femoral arteries and surrounding tissues as well. Valves in the femoral veins were preserved.
Animal Behavior and Symptom Monitoring
During anesthesia, none of the animals showed clinical signs of distress due to the clot formation or the thrombolysis treatment. All recovery animals showed no significant pain levels post-treatment and returned to normal activity levels and feeding activity upon full recovery from anesthesia. No evidence of abnormal bleeding was observed in any of the recovered animals. No change in general behavior was observed during the course of two weeks.
Discussion
This study demonstrated the efficacy and safety of microtripsy-mediated thrombolysis in a porcine DVT model. Microtripsy reliably generated well-controlled acoustic cavitation in the pig femoral vein to perform thrombolysis treatments. In 13 out of the 14 thrombolysis treatments conducted in this study, a flow channel was generated through the completely occlusive or partially occlusive clot by microtripsy and blood flows were successfully restored or significantly improved. The flow channel diameters generated were increased by an average of 3.3 ± 1.0 mm, in comparison to an average vessel diameter of 6.7 mm. The average treatment time was 16.6 minutes per cm-long thrombus. Only mild intravascular hemolysis was induced during microtripsy thrombolysis. No damage was observed on vessel walls after two weeks of recovery, venous valves were preserved, and there was no sign of pulmonary embolism.
Currently, the first steps in the treatment of deep vein thrombosis generally include therapeutic drugs such as recombinant tissue plasminogen activator (rt-PA), which is a fibrin-specific thrombolytic (Higgins and Bennett 1990). However, drug-based therapy is not effective in retracted clots, and stringent dose-dependent guidelines for the use of rt-PA and other drugs due to the risk of serious systemic bleeding exist, which prevent them from being used in many cases (Turi et al. 1993). Therefore, many local interventions have been applied such as direct application of therapeutic agents to the clot or mechanical stimulation and disruption of the thrombus via direct contact (Mewissen et al. 1999; O’Sullivan et al. 2007). However, these methods are inherently invasive and even the local application of pharmaceuticals carries with it the risk of systemic side effects. Sonothrombolysis employs low intensity ultrasound to improve the efficacy of thrombolytic agents such as rt-PA or injected microbubbles for contrast enhancement (Porter et al. 1996; Behrens et al. 1999; Datta et al. 2008; Holland et al. 2008; Brown et al. 2011; Hitchcock et al. 2011). Various sonothrombolysis therapies have shown recent successes in the treatment of DVT and stroke in both animal and clinical trials in recent years (Raabe 2006; Bader et al. 2016). However, pharmaceutical thrombolysis has been shown to be ineffective in the treatment of retracted clots (Blinc et al. 1992; Kunitada et al. 1992; Sutton et al. 2013). Additionally, the application of ultrasound to pharmaceutical thrombolysis does not mitigate the risks of using such drugs, so microtripsy thrombolysis offers the distinct advantage of being effective for retracted clots and not requiring injected therapeutics. Another ultrasonic technique that has been applied to DVT is high-intensity focused ultrasound (HIFU), which generally employs the thermal effects of ultrasound to destroy unwanted tissue (Siegel and Luo 2008; Burgess et al. 2012; Wright et al. 2012). However, HIFU generally carries with it the potential for damage of surrounding tissue due to diffusive heating patterns (Church 2007). While all of the aforementioned technologies have shown very promising results in the treatment of DVT, microtripsy thrombolysis hold distinct advantages over other ultrasonic thrombolysis modalities in that it is non-invasive, non-thermal, does not require injection of microbubbles or other therapeutic agents and is also effective for retracted clot (Zhang et al. 2015a; Zhang et al. 2015c; Zhang et al. 2016).
Microtripsy and shock-scattering histotripsy are two different versions of histotripsy. They both utilize cavitation to fractionate soft tissues, but the mechanisms to generate cavitation are different (Maxwell et al. 2011b; Lin et al. 2014). They share some common advantages of histotripsy, such as no heating on overlay tissue and easy image-guidance (Kim et al. 2011; Vlaisavljevich et al. 2013; Kim et al. 2014). But Microtripsy is more reliable and has a higher precision. Compared to shock-scattering histotripsy, which relies on weak cavitation nuclei to generate the initial spare individual bubbles from which to scatter the shockwave, microtripsy uses the stable cavitation nuclei intrinsic to the tissue or blood. As a result, microtripsy can generate cavitation near tissue interfaces (e.g., tissue-fluid such as vessel wall, tissue-bone interface such as skull and rib surface, etc.), without forming cavitation on these interfaces and results in reliable cavitation generation. In contrast, shock-scattering cavitation often forms pre-focal cavitation on these tissue interfaces, thus shielding the focal cavitation. The eroded volume in tissue due to microtripsy matches well with the focal volume where the peak negative pressure exceeds the cavitation intrinsic threshold. When using a pressure just above the intrinsic threshold, the resulting cavitation zone can be much smaller than the focal zone, resulting in high treatment precision controlled by the pressure. For shock-scattering histotripsy, the size of the cavitation cloud depends on the layers of cavitation formed by shock-scattering, which is controlled by the number of acoustic cycles applied in the ultrasound pulse and is typically equal to or larger than the focal zone in size. We have previously published three in vitro studies (Zhang et al. 2015a; Zhang et al. 2015c; Zhang et al. 2016) taking incremental steps to investigate and demonstrate the advantages of microtripsy. This study is the first in vivo validation of microtripsy thrombolysis. The differences and significant improvements in comparison with the previously published in vivo shock-scattering histotripsy thrombolysis study are summarized in Table 4. In both in vivo studies, the same deep vein thrombosis porcine model was used.
Table 4.
Comparison of In vivo Thrombolysis Treatment Results in Porcine DVT Model
| Shock-scattering Histotripsy (Maxwell et al 2011) | Microtripsy | |
|---|---|---|
| Central Frequency | 1 MHz | 1 MHz |
| Cycles per Pulse | 5 | 1.5 |
| PRF | 1000 Hz | 100 Hz |
| P-* | ~17 MPa | ~35 MPa |
| Recanalization Success Rate | 58.3% (7 of 12) | 92.8% (13 of 14) |
| Increase in Channel Diameter** | 3.0 ± 0.8 mm | 3.3 ± 1.0 mm |
| Increase in Flow Rate | N/A | 4.74 ± 2.08 cm/s |
| Restored Flow | Less Continuous | Strong and Continuous |
| Treatment Time | ~ 5.2 min/cm | 16.6 min/cm |
| Estimated # of Pulses Delivered per Treatment | 625,000 | 180,000 |
| Increase in Free Hemoglobin*** | > 300 mg/dL | < 70 mg/dL |
| Endothelium Damage of Femoral Vein | Observed | Not Observed |
| Hemorrhage | Medium | Minor |
| Vessel Wall Perforation | No | No |
Estimated peak negative pressure at the target with assumptions of 2cm-thick overlaying tissue and 0.5 dB/cm/MHz acoustic attenuation.
Only include the treatments where recanalization was successful. 7 treatments for shock-scattering histotripsy and 13 treatments for microtripsy.
Free hemoglobin is an indication of the degree of hemolysis. Microtripsy caused less hemolysis than shock scattering histotripsy.
The efficacy of microtripsy-mediated thrombolysis was shown to be significantly improved from the previous histotripsy-mediated thrombolysis using the shock scattering mechanism. Using a similar porcine DVT model, microtripsy achieved 13 successful recanalizations in the total 14 treatments, whereas the shock-scattering histotripsy only achieved successful recanalizations in 7 out of 12 treatments [11]. Only one microtripsy treatment in this study was unable to restore flow, due to the unintentional extension of the thrombus to ultrasound-inaccessible regions. The difference in treatment efficacy is likely related to the different mechanisms that the two approaches use to generate cavitation. Microtripsy uses the intrinsic threshold mechanism where acoustic cavitation is generated when the negative pressure phase of a single-cycle ultrasound pulse directly exceeds a cavitation threshold intrinsic to the media, and shock-scattering histotripsy uses multi-cycle ultrasound pulses and relies on pre-existing weak nuclei to reflect and invert positive pressure phases to combine with the incident negative pressure phases to generate acoustic cavitation. Weak nuclei often reside on tissue interfaces, such as vessel walls, so cavitation generated by shock-scattering histotripsy is easily formed on vessel walls instead of at target thrombus inside the vessels. In contrast, cavitation generated by microtripsy is more confined at its desired focus and less affected by surrounding tissue interfaces than shock-scattering histotripsy, resulting in more reliable and continuous fractionations in target thrombi.
The safety of microtripsy thrombolysis was also shown to be improved from the previous shock-scattering histotripsy. Hemolysis and vessel damage were both greatly reduced using microtripsy. Only minor hemolysis was observed during microtripsy thrombolysis treatments in this study. In the free-flow treatments, hemolysis induced by microtripsy was about 15 times less than that induced by shock-scattering histotripsy [12]. The lower PRF used in microtripsy thrombolysis is expected to play an important role in reducing hemolysis. In microtripsy thrombolysis, a higher pressure (~30 MPa P-) and a lower PRF (100 Hz) was used in comparison to the previous shock-scattering histotripsy study (~17 MPa P- and 500 Hz PRF). With relatively slow blood flow, the same volume of blood is exposed to cavitation more times using a higher PRF, resulting in the rupturing of more erythrocytes using the shock-scatter approach. The natures of the two cavitation mechanisms may also make a difference in hemolysis. The less-confined, larger cavitation bubble clouds generated by shock-scattering histotripsy may affect a larger volume of blood in the flow than the better-confined, smaller cavitation generated by microtripsy. In addition, no vessel damage was observed after two weeks of recovery from the microtripsy-mediated thrombolysis treatments. There was no damage observed in the endothelial cells of the targeted femoral veins or adjacent femoral arteries. Focal hemorrhage observed in the tunica adventitia disappeared completely after two weeks of recovery. This improvement was attributed to the better-confined cavitation in the vessel lumen generated by microtripsy, as shock-scattering histotripsy tends to generate cavitation on vessel walls where weak nuclei reside and therefore cause damage. Microtripsy generates more reproducible and predictable cavitation at the desired target without contacting the vessel walls so that vessel damage can be minimized. In addition, the new design of the therapy transducer also contributed to the reduced vessel damage. The therapy transducer used in this study was designed for DVT treatment to have the focal zone smaller than the lumen of the femoral vein, while the therapy transducer used in the previous study had a focal zone larger than the femoral vein.
Exposure of microtripsy in free blood flow in the femoral vein was considered to be the worst case test to further evaluate the safety of microtripsy-mediated thrombolysis treatment, as it maximized the potential contact between cavitation and vessel wall without a thrombus barrier, thus resulting in maximal vessel damage. In addition, without the thrombus barrier, a higher volume of free blood was expected to be exposed to cavitation in the flow, leading to a greater risk of hemolysis. The histological results showed no vessel perforation or major endothelial damage in any samples treated in free blood flow, and there was only microscopic hemorrhage located in the tunica adventitia of the target femoral veins. The hematological results also showed no statistically significant increase in free Hgb, LDH, potassium, or serum hematocrit in blood before and after the free flow treatments, suggesting that only slight hemolysis was induced during the free-flow treatments. All of these results from the free-flow scenario provide further evidence to support the safety of microtripsy thrombolysis treatment. A surprising result was that a lesser degree of hemolysis was observed in the free-flow treatments than that induced during the thrombolysis treatments with clots. One explanation is that in the thrombolysis treatment, cavitation was reliably and robustly generated in the targeted clots, and dissolving the targeted clots mostly consisting of red blood cells would lead to an increase in free hemoglobin. Another reason is associated with the different behavior of acoustic cavitation generated in free blood flow than in static tissue. Although the therapy system was consistently targeted at the center of vessel lumen, the cavitation tended to form at the bottom of the vessel wall in the flowing environment. Therefore, blood cells passing though the target may not get full cavitation exposure to be fractionated. Cavitation also appeared smaller and sparser in the free flow environment than in the thrombus on the ultrasound images, which could further reduce the blood cell lysis. In the flow environment, residual bubble nuclei from cavitational bubble collapse are immediately flushed away with blood flow and cannot serve as “seeds” to enhance the following cavitation generation. Therefore the cavitation observed in the free-flow veins was smaller and sparser and lyzed less blood cells.
There are notable differences between this porcine DVT model and a human DVT: available acoustic access, thrombi age, thrombi length and localization of thrombi. There is better acoustic access to DVT sites in human legs than in pig legs. Pig legs are short and the femoral veins are very close to the abdomen, leaving us with a limited acoustic window for ultrasound to transmit to the target in the veins. Human legs are longer, and DVT sites are usually accessible from more angles and locations. Therefore, thrombolysis treatments in human legs could potentially be more effective with a therapy transducer with a larger aperture and more transmitting area than the transducer used in this study. Another consideration about this study is that the age of thrombi in human femoral veins varies from several days to weeks, but we were only able to form and treat very acute thrombi using this porcine model in this study. We are currently working on developing a new porcine model to form chronic thrombi inside femoral veins. We are also developing a real-time thrombolysis monitoring algorithm to adaptively adjust therapy doses and strategies for thrombi with different ages (Zhang et al. 2015b; Zhang et al. 2016). In some cases, the thrombi formed in human legs can be extended to over 10 cm long, whereas the thrombi formed in our study were less than 3 cm. Treating a long thrombus may be challenging because re-clotting may occur before complete recanalization is achieved. Localization of thrombi in humans may also be a challenge. In some cases, the thrombi (especially in the chronic phase) are not easily visualized on ultrasound images due to the lack of red blood cells inside the thrombi. Pre-treatment planning based on other thrombus localization methods, such as angiography or magnetic resonance imaging, may be needed. We believe the potential issues from these differences are solvable, and the application of microtripsy-mediated thrombolysis for human DVT therapy is promising.
It should be noted that our study has certain limitations that are currently being improved upon. First, only H&E staining was used for histology evaluation in this study. Additional types of staining, such as Evans Blue, may provide us with a more comprehensive evaluation of damage to vessels and surrounding tissue. Second, to avoid vessel damage, microtripsy was used only to open a channel in the thrombus rather than used for full thrombolysis. This study focused on the clinical situation in which only recanalization is required for blood circulation restoration. There may be cases that require complete removal of a thrombus due to high risks of thrombus detachment and re-embolism, which will be investigated in the future. Third, the treatment speed in this study was slower than desired. We are investigating the feasibility of using higher PRFs (>100 Hz) and optimized treatment strategies to accelerate thrombolysis treatments. The number of pulses applied at each treatment location (the dosage) could be also reduced by increasing the peak negative pressure of each ultrasound pulse to further increase treatment speed. Fourth, in one of the thrombolysis treatments in this study, recanalization could not be achieved due to the extension of the thrombus to a proximal region of the femoral vein that was blocked by the pelvic bone and could not be reached by ultrasound. However, the transducer used in this study was specifically designed for treatment of DVT in an open region of the femoral vein, and we feel that microtripsy thrombolysis to a region that was blocked from one location may be achievable by using the right transducer that is able to access the clot from another location.
Supplementary Material
Acknowledgments
This work is supported by grants from the National Institute of Biomedical Imaging and Bioengineering, under Award R01 EB008998.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams HP, Brott TG, Furlan AJ, Gomez CR, Grotta J, Helgason CM, Kwiatkowski T, Lyden PD, Marler JR, Torner J. Guidelines for thrombolytic therapy for acute stroke: a supplement to the guidelines for the management of patients with acute ischemic stroke a statement for healthcare professionals from a special writing group of the stroke council, American heart association. Circulation. 1996;94:1167–74. doi: 10.1161/01.cir.94.5.1167. [DOI] [PubMed] [Google Scholar]
- Bader KB, Bouchoux G, Holland CK. Therapeutic Ultrasound. Springer; 2016. Sonothrombolysis; pp. 339–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates SM, Ginsberg JS. Treatment of deep-vein thrombosis. New England Journal of Medicine. 2004;351:268–77. doi: 10.1056/NEJMcp031676. [DOI] [PubMed] [Google Scholar]
- Beckman MG, Hooper WC, Critchley SE, Ortel TL. Venous thromboembolism: a public health concern. American journal of preventive medicine. 2010;38:S495–S501. doi: 10.1016/j.amepre.2009.12.017. [DOI] [PubMed] [Google Scholar]
- Behrens S, Daffertshofer M, Spiegel D, Hennerici M. Low-frequency, low-intensity ultrasound accelerates thrombolysis through the skull. Ultrasound in medicine & biology. 1999;25:269–73. doi: 10.1016/s0301-5629(98)00158-6. [DOI] [PubMed] [Google Scholar]
- Blinc A, Keber D, Lahajnar G, Zupančič I, Zorec-Karlovšek M, Demsar F. Magnetic resonance imaging of retracted and nonretracted blood clots during fibrinolysis in vitro. Pathophysiology of Haemostasis and Thrombosis. 1992;22:195–201. doi: 10.1159/000216319. [DOI] [PubMed] [Google Scholar]
- Brown AT, Flores R, Hamilton E, Roberson PK, Borrelli MJ, Culp WC. Microbubbles improve sonothrombolysis in vitro and decrease hemorrhage in vivo in a rabbit stroke model. Investigative radiology. 2011:46. doi: 10.1097/RLI.0b013e318200757a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess A, Huang Y, Waspe AC, Ganguly M, Goertz DE, Hynynen K. High-intensity focused ultrasound (HIFU) for dissolution of clots in a rabbit model of embolic stroke. PloS one. 2012;7:e42311. doi: 10.1371/journal.pone.0042311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Coussios C-C, Ammi AY, Mast TD, de Courten-Myers GM, Holland CK. Ultrasound-enhanced thrombolysis using Definity® as a cavitation nucleation agent. Ultrasound in medicine & biology. 2008;34:1421–33. doi: 10.1016/j.ultrasmedbio.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devanagondi R, Zhang X, Xu Z, Ives K, Levin A, Gurm H, Owens GE. Hemodynamic and Hematologic Effects of Histotripsy of Free-Flowing Blood: Implications for Ultrasound-Mediated Thrombolysis. Journal of vascular and interventional radiology. 2015;26:1559–65. doi: 10.1016/j.jvir.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman HS, Koroshetz W, Qureshi N. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1996;334:1405. [PubMed] [Google Scholar]
- Higgins DL, Bennett WF. Tissue plasminogen activator: the biochemistry and pharmacology of variants produced by mutagenesis. Annual review of pharmacology and toxicology. 1990;30:91–121. doi: 10.1146/annurev.pa.30.040190.000515. [DOI] [PubMed] [Google Scholar]
- Hitchcock KE, Ivancevich NM, Haworth KJ, Stamper DNC, Vela DC, Sutton JT, Pyne-Geithman GJ, Holland CK. Ultrasound-enhanced rt-PA thrombolysis in an ex vivo porcine carotid artery model. Ultrasound in medicine & biology. 2011;37:1240–51. doi: 10.1016/j.ultrasmedbio.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland CK, Vaidya SS, Datta S, Coussios C-C, Shaw GJ. Ultrasound-enhanced tissue plasminogen activator thrombolysis in an in vitro porcine clot model. Thrombosis research. 2008;121:663–73. doi: 10.1016/j.thromres.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karthikesalingam A, Young E, Hinchliffe R, Loftus I, Thompson M, Holt P. A systematic review of percutaneous mechanical thrombectomy in the treatment of deep venous thrombosis. European Journal of Vascular and Endovascular Surgery. 2011;41:554–65. doi: 10.1016/j.ejvs.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Kim Y, Gelehrter S, Fifer C, Lu J, Owens G, Berman D, Williams J, Wilkinson J, Ives K, Xu Z. Non - invasive pulsed cavitational ultrasound for fetal tissue ablation: feasibility study in a fetal sheep model. Ultrasound in Obstetrics & Gynecology. 2011;37:450–7. doi: 10.1002/uog.8880. [DOI] [PubMed] [Google Scholar]
- Kim Y, Vlaisavljevich E, Owens G, Allen S, Cain C, Xu Z. In vivo transcostal histotripsy therapy without aberration correction. Physics in medicine and biology. 2014;59:2553. doi: 10.1088/0031-9155/59/11/2553. [DOI] [PubMed] [Google Scholar]
- Kunitada S, FitzGerald G, Fitzgerald D. Inhibition of clot lysis and decreased binding of tissue-type plasminogen activator as a consequence of clot retraction. Blood. 1992;79:1420–7. [PubMed] [Google Scholar]
- Lin K-W, Kim Y, Maxwell AD, Wang T-Y, Hall T, Xu Z, Fowlkes JB, Cain C. Histotripsy beyond the intrinsic cavitation threshold using very short ultrasound pulses: microtripsy. Ultrasonics, Ferroelectrics, and Frequency Control, IEEE Transactions on. 2014;61:251–65. doi: 10.1109/TUFFC.2014.6722611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell AD, Cain CA, Duryea AP, Yuan L, Gurm HS, Xu Z. Noninvasive thrombolysis using pulsed ultrasound cavitation therapy–histotripsy. Ultrasound in medicine & biology. 2009;35:1982–94. doi: 10.1016/j.ultrasmedbio.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell AD, Cain CA, Hall TL, Fowlkes JB, Xu Z. Probability of cavitation for single ultrasound pulses applied to tissues and tissue-mimicking materials. Ultrasound in medicine & biology. 2013;39:449–65. doi: 10.1016/j.ultrasmedbio.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell AD, Owens G, Gurm HS, Ives K, Myers DD, Xu Z. Noninvasive treatment of deep venous thrombosis using pulsed ultrasound cavitation therapy (histotripsy) in a porcine model. Journal of vascular and interventional radiology. 2011a;22:369–77. doi: 10.1016/j.jvir.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell AD, Wang T-Y, Cain CA, Fowlkes JB, Sapozhnikov OA, Bailey MR, Xu Z. Cavitation clouds created by shock scattering from bubbles during histotripsy. The Journal of the Acoustical Society of America. 2011b;130:1888–98. doi: 10.1121/1.3625239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson RA, Pincus MR. Henry’s clinical diagnosis and management by laboratory methods. Elsevier Health Sciences. 2016 [Google Scholar]
- Mewissen MW, Seabrook GR, Meissner MH, Cynamon J, Labropoulos N, Haughton SH. Catheter-directed Thrombolysis for Lower Extremity Deep Venous Thrombosis: Report of a National Multicenter Registry 1. Radiology. 1999;211:39–49. doi: 10.1148/radiology.211.1.r99ap4739. [DOI] [PubMed] [Google Scholar]
- O’Sullivan GJ, Lohan DG, Gough N, Cronin CG, Kee ST. Pharmacomechanical thrombectomy of acute deep vein thrombosis with the Trellis-8 isolated thrombolysis catheter. Journal of vascular and interventional radiology. 2007;18:715–24. doi: 10.1016/j.jvir.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Porter TR, LeVeen RF, Fox R, Kricsfeld A, Xie F. Thrombolytic enhancement with perfluorocarbon-exposed sonicated dextrose albumin microbubbles. American heart journal. 1996;132:964–8. doi: 10.1016/s0002-8703(96)90006-x. [DOI] [PubMed] [Google Scholar]
- Raabe RA. Ultrasound facilitated thrombolysis in treating DVT. Endovascular Today. 2006;4:1–14. [Google Scholar]
- Ryan EA, Mockros LF, Weisel JW, Lorand L. Structural origins of fibrin clot rheology. Biophysical journal. 1999;77:2813–26. doi: 10.1016/S0006-3495(99)77113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharafuddin MJ, Sun S, Hoballah JJ, Youness FM, Sharp WJ, Roh B-S. Endovascular management of venous thrombotic and occlusive diseases of the lower extremities. Journal of vascular and interventional radiology. 2003;14:405–23. doi: 10.1097/01.rvi.0000064849.87207.4f. [DOI] [PubMed] [Google Scholar]
- Siegel RJ, Luo H. Ultrasound thrombolysis. Ultrasonics. 2008;48:312–20. doi: 10.1016/j.ultras.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Sutton JT, Ivancevich NM, Perrin SR, Vela DC, Holland CK. Clot retraction affects the extent of ultrasound-enhanced thrombolysis in an ex vivo porcine thrombosis model. Ultrasound in medicine & biology. 2013;39:813–24. doi: 10.1016/j.ultrasmedbio.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turi ZG, Goldberg S, LittleJohn JK, VanderArk C, Shadoff N, Karlsberg R, Williams J, Butman S, Stadius ML, Wise K. Dose-related efficacy and bleeding complications of double-chain tissue plasminogen activator in acute myocardial infarction. The American journal of cardiology. 1993;71:1009–14. doi: 10.1016/0002-9149(93)90564-s. [DOI] [PubMed] [Google Scholar]
- Vlaisavljevich E, Kim Y, Allen S, Owens G, Pelletier S, Cain C, Ives K, Xu Z. Image-guided non-invasive ultrasound liver ablation using histotripsy: Feasibility study in an in vivo porcine model. Ultrasound in medicine & biology. 2013;39:1398–409. doi: 10.1016/j.ultrasmedbio.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright C, Hynynen K, Goertz D. In vitro and in vivo high intensity focused ultrasound thrombolysis. Investigative radiology. 2012;47:217. doi: 10.1097/RLI.0b013e31823cc75c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Hall TL, Fowlkes JB, Cain CA. Effects of acoustic parameters on bubble cloud dynamics in ultrasound tissue erosion (histotripsy) The Journal of the Acoustical Society of America. 2007;122:229–36. doi: 10.1121/1.2735110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Owens G, Gordon D, Cain C, Ludomirsky A. Noninvasive creation of an atrial septal defect by histotripsy in a canine model. Circulation. 2010;121:742–9. doi: 10.1161/CIRCULATIONAHA.109.889071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Raghavan M, Hall T, Mycek M-A, Fowlkes JB, Cain C. Evolution of bubble clouds induced by pulsed cavitational ultrasound therapy-histotripsy. Ultrasonics, Ferroelectrics, and Frequency Control, IEEE Transactions on. 2008;55:1122–32. doi: 10.1109/TUFFC.2008.764. [DOI] [PubMed] [Google Scholar]
- Zhang X, Cain C, Owens G, Gurm H, Macoskey J, Xu Z. Histotripsy Thrombolysis on Retracted Clot. Ultrasound in Medicine & Biology. 2016 doi: 10.1016/j.ultrasmedbio.2016.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Jin L, Vlaisavljevich E, Owens GE, Gurm HS, Cain CA, Xu Z. Noninvasive thrombolysis using microtripsy: a parameter study. Ultrasonics, Ferroelectrics, and Frequency Control, IEEE Transactions on. 2015a;62:2092–105. doi: 10.1109/TUFFC.2015.007268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Miller RM, Lin K-W, Levin AM, Owens GE, Gurm HS, Cain CA, Xu Z. Real-time feedback of histotripsy thrombolysis using bubble-induced color Doppler. Ultrasound in medicine & biology. 2015b;41:1386–401. doi: 10.1016/j.ultrasmedbio.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Owens GE, Gurm HS, Ding Y, Cain CA, Xu Z. Noninvasive thrombolysis using histotripsy beyond the intrinsic threshold (microtripsy) Ultrasonics, Ferroelectrics, and Frequency Control, IEEE Transactions on. 2015c;62:1342–55. doi: 10.1109/TUFFC.2015.007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.