Abstract
All organisms need to be capable of adapting to changes in the availability and composition of nutrients. Over 75 years ago, researchers discovered that a calorie restricted (CR) diet could significantly extend the lifespan of rats, and since then a CR diet has been shown to increase lifespan and healthspan in model organisms ranging from yeast to non-human primates. In this review, we discuss the effects of a CR diet on metabolism and healthspan, and highlight emerging evidence that suggests that dietary composition – the precise macronutrients that compose the diet – may be just as important as caloric content. In particular, we discuss recent evidence that suggests protein quality may influence metabolic health. Finally, we discuss key metabolic pathways which may influence the response to CR diets and altered macronutrient composition. Understanding the molecular mechanisms responsible for the effects of CR and dietary composition on health and longevity may allow the design of novel therapeutic approaches to age-related diseases.
Calorie restriction promotes health and longevity
Calorie restriction (CR), a dietary intervention in which calories are reduced while maintaining adequate levels of micronutrients, was first discovered to extend the lifespan of rats more than 75 years ago (McCay et al., 1935). Since that time, a CR diet has been shown to extend lifespan in many model organisms including yeast, worms, flies, rodents, and non-human primates (Colman et al., 2014; Greer and Brunet, 2009; Lin et al., 2000; Rogina and Helfand, 2004; Weindruch et al., 1986). The precise degree of restriction, as well as the precise macronutrient composition of the CR regimen utilized, that maximizes lifespan extension may vary between individuals and species (Mair et al., 2005; Piper et al., 2005). A recent study of a 40% CR diet – typically utilized in mouse studies of CR – across 41 strains of recombinant inbred mice found a range of responses including strains with decreased lifespan, and it has been proposed that the response to CR and/or the optimal degree of restriction to promote longevity may vary according to genotype (Liao et al., 2010; Mitchell et al., 2016). While some have argued that the beneficial effects of CR in laboratory animals is an effect of overfeeding in the lab, CR extends maximum lifespan and robustly inhibits cancer in wild-type mice (Harper et al., 2006).
The specific mechanism by which CR extends lifespan and promotes health is unknown, but as the gold standard for anti-aging interventions, understanding the molecular and physiological mechanisms that underlie the effects of CR has been a priority of researchers for many years. Understanding the mechanisms that underlie the beneficial effects of a CR diet may enable the creation of dietary or pharmaceutical interventions that will permit those incapable of adhering to a CR diet to achieve some of the same benefits to health and longevity. The physiological effects of CR have been extensively cataloged; some of the changes which are believed to be beneficial include reduced inflammation (Chung et al., 2001), reduced oxidative stress (Sohal and Weindruch, 1996), altered neuroendocrine and sympathetic nervous system function, reduced energy expenditure, and improved metabolic flexibility (Bordone and Guarente, 2005; Heilbronn and Ravussin, 2003). CR also alters metabolism at the transcriptional level, and several studies show that CR can reverse many of the transcriptional changes associated with aging (Lee et al., 1999; Park et al., 2009; Pearson et al., 2008; Weindruch et al., 2001). Importantly, CR has been shown to not only extend lifespan, but also to improve healthspan, with animals on CR diets showing decreased rates of cancer, cardiovascular disease, and diabetes (Berrigan et al., 2002; Colman et al., 2014; Lamming and Anderson, 2014). A CR diet also promotes cognition in mouse models of neurodegeneration and Alzheimer’s disease (Graff et al., 2013; Halagappa et al., 2007).
Ongoing studies of CR in non-human primates suggest that CR promotes longevity and prevents age associated diseases such as cancer, type 2 diabetes, cardiovascular disease, brain atrophy, and osteoporosis (Colman et al., 2014; Cruzen and Colman, 2009; Mattison et al., 2012b). CR studies performed in non-human primates also show improved metabolic parameters, including increased insulin sensitivity and glucose tolerance as well as reduced energy expenditure, similar to their rodent counterparts (Kemnitz, 2011). While study design, husbandry, and dietary composition appear to impact the effect of CR on the longevity of primates (Colman et al., 2014), CR clearly has a dramatic effect upon the prevalence and severity of age-related diseases (Colman et al., 2009; Mattison et al., 2012a). These studies suggest that the fundamental mechanisms engaged by CR are preserved all the way from yeast to primates (Colman and Anderson, 2011; Fontana and Partridge, 2015).
While the benefit of a CR diet to human lifespan is unknown, the physiological and metabolic effects of CR in humans appear to be similar to those observed in non-human primates. Humans who consumed a low calorie, nutrient dense diet for approximately two years in Biosphere 2 showed physiologic, hematologic, hormonal, and biochemical changes resembling those of rodents and monkeys on such diets (Walford et al., 2002). More recently, a randomized clinical trial of a 25% CR diet in non-obese adults was shown to result in significant weight loss, primarily due to loss of adiposity through decreased visceral fat (Fontana et al., 2016b). Other studies on the effect of a CR diet on humans has demonstrated that CR can improve age-associated changes in blood pressure, systemic inflammation, and myocardial fibrosis (Meyer et al., 2006), protect against atherosclerosis (Fontana et al., 2004), and reduce cardiovascular disease instance and risk (Cruzen and Colman, 2009). Similar to rodents and non-human primates placed on a CR diet, humans placed on a CR diet also have increased levels of adiponectin and improved insulin sensitivity (Fontana et al., 2009). Given these favorable effects, while the jury remains out on the effect of a CR diet on human lifespan, it appears very likely that a CR diet has the potential to improve healthspan, and perhaps prevent or delay many age-associated diseases.
While CR diets will likely prove effective in preventing age-related disease, they are difficult to sustain for all but a handful of individuals. There is therefore great interest in developing dietary strategies that are easier to follow that mimic the beneficial effects of a CR diet. One of the most popularly researched and implemented CR mimetic dietary strategies is feeding intermittently – either restricting the time of day during which feeding is permitted, or fasting intermittently. As similar dietary regimens have been followed by many for religious reasons for centuries or millennia, they may be more sustainable. The cellular responses to fasting are similar to CR but include reduced oxidative damage, improved energy metabolism, and overall protection. Physiologically, fasting can improve longevity and prevent against many diseases such as diabetes, heart disease, cancers, obesity, hypertension, asthma, rheumatoid arthritis, and neurodegeneration (Longo and Mattson, 2014). This may occur through a shift to fat and ketone metabolism during a fast, as well as through adaptive changes to stress and repair of cellular damage (Mattson et al., 2014). Restricting feeding to four short time periods per day can significantly extend the lifespan of rats (Deerberg et al., 1990), and in mice limiting food access to an eight hour window each day can improve metabolic disease even in consumption of diabetogenic diets (Chaix et al., 2014). However, human data on the benefits of time-restricted feeding suggests that the benefits of time-restricted feeding may depend on the exact composition of the diet (Mattson et al., 2014), and thus it is not clear that this type of dietary intervention will promote healthy aging in humans, or if CR mimetics will be as effective as they are in model organisms.
Dietary composition – when is a calorie not just a calorie?
Unfortunately, while a CR diet may be an effective method in reducing age-associated diseases and possibly extending lifespan, even a slight reduction in caloric intake or occasional fasting may be difficult in present-day Western society, in which we are surrounded by cheap and readily available calories and struggling with obesity. Intriguingly, despite significant interest in the biological mechanisms engaged by CR, until recently it has been unclear what macronutrients in a CR diet are responsible for its beneficial effects. While CR regimens typically decrease protein, fat, and carbohydrates, research suggests the reduced consumption of these macronutrients may not contribute equally to CR’s benefits.
Many of the first experiments which investigated the effect of restricting individual macronutrients, particularly protein, on rat lifespan observed varied results (Weindruch and Walford, 1988). In retrospect, these difficulties may have been due to a combination of uncertainty regarding the exact nutritional needs of rats, and the use of dietary protein from different sources with varying amino acid composition. These problems began to be resolved in the 1980s, and experiments using Fisher 344 rats determined that restriction of dietary fat in the context of an isocaloric diet did not extend lifespan, but that the source of dietary protein greatly impacted lifespan (Iwasaki et al., 1988a, b). More recent experiments conducted in Drosophila melanogaster have shown that restriction of either dietary sugar or yeast will extend lifespan, but that calorie-for-calorie, restriction of yeast has a much greater impact than restriction of sugar (Mair et al., 2005). Another Drosophila study showed that essential amino acid balance changes can reverse the effects of DR, even though calorie level is restricted (Grandison et al., 2009).
Since these initial experiments, dietary protein has been revealed as a key determinant of the effects of a CR diet on rodent metabolism and lifespan. In a series of experiments during the last decade, a nutritional Geometric Framework approach was used to analyze the lifespan of first Drosophila and more recently C57BL/6J mice fed isocaloric diets with many possible ratios of protein, fat, and carbohydrates (Lee et al., 2008; Solon-Biet et al., 2014). This approach takes into account that in diet studies, shifting ratios in diet always affects at least two macronutrients, and may affect the influence of the third. The Geometric Framework therefore permits the consideration of altered macronutrient ratios and total energy intake in their full spectrum. In Drosophila, this approach was used to determine the lifespan of over 1,000 flies fed one of 28 diets with varying concentrations of sugar and yeast, and Lee and colleagues determined that “lifespan declined from the maximum as protein intake increased and as carbohydrate intake fell” (Lee et al., 2008). Similarly, a recent study determined the lifespan of C57BL/6J mice placed on one of 25 different diets with varying concentrations of protein, carbohydrates, and fat, and determined that mice fed a low protein diet lived the longest, with mice fed a low protein, high carbohydrate diet living the very longest (Solon-Biet et al., 2014). Importantly, this study also found that CR in the context of high protein did not extend lifespan. Further work by Solon-Biet and colleagues demonstrated that low protein, high carbohydrate diets produce metabolic benefits comparable to CR with respect to blood levels of glucose, insulin and lipids. In addition, low protein high carbohydrate diets did not produce additional benefits when fed at CR levels (Solon-Biet et al., 2015a).
While the mechanisms downstream of a low protein diet that mediate these beneficial effects are still being determined, protein consumption in mice correlates with blood levels of the three branched-chain amino acids (BCAAs), leucine, isoleucine, and valine (Solon-Biet et al., 2014). Specifically restricting dietary levels of the BCAAs to the levels found in a low protein diet improves many aspects of mouse metabolic health to the same extent as a low protein diet (Fontana et al., 2016a). Interestingly, as opposed to mice on a CR diet that conserve energy, mice on a low protein, high carbohydrate diet have increased energy expenditure. Mice fed a low protein diet have increased levels of the fasting-induced insulin-sensitizing hormone FGF21, which is believed to mediate the increased energy expenditure of mice on a low protein diet (Fontana et al., 2016a; Laeger et al., 2014). Intriguingly, restriction of BCAAs alone does not induce FGF21 or increase energy expenditure (Fontana et al., 2016a), demonstrating that at least some aspects of the improved metabolic health associated with a low protein diet are independent of FGF21.
While restriction of the BCAAs does not induce FGF21, restriction of another essential amino acid, methionine, does induce FGF21 (Lees et al., 2014). Methionine is a particularly interesting amino acid, as it is essential for translation initiation as well as processes that require donation of methyl groups in methylation. Methionine restriction extends the lifespan of flies, rats and mice (Lee et al., 2014; Miller et al., 2005; Orentreich et al., 1993), inducing a host of metabolic adaptations, such as beneficial changes in body weight, composition and glycemic control. Restriction of dietary methionine also reverses age-associated metabolic dysfunction in aged mice (Lees et al., 2014; Perrone et al., 2013). We did not identify changes in blood levels of methionine in humans on a protein-restricted diet (Fontana et al., 2016a), making it unclear if methionine restriction normally contributes to the benefits of a low protein or CR diet. However, vegan diets may have naturally low levels of methionine (McCarty et al., 2009), and further investigation of the effects of these diets on methionine levels and metabolic health may be warranted.
Unlike CR, there has been a great number of observational studies of the effects of low and high protein diets in humans. One such recent study with over 38,000 participants found that humans in the highest quartile of protein consumption had more than twice the risk of developing diabetes than humans in the lowest quartile (Sluijs et al., 2010). While a high protein diet is associated with increased cardiovascular mortality (Lagiou et al., 2007), reduced consumption of dietary protein is associated with decreased cancer incidence as well decreased mortality rates in individuals under 65 (Levine et al., 2014). Though few randomized controlled trials of protein restriction have been performed in humans, we recently found that humans fed a diet in which 7–9% of calories were derived from protein for only 6 weeks had improved metabolic health. Subjects randomized to this low protein diet had significant decreases in fasting blood glucose and circulating levels of BCAAs, and lost over five pounds, more than half of which was fat mass (Fontana et al., 2016a).
Carbohydrate restriction has also been an interest to researchers, as it has been assumed that decreased dietary carbohydrates would benefit both blood glucose and obesity. Research into restriction of dietary carbohydrates in rats has found that restricting carbohydrates as a means of restricting energy intake improves metabolic health, lowering total cholesterol, serum triglycerides, and HOMA2-IR, and increases expression of PPARγ and adiponectin in adipose tissue (Chen et al., 2015). However, as opposed to the effects of protein or amino acid restriction, these effects were only proportional to the level of energy restricted. In humans, recent studies suggest that the benefits of a low carbohydrate diet are likely minimal. While carbohydrate restriction initially lowered weight and fat mass in obese humans, over time this effect diminished, and weight benefits were not maintained (Hashimoto et al., 2016). Moreover, a recent meta-analysis of randomized control trials for low carbohydrate diets in subjects with type 2 diabetes suggests that a low carbohydrate diet does not improve glycemic control or other metabolic outcomes (van Wyk et al., 2016).
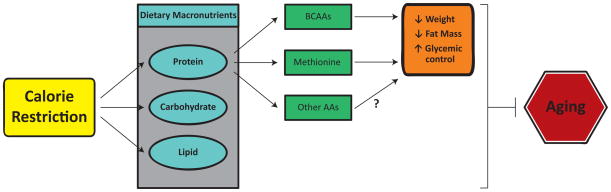
In conclusion, although much research remains to be done, it appears that restriction of dietary protein contributes to the metabolic benefits of a CR diet, and that alterations in protein quality may mimic some of the beneficial effects of a CR diet (Figure 1). This type of intervention, which does not limit calorie intake, may allow the benefits of CR to be achieved by a much broader population. Restriction of dietary protein alone, or even of specific essential amino acids, promotes many of the same metabolic benefits as CR, and could conceivably be a translatable intervention to prevent or delay age-related disease. However, CR or protein restriction in the elderly will need to be approached cautiously; the preservation of muscle and bone mass is a priority in the aged, as sarcopenia is a major driver of frailty and loss of independence (Jensen et al., 2014; Landi et al., 2015). Indeed, a growing body of research suggests that protein intake by the elderly may need to be increased in order to preserve muscle mass (Nowson and O’Connell, 2015; Paddon-Jones and Rasmussen, 2009; Wolfe et al., 2008), and low protein diets may not decrease mortality in people over the age of 65 (Levine et al., 2014). However, lifelong CR diet feeding preserves muscle mass in rodents and non-human primates despite an absolute reduction in protein intake (Aspnes et al., 1997; Colman et al., 2008; McKiernan et al., 2012; Mercken et al., 2012; van Norren et al., 2015), suggesting that these types of interventions may reduce the need to increase protein intake during aging. Further research will be required to fully understand how age, as well as genetic background, activity level, and health impact the response to altered protein quality.
Figure 1. Protein quality may contribute to the effects of calorie restriction.
CR usually involves the restriction of all macronutrients, including dietary protein. We and others have found that the specific amino acid composition of the diet (protein quality) regulates metabolism, health, and longevity, and partially mimics the effects of a CR diet. Restriction of dietary branched chain amino acids (BCAAs) promotes leanness and glucose tolerance, while restriction of dietary methionine promotes leanness, insulin sensitivity, and lifespan. We propose that the beneficial effects of a CR diet on metabolic health and aging is mediated not simply by a reduction in caloric intake, but by the restriction of specific macronutrients, including BCAAs and methionine.
The mechanistic Target Of Rapamycin - a central regulator of longevity
To truly harness the beneficial effects of CR or protein restriction, we will need to understand the molecular mechanisms that drive these effects. One of the most conserved responses to CR in mammals, including humans, is increased insulin sensitivity, which is generally believed to be beneficial in terms of health and longevity (reviewed in (Lamming, 2014)). Conversely, overnutrition is associated with decreased insulin sensitivity, decreased lifespan, and an increased risk of several age-related diseases. A logical assumption about CR is that increased insulin sensitivity may mediate many of the beneficial effects of CR. Indeed, some long-lived mouse mutants have increased insulin sensitivity – e.g., mice lacking S6K1, mice in which the insulin receptor has been specifically deleted in adipose tissue (FIRKO), and Ames dwarf mice (Bluher et al., 2003; Masternak et al., 2009; Selman et al., 2009).
However, many other long-lived mouse mutants do not have increased insulin sensitivity, including mice heterozygous for both mTOR and mLST8, mice expressing a hypomorphic allele of mTOR, mice heterozygous for either Igf1r or Akt1, and mice in which IRS2 was deleted specifically in the brain (Bokov et al., 2011; Lamming et al., 2012; Nojima et al., 2013; Taguchi et al., 2007; Wu et al., 2013). Moreover, the most robust pharmaceutical intervention that extends lifespan is rapamycin, an inhibitor of the mTOR (mechanistic Target Of Rapamycin) protein kinase, which induces insulin resistance (Lamming et al., 2012). These conflicting observations, as well data from C. elegans and other model organisms, can be reconciled by noting that what all of these interventions, including rapamycin and CR, have in common is decreased PI3K/Akt/mTOR pathway signaling (Blagosklonny, 2012; Lamming, 2014; Mercken et al., 2013). Other energy sensing pathways implicated in the response to CR include AMPK and the sirtuin family of histone deacetylases, the activation of which may inhibit mTOR pathway signaling (Ghosh et al., 2010; Gwinn et al., 2008; Wang et al., 2011).
mTOR is found in two distinct complexes; mTOR complex 1 (mTORC1) functions as a central regulator of metabolism and growth, integrating information about the availability of many distinct nutrients including amino acids, glucose, and oxygen as well as hormonal cues. In contrast, mTORC2 is insensitive to amino acids and serves as a key effector of PI3K signaling. mTOR signaling plays a key role in the regulation of mammalian physiology and metabolism, which we have discussed in depth in a recent review (reviewed in (Kennedy and Lamming, 2016)). However, the role of mTOR in the response to CR, and in particular to restriction of dietary protein and amino acids, deserves consideration.
The mTOR protein kinase is one of two evolutionarily conserved amino acid responsive kinases, the other being GCN2, which senses an accumulation of uncharged transfer RNAs that indicates a deficiency in one or more amino acids (Gallinetti et al., 2013). In contrast to GCN2, which is more sensitive to the essential amino acids, mTOR activity is especially sensitive to a few key amino acids. mTORC1 is particularly sensitive to the BCAAs, which potently activate mTORC1 in vitro in cell culture, and in vivo in both rodents and humans (Blomstrand et al., 2006; Gallinetti et al., 2013; Li et al., 2011; Moberg et al., 2014; Sans et al., 2006; Xiao et al., 2011). It is therefore logical to consider if restriction of dietary protein may promote longevity in part through inhibition of mTOR signaling, particularly in light of recent studies demonstrating that mTORC1 inhibition is required for the beneficial effects of protein restriction on stress resistance after hepatic injury (Harputlugil et al., 2014). mTORC1 inhibition likewise is associated with the ability of protein restriction to protect from renal ischemia reperfusion injury (Robertson et al., 2015).
In the above-referenced study showing that lifespan is the longest in mice consuming the lowest percentage of calories from protein, hepatic mTORC1 activity correlated with protein intake, although the overall effect was small (Solon-Biet et al., 2014). However, plasma BCAA levels showed a very strong correlation with mTORC1 activity. In order to determine if reduced dietary protein can inhibit mTORC1 signaling in vivo, we recently fed mice a low protein diet for approximately three months as part of a tumor xenograft study. We found that a low protein diet significantly inhibited tumor growth, but more stunningly, specifically inhibited mTORC1 activity in both the tumor and somatic tissues (Lamming et al., 2015). The mechanism behind this effect is unclear; decreased mTORC1 activity could be due to a decrease in specific amino acids, or due to a decrease in total amino acids. Recent developments have shed new light on our understanding of how amino acids – and in particular, the branched chain amino acids – regulate mTORC1 activity at the molecular level.
Regulation of mTORC1 by amino acids
The past few years have seen a dramatic advance in our understanding of how amino acids regulate mTORC1 function (Figure 2). While we and others have described the activation of mTORC1 by amino acids in detail elsewhere (Goberdhan et al., 2016; Kennedy and Lamming, 2016), the essential breakthrough was the realization that in response to stimulation by amino acids, mTORC1 is recruited to the lysosome by the Rag family of small GTPases – RagA, RagB, RagC, and RagD (Sancak et al., 2010; Sancak et al., 2008). Rag A is critical for mTORC1 response to growth factors, and its deletion result in embryonic lethality, while a constitutively activated RagA mutant results in neonatal lethality, proving how essential proper mTORC1 regulation is (Efeyan et al., 2014; Efeyan et al., 2013). While the association of mTORC1 with the lysosome was originally unexpected, it may be efficient to coordinate the site of amino acid recycling with mTORC1 activation, given that the complex serves as a suppressor of autophagy. The nucleotide loading state of the Rags regulates their function and ability to recruit mTORC1 to the lysosome, with GTP-loaded RagA/B and GDP-loaded RagC/D binding to and activating mTORC1 (Sancak et al., 2008).
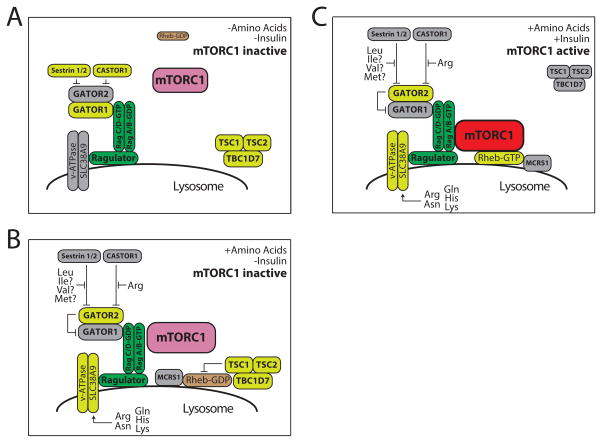
Figure 2. Model for the recruitment and activation of mTORC1 activity by amino acids.
Both amino acids and growth factor (e.g., insulin) signaling are required to activate mTORC1. Activation of mTORC1 requires the co-localization of mTORC1 with Rheb-GTP. A) In the absence of amino acids and insulin, neither mTORC1 or Rheb localize to the lysosome. B) Amino acids promote the localization of mTORC1 to the lysosome, in part by relieving the inhibition of Sestrin1/2 and CASTOR1 on GATOR2, which permits GATOR2 to inhibit the GAP activity of GATOR1. Amino acids also promote the lysosomal recruitment of Rheb by MCRS1. TSC, which is localized to the lysosome under conditions of stress, continues to inhibit Rheb, and thus mTORC1 remains inactive. C) Insulin induces TSC to leave the lysosome, permitting Rheb to bind GTP; mTORC1 can then interact with GTP-bound Rheb and becomes active. Adapted from Kennedy and Lamming, 2016, Cell Metabolism, with permission.
There has been a significant amount of effort to define the guanine nucleotide exchange factor (GEF) and GTPase-activating protein (GAP) for the Rags that thereby regulate mTORC1 activity. Two major regulatory pathways have been defined; the first, the Ragulator complex, is a collection of proteins at the lysosomal surface that interacts with the vacuolar H(+)-adenosine triphosphatase(v-ATPase) and has GEF activity towards RagA and Rag B (Bar-Peled et al., 2012). The Ragulator and the v-ATPase interact with SLC38A9, a lysosomal low-affinity amino acid transporter that is required for the normal activation of mTORC1 by certain amino acids including arginine and lysine (Jung et al., 2015; Rebsamen et al., 2015; Wang et al., 2015). SLC38A9 may function by regulating the GEF activity of the Ragulator, or the ability of the Ragulator to recruit the Rags to the lysosomal surface. The second major Rag regulatory pathway is served by the GATOR complex, which is composed of two subunits, GATOR1 and GATOR2. GATOR1 functions as a GAP for RagA and RagB, inhibiting their ability to recruit mTORC1 to the lysosome, while GATOR2 functions as a negative regulator of GATOR1 (Bar-Peled et al., 2013).
Recent discoveries have determined that the regulation of GATOR2 activity is a major mechanism by which amino acids regulate mTORC1 activity. The ability of GATOR2 to inhibit GATOR1 is inhibited by binding to Sestrin2 and CASTOR1 (Chantranupong et al., 2016; Chantranupong et al., 2014; Kim et al., 2015; Saxton et al., 2016; Wolfson et al., 2016). Sestrin2 acts as a sensor for leucine, and possibly also for the other BCAAs as well as methionine, which can all bind to a pocket on Sestrin 2. Amino acid binding by Sestrin2, particularly with respect to leucine, is reported to disrupt the interaction of Sestrin2 and GATOR2, freeing GATOR2 to inhibit GATOR1 (Saxton et al., 2016; Wolfson et al., 2016). This model has been recently questioned as potentially inconsistent with previous studies, which reported that Sestrins in Drosophila and mice can inhibit mTORC1 in vivo in the presence of physiological levels of amino acids (reviewed in (Lee et al., 2016)). Another protein, CASTOR1, was recently reported to act similarly to Sestrin2, but with respect to arginine, which disrupts the interaction of CASTOR1 (or its homolog CASTOR2) with GATOR2 (Chantranupong et al., 2016).
Other molecular mechanisms for Rag-dependent amino acid sensing have also been described, mediated by other proteins including transporters and ubiquitin ligases (reviewed in (Kennedy and Lamming, 2016)). While the molecular mechanisms by which other amino acids regulate the nucleotide loading of the Rags remains to be defined – in particular, understanding how the nucleotide loading of RagC and RagD is regulated by amino acids - the activation of mTORC1 at the lysosomal surface requires the presence of GTP-bound Rheb. It was recently discovered that amino acids stimulate the binding of Rheb to microspherule protein 1 (MCRS1), and localization of Rheb-MCRS1 to the lysosome (Fawal et al., 2015). At the lysosomal surface, Rheb is inactivated by the presence of the tuberous sclerosis complex (TSC), which is a GAP for Rheb. Insulin stimulates the AKT-dependent phosphorylation of TSC2, stimulating the departure of TSC from the lysosome, and permitting Rheb-GTP to activate mTORC1 (Menon et al., 2014). While the ability of TSC to inhibit mTORC1 signaling has always been thought of as amino-acid independent, recent findings suggest that amino acid deprivation (as well as other cellular stresses) also promotes the lysosomal localization of TSC (Demetriades et al., 2014; Demetriades et al., 2016). Thus, amino acids regulate almost every aspect of mTORC1 signaling.
Branched chain amino acids regulate metabolic health
While all of the standard twenty amino acids are necessary for protein translation, not all amino acids activate mTORC1 to the same extent. As summarized by Christopher Lynch over a decade ago, experiments in a variety of cell types have demonstrated that “most of the effects of amino acids on mTOR signaling are abolished by lowering the concentration of leucine, or mimicked by adding leucine and, to a lesser extent, the other branched-chained amino acids” (Lynch, 2001). Indeed, the three BCAAs potently activate mTORC1 activity in not only in cell culture, but also in vivo in rodents and humans (Blomstrand et al., 2006; Gallinetti et al., 2013; Li et al., 2011; Moberg et al., 2014; Sans et al., 2006; Xiao et al., 2011).
Just as CR and PR inhibit mTORC1 signaling, chronic overnutrition is associated with increased mTORC1 signaling, and it is now clear that activation of mTORC1 is associated with the development of insulin resistance (Khamzina et al., 2005). At the molecular level, this is mediated by the activation of S6K1 and the resultant inhibitory phosphorylation of IRS1 (Tremblay et al., 2007), as well as by the direct phosphorylation of the adaptor protein Grb10 by mTORC1 (Hsu et al., 2011). Myotubes from humans with type 2 diabetes have increased phosphorylation of IRS1, decreased PI3K activity and limited association of PI3K with IRS1 compared to controls (Bouzakri et al., 2003). In addition to the chronic effects of overnutrition on mTORC1, the acute effects of mTORC1 activation can be observed in humans; infusing amino acids to activate mTORC1 increases S6K1 phosphorylation and inhibitory phosphorylation of IRS1 in skeletal muscle, leading to insulin resistance (Tremblay et al., 2007). Because the BCAAs potently activate mTORC1, BCAA restriction may therefore be a means of restricting mTORC1 activity to improve metabolic health and longevity.
We discussed above our recent findings that a PR or BCAA restricted diet can improve certain aspects of metabolic health. Specifically, PR in either humans and mice, or specific restriction of the dietary BCAAs in mice, improves glucose tolerance and decreases fasting blood glucose levels while decreasing weight gain and fat mass gain in young growing mice (Fontana et al., 2016a). This is the first evidence that a physiological and sustainable reduction of dietary BCAAs can promote health and glycemic control, but it is not the first evidence that dietary BCAAs have an important role in metabolic health. Several studies have observed increased serum levels of the three BCAAs in insulin-resistant humans and animals, and BCAA levels are predictive of development of type 2 diabetes (reviewed in (Lynch and Adams, 2014)), while feeding mice a diet entirely lacking in either leucine, isoleucine, or valine promotes insulin sensitivity (Xiao et al., 2011; Xiao et al., 2014). Feeding a diet with reduced levels of BCAAs to Zucker fatty rats likewise improves skeletal muscle insulin sensitivity (White et al.). Indeed, in addition to BCAAs strongly correlating with insulin resistance and diabetes, a possible causal role for BCAAs in type 2 diabetes has been suggested (Newgard et al., 2009).
However, despite the clear links between branched chain amino acids and mTORC1 activity, and mTORC1 activity and insulin resistance, it is not clear that the metabolic effects of the BCAAs are mediated by mTORC1; in humans fed a protein restricted diet, the levels of plasma BCAAs are only altered by approximately 10–20% (Fontana et al., 2016a). Intriguingly, mice in which the gene encoding Bcat2, the mitochondrial branched chain aminotransferase, is deleted have improved glucose tolerance, insulin sensitivity, and energy expenditure, as well as decreased body weight, despite increased plasma levels of the BCAAs (She et al., 2007). This data suggests that either BCAA metabolites or the downstream effects of BCAA catabolism on physiology mediate the negative impact of high blood levels of the BCAAs. In support of this model, a recent study of insulin resistance in mice and humans suggests that 3-hydroxyisobutyrate, a product of valine metabolism produced in skeletal muscle, stimulates the uptake of fatty acids by skeletal muscle, directly promoting lipid accumulation and insulin resistance (Jang et al., 2016).
A complicating factor in determining how BCAAs as well as other metabolically important amino acids such as methionine may regulate metabolic health is that mTORC1 is not the sole kinase involved in the response to the availability of amino acids. As discussed above, GCN2 is a major sensor of amino acid deficiency which regulates translation via the phosphorylation of eIF2α. The catabolism of BCAAs is also a major source of energy, and restriction of either dietary protein or of the BCAAs specifically can result in the activation of AMPK (Chotechuang et al., 2009; Xiao et al., 2014). Likewise, while methionine is essential for protein translation and can be utilized for energy, it also has a key role in numerous cellular processes following conversion to the methyl donor S-adenosylmethionine (SAM) (Brosnan and Brosnan, 2006).
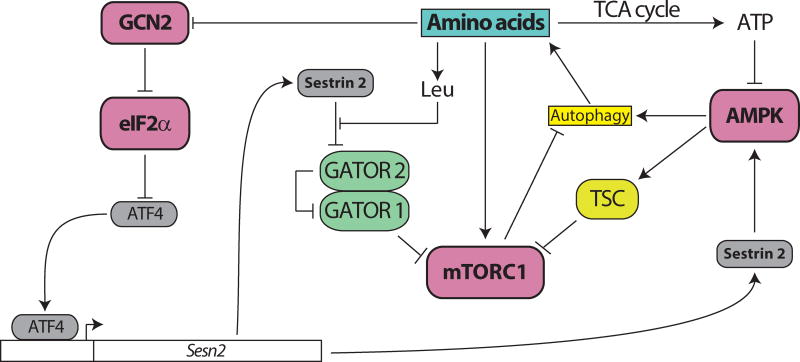
The interaction of these core energy and nutrient sensing pathways is complex (Figure 3). In addition to the effects of amino acids that are mediated directly by mTORC1, there is also crosstalk between mTORC1 and GCN2. Upon amino acid depletion, GCN2 inhibits eIF2α, which induces translation of Atf4; Atf4 in turn stimulates expression of Sestrin2, which represses mTORC1 by blocking lysosomal localization (Ye et al., 2015). Sestrin2 also indirectly inhibits mTORC1 through the activation of AMPK; AMPK inhibits mTORC1 both through an inhibitory phosphorylation of the mTORC1 subunit Raptor and an activating phosphorylation of TSC2 (Budanov and Karin, 2008; Gwinn et al., 2008; Inoki et al., 2002; Lee et al., 2010; Lee et al., 2012). AMPK activity is also repressed by mTORC1 activity via the S6K1-mediated phosphorylation of AMPK (Dagon et al., 2012). Further integrating the GCN2 and mTORC1 pathways, it was discovered earlier this year that mTORC1 stimulates the phosphorylation of eIF2β, and that this leads to the dephosphorylation of eIF2α, likely through recruitment of a phosphatase (Gandin et al., 2016). Until recently, dissecting out the role of individual amino acids in regulating this network and impacting health and metabolism has been infeasible. With the recent identification of the amino acid sensors that regulate mTORC1, as well as the development of metabolomics, genome editing, and tissue-specific models of altered mTOR and GCN2 function, this goal may finally be achievable.
Figure 3. Amino acids regulate multiple, interacting nutrient sensing pathways.
Amino acid availability is sensed by multiple kinases, including mTORC1, GCN2, and AMPK. The high integration and feedback signaling between these pathways has made understanding the molecular basis for the physiological effects of protein quality infeasible until recently.
Conclusions
For over three quarters of a century, the seminal observations by Clive McCay and colleagues have served to tantalize scientists seeking to preserve health into old age and to extend lifespan. This quest has taken on increased urgency with the realization that a “silver tsunami” of age-related diseases is rapidly approaching as the percentage of the population over the age of 65 booms. In particular, the concept of a “metabolic clock” that links metabolism and aging has become increasing popular as evidence mounts that our Western diet and lifestyle may not only be impairing our health, but also actively accelerating aging itself (reviewed in (López-Otín et al., 2016)). Yet, despite this urgency and the promise of better health through a calorie restricted diet, the ability to adhere to a rigorous CR diet is beyond the scope of a population that has broad access to relatively cheap calories. While scientific research has shed a great deal of light on the biological pathways that are altered by a CR diet and mediate its beneficial effects, progress in identifying a small molecule that can provide the myriad benefits of CR has been slow. The best molecular mimic of CR – the mTOR inhibitor rapamycin – extends lifespan, but its effect on healthspan are uncertain and debated in literature (Johnson et al., 2013; Neff et al., 2013; Wilkinson et al., 2012; Zhang et al., 2014) and its side effects numerous (Arriola Apelo and Lamming, 2016).
Recent findings suggest that a better way forward exists – diets with altered macronutrient content promote metabolic health in both humans and mice, and low protein diets are associated with longevity in both species. Moreover, diets with alterations in macronutrient content show positive effects on both metabolism and, in the case of methionine restriction, longevity, demonstrating that dietary protein quality is a powerful regulator of longevity. While the molecular mechanisms affected by these diets are – much like CR – varied and numerous, precise manipulation of dietary amino acid content and protein quality may allow the dissection of the molecular mechanisms that drive the benefits of altered consumption of specific amino acids.
Developing small molecules that harness our emerging knowledge of molecular targets such as the Sestrin2/GATOR2/mTORC1 signaling pathway and translating these molecules to the clinic is likely to be a protracted process, and potentially lead to unanticipated side effects. In contrast, FDA-approved medical foods lacking specific amino acids are already available for the treatment of people with inborn errors of metabolism such as maple syrup urine disease and homocystinuria. In the short-term, these types of medical foods could potentially be utilized as sustainable therapies for the treatment of diabetes and other metabolic diseases, while in the long term, interventions that inhibit the uptake or catabolism of specific dietary amino acids may prove to be useful in treatment of other diseases of aging.
Highlights.
Many of the benefits of calorie restriction are mediated by reduced protein.
Restriction of specific dietary amino acids promotes metabolic health.
The protein kinases mTORC1 and GCN2 respond to altered dietary amino acids.
Altering dietary macronutrients may be a translatable intervention for healthy aging.
Acknowledgments
We would like to thank all the members of the Lamming lab for their support. The Lamming lab is supported in part by grants from the NIH/NIA (R00 AG041765 to D.W.L. and R21 AG050135 jointly to D.W.L. and Dr. Matthew J. Merrins), a New Investigator Program Award from the Wisconsin Partnership Program, and a Glenn Foundation Award for Research in the Biological Mechanisms of Aging, as well as startup funds from the UW-Madison School of Medicine and Public Health and the UW-Madison Department of Medicine. This review was prepared while D.W.L. was an AFAR Research Grant recipient from the American Federation for Aging Research. N.E.C. is supported in part by a training grant from the UW Institute on Aging (NIA T32 AG000213). This work was supported using facilities and resources from the William S. Middleton Memorial Veterans Hospital. This work does not represent the views of the Department of Veterans Affairs or the United States Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arriola Apelo SI, Lamming DW. Rapamycin: an inhibitor of aging emerges from the soil of Easter island. J Gerontol A Biol Sci Med Sci. 2016;71:841–849. doi: 10.1093/gerona/glw090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspnes LE, Lee CM, Weindruch R, Chung SS, Roecker EB, Aiken JM. Caloric restriction reduces fiber loss and mitochondrial abnormalities in aged rat muscle. FASEB J. 1997;11:573–581. doi: 10.1096/fasebj.11.7.9212081. [DOI] [PubMed] [Google Scholar]
- Bar-Peled L, Chantranupong L, Cherniack AD, Chen WW, Ottina KA, Grabiner BC, Spear ED, Carter SL, Meyerson M, Sabatini DM. A Tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science. 2013;340:1100–1106. doi: 10.1126/science.1232044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Peled L, Schweitzer LD, Zoncu R, Sabatini DM. Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell. 2012;150:1196–1208. doi: 10.1016/j.cell.2012.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrigan D, Perkins SN, Haines DC, Hursting SD. Adult-onset calorie restriction and fasting delay spontaneous tumorigenesis in p53-deficient mice. Carcinogenesis. 2002;23:817–822. doi: 10.1093/carcin/23.5.817. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV. Once again on rapamycin-induced insulin resistance and longevity: despite of or owing to. Aging (Albany NY) 2012;4:350–358. doi: 10.18632/aging.100461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomstrand E, Eliasson J, Karlsson HK, Kohnke R. Branched-chain amino acids activate key enzymes in protein synthesis after physical exercise. The Journal of nutrition. 2006;136:269S–273S. doi: 10.1093/jn/136.1.269S. [DOI] [PubMed] [Google Scholar]
- Bluher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- Bokov AF, Garg N, Ikeno Y, Thakur S, Musi N, DeFronzo RA, Zhang N, Erickson RC, Gelfond J, Hubbard GB, et al. Does reduced IGF-1R signaling in Igf1r+/− mice alter aging? PLoS One. 2011;6:e26891. doi: 10.1371/journal.pone.0026891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nature Reviews Molecular Cell Biology. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- Bouzakri K, Roques M, Gual P, Espinosa S, Guebre-Egziabher F, Riou JP, Laville M, Le Marchand-Brustel Y, Tanti JF, Vidal H. Reduced Activation of Phosphatidylinositol-3 Kinase and Increased Serine 636 Phosphorylation of Insulin Receptor Substrate-1 in Primary Culture of Skeletal Muscle Cells From Patients With Type 2 Diabetes. Diabetes. 2003;52:1319–1325. doi: 10.2337/diabetes.52.6.1319. [DOI] [PubMed] [Google Scholar]
- Brosnan JT, Brosnan ME. The sulfur-containing amino acids: an overview. The Journal of nutrition. 2006;136:1636S–1640S. doi: 10.1093/jn/136.6.1636S. [DOI] [PubMed] [Google Scholar]
- Budanov AV, Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134:451–460. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaix A, Zarrinpar A, Miu P, Panda S. Time-Restricted Feeding Is a Preventative and Therapeutic Intervention against Diverse Nutritional Challenges. Cell Metabolism. 2014;20:991–1005. doi: 10.1016/j.cmet.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantranupong L, Scaria SM, Saxton RA, Gygi MP, Shen K, Wyant GA, Wang T, Harper JW, Gygi SP, Sabatini DM. The CASTOR Proteins Are Arginine Sensors for the mTORC1 Pathway. Cell. 2016;165:153–164. doi: 10.1016/j.cell.2016.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantranupong L, Wolfson RL, Orozco JM, Saxton RA, Scaria SM, Bar-Peled L, Spooner E, Isasa M, Gygi SP, Sabatini DM. The Sestrins interact with GATOR2 to negatively regulate the amino-acid-sensing pathway upstream of mTORC1. Cell reports. 2014;9:1–8. doi: 10.1016/j.celrep.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JH, Ouyang C, Ding Q, Song J, Cao W, Mao L. A Moderate Low-Carbohydrate Low-Calorie Diet Improves Lipid Profile, Insulin Sensitivity and Adiponectin Expression in Rats. Nutrients. 2015;7:4724–4738. doi: 10.3390/nu7064724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotechuang N, Azzout-Marniche D, Bos C, Chaumontet C, Gausseres N, Steiler T, Gaudichon C, Tome D. mTOR, AMPK, and GCN2 coordinate the adaptation of hepatic energy metabolic pathways in response to protein intake in the rat. Am J Physiol Endocrinol Metab. 2009;297:E1313–1323. doi: 10.1152/ajpendo.91000.2008. [DOI] [PubMed] [Google Scholar]
- Chung HY, Kim HJ, Kim JW, Yu BP. The Inflammation Hypothesis of Aging. Annals of the New York Academy of Sciences. 2001;928:327–335. [PubMed] [Google Scholar]
- Colman RJ, Anderson RM. Nonhuman Primate Calorie Restriction. Antioxidants & Redox Signaling. 2011;14:229–239. doi: 10.1089/ars.2010.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Beasley TM, Allison DB, Weindruch R. Attenuation of sarcopenia by dietary restriction in rhesus monkeys. J Gerontol A Biol Sci Med Sci. 2008;63:556–559. doi: 10.1093/gerona/63.6.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, Anderson RM. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nature communications. 2014;5:3557. doi: 10.1038/ncomms4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruzen C, Colman RJ. Effects of Caloric Restriction on Cardiovascular Aging in Non-human Primates and Humans. Clinics in geriatric medicine. 2009;25:733–743. doi: 10.1016/j.cger.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagon Y, Hur E, Zheng B, Wellenstein K, Cantley LC, Kahn BB. p70S6 kinase phosphorylates AMPK on serine 491 to mediate leptin’s effect on food intake. Cell Metab. 2012;16:104–112. doi: 10.1016/j.cmet.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deerberg F, Rapp KG, Kaspareit-Rittinghausen J, Lorcher K. The effect of food restriction by time-scheduled feeding on the development of body-weight, lifespan and incidence of spontaneous tumours and diseases in male Han:SPRD rats. Z Versuchstierkd. 1990;33:9–17. [PubMed] [Google Scholar]
- Demetriades C, Doumpas N, Teleman AA. Regulation of TORC1 in response to amino acid starvation via lysosomal recruitment of TSC2. Cell. 2014;156:786–799. doi: 10.1016/j.cell.2014.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetriades C, Plescher M, Teleman AA. Lysosomal recruitment of TSC2 is a universal response to cellular stress. Nature communications. 2016;7:10662. doi: 10.1038/ncomms10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efeyan A, Schweitzer LD, Bilate AM, Chang S, Kirak O, Lamming DW, Sabatini DM. RagA, but Not RagB, Is Essential for Embryonic Development and Adult Mice. Dev Cell. 2014 doi: 10.1016/j.devcel.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efeyan A, Zoncu R, Chang S, Gumper I, Snitkin H, Wolfson RL, Kirak O, Sabatini DD, Sabatini DM. Regulation of mTORC1 by the Rag GTPases is necessary for neonatal autophagy and survival. Nature. 2013;493:679–683. doi: 10.1038/nature11745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Cummings NE, Arriola Apelo SI, Neuman JC, Kasza I, Schmidt BA, Cava E, Spelta F, Tosti V, Syed FA, et al. Decreased consumption of branched-chain amino acids improves metabolic health. Cell Rep. 2016a;16:520–530. doi: 10.1016/j.celrep.2016.05.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Klein S, Holloszy JO. Effects of long-term calorie restriction and endurance exercise on glucose tolerance, insulin action, and adipokine production. Age. 2009;32:97–108. doi: 10.1007/s11357-009-9118-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci U S A. 2004;101:6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Partridge L. Promoting Health and Longevity through Diet: From Model Organisms to Humans. Cell. 2015;161:106–118. doi: 10.1016/j.cell.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Villareal DT, Das SK, Smith SR, Meydani SN, Pittas AG, Klein S, Bhapkar M, Rochon J, Ravussin E, et al. Effects of 2-year calorie restriction on circulating levels of IGF-1, IGF-binding proteins and cortisol in nonobese men and women: a randomized clinical trial. Aging Cell. 2016b;15:22–27. doi: 10.1111/acel.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallinetti J, Harputlugil E, Mitchell JR. Amino acid sensing in dietary-restriction-mediated longevity: roles of signal-transducing kinases GCN2 and TOR. Biochem J. 2013;449:1–10. doi: 10.1042/BJ20121098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandin V, Masvidal L, Cargnello M, Gyenis L, McLaughlan S, Cai Y, Tenkerian C, Morita M, Balanathan P, Jean-Jean O, et al. mTORC1 and CK2 coordinate ternary and eIF4F complex assembly. Nature communications. 2016;7:11127. doi: 10.1038/ncomms11127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh HS, McBurney M, Robbins PD. SIRT1 negatively regulates the mammalian target of rapamycin. PLoS One. 2010;5:e9199. doi: 10.1371/journal.pone.0009199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goberdhan DC, Wilson C, Harris AL. Amino Acid Sensing by mTORC1: Intracellular Transporters Mark the Spot. Cell Metab. 2016;23:580–589. doi: 10.1016/j.cmet.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff J, Kahn M, Samiei A, Gao J, Ota KT, Rei D, Tsai LH. A dietary regimen of caloric restriction or pharmacological activation of SIRT1 to delay the onset of neurodegeneration. J Neurosci. 2013;33:8951–8960. doi: 10.1523/JNEUROSCI.5657-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandison RC, Piper MDW, Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature. 2009;462:1061–1064. doi: 10.1038/nature08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Brunet A. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell. 2009;8:113–127. doi: 10.1111/j.1474-9726.2009.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halagappa VK, Guo Z, Pearson M, Matsuoka Y, Cutler RG, Laferla FM, Mattson MP. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease. Neurobiol Dis. 2007;26:212–220. doi: 10.1016/j.nbd.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Harper JM, Leathers CW, Austad SN. Does caloric restriction extend life in wild mice? Aging cell. 2006;5:441–449. doi: 10.1111/j.1474-9726.2006.00236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harputlugil E, Hine C, Vargas D, Robertson L, Manning Brendan D, Mitchell James R. The TSC Complex Is Required for the Benefits of Dietary Protein Restriction on Stress Resistance In Vivo. Cell Reports. 2014;8:1160–1170. doi: 10.1016/j.celrep.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y, Fukuda T, Oyabu C, Tanaka M, Asano M, Yamazaki M, Fukui M. Impact of low-carbohydrate diet on body composition: meta-analysis of randomized controlled studies. Obesity Reviews. 2016;17:499–509. doi: 10.1111/obr.12405. [DOI] [PubMed] [Google Scholar]
- Heilbronn LK, Ravussin E. Calorie restriction and aging: review of the literature and implications for studies in humans. The American Journal of Clinical Nutrition. 2003;78:361–369. doi: 10.1093/ajcn/78.3.361. [DOI] [PubMed] [Google Scholar]
- Hsu PP, Kang SA, Rameseder J, Zhang Y, Ottina KA, Lim D, Peterson TR, Choi Y, Gray NS, Yaffe MB, et al. The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science. 2011;332:1317–1322. doi: 10.1126/science.1199498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- Iwasaki K, Gleiser CA, Masoro EJ, McMahan CA, Seo EJ, Yu BP. The influence of dietary protein source on longevity and age-related disease processes of Fischer rats. J Gerontol. 1988a;43:B5–12. doi: 10.1093/geronj/43.1.b5. [DOI] [PubMed] [Google Scholar]
- Iwasaki K, Gleiser CA, Masoro EJ, McMahan CA, Seo EJ, Yu BP. Influence of the restriction of individual dietary components on longevity and age-related disease of Fischer rats: the fat component and the mineral component. J Gerontol. 1988b;43:B13–21. doi: 10.1093/geronj/43.1.b13. [DOI] [PubMed] [Google Scholar]
- Jang C, Oh SF, Wada S, Rowe GC, Liu L, Chan MC, Rhee J, Hoshino A, Kim B, Ibrahim A, et al. A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat Med. 2016;22:421–426. doi: 10.1038/nm.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Journal of the American College of Cardiology. 2014;63:2985–3023. doi: 10.1016/j.jacc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Martin GM, Rabinovitch PS, Kaeberlein M. Preserving Youth: Does Rapamycin Deliver? Science Translational Medicine. 2013;5:211fs240–211fs240. doi: 10.1126/scitranslmed.3007316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J, Genau HM, Behrends C. Amino Acid-Dependent mTORC1 Regulation by the Lysosomal Membrane Protein SLC38A9. Mol Cell Biol. 2015;35:2479–2494. doi: 10.1128/MCB.00125-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemnitz JW. Calorie Restriction and Aging in Nonhuman Primates. Ilar Journal. 2011;52:66–77. doi: 10.1093/ilar.52.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy BK, Lamming DW. The Mechanistic Target of Rapamycin: The Grand ConducTOR of Metabolism and Aging. Cell Metab. 2016;23:990–1003. doi: 10.1016/j.cmet.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khamzina L, Veilleux A, Bergeron S, Marette A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology. 2005;146:1473–1481. doi: 10.1210/en.2004-0921. [DOI] [PubMed] [Google Scholar]
- Kim JS, Ro SH, Kim M, Park HW, Semple IA, Park H, Cho US, Wang W, Guan KL, Karin M, et al. Sestrin2 inhibits mTORC1 through modulation of GATOR complexes. Scientific reports. 2015;5:9502. doi: 10.1038/srep09502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laeger T, Henagan TM, Albarado DC, Redman LM, Bray GA, Noland RC, Munzberg H, Hutson SM, Gettys TW, Schwartz MW, et al. FGF21 is an endocrine signal of protein restriction. J Clin Invest. 2014;124:3913–3922. doi: 10.1172/JCI74915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagiou P, Sandin S, Weiderpass E, Lagiou A, Mucci L, Trichopoulos D, Adami HO. Low carbohydrate-high protein diet and mortality in a cohort of Swedish women. J Intern Med. 2007;261:366–374. doi: 10.1111/j.1365-2796.2007.01774.x. [DOI] [PubMed] [Google Scholar]
- Lamming DW. Diminished mTOR signaling: a common mode of action for endocrine longevity factors. SpringerPlus. 2014;3:735. doi: 10.1186/2193-1801-3-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamming DW, Anderson RM. eLS. Chichester: John Wiley & Sons, Ltd; 2014. Metabolic Effects of Caloric Restriction. [Google Scholar]
- Lamming DW, Cummings NE, Rastelli AL, Gao F, Cava E, Bertozzi B, Spelta F, Pili R, Fontana L. Restriction of dietary protein decreases mTORC1 in tumors and somatic tissues of a tumor-bearing mouse xenograft model. Oncotarget. 2015;6:31233–31240. doi: 10.18632/oncotarget.5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, Davis JG, Salmon AB, Richardson A, Ahima RS, et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335:1638–1643. doi: 10.1126/science.1215135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi F, Calvani R, Cesari M, Tosato M, Martone AM, Bernabei R, Onder G, Marzetti E. Sarcopenia as the Biological Substrate of Physical Frailty. Clin Geriatr Med. 2015;31:367–374. doi: 10.1016/j.cger.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Lee BC, Kaya A, Ma S, Kim G, Gerashchenko MV, Yim SH, Hu Z, Harshman LG, Gladyshev VN. Methionine restriction extends lifespan of Drosophila melanogaster under conditions of low amino-acid status. Nature communications. 2014;5:3592. doi: 10.1038/ncomms4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CK, Klopp RG, Weindruch R, Prolla TA. Gene Expression Profile of Aging and Its Retardation by Caloric Restriction. Science. 1999;285:1390–1393. doi: 10.1126/science.285.5432.1390. [DOI] [PubMed] [Google Scholar]
- Lee JH, Budanov AV, Park EJ, Birse R, Kim TE, Perkins GA, Ocorr K, Ellisman MH, Bodmer R, Bier E, et al. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science. 2010;327:1223–1228. doi: 10.1126/science.1182228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Budanov AV, Talukdar S, Park EJ, Park HL, Park HW, Bandyopadhyay G, Li N, Aghajan M, Jang I, et al. Maintenance of metabolic homeostasis by Sestrin2 and Sestrin3. Cell Metab. 2012;16:311–321. doi: 10.1016/j.cmet.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Cho US, Karin M. Sestrin regulation of TORC1: Is Sestrin a leucine sensor? Sci Signal. 2016;9:re5. doi: 10.1126/scisignal.aaf2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KP, Simpson SJ, Clissold FJ, Brooks R, Ballard JW, Taylor PW, Soran N, Raubenheimer D. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc Natl Acad Sci U S A. 2008;105:2498–2503. doi: 10.1073/pnas.0710787105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees EK, Krol E, Grant L, Shearer K, Wyse C, Moncur E, Bykowska AS, Mody N, Gettys TW, Delibegovic M. Methionine restriction restores a younger metabolic phenotype in adult mice with alterations in fibroblast growth factor 21. Aging Cell. 2014;13:817–827. doi: 10.1111/acel.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ME, Suarez JA, Brandhorst S, Balasubramanian P, Cheng CW, Madia F, Fontana L, Mirisola MG, Guevara-Aguirre J, Wan J, et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014;19:407–417. doi: 10.1016/j.cmet.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Yin Y, Tan B, Kong X, Wu G. Leucine nutrition in animals and humans: mTOR signaling and beyond. Amino Acids. 2011;41:1185–1193. doi: 10.1007/s00726-011-0983-2. [DOI] [PubMed] [Google Scholar]
- Liao CY, Rikke BA, Johnson TE, Diaz V, Nelson JF. Genetic Variation in the Murine Lifespan Response to Dietary Restriction: from Life Extension to Life Shortening. Aging cell. 2010;9:92–95. doi: 10.1111/j.1474-9726.2009.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- Longo VD, Mattson MP. Fasting: molecular mechanisms and clinical applications. Cell Metab. 2014;19:181–192. doi: 10.1016/j.cmet.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Otín C, Galluzzi L, Freije José MP, Madeo F, Kroemer G. Metabolic Control of Longevity. Cell. 2016;166:802–821. doi: 10.1016/j.cell.2016.07.031. [DOI] [PubMed] [Google Scholar]
- Lynch CJ. Role of leucine in the regulation of mTOR by amino acids: revelations from structure-activity studies. The Journal of nutrition. 2001;131:861S–865S. doi: 10.1093/jn/131.3.861S. [DOI] [PubMed] [Google Scholar]
- Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nature reviews Endocrinology. 2014;10:723–736. doi: 10.1038/nrendo.2014.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair W, Piper MDW, Partridge L. Calories Do Not Explain Extension of Life Span by Dietary Restriction in Drosophila. PLoS Biol. 2005;3:e223. doi: 10.1371/journal.pbio.0030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masternak MM, Panici JA, Bonkowski MS, Hughes LF, Bartke A. Insulin sensitivity as a key mediator of growth hormone actions on longevity. J Gerontol A Biol Sci Med Sci. 2009;64:516–521. doi: 10.1093/gerona/glp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012a;489:318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012b;489:318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Allison DB, Fontana L, Harvie M, Longo VD, Malaisse WJ, Mosley M, Notterpek L, Ravussin E, Scheer FAJL, et al. Meal frequency and timing in health and disease. Proceedings of the National Academy of Sciences. 2014;111:16647–16653. doi: 10.1073/pnas.1413965111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty MF, Barroso-Aranda J, Contreras F. The low-methionine content of vegan diets may make methionine restriction feasible as a life extension strategy. Medical hypotheses. 2009;72:125–128. doi: 10.1016/j.mehy.2008.07.044. [DOI] [PubMed] [Google Scholar]
- McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. Journal of Nutrition. 1935;10:63–79. [PubMed] [Google Scholar]
- McKiernan SH, Colman RJ, Aiken E, Evans TD, Beasley TM, Aiken JM, Weindruch R, Anderson RM. Cellular adaptation contributes to calorie restriction-induced preservation of skeletal muscle in aged rhesus monkeys. Exp Gerontol. 2012;47:229–236. doi: 10.1016/j.exger.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon S, Dibble CC, Talbott G, Hoxhaj G, Valvezan AJ, Takahashi H, Cantley LC, Manning BD. Spatial control of the TSC complex integrates insulin and nutrient regulation of mTORC1 at the lysosome. Cell. 2014;156:771–785. doi: 10.1016/j.cell.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercken EM, Carboneau BA, Krzysik-Walker SM, de Cabo R. Of mice and men: the benefits of caloric restriction, exercise, and mimetics. Ageing Res Rev. 2012;11:390–398. doi: 10.1016/j.arr.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercken EM, Crosby SD, Lamming DW, JeBailey L, Krzysik-Walker S, Villareal DT, Capri M, Franceschi C, Zhang Y, Becker K, et al. Calorie restriction in humans inhibits the PI3K/AKT pathway and induces a younger transcription profile. Aging Cell. 2013;12:645–651. doi: 10.1111/acel.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TE, Kovács SJ, Ehsani AA, Klein S, Holloszy JO, Fontana L. Long-Term Caloric Restriction Ameliorates the Decline in Diastolic Function in Humans. Journal of the American College of Cardiology. 2006;47:398–402. doi: 10.1016/j.jacc.2005.08.069. [DOI] [PubMed] [Google Scholar]
- Miller RA, Buehner G, Chang Y, Harper JM, Sigler R, Smith-Wheelock M. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell. 2005;4:119–125. doi: 10.1111/j.1474-9726.2005.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, Madrigal-Matute J, Scheibye-Knudsen M, Fang E, Aon M, Gonzalez-Reyes JA, Cortassa S, Kaushik S, Gonzalez-Freire M, Patel B, et al. Effects of Sex, Strain, and Energy Intake on Hallmarks of Aging in Mice. Cell Metab. 2016;23:1093–1112. doi: 10.1016/j.cmet.2016.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberg M, Apro W, Ohlsson I, Ponten M, Villanueva A, Ekblom B, Blomstrand E. Absence of leucine in an essential amino acid supplement reduces activation of mTORC1 signalling following resistance exercise in young females. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2014;39:183–194. doi: 10.1139/apnm-2013-0244. [DOI] [PubMed] [Google Scholar]
- Neff F, Flores-Dominguez D, Ryan DP, Horsch M, Schroder S, Adler T, Afonso LC, Aguilar-Pimentel JA, Becker L, Garrett L, et al. Rapamycin extends murine lifespan but has limited effects on aging. J Clin Invest. 2013;123:3272–3291. doi: 10.1172/JCI67674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojima A, Yamashita M, Yoshida Y, Shimizu I, Ichimiya H, Kamimura N, Kobayashi Y, Ohta S, Ishii N, Minamino T. Haploinsufficiency of akt1 prolongs the lifespan of mice. PLoS One. 2013;8:e69178. doi: 10.1371/journal.pone.0069178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowson C, O’Connell S. Protein Requirements and Recommendations for Older People: A Review. Nutrients. 2015;7:6874–6899. doi: 10.3390/nu7085311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orentreich N, Matias JR, DeFelice A, Zimmerman JA. Low methionine ingestion by rats extends life span. The Journal of nutrition. 1993;123:269–274. doi: 10.1093/jn/123.2.269. [DOI] [PubMed] [Google Scholar]
- Paddon-Jones D, Rasmussen BB. Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care. 2009;12:86–90. doi: 10.1097/MCO.0b013e32831cef8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, Kim K, Page GP, Allison DB, Weindruch R, Prolla TA. Gene expression profiling of aging in multiple mouse strains: identification of aging biomarkers and impact of dietary antioxidants. Aging Cell. 2009;8:484–495. doi: 10.1111/j.1474-9726.2009.00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone CE, Malloy VL, Orentreich DS, Orentreich N. Metabolic adaptations to methionine restriction that benefit health and lifespan in rodents. Exp Gerontol. 2013;48:654–660. doi: 10.1016/j.exger.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Piper MD, Mair W, Partridge L. Counting the calories: the role of specific nutrients in extension of life span by food restriction. J Gerontol A Biol Sci Med Sci. 2005;60:549–555. doi: 10.1093/gerona/60.5.549. [DOI] [PubMed] [Google Scholar]
- Rebsamen M, Pochini L, Stasyk T, de Araujo ME, Galluccio M, Kandasamy RK, Snijder B, Fauster A, Rudashevskaya EL, Bruckner M, et al. SLC38A9 is a component of the lysosomal amino acid sensing machinery that controls mTORC1. Nature. 2015;519:477–481. doi: 10.1038/nature14107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson LT, Trevino-Villarreal JH, Mejia P, Grondin Y, Harputlugil E, Hine C, Vargas D, Zheng H, Ozaki CK, Kristal BS, et al. Protein and Calorie Restriction Contribute Additively to Protection from Renal Ischemia Reperfusion Injury Partly via Leptin Reduction in Male Mice. The Journal of nutrition. 2015;145:1717–1727. doi: 10.3945/jn.114.199380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci U S A. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sans MD, Tashiro M, Vogel NL, Kimball SR, D’Alecy LG, Williams JA. Leucine activates pancreatic translational machinery in rats and mice through mTOR independently of CCK and insulin. The Journal of nutrition. 2006;136:1792–1799. doi: 10.1093/jn/136.7.1792. [DOI] [PubMed] [Google Scholar]
- Saxton RA, Knockenhauer KE, Wolfson RL, Chantranupong L, Pacold ME, Wang T, Schwartz TU, Sabatini DM. Structural basis for leucine sensing by the Sestrin2-mTORC1 pathway. Science. 2016;351:53–58. doi: 10.1126/science.aad2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140–144. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She P, Reid TM, Bronson SK, Vary TC, Hajnal A, Lynch CJ, Hutson SM. Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metab. 2007;6:181–194. doi: 10.1016/j.cmet.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluijs I, Beulens JW, van der AD, Spijkerman AM, Grobbee DE, van der Schouw YT. Dietary intake of total, animal, and vegetable protein and risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-NL study. Diabetes Care. 2010;33:43–48. doi: 10.2337/dc09-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. Oxidative Stress, Caloric Restriction, and Aging. Science (New York, NY) 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solon-Biet Samantha M, McMahon Aisling C, Ballard J, William O, Ruohonen K, Wu Lindsay E, Cogger Victoria C, Warren A, Huang X, Pichaud N, Melvin Richard G, et al. The Ratio of Macronutrients, Not Caloric Intake, Dictates Cardiometabolic Health, Aging, and Longevity in Ad Libitum-Fed Mice. Cell Metabolism. 2014;19:418–430. doi: 10.1016/j.cmet.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A, Wartschow LM, White MF. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science. 2007;317:369–372. doi: 10.1126/science.1142179. [DOI] [PubMed] [Google Scholar]
- Tremblay F, Brule S, Hee Um S, Li Y, Masuda K, Roden M, Sun XJ, Krebs M, Polakiewicz RD, Thomas G, et al. Identification of IRS-1 Ser-1101 as a target of S6K1 in nutrient- and obesity-induced insulin resistance. Proc Natl Acad Sci U S A. 2007;104:14056–14061. doi: 10.1073/pnas.0706517104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Norren K, Rusli F, van Dijk M, Lute C, Nagel J, Dijk FJ, Dwarkasing J, Boekschoten MV, Luiking Y, Witkamp RF, et al. Behavioural changes are a major contributing factor in the reduction of sarcopenia in caloric-restricted ageing mice. J Cachexia Sarcopenia Muscle. 2015;6:253–268. doi: 10.1002/jcsm.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wyk HJ, Davis RE, Davies JS. A critical review of low-carbohydrate diets in people with Type 2 diabetes. Diabetic Medicine. 2016;33:148–157. doi: 10.1111/dme.12964. [DOI] [PubMed] [Google Scholar]
- Walford RL, Mock D, Verdery R, MacCallum T. Calorie restriction in biosphere 2: alterations in physiologic, hematologic, hormonal, and biochemical parameters in humans restricted for a 2-year period. J Gerontol A Biol Sci Med Sci. 2002;57:B211–224. doi: 10.1093/gerona/57.6.b211. [DOI] [PubMed] [Google Scholar]
- Wang RH, Kim HS, Xiao C, Xu X, Gavrilova O, Deng CX. Hepatic Sirt1 deficiency in mice impairs mTorc2/Akt signaling and results in hyperglycemia, oxidative damage, and insulin resistance. J Clin Invest. 2011;121:4477–4490. doi: 10.1172/JCI46243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Tsun ZY, Wolfson RL, Shen K, Wyant GA, Plovanich ME, Yuan ED, Jones TD, Chantranupong L, Comb W, et al. Metabolism. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science. 2015;347:188–194. doi: 10.1126/science.1257132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R, Kayo T, Lee CK, Prolla TA. Microarray Profiling of Gene Expression in Aging and Its Alteration by Caloric Restriction in Mice. The Journal of nutrition. 2001;131:918S–923S. doi: 10.1093/jn/131.3.918S. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Walford RL. The retardation of aging and disease by dietary restriction. Springfield, Ill., U.S.A: C.C. Thomas; 1988. [Google Scholar]
- Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. The Journal of nutrition. 1986;116:641–654. doi: 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- White PJ, Lapworth AL, An J, Wang L, McGarrah RW, Stevens RD, Ilkayeva O, George T, Muehlbauer MJ, Bain JR, et al. Branched-chain amino acid restriction in Zucker-fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl-glycine export. Molecular metabolism. doi: 10.1016/j.molmet.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson JE, Burmeister L, Brooks SV, Chan CC, Friedline S, Harrison DE, Hejtmancik JF, Nadon N, Strong R, Wood LK, et al. Rapamycin slows aging in mice. Aging Cell. 2012;11:675–682. doi: 10.1111/j.1474-9726.2012.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe RR, Miller SL, Miller KB. Optimal protein intake in the elderly. Clinical Nutrition. 2008;27:675–684. doi: 10.1016/j.clnu.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Wolfson RL, Chantranupong L, Saxton RA, Shen K, Scaria SM, Cantor JR, Sabatini DM. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science. 2016;351:43–48. doi: 10.1126/science.aab2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JJ, Liu J, Chen EB, Wang JJ, Cao L, Narayan N, Fergusson MM, Rovira II, Allen M, Springer DA, et al. Increased mammalian lifespan and a segmental and tissue-specific slowing of aging after genetic reduction of mTOR expression. Cell reports. 2013;4:913–920. doi: 10.1016/j.celrep.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F, Huang Z, Li H, Yu J, Wang C, Chen S, Meng Q, Cheng Y, Gao X, Li J, et al. Leucine deprivation increases hepatic insulin sensitivity via GCN2/mTOR/S6K1 and AMPK pathways. Diabetes. 2011;60:746–756. doi: 10.2337/db10-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F, Yu J, Guo Y, Deng J, Li K, Du Y, Chen S, Zhu J, Sheng H, Guo F. Effects of individual branched-chain amino acids deprivation on insulin sensitivity and glucose metabolism in mice. Metabolism. 2014;63:841–850. doi: 10.1016/j.metabol.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Ye J, Palm W, Peng M, King B, Lindsten T, Li MO, Koumenis C, Thompson CB. GCN2 sustains mTORC1 suppression upon amino acid deprivation by inducing Sestrin2. Genes Dev. 2015;29:2331–2336. doi: 10.1101/gad.269324.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Bokov A, Gelfond J, Soto V, Ikeno Y, Hubbard G, Diaz V, Sloane L, Maslin K, Treaster S, et al. Rapamycin extends life and health in C57BL/6 mice. J Gerontol Ser A Biol Sci Med Sci. 2014;69A:119–130. doi: 10.1093/gerona/glt056. [DOI] [PMC free article] [PubMed] [Google Scholar]