Abstract
Background
Patients with atrial fibrillation (AF) experience an increased risk of heart failure (HF). However, data are lacking on current trends in the risk of HF after AF.
Objective
To describe the temporal trends in HF occurrence after AF in a community cohort of patients with incident AF from 2000–2013.
Methods
Cox regression examined the association of year of AF diagnosis with HF and the predictors of developing HF after AF.
Results
Among 3491 AF patients without prior HF, 750 (21%) developed incident HF over a mean follow-up of 3.7 years. Among those with an echocardiogram, 422 (61%) had HF with preserved ejection fraction (HFpEF) and 270 (39%) had HF with reduced ejection fraction (HFpEF). After adjusting for demographics and comorbidities, the risk of developing HF did not change over time (hazard ratio [HR] (95% CI) per year of AF diagnosis: 1.01 (0.98–1.03) overall; 1.00 (0.98–1.03) for HFpEF; 1.00 (0.96–1.03) for HFrEF). Increasing age, obesity, smoking, diabetes, chronic pulmonary disease, and renal disease were predictors of developing HF. Compared to the Olmsted County, MN population, a substantial excess risk of developing HF was observed after AF diagnosis (standardized morbidity ratio (95% CI) 9.60 (7.44–12.19), 2.13 (1.56–2.84), and 1.70 (1.34–2.14) at 90 days, 1 year, and 3 years after diagnosis).
Conclusion
In the community, HF is a frequent adverse outcome among patients with AF, and HFpEF is more common than HFrEF. The rates of HF after AF have not declined, highlighting the importance of continued efforts to improve outcomes in AF.
Keywords: atrial fibrillation, atrial flutter, heart failure, epidemiology, outcomes
INTRODUCTION
Atrial fibrillation (AF) is the most common cardiac arrhythmia and disproportionately affects the elderly. AF is associated with substantial morbidity and mortality, and one of the most common consequences of AF is the development of heart failure (HF).1 AF and HF share common risk factors and each condition predisposes a patient to the development of the other condition;2–6 the interaction of the 2 conditions has been described as a ‘vicious electromechanical cycle.’7 Notably, AF patients experience an approximately 3-fold increased risk of developing HF;5, 6, 8–10 yet, it is unknown whether improvements over time in the risk of HF after AF have occurred. A study in Olmsted County, MN residents with incident AF between 1980 and 2000 reported no improvement in the risk of HF over time in age- and sex-adjusted models, but a small decline after adjustment for comorbidities.11 Recent evidence from the Framingham Heart Study suggests that AF patients have differential risks for developing each type of HF, with greater risks for developing HF with preserved ejection fraction (HFpEF) than HF with reduced ejection fraction (HFpEF).5 In addition, although the incidence of HF has declined over time, a shift in case mix of HF over the recent decade has resulted in an increased proportion of HFpEF.12 Taken collectively, these findings suggest that the trends in developing HF after AF may differ over time by type of HF. However, contemporary data on the temporal trends in the incidence of HF after AF, specifically delineating differences over time by type of HF, are not described. Our objective was to provide contemporary data on trends for all HF, HFpEF, and HFrEF in a community cohort of patients with incident AF from 2000 to 2013.
METHODS
Study Population
This study was conducted in Olmsted County, Minnesota utilizing the resources of the Rochester Epidemiology Project (REP), a records-linkage system allowing virtually complete capture of health care in county residents.13 This record linkage system encompasses more than 6 million person-years of follow-up in over 500,000 unique individuals since 1966, enabling virtually complete capture of outcomes in Olmsted County, MN residents.13 Demographic and ethnic characteristics of Olmsted County are representative of the state of Minnesota and the Midwest region of the US, and age- and sex-specific mortality rates are similar to national data, supporting the generalizability of REP data.14 This study was approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards.
Incident Atrial Fibrillation Cohort
Adults (aged ≥18 years) with incident AF or atrial flutter occurring between 2000 and 2013 were identified using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes 427.31 and 427.32 from all providers in the REP, as well as Mayo Clinic electrocardiograms (ECGs). Diagnostic codes and ECGs from both inpatient and outpatient encounters were captured. The medical records for all patients were manually reviewed to validate the events, requiring evidence of first-ever AF or atrial flutter on an ECG, monitoring device, echocardiogram, or a physician diagnosis, as previously described.15 AF that occurred within 30 days after a cardiac surgery was excluded. However, among these individuals with post-operative AF, we continued to review the medical record for another episode of AF not associated with a surgery, which we considered incident AF.
Ascertainment of Heart Failure
HF events were identified using ICD-9-CM code 428 assigned during an inpatient or outpatient encounter at any provider in the REP. Trained nurse abstractors reviewed the medical records of these patients and validated the HF events using the Framingham Criteria.16 The incident date of HF was obtained and patients with HF occurring prior to the date of incident AF were considered prevalent and excluded. Only first-ever (incident) HF events occurring after the index AF date were included as outcomes in the analysis. The closest echocardiogram within 1 year of the date of HF was obtained to characterize the type of HF. Patients with EF greater than or equal to 50% were categorized as HFpEF and those with EF less than 50% were categorized as HFrEF.17
Clinical Data Collection
Height, weight, and smoking status at the time of AF diagnosis were manually abstracted from the medical records. Body mass index (BMI) was calculated as weight (in kg) divided by height (in meters) squared. Current, former, and never smoking status were collected and those who had smoked within 6 months prior to AF diagnosis were considered current smokers. Estimated glomerular filtration rate (eGFR) was calculated using the closest serum creatinine value within 90 days of the index AF date.18 The remaining covariates were ascertained electronically, implementing rules as previously described to reduce the capture of false positives due to rule out and suspect diagnoses by requiring 2 occurrences of a code within the 5 years prior to AF.15 The ICD-9 diagnostic codes used to define the comorbidities are included in Supplemental Table 1. Finally, outpatient prescription data is not routinely available in the REP prior to 2004. In the subset of our cohort with AF diagnosed between 2004 and 2013, rhythm control treatment approaches were defined as outpatient prescriptions for antiarrhythmic medications, direct current cardioversions, catheter ablation procedures, or Maze procedures.
Statistical Analysis
Analyses were performed using SAS, version 9.4. Patients with prevalent HF at baseline or with HF occurring the same day as AF were excluded. Characteristics of the cohort are presented as means and proportions; trends across year of AF diagnosis groups (2000–2003, 2004–2007, 2008–2010, 2011–2013) were compared using linear regression with year group modeled as a 3-level categorical variable and Mantel-Haenszel chi-square tests. Cumulative incidence curves for HF across year of AF diagnosis categories were constructed treating death as a competing risk.19 P-values comparing the cumulative incidence curves were obtained using the method by Gray.20 Curves were constructed for all HF, HFpEF, and HFrEF.
Cox proportional hazards regression models were used to determine the association of calendar year of AF diagnosis with the risk of any HF, HFpEF, and HFrEF. Follow-up time was calculated from the index date of AF until diagnosis of HF, death, last clinical encounter, or December 31, 2013, whichever came first. Year was treated continuously in one set of models to obtain p-values for time trends; a second set of models categorized year of AF diagnosis into groups with 2000–2003 serving as the reference to aid in interpretation. Models were adjusted for age, sex, and comorbidities that differed across year of AF diagnosis, including BMI, hypertension, diabetes, chronic pulmonary disease, peripheral vascular disease, and renal disease. In sensitivity analyses, we adjusted for eGFR (instead of renal disease using diagnostic codes) among the patients with an eGFR measured within ±90 days of index (n=3261). Results from our sensitivity analysis were similar. To avoid excluding patients from the analysis due to missing eGFR data, our main models included renal disease captured by diagnostic codes. We tested age*year of AF and sex*year of AF interactions and found that the trends in HF after AF did not differ by age or sex. Sub-group analyses among patients with available rhythm control treatment approaches were also conducted, adjusting for rhythm control as a time-dependent variable as well as testing a rhythm control*year of AF interaction. The proportional hazards assumption was tested using scaled Schoenfeld residuals and found to be valid. In addition, Cox regression examined associations of patient characteristics with the occurrence of any HF, HFpEF, and HFrEF after adjusting for age and sex. Finally, we determined the associations of HF with death by modeling HF as a time-dependent variable in the Cox model.
The incident HF rate after AF was compared with the incident HF rate in the population of Olmsted County, Minnesota12 to calculate standardized morbidity ratios. Expected HF rates were calculated by applying sex-, age- and period-specific HF incidence rates in the general population of Olmsted County to the person-time follow-up of the study cohort. Standardized morbidity ratios were calculated for discrete time periods over follow-up. For each time period, the standardized morbidity ratio was calculated by dividing the number of observed incident HF cases in the AF cohort for that time period by the expected number of HF cases. Confidence intervals were calculated according to the Poisson distribution. This analysis was repeated for HFpEF and HFrEF separately.
RESULTS
Between 2000 and 2013, 4431 patients with incident AF were identified. Of these, 640 had prevalent HF and 300 had HF occurring the same day as AF diagnosis and were excluded, resulting in 3491 AF patients. The mean (standard deviation) age at time of incident AF was 71.0 (14.9) years and 54.5% were male (Table 1). The most common comorbidities were hypertension (66%), diabetes (20%), and malignancy (19%). Patients diagnosed with AF in more recent years were more likely to be male, obese, and had higher prevalences of hypertension, diabetes, peripheral vascular disease, and renal disease compared to patients diagnosed with AF in earlier years.
Table 1.
Baseline characteristics of the incident atrial fibrillation patients
| Overall (N=3491) | 2000–2003 (N=808) | 2004–2007 (N=944) | 2008–2010 (N=769) | 2011–2013 (N=970) | P-value for trend | |
|---|---|---|---|---|---|---|
| Age, mean (SD) | 71.0 (14.9) | 71.1 (14.9) | 71.0 (15.1) | 70.5 (14.8) | 71.4 (14.7) | 0.671 |
| Male | 1901 (54.5) | 420 (52.0) | 491 (52.0) | 435 (56.6) | 555 (57.2) | 0.007 |
| Body mass index, kg/m2 | 0.010 | |||||
| <25 | 985 (28.2) | 226 (28.0) | 295 (31.3) | 211 (27.4) | 253 (26.1) | |
| 25 to <30 | 1153 (33.1) | 283 (35.0) | 312 (33.1) | 253 (32.9) | 305 (31.5) | |
| ≥ 30 | 1350 (38.7) | 299 (37.0) | 335 (35.6) | 305 (39.7) | 411 (42.4) | |
| Smoking status | 0.093 | |||||
| Current | 372 (10.7) | 90 (11.1) | 92 (9.8) | 87 (11.3) | 103 (10.6) | |
| Former | 1428 (40.9) | 317 (39.2) | 374 (39.6) | 327 (42.5) | 410 (42.3) | |
| Never | 1691 (48.4) | 401 (49.6) | 478 (50.6) | 355 (46.2) | 457 (47.1) | |
| Hypertension | 2302 (65.9) | 473 (58.5) | 623 (66.0) | 536 (69.7) | 670 (69.1) | <0.001 |
| Myocardial infarction | 331 (9.5) | 74 (9.2) | 79 (8.4) | 70 (9.1) | 108 (11.1) | 0.103 |
| Diabetes | 686 (19.7) | 117 (14.5) | 172 (18.2) | 179 (23.3) | 218 (22.5) | <0.001 |
| Chronic pulmonary disease | 430 (12.3) | 129 (16.0) | 107 (11.3) | 78 (10.1) | 116 (12.0) | 0.015 |
| Peripheral vascular disease | 254 (7.3) | 45 (5.6) | 71 (7.5) | 58 (7.5) | 80 (8.3) | 0.045 |
| Dementia | 182 (5.2) | 36 (4.5) | 43 (4.6) | 54 (7.0) | 49 (5.1) | 0.253 |
| Malignancy | 671 (19.2) | 167 (20.7) | 153 (16.2) | 159 (20.7) | 192 (19.8) | 0.686 |
| Metastatic solid tumor | 178 (5.1) | 40 (5.0) | 44 (4.7) | 51 (6.6) | 43 (4.4) | 0.996 |
| Renal disease | 194 (5.6) | 30 (3.7) | 33 (3.5) | 44 (5.7) | 87 (9.0) | <0.001 |
| eGFR, mean (SD), mL/min per 1.73 m2* | 65.9 (26.2) | 59.0 (19.9) | 63.0 (23.4) | 70.6 (29.0) | 70.6 (29.1) | <0.001 |
| Rheumatologic disease | 166 (4.8) | 40 (5.0) | 42 (4.5) | 35 (4.6) | 49 (5.1) | 0.854 |
| Ischemic stroke or TIA | 404 (11.6) | 90 (11.1) | 110 (11.7) | 89 (11.6) | 115 (11.9) | 0.672 |
| Liver disease | 65 (1.9) | 9 (1.1) | 20 (2.1) | 13 (1.7) | 23 (2.4) | 0.104 |
| CHA2DS2-VASc, mean (SD) | 2.9 (1.8) | 2.8 (1.8) | 2.9 (1.8) | 2.9 (1.8) | 3.0 (1.8) | 0.079 |
All results are reported as n (%) unless otherwise specified.
TIA, transient ischemic attack; eGFR, estimated glomerular filtration rate.
Among 3261 patients with eGFR measured within ±90 days of index.
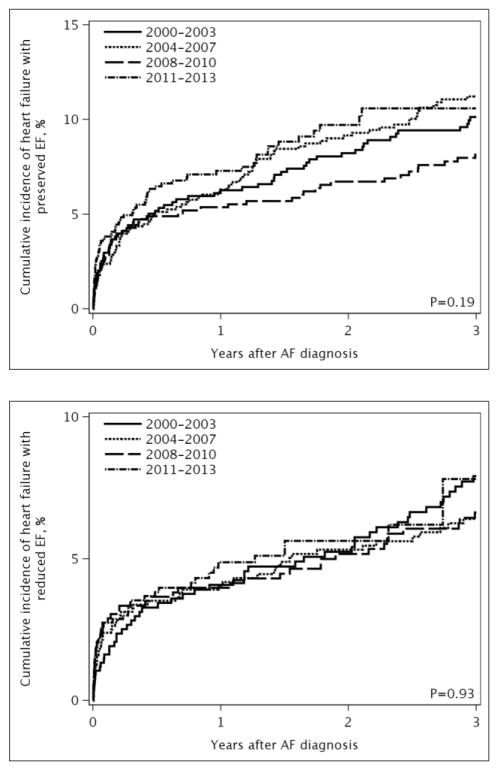
Over a mean follow-up of 3.7 years, 750 (21%) developed incident HF. The type of HF was known for 692 (92%) patients: 422 had HFpEF and 270 had HFrEF. The incidence rates (95% CI) of HF per 100 person-years were 5.84 (5.43–6.27) for all HF, 3.32 (3.01–3.66) for HFpEF, and 2.13 (1.88–2.40) for HFrEF. Treating death as a competing risk, no temporal trend in the risk of developing HF after AF was observed (Figure 1).
Figure 1. Cumulative incidence of heart failure by year of atrial fibrillation diagnosis.
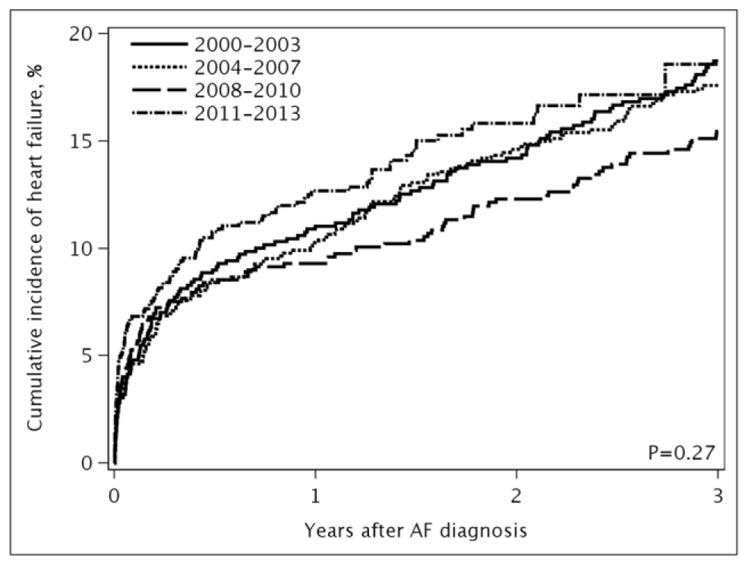
Panel A, all heart failure; Panel B, heart failure with preserved ejection fraction; Panel C, heart failure with reduced ejection fraction. The cumulative incidence curves were adjusted for death as a competing event.
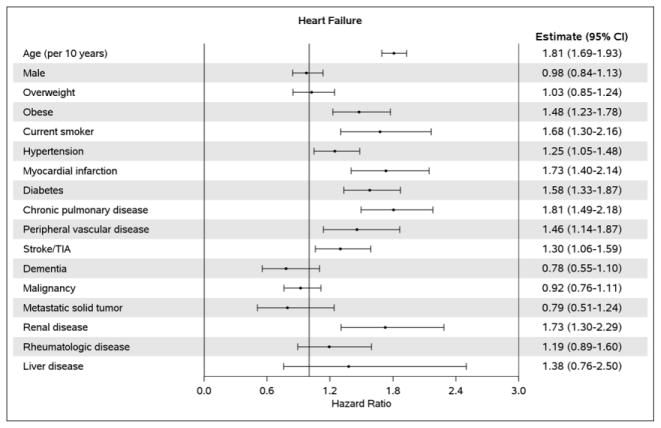
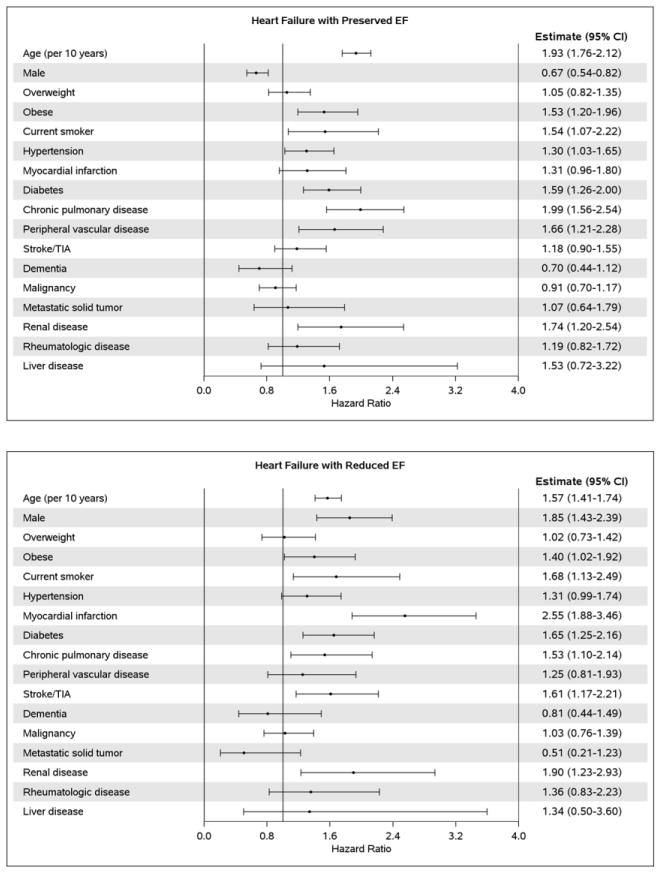
After adjustment for demographics and comorbidities, the hazard ratio (HR) (95% CI) for HF per year of AF diagnosis was 1.00 (0.98–1.03); p-trend=0.695 (Table 2). Likewise, no difference over time was observed for HFpEF (HR 1.00, 95% CI 0.98–1.03) or HFrEF (HR 1.00, 95% CI 0.96–1.03). In a sensitivity analysis, HF occurring the same day as AF (n=300) were counted as events and results were consistent (HR (95% CI) for HF per year of AF diagnosis: 1.00 (0.98–1.02), 0.99 (0.98–1.01), and 1.00 (0.98–1.02) for all HF, HFpEF, and HFrEF, respectively). Finally, in subgroup analyses among patients with AF diagnosed between 2004 and 2013, adjustment for rhythm control treatment approaches did not change the results (HR (95% CI) for HF per year of AF diagnosis: 1.01 (0.97–1.04), 1.00 (0.95–1.05), and 1.01 (0.95–1.08) for all HF, HFpEF, and HFrEF, respectively). In addition, no significant interactions between rhythm control and year of AF diagnosis were observed, indicating that the trends in HF risk over time did not differ between patients treated with rhythm and rate control. After adjusting for sex, older age at the time of incident AF was associated with an increased risk of developing HF (Figure 2A). After adjustment for age and sex, the following conditions were significant predictors of developing subsequent HF: obesity, current smoking status, hypertension, myocardial infarction, diabetes, chronic pulmonary disease, peripheral vascular disease, stroke/transient ischemic attack (TIA), and renal disease. Predictors of developing HFpEF were similar, with the exception of myocardial infarction and stroke/TIA which were not predictors of HFpEF, and female sex which was a predictor of HFpEF (Figure 2B). Increasing age, male sex, obesity, current smoking status, myocardial infarction, diabetes, chronic pulmonary disease, stroke/TIA, and renal disease were predictors of developing HFrEF, and hypertension was borderline significant (Figure 2C).
Table 2.
Hazard ratios (95% confidence intervals) for incident heart failure by year of atrial fibrillation diagnosis
| All heart failure | Heart failure with preserved ejection fraction | Heart failure with reduced ejection fraction | |
|---|---|---|---|
| Per year | 1.00 (0.98–1.03) | 1.00 (0.98–1.03) | 1.00 (0.96–1.03) |
| P time trend* | 0.695 | 0.805 | 0.919 |
| By year group | |||
| 2000–2003 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 2004–2007 | 1.07 (0.89–1.29) | 1.15 (0.90–1.47) | 0.96 (0.70–1.32) |
| 2008–2010 | 0.94 (0.75–1.18) | 0.89 (0.65–1.22) | 0.96 (0.67–1.39) |
| 2011–2013 | 1.07 (0.84–1.36) | 1.10 (0.80–1.51) | 0.93 (0.62–1.40) |
Adjusted for age, sex, body mass index, hypertension, diabetes, chronic pulmonary disease, peripheral vascular disease, and renal disease.
P time trend is the 1df p-value for year based on the model treating year as a continuous variable.
Figure 2. Age- and sex-adjusted predictors of heart failure.
Panel A, all heart failure; Panel B, heart failure with preserved ejection fraction; Panel C, heart failure with reduced ejection fraction. COPD, chronic obstructive pulmonary disease; TIA, transient ischemic attack; EF, ejection fraction. The estimate for age is adjusted for sex and the estimate for sex is adjusted for age; all other estimates are adjusted for age and sex.
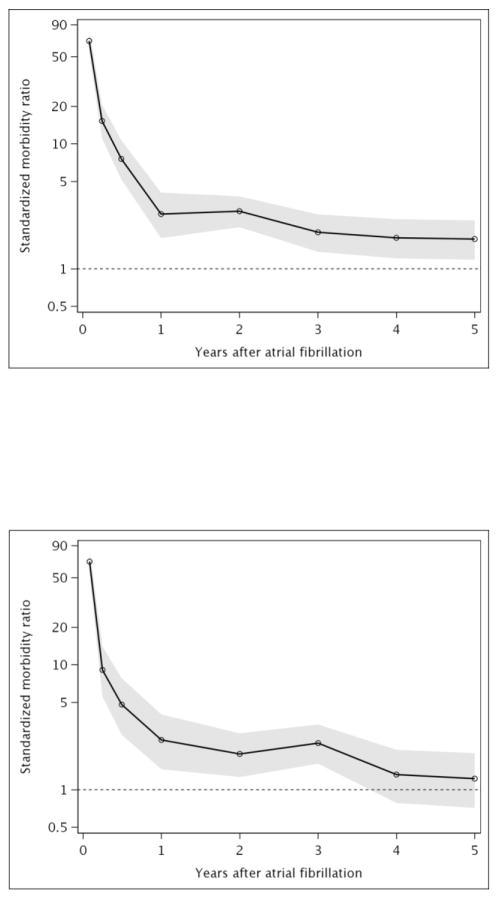
Standardized morbidity ratios comparing the risk of incident HF for AF patients to the Olmsted County, MN population of a similar age and sex distribution showed a dramatic excess risk of HF observed early after AF diagnosis (Figure 3A). Overall, AF patients exhibited an elevated risk of incident HF through 3 years after diagnosis, but no increased risk of HF after 4 years. Standardized morbidity ratios (95% CI) were 9.60 (7.44–12.19) at 31–90 days after diagnosis, 2.13 (1.56–2.84) at 181 days-1 year and 1.70 (1.34–2.14) at 2–3 years after AF diagnosis. Similar patterns were observed for HFpEF (Figure 3B) and HFrEF (Figure 3C), except the risk of HFpEF remained elevated through 5 years of follow-up in patients with AF compared to the Olmsted County population.
Figure 3. Standardized morbidity ratios of heart failure after incident atrial fibrillation.
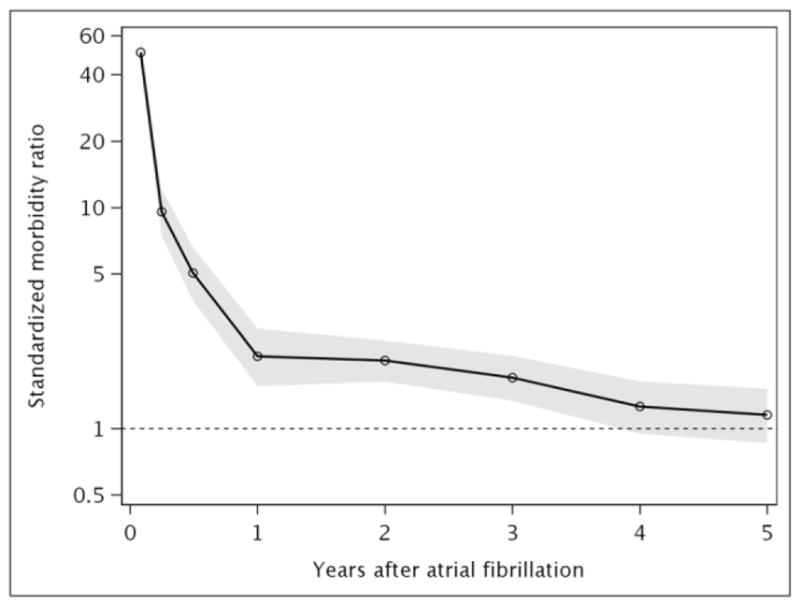
Panel A, all heart failure; Panel B, heart failure with preserved ejection fraction; Panel C, heart failure with reduced ejection fraction. Shaded area indicates 95% confidence intervals.
We also assessed the impact of HF on death among the 3491 AF patients free of HF at the time of AF diagnosis, by treating HF as a time-dependent variable in a Cox model. After adjustment for demographics and comorbidities, patients who developed HF over follow-up had more than a 2-fold increased risk of death than those without HF (HR 2.68, 95% CI 2.35–3.06). Associations were similar for HFpEF (HR 2.54, 95% CI 2.16–2.99) and HFrEF (HR 2.52, 95% CI 2.08–3.05).
DISCUSSION
In this geographically-defined community, the development of HF after AF was common, with more than one-fifth of AF patients developing HF over 3.7 years of follow-up. No decline in the risk of HF after AF was observed since 2000. Developing HFpEF was more common than HFrEF. A substantial excess risk of developing HF was observed for AF patients compared to the Olmsted County population of similar age and sex. Finally, AF patients who developed HF exhibited a more than 2-fold increased risk of death than AF patients who did not develop HF.
Heart Failure Risk after Atrial Fibrillation
Although HF commonly occurs in AF patients, little data exist on temporal trends in HF after AF. A prior study in Olmsted County, MN residents with incident AF between 1980 and 2000 reported no improvement in the risk of HF over time in age- and sex-adjusted models.11 After adjustment for comorbidities, a decline of 2% per calendar year of AF was observed in the risk of HF (adjusted HR 0.98, 95% CI 0.97–0.99). Our updated results in Olmsted County, MN residents with AF between 2000 and 2013 indicate no difference in the risk of HF after AF since 2000. In addition, no differences were observed over time for either type of HF. However, the rates of HFpEF were higher than for HFrEF, which is consistent with findings from the Framingham Study.5
Interestingly, we observed that the rates of HF were more than 2-fold higher than rates of stroke/TIA observed among patients with AF in our community,21 with incidence rates per 100 person-years of 5.84 for HF and 2.14 for ischemic stroke/TIA. This finding is consistent with a recent study among Medicare beneficiaries that identified HF as the most common non-fatal cardiovascular event occurring after AF.22 However, in our cohort, 21% of AF patients developed HF over a mean follow-up of 3.7 years, whereas only 13.7% of Medicare beneficiaries developed HF in the first 5 years after AF.22 The larger proportion of patients developing HF in our cohort is likely due to differences in ascertainment; we identified HF occurring in both inpatient and outpatient settings, whereas only inpatient or emergency claims identified HF outcomes in the Medicare study. Furthermore, our rates are in line with recent data from the Framingham Heart Study, which reported that 28% of AF patients developed HF the same day or after AF.5
Factors associated with an increased risk of developing HF included increasing age, obesity, current smoking status, hypertension, prior myocardial infarction, diabetes, chronic pulmonary disease, peripheral vascular disease, prior stroke or TIA, and renal disease. These findings are consistent with the knowledge that many of the underlying risk factors and mechanisms for AF and HF are the same.23 However, our study identified male sex as a risk factor for only HFrEF; female sex was a risk factor for HFpEF, which has been previously been reported in the Framingham Heart Study.5 In addition, peripheral vascular disease was associated with the development of HFpEF but not HFrEF, and myocardial infarction and stroke/TIA were associated with the development of HFrEF only. Finally, consistent with previous reports from the Framingham Heart Study,5, 6 we observed that the development of HF after AF conferred more than a 2-fold increased risk of death compared to patients who did not develop HF after AF.
Clinical Implications
Our findings augment important evidence that outcomes after AF have not improved over recent years despite continued emphasis on improving AF therapies. Previous data from our cohort showed that the risk of ischemic stroke/TIA21 and survival15 after AF have not improved since 2000. The current study now demonstrates that the risk of HF after AF is also stagnating. The lack of improvement in major cardiovascular events after AF indicate that efforts are urgently needed to improve AF outcomes. The importance of studying coexisting conditions and addressing comorbidities in treatment guidelines for patients with cardiovascular disease has been recently emphasized.24 Yet, while current AF guidelines provide treatment recommendations for patients with AF and concomitant HF,25 no recommendations exist for the prevention of HF in patients with AF. To reduce adverse clinical outcomes in AF, interventions should not only treat the AF itself, but should address the many comorbidities that commonly occur in elderly AF patients. For example, interventions to improve modifiable risk factors, including weight loss26 and improvements in cardiorespiratory fitness,27 which are associated with reduction in AF burden and maintenance of sinus rhythm, may reduce adverse outcomes in AF. Thus, future studies should address not only short term reduction in AF burden but the influence of weight loss and improvements in cardiorespiratory fitness on long term clinical outcomes, such as the development of HF.
Limitations and Strengths
Some limitations deserve mention. First, we did not have sufficient information to stratify results by type of AF (paroxysmal, persistent, permanent). Second, we may have missed some HF events if the diagnosis occurred outside of Olmsted County. Finally, our results may not be generalizable to the entire US, although Olmsted County is representative of the state of Minnesota and the Upper Midwest region of the US.14 Importantly, our data represent the experience of a community and includes all residents with AF without restriction on age, sex, other patient characteristics, treatment setting, or insurance provider. We captured and manually validated AF and HF occurring in both inpatient and outpatient settings from multiple providers in the area, allowing for near complete ascertainment of AF and outcomes.
Conclusion
In the community, 21% of AF patients developed HF over 3.7 years of mean follow-up, with HFpEF being more common than HFrEF. Patients with AF have an excess risk of developing HF, especially early after AF diagnosis, and those who develop HF experience more than a 2-fold increased risk of death compared to AF patients who do not develop HF. Finally, the rates of developing HF after AF have not improved since 2000. Thus, continued efforts to optimize the management of AF to improve outcomes are needed.
Supplementary Material
Acknowledgments
We thank Kay Traverse, Susan Stotz, and Dawn Schubert, for assistance with data collection and Deborah Strain for secretarial assistance.
FUNDING SOURCES
This work was supported by grants from the American Heart Association (11SDG7260039) and the National Institute on Aging (R01 AG034676). Additional support was provided by the American Heart Association (16EIA26410001) to Dr. Alonso.
Footnotes
Conflict of Interest: Alanna M. Chamberlain is a Co-Investigator of the Rochester Epidemiology Project (R01 AG034676). All other authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–60. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Anter E, Jessup M, Callans DJ. Atrial fibrillation and heart failure: treatment considerations for a dual epidemic. Circulation. 2009;119:2516–2525. doi: 10.1161/CIRCULATIONAHA.108.821306. [DOI] [PubMed] [Google Scholar]
- 3.McManus DD, Shaikh AY, Abhishek F, Vasan RS. Atrial fibrillation and heart failure parallels: lessons for atrial fibrillation prevention. Crit Pathways in Cardiol. 2011;10:46–51. doi: 10.1097/HPC.0b013e31820e1a4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrison TB, Bunch TJ, Gersh BJ. Pathophysiology of concomitant atrial fibrillation and heart failure: implications for management. Nat Clin Pract Cardiovasc Med. 2009;6:46–56. doi: 10.1038/ncpcardio1414. [DOI] [PubMed] [Google Scholar]
- 5.Santhanakrishnan R, Wang N, Larson MG, et al. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation. 2016;133:484–492. doi: 10.1161/CIRCULATIONAHA.115.018614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D'Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 7.Cha YM, Redfield MM, Shen WK, Gersh BJ. Atrial fibrillation and ventricular dysfunction: a vicious electromechanical cycle. Circulation. 2004;109:2839–2843. doi: 10.1161/01.CIR.0000132470.78896.A8. [DOI] [PubMed] [Google Scholar]
- 8.Stewart S, Hart CL, Hole DJ, McMurray JJ. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. 2002;113:359–364. doi: 10.1016/s0002-9343(02)01236-6. [DOI] [PubMed] [Google Scholar]
- 9.Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med. 1995;98:476–484. doi: 10.1016/S0002-9343(99)80348-9. [DOI] [PubMed] [Google Scholar]
- 10.Vermond RA, Geelhoed B, Verweij N, Tieleman RG, Van der Harst P, Hillege HL, Van Gilst WH, Van Gelder IC, Rienstra M. Incidence of atrial fibrillation and relationship with cardiovascular events, heart failure, and mortality: a community-based study from the Netherlands. J Am Coll Cardiol. 2015;66:1000–1007. doi: 10.1016/j.jacc.2015.06.1314. [DOI] [PubMed] [Google Scholar]
- 11.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna W, Seward JB, Iwasaka T, Tsang TS. Incidence and mortality risk of congestive heart failure in atrial fibrillation patients: a community-based study over two decades. Eur Heart J. 2006;27:936–941. doi: 10.1093/eurheartj/ehi694. [DOI] [PubMed] [Google Scholar]
- 12.Gerber Y, Weston SA, Redfield MM, Chamberlain AM, Manemann SM, Jiang R, Killian JM, Roger VL. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med. 2015;175:996–1004. doi: 10.1001/jamainternmed.2015.0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ., 3rd History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87:151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chamberlain AM, Gersh BJ, Alonso A, Chen LY, Berardi C, Manemann SM, Killian JM, Weston SA, Roger VL. Decade-long trends in atrial fibrillation incidence and survival: a community study. Am J Med. 2015;128:260–267. doi: 10.1016/j.amjmed.2014.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88:107–115. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 17.Yturralde RF, Gaasch WH. Diagnostic criteria for diastolic heart failure. Prog Cardiovasc Dis. 2005;47:314–319. doi: 10.1016/j.pcad.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Coresh J, Greene T, Stevens LA, Zhang Y, Hendriksen S, Kusek JW, Van Lente F. Using standardized serum creatinine values in the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 19.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 20.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 21.Chamberlain AM, Brown RD, Jr, Alonso A, Gersh BJ, Killian JM, Weston SA, Roger VL. No decline in the risk of stroke following incident atrial fibrillation since 2000 in the community: a concerning trend. J Am Heart Assoc. 2016;5:e003408. doi: 10.1161/JAHA.116.003408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piccini JP, Hammill BG, Sinner MF, Hernandez AF, Walkey AJ, Benjamin EJ, Curtis LH, Heckbert SR. Clinical course of atrial fibrillation in older adults: the importance of cardiovascular events beyond stroke. Eur Heart J. 2014;35:250–256. doi: 10.1093/eurheartj/eht483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lubitz SA, Benjamin EJ, Ellinor PT. Atrial fibrillation in congestive heart failure. Heart Fail Clin. 2010;6:187–200. doi: 10.1016/j.hfc.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnett DK, Goodman RA, Halperin JL, Anderson JL, Parekh AK, Zoghbi WA. AHA/ACC/HHS strategies to enhance application of clinical practice guidelines in patients with cardiovascular disease and comorbid conditions: from the American Heart Association, American College of Cardiology, and US Department of Health and Human Services. Circulation. 2014;130:1662–1667. doi: 10.1161/CIR.0000000000000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–267. doi: 10.1161/CIR.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pathak RK, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Wong CX, Twomey D, Elliott AD, Kalman JM, Abhayaratna WP, Lau DH, Sanders P. Long-term effect of goal-directed weight management in an atrial fibrillation cohort: a long-term follow-up study (LEGACY) J Am Coll Cardiol. 2015;65:2159–2169. doi: 10.1016/j.jacc.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Pathak RK, Elliott A, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Hendriks JM, Twomey D, Kalman JM, Abhayaratna WP, Lau DH, Sanders P. Impact of CARDIOrespiratory FITness on arrhythmia recurrence in obese individuals with atrial fibrillation: the CARDIO-FIT Study. J Am Coll Cardiol. 2015;66:985–996. doi: 10.1016/j.jacc.2015.06.488. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.