Abstract
Chemotherapy-induced neurotoxicity of peripheral nervous system (PNS) hinders efficacy of cancer treatments. Mechanisms initiating PNS injury by anticancer drugs are incompletely understood delaying development of effective management strategies. To understand events triggered in PNS by cancer drugs, we exposed dorsal root ganglion (DRG) neurons to cisplatin, a drug from platinum based class of chemotherapeutics frequently implicated in peripheral neuropathies. While cisplatin enters cancer cells and forms cisplatin:DNA crosslinks that block cell proliferation, circulating cisplatin can also reach the PNS and produce crosslinks that impede critical DNA transactions in postmitotic neurons. Cisplatin forms crosslinks with both, nuclear and mitochondrial DNA (mtDNA). Crosslinks are repairable primarily via the nucleotide excision repair (NER) pathway, which is present in nuclei but absent from mitochondrial compartment. Hence, high mitochondrial content as well as limited shielding by blood nerve barrier make DRG neurons particularly vulnerable to mitochondrial injury by cisplatin. We report that in DRG neurons cisplatin elevates reactive oxygen species, depletes mtDNA and impairs mitochondrial respiration, whereas concomitant meclizine supplementation preserves redox balance, attenuates mitochondrial compromise and augments DNA repair. Meclizine is an antihistamine drug recently implicated in neuroprotection via modulation of energy metabolism. Our data demonstrate that in the mitochondria-rich DRG neurons, meclizine mitigates cisplatin induced mitochondrial compromise via enhancement of pentose phosphate pathway and repletion of nicotinamide adenine dinucleotide phosphate (NADPH) and glutathione stores. The findings suggest that meclizine mediated preservation of redox balance sustains mitochondrial respiration and supports execution of cellular processes, including timely removal of cisplatin crosslinks from nuclear DNA, thereby attenuating cisplatin toxicity in DRG neurons. Collectively, the findings reveal potential for pharmacologic modulation of dorsal root ganglion neurons metabolism for protection against toxicity of chemotherapeutic drugs.
Keywords: cisplatin, DNA damage, DNA repair, dorsal root ganglion neurons, meclizine, mitochondrial respiration, mtDNA, pentose phosphate pathway, ROS
INTRODUCTION
Chemotherapy-induced neurotoxicity of the peripheral nervous system (PNS) hinders therapeutic potential of many anti cancer compounds, including the platinum based class of antimitotic drugs. The mechanisms by which platinum drugs cause peripheral toxicity [1,2] remain incompletely understood, but are thought to involve nuclear, cytoplasmic and mitochondrial components [1–8]. Cisplatin enters cells by diffusion. Its chlorides are replaced with water forming the active, positively charged species, which rapidly react with DNA to form cisplatin:DNA adducts (intra- and interstrand crosslinks) blocking DNA replication in proliferating cancer cells [9]. Cisplatin:DNA adducts in nuclear DNA are repairable via the ubiquitous nucleotide excision repair (NER) pathway, which accounts in part, for resumption of cancer cell proliferation and development of cisplatin resistance [10,11]. Capacity for repair of cisplatin:DNA adducts has been reported in neural tissues of mice with proficient NER pathway [2]. In contrast, however, cisplatin adducts are likely to persist in mtDNA due to the absence of NER pathway in mitochondrial compartment [12–14]. Because dorsal root ganglion (DRG) neurons primarily rely on mitochondria for energy production and impaired mitochondria are a source of deleterious reactive oxygen species (ROS), it is plausible that in peripheral neurons, mitochondrial dysfunction significantly contributes to the development of cisplatin-induced neurotoxicity [5,7]. Notwithstanding, recent reports revealed that sensory neurons have inherent capacity to utilize glycolytic energy for axonal transport [15] raising the question of whether metabolic reprogramming to augment glycolytic metabolism and improve redox balance might be viable strategy for protection against mitochondrial compromise-associated peripheral neurotoxicity. Specifically, whether reprogramming of energy metabolism to enhance the oxidative phase of pentose phosphate pathway to maintain favorable NADP+/NADPH ratios and preserve antioxidant defenses and thereby mitochondrial status [16,17], might serve as strategy for protection of DRG neurons from injury incidental to mitochondria-disrupting effects of chemotherapeutic drugs [1,18–20]. To examine this premise we adapted an in vitro system of cultured DRG neurons [21] to study the effects of sub lethal doses of cisplatin that support investigation of cisplatin-induced molecular, metabolic and morphologic changes without triggering significant death and loss of neurons. To determine to what extent the adverse effects of cisplatin could be relieved, we used meclizine, an ‘old’ antihistamine drug recently implicated in reprogramming of energy metabolism and neuroprotection in models of ischemia/reperfusion, Huntington’s and Parkinson’s disease and hypoxic stress [22–25]. The mechanism by which meclizine exerts neuroprotection are presently not well understood. The studies that first linked meclizine to energy metabolism suggested that it might protect cells by lessening mitochondrial respiration [23], possibly via inhibition of the ethanolamine branch of Kennedy pathway [26]. Subsequent studies in other models demonstrated that meclizine can rapidly stimulate glycolytic metabolism in cultured neuronal cells [24,25], suggesting that meclizine targets may differ among cell types. Here, we report that cisplatin exposures compromise mitochondrial function of DRG neurons and that meclizine mitigates cisplatin-induced mitotoxicity and improves neuronal homeostasis and function.
METHODS
Culture and treatments of adult mouse DRG neurons
The Institutional Animal Care and Use Committee of the University of Texas Medical Branch in Galveston approved the mouse-handling procedures. Dorsal root ganglion neurons were isolated from male C57BL/6 mice 3–4 months old (Harlan Laboratories, USA) as previously described [21,27–30]. Ganglia obtained from all spinal levels were placed in cold dissecting solution (130 mM NaCl, 5 mM KCl, 2 mM KH2PO4, 1.5 mM CaCl2, 6 mM MgCl2, 10 mM glucose and 10 mM Hepes, pH 7.2), incubated with collagenase type A (Roche) and trypsin (1 h/37°C) followed by DNase I (Roche), dissociated by 20 triturations and spun (168 g/3 min). Pellets were passed through 70 μm strainer, spun and re-suspended in DMEM/F12 (Sigma) with 10% FBS, 10 ng/ml nerve growth factor (Sigma) and penicillin/streptomycin. Cells were seeded on coated (10 μg/ml laminin and 100 μg/ml poly-L-ornithine, Sigma) glass coverslips or in 96- or 6-well plates at (3–4)×103 and (3–4)×104/cm2, respectively. Treatments were initiated 24–30 hours later after neurite network has been established. Meclizine dihydrochloride (#4245 R&D, Minneapolis, MN) stock solution was made 10 mM in dimethyl sulfoxide; 15-μM dose was found adequate for protection against cisplatin; DMSO was added to all cultures at matching concentration. 6-aminonicotinamide (#AC104150010, Acros Organics) was used at 100 μM. Low, 10 μM/24-hour dose of cisplatin (Cis-Diammineplatinum (II) dichloride Sigma P4394) was selected to enable investigation of cisplatin effects without significant loss of DRG neurons. This dose was selected based on assessments of DRG neurons survival following exposure to a range of cisplatin concentrations as determined by trypan blue exclusion and the CellTiter 96® (#G3580, Promega) cell viability kit (Supplementary Figure 1). The selected dose agrees with earlier reports on viability of DRG neurons exposed to varying concentrations of cisplatin [4].
Immunofluorescent staining and neurite lengths measurements
DRG neurons were seeded on coverslips and processed as we described [31,32]. Briefly, cultures were fixed in 4% paraformaldehyde, permeabilized with 0.1% Triton X-100/0.1% sodium citrate in PBS and blocked with 3% BSA (w/v)/1% Donkey serum (v/v) in PBS. Primary antibodies were: rabbit anti-Neurofilament 200 (1:20,000, Sigma-N4142), mouse anti γH2AX (1:4,000, Millipore #05-636), mouse anti DNA (single and double stranded) used at 1:500 (Chemicon #CBL186; clone AC-30-10) [33] and rabbit anti-TFAM (1:2,000, #GTX103231 Genetex). Following 2 hours with primary antibodies and 3x washes in 1% BSA, slides were incubated 45 min with goat-anti mouse 488, goat anti-rabbit 594 or goat anti mouse IgM (#A21042) AlexaFluor secondary antibodies mounted with Prolong® Gold Anti-fade with DAPI and viewed with 40x objective on Olympus IX71 with QIC-F-M-12-C cooled camera (QImaging, Surrey, BC) and QCapture Pro (QImaging) software (for DRG neuron culture purity and status see Supplementary Figure 2). DRG neurite lengths were measured by tracing immunofluorescence of the DRG-specific cytoskeleton protein NF200. Tracing and measurements were done using 10x objective and NIH ImageJ software. For each experimental condition, neurites extending from somas of at least 10 DRG neurons were analyzed for each experimental condition; for each DRG cell body 4–5 neurites were traced. Three sets of independent biological experiments were done and mean±SEM values were calculated for 120–150 neurites for each condition [20,34,35]. For imaging mtDNA distribution in neurites, slides were viewed with 60x oil objective on Nikon Eclipse E600 fluorescent microscope.
Alkaline comet assay
A modification of the Single Cell Gel Electrophoresis (alkaline comet) assay [36,37] was used to assess cisplatin crosslinks removal [38–40] from nuclear DNA of DRG neurons. DRG neurons were seeded in 24-well plates and subjected to different treatments as indicated. Cisplatin was applied at 25 μM for 1 hour (note: apart for this experiment, all cisplatin exposures in this study were carried out with 10 μM cisplatin for 24 h). After 1-hour medium was replaced and DRG were let recover overnight in fresh medium with/without 15 μM meclizine. Upon termination of treatments, cultures were exposed to H2O2 (30 μM/30 min) to generate single strand breaks, which underlie the tail formation in alkaline comet assay. Cultures were then processed as we described previously [41]. Briefly, DRGs were collected, mixed with 50 μl 1% low melting agarose, spread onto agarose pre-coated glass slides and placed at 4°C for agarose to solidify. After coverslips removal, slides were incubated (1 h/4°C) in alkaline lysis buffer (2.5 M NaCl, 0.1 M EDTA, 10 mM Tris, pH 10, 1% Triton X-100) followed by 40-min/25°C in alkaline electrophoresis buffer (0.3 M NaOH, 1 mM EDTA) and 20 min electrophoresis at 22 V. Slides were neutralized (0.4 M Tris pH 7.5), washed, stained with ethidium bromide, air dried, viewed with IX71 Olympus microscope and images were taken with QIC-F-M-12-C cooled camera. Olive tail moments (OTM) were analyzed using the Casp software [42]. At least 20 nuclei were analyzed for each treatment condition for three independent biological sets of experiments; OTM data are mean±SEM and significance (P ≤ 0.05) was determined using the Student’s t-test.
Measurement of oxygen consumption rates (OCR)
XF24 extracellular flux analyzer (Seahorse Bioscience) was used to measure oxygen consumption rates (OCR) using established protocols [43–45] and as we previously described [25,31,32]. DRG neurons seeded in XF24 plates ([1–1.3] ×104/well) were cultured as described above. Prior to XF24 measurements medium was replaced with un-buffered Dulbecco’s Modified Eagle’s medium (Sigma, D5030) supplemented with 5 mM pyruvate/15 mM glucose/2 mM GlutaMAX (#35050, Invitrogen) adjusted to pH 7.4 and equilibrated in CO2 free incubator at 37°C. Assay protocols were executed by XF24 software. Sequential additions of mitochondrial effectors were through ports of XF24 cartridges; concentrations of effectors were previously optimized for DRG neurons [25] to 2 μM oligomycin (O4876, Sigma), 2 μM carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP), (C2920, Sigma) and 1.8 μM antimycin A (A8674, Sigma). Changes in OCR in response to additions of mitochondrial effectors served to compare respiratory parameters between control and test groups [43,44]. Baseline OCR was calculated by subtraction of non-mitochondrial OCR, i.e., the portion retained after addition of antimycin A. Each parameter calculated for control cultures was assigned the value of 100%; effects of treatments were calculated as percent change relative to each respective control.
Measurement of NADP+/NADPH ratios
Ratios of intracellular NADP+/NADPH were determined using NADP+/NADPH-Glo™ kit (#G9081, Promega) according to manufacturer’s guide. Briefly, DRGs were seeded at 104/well in 96-well plates. After treatment, DRGs were lysed in either NADP+ or NADPH lysis buffer (60°C/15 min). Lysates were neutralized, spun at 13,000 g/2 min to remove debris; supernatants were collected, loaded into white wall 96-well plate, mixed with detection reagent and incubated at 22°C/30 min in the dark. Four sets of independent experiments were carried out and mean±SEM values were calculated. Luminescence was read on TECAN FL200 plate reader. NADP+ and NADPH concentrations were derived from standard curves (Magellan™ software, TECAN, San Jose, CA).
Measurement of intracellular thiols levels
Assessment of reduced intracellular glutathione (GSH) was with Thioltracker Violet (Life Technologies) cell-permeant thiol-reactive fluorescent probe as described [46,47]. DRG neurons were seeded in black wall 96-well plates (104/well). After treatments, cultures were washed with PBS. Freshly prepared 10 μM Thioltracker Violet in DPBS was added for 30 min/37°C incubation in the dark. Fluorescence was measured using the FL200 Tecan (excitation at 415 nm/emission 530 nm). Four sets of independent experiments were carried out and mean±SEM values were calculated.
In situ imaging of superoxide mediated dihydroethidium oxidation
Superoxide generation as surrogate for ROS was assessed in live DRG neurons by imaging superoxide-mediated oxidation of dihydroethidium (#D23107, Invitrogen) to 2-hydroxyethidium [48,49] and as we described [50]. Upon termination of treatments, dihydroethidium was added to cultured DRG neurons at final concentration of 100 nM for 20 min in the dark. Incubation was terminated by quick washes with PBS. Cultures were observed with Olympus IX71 fluorescence microscope and images were captured sequentially with QIC-F-M-12-C cooled camera fitted with the QCapture Pro software. Fluorescence intensity of individual DRG neurons demarcated by circular boundaries was scored with ImageJ software (NIH) and exported to Excel for determination of average intensity and further analysis. At least 20 DRG neurons obtained in 3 independent experimental sets were scored for each condition.
mtDNA copy number determination by Real-Time qPCR
Total DNA was isolated from DRG neurons (3×104) using easy DNA isolation kit (Life Technologies) and RNA was digested with RNase A (40 μg/ml/30 min/37°C). Real-time qPCR reactions were assembled in duplicates with SSO FAST Evagreen supermix (Biorad, Hercules, CA). The single-copy nuclear gene beta-2-microglobulin (B2M) was used as reference for determination of mtDNA copy number. A 180-nucleotide fragment of mitochondrial gene encoding cytochrome oxidase subunit III (Cox III) served amplification target after validation of qPCR data with two additional mitochondria encoded genes, Cox1 and ND1 that yielded similar results. mtDNA copy number was calculated using the formula: mtDNA copy number =2×2(CTCoxIII − CTB2M) [51,52]. Primers for CoxIII (MGI:102502) were F-caattacatgagctcatcatagc and R-ccatggaatccagtagcca and for B2M (NM_009735) F-atccaaatgctgaagaacgg and R-atcagtctcagtgggggtga. Three sets of independent experiments were carried out and mean±SEM values for mtDNA copy number were calculated.
Statistical analysis
Data are given as mean±SEM obtained from 3–5 independent biological experiments, as indicated. One-way ANOVA was employed to compare the means among groups followed by post-test Tukey’s analysis to determine differences in means of multiple groups or as indicated. P<0.05 was considered statistically significant. MegaStat® package for Excel was used.
RESULTS
Meclizine lessens cisplatin-induced shortening of DRGs neurites
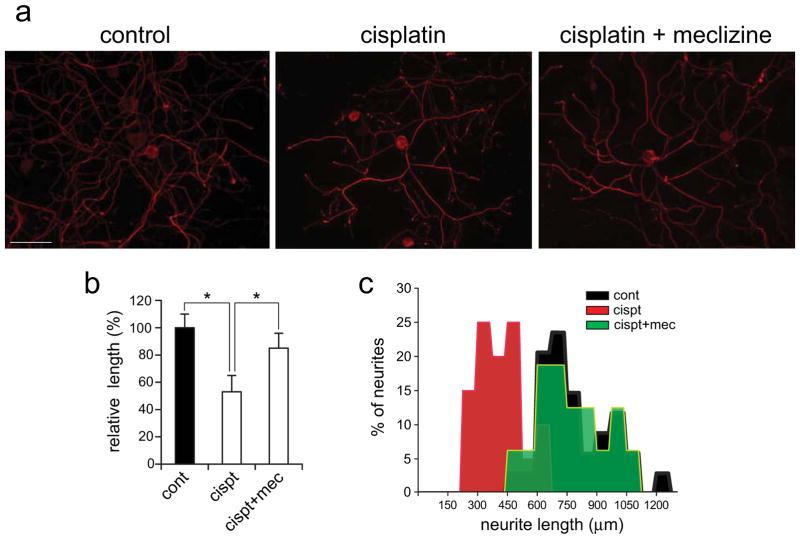
To assess the impact of cisplatin exposures on DRG neurons, neurite lengths were compared between controls and cisplatin exposed cultures. The sub-lethal dose of 10 μM/24 hours was selected to enable investigation of molecular, metabolic and morphologic changes, induced by cisplatin, while averting significant loss of DRG neurons. Neurites lengths were measured in controls and cultures exposed to cisplatin with/out meclizine supplementation. Meclizine supplementation significantly lessened cisplatin-induced neurite shortening [Fig 1]. Neurites were observed by immunofluorescence (IF) of the DRG-specific cytoskeleton protein, neurofilament 200 (NF200) [Fig 1a]. Neurite outgrowth from cell bodies was traced and measured using the ImageJ software. Bar graphs and frequency histograms of mean neurites lengths show marked shortening by cisplatin and a shift towards normal neurites lengths when cultures are supplemented with meclizine in the course of cisplatin exposure [Fig 1b and c].
Fig. 1. Meclizine lessens cisplatin induced shortening of DRG neurites.
Neurite outgrowth from DRG somas was measured in controls and cultures incubated for 24 hours with 10 μM cisplatin with/out meclizine supplementation. DRG cell bodies and neurites are visualized by immunofluorescence of the DRG specific cytoskeleton protein NF200 [red]. Individual neurites extending from cell body were traced and measured using ImageJ software [scale bar=100 μm]. (b) ImageJ measurements of neurite lengths are presented as relative changes in lengths (mean±SEM; * different from cisplatin; P< 0.05) and as length frequency histograms (c).
Cisplatin crosslinks removal from nuclear DNA is accelerated in the presence of meclizine
Upon entering cells, cisplatin forms cisplatin:DNA crosslinks and timely crosslinks removal is consistent with normal cellular function. We compared removal of crosslinks from nuclear DNA of DRG neurons incubated in the presence or absence of meclizine. Removal was assessed using a modification of the alkaline comet assay, which detects single strand breaks (SSBs) in nuclear DNA [41,53]. After one-hour incubation with cisplatin, medium was replaced and DRGs were kept overnight to allow for DNA repair with/out meclizine. Following repair incubation, DRGs were treated with H2O2 (30 μM/30 min) to produce SSBs, that underlie the comet tail formation in alkaline comet assay. Tail formation, however, is prevented by crosslinks. Accordingly, in the aftermath of cisplatin exposure, the extent of comet tail formation by H2O2 is inversely proportional to levels of cisplatin crosslinks remaining in nuclear DNA [38–40]. We found that compared to comet tails induced by H2O2 without prior cisplatin exposure, tails induced by H2O2 after cisplatin, were markedly shorter [Fig 2]. When meclizine was added in the course of post-cisplatin recovery of DRG neurons, tail lengths increased, reflecting more efficient crosslinks clearance in the presence of meclizine. Tail parameters were quantified using the Casp software [42] and data are presented as mean±SEM [Fig 2].
Fig. 2. Removal of cisplatin crosslinks from nuclear DNA is accelerated in the presence of meclizine.
Crosslinks removal was assessed using a modification of the comet assay where decreases in H2O2 generated comet tails are inversely proportional to levels of cisplatin crosslinks present in nuclear DNA. Comet formation was assessed in controls and immediately after exposure to cisplatin or H2O2 [upper panel]. Lower panel shows comet assays of DRGs, which were left to recover in presence/absence of meclizine after cisplatin exposure and subsequently were incubated with H2O2. Resultant comet olive tail moments (OTM) were captured by camera and analyzed with Casp software. Data from 3 sets of experiments are presented as mean±SEM; *indicates different from recovery after cisplatin in the absence of meclizine; P<0.05.
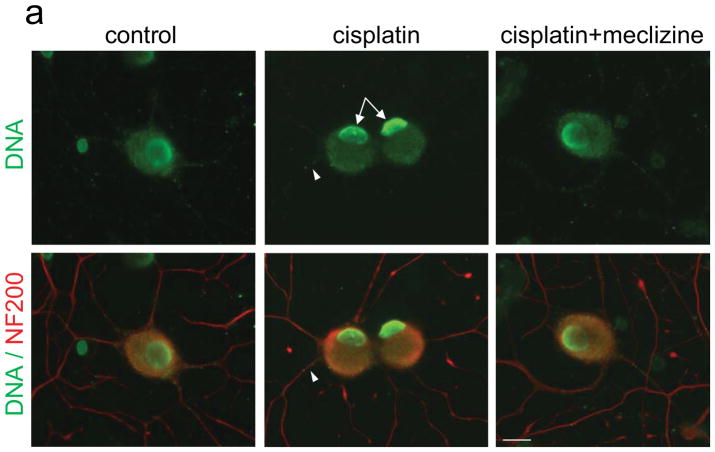
Differential chromatin rearrangements in DRG neurons exposed to cisplatin in the presence or absence of meclizine
Altered patterns of nuclear γH2AX foci reflect chromatin rearrangements associated with stress, DNA damage and DNA repair processes [54]. Here, the density of cisplatin-induced γH2AX foci was significantly lower in nuclei of DRG neurons exposed to cisplatin in the presence compared to exposure without meclizine [Fig 3, green]; DRG somas and neurites are visualized by NF200 immunofluorescence [red]. Lower density of γH2AX foci is consistent with diminished chromatin rearrangement and suggestive of lower levels of persisting cisplatin:DNA adducts in the presence of meclizine. Chromatin rearrangements were also probed using an anti-DNA antibody [33], which under normal conditions due to limited accessibility imposed by chromatin packaging, reacts only marginally with nuclear DNA. Formation of cisplatin crosslinks in nuclear DNA triggers changes in chromatin packaging that facilitate access of damage recognition and repair proteins to DNA. While chromatin rearrangements are commonly monitored via phosphorylation levels of the variant histone protein, H2AX [Fig 3], we show that these rearrangements also facilitate accessibility of the anti-DNA antibody to nuclear DNA [Fig 4A, green, arrows]. Notably, when exposures are done in the presence of meclizine [right panel], nuclear DNA immunoreactivity is significantly reduced [Fig 4A, right panel]. Reduced accessibility of the anti-DNA antibody to nuclear DNA is suggestive of lesser residual DNA damage and partial restoration of chromatin packaging. To our knowledge, this is novel utilization of anti-DNA antibodies to help gauge chromatin rearrangements and serve as independent tool for monitoring formation of DNA damage and progression of the repair process.
Fig. 3. Density of cisplatin-induced nuclear γH2AX foci is reduced by meclizine.
γH2AX foci are visualized by IF of γH2AX [green], while DRG cell bodies and neurites are observed by NF200 [red, scale bar=10 μm]; foci density normalized to nuclear surface area was quantified with ImageJ. Lower density of γH2AX foci was observed following cisplatin exposure in the presence of meclizine. Data are mean±SEM densities calculated for each treatment for 4 sets of independent experiments. *indicates different from control or different from cisplatin + meclizine; P<0.05.
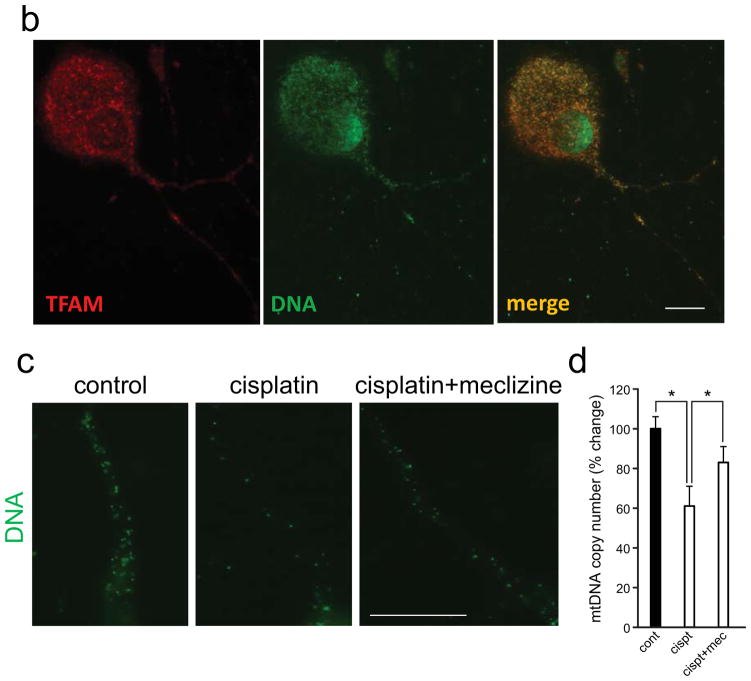
Fig. 4. Meclizine attenuates cisplatin-induced decline in mtDNA copy number, depletion of mtDNA in neurites and accessibility of anti-DNA antibody to nuclear DNA in DRG neurons.
(a) DNA immunofluorescence is green and cell somas and neurites are visualized by NF200 [red]. In control DRGs, DNA immunoreactivity is observed mainly in nuclear periphery, whereas after cisplatin, strong IF is observed throughout the nucleus [center panel, green, arrows]. Exposure in the presence of meclizine results in reduced IF of nuclear DNA [right panel, green]. IF of mtDNA [green] observed in somas and neurites of control DRGs is dense and uniformly distributed [left panel]. IF is reduced, with more sparsely distributed punctate pattern observed in cisplatin exposed DRG neurites [center, green, arrowhead]. Images in panel (b) demonstrate that in somas and neurites immunoreactivity of mtDNA [green] coincides with that of the mitochondrial transcription factor TFAM [red], which coats the mitochondrial genome (merge). (c) High magnification images of neurites [captured with 60x oil objective, green] reveal changes in mtDNA distribution, with reduced density of mtDNA IF following cisplatin exposure [center] and partial restoration of control like distribution pattern in the presence of meclizine. (a, b, c) scale bar=10 μm. (d) Depletion of mtDNA copy number by cisplatin is partially averted by meclizine: qPCR analyses; data are presented as % change relative to control (mean±SEM for 3 experiments); *indicates different from control or different from cisplatin + meclizine; P<0.05.
Meclizine attenuates cisplatin-induced decreases in mtDNA copy number and diminution of mtDNA immunoreactivity in DRG neurites
Since cisplatin forms crosslinks also with mtDNA [5], we compared the effects of cisplatin on mtDNA of DRG neurons exposed with/out meclizine. Two types of analyses were used, real-time qPCR to measure changes in mtDNA copy number and immunofluorescent imaging to characterize alterations in levels and distribution of mtDNA in DRG neurites. Immunofluorescent imaging was done with anti-DNA antibody, which readily reacts with mtDNA of mitochondria populating cell bodies and neurites of DRG neurons [Fig 4a and b, green]. The high mitochondrial content of DRG neurons is reflected in robust immunofluorescence of mtDNA as well as that of TFAM, a mitochondrial transcription factor, which coats mitochondrial genomes and is the main protein component of mtDNA nucleoids [55]. As expected TFAM immunoreactivity co-localized with that of mtDNA in DRG somas and neurites [Fig 4b, merge]. Depletion of mtDNA immunofluorescence was readily detectable following cisplatin exposure [Fig 4a and c]. The robust immunoreactivity of mtDNA observed in control cultures [Fig 4a, green, left panel, somas and neurites] was reduced following cisplatin exposure, revealing sparse punctate mtDNA distribution pattern in neurites (center, arrowhead]. In cultures exposed to cisplatin in the presence of meclizine, immunofluorescence patterns were closer to controls, suggesting lesser depletion of mtDNA [right panel]. Immunofluorescence of mtDNA observed at higher magnification [Fig 4c, green], confirmed that high density and uniform distribution of mtDNA seen in control neurites was depleted by cisplatin exposure (center). A more uniform distribution pattern of mtDNA in neurites was preserved when meclizine was supplemented in the course of cisplatin exposure (right).
Alterations in mtDNA content in DRG neurons were independently assessed by real-time qPCR [56]. Quantification analyses of mtDNA copy number revealed a nearly 40% decrease in mtDNA copy number after 24-hour exposure of DRG neurons to cisplatin. Reduction was partially averted by meclizine supplementation during exposure, with mtDNA copy number preserved at ~80% of control levels [Fig 4d].
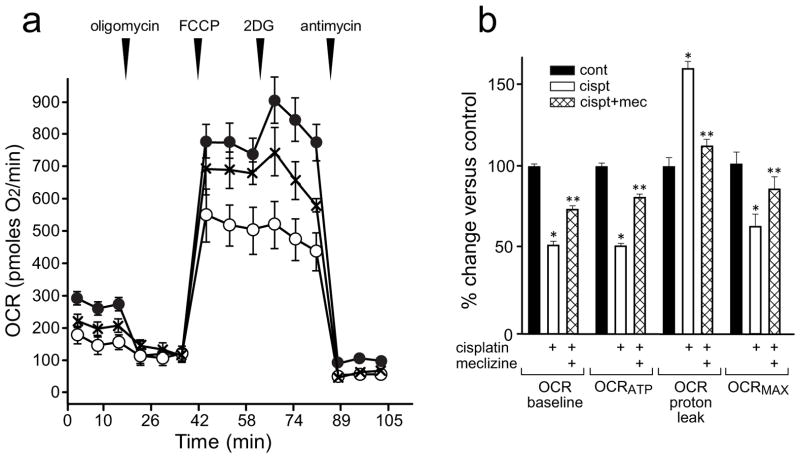
Meclizine lessens cisplatin-induced compromise of mitochondrial respiration in DRG neurons
To determine whether in DRG neurons mitochondrial function might be compromised by cisplatin and to what extent compromise is preventable by meclizine; mitochondrial respiratory parameters were assessed using the extracellular flux analyzer, XF24, which measures OCR in cultured intact cells [Fig 5]. In XF implemented assays, the alterations in OCR produced by mitochondrial effectors reveal changes in respiratory parameters indicative of mitochondrial status in the aftermath of different treatments [43]. Sequential ‘in-port’ addition of oligomycin, FCCP, 2-deoxyglucose (2DG) and antimycin A are done in specialty 24-well plates equipped with oxygen sensors. Control and treated DRG cultures are processed in parallel as we described [25,31,32]. Sequential additions of effectors revealed that under control conditions ~85% of OCR feeds mitochondrial respiration [Fig 5, solid circles], ~70% of mitochondrial OCR supports ATP synthesis, whereas ~30% is lost to proton leak as revealed by the addition of oligomycin, an inhibitor of ATPase. The increase in OCR upon the addition of FCCP is defined as maximal respiration that reflects the cell type specific capacity for substrate oxidation, and is considered an indicator of cell ability to meet changing metabolic demands. Under normal conditions, maximal respiration of DRG neurons is ~3-fold higher than baseline respiration [25]. Following cisplatin challenge, respiratory parameters of DRG mitochondria were compromised [Fig 5, empty circles]: baseline OCR and ATP synthesis-linked OCR decreased by 50%, OCR feeding the proton leak increased by ~65% and maximal respiration decreased by 45% compared to control conditions. Meclizine supplementation attenuated adverse effects of cisplatin [Fig 5, crosses]: the reduction of baseline respiration by cisplatin, decreased from 50 to 25%, reduction in ATP synthesis-linked OCR decreased from 50 to 15%, proton leak decreased from 65 to 15% and maximal respiration was partially restored (from 55 to 80% of control levels) [Fig 5b]. As expected under conditions of mitochondrial compromise, DRGs ability to respond to 2-deoxyglucose (2DG) by increasing mitochondrial OCR was abrogated by cisplatin. When meclizine was present during exposure, DRGs retained the normal response to 2DG, albeit without reaching significance. 2DG inhibits hexokinase, the enzyme, which catalyzes the first step of glycolysis leading to suppression of glycolytic metabolism. Under normal conditions, healthy mitochondria compensate by increasing utilization of pyruvate and glutamine (supplemented in assay medium) as reflected in increases in mitochondrial OCR.
Fig. 5. Meclizine partially alleviates cisplatin induced mitotoxicity in DRG neurons.
Mitochondrial respiratory parameters were assessed using the XF24 analyzer. DRG neurons were incubated under control conditions (vehicle, solid circles) and in the presence of cisplatin without/with meclizine supplementation (empty circles/crosses). Baseline oxygen consumption rate (OCR) was reduced by cisplatin and partially restored by meclizine. Sequential, in port additions of mitochondrial effectors (vertical arrowheads) revealed a 50% decrease in ATP synthesis linked OCR (OCRATP) with partial restoration by meclizine, as well as a 65% increase in OCR feeding the proton leak, which was reduced to 15% in the presence of meclizine (b). Maximal respiration (OCRMAX) was reduced by 45% by cisplatin and preserved at 80% of control level by meclizine (crosshatch). Changes in respiratory parameters were calculated relatively to respective controls and are given as mean±SEM for 5 sets of independent biological experiments. *indicates different from respective control; **indicates different from cisplatin without meclizine supplementation; P<0.05.
Enhancement of pentose phosphate pathway by meclizine is indispensable for preservation of NADP+/NADPH ratios and GSH stores and for diminution of ROS during cisplatin exposure
To delineate the mechanisms that underlie protection of DRG neurons by meclizine in the setting of cisplatin exposure, NADP+ and NADPH levels were measured in controls and in cultures exposed for 24 hours to cisplatin with/without meclizine supplementation [Fig 6a]. Cisplatin exposures decreased NADPH and increased NADP+ levels by ~25%, indicative of depletion of reducing equivalents and diminished antioxidant capacity in cisplatin challenged DRG neurons. When cultures were supplemented with meclizine in the course of exposure, NADPH was maintained at near control levels. The resultant more favorable NADP+/NADPH ratio [Fig 6b] suggests that meclizine helps sustain redox balance by enhancement of the pentose phosphate branch of glycolytic metabolism. To test this possibility, 6-aminonicotinamide (6AN) was used. 6AN is a competitive inhibitor of glucose-6-phosphate dehydrogenase (G6PD) and 6-phosphogluconate, the first two enzymes of the oxidative branch of pentose phosphate pathway (PPP). G6PD catalyzes the conversion of glucose 6-phosphate to 6-phosphogluconolactone, which is the rate limiting step of PPP [57,58]. When 6AN was given in conjunction with meclizine during cisplatin exposure, protective effects of meclizine were abrogated and NADPH depletion as well as unfavorable NADP+/NADPH ratios persisted [Fig 6a & b], suggestive of impaired antioxidant capacity under these conditions [17]. To further examine the involvement of pentose phosphate pathway in protection of DRG neurons, a downstream system that uses NADPH to maintain reduced glutathione stores was also assessed [Fig 6c]. Glutathione reductases utilize NADPH equivalents to maintain GSH that is used in different downstream reducing systems. Meclizine increased GSH levels under control conditions and prevented GSH depletion by cisplatin [Fig 6c]; the nearly 40% GSH reduction by cisplatin was restored to ~85% of control in the presence of meclizine. However, preservation of glutathione stores by meclizine during cisplatin exposure was abrogated by addition of 6AN that inhibits the pentose phosphate pathway [Fig 6c]. Adequate levels of reduced glutathione are requisite for antioxidant defenses, redox balance and maintenance of homeostasis under normal and injurious conditions.
Fig. 6. Meclizine improves NADP+/NADPH ratios and GSH stores and attenuates cisplatin-induced ROS.
(a, b) Meclizine ameliorates the cisplatin-induced increases in NADP+, decreases in NADPH and increases of NADP+/NADPH ratios. Concomitant addition of 6AN abrogated these effects of meclizine. Data are mean±SEM for 4 independent experiments; *indicates different from control or from cisplatin + meclizine; P< 0.05. (c) Meclizine prevents cisplatin associated depletion of GSH. GSH levels depleted by cisplatin exposure were partially restored in the presence of meclizine. GSH restoration was abrogated by 6AN (N.S.; not different from cisplatin alone). Data are given as mean±SEM calculated from 4 independent experiments; *indicates different from control or from cisplatin + meclizine; P< 0.05. (d) Meclizine attenuates cisplatin-induced ROS formation. ROS levels were assessed by imaging of superoxide-mediated oxidation of dihydroethidium to 2-hydroxyethidium observed as red fluorescence. Fluorescence intensity normalized to cell body surface area was quantified by ImageJ and presented as mean±SEM of 3 sets of experiments; in each set, 5–8 DRG neurons were sequentially scored for each condition (≥20 DRG neurons for each condition). Meclizine attenuated cisplatin-induced fluorescence intensity. This effect was abrogated by concomitant addition of 6AN; *indicates different from control or different from cisplatin + meclizine; P< 0.05. N.S. indicates not significant.
To determine to what extent meclizine-mediated favorable changes in NADP+/NADPH ratios and GSH levels during cisplatin exposures translate into better ROS control in DRG neurons, ROS levels were assessed in situ by monitoring superoxide-mediated oxidation of dihydroethidium to 2-hydroxyethidium [Fig 6d] as we previously described [50]. Increases in dihydroethidium oxidation by superoxide, indicative of elevated ROS, were observed in cisplatin exposed DRG neurons whereas meclizine supplementation during exposure, reduced signal intensity (fluorescence intensity normalized to cell surface area was quantified by ImageJ). When 6AN, an inhibitor of PPP was added in the course of cisplatin exposure, ROS control by meclizine was abrogated.
DISCUSSION
Meclizine, a histamine type H1 receptor antagonist [59,60] has been recently implicated in metabolic reprogramming and found neuroprotective in settings of disrupted oxygen supply and excessive oxidative stress [22–25]. To date, the mechanisms underlying neuroprotection by meclizine are incompletely understood, but thought to involve a shift towards glycolytic metabolism to augment ATP production and redox balance under compromised conditions [22–25]. Our recent study revealed that enhancement of glycolytic metabolism by meclizine is indispensable for protection of DRG neurons mitochondria from hypoxic stress [25]. Likewise meclizine mediated enhancement of glycolysis was found requisite for protection of neurons against 6-hydroxydopamine in an in vitro model of Parkinson’s disease [24] and upregulation of glycolysis by meclizine rescued kidney tubular epithelial cells from chemical anoxia in vitro [61]. While the ‘off target’ actions of meclizine are presently not fully understood, emerging reports on glycolytic metabolism augmentation by meclizine observed in diverse models warrant closer investigation. Although, traditionally neuronal cells have not been associated with metabolic plasticity, recent studies suggested that neurons can utilize glycolytic metabolism under stressful conditions [62–66] and sensory neurons use glycolysis to support axonal transport [15]. In context of these findings, we asked whether metabolic reprogramming could protect the peripheral nervous system against chemotherapy-associated neurotoxicity. We report that enhancement of the pentose phosphate branch of glycolytic metabolism by meclizine, improves redox balance and antioxidant capacity and thereby lessens DRG neurons injury by cisplatin, an antimitotic cancer drug frequently associated with peripheral neuropathy [3].
Cisplatin toxicity involves nuclear and cytoplasmic events [6,67] initiated by rapid formation of crosslinks with nuclear and mtDNA [9,68]. Cisplatin crosslinks are repairable via the NER pathway that catalyzes removal of cisplatin crosslinks from nuclear DNA [69,70]. NER pathway is ubiquitous in mammalian cells and active also in DRG neurons [2,71]. In contrast, the NER pathway is absent from mitochondrial compartment [12] and contribution of other mitochondrial DNA repair pathways to removal of cisplatin adducts is predicted to be marginal [14]. This renders neuronal mitochondria critically sensitive to cisplatin, as adducts are likely to persist longer, hindering mitochondrial function and escalating generation of ROS [7]. Because neurons rely largely on mitochondrial energy production, mitochondrial impairments by cisplatin are predicted to compromise neuronal functions, including DNA repair processes, which are ATP dependent [5,72]. We found that cisplatin-induced decreases in mtDNA copy number and depletion of mtDNA in DRG neurites are partially averted in the presence of meclizine. Moreover, cisplatin-induced compromise of mitochondrial respiratory parameters, including oxygen consumption linked with ATP synthesis, is alleviated when DRG neurons are supplemented with meclizine. Importantly, we show that meclizine improves cisplatin crosslinks removal rates, as evidenced in alkaline comet assays, diminished density of nuclear γH2AX foci and diminished accessibility of anti-DNA antibody to nuclear DNA in cisplatin exposed DRG neurons. Temporal changes in patterns of γH2AX foci reflect chromatin rearrangements associated with DNA damage and subsequent progression of DNA repair processes [73]. Diminished density of γH2AX foci and diminished accessibility of the anti-DNA antibody to nuclear DNA in the presence of meclizine is suggestive of lesser residual cisplatin-induced DNA damage and partial restoration of chromatin packaging. When considered together, these findings are consistent with the observed lesser dysfunction and improved neurite morphology of DRG neurons challenged with cisplatin in the presence of meclizine.
Because meclizine has been previously linked to metabolic reprogramming and we found that it augments NADPH levels, we considered the possibility that pentose phosphate pathway, which is a significant, although not exclusive source of NADPH, might be involved in neuroprotection by meclizine. This was tested using 6AN, an inhibitor of glucose-6-phosphate dehydrogenase (G6PD), the first and rate limiting enzyme of PPP [57,58]. Addition of 6AN abrogated meclizine-mediated preservation of NADPH levels in DRG neurons exposed to cisplatin. Likewise, 6AN abrogated restoration of GSH, a component of a downstream cofactor system coupled with NADPH, jointly implicating involvement of the pentose phosphate branch of glycolytic metabolism. NADPH and GSH cofactor systems are central to redox balance and antioxidant capacity in nerve cells [74,75] and critical in DRG neurons, whose inherent features, i.e., limited shielding from circulating compounds [76] and high mitochondrial content necessitated by metabolic demands, exacerbate vulnerability to toxins and oxidative stress. To ascertain that favorable NADP+/NADPH ratios and GSH levels preserved by meclizine during cisplatin exposures indeed translate into improved ROS control, ROS levels were assessed. Cisplatin-induced ROS was lowered in the presence of meclizine, but not when 6AN, the PPP inhibitor was added, indicating that the PPP was required for protection of DRG neurons by meclizine. Importantly, ROS control exerted by meclizine mitigated cisplatin-induced depletion of mtDNA and lessened respiratory impairments in DRG neurons.
Our study reveals potential for pharmacologic harnessing of a central endogenous defense mechanism, i.e., augmentation of redox balance via enhancement of the pentose phosphate branch of glycolytic metabolism for neuroprotection of peripheral nervous system neurons from circulating neurotoxic antimitotic drugs. The premise that PNS injury by antimitotic drugs involves mitochondria and that features inherent to DRG neurons, predispose them to damage by antimitotic drugs is understudied. Our findings link compromised mitochondrial respiration with reduced capacity for nuclear DNA repair and conversely, link metabolic reprogramming by meclizine with improved efficacy of DNA repair and reduced cisplatin genotoxicity in DRG neurons. Together, data reveal potential for exploiting metabolic plasticity of DRG neurons for protection of the peripheral nervous system against collateral damage by antimitotic chemotherapeutic drugs.
Supplementary Material
Viability of DRG neurons exposed to a range of cisplatin concentrations was assessed by the trypan blue exclusion assay and the CellTiter 96® (#G3580, Promega) viability kit (MTS). After seeding DRG neurons were incubated for 24 hours to allow for neurites outgrowth and then exposed to a range of cisplatin concentration (5–150 μM) for the duration of 24 hours prior to viability assessments. (1a) At termination of the 24-hour exposure, cultures were incubated for 10 min with Trypan blue (Cellgro), washed with PBS and fixed with 4% paraformaldehyde. At least 100–150 cells were evaluated for each condition. Data from three sets of independent biological experiments (300–450 cells/condition) are presented as mean±SEM (Supp Fig 1a).
(1b) CellTiter 96® viability assay is based on a tetrazolium compound (MTS) that is reduced by living cells into a colored formazan product measured by absorbance at 490 nm. DRG neurons grown in 96-well plates were exposed to cisplatin concentrations ranging 5–150 μM in triplicates. Following treatment cultures were processed according to manufacturer’s protocol and absorbance read using TECAN F200 Pro plate Reader (TECAN, San Jose, CA). Viability was calculated relative to control cultures and data from 3 independent biological experiments done in triplicates are presented as mean±SEM [Supp Fig 1b]. In the case of trypan blue exclusion, only marginal loss of cell viability was detected with 10 μM cisplatin. Interestingly, with low cisplatin concentrations, greater sensitivity was attained using the MTS assay (CellTiter 96®). MTS which measures reductive activity of metabolically active cells, revealed a 12% decrease following exposure to 10 μM cisplatin whereas only 2% decrease was measured at this concentration by trypan blue which identifies dead cells. Both assays support the selection of 10 μM dose for studying effects of cisplatin while avoiding significant loss of DRG neurons (Supplementary Fig 1a and 1b, respectively).
Status and purity of DRG neuron cultures was assessed by labeling with DAPI (blue) in conjunction with the DRD specific high molecular weight cytoskeleton protein, NF200 [red]. Immunoreactivity of the anti-DNA antibody [green] served surrogate for the extent of nuclear DNA damage. Strong immunofluorescence of nuclear DNA [green, center panel] indicates chromatin rearrangements that facilitate greater accessibility of the anti-DNA antibody to nuclear DNA. Immunoreactivity is reduced if meclizine is preset during exposure, suggestive of lesser DNA damage. Merged images are shown in the bottom panel.
Acknowledgments
This work was supported by grants from the National Institutes of Health (ES014613) and Shriners Hospitals for Children (86700) and Surgery Department at UTMB to EWE. We thank Steve Schuenke and Eileen Figueroa for assistance with manuscript preparation.
Abbreviations
- 6AN
6-aminonicotinamide
- DRG
dorsal root ganglion
- mtDNA
mitochondrial DNA
- NER
nucleotide excision repair
- NADP+
nicotinamide adenine dinucleotide phosphate
- NADPH
nicotinamide adenine dinucleotide phosphate, reduced form
- OCR
oxygen consumption rate
- PPP
pentose phosphate pathway
- PNS
peripheral nervous system
- GSH
reduced glutathione
- ROS
reactive oxygen species
Footnotes
Conflict of Interest/Disclosure: Authors declare no conflicts of interest.
References
- 1.Bennett GJ, Doyle T, Salvemini D. Mitotoxicity in distal symmetrical sensory peripheral neuropathies. Nat Rev Neurol. 2014;10(6):326–336. doi: 10.1038/nrneurol.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dzagnidze A, Katsarava Z, Makhalova J, Liedert B, Yoon MS, Kaube H, Limmroth V, Thomale J. Repair capacity for platinum-DNA adducts determines the severity of cisplatin-induced peripheral neuropathy. J Neurosci. 2007;27(35):9451–9457. doi: 10.1523/JNEUROSCI.0523-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boehmerle W, Huehnchen P, Peruzzaro S, Balkaya M, Endres M. Electrophysiological, behavioral and histological characterization of paclitaxel, cisplatin, vincristine and bortezomib-induced neuropathy in C57Bl/6 mice. Sci Rep. 2014;4:6370. doi: 10.1038/srep06370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelley MR, Jiang Y, Guo C, Reed A, Meng H, Vasko MR. Role of the DNA base excision repair protein, APE1 in cisplatin, oxaliplatin, or carboplatin induced sensory neuropathy. PLoS One. 2014;9(9):e106485. doi: 10.1371/journal.pone.0106485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Podratz JL, Knight AM, Ta LE, Staff NP, Gass JM, Genelin K, Schlattau A, Lathroum L, Windebank AJ. Cisplatin induced mitochondrial DNA damage in dorsal root ganglion neurons. Neurobiol Dis. 2011;41(3):661–668. doi: 10.1016/j.nbd.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sancho-Martinez SM, Prieto-Garcia L, Prieto M, Lopez-Novoa JM, Lopez-Hernandez FJ. Subcellular targets of cisplatin cytotoxicity: an integrated view. Pharmacol Ther. 2012;136(1):35–55. doi: 10.1016/j.pharmthera.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Xiao WH, Bennett GJ. Effects of mitochondrial poisons on the neuropathic pain produced by the chemotherapeutic agents, paclitaxel and oxaliplatin. Pain. 2012;153(3):704–709. doi: 10.1016/j.pain.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao WH, Zheng H, Bennett GJ. Characterization of oxaliplatin-induced chronic painful peripheral neuropathy in the rat and comparison with the neuropathy induced by paclitaxel. Neuroscience. 2012;203:194–206. doi: 10.1016/j.neuroscience.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eastman A. The formation, isolation and characterization of DNA adducts produced by anticancer platinum complexes. Pharmacol Ther. 1987;34(2):155–166. doi: 10.1016/0163-7258(87)90009-x. [DOI] [PubMed] [Google Scholar]
- 10.Mendoza J, Martinez J, Hernandez C, Perez-Montiel D, Castro C, Fabian-Morales E, Santibanez M, Gonzalez-Barrios R, Diaz-Chavez J, Andonegui MA, Reynoso N, Onate LF, Jimenez MA, Nunez M, Dyer R, Herrera LA. Association between ERCC1 and XPA expression and polymorphisms and the response to cisplatin in testicular germ cell tumours. Br J Cancer. 2013;109(1):68–75. doi: 10.1038/bjc.2013.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Usanova S, Piee-Staffa A, Sied U, Thomale J, Schneider A, Kaina B, Koberle B. Cisplatin sensitivity of testis tumour cells is due to deficiency in interstrand-crosslink repair and low ERCC1-XPF expression. Mol Cancer. 2010;9:248. doi: 10.1186/1476-4598-9-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clayton DA, Doda JN, Friedberg EC. The absence of a pyrimidine dimer repair mechanism in mammalian mitochondria. Proc Natl Acad Sci U S A. 1974;71(7):2777–2781. doi: 10.1073/pnas.71.7.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Croteau DL, Stierum RH, Bohr VA. Mitochondrial DNA repair pathways. Mutat Res. 1999;434(3):137–148. doi: 10.1016/s0921-8777(99)00025-7. [DOI] [PubMed] [Google Scholar]
- 14.Liu P, Demple B. DNA repair in mammalian mitochondria: Much more than we thought? Environ Mol Mutagen. 2010;51(5):417–426. doi: 10.1002/em.20576. [DOI] [PubMed] [Google Scholar]
- 15.Zala D, Hinckelmann MV, Yu H, Lyra da Cunha MM, Liot G, Cordelieres FP, Marco S, Saudou F. Vesicular glycolysis provides on-board energy for fast axonal transport. Cell. 2013;152(3):479–491. doi: 10.1016/j.cell.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 16.Quaegebeur A, Segura I, Schmieder R, Verdegem D, Decimo I, Bifari F, Dresselaers T, Eelen G, Ghosh D, Davidson SM, Schoors S, Broekaert D, Cruys B, Govaerts K, De Legher C, Bouche A, Schoonjans L, Ramer MS, Hung G, Bossaert G, Cleveland DW, Himmelreich U, Voets T, Lemmens R, Bennett CF, Robberecht W, De Bock K, Dewerchin M, Ghesquiere B, Fendt SM, Carmeliet P. Deletion or inhibition of the oxygen sensor PHD1 protects against ischemic stroke via reprogramming of neuronal metabolism. Cell Metab. 2016;23(2):280–291. doi: 10.1016/j.cmet.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dey S, Sidor A, O’Rourke B. Compartment-specific control of reactive oxygen species scavenging by antioxidant pathway enzymes. J Biol Chem. 2016;291(21):11185–11197. doi: 10.1074/jbc.M116.726968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett GJ, Liu GK, Xiao WH, Jin HW, Siau C. Terminal arbor degeneration--a novel lesion produced by the antineoplastic agent paclitaxel. Eur J Neurosci. 2011;33(9):1667–1676. doi: 10.1111/j.1460-9568.2011.07652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallace KB. Mitochondrial off targets of drug therapy. Trends Pharmacol Sci. 2008;29(7):361–366. doi: 10.1016/j.tips.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Yan F, Liu JJ, Ip V, Jamieson SM, McKeage MJ. Role of platinum DNA damage-induced transcriptional inhibition in chemotherapy-induced neuronal atrophy and peripheral neurotoxicity. J Neurochem. 2015;135(6):1099–1112. doi: 10.1111/jnc.13355. [DOI] [PubMed] [Google Scholar]
- 21.Owen DE, Egerton J. Culture of dissociated sensory neurons from dorsal root ganglia of postnatal and adult rats. Methods Mol Biol. 2012;846:179–187. doi: 10.1007/978-1-61779-536-7_16. [DOI] [PubMed] [Google Scholar]
- 22.Gohil VM, Offner N, Walker JA, Sheth SA, Fossale E, Gusella JF, MacDonald ME, Neri C, Mootha VK. Meclizine is neuroprotective in models of Huntington’s disease. Hum Mol Genet. 2011;20(2):294–300. doi: 10.1093/hmg/ddq464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gohil VM, Sheth SA, Nilsson R, Wojtovich AP, Lee JH, Perocchi F, Chen W, Clish CB, Ayata C, Brookes PS, Mootha VK. Nutrient-sensitized screening for drugs that shift energy metabolism from mitochondrial respiration to glycolysis. Nat Biotechnol. 2010;28(3):249–255. doi: 10.1038/nbt.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong CT, Chau KY, Schapira AH. Meclizine-induced enhanced glycolysis is neuroprotective in Parkinson disease cell models. Sci Rep. 2016;6:25344. doi: 10.1038/srep25344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhuo M, Gorgun MF, Englander EW. Augmentation of glycolytic metabolism by meclizine is indispensable for protection of dorsal root ganglion neurons from hypoxia-induced mitochondrial compromise. Free Radic Biol Med. 2016;22:20–31. doi: 10.1016/j.freeradbiomed.2016.1007.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gohil VM, Zhu L, Baker CD, Cracan V, Yaseen A, Jain M, Clish CB, Brookes PS, Bakovic M, Mootha VK. Meclizine inhibits mitochondrial respiration through direct targeting of cytosolic phosphoethanolamine metabolism. J Biol Chem. 2013;288(49):35387–35395. doi: 10.1074/jbc.M113.489237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernyhough P. Mitochondrial dysfunction in diabetic neuropathy: a series of unfortunate metabolic events. Curr Diab Rep. 2015;15(11):89. doi: 10.1007/s11892-015-0671-9. [DOI] [PubMed] [Google Scholar]
- 28.Huang LY, Neher E. Ca(2+)-dependent exocytosis in the somata of dorsal root ganglion neurons. Neuron. 1996;17(1):135–145. doi: 10.1016/s0896-6273(00)80287-1. [DOI] [PubMed] [Google Scholar]
- 29.Lindsay RM. Nerve growth factors (NGF, BDNF) enhance axonal regeneration but are not required for survival of adult sensory neurons. J Neurosci. 1988;8(7):2394–2405. doi: 10.1523/JNEUROSCI.08-07-02394.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malin SA, Davis BM, Molliver DC. Production of dissociated sensory neuron cultures and considerations for their use in studying neuronal function and plasticity. Nat Protoc. 2007;2(1):152–160. doi: 10.1038/nprot.2006.461. [DOI] [PubMed] [Google Scholar]
- 31.Singh S, Englander EW. Nuclear depletion of apurinic/apyrimidinic endonuclease 1 (Ape1/Ref-1) is an indicator of energy disruption in neurons. Free Radic Biol Med. 2012;53(9):1782–1790. doi: 10.1016/j.freeradbiomed.2012.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh S, Zhuo M, Gorgun FM, Englander EW. Overexpressed neuroglobin raises threshold for nitric oxide-induced impairment of mitochondrial respiratory activities and stress signaling in primary cortical neurons. Nitric Oxide. 2013;32:21–28. doi: 10.1016/j.niox.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheer U, Messner K, Hazan R, Raska I, Hansmann P, Falk H, Spiess E, Franke WW. High sensitivity immunolocalization of double and single-stranded DNA by a monoclonal antibody. Eur J Cell Biol. 1987;43(3):358–371. [PubMed] [Google Scholar]
- 34.Fernyhough P, Willars GB, Lindsay RM, Tomlinson DR. Insulin and insulin-like growth factor I enhance regeneration in cultured adult rat sensory neurones. Brain Res. 1993;607(1–2):117–124. doi: 10.1016/0006-8993(93)91496-f. [DOI] [PubMed] [Google Scholar]
- 35.Habash T, Saleh A, Roy Chowdhury SK, Smith DR, Fernyhough P. The proinflammatory cytokine, interleukin-17A, augments mitochondrial function and neurite outgrowth of cultured adult sensory neurons derived from normal and diabetic rats. Exp Neurol. 2015;273:177–189. doi: 10.1016/j.expneurol.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 36.Olive PL, Frazer G, Banath JP. Radiation-induced apoptosis measured in TK6 human B lymphoblast cells using the comet assay. Radiat Res. 1993;136(1):130–136. [PubMed] [Google Scholar]
- 37.Singh NP, Tice RR, Stephens RE, Schneider EL. A microgel electrophoresis technique for the direct quantitation of DNA damage and repair in individual fibroblasts cultured on microscope slides. Mutat Res. 1991;252(3):289–296. doi: 10.1016/0165-1161(91)90008-v. [DOI] [PubMed] [Google Scholar]
- 38.Almeida GM, Duarte TL, Steward WP, Jones GD. Detection of oxaliplatin-induced DNA crosslinks in vitro and in cancer patients using the alkaline comet assay. DNA Repair (Amst) 2006;5(2):219–225. doi: 10.1016/j.dnarep.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 39.Spanswick VJ, Hartley JM, Hartley JA. Measurement of DNA interstrand crosslinking in individual cells using the Single Cell Gel Electrophoresis (Comet) assay. Methods Mol Biol. 2010;613:267–282. doi: 10.1007/978-1-60327-418-0_17. [DOI] [PubMed] [Google Scholar]
- 40.Hartley JM, Spanswick VJ, Hartley JA. Measurement of DNA damage in individual cells using the Single Cell Gel Electrophoresis (Comet) assay. Methods Mol Biol. 2011;731:309–320. doi: 10.1007/978-1-61779-080-5_25. [DOI] [PubMed] [Google Scholar]
- 41.Li H, Swiercz R, Englander EW. Elevated metals compromise repair of oxidative DNA damage via the base excision repair pathway: implications of pathologic iron overload in the brain on integrity of neuronal DNA. J Neurochem. 2009;110(6):1774–1783. doi: 10.1111/j.1471-4159.2009.06271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Konca K, Lankoff A, Banasik A, Lisowska H, Kuszewski T, Gozdz S, Koza Z, Wojcik A. A cross-platform public domain PC image-analysis program for the comet assay. Mutat Res. 2003;534(1–2):15–20. doi: 10.1016/s1383-5718(02)00251-6. [DOI] [PubMed] [Google Scholar]
- 43.Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J. 2011;435(2):297–312. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dranka BP, Benavides GA, Diers AR, Giordano S, Zelickson BR, Reily C, Zou L, Chatham JC, Hill BG, Zhang J, Landar A, Darley-Usmar VM. Assessing bioenergetic function in response to oxidative stress by metabolic profiling. Free Radic Biol Med. 2011;51(9):1621–1635. doi: 10.1016/j.freeradbiomed.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerencser AA, Neilson A, Choi SW, Edman U, Yadava N, Oh RJ, Ferrick DA, Nicholls DG, Brand MD. Quantitative microplate-based respirometry with correction for oxygen diffusion. Anal Chem. 2009;81(16):6868–6878. doi: 10.1021/ac900881z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mandavilli BS, Janes MS. Detection of intracellular glutathione using ThiolTracker violet stain and fluorescence microscopy. Curr Protoc Cytom. 2010;Chapter 9(Unit 9):35. doi: 10.1002/0471142956.cy0935s53. [DOI] [PubMed] [Google Scholar]
- 47.Santambrogio P, Dusi S, Guaraldo M, Rotundo LI, Broccoli V, Garavaglia B, Tiranti V, Levi S. Mitochondrial iron and energetic dysfunction distinguish fibroblasts and induced neurons from pantothenate kinase-associated neurodegeneration patients. Neurobiol Dis. 2015;81:144–153. doi: 10.1016/j.nbd.2015.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bindokas VP, Jordan J, Lee CC, Miller RJ. Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine. J Neurosci. 1996;16(4):1324–1336. doi: 10.1523/JNEUROSCI.16-04-01324.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Forkink M, Willems PH, Koopman WJ, Grefte S. Live-cell assessment of mitochondrial reactive oxygen species using dihydroethidine. Methods Mol Biol. 2015;1264:161–169. doi: 10.1007/978-1-4939-2257-4_15. [DOI] [PubMed] [Google Scholar]
- 50.Englander EW, Hu Z, Sharma A, Lee HM, Wu ZH, Greeley GH. Rat MYH, a glycosylase for repair of oxidatively damaged DNA, has brain-specific isoforms that localize to neuronal mitochondria. J Neurochem. 2002;83(6):1471–1480. doi: 10.1046/j.1471-4159.2002.01259.x. [DOI] [PubMed] [Google Scholar]
- 51.Dimmock D, Tang LY, Schmitt ES, Wong LJ. Quantitative evaluation of the mitochondrial DNA depletion syndrome. Clin Chem. 2010;56(7):1119–1127. doi: 10.1373/clinchem.2009.141549. [DOI] [PubMed] [Google Scholar]
- 52.Venegas V, Wang J, Dimmock D, Wong LJ. Real-time quantitative PCR analysis of mitochondrial DNA content. Curr Protoc Hum Genet. 2011;Chapter 19(Unit 19):17. doi: 10.1002/0471142905.hg1907s68. [DOI] [PubMed] [Google Scholar]
- 53.Lee HM, Greeley GH, Jr, Englander EW. Transgenic overexpression of neuroglobin attenuates formation of smoke-inhalation-induced oxidative DNA damage, in vivo, in the mouse brain. Free Radic Biol Med. 2011;51(12):2281–2287. doi: 10.1016/j.freeradbiomed.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Atsumi Y, Inase A, Osawa T, Sugihara E, Sakasai R, Fujimori H, Teraoka H, Saya H, Kanno M, Tashiro F, Nakagama H, Masutani M, Yoshioka K. The Arf/p53 protein module, which induces apoptosis, down-regulates histone H2AX to allow normal cells to survive in the presence of anti-cancer drugs. J Biol Chem. 2013;288(19):13269–13277. doi: 10.1074/jbc.M112.402560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang YE, Marinov GK, Wold BJ, Chan DC. Genome-wide analysis reveals coating of the mitochondrial genome by TFAM. PLoS One. 2013;8(8):e74513. doi: 10.1371/journal.pone.0074513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song L, Shan Y, Lloyd KC, Cortopassi GA. Mutant Twinkle increases dopaminergic neurodegeneration, mtDNA deletions and modulates Parkin expression. Hum Mol Genet. 2012;21(23):5147–5158. doi: 10.1093/hmg/dds365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hothersall JS, Gordge M, Noronha-Dutra AA. Inhibition of NADPH supply by 6-aminonicotinamide: effect on glutathione, nitric oxide and superoxide in J774 cells. FEBS Lett. 1998;434(1–2):97–100. doi: 10.1016/s0014-5793(98)00959-4. [DOI] [PubMed] [Google Scholar]
- 58.Tyson RL, Perron J, Sutherland GR. 6-Aminonicotinamide inhibition of the pentose phosphate pathway in rat neocortex. Neuroreport. 2000;11(9):1845–1848. doi: 10.1097/00001756-200006260-00009. [DOI] [PubMed] [Google Scholar]
- 59.Kubo N, Shirakawa O, Kuno T, Tanaka C. Antimuscarinic effects of antihistamines: quantitative evaluation by receptor-binding assay. Jpn J Pharmacol. 1987;43(3):277–282. doi: 10.1254/jjp.43.277. [DOI] [PubMed] [Google Scholar]
- 60.Cohen B, DeJong JM. Meclizine and placebo in treating vertigo of vestibular origin. Relative efficacy in a double-blind study. Arch Neurol. 1972;27(2):129–135. doi: 10.1001/archneur.1972.00490140033006. [DOI] [PubMed] [Google Scholar]
- 61.Kishi S, Campanholle G, Gohil VM, Perocchi F, Brooks CR, Morizane R, Sabbisetti V, Ichimura T, Mootha VK, Bonventre JV. Meclizine Preconditioning Protects the Kidney Against Ischemia-Reperfusion Injury. EBioMedicine. 2015;2(9):1090–1101. doi: 10.1016/j.ebiom.2015.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jang S, Nelson JC, Bend EG, Rodriguez-Laureano L, Tueros FG, Cartagenova L, Underwood K, Jorgensen EM, Colon-Ramos DA. Glycolytic Enzymes Localize to Synapses under Energy Stress to Support Synaptic Function. Neuron. 2016;90(2):278–291. doi: 10.1016/j.neuron.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karaca M, Frigerio F, Migrenne S, Martin-Levilain J, Skytt DM, Pajecka K, Martin-del-Rio R, Gruetter R, Tamarit-Rodriguez J, Waagepetersen HS, Magnan C, Maechler P. GDH-Dependent Glutamate Oxidation in the Brain Dictates Peripheral Energy Substrate Distribution. Cell Rep. 2015;13(2):365–375. doi: 10.1016/j.celrep.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 64.Lee do Y, Xun Z, Platt V, Budworth H, Canaria CA, McMurray CT. Distinct pools of non-glycolytic substrates differentiate brain regions and prime region-specific responses of mitochondria. PLoS One. 2013;8(7):e68831. doi: 10.1371/journal.pone.0068831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lundgaard I, Li B, Xie L, Kang H, Sanggaard S, Haswell JD, Sun W, Goldman S, Blekot S, Nielsen M, Takano T, Deane R, Nedergaard M. Direct neuronal glucose uptake heralds activity-dependent increases in cerebral metabolism. Nat Commun. 2015;6:6807. doi: 10.1038/ncomms7807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patel AB, Lai JC, Chowdhury GM, Hyder F, Rothman DL, Shulman RG, Behar KL. Direct evidence for activity-dependent glucose phosphorylation in neurons with implications for the astrocyte-to-neuron lactate shuttle. Proc Natl Acad Sci U S A. 2014;111(14):5385–5390. doi: 10.1073/pnas.1403576111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim HS, Guo C, Thompson EL, Jiang Y, Kelley MR, Vasko MR, Lee SH. APE1, the DNA base excision repair protein, regulates the removal of platinum adducts in sensory neuronal cultures by NER. Mutat Res. 2015;779:96–104. doi: 10.1016/j.mrfmmm.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McDonald ES, Randon KR, Knight A, Windebank AJ. Cisplatin preferentially binds to DNA in dorsal root ganglion neurons in vitro and in vivo: a potential mechanism for neurotoxicity. Neurobiol Dis. 2005;18(2):305–313. doi: 10.1016/j.nbd.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 69.Moggs JG, Szymkowski DE, Yamada M, Karran P, Wood RD. Differential human nucleotide excision repair of paired and mispaired cisplatin-DNA adducts. Nucleic Acids Res. 1997;25(3):480–491. doi: 10.1093/nar/25.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cleaver JE, Lam ET, Revet I. Disorders of nucleotide excision repair: the genetic and molecular basis of heterogeneity. Nat Rev Genet. 2009;10(11):756–768. doi: 10.1038/nrg2663. [DOI] [PubMed] [Google Scholar]
- 71.Englander EW. DNA damage response in peripheral nervous system: coping with cancer therapy-induced DNA lesions. DNA Repair (Amst) 2013;12(8):685–690. doi: 10.1016/j.dnarep.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tomkinson AE, Howes TR, Wiest NE. DNA ligases as therapeutic targets. Transl Cancer Res. 2013;2(3) pii: 1219. [PMC free article] [PubMed] [Google Scholar]
- 73.Lukas J, Lukas C, Bartek J. More than just a focus: The chromatin response to DNA damage and its role in genome integrity maintenance. Nat Cell Biol. 2011;13(10):1161–1169. doi: 10.1038/ncb2344. [DOI] [PubMed] [Google Scholar]
- 74.Smeyne M, Smeyne RJ. Glutathione metabolism and Parkinson’s disease. Free Radic Biol Med. 2013;62:13–25. doi: 10.1016/j.freeradbiomed.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fernandez-Fernandez S, Almeida A, Bolanos JP. Antioxidant and bioenergetic coupling between neurons and astrocytes. Biochem J. 2012;443(1):3–11. doi: 10.1042/BJ20111943. [DOI] [PubMed] [Google Scholar]
- 76.Weerasuriya A, Mizisin AP. The blood-nerve barrier: structure and functional significance. Methods Mol Biol. 2011;686:149–173. doi: 10.1007/978-1-60761-938-3_6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Viability of DRG neurons exposed to a range of cisplatin concentrations was assessed by the trypan blue exclusion assay and the CellTiter 96® (#G3580, Promega) viability kit (MTS). After seeding DRG neurons were incubated for 24 hours to allow for neurites outgrowth and then exposed to a range of cisplatin concentration (5–150 μM) for the duration of 24 hours prior to viability assessments. (1a) At termination of the 24-hour exposure, cultures were incubated for 10 min with Trypan blue (Cellgro), washed with PBS and fixed with 4% paraformaldehyde. At least 100–150 cells were evaluated for each condition. Data from three sets of independent biological experiments (300–450 cells/condition) are presented as mean±SEM (Supp Fig 1a).
(1b) CellTiter 96® viability assay is based on a tetrazolium compound (MTS) that is reduced by living cells into a colored formazan product measured by absorbance at 490 nm. DRG neurons grown in 96-well plates were exposed to cisplatin concentrations ranging 5–150 μM in triplicates. Following treatment cultures were processed according to manufacturer’s protocol and absorbance read using TECAN F200 Pro plate Reader (TECAN, San Jose, CA). Viability was calculated relative to control cultures and data from 3 independent biological experiments done in triplicates are presented as mean±SEM [Supp Fig 1b]. In the case of trypan blue exclusion, only marginal loss of cell viability was detected with 10 μM cisplatin. Interestingly, with low cisplatin concentrations, greater sensitivity was attained using the MTS assay (CellTiter 96®). MTS which measures reductive activity of metabolically active cells, revealed a 12% decrease following exposure to 10 μM cisplatin whereas only 2% decrease was measured at this concentration by trypan blue which identifies dead cells. Both assays support the selection of 10 μM dose for studying effects of cisplatin while avoiding significant loss of DRG neurons (Supplementary Fig 1a and 1b, respectively).
Status and purity of DRG neuron cultures was assessed by labeling with DAPI (blue) in conjunction with the DRD specific high molecular weight cytoskeleton protein, NF200 [red]. Immunoreactivity of the anti-DNA antibody [green] served surrogate for the extent of nuclear DNA damage. Strong immunofluorescence of nuclear DNA [green, center panel] indicates chromatin rearrangements that facilitate greater accessibility of the anti-DNA antibody to nuclear DNA. Immunoreactivity is reduced if meclizine is preset during exposure, suggestive of lesser DNA damage. Merged images are shown in the bottom panel.